Abstract
BACKGROUND:
Osteoarthritis (OA) is one of the most common diseases among the elderly. OA occurs due to an imbalance between degradation and synthesis in articular joint tissue, causing changes in joint components such as cells, matrices and molecular production. Therefore, knowledge of cartilage-degrading enzymes such as ADAMTS-4 and iNOS is needed.
AIM:
This study aims to prove the effect of Mesenchymal Stem Cell Wharton Jelly on decreasing ADAMTS-4 levels as cartilage-degrading enzymes and increasing levels of iNOS which showed the immunosuppressive potential of MSC-WJ in cases of osteoarthritis in vivo.
MATERIAL AND METHODS:
This research is an experimental study with the design of Post-test-Only Control Group Design. The sample consisted of 16 OA rats as a control group and 16 OA rats treated with MSC-WJ as a treatment group. OA induction is done by injection of monosodium iodoacetate (MIA) into the intra-articular right knee. Giving MSC-WJ is done in the third week after MIA induction. The serum ADAMTS-4 and iNOS levels were measured after 3 weeks treated with MSC-WJ using the ELISA method. The statistical test used is an independent t-test. The value of p < 0.05 was said to be statistically significant.
RESULT:
The result showed that serum ADAMTS-4 levels were lower in the group treated with MSC-WJ than in the control group, but not statistically significant (p > 0.05). Serum iNOS levels were higher in the group treated with MSC-WJ than in the control group (p < 0.05).
CONCLUSION:
This study concluded that MSC-WJ significantly reduced ADAMTS-4 levels and increased the serum iNOS levels of OA rats.
Keywords: ADAMTS-4, iNOS, Mesenchymal Stem Cell Wharton Jelly, Osteoarthritis
Introduction
Osteoarthritis (OA) is a disorder of the joint joints characterised by cell stress and extracellular matrix degradation triggered by micro and macro injuries. This disease manifests first as molecular disorder followed by anatomical abnormalities, and physiology (characterised by cartilage degradation, bone remodelling, osteophyte formation, joint inflammation and loss of normal joint function), which can lead to disease [1].
Molecular disorders in osteoarthritis activate the pro-inflammatory pathway, causing an increase in expression of inflammatory mediators such as IL-1β and TNF-α. A Disintegrin-like and Metalloproteinases with Thrombospondin Motifs-4 (ADAMTS-4) is aggrecanase which is responsible for aggrecanolysis in OA [2], [3], [4]. Fan et al., (2005) reported that IL-1β can improve ADAMTS-4 regulation in both normal human chondrocytes and OA [5]. Bondeson et al., (2007) showed that IL-1β increased regulation of ADAMTS-4 in synovial OA in human fibroblasts [6].
Mesenchymal Stem Cells (MSC) has the potential for multipotent differentiation for regenerative medicine [7] and has the strong immunosuppressive capacity, so it has therapeutic potential for various inflammatory-related diseases [8], [9], [10]. The immunosuppressive ability of MSC is shown by the increase in nitric oxide (NO) products which play a major role in inhibiting T-cell proliferation [10]. Inducible nitric oxide synthase (iNOS) is an enzyme that plays a role in NO synthesis. Inducible nitric-oxide synthase induced MSC after activation by IFN IF and TNFα, IL-1α or IL-1β. MSC of iNOS-/- rat has a low ability to suppress T-cell proliferation [8].
This study aims to prove the effect of Mesenchymal Stem Cell Wharton Jelly on decreasing ADAMTS-4 levels as cartilage-degrading enzymes and increasing levels of iNOS which showed the immunosuppressive potential of MSC-WJ in cases of osteoarthritis in vivo.
Material and Methods
Animal and Experimental Design
Male white rats (Rattus novergicus) with a weight ranging from 200-250 grams as experimental animals placed in clean, disinfected and pathogen-free cages and given standard food in the form of pellets and drinking in ad libitum. Trial animals adapted first for 1 week before treatment. Induction of osteoarthritis conducted with 300 μg intra-articular injection of monosodium iodoacetate (MIA) (Sigma Aldric, USA) in 50 μl of saline solution (0.9% NaCl) sterile [11] singly into the right knee joint rats anaesthetized by intraperitoneal injection of xylazine 10 mg/kg and ketamine 20 mg/kg uses insulin syringe with a needle (needle) 27G [12]. 32 osteoarthritis male white rats (three weeks after MIA induction) were divided into 2 treatment groups (n = 16): Control group and MSC-WJ group. MSC-WJ group is given 50 μl MSC-WJ with a dose of 1 x 106 cells into the right knee joint and a control group given 50 μl complete medium after anaesthetized. Rats were sacrificed after 3 weeks of treatment. Serum and knee joint were taken and then analyzed.
Analysis of Flow Cytometry
Mesenchymal Stem Cell Wharton Jelly was obtained from the Indonesian Medical Education and Research Institute (IMERI) Faculty of Medicine, University of Indonesia. Based on the analysis of flow cytometry, MSC-WJ used for this therapy had CD73-APC cell surface expression 99.8%, CD105-PerCP-Cys5.5 95% and CD90-FITC 99.9%. Photocell was taken use Nikon Ti-S microscope. Scale bar: 500 μm.
Measurement of serum ADAMTS-4 and iNOS by ELISA
Blood was taken from sinus periorbital and centrifuged at 3000 rpm for 15 minutes. The collected serum was stored at -80°C until measurement. Serum ADAMTS-4 and iNOS levels were measured by an ELISA kit (Bioassay Technology Laboratory, China). All samples are measured in duplicate.
Examination of ADAMTS-4 Levels (Work protocol based on rat ADAMTS-4 ELISA Kit)
Prepare all reagents, standard solutions and samples as instructed. Bring all reagents to room temperature before use. The assay is performed at room temperature. Determine the number of strips required for the assay. Insert the strips in the framers for use. The unused strips should be stored at 2-8°C. Add 50 µL standard well. Add 40 µL sample to sample wells and then add 10 µL anti-ADAMTS-4 antibody to sample wells, then add 50 µL streptavidin-HRP to sample wells and standard wells (Not blank control well). Mix well. Cover the plate with a shaker. Incubate 60 minutes at 37°C. Removed the sealer and wash the plate 5 times with wash buffer. Soak wells with at least 0,35 ml wash buffer for 30 seconds to minute for each wash. For automated washing, aspirate all wells and wash 5 times with wash buffer, overfilling wells with wash buffer. Blot the plate onto paper towels or other absorbent material. Add 50 µL substrate solution A to each well and then add 50 µL substrate solution B to each well. Incubate plate covered with a new sealer for 10 minutes at 37°C in the dark. Add 50 µL stop solution to each well; the blue colour will change into yellow immediately. Determine the optical density (OD value) of each well immediately using a microplate reader set a 450 nm within 30 min after adding the stop solution.
Examination of iNOS Levels (Work protocol based on rat iNOS ELISA Kit)
Prepare all reagents, standard solutions and samples as instructed. Bring all reagents to room temperature before use. The assay is performed at room temperature. Determine the number of strips required for the assay. Insert the strips in the framers for use. The unused strips should be stored at 2-8°C. Add 50 µL standard well. Add 40 µL sample to sample wells and then add 10 µL anti-iNOS antibody to sample wells, then add 50 µL streptavidin-HRP to sample wells and standard wells (Not blank control well). Mix well. Cover the plate with a shaker. Incubate 60 minutes at 37°C. Removed the sealer and wash the plate 5 times with wash buffer. Soak wells with at least 0,35 ml wash buffer for 30 seconds to minute for each wash. For automated washing, aspirate all wells and wash 5 times with wash buffer, overfilling wells with wash buffer. Blot the plate onto paper towels or other absorbent material. Add 50 µL substrate solution A to each well and then add 50 µL substrate solution B to each well. Incubate plate covered with a new sealer for 10 minutes at 37°C in the dark. Add 50 µL stop solution to each well; the blue colour will change into yellow immediately. Determine the optical density (OD value) of each well immediately using a microplate reader set a 450 nm within 30 min after adding the stop solution.
Research Ethics
This study was already passed the ethics clearance and has been approved by the Ethics Committee of the Faculty of Medicine, Andalas University, Padang with registration number: 549/KEP/FK/2017.
Statistical analysis
Data are presented in mean and elementary forms. The statistical analysis used is SPSS 18.0. The statistical test used is an independent t-test. The value of p <0.05 was said to be statistically significant.
Results
OA rats were divided into 2 groups, namely the control group and the group treated with MSC-WJ (Figure 1). Examination of the levels of ADAMTS-4 and iNOS was carried out in the serum of rats by ELISA.
Figure 1.
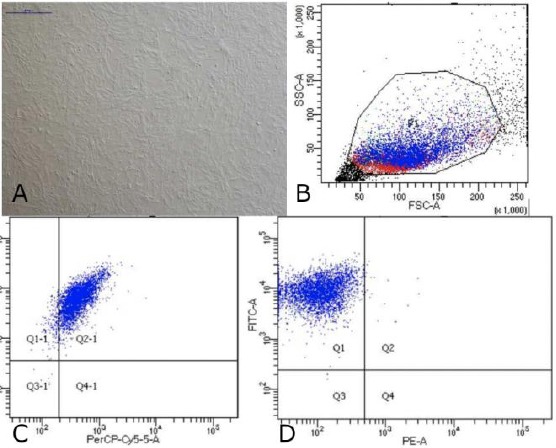
Data on Characteristics of Mesenchymal Stem Cells Wharton Jelly; A) Cells MSC-WJ reach confluence. Scale bar: 500 μM. Photographs of cells taken using a Nikon Ti-S microscope; B) Data flow cytometry. Forward scatter (FCS) plot&side scatter (SSC) plot. Population gated events (P1): 20,000; C) Cell surface markers expression: CD73-APC 99.8% and CD105- PerCP-Cy5.5 95%; D) Cell surface markers expression: CD90-FITC 99.9% and Lin (-) - PE 0.4%
ELISA examination
The blood obtained from the centrifuged animal is then obtained serum. Serum before analysis was stored in a refrigerator temperature of -80°C. The serum obtained was determined by ADAMTS-4 and iNOS levels, carried out in the Biomedical laboratory, Faculty of Medicine, Andalas University.
The results of the measurement of ADAMTS-4 and iNOS levels were carried out in normal rat, and the mean levels of ADAMTS-4 and iNOS were 27.92 ng/ml and 20.86 ng/ml. Based on the results of the normality test the data shows that the two research variables namely ADAMTS-4 and iNOS are normally distributed (p > 0.05). Thus, the parametric test (free t-test) can then be carried out.
Effect of MSC-WJ on ADAMTS-4 levels in serum of OA rats
The results of the measurement of ADAMTS-4 levels by ELISA method showed that the serum ADAMTS-4 levels of OA rats treated with MSC-WJ were lower than those not treated which can be seen in Figure 2.
Figure 2.
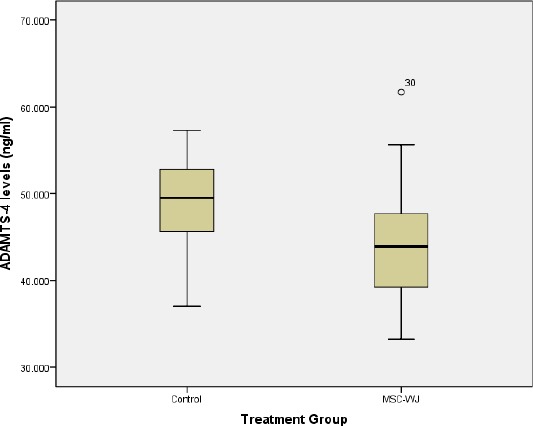
Boxplot graph of rat serum ADAMTS-4 levels
The difference in ADAMTS-4 levels between the serum of rats treated with MSC-WJ and not treated bivariate test can be seen in Table 1.
Table 1.
Mean differences in ADAMTS-4 levels by group
| Groups | ADAMTS-4 Levels (ng/ml) (Mean ± SD) |
P value |
|---|---|---|
| Control | 47.63 ± 5.32 | 0.114 |
| MSC-WJ | 43.89 ± 7.50 |
Table 1 showed that there are differences in levels of ADAMTS-4 based on treatment. Decreased levels of ADAMTS-4 in the group treated with MSC-WJ from the control group. Statistically, the differences were not significant (p > 0.05).
Effect of MSC-WJ on iNOS levels in serum of OA rats
The results of measurement of iNOS levels by ELISA method showed that the serum iNOS levels of OA rats treated with MSC-WJ were lower than those not treated with bivariate tests which can be seen in Figure 3.
Figure 3.
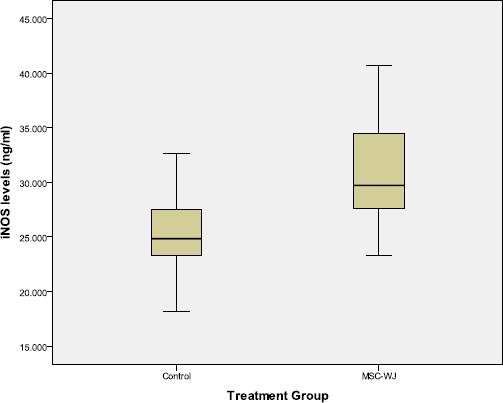
Boxplot graph of rat serum iNOS levels
The difference in iNOS levels between the serum of rats treated with MSC-WJ and not treated bivariate test can be seen in Table 1.
Table 2 showed that there are differences in levels of iNOS based on treatment. Increased levels of iNOS in the group treated with MSC-WJ from the control group. Statistically, the differences were significant (p <0.05).
Table 2.
Mean differences in iNOS levels by group
| Groups | iNOS Levels (ng/ml) (Mean ± SD) |
P value |
|---|---|---|
| Control | 24.96 ± 3.56 | 0.000 |
| MSC-WJ | 30.79 ± 4.64 |
Discussion
Aggrecanase-1 (ADAM-TS-4)
Aggrecanase-1 (ADAMTS-4) is mainly expressed in the active form in the osteoarthritis cartilage and plays an important role in the degradation of aggrecan in the cartilage of human osteoarthritis. ADAMTS-4 is overexpressed in human cartilage OA, and the expression of ADAMTS-4 in articular chondrocytes directly correlates with the degree of damage to the articular cartilage in OA. According to Naito et al., (2007), ADAMTS-4 is an aggrecanase expressed in human OA cartilage and plays a key role in aggrecan degradation in humans OA [13].
Increased regulation of the expression of the ADAMTS-4 gene (aggrecanase-1) in OA was induced by IL-1. Interleukin-1β activates the NF-kB cascade in chondrocytes and kills almost all anabolic pathways, including collagen type II and aggrecan synthesis [14] and increases catabolic pathways.
The results of this study indicate that the levels of ADAMTS-4 serum OA rats treated with MSC-WJ were lower than those not treated, but the difference was not significant. While the results of the research by Shu et al., (2016) showed that synovial ADAMTS-4 expression decreased significantly in OA joints after 6 weeks injected MSC compared with those not injected MSC [15].
The results of van Buul et al., (2012) show that MSC can reduce IL-1β gene expression in synovial and cartilage tissue (16). MSC can increase anti-inflammatory cytokines which can inhibit the NF-kB cascade, thereby reducing catabolic pathways [17]. This causes MSC-WJ to have the ability to reduce serum ADAMTS-4 levels, which is one of the catabolic factors responsible for the occurrence of OA [17]. Although in this study there was a tendency to decrease ADAMTS-4 levels after MSC-WJ treatment, it did not reach statistical significance. This is probably due to the not optimal incubation time for MSC-WJ.
Inducible nitric oxide synthase (iNOS)
Inducible nitric oxide synthase (iNOS) is an enzyme responsible for the production of nitric oxide (NO), the main proinflammatory and destructive mediator in osteoarthritis (OA). INOS expression increases regulation by inflammatory cytokines including IL-1β, IL-17, TNF-α, ΙFN-γ [18].
Mesenchymal stem cells can act as an anti-inflammatory by reducing the production of proinflammatory cytokines which will directly inhibit the function and proliferation of T cells. Also, MSC has a potent immunosuppressive capacity. In inflammatory conditions, MSC of rats expresses high levels of iNOS, which inhibits immune cell proliferation and function [19]. Immunosuppressive effects occur through enzymatic actions such as inducible nitric oxide synthase (iNOS) and Indoleamine 2, 3-dioxygenase (IDO), and through the production of human leukocyte antigen class I (HLA-G) and prostaglandin E2 (PGE2) [20], [21].
The results showed that the iNOS levels of serum OA mice treated with MSC-WJ were higher than those not treated. Research by Li et al., (2013) found that iNOS expression peaked at 1 week after being given HUC-MSC transplants in acute tubular necrosis (ATN) rat and then decreased to near normal values after 4 weeks of transplantation [22]. Yun et al., (2016) in their study found that MSC treatment of animals trying OA after 2 months can stimulate a decrease in the regulation of expression of inflammatory cytokines such as iNOS [17]. While the results of the study of Cosenza et al., (2017) in vitro, Mesenchymal stem cells reduce iNOS gene expression [23]. Xu et al., (2018) also obtained results of a decrease in iNOS expression using umbilical cord mesenchymal stem cells (UC-MSC) [24].
The increase in iNOS levels in this study was due to the immunosuppressive nature of MSC. The immunosuppressive function of MSC is caused by IFNγ along with one of three other proinflammatory cytokines, TNFα, IL-1α, or IL-1β. This cytokine combination provokes the expression of several chemokines and inducible nitric oxide synthase (iNOS) by MSC [9].
The presence of proinflammatory cytokines, MSC facilitates the high expression of iNOS which stimulates NO secretion, thus causing inhibition of T cell proliferation [25]. According to Ren et al., (2008), both in vivo and in vitro studies showed that iNOS-deficient MSC showed reduced inhibiting ability [9]. High NO concentrations can suppress immunity modulation and cause immune cell apoptosis through inhibition in the cell cycle phase G0/G1 [26], inhibition of phosphorylation of the transducer signal and activator of transcription 5 (STAT5) and signal transducers in T cells [27]. The results of this study showed an increase in iNOS levels after being treated by MSC-WJ. This situation shows that MSC-WJ is immunosuppressive.
This study concluded that MSC-WJ significantly reduced ADAMTS-4 levels and increased the serum iNOS levels of OA rats.
Footnotes
Funding: This research was funded by DIPA PNBP Medical Faculty of Andalas University, Ministry of Research, Technology and Higher Education with Research Contract Number: 90/BBPT/PNP/FK-UNAND-2018 Budget Year 2018
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Kraus VB, Blanco FJ, Englund M, Karsdal MA, Lohmander LS. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis Cartilage. 2015;23(8):1233–41. doi: 10.1016/j.joca.2015.03.036. https://doi.org/10.1016/j.joca.2015.03.036 PMid:25865392 PMCid:PMC4516635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bondeson J, Lauder S, Wainwright S, et al. Adenoviral gene transfer of the endogenous inhibitor IkBa into human osteoarthritis synovial fibroblasts demonstrates that several matrix metalloproteinases and aggrecanases are nuclear factor-kB-dependent. J Rheumatol. 2007;34:523–33. [PubMed] [Google Scholar]
- 3.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease :Potential Implication for the Selection of New Therapeutic Targets. Arthritis Rheum. 2001;44:1237–47. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. https://doi.org/10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Benito MJ, Veale DJ, Fitzgerald O, Van Den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–7. doi: 10.1136/ard.2004.025270. https://doi.org/10.1136/ard.2004.025270 PMid:15731292 PMCid:PMC1755629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan Z, Bau B, Yang H, Soeder S, Aigner T. Freshly isolated osteoarthritic chondrocytes are catabolically more active than normal chondrocytes, but less responsive to catabolic stimulation with interleukin-1ß. Arthritis Rheum. 2005;52:136–43. doi: 10.1002/art.20725. https://doi.org/10.1002/art.20725 PMid:15641077. [DOI] [PubMed] [Google Scholar]
- 6.Bondeson J, Lauder S, Wainwright S, et al. Adenoviral gene transfer of the endogenous inhibitor IkBa into human osteoarthritis synovial fibroblasts demonstrates that several matrix metalloproteinases and aggrecanases are nuclear factor-kB-dependent. J Rheumatol. 2007;34:523–33. [PubMed] [Google Scholar]
- 7.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–34. doi: 10.1002/jcp.21200. https://doi.org/10.1002/jcp.21200 PMid:17620285. [DOI] [PubMed] [Google Scholar]
- 8.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. https://doi.org/10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 9.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal Stem Cell-Mediated Immunosuppression Occurs via Concerted Action of Chemokines and Nitric Oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. https://doi.org/10.1016/j.stem.2007.11.014 PMid:18371435. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X, Huang Y, Zhu Z, Huang X, Han X, Xie N, Ren G. Mesenchymal stem cells:a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510–518. doi: 10.1038/cr.2010.44. https://doi.org/10.1038/cr.2010.44 PMid:20368733. [DOI] [PubMed] [Google Scholar]
- 11.van Buul GM, Siebelt M, Leijs MJC, Bos PK, Waarsing JH, Kops N, et al. Mesenchymal Stem Cells Reduce Pain But Not Degenerative Changes in a Mono-Iodoacetate Rat Model of Osteoarthritis. J Orthop Res. 2014;32:1167–1174. doi: 10.1002/jor.22650. https://doi.org/10.1002/jor.22650 PMid:24839120. [DOI] [PubMed] [Google Scholar]
- 12.Javanmard MZ, Asgari D, Karimipour M, Atabaki F, Farjah G, Niakani A. Mesenchymal Stem Cells Inhibit Proteoglycan Degeneration in a Rat Model of Osteoarthritis. Gene Cell Tisue. 2015;2(4) e31011:1–5. https://doi.org/10.17795/gct-31011. [Google Scholar]
- 13.Naito S, Shiomi T, Okada A, Kimura T, Chijiiwa M, Fujita Y, Yatabe T, Komiya K, Enomoto H, Fujikawa K, Okada Y. Expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic cartilage. Pathology International. 2007;57:703–711. doi: 10.1111/j.1440-1827.2007.02167.x. https://doi.org/10.1111/j.1440-1827.2007.02167.x PMid:17922681. [DOI] [PubMed] [Google Scholar]
- 14.Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013;141:1–10. doi: 10.1038/nrrheum.2013.141. https://doi.org/10.1038/nrrheum.2013.141. [DOI] [PubMed] [Google Scholar]
- 15.Shu C C, Ravi V, Zaki S, Smith S M, Schiavinato A, Smith MM, Little CB. The Effects Of Intra-Articular Injection Of Mesenchymal Stem Cells Versus Hyaluronan Hexadecylamide- Derivative on Post-Traumatic OA:The Relationship Between Synovial Inflammation, Structural Pathology and Pain Sensitisation. Osteoarthritis and Cartilage. 2016;24:S63–S534. https://doi.org/10.1016/j.joca.2016.01.580. [Google Scholar]
- 16.van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, et al. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthritis Cartilage. 2012;20:1186–1196. doi: 10.1016/j.joca.2012.06.003. https://doi.org/10.1016/j.joca.2012.06.003 PMid:22771777. [DOI] [PubMed] [Google Scholar]
- 17.Yun S, Ku SK, Kwon YS. Adipose-derived mesenchymal stem cells and platelet-rich plasma synergistically ameliorate the surgical-induced osteoarthritis in Beagle dogs. Journal of Orthopaedic Surgery and Research. 2016;11(9):1–12. doi: 10.1186/s13018-016-0342-9. https://doi.org/10.1186/s13018-016-0342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonidou A, Lepetsos P, Mintzas M, Kenanidis E, Macheras G, Tzetis M, Potoupnis M, Tsiridis E. Inducible nitric oxide synthase as a target for osteoarthritis treatment. Expert Opinion on Therapeutic Targets. 2018:1–20. doi: 10.1080/14728222.2018.1448062. https://doi.org/10.1080/14728222.2018.1448062. [DOI] [PubMed] [Google Scholar]
- 19.Xu C, Ren G, Cao G, Chen Q, Shou P, Zheng C, Du L, Han X, Jiang M, Yang Q, Lin L. miR-155 regulates immune modulatory properties of mesenchymal stem cells by targeting TAK1-binding protein 2. Journal of Biological Chemistry. 2013;288(16):11074–9. doi: 10.1074/jbc.M112.414862. https://doi.org/10.1074/jbc.M112.414862 PMid:23449975 PMCid:PMC3630877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouffi C, Djouad F, Mathieu M, Noel D, Jorgensen C. Multipotent mesenchymal stromal cells and rheumatoid arthritis:risk or benefit? Rheumatology. 2009;48(10):1185–9. doi: 10.1093/rheumatology/kep162. https://doi.org/10.1093/rheumatology/kep162 PMid:19561159. [DOI] [PubMed] [Google Scholar]
- 21.Gieseke F, Böhringer J, Bussolari R, Dominici M, Handgretinger R, Müller I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood. 2010;116:3770–3779. doi: 10.1182/blood-2010-02-270777. https://doi.org/10.1182/blood-2010-02-270777 PMid:20644118. [DOI] [PubMed] [Google Scholar]
- 22.Li F, Xiong F, Zhang Y, Li Y, Zhao H, Cho SC, Ichim TE, Yang X, Hu X. Therapeutic effects of human umbilical cord-derived mesenchymal stem cells against acute tubular necrosis quantified through measures of iNOS, BMP-7 and Bcl-2. Open Journal of Regenerative Medicine. 2013;2(02):31–38. https://doi.org/10.4236/ojrm.2013.22006. [Google Scholar]
- 23.Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Scientific Reports. 2017;7(1):16214. doi: 10.1038/s41598-017-15376-8. https://doi.org/10.1038/s41598-017-15376-8 PMid:29176667 PMCid:PMC5701135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Luo H, Chen F, Shi Y, Sun M. Human umbilical cord mesenchymal stem cells polarize RAW264.7 macrophages to an anti-inflammatory subpopulation. Int J Clin Exp Pathol. 2018;11(3):1446–1452. [PMC free article] [PubMed] [Google Scholar]
- 25.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. https://doi.org/10.1182/blood-2006-02-002246 PMid:16985180. [DOI] [PubMed] [Google Scholar]
- 26.Carrade Holt DD, Wood JA, Granick JL, Walker NJ, Clark KC, Borjesson DL. Equine mesenchymal stem cells inhibit T cell proliferation through different mechanisms depending on tissue source. Stem Cells Dev. 2014;23:1258–1265. doi: 10.1089/scd.2013.0537. https://doi.org/10.1089/scd.2013.0537 PMid:24438346. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Yuan Q, Xie L. Mesenchymal Stem Cell-Based Immunomodulation:Properties and Clinical Application. Stem Cells International. 2018 doi: 10.1155/2018/3057624. https://doi.org/10.1155/2018/3057624. [DOI] [PMC free article] [PubMed] [Google Scholar]


