Abstract
Background
White matter hyperintensities (WMH) represent ischemic white matter damage in late-life depression (LLD) and are associated with cognitive control dysfunction. Understanding the impact of WMH on the structural connectivity of gray matter and the cognitive control correlates of WMH-related structural dysconnectivity can provide insight into the pathophysiology of LLD.
Methods
We compared WMH burden and performance on clinical measures of cognitive control in patients with LLD (N = 44) and a control group of non-depressed older adults (N = 59). We used the Network Modification (NeMo) Tool to investigate the impact of WMH on structural dysconnectivity in specific gray matter regions, and how such connectivity was related to cognitive control functions.
Results
Compared to the control group, LLD participants had greater WMH burden, poorer performance on Trail Making Test (TMT) A & B, and greater self-reported dysexecutive behavior on the Frosntal Systems Behavior Scale-Executive Function subscale (FrSBe-EF). Within the LLD group, disrupted connectivity in the left supramarginal gyrus, paracentral lobule, thalamus, and pallidum was associated with psychomotor slowing (TMT-A). Altered connectivity in the left supramarginal gyrus, paracentral lobule, precentral gyrus, postcentral gyrus, thalamus, and pallidum was associated with poor attentional set-shifting (TMT-B). A follow-up analysis that isolated set-shifting ability (TMT-B/A ratio) confirmed the association with dysconnectivity in the bilateral paracentral lobule, right thalamus, left precentral gyrus, postcentral gyrus, and pallidum; additionally, it revealed associations with dysconnectivity in the right posterior cingulate, and left anterior cingulate, middle frontal cortex, and putamen.
Conclusions
In LLD, WMH are associated with region-specific disruptions in cortical and subcortical gray matter areas involved in attentional aspects of cognitive control systems and sensorimotor processing, which in turn are associated with slower processing speed, and reduced attentional set-shifting.
Clinical trials registration
Keywords: Late life depression, cognitive control, executive functions; White matter hyperintensities; Structural connectivity; MRI
Abbreviations: ARWMC, Age-Related White Matter Changes scale; ChaCo, Change in Connectivity; DRS-2, Dementia Rating Scale-2; FDR, False Discovery Rate; FrSBe-EF, Frontal System Behavioral Scale-Executive Function; GAD, General Anxiety Disorder; HDRS, Hamilton Depression Rating Scale; ICC, Intraclass correlation coefficient; LLD, Late-life depression; MDD, Major Depressive Disorder; MMSE, Mini Mental State Examination; NeMo, Network Modification tool; PLSR, Partial least square regression; TMT, Trail Making Test; TMT-B/A, Trail Making Test, ratio of B over A; VIP, Variable importance in the projection; WMH, White Matter Hyperintensities; WMHr, White Matter Hyperintensities ratio
Highlights
-
•
Older depressed adults demonstrate psychomotor slowing and poor set-shifting
-
•
Psychomotor slowing and poor set-shifting relate to WMH-linked dysconnectivity
-
•
Structural dysconnectivity in dorsal attention, cognitive-control, and sensorimotor regions implicated.
1. Introduction
Intact white matter consisting of myelinated axons allows efficient information transfer between brain regions and supports cognitive and emotional processes (Filley, 2005). White matter is particularly vulnerable to aging (Bennett and Madden, 2014; Hinman and Abraham, 2007), and disruption of white matter has been documented in a variety of mood disorders (Fields, 2008; Filley and Fields, 2016). In particular, late-life depression (LLD) has been conceptualized as a “disconnection syndrome” (Taylor et al., 2013) originating from white matter pathology contributed by vascular, inflammatory, and other processes (Aizenstein et al., 2011; Alexopoulos et al., 2012).
White matter hyperintensities (WMH) are common, aging-related, magnetic resonance markers of white matter disruption (Aizenstein et al., 2011; Reijmer et al., 2015; Taylor et al., 2001). Both post mortem and neuroradiological studies have reported high WMH burden in LLD (Gunning-Dixon et al., 2010; Krishnan et al., 1997; van Agtmaal et al., 2017), which is often of cerebrovascular origin and reflects ischemic processes (Thomas et al., 2002). Investigation of the impact of WMH on structural network connectivity may clarify some of the mechanisms by which WMH are related to the frequent cognitive control abnormalities of LLD that confer risk for poor antidepressant response and poor functional outcomes.
One prominent clinical expression of inefficient cognitive control in LLD is executive dysfunction. While LLD is associated with deficits in a broad array of cognitive domains (Bhalla et al., 2006), converging data have established a particular relation between increased WMH and executive dysfunction in LLD both cross-sectionally (Vasudev et al., 2012; Lesser et al., 1996) and longitudinally (Kohler et al., 2010). The executive dysfunction of LLD is characterized by a reduced ability to process information efficiently, think flexibly and adaptively, and initiate goal-directed activities while inhibiting irrelevant stimuli (Kohler et al., 2010). Executive dysfunction of LLD has a negative impact on activities of daily living (Alexopoulos et al., 2002) and increases disability (Naarding et al., 2007).
Recent studies have investigated the impact of WMH in older adults on connectivity at the network level. These studies have begun to investigate how network disruptions contribute to poor executive functions. Among non-depressed older adults, WMH are associated with altered functional connectivity in the frontoparietal attention network (Lockhart et al., 2015) and in the default mode network (Wu et al., 2011), the latter of which is linked to executive dysfunction (Reijmer et al., 2015). At the structural network level, WMH burden in LLD decreases the “resilience” of structural networks as defined by a graph-theoretical metric (Ajilore et al., 2014). In LLD, WMH may preferentially affect specific white matter fibers tracts, such as the superior longitudinal fasciculus and the uncinate fasciculus (Sheline et al., 2008). WMH in these two fiber tracts are tied to poor executive functions (Sheline et al., 2008) and reward learning (Dombrovski et al., 2015).
Understanding the impact of WMH on structural connectivity at the network level may elucidate the specific cortical and subcortical gray matter networks contributing to the executive difficulties often observed in LLD. Thus, the primary goal of this study was to identify WMH-linked, region-specific alterations in structural connectivity and their associations with executive performance and self-reported dysexecutive behavior in older adults with major depression. We hypothesized that relative to non-depressed participants, LLD patients would have poorer executive functions and higher WMH burden. We further hypothesized that within the LLD group, WMH-related dysconnectivity in gray matter regions comprising aspects of cognitive control networks would be associated with slowed processing speed, poorer attentional set-shifting and cognitive inhibition, and dysexecutive behavior.
2. Materials and methods
2.1. Participants
This study included participants with LLD and non-depressed participants, aged 60 to 85 years. The LLD group met DSM-IV criteria for major depression without psychotic features, had a 24-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960) score ≥ 18, and had a Mini Mental Status Examination (MMSE) score ≥ 26 (Folstein et al., 1975). Participants in the comparison group had no history or presence of any psychiatric disorder. All subjects were recruited through flyers and advertisements and all signed informed consent approved by the Weill Cornell Medical College and the Nathan Kline Institute Institutional Review Boards. Exclusion criteria were: any current or past Axis I psychiatric disorder other than Major Depressive Disorder (MDD) or Generalized Anxiety Disorder (GAD); high suicide risk; dementia by DSM-IV criteria; acute or severe medical illness; history of neurological diseases; history of electroconvulsive therapy; ongoing treatment with drugs associated with depression (e.g. steroids, alpha-methyl-dopa, clonidine, reserpine, tamoxifen, or cimetidine); and any contraindications to MRI scanning.
2.2. Assessment
Each depressed participant was evaluated by a study psychologist and study psychiatrist. LLD patients were not on antidepressants at the time of study procedures. Those who were previously prescribed an antidepressant underwent a one-week washout procedure under the care of a study psychiatrist. Research assistants trained and certified by the Weill Cornell Institute of Geriatric Psychiatry administered the clinical rating scales and the neuropsychological tests.
DSM-IV diagnosis was assigned based on the Structured Clinical Interview for DSM-Revised. Severity of depression was based on the HDRS (Hamilton, 1960), and overall cognitive impairment was assessed with the MMSE (Folstein et al., 1975). Upon entering the study, participants were administered the Trail Making Test (TMT) to assess psychomotor speed (TMT-A) and attentional set-shifting (TMT-B) (Reitan, 1958). The Stroop Color Word Test, interference index (Stroop, 1935) was used to assess cognitive inhibition (Golden and Freshwater, 2002). The interference index was calculated based on the following established formula, ColorWord – [(Word x Color) / (Word + Color)]. The Dementia Rating Scale-2 (DRS-2), Initiation/Perseveration scale was also used as a measure of executive function. Dysexecutive behavior was rated with the self-reported, executive function subscale from the Frontal Systems Behavior Scale (FrSBe-EF) (Grace, 2011). The FrSBe is a self-report inventory that assesses a broad range of behavior associated with frontal network and frontal-subcortical systems dysfunction. Behavior is rated within the domains of apathy, disinhibition, and executive dysfunction. Frequency of behavior is rated on five-point Likert-scale items. Example items from the EF subscale include “cannot do two things at once” and “am inflexible, unable to change routines”.
2.3. MRI data acquisition
MRI scans were acquired on a 3 T Siemens TiM Trio (Erlangen, Germany) equipped with a 32-channel head coil at the Center for Biomedical Imaging and Neuromodulation of the Nathan Kline Institute for Psychiatric Research. Anatomical imaging included a turbo dual echo scan, high-resolution whole brain images acquired using a 3D T1-weighted MPRAGE and T2-weighted FLAIR images. The acquisition parameters were respectively: MPRAGE: TR = 2500 ms, TE = 3.5 ms, slice thickness = 1 mm, TI = 1200 ms, 192 axial slices, matrix = 256 × 256 (voxel size = 1 mm isovoxel), FOV = 256 mm, IPAT = 2, flip angle = 8 degrees; FLAIR: TR = 9000 ms, TE = 111 ms, TI = 2500 ms, FOV = 192 × 256 mm, matrix 192 × 256, slice thickness = 2.5 mm, number of slices = 64, IPAT = 2, flip angle = 120 degrees. The FLAIR sequence had an in-plane resolution of 1x1mm.
2.4. WMH segmentation and WMH burden estimation
First, we performed a visual check on both T1 and FLAIR images to exclude subjects with macroscopic artifacts or neurological sequelae. Two raters (M.R., M.S.) independently performed a group-blinded visual rating of WMH severity using the operational criteria of the Age-Related White Matter Change scale (ARWMC) (Wahlund et al., 2001; Xiong et al., 2011). The inter-rater agreement was measured as intraclass correlation coefficient (ICC = 0.95). Imaging data analysis were performed using FSL (Jenkinson et al., 2012). FSL's BIANCA was used to segment WMH lesions (Griffanti et al., 2016). A training dataset of manually segmented WMH masks was created from 12 subjects' FLAIR images and used by BIANCA to segment WMH lesions in all subjects using a threshold of 0.8 for visually-defined high WMH burden, 0.9 for moderate and 0.99 for mild, based on ARWMC scores (<5, 5–9, ≥10). WMH lesions smaller than 3 voxels were deleted (Wahlund et al., 2001). A visual check was performed on each individual WMH mask for minimal manual adjustments. The WMH burden was calculated as the log transformed percentage of the ratio between WMH volume and intracranial volume (normalized for head size). Intracranial volume was obtained through a sum of gray matter, white matter, and ventricular cerebrospinal fluid (Heinen et al., 2016). Because WMH burden generally increases as a function of age, we examined if our WMH burden estimation was correlated with age and visual WMH rating using Spearman's Rank-Order Correlation method.
2.5. Estimation of WMH-associated connectome disruptions using the network modification tool
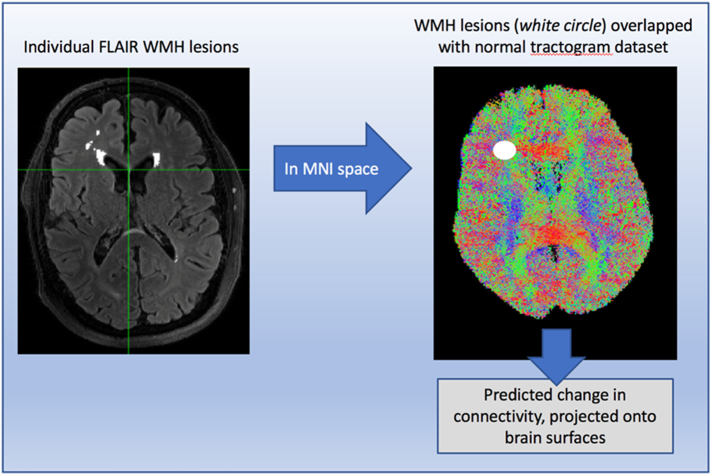
We applied the “Network Modification Tool” (NeMo) (Kuceyeski et al., 2013), a recently developed, Matlab-based software, which estimates the structural alterations in connectivity associated with WMH. Each final WMH mask was nonlinearly registered to MNI space, binarized, converted to its negative (0 outside, −1 inside the lesions) and then entered as input in NeMo. NeMo uses a free-access dataset of 73 healthy subjects tractograms (Tractogram Reference Set) to estimate the brain network's dysconnectivity pattern inferred by each set of WMH lesions within each patient. Through this process, NeMo estimates the white matter fibers that would be damaged by each set of lesions in normal tractograms, and provides this “loss in connectivity” for an atlas of cortical and subcortical regions. This alteration in connectivity is expressed as “Change in Connectivity” (ChaCo) scores, or the percent of streamlines connecting to a given region that pass through the WMH lesion. The “ChaCo” term was used to be consistent with prior studies that used the NeMo Tool, despite the cross-sectional nature and absence of longitudinal analysis in this study. We calculated ChaCo scores for all cortical and subcortical gray matter surfaces based on an 86-region Freesurfer parcellation. Details on the specific steps performed by NeMo are fully described in the original paper (Kuceyeski et al., 2013). A streamlined visual summary of the procedure is described in Fig. 1. More negative ChaCo values indicate lower connectivity relative to the reference tractogram set.
Fig. 1.
The Network Modification Tool.
2.6. Statistical analyses
Statistical analyses were performed on IBM SPSS Statistics version 25 and in Matlab R2017b. Independent samples t-tests and chi-square tests were used to investigate group differences in demographic and clinical variables, as well as for WMH burden. Two-tailed Spearman rho was calculated for correlations of WMH burden with age and ARWMC visual score. Within the LLD group, one-tailed Spearman correlations were performed between ChaCo scores and those cognitive variables that demonstrated a statistically significant group difference with the comparison group. We used one-tailed tests because our hypotheses were directional, i.e. lower connectivity (relative to the reference tractogram set) would be associated with poorer executive performance. To reduce the dimensionality of the correlation matrix (ChaCo scores for each of the 86 parcellated regions x behavioral performances on multiple executive functioning measures), as well as to reduce the noise of regions with minimal dysconnectivity (Kuceyeski et al., 2013; Pardini et al., 2015), we selected only regions: 1) with ChaCo scores greater than or equal to a minimum threshold of 1% in average connectivity disruption (Pardini et al., 2015); and 2) located within networks hypothesized to be associated with our outcome measures. These included cortical regions in the frontoparietal network, dorsal and ventral attention networks, and the sensorimotor network, as well as select subcortical regions (thalamus and basal ganglia). This process resulted in 29 regions of interest. All p-values were subjected to Benjamini Hochberg's false-discovery rate (FDR) correction (q < 0.05) (Benjamini and Hochberg, 1995) and those that survived are reported as uncorrected p values. Correlations between these 29 regions and behavior were repeated in the healthy control group.
Regions that survived FDR correction were, then, entered into separate partial least square regression (PLSR) models with covariates of age, education, and depression severity (HDRS). PLSR accounts for a large number of predictors and multicollinearity among predictor variables by extracting independent components. These components are subsequently regressed on the outcome of interest. PLSR is commonly used with the ChaCo metric (Kuceyeski et al., 2015; Kuceyeski et al., 2018). After computing PLSR models, in order to minimize model over-fitting, we chose the factor solution that minimized the mean square error using leave-one-out cross-validation, i.e., K-fold cross-validation where K was equal to the number of data points (44 for TMT-A and 43 for TMT-B). Predictor variables that loaded onto the components of interest were determined by inspecting the SPSS output factor loadings and variable importance in the projection (VIP). Regions of interest that had VIP values close to or exceeding 1 were considered to contribute to a given component.
3. Results
3.1. Descriptive findings
One hundred and three older adults were studied. Of these, 44 were participants with LLD and 59 were non-depressed older adults. One individual with LLD did not complete the TMT-B and three individuals with LLD did not complete the FrsBe-EF. Age and gender did not significantly differ between the groups (Table 1). LLD participants were less educated than the non-depressed group and had greater psychomotor slowing on the TMT-A, poorer executive performance (attentional set-shifting) on the TMT-B, and greater dysexecutive behavior on the FrSBe-EF. There was no statistically significant group difference in Stroop Interference, or on the DRS-2 Initiation/Perseveration subscale. Thus, TMT-A, TMT-B, and the FrSBe-EF were used in subsequent correlation and regression analyses.
Table 1.
Demographic and clinical characteristics of patients with late-life depression (LLD) and healthy control participants.a
| LLD | Healthy Control | Statistic | Significance (p) | |
|---|---|---|---|---|
| Age (years) | 72.5 (6.5) | 72.8 (6.0) | t = 0.26 | 0.79 |
| Education (years) | 15.0 (2.7) | 16.9 (2.2) | t = 3.81 | < 0.001a |
| Gender (M/F, number) | 16 / 28 | 26 / 33 | χ2 = 0.62 | 0.43 |
| HDRS (total) | 23.5 (4.5) | 1.3 (1.3) | t = 31.42 | < 0.001a |
| TMT-A (s) | 57.9 (45.4) | 40.5 (13.9) | t = 2.45 | 0.02a |
| TMT-B (s) | 127.5 (66.8) | 90.7 (36.5) | t = 3.27 | 0.002a |
| DRS-2 I/P (raw) | 36.3 (1.3) | 36.0 (3.1) | t = 0.72 | 0.47 |
| Stroop Interference | −3.3 (7.6) | −3.8 (7.7) | t = 0.29 | 0.78 |
| FrSBe-EF | 43.4 (11.5) | 25.8 (5.6) | t = 9.06 | < 0.001a |
| ARWMC Ratings | 4.7 (4.3) | 2.7 (3.0) | t = 2.65 | 0.01a |
| Log of WMHr percentage | −1.9 (1.1) | −2.4 (1.0) | t = 2.65 | 0.009a |
Denotes statistically significant difference. Values shown are mean (standard deviation) unless otherwise noted. ARWMC = age-related white matter changes scale; DRS-2 I/P = Dementia Rating Scale-2 Initiation/Perseveration scale; FrSBe-EF = Frontal Systems Behavior Scale-Executive Functions subscale; HDRS = Hamilton Depression Rating Scale; TMT = Trial Making Test; WMHr = White Matter Hyperintensities ratio.
3.2. Group differences in WMH
Relative to comparison participants, LLD patients had greater WMH burden as assessed through both visual ratings (ARWMC scale score) (t = 2.65, p = .01) and BIANCA automatic segmentation (expressed as natural log of WMH ratio percentage) (t = 2.65, p < .01). In the combined group of all participants, WMH burden was correlated with both visual ratings (rs = 0.83; p < .001) and age (rs = 0.33, p = .001). Similar correlations of WMH burden occurred within the LLD group (with visual rating: rs = 0.83, p < .001; with age: rs = 0.40, p = .007) and within the comparison group (with visual rating: rs = 0.84, p < .001; with age: rs = 0.32, p = .013).
3.3. Correlations between region-specific ChaCo scores and executive functions
Based on our a priori selection criteria, the following regions of interest were included in the correlation analyses: Bilateral caudal anterior cingulate, caudal middle frontal gyrus, paracentral lobule, pars opercularis, postcentral gyrus, precentral gyrus, rostral middle frontal gyrus, superior parietal cortex, supramarginal gyrus, insula, thalamus, caudate, putamen, and pallidum. The right posterior cingulate was also included. Within the LLD group, after FDR correction (q < 0.05), the following correlations between ChaCo and executive functioning were statistically significant. TMT-A correlated with ChaCo in the left supramarginal gyrus (rs = −0.49, p < .001), left paracentral lobule (rs = −0.43, p = .002), left precentral gyrus (rs = −0.42, p = .002), left postcentral gyrus (rs = −0.45, p = .001), left thalamus (rs = −0.44, p = .002), and left pallidum (rs = −0.38, p = .006). As depicted in Fig. 2, TMT-B was also associated with ChaCo in the left supramarginal gyrus (rs = −0.39, p = .005), left paracentral lobule (rs = −0.42, p = .002), left precentral gyrus (rs = −0.39, p = .005), left postcentral gyrus (rs = −0.40, p = .004), left thalamus (rs = −0.40, p = .004), and left pallidum (rs = −0.39, p = .005). The direction of all correlation coefficients indicated that greater alteration in connectivity to the above regions was associated with slower psychomotor speed and greater difficulty in set-shifting.
Fig. 2.
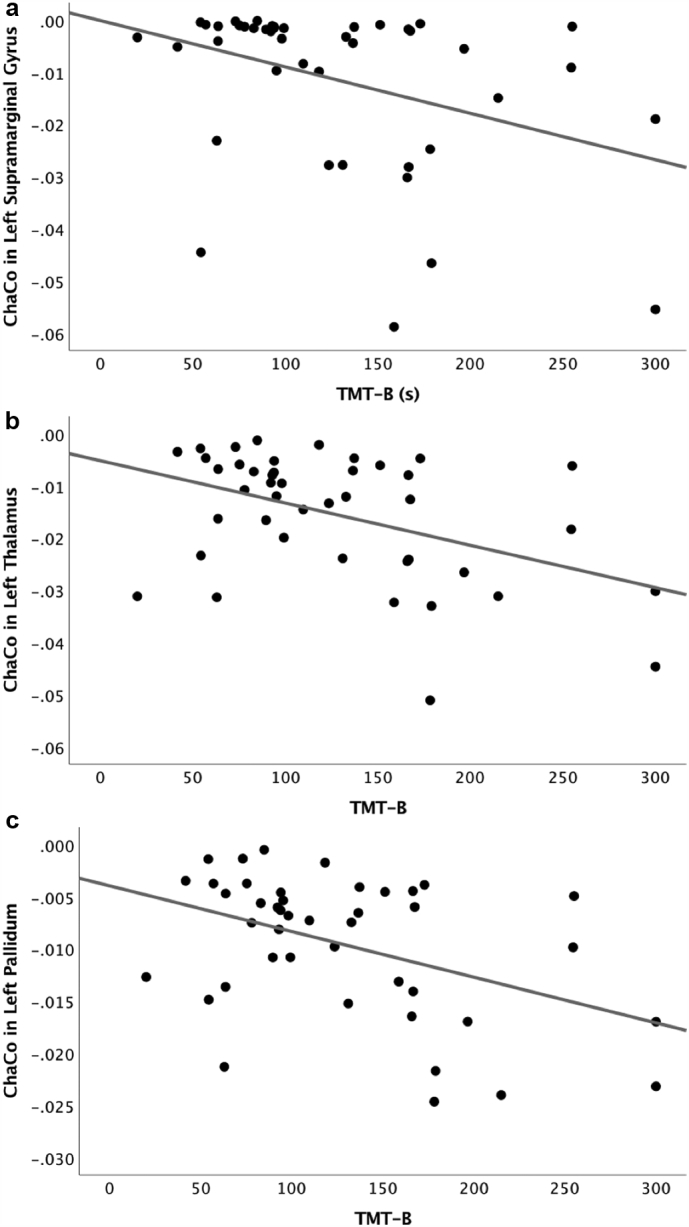
Scatterplots depicting the relationship between Trail Making Test-B (TMT-B) performance in seconds and change in connectivity (ChaCo) in the (a) left supramarginal gyrus, (b) left thalamus, and (c) left pallidum.
No correlations survived FDR correction for the FrSBe-EF in patients with LLD; thus, we report exploratory analyses for the FrSBe-EF in Supplement 1. No correlations were significant at the FDR-corrected threshold for TMT-A, TMT-B, or FrSBe-EF in the healthy control group.
3.4. Regression models
The PLSR model with TMT-A as the outcome revealed a one component solution, though the overall variance accounted for in TMT-A performance was relatively small (7%; Table 2). This component included ChaCo in the left supramarginal gyrus, left paracentral lobule, left thalamus, and left pallidum. With TMT-B as the outcome, results of the PLSR model indicated an optimal solution with two components. The first component accounted for the majority of variance (35%) and included ChaCo in the left supramarginal gyrus, left paracentral lobule, left precentral gyrus, left postcentral gyrus, left thalamus, left pallidum, and age. The second component accounted for an independent 17% of the variance and included years of education and depression severity (HDRS).
Table 2.
Results of partial least squares regression (PLSR) models with Trail Making Test A & B as the outcome variables. Age, education, and depression severity (Hamilton Depression Rating Scale [HDRS]) were entered into the models as covariates.
| Model | Number of PLSR Components | Adjusted R2 | PLSR Component Variables (Beta weights [association with outcome] in parentheses) |
|---|---|---|---|
| Trail Making Test A | 1 | 0.07 | Component 1: Left paracentral lobule (β = 0.06), left supramarginal gyrus (β = 0.60), left thalamus (β = 0.18), left pallidum (β = 0.24). |
| Trail Making Test B | 2 | 0.35 | Component 1: Left paracentral lobule (β = 0.12), left supramarginal gyrus (β = 0.34), left precentral gyrus (β = 0.03), left postcentral gyrus (β = 0.15), left thalamus (β = 0.23), left pallidum (β = 0.26), age (β = 0.21) |
| 0.17 | |||
| Component 2: Years of education (β = 0.44), depression severity (HDRS; β = 0.27) | |||
| Trail Making Test B/A Ratio | 1 | 0.13 | Component 1: right paracentral lobule (β = 0.12), left paracentral lobule (β = 0.36), left precentral cortex (β = 0.31), left postcentral cortex (β = 0.43), right posterior cingulate (β = 0.22), left caudal anterior cingulate (β = 0.23), left caudal middle frontal cortex (β = 0.32), right thalamus (β = 0.48), left putamen (β = 0.07), and left pallidum (β = 0.17) |
Because dysconnectivity in largely overlapping regions predicted performance on both TMT-A and TMT-B, as a follow-up analysis we verified the effect of dysconnectivity on set-shifting (separate from psychomotor speed) by conducting correlations and PLSR with the ratio of TMT-B/A as the outcome variable (Drane et al., 2002; Salthouse, 2011). We found significant correlations for the following ROIs that were largely consistent with our original TMT-B results: left supramarginal gyrus (rs = −0.27, p = .05), left paracentral lobule (rs = −0.36, p = .013), left precentral gyrus (rs = −0.38, p = .009), left postcentral gyrus (rs = −0.36, p = .013), and left pallidum (rs = −0.27, p = .045). Additional ROIs in cognitive control and default mode regions emerged as significantly associated with the B/A ratio (Fig. 3): left dorsal anterior cingulate cortex (rs = −0.27, p = .05), left middle frontal cortex (rs = −0.27, p = .05), left superior parietal cortex (rs = −0.33, p = .021), right posterior cingulate (rs = −0.33, p = .02), right thalamus (rs = −0.32, p = .03), right pallidum (rs = −0.29, p = .04), left putamen (rs = −0.27, p = .046), and right paracentral lobule (rs = −0.31, p = .03). We then conducted a follow-up PLSR model with TMT-B/A as the outcome, and these ROIs, age, education and depression as predictors. Cross-validated results indicated a one component solution accounting for 13% of the variance and comprised of ChaCo in the right paracentral lobule, left paracentral lobule, left precentral cortex, left postcentral cortex, right posterior cingulate cortex, left anterior cingulate cortex, left middle frontal cortex, right thalamus, left putamen, and left pallidum (Table 2).
Fig. 3.
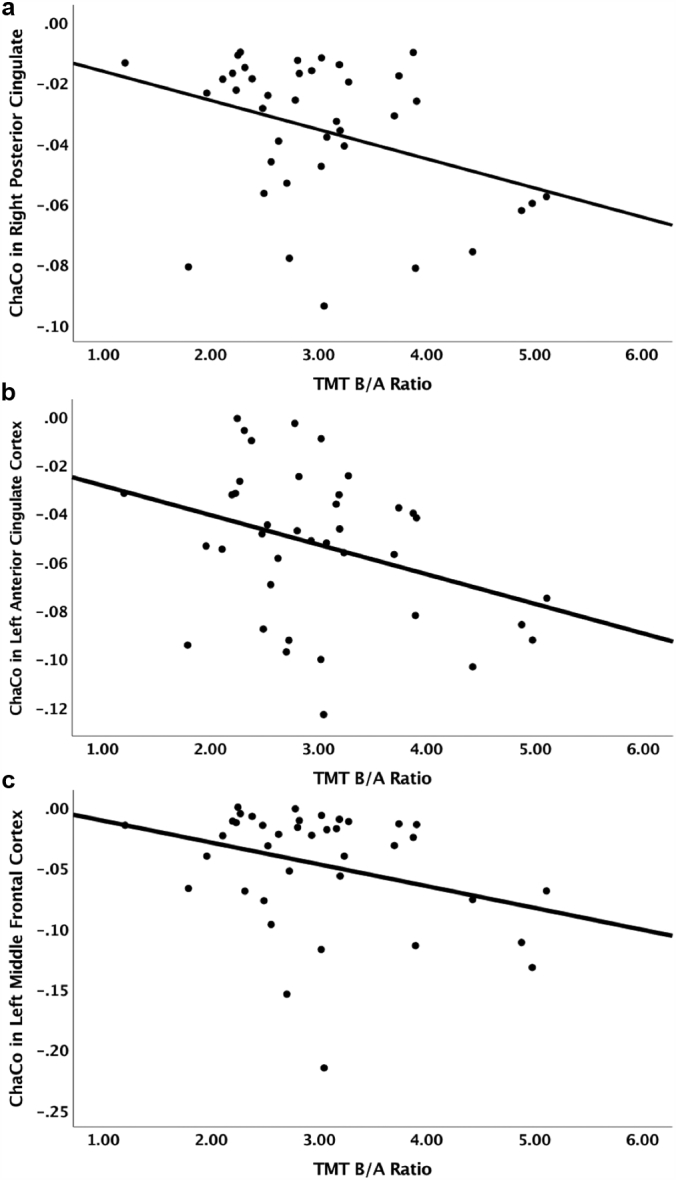
Scatterplots depicting the relationship between Trail Making Test-B/A ratio (TMT-B/A) and change in connectivity (ChaCo) in the (a) right posterior cingulate, (b) left anterior cingulate, and (c) left middle frontal cortex.
4. Discussion
The principal finding of this study is that WMH-related structural dysconnectivity in gray matter regions of cognitive-control, sensorimotor, and attentional networks is associated with poorer attentional set-shifting, slower processing speed, and dysexecutive behavior in individuals with LLD. To our knowledge this is the first study in LLD to identify relationships among WMH, associated structural gray matter dysconnectivity, and executive functions at both the performance and self-report levels of analysis.
Consistent with our hypotheses, the regions with altered connectivity that were associated with poorer executive functions were nodes of networks supporting attentional aspects of cognitive control. Regions of the sensorimotor network also emerged as predictors of executive functions. Notably, the variance accounted for in TMT-B was substantially greater than that accounted for in TMT-A, indicating that gray matter structural connectivity in these regions was more strongly associated with attentional set-shifting than with processing speed. Further analysis that attempted to better isolate the effects of set-shifting using the TMT-B/A ratio identified additional cognitive control and default mode network regions in which dysconnectivity was associated with poorer set-shifting performance.
Our results extend prior work on the cerebral structural correlates of the poor executive functions found in LLD to demonstrate that WMH are related to lower gray matter connectivity of the attention, sensorimotor, and select subcortical regions. Set-shifting, assessed by the TMT-B/A ratio score, was associated with regions known to be relevant in attentional aspects of cognitive control. The posterior cingulate cortex is a key node in the default mode network and is thought to be involved in arousal, attentional focus, and the shifting of attention from internal states to external cues (Leech and Sharp, 2014). The anterior cingulate cortex is a critical region of the cognitive control network relevant to salience processing, effortful regulation of attention, and error awareness and detection (Orr and Hester, 2012; Braver et al., 2001; Gasquoine, 2013). It has previously been implicated in animal studies of set-shifting (Bissonette et al., 2013). In human studies, white matter abnormalities have been demonstrated in the anterior cingulate in LLD (Gunning-Dixon et al., 2008). The middle frontal gyrus is associated with set shifting (Aron et al., 2004) and with resolving cognitive interference (Rahm et al., 2013).
Additionally, we found that dysconnectivity in the left supramarginal gyrus, a region within the inferior parietal lobe, emerged as a predictor of psychomotor processing speed and attentional set-shifting. The supramarginal gyrus has been previously implicated in attention difficulties in patients with LLD (Wang et al., 2008), as well as in post-stroke depression (Zhang et al., 2018). Further, the role of the supramarginal gyrus is consistent with prior work implicating the role of the superior longitudinal fasciculus in executive functioning in LLD, as this fiber tract connects frontal and parietal regions (Sheline et al., 2008).
Our results are also consistent with prior findings on the importance of the cortico-basal ganglia-thalamocortical loop to executive functions (Cummings, 1993; Pugh and Lipsitz, 2002), including in LLD (Sexton et al., 2013). In our study, altered thalamic, putamen, and pallidum connectivity emerged as significant predictors of attentional set-shifting. The thalamus is a well-known region implicated in executive control (Marzinzik et al., 2008). Altered thalamic functioning has been associated with executive dysfunction in lesion studies after stroke (Van Der Werf et al., 2003) and psychiatric disorders such as schizophrenia (Minzenberg et al., 2009). Similarly, pallidum projections to the striatum have been implicated in executive control performance (Wei and Wang, 2016), including set-shifting (Scott et al., 2002). Ischemic infarcts in the pallidum are associated with depression and executive dysfunction post-stroke (Vataja et al., 2005).
Dysconnectivity to gray matter regions of the sensorimotor network were also associated with set shifting in LLD. The sensorimotor network, which comprises regions involved in sensory, motor, and cognitive performance, is vulnerable to aging (Cassady et al., 2019). Individuals with greater WMH burden have been shown to express disrupted sensorimotor network functional connectivity (Wu et al., 2015). Our results suggest that dysconnectivity within sensorimotor regions is implicated perhaps because of the perceptual and motor demands that these tasks require in addition to attentional and executive control processes.
We observed preliminary evidence for an association between WMH-related structural dysconnectivity in the left superior parietal lobe and dysexecutive behavior (Supplement 1). This relationship did not survive FDR correction, and, thus, warrants replication. The left superior parietal region is critical to top-down, goal-driven attentional control (Corbetta et al., 1995; Alnæs et al., 2015), and has been previously implicated in dysexecutive behavior on the FrSBe (Alexopoulos et al., 2012). In our study, the region associated with dysexecutive behavior (FrSBe-EF) was distinct from those associated with executive functions on a performance-based measure (Trail Making Test), which is consistent with the notion that subjective and objective measures of executive impairment only partially overlap (Fava et al., 2018) and may be sustained by different underlying brain circuit pathology.
Taken together, our findings suggest broader implications for the treatment of LLD. WMH, poor executive performance, and self-reported dysexecutive behavior are all associated with poorer and slower response to antidepressants (Pimontel et al., 2016; Manning et al., 2015). Greater understanding of the gray matter networks affected by WMH-related structural dysconnectivity can inform clinical interventions designed to ameliorate cognitive control weaknesses. Such interventions can specifically target these network abnormalities using either cognitive (Anguera et al., 2017; Morimoto et al., 2016) and/or device-based neuromodulatory approaches (Dubin et al., 2017; Drysdale et al., 2017).
The limitations of our study include a relatively small sample size; further investigation with a larger sample is needed to confirm its findings. The battery of executive functions was rather limited and had minimal redundancy in tests tapping similar executive functions. However, the tests chosen have been extensively validated and are frequently used in neuropsychological evaluations in clinical settings. Further, the structural network disruption quantified by the NeMo Tool is based on a reference tractogram dataset from healthy adults. Nonetheless, prior work has used the same tractogram dataset to accurately quantify structural network dysfunction and related behavioral changes in geriatric populations including stroke, Alzheimer's disease, and frontotemporal dementia (Kuceyeski et al., 2013; Kuceyeski et al., 2015; Kuceyeski et al., 2012). Finally, because our sample of individuals with LLD were actively depressed, it is difficult to ascertain whether slowed processing, poor set-shifting, and dysexecutive behavior were secondary to depression symptoms or underlying cognitive impairment that would remain even after remission. A question for future research is whether our findings can be replicated in remitted adults with LLD.
4.1. Conclusions
In summary, this study demonstrated a link between WMH-related region-specific structural dysconnectivity in sensorimotor networks and cortical and subcortical regions involved in attentional aspects of cognitive control, and behavioral measures of attentional set-shifting and psychomotor speed. Our findings provide evidence for how cerebrovascular pathology may ultimately manifest as clinical symptoms of disrupted cognitive control networks and may inform novel treatment targets.
Acknowledgments
Acknowledgements
The authors thank Cristina Pollari for database management and Naib Chowdhury for assistance with behavioral data acquisition.
Financial disclosures/conflicts of interest
G.S. Alexopoulos has served on the speakers' bureaus of Allergan, Lundbeck, Otsuka, and Takeda. Drs. Respino, Jaywant, Kuceyeski, Hoptman, Victoria, Scult, Pimontel, Sankin, Liston, Belvederi Murri, and Gunning report no financial disclosures.
Roles of the funding sources
This work was supported by the National Institute of Mental Health (NIMH) grants R01 MH097735 (Gunning) and T32 MH019132 (Alexopoulos). The sponsor did not have any role in the study design, the acquisition, analysis, or interpretation of the data, the writing of the report, or the decision to submit the manuscript for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101852.
Appendix A. Supplementary data
Exploratory Analysis of the FrSBe-EF
References
- Aizenstein H.J., Andreescu C., Edelman K.L., Cochran J.L., Price J., Butters M.A. fMRI correlates of white matter hyperintensities in late-life depression. Am. J. Psychiatry. 2011;168:1075–1082. doi: 10.1176/appi.ajp.2011.10060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajilore O., Lamar M., Leow A., Zhang A., Yang S., Kumar A. Graph theory analysis of cortical-subcortical networks in late-life depression. Am. J. Geriatr. Psychiatry. 2014;22(2):195–206. doi: 10.1016/j.jagp.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos G.S., Kiosses D.N., Klimstra S., Kalayam B., Bruce M.L. Clinical presentation of the “depression-executive dysfunction syndrome” of late life. Am. J. Geriatr. Psychiatry. 2002;10(1):98–106. [PubMed] [Google Scholar]
- Alexopoulos G.S., Hoptman M.J., Kanellopoulos D., Murphy C.F., Lim K.O., Gunning F.M. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J. Affect. Disord. 2012;139(1):56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnæs D., Sneve M.H., Richard G., Skåtun K.C., Kaufmann T., Nordvik J.E. Functional connectivity indicates differential roles for the intraparietal sulcus and the superior parietal lobule in multiple object tracking. Neuroimage. 2015;123:129–137. doi: 10.1016/j.neuroimage.2015.08.029. [DOI] [PubMed] [Google Scholar]
- Anguera J.A., Gunning F.M., Areán P.A. Improving late life depression and cognitive control through the use of therapeutic video game technology: a proof-of-concept randomized trial. Depress Anxiety. 2017;34(6):508–517. doi: 10.1002/da.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Monsell S., Sahakian B.J., Robbins T.W. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127(7):1561–1573. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995;57(1):289–300. [Google Scholar]
- Bennett I.J., Madden D.J. Disconnected aging: cerebral white matter integrity and age-related differences in cognition. Neuroscience. 2014;276:187–205. doi: 10.1016/j.neuroscience.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla R.K., Butters M.A., Mulsant B.H., Begley A.E., Zmuda M.D., Schoderbek B. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am. J. Geriatr. Psychiatry. 2006;14(5):419–427. doi: 10.1097/01.JGP.0000203130.45421.69. Available from. [DOI] [PubMed] [Google Scholar]
- Bissonette G.B., Powell E.M., Roesch M.R. Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex. Behav. Brain Res. 2013;250:91–101. doi: 10.1016/j.bbr.2013.04.037. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver T.S., Barch D.M., Gray J.R., Molfese D.L., Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb. Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Cassady K., Gagnon H., Lalwani P., Simmonite M., Foerster B., Park D. Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. Neuroimage. 2019;186:234–244. doi: 10.1016/j.neuroimage.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L., Miezin F.M., Petersen S.E. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270(5237):802–805. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- Cummings J.L. Frontal-subcortical circuits and human behavior. Arch. Neurol. 1993;50(8):873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Dombrovski A.Y., Szanto K., Clark L., Aizenstein H.J., Chase H.W., Reynolds C.F. Corticostriatothalamic reward prediction error signals and executive control in late-life depression. Psychol. Med. 2015;45(7):1413–1424. doi: 10.1017/S0033291714002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane D.L., Yuspeh R.L., Huthwaite J.S., Klingler L.K. Demographic characteristics and normative observations for Derived-Trail making test indices. Neuropsychiatry Neuropsychol. Behav. Neurosci. 2002;15(1):39–43. [PubMed] [Google Scholar]
- Drysdale A.T., Grosenick L., Downar J., Dunlop K., Mansouri F., Meng Y. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 2017;23(1):28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin M.J., Liston C., Avissar M.A., Ilieva I., Gunning F.M. Network-guided transcranial magnetic stimulation for depression. Curr. Behav. Neurosci. Rep. 2017;4(1):70–77. doi: 10.1007/s40473-017-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M., Mahableshwarkar A.R., Jacobson W., Zhong W., Keefe R.S., Olsen C.K. What is the overlap between subjective and objective cognitive impairments in MDD? Ann. Clin. Psychiatry. 2018;30(3):176–184. [PubMed] [Google Scholar]
- Fields R.D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley C.M. White matter and behavioral neurology. Ann. N. Y. Acad. Sci. 2005;1064:162–183. doi: 10.1196/annals.1340.028. [DOI] [PubMed] [Google Scholar]
- Filley C.M., Fields R.D. White matter and cognition: making the connection. J. Neurophysiol. 2016;116(5):2093–2104. doi: 10.1152/jn.00221.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R., Roth M., Shapiro M.B., Post F. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gasquoine P.G. Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neurosci. Biobehav. Rev. 2013;37(3):340–348. doi: 10.1016/j.neubiorev.2013.01.002. [Internet]. Elsevier Ltd. Available from: [DOI] [PubMed] [Google Scholar]
- Golden C.J., Freshwater S.M. Stoelting Co.; Wood Dale, IL: 2002. The Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. [Google Scholar]
- Grace J. Encyclopedia of Clinical Neuropsychology. 2011. Frontal systems behavior scale; pp. 1090–1093. [Google Scholar]
- Griffanti L., Zamboni G., Khan A., Li L., Bonifacio G., Sundaresan V. BIANCA (brain intensity AbNormality classification algorithm): a new tool for automated segmentation of white matter hyperintensities. Neuroimage. 2016;141:191–205. doi: 10.1016/j.neuroimage.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon F.M., Hoptman M.J., Lim K.O., Murphy C.F., Klimstra S., Latoussakis V. Macromolecular white matter abnormalities in geriatric depression: a magnetization transfer imaging study. Am. J. Geriatr. Psychiatry. 2008;16(4):255–262. doi: 10.1097/JGP.0b013e3181602a66. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon F.M., Walton M., Cheng J., Acuna J., Klimstra S., Zimmerman M.E. MRI signal hyperintensities and treatment remission of geriatric depression. J. Affect. Disord. 2010;126(3):395–401. doi: 10.1016/j.jad.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M.C. Hamilton depression rating scale (HAM-D) Redloc. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen R., Bouvy W., Mendrik A., Viergever M., Biessels G., De Bresser J. Robustness of automated methods for brain volume measurements across different MRI field strengths. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0165719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman J.D., Abraham C.R. What's behind the decline? The role of white matter in brain aging. Neurochem. Res. 2007;32:2023–2031. doi: 10.1007/s11064-007-9341-x. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kohler S., Thomas A.J., Lloyd A., Barber R., Almeida O.P., O'Brien J.T. White matter hyperintensities, cortisol levels, brain atrophy and continuing cognitive deficits in late-life depression. Br. J. Psychiatry. 2010;196(2):143–149. doi: 10.1192/bjp.bp.109.071399. [DOI] [PubMed] [Google Scholar]
- Krishnan K.R., Hays J.C., Blazer D.G. MRI-defined vascular depression. Am. J. Psychiatry. 1997;154(4):497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- Kuceyeski A., Zhang Y., Raj A. Linking white matter integrity loss to associated cortical regions using structural connectivity information in Alzheimer's disease and fronto-temporal dementia: the loss in connectivity (LoCo) score. Neuroimage. 2012;61(4):1311–1323. doi: 10.1016/j.neuroimage.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuceyeski A., Maruta J., Relkin N., Raj A. The network modification (NeMo) tool: elucidating the effect of white matter integrity changes on cortical and subcortical structural connectivity. Brain Connect. 2013;3(5):451–463. doi: 10.1089/brain.2013.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuceyeski A., Navi B.B., Kamel H., Relkin N., Villanueva M., Raj A. Exploring the brain's structural connectome: a quantitative stroke lesion-dysfunction mapping study. Hum. Brain Mapp. 2015;36:2147–2160. doi: 10.1002/hbm.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuceyeski A., Monohan E., Morris E., Fujimoto K., Vargas W., Gauthier S.A. Baseline biomarkers of connectome disruption and atrophy predict future processing speed in early multiple sclerosis. NeuroImage Clin. 2018;19(May):417–424. doi: 10.1016/j.nicl.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser I.M., Boone K.B., Mehringer C.M., Wohl M.A., Miller B.L., Berman N.G. Cognition and white matter hyperintensities in older depressed patients. Am. J. Psychiatry. 1996;153(10):1280–1287. doi: 10.1176/ajp.153.10.1280. [DOI] [PubMed] [Google Scholar]
- Lockhart S.N., Luck S.J., Geng J., Beckett L., Disbrow E.A., Carmichael O. White matter hyperintensities among older adults are associated with futile increase in frontal activation and functional connectivity during spatial search. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0122445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning K.J., Alexopoulos G.S., Banerjee S., Morimoto S.S., Seirup J.K., Klimstra S.A. Executive functioning complaints and escitalopram treatment response in late-life depression. Am. J. Geriatr. Psychiatry. 2015;23(5):440–445. doi: 10.1016/j.jagp.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzinzik F., Wahl M., Schneider G.H., Kupsch A., Curio G., Klostermann F. The human thalamus is crucially involved in executive control operations. J. Cogn. Neurosci. 2008;20(10):1903–1914. doi: 10.1162/jocn.2008.20124. [DOI] [PubMed] [Google Scholar]
- Minzenberg M.J., Laird A.R., Thelen S., Carter C.S., Glahn D.C. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch. Gen. Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto S.S., Gunning F.M., Wexler B.E., Hu W., Ilieva I., Liu J. Executive dysfunction predicts treatment response to neuroplasticity-based computerized cognitive remediation (nCCR-GD) in elderly patients with major depression. Am. J. Geriatr. Psychiatry. 2016;24(10):816–820. doi: 10.1016/j.jagp.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naarding P., Tiemeier H., Breteler M.M.B., Schoevers R.A., Jonker C., Koudstaal P.J. Clinically defined vascular depression in the general population. Psychol. Med. 2007;37(3):383–392. doi: 10.1017/S0033291706009196. [DOI] [PubMed] [Google Scholar]
- Orr C., Hester R. Error-related anterior cingulate cortex activity and the prediction of conscious error awareness. Front. Hum. Neurosci. 2012;6:1–12. doi: 10.3389/fnhum.2012.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini M., Bonzano L., Bergamino M., Bommarito G., Feraco P., Murugavel A. Cingulum bundle alterations underlie subjective fatigue in multiple sclerosis. Mult. Scler. J. 2015;21(4):442–447. doi: 10.1177/1352458514546791. [DOI] [PubMed] [Google Scholar]
- Pimontel M.A., Rindskopf D., Rutherford B.R., Brown P.J., Roose S.P., Sneed J.R. A meta-analysis of executive dysfunction and antidepressant treatment response in late-life depression. Am. J. Geriatr. Psychiatry. 2016;24(1):31–41. doi: 10.1016/j.jagp.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh K.G., Lipsitz L.A. The microvascular frontal-subcortical syndrome of aging. Neurobiol. Aging. 2002;23(3):421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Rahm C., Liberg B., Wiberg-Kristoffersen M., Aspelin P., Msghina M. Rostro-caudal and dorso-ventral gradients in medial and lateral prefrontal cortex during cognitive control of affective and cognitive interference. Scand. J. Psychol. 2013;54:66–71. doi: 10.1111/sjop.12023. [DOI] [PubMed] [Google Scholar]
- Reijmer Y.D., Schultz A.P., Leemans A., O'Sullivan M.J., Gurol M.E., Sperling R. Decoupling of structural and functional brain connectivity in older adults with white matter hyperintensities. Neuroimage. 2015;117:222–229. doi: 10.1016/j.neuroimage.2015.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R.M. Validity of the trail making test as an Indicator of organic brain damage. Percept. Mot. Skills. 1958;8(3):271–276. [Google Scholar]
- Salthouse T.A. What cognitive abilities are involved in trail-making performance? Intelligence. 2011;39(4):222–232. doi: 10.1016/j.intell.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R.B., Harrison J., Boulton C., Wilson J., Gregory R., Parkin S. Global attentional-executive sequelae following surgical lesions to globus pallidus interna. Brain. 2002;125(3):562–574. doi: 10.1093/brain/awf046. [DOI] [PubMed] [Google Scholar]
- Sexton C.E., MacKay C.E., Ebmeier K.P. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am. J. Geriatr. Psychiatry. 2013;21(2):184–195. doi: 10.1016/j.jagp.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Price J.L., Vaishnavi S.N., Mintun M.A., Barch D.M., Epstein A.A. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am. J. Psychiatry. 2008;165:524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop J.R. Stroop color word test. J. Exp. Physiol. 1935;18:643–662. [Google Scholar]
- Taylor W.D., Payne M.E., Krishnan K.R.R., Wagner H.R., Provenzale J.M., Steffens D.C. Evidence of white matter tract disruption in MRI hyperintensities. Biol. Psychiatry. 2001;50(3):179–183. doi: 10.1016/s0006-3223(01)01160-x. [DOI] [PubMed] [Google Scholar]
- Taylor W.D., Aizenstein H.J., Alexopoulos G.S. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol. Psychiatry. 2013;18(9):963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.J., O'Brien J.T., Davis S., Ballard C., Barber R., Kalaria R.N. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch. Gen. Psychiatry. 2002;59(9):785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- van Agtmaal M.J.M., Houben A.J.H.M., Pouwer F., Stehouwer C.D.A., Schram M.T. Association of microvascular dysfunction with late-life depression: A systematic review and meta-analysis. JAMA Psychiatry. 2017;74(7):729–739. doi: 10.1001/jamapsychiatry.2017.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf Y.D., Scheltens P., Lindeboom J., Witter M.P., Uylings H.B.M., Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003;41(10):1330–1344. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Vasudev A., Saxby B.K., O'Brien J.T., Colloby S.J., Firbank M.J., Brooker H. Relationship between cognition, magnetic resonance white matter hyperintensities, and cardiovascular autonomic changes in late-life depression. Am. J. Geriatr. Psychiatry. 2012;20(8):691–699. doi: 10.1097/JGP.0b013e31824c0435. [DOI] [PubMed] [Google Scholar]
- Vataja R., Pohjasvaara T., Mäntylä R., Ylikoski R., Leskelä M., Kalska H. Depression–executive dysfunction syndrome in stroke patients. Am. J. Geriatr. Psychiatry. 2005;13(2):99–107. doi: 10.1176/appi.ajgp.13.2.99. [DOI] [PubMed] [Google Scholar]
- Wahlund L.O., Barkhof F., Fazekas F., Bronge L., Augustin M., Sjögren M. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32(6):1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- Wang L., Krishnan K.R., Steffens D.C., Potter G.G., Dolcos F., McCarthy G. Depressive state- and disease-related alterations in neural responses to affective and executive challenges in geriatric depression. Am. J. Psychiatry. 2008;165(7):863–871. doi: 10.1176/appi.ajp.2008.07101590. [DOI] [PubMed] [Google Scholar]
- Wei W., Wang X.J. Inhibitory control in the Cortico-basal ganglia-Thalamocortical loop: complex regulation and interplay with memory and decision processes. Neuron. 2016;92(5):1093–1105. doi: 10.1016/j.neuron.2016.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Andreescu C., Butters M.A., Tamburo R., Reynolds C.F., 3rd, Aizenstein H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res. 2011;194(1):39–46. doi: 10.1016/j.pscychresns.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Lai Y., Zhang Y., Yao L., Wen X. Breakdown of sensorimotor network communication in leukoaraiosis. Neurodegener. Dis. 2015;15(6):322–330. doi: 10.1159/000435918. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Yang J., Wong A., Wong C.H.K., Chan S.S.W., Li H.H.S. Operational definitions improve reliability of the age-related white matter changes scale. Eur. J. Neurol. 2011;18(5):744–749. doi: 10.1111/j.1468-1331.2010.03272.x. [DOI] [PubMed] [Google Scholar]
- Zhang P., Wang J., Xu Q., Song Z., Dai J., Wang J. Altered functional connectivity in post-ischemic stroke depression: a resting-state functional magnetic resonance imaging study. Eur. J. Radiol. 2018;100:156–165. doi: 10.1016/j.ejrad.2018.01.003. June 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exploratory Analysis of the FrSBe-EF