Summary
Two types of extracellular vesicles (EVs), exosomes and ectosomes, are generated and released by all cells, including immune cells. The two EVs appear different in many properties: size, mechanism and site of assembly, composition of their membranes and luminal cargoes, sites and processes of release. In functional terms, however, these differences are minor. Moreover, their binding to and effects on target cells appear similar, thus the two types are considered distinct only in a few cases, otherwise they are presented together as EVs. The EV physiology of the various immune cells differs as expected from their differential properties. Some properties, however, are common: EV release, taking place already at rest, is greatly increased upon cell stimulation; extracellular navigation occurs adjacent and at distance from the releasing cells; binding to and uptake by target cells are specific. EVs received from other immune or distinct cells govern many functions in target cells. Immune diseases in which EVs play multiple, often opposite (aggression and protection) effects, are numerous; inflammatory diseases; pathologies of various tissues; and brain diseases, such as multiple sclerosis. EVs also have effects on interactive immune and cancer cells. These effects are often distinct, promoting cytotoxicity or proliferation, the latter together with metastasis and angiogenesis. Diagnoses depend on the identification of EV biomarkers; therapies on various mechanisms such as (1) removal of aggression‐inducing EVs; (2) EV manipulations specific for single targets, with insertion of surface peptides or luminal miRNAs; and (3) removal or re‐expression of molecules from target cells.
Keywords: cancer, cell trafficking, inflammation, signal, transduction, transcription factors
Origin and structure of the two types of extracellular vesicles
The presence in the extracellular space of membrane structures, part of which look like small vesicles, has long been known to electron microscopists. For decades, however, these structures were mostly interpreted as fragments of cells and their membranes, released during degeneration, cell death or simple fixation. More than 30 years ago, however, during in‐vitro incubation of erythrocytes, vesicles visible using the light microscope, and thus of considerable size, were released by shedding from small areas of the plasma membrane 1. These vesicles, although variously named, including shedding vesicles and microvesicles, are also called ectosomes (i.e. vesicles released from the surface of the cell) 2, 3 (Fig. 1), a nomenclature employed in this review.
Figure 1.
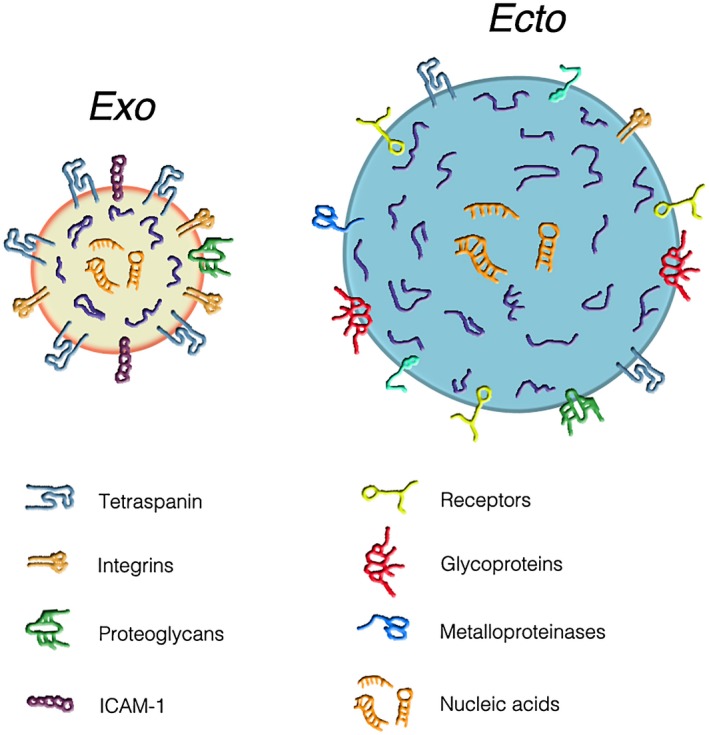
Structure and composition of the two types of extracellular vesicles (EVs): exosomes (left) and ectosomes (right). The comparative analysis shows that, in addition to their differences in size (with diameters of 50–150 and 150–600 nm, respectively), the two types of vesicles exhibit differences in both membranes and cargoes. In exosomes (gold background), the membranes are rich in tetraspanins, a tetrameric protein complex with critical importance for the trapping of both membrane and luminal proteins. Tetraspanins are also present in the ectosome (sky‐blue background) membrane, however at lower density. A similar partial difference is true also for integrins and proteoglycans. In contrast, the adhesion protein, intercellular adhesion molecule 1 (ICAM‐1), is present only in the exosome membrane. The ectosome membrane is rich in other proteins: receptors, glycoproteins, metalloproteinases and others. The luminal cargoes of both EV types are similar in the two EV types. They contain many typical proteins (blue strings), some of which are anchored (by myristoylation, palmitoylation or other sequences) to the membrane, together with low concentrations of cytosolic proteins. The lumena of both EV types show various types of orange sequences composed by nucleic acids, i.e. mRNAs, miRNAs and DNA sequences. From Meldolesi [3].
Ectosomes are released by all living cells 2, 3, 4. For almost 2 decades, these vesicles remained largely unknown. A detailed level of knowledge about them was reached only 10 years ago 3, 4, 5, 6. In the meantime, intracellular vacuoles, named multi‐vesicular bodies (MVBs), present in all cells, were recognized as endosome cisternae recycled from the cell surface. Compared to the other endosome cisternae, the MVBs exhibit a distinctive property: the occurrence within their lumen of many small vesicles generated at their membrane by reverse exocytosis. Similar to many other endosome cisternae, a fraction of MVBs undergo surface exocytosis reinforced by cell stimulation. By such exocytosis the MVB lumen, including the intact vesicles, are released to the extracellular space. Upon their release these vesicles assume the name of exosomes 2, 3 (Fig. 1).
For more than 20 years, the two families of extracellular vesicles (EVs), large ectosomes and small exosomes (with diameters of 100–600 and 50–150 nm, respectively) 3 have been known to be delimited by membranes that include some molecules typical of their membranes of origin, plasma membranes and endosomal membranes. Together with molecules of their membrane of origin the EVs include many other distinct molecules, concentrated here during the course of their assembly. Within their lumen, both EV types contain largely specific cargoes primarily composed of different proteins, glycans, lipids and nucleotides. The latter include many RNAs, some coding (mRNAs), the others non‐encoding, especially microRNAs (miRNAs) 1, 2, 4, 6, 7, 8. In addition, the EVs can contain short DNA sequences (Fig. 1). Further information about these vesicles can be found in recent reviews 2, 3, 5, 7.
Extracellular vesicles: functions in general cell biology and immunology
The EVs of the two types, initially discovered by studies of their membranes and cargoes, were considered physiological structures competent for exchange of distinct specific molecules from and to various types of cells (see [2, 3, 6]). However, recent studies have clarified that the various proteins and miRNAs, abundant in one type of EV, are also present in the other type, although at lower levels. In other words, ectosomes and exosomes are more similar than previously believed 3, 9. In addition, many EV populations published so far are reported to include only one of the two types, and not the other. In many other studies, however, they include mixtures of the two. For these considerations, the distinct nomenclature of ectosomes and exosomes is employed only rarely in the present review, where both vesicles are presented together as EVs. This does not mean that the EV populations are always homogeneous. In fact, EV heterogeneities are common – dependent, however, not only from their different origin, but also from the functional state, variable in both EV assembly and release. As an example of functional heterogeneity consider the release of EVs, rare when the cells are at rest, increased to a large extent, with an at least partially different composition, when the same cells are appropriately stimulated. Similar results, demonstrated in vivo in mice and humans, have also been reported in single cells grown in vitro, when their fluids are significantly changed 2, 3, 9, 10.
Upon their release, navigating EVs can have a different fate. A small fraction of them dissolves, with solution of their cargo components into the extracellular fluids. Most EVs, however, interact with specific types of cells, not only in the proximity, but in some cases also at large distance from their site of release. The binding of EVs to target cells does not take place at random but depends on the expression, at the surface of the cells, of specific receptors, many of which are not yet identified 3, 5 (Fig. 2). Upon such binding, some EVs induce activation of intracellular signalling processes. Upon binding, other EVs undergo reverse exocytic fusion of their membrane, taking place either at the surface or upon EV internalization by endocytosis or phagocytosis. The result of such fusions is the release of EV cargoes to the cytosol of target cells (Fig. 2). The cargo molecules received by target cells do not necessarily remain there. Upon their mixing with local molecules, they can participate in the generation of other EVs, which are released and undergo fusion with other cells by a process defined as recycling of intercellular communications 3, 5, 11 (Fig. 2).
Figure 2.
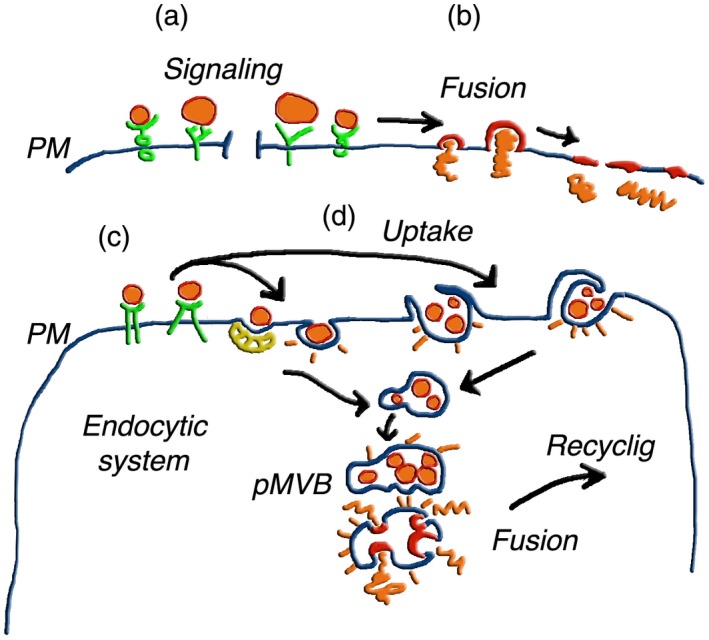
Interaction and fusion of extracellular vesicles (EVs) with target cells. The top images (a,b) illustrate the interaction of the two EV types, distinguished by their largely different size, with the plasma membrane of a target cell 2, 3, 4, 5, 6. In (a) an exosome and an ectosome are directly bound to cell surface signalling receptors. This process, not frequent, results in the activation of the receptors with ensuing generation of intracellular signals (not shown); (b) begins with EV binding to receptors structurally analogous to those shown in (a). Such binding, however, is followed by fusion of EVs with the plasma membrane of target cells, followed by integration of their membranes into the cell surface, and discharge of their luminal cargoes into the cell cytosol 2, 3, 6. (c,d) Fusion events that, upon EV binding to the plasma membrane (c), occur in the endocytic system (d). The two left uptake processes are mediated by clathrin‐surfaced vesicles and caveolae. Those to the right depend on phagocytosis and macropinocytosis. In the following steps, intact EVs accumulate, first within endocytic vesicles, then within cisternae. Accumulation within a pseudo‐multivesicular body (pMVB) is followed by EV membrane fusion, with discharge of cargoes into the cytosol 2, 3. The EV membranes and cargoes could then undergo redistribution within the cell, with ensuing recycling of either exosomes or ectosomes assembled at MVBs or plasma membrane, respectively. Recycled EVs are finally released to the extracellular fluid. Summing up, the same cell, which first operates as a target, then contributes as a parent cell to the generation and discharge of EVs 3, 6. From Meldolesi [3].
Several papers concerning basic EV processes reported so far illustrate many cell types, including immune cells 7, 8, 9, 10, 11, 12. In many cases, in fact, the latter cells operate by the described basic and functional mechanisms, analogous to the cells of other types. In view of this analogy, this review is focused primarily on the events of physiological and pathological relevance taking place in cells of the immune system, in particular in B and T lymphocytes, dendritic cells, granulocytes and macrophages. A subsequent section also concerns specific diseases, including cancer, in which immune cells often play key roles. A final section deals with the relevance of EVs in the diagnosis and therapy of those diseases 3, 5, 6, 7, 12. The results reported in this review illustrate many recent developments of the EV role in immune cells, presented in general terms. For further details, readers are addressed to articles and reviews listed in the Reference section and in the rest of the literature.
Physiological EV effects in immunology
Until approximately 2 decades ago, most connections among distinct immune cells, necessary for the acquisition and maintenance of adaptive immunity, were attributed to two distinct mechanisms: direct connection between different cells and secretion of specific molecules released by cells upon appropriate stimulation. A third mechanism recognized now is due to EVs released from, and then fused to, target cells. In this case, the effects are due to the intercellular exchange of specific membrane and cargo molecules 7, 8, 9, 10, 11, 12. The functional relevance of such mechanism depends on the age of the participating animals and humans. In particular, the blood concentration of EVs decreases with advancing age due to their increased internalization 13.
Together with unique proteins and lipids, the EV cargoes are rich in various miRNAs. When transferred to the cytoplasm of immune target cells these RNAs govern various processes, including expression of immune genes and proteins relevant for development and function 12, 14. The human blood plasma contains many EVs rich in miRNAs of various types. Among these miRNAs, some are targeted from donors to distinct immunological cells; for example, monocytes and lymphocytes 15, 16, 17. miRNAs also appear critical for dendritic cells, with ensuing anti‐pathogenic effects and tolerant phenotypes of T lymphocyte target cells 18, 19, 20.
Binding of EVs to their specific target cells takes place by a type of agonist–receptor interaction, depending on particular proteins present in the two interacting surfaces. Such interactions justify the selective effects of EVs to their target cells. For example, the EVs released by human platelets, targeted to granulocytes and monocytes, do not have such an effect on lymphocytes of all types. Interestingly, this distinction depends on the affinity of the binding, which is approximately 100‐fold higher for granulocytes with respect to lymphocytes 15, 20, 21.
Immune cell diseases
Considered together, the EVs released from immune cells modulate ample aspects of the system by either enhancing or suppressing its various activities 22, 23. This role of EVs has been confirmed by in‐vitro studies, where the cells employed for their release are often of a single type, and target cells are known. EVs are often important for animals and humans, especially when afflicted by infectious diseases. The EVs from pathogens are relevant for various reasons: on one hand, for the co‐ordinated activity of bacteria; on the other hand, for lesions of host cells, including alterations of immune recognition and cellular responses 24, 25, 26. The EVs most effective for intervention in the latter responses are those derived from mesenchymal stromal cells (MSCs) 26. When the investigation of inflammation depends on the analysis of EVs from the blood plasma, the origin of these vesicles includes various types of cells, especially platelets, endothelia and leucocytes. The effects of EVs revealed by these studies include increases of inflammation, with ensuing increases of pathology and various disorders. However, effects induced by miscellaneous EVs have also been of anti‐inflammatory type. It appears, therefore, that the EV cocktails from the blood or other origin can induce apparently opposite effects, programmed towards pro‐ or anti‐inflammatory cell phenotypes 23, 27, 28, 29, 30, 31, 32.
EVs also operate in tissue pathology. Extracellular vesicles from human liver stem cells reduce injury in an ex‐vivo normo‐thermic hypoxic rat liver perfusion model 33. In the lung, anti‐inflammatory EVs addressed to alveolar macrophages result in the protection of epithelial cells 34. Upon heart infarction, stem cell therapy, induced by myocardial or myocyte progenitor cells, is mediated by factors affecting T cell proliferation and function 35, 36. Among the intermediate mechanisms activated within T cells are the up‐ and down‐regulation of several intracellular pathways. The success of the progenitor cells employed depends on their strong capacity for immunosuppression 34, 35, 36.
Brain diseases are also modulated by EVs. EVs established between neurones and glial cells induce inflammation and alteration of synapses 37, 38. Among the effects induced by brain injury are neuronal degeneration, microgliosis and astrocytosis, all reduced by treatment with EVs generated by MSC 18, 20, 38. In multiple sclerosis, an autoimmune disease of the central nervous system characterized by localized neuro‐axonal degenerations, the function of regulatory T cells is impaired. EVs generated by restimulation of such cells regulate the disease development 39. Analysis of cerebrospinal fluid in multiple sclerosis has demonstrated an enrichment of EVs in the plasma, which is important for the disease 40, while EVs containing interleukin (IL)‐4 modulate neuro‐inflammation 41. A potential for multiple sclerosis and its therapy is also demonstrated by EVs generated from human MSCs 42. In another disease of the central nervous system, amyotrophic lateral sclerosis, neuronal degeneration is located in multiple areas. Such disease depends, at least in part, on EVs enriched in toxic proteins, released by blood leucocytes from sporadic patients 43. Finally, wound healing remains a challenging clinical problem. In this case, the challenge is to consider the role of leucocyte EVs in the generation of the process and in its therapy 44.
Immune cell–cancer cell interactions
As emphasized in publications already mentioned, various types of EV play critical roles in the interaction of cancer cells with the immune system (see, for example, [7, 14, 20, 21]). Recent evidence has demonstrated that the EVs of both exosomes and ectosomes originate not only from immune but also from cancer cells. Depending on their origin, these EVs exhibit profound heterogeneities of both their membranes and luminal cargoes, affecting highly important molecules such as growth factors, cytokines, chemokines, various receptors, oncoproteins, oncogenes and multiple miRNAs. EVs from both immune and cancer cells transfer immunologically active molecules that influence physiological and pathological processes. EV heterogeneities participate, therefore, in the generation and control of the intricate complexity of cancer biology. In addition to processes already mentioned in this review, the EVs involved in back‐and‐forth traffic from immune and cancer cells have a role in external processes. Among these processes are the development of angiogenesis and the establishment of distant pro‐metastatic cell niches 45, 46, 47 (Fig. 3).
Figure 3.
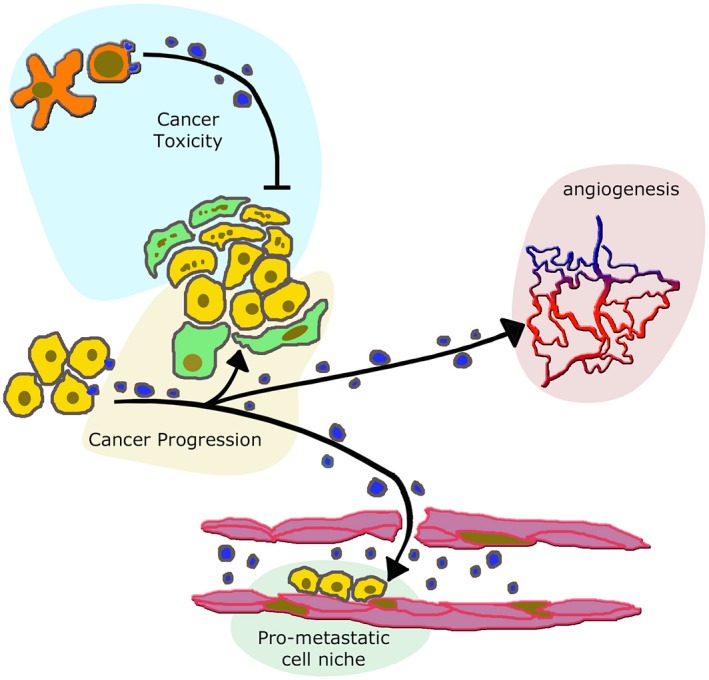
Extracellular vesicles (EVs) from immune and cancer cells induce different effects on cancer and peripheral structures. EVs of variable size, released from lymphocytes activated by dendrites (red cytoplasm over light blue background distributed at the top of the figure) can induce cell toxicity affecting both adjacent cells (here fibroblasts and mesenchymal stromal cells (MSCs) labelled by several points, distributed over a green cytoplasm) and cancer cells (points over yellow background) 18, 19, 20. In contrast, EVs released from unaffected cancer cells reinforce the adjacent fibroblast (green) and cancer (yellow) cells, distributed over the central golden background. This effect of EVs induces cancer progression 49, 50, 51, 52, 53, 54, 55. Moreover, the EVs of cancer cell origin move to the proximity of neighbouring vessels, thus inducing stimulation of angiogenesis (red of the vessel system over the pink background to the right) 46. EVs of cancer origin penetrate within vessels (bottom, light green background), where they induce the intra‐lumenal assembly of pre‐metastatic cell niches 45, 46, 47, destined to develop into metastases.
A key function played by EVs in the cancer environment is modification of the phenotype and function of cancer cells. The result of these effects can be either the progression or depletion of cancer cells 48, 49. The latter effect is induced by EVs from CD8+ T cells activated by dendritic cells. Upon their infiltration into tumour lesions, these EVs participate in the elimination of non‐cancer cells, such as fibroblasts and MSCs, after which they attack tumour cells. These EVs can thus exhibit direct cytotoxicity against tumour cells 49, 50, 51, 52 (Fig. 3). However, EVs derived from immune cells can also reinforce cancer progression 49, 53, 54, 55 (Fig. 3).
Many other EVs, often concentrated in the hypoxic cancer environment or in the blood plasma, induce effects such as cell proliferation and invasion, which promote cancer progression. In this case, the EVs, mostly of tumour origin, play a role in the targeting of non‐malignant or other cancer cells 56. Upon their generation from many cells of single cancers, targeting of these EVs is addressed not only to nearby cells of origin, but also to others at greater distance. In addition to angiogenesis and metastasis 45, 46, 47, targeting occurs during cell migration and invasion 53, 54. A mechanism of these EV effects is immune suppression of lymphocytes, such as CD8+ T, or macrophages 55, 56, 57, 58, 59, possibly dependent on the protein 14‐3‐3ζ over‐expressed at the surface of the vesicles 60. Within cancer target cells, the operative effects induced by the EV binding include the activation of specific signalling pathways 61.
Diagnosis and therapy
Information developed concerning EVs offers opportunities for their use in diagnosis and therapy. Important for these studies is the identification of biomarkers, useful for the strategy of medical action. Such identification, based on the analyses of plasma EVs, has led to the revelation (or confirmation) of their involvement in specific cancers. Among biomarkers, some correspond to whole EVs, known to be released from particular cancer cells 45, 46, 47; others are based on EV surface proteins, such as tetraspanins and 14‐3‐3ζ 3, 60 or on miRNAs, such as miR‐301a‐3p and many others, abundant in the lumen of cancer EVs 46, 48. These biomarkers are tools to be employed, together with the classical tools of medicine, in the diagnosis of various cancers and in the evaluation and prediction of their development 20, 51, 57, 62.
The relevance of EVs in therapy, already established in the last few years, is still growing, promising important developments for next future. Various approaches have already been developed. MSC‐derived EVs have attracted attention because of their ability to migrate towards inflammatory areas, including tumours and other diseases. Distinct types of EVs, such as those of cancer origin, can exacerbate the cell microenvironment. In this case, a considered therapeutic approach has been EV removal carried out by affinity‐based methods. An additional approach, considered of great interest, is the manipulation of exosomes and/or ectosomes carried out by insertion at their surface of peptide ligands specific for target cell receptors, within the lumen, of appropriate miRNAs and/or chemotherapeutic drugs. A final possibility is another manipulation, making it possible for the EVs to remove molecules from their target cells 6, 62, 63.
At present, therapy with EVs takes place not only for cancer but also for other diseases. In infectious diseases, EVs from MSCs are employed as novel therapeutic tools. In fact, they suppress proinflammatory processes, also reducing oxidative stress and fibrosis with concomitant tissue regeneration, allowing endogenous stem and progenitor cells to repair affected tissues. Such approaches, not yet fully standardized, have already been successfully employed in humans 30, 64. EVs from MSCs are also of interest for treatment of brain degenerative diseases. Specifically, these treatments induce protection from both short‐term defects of myelination and long‐term lesions of white matter, accompanied by reduction of various types of alteration: neuronal degeneration, microgliosis and reactive astrocytosis. Thus, EVs from MSCs induce considerable structural and functional improvements in the brain 37, 64. In addition, the same EVs demonstrate therapeutic potential not only in brain diseases, but also in biological processes. Among these is wound‐healing taking place in many tissues, including kidney, heart and liver 37, 64.
Cancer therapy governed by EVs has been the most extensively investigated process. A few years ago, significant differences had already been reported between the EVs released by tumour cells, which induce immunoregulation, compared to the EVs from immune cells, which induce immunomodulation 65. At the time, progress was expected particularly for the EV‐based immunotherapies 65, 66, 67. Improvements developed recently are focused on the treatment of single or various cancers. A few examples have already been reported in this review 48, 50, 62. Consider now three other examples specific for single cancers. A permissive anti‐tumoral approach is specific for a brain cancer, the glioblastoma multiforme. The therapeutic mechanism is based on the re‐expression, within these cancer cells, of leucine‐rich repeat C4 (LRRC4), a tumour suppressor gene. When transported by EVs, this gene can ultimately inhibit the proliferation of regulatory T cells that infiltrate the tumour 68. Another approach, already promising for in‐vivo therapy of breast cancer, is based on the engineering of MSC cells competent for the release of EVs enriched in the microRNA, miR‐379. The systemic administration of such EVs has demonstrated therapeutic effects in cancer and its metastases 69.
Another interesting therapeutic process has been developed for hepatocellular carcinoma cells. In this case, EVs derived from stellate cells (1) could be loaded with an miRNA of choice (miR‐335‐5p); (2) were taken up by hepatocellular carcinoma cells in vitro and, more importantly, also in vivo; and (3) were found to inhibit proliferation and invasion of hepatocellular carcinoma cells in vitro and to induce their tumour shrinkage in vivo. The summing‐up of this system is highly promising for new therapeutic strategies 70.
Most of the EV‐governed anti‐cancer processes are recommended not to be used alone, but in combination with classical chemotherapeutic drugs 63, 71. This combination, however, is not always successful. EVs from various origins operate in chemotherapy drug resistance by participating in various processes, such as expression of glutathione S‐transferase or other enzymes; sequestration of cytotoxic drugs; capture of monoclonal antibodies by their binding to membrane proteins; and release to the cancer cell cytoplasm of specific proteins together with various coding and non‐coding RNAs (see, among others, [72, 73]). These effects are at least potentially critical because, together with the cross‐talk of cancer cells with stromal cells, they play substantial roles in the establishment of cancer. Therapy in this case consists of the prevention or elimination of the EV mechanisms of action 62, 74.
Conclusions
Both types of EV, the exosomes and ectosomes, participate in numerous processes and are therefore of high importance. The 2–3‐decade history of these vesicles, which appears recent when compared to other organelles, is indeed complex and innovative. Their discovery and the initial identification of their functions in a limited number of cells were unexpected in basic cell biology. Rapid release, targeting and fusion of EVs were recognized to involve many, and then all, types of cells. In parallel, the accumulation of knowledge concerning these vesicles revealed that their activity is not limited to physiology, but is also relevant for a growing number of diseases. Currently, mechanisms have been developed for the diagnosis of specific diseases and, even more importantly, for the progress of new, highly promising therapies. It can be concluded that medicine is the main field of current EV research, carried out by basic scientists in collaboration with highly qualified medical specialists of the field. This explains why the number of publications concerning these vesicles has grown so much during the last 10 years: from 200 in 2007 to more than 2000 in 2017.
Immunity is one of the fields of great interest for EV research. Experimental studies have revealed the critical role of these vesicles and the mechanisms of their action in processes that, until recently, were believed to depend on different mechanisms. Many results and conclusions along these lines, often focused on specific areas of immunity, have been convincing. Therefore, they have been the target of immune reviews, and have also been included in specific immunology publications. Approaches of this type have become potentially valuable for specialists in specific areas.
In the present review, many areas of the immune EV functions have been illustrated. Interest has been directed to mechanistic analogies of the latter functions with respect to those of other cell types. The physiological properties of EVs have been followed by properties specific for a number of diseases, including the interactions of various EVs from and with immune and cancer cells. The presentation has been focused mainly on processes that have emerged during the last few years, including those that have contributed new ideas. Current developments expected for the near future include the molecular characterization of EVs released from various types of immune cells and their interaction with specific target cells. Another group of intensely investigated areas includes therapeutic EVs composed by appropriately addressed membranes together with their specific, drug‐containing cargoes 3, 75, 76, 77, 78.
In conclusion, it is hoped that this review will be useful to immune scientists interested in the updating of existing information combined with the development of innovative studies, currently under operation and/or approval. In other words, it is hoped that information on EV functions in immune cells will be useful to readers interested in EV activities and their expected development during the next few years.
Disclosures
The extracellular vesicles, presented here for immune vesicles, have been previously investigated by the author, specifying the general criteria and the interests specific for neural cells.
References
- 1. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of membrane activities with released vesicles (ectosomes). J Bio Chem 1987; 262:9412–20. [PubMed] [Google Scholar]
- 2. Colombo M, Raposo G, Thery C. Biogenesis secretion and intercellular interactions of exosomes and other extracellular vesicles. Ann Rev Cell Dev Biol 2014; 30:255–89. [DOI] [PubMed] [Google Scholar]
- 3. Meldolesi J. Exosomes and ectosomes in intercellular communication. Curr Biol 2018; 28:435–44. [DOI] [PubMed] [Google Scholar]
- 4. Pollet H, Conrad L, Cloos AS et al Plasma membrane lipid domain platforms for vesicle biogenesis and shedding? Biomolecules 2018; 14:5 https://doi.org/103390/biom8030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sedwick AE, D’Souza‐Schorey C. The biology of extracellular microvesicles. Traffic 2018; 19:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vesicles. Trends Cell Biol 2017; 27:172–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014; 14:195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Driedonks TAP, Van der Grein SG, Ariyurek Y et al Immune stimuli shape the small non‐coding transcriptome of extracellular vesicles released by dendritic cells. Cell Mol Life Sci 2018; 75:3857–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tucher C, Bode K, Schiller P et al Extracellular vesicle subtypes released from activated or apoptotic T‐lymphocytes carry specific and stimulus‐dependent protein cargo. Front Immunol 2018; 9:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tkach M, Kowal J, Zucchetti AE et al Quantitative differences in T cell activation by dendritic cell‐derived extracellular vesicle subtypes. EMBO J 2017; 36:3012–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buzas EI, Toth EA, Sodar BW, Szabò Taylor KE. Molecular interaction at the surface of extracellular vesicles. Semin Immunopathol 2018; 40:453–64. 10.1007/s0028-018-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Momen‐Heravi F, Bala S. miRNA regulation of innate immunity. J Leukoc Biol 2018; 10.1002/JLB.3MIR1117-459R. [DOI] [PubMed] [Google Scholar]
- 13. Eytan E, Green EE, Bodogal M et al Age related changes in plasma extracellular vesicle characteristics and internalization by leukocytes. Sci Rep 2017; 7:1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lindenbergh MFS, Storvogel W. Antigen presentation by extracellular vesicles from professional antigen presenting cells. Ann Rev Immunol 2018; 36:435–59. [DOI] [PubMed] [Google Scholar]
- 15. Weiss R, Gröger M, Rauscher S et al Differential interaction of platelet‐derived extracellular vesicles with leukocyte subsets in human whole blood. Sci Rep 2018; 8:6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Classen L, Tykocinski LO, Wiedemann F et al Extracellular vesicles mediate intercellular communication: transfer of functionally active microRNAs by microvesicles into phagocytes. Eur J Immunol 2017; 47:1535–49. [DOI] [PubMed] [Google Scholar]
- 17. Hsin JP, Lu Y, Loeb GB et al The effect of cellular context on miR‐155‐mediated gene regulation in four major immune cell types. Nat Immunol 2018; 19:1137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smyth LA, Boardman DA, Tung SL et al Micro RNAs affect dendritic cell function and phenotype. Immunol 2015; 144:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tung SL, Boardman DA, Sen M et al Regulatory T cell‐derived extracellular vesicles modify dendritic cell function. Sci Rep 2018; 8:6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Torralba D, Baixauli F, Villarroya‐Beltri C et al Priming of dendritic cells by DNA‐containing extracellular vesicles from activated T cells through antigen‐driven contacts. Nature Comm 2018; 9:2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smyth TH, Redzic JS, Graner MW, Anchordoquy TJ. Examination of the specificity of tumor cell‐derived exosomes with tumor cells in vitro . Biochim Biophys Acta 2014; 1838:2954–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wen C, Seeger RC, Fabbri M et al Biological role and potential applications of immune cell‐derived extracellular vesicles. J Extracell Vesicles 2017; 6:1400370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rhys HI, Dell’Accio F, Pitzalis C et al Neutrophil microvesicles from healthy controls and rheumatoid arthritis patients prevent the inflammatory activation of macrophages. BioMedicine 2018; 29:60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cipriano MJ, Hajduk SL. Drivers of persistent infection: pathogen‐induced extracellular vesicles. Essays Biochem 2018; 62:135–47. [DOI] [PubMed] [Google Scholar]
- 25. Hosseini‐Beheshti E, Grau GER. Extracellular vesicles as mediators of immune‐pathology in infectious diseases. Immunol Cell Biol 2018; 10.1111/imcb.12044. [DOI] [PubMed] [Google Scholar]
- 26. Mardpour S, Hamidieh AA, Teleahmad S et al Interaction between mesenchymal stromal cell‐derived extracellular vesicles and immune cells by distinct protein content. J Cell Physiol 2018; 10.1002/cp.27669. [DOI] [PubMed] [Google Scholar]
- 27. Hzelton I, Yates A, Dale A et al Exacerbation of acute traumatic brain injury by circulating extracellular vesicles. J Neurotrauma 2018; 35:639–51. [DOI] [PubMed] [Google Scholar]
- 28. Xu J, Feng Y, Jeyaram A et al Circulating plasma extracellular vesicles from septic mice induce inflammation via microRNA‐ and TLR7‐dependent mechanisms. J Immunol 2018; 201:3392–400. 10.4048/jmmunol.1801008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shi C, Ule‐Lemee A, Deng J et al Characterization of heath shock protein 27 in extracellular vesicles: a potential anti‐inflammatory therapy. FASEB J 2018; 33:1617–30. 10.1096/fj201899987R. [DOI] [PubMed] [Google Scholar]
- 30. Slomka A, Urban SK, Lukacs‐Kornek V et al Large extracellular vesicles: have we found the holy grail of inflammation? Front Immunol 2018 ; 9:2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hosseinkhani B, Kuypers S, van den Akker NMS et al Extracellular vesicles work as a functional inflammatory mediator between vascular endothelial cells and immune cells. Front Immunol 2018; 9:1789 https://doi.org/103389/fimmu.2018.01789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karasu E, Eisenhardt SU, Harant J, Huber‐Lang M. Extracellular vesicles: packages set with complement. Front Immunol 2018; 9:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rigo F, De Stefano N, Navarro‐Tableros V et al Extracellular vesicles from human liver stem cells reduce injury in an ex vivo normo‐thermic hypoxic rat liver perfusion model. Transplantation 2018; 102:e205–10. [DOI] [PubMed] [Google Scholar]
- 34. Haggadone MD, Peters‐Golden M. Micro‐environmental influences on extracellular vesicles‐mediated communication in the lung. Trends Mol Med 2018; 24:963–75. [DOI] [PubMed] [Google Scholar]
- 35. Loyer X, Zlatanova I, Devue C et al Intra‐cardiac release of extracellular vesicles shaped inflammation following myocardial infarction. Circ Res 2018; 123:100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van den Akker F, Frijsen KR, Deddens JC et al Suppression of T cells by mesenchymal and cardiac progenitor cells is partly mediated via extracellular vesicles. Heliyon 2018; 4:e00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Drommelschmidt K, Sedar M, Bendix I et al Mesenchymal stem cell‐derived extracellular vesicles ameliorate inflammation‐induced preterm brain injury. Brain Behav Immunol 2017; 60:220–32. [DOI] [PubMed] [Google Scholar]
- 38. Prada I, Gabrielli M, Turola E et al Glia‐to neuron transfer of mRNAs via extracellular vesicles: a new mechanism underlying inflammation‐induces synaptic alterations. Acta Neuropathol 2018; 135:529–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Azimi M, Ghabae M, Moghadasi AN et al Immunomodulatory function of Treg‐derived exosomes is impaired in patients with relapsing‐remitting multiple sclerosis. Immunol Res 2018; 66:513–20. 10.1007/s12026-018-9008-5. [DOI] [PubMed] [Google Scholar]
- 40. Welton JL, Loveless S, Stone T et al Cerebrospinal fluid extracellular vesicle enrichment for protein biomarker discovery in neurological disease, multiple sclerosis. J Extracell Vesicles 2017; 6:1569805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Casella G, Colombo F, Finardi A et al Extracellular vesicles containing IL‐4 modulate neuro‐inflammation in a mouse model of multiple sclerosis. Mol Ther 2018; 26:2107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laso‐Garcia F, Ramos‐Cejudo J, Carillo‐Salinas FJ et al Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLOS ONE 2018; 13:e202590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sproviero D, La Salvia S, Giannini M et al Pathological proteins are transported by extracellular vesicles of sporadic amyotrophic lateral sclerosis. Front Neurosci 2018; 12:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Golchin A, Hosseinzadeh S, Ardenhirilajimi A. The exosomes released from different cell types and their effects in wound healing. J Cell Biochem 2018; 119:5043–52. [DOI] [PubMed] [Google Scholar]
- 45. Greening DW, Gopal SK, Xu R et al Exosomes and their roles in immune regulation and cancers. Semin Cells Dev Biol 2015; 40:72–81. [DOI] [PubMed] [Google Scholar]
- 46. Willmes E, Cabanas C, Mager J et al Extracellular vesicle heterogeneity: sub‐populations, isolation, technique, and diverse functions in cancer progression. Front Immunol 2018; 9:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weidle UH, Birzele F, Kollmorgen G, Ruger R. The multiple roles of exosome in metastasis. Cancer Genomics Proteomics 2017; 14:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dorsam B, Reitners KS, von Strandmann EP. Cancer‐derived extracellular vesicles: friend and foe of tumor immune‐surveillance. Phil Trans R Soc B Biol Sci 2018; 373:20160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shen M, Ren X. New insights into the biological impacts of immune cell derived exosomes within the tumor environment. Cancer Lett 2018; 431:115–22. [DOI] [PubMed] [Google Scholar]
- 50. Seo N, Shirakura Y, Tahara Y et al Activated CD8+ T cell extracellular vesicles prevent tumor progression by targeting of lesional mesenchymal cells. Nat Commun 2018; 9:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang X, Luo G, Zhang K et al Hypoxic tumor‐derived exosomal Mir‐301a mediates M2 macrophage polarization via PTEN‐P3K gamma to promote pancreatic cancer metastasis. Cancer Res 2018; 78:4586–98. [DOI] [PubMed] [Google Scholar]
- 52. Sullivan R, Maresh G, Zhang X et al The emerging role of extracellular vesicles as communication vehicles within the tumor microenvironment and beyond. Front Endocrinol 2017; 8:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Whiteside TL. Exosomes in cancer. Another mechanism of tumor‐induced immune suppression. Adv Exp Med Biol 2017; 1036:81–9. [DOI] [PubMed] [Google Scholar]
- 54. Li M, Lu Y, Xu W et al Horizontal transfer of hexosomal CXCR54 promotes murine hepatocarcinoma cell migration, invasion and lymphoangiogenesis. Gene 2018; 676:101–9. [DOI] [PubMed] [Google Scholar]
- 55. Li Y, Yang Y, Xiong A et al Comparative gene expression analysis of lymphocytes treated with exosomes derived from ovarian cancer and ovarian cysts. Front Immunol 2017; 8:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ludwig S, Floros T, Theodoraki MN et al Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin Cancer Res 2017; 23:4843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Miyazaki T, Ikeda K, Sato W et al Extracellular vesicle‐mediated EBAG9 transfer from cancer cells to tumor microenvironment promotes immune escape and tumor progression. Oncogenesis 2018; 7:7 10.1038/s41389-017-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lawson J, Dickman C, Towle R et al Extracellular vesicle secretion of miR‐142‐3p from lung adenocarcinoma cells induces tumor‐promoting changes in the stroma through cell–cell communication. Mol Carcinog 2019; 58:376–87. [DOI] [PubMed] [Google Scholar]
- 59. Maybruck BD, Pfannenstiel LW, Diaz‐Montero M, Gastman BR. Tumor‐derived exosomes induce CD8+T cell suppressors. J Immunother Cancer 2017; 5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang X, Shen H, Zahangyuan G et al 14‐3‐3ζ delivered by hepatocellular carcinoma‐derived exosomes impaired anti‐tumor function of tumor‐infiltrating T lymphocytes. Cell Death Dis 2018; 9:159.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wu L, Zhang X, Zhang B et al Exosomes derived from gastric cancer cells activate NF‐kB pathway in macrophages to promote cancer progression. Tumor Biol 2016; 37:12169–80. [DOI] [PubMed] [Google Scholar]
- 62. Huber V, Vallacchi V, Fleming V et al Tumor derived miRNAs induce myeloid suppressor cells and predict immunotherapy resistance in melanoma. J Clin Invest 2018. 10.1172/JCI98060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moore C, Kosgodace U, Lange S, Inal JM. The emerging role of exosomes and microvesicles (EMV‐) based cancer therapeutics and immunotherapy. Int J Cancer 2017; 141:428–36. [DOI] [PubMed] [Google Scholar]
- 64. Borger V, Bremer M, Ferrer‐Tur R et al Mesenchymal stem‐stromal cell‐derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci 2017; 18:E1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang X, Pei Z, Chen J et al Exosomes for immune‐regulation and therapeutic intervention in cancer. J Cancer 2016; 7:1081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nawaz M, Fatima F, Nazarenko I et al Extracellular vesicles in ovarian cancer: applications to tumor biology, immunotherapy and biomarker discovery. Expert Rev Proteomics 2016; 13:395–405. [DOI] [PubMed] [Google Scholar]
- 67. Li CH, Im EJ, Moon PG, Baek MC. Discovery of biomarker for colon cancer through proteomic profiling of small extracellular vesicles. BMC Cancer 2018; 18:1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li P, Feng J, Liu Y et al Novel therapy for glioblastoma multiforme by restoring LRRC4 in tumor cells: LRRC4 inhibits tumor‐infiltrating regulatory T cells by cytokine and programmed cell death 1‐containing exosomes. Front Immunol 2017; 8:1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. O’Brien KP, Khan S, Gilligan KE et al Employing mesenchymal stem cells to support tumor‐targeted delivery of extracellular vesicle (EV)‐encapsulated microRNA‐379. Oncogene 2018; 37:2137–49. [DOI] [PubMed] [Google Scholar]
- 70. Wang F, Li L, Piontek K et al Exosome miR‐335 as a novel therapeutic strategy in hepato‐cellular carcinoma. Hepatobiology 2018; 67:940–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yang SJ, Wang DD, Li J et al Predictive role of GSTP1‐containg exosomes in chemotherapy resistant breast cancer. Gene 2017; 623:5–14. [DOI] [PubMed] [Google Scholar]
- 72. Wang X, Xu C, Hua Y et al Exosomes play an important role in the process of psoralen reverse multidrug resistance of breast cancer. J Exp Clin Cancer Res 2016; 35:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kwok HH, Ning Z, Chong P et al Transfer of extracellular vesicle‐associated‐RNAs induces drug resistance in ALK‐translocated lung adenocarcinoma. Cancers (Basel) 2019; 11:pii: E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Samuel P, Fabbri M, Carter DRF. Mechanisms of drug resistance in cancer: the role of extracellular vesicles. Proteomics 2017; 17:1600375. [DOI] [PubMed] [Google Scholar]
- 75. Das CK, Jena BC, Banerjee I et al. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol Pharm 2018; 10.1021/acs.molpharmaceut.8b00901. [DOI] [PubMed] [Google Scholar]
- 76. Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ . Extracellular vesicles in cancer ‐ implications for future improvements in cancer care. Nat Rev Clin Oncol 2018;15:617–38. [DOI] [PubMed] [Google Scholar]
- 77. Arenaccio C, Chiozzini C, Ferrantelli F, Leone P, Olivetta E, Federico M. Exosomes in therapy: engineering, pharmacokinetics and future applications. Curr Drug Targets 2019; 20:87–95. [DOI] [PubMed] [Google Scholar]
- 78. Liao W, Du Y, Zhang C et al Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater 2019; 86:1–14. [DOI] [PubMed] [Google Scholar]


