Summary
B cells have various functions, besides being plasma cell precursors. We determined the presence of intragraft B cells at time of acute rejection (AR) and looked for correlates of B cell involvement in peripheral blood. Renal biopsies at time of AR or stable graft function were analysed for the presence of B cells and B cell‐related gene expression, as well as C4d staining. Peripheral blood B cell subset distribution was analysed at various time‐points in patients with AR and controls, alongside serum human leucocyte antigen (HLA) antibodies. AR was accompanied by intragraft CD20+ B cells, as well as elevated CD20 (MS4A1) and CD19 gene expression compared to controls. B cell infiltrates were proportional to T cells, and accompanied by the chemokine pair C‐X‐C motif chemokine ligand 13 (CXCL13)–C‐X‐C motif chemokine receptor 5 (CXCR5) and B cell activating factor (BAFF). Peripheral blood memory B cells were decreased and naive B cells increased at AR, in contrast to controls. While 22% of patients with AR and 5% of controls showed de‐novo donor‐specific antibodies (DSA), all biopsies were C4d‐negative. These results suggest a role for B cells in AR by infiltrating the graft alongside T cells. We hypothesize that the shift in peripheral blood B cell composition is related to the graft infiltration at time of AR.
Keywords: B cell infiltration, flow cytometry, memory B cell, T cell/B cell interaction
Introduction
B cells have long been regarded as merely giving rise to antibody‐producing plasma cells upon antigen recognition and T cell help. In renal transplantation, donor‐specific antibody (DSA) formation is undoubtedly an important aspect in the alloimmune response, resulting in inferior graft survival 1. However, B cells possess additional features that may place them more centrally in the alloimmune response. B cells produce many cytokines, both pro‐ and anti‐inflammatory, by which they potentially regulate immune responses 2. In addition, B cells are potent antigen‐presenting cells (APCs) that can efficiently take up alloantigen by means of their B cell receptor (BCR) 3. These alloantigens are transported to the endosomal compartment and loaded onto major histocompatibility complex (MHC) class II molecules for presentation to T cells via the indirect pathway 4. Uniquely, B cells are the only APCs that have the capacity to clonally expand. There is increasing evidence for an important role of B cell antigen presentation in the immune response towards solid organ transplants. For example, in a mouse model of cardiac allograft rejection, the selective absence of MHC class II on B cells resulted in prolonged graft survival 5, whereas in a skin transplant model, the development of alloreactive memory T cells was impaired in the absence of antigen presentation by B cells 6.
In the clinical setting, infiltration of B cells has been well described for kidney allografts undergoing chronic rejection. This can be accompanied by infiltration of several other immune cells, giving rise to the formation of ectopic lymphoid tissue 7. The ectopic B cell clusters developing during chronic rejection have been shown to be clonal, suggesting local expansion of infiltrating B cells 8, 9. Similarly, during acute cellular rejection, a proportion of kidney allograft recipients showed extensive B cell infiltrates in biopsies with rejection, which was associated with steroid resistance and inferior outcome 10. As the occurrence of these infiltrates was generally not accompanied by alloantibody production, nor by C4d deposition, it is thought that the infiltrating MHC class II‐positive B cells were acting as APCs for T cells 10, 11. Others have corroborated the presence of B cells during acute cellular rejection 12, 13, 14, probably attracted by the chemokine C‐X‐C motif chemokine ligand 13 (CXCL13) 11, 15. Besides reports describing B cell infiltrates in biopsies, there are additional data supporting a role of B cells during acute cellular rejection. For example, induction therapy with the B cell‐depleting agent rituximab in cross‐match negative, DSA‐positive kidney transplant recipients resulted not only in a decrease of acute antibody‐mediated rejection, but also of T cell‐mediated rejection 16.
In the current study, we aimed to determine whether B cells infiltrate renal grafts at time of acute rejection (AR), and whether we could find evidence for the involvement of peripheral blood B cells in this process.
Materials and methods
Patients
We selected 18 kidney transplant recipients who experienced a biopsy‐proven AR episode within 6 months after transplantation, as well as 22 patients with stable graft course (all transplanted between 2007 and 2011), and 20 healthy controls. Selection was based on the availability of peripheral blood samples taken before transplantation, at time of hospital discharge after transplantation and at the time of AR, prior to the initiation of anti‐rejection therapy. All patients were non‐immunized at time of transplantation and were transplanted with a negative complement‐dependent cytotoxicity (CDC) cross‐match. For the patients with stable graft function, follow‐up time‐points matching the rejection time‐points were selected (Table 1). Sufficient biopsy material was available for 16 patients in the AR group [12 T cell‐mediated rejection (TCMR) I, four TCMR II, according to the 2015 BANFF classification 17], whereas control biopsies (n = 14) were from an independent group of patients from whom a protocol biopsy or a biopsy for cause without any sign of rejection was obtained, as well as from a group of patients with acute tubular necrosis (ATN, n = 7) after transplantation. An independent validation cohort for the biopsy studies was selected and comprised 17 patients with AR and eight patients with stable graft function (Supporting information, Table S1).
Table 1.
Patient demographics
| AR | No AR | P‐value | |
|---|---|---|---|
| Number of subjects (n) | 18 | 22 | |
| Age at time of Tx (years)* | 39 (20–74) | 59 (31–74) | 0·005 |
| Transplant number (first/second) | 17/1 | 20/2 | 1·000 |
| Gender (M/F) | 10/8 | 16/6 | 0·327 |
| Donor type (living/HBD/NHBD) | 10/5/3 | 8/8/6 | 0·525 |
| Donor age (years)* | 48 (35–79) | 49 (18–77) | 0·468 |
| Total HLA‐A, ‐B, ‐DR mismatch (n)* | 3 (2–5) | 3 (0–6) | 0·602 |
| Graft function (direct/delayed) | 15/3 | 17/5 | 0·709 |
| Time to discharge (d)* | 7 (5–14) | 10 (4–20) | 0·058 |
| Time after transplantation (m)*, † | 1·6 (0–6) | 1·5 (1–6) | 0·994 |
| CMV–/– (n) | 4 | 2 | 0·381 |
HBD = heart‐beating donation; NHBD = non‐heart‐beating donation; Tx = transplant.
Mann–Whitney U‐test: age, mm, time to discharge and time after transplantation.
Fisher’s exact test: transplant number, gender, donor type, graft function, cytomegalovirus (CMV); AR = acute rejection; HLA = human leucocyte antigen; HBD = heart‐beating donation; NHBD = non‐heart‐beating donation; M/F = male/female.
Median (range).
Time‐point of rejection or follow‐up sample.
All patients received induction therapy with a CD25 blocking antibody (either dacluzimab or basiliximab) and maintenance immunosuppression with prednisone, calcineurin inhibitor (tacrolimus or cyclosporin) and mycophenolate mofetil (MMF), as previously described 18. Patients were treated routinely with oral (tablet) valganciclovir prophylaxis for 3 months, except in the case of a CMV‐negative donor recipient combination. Patient demographics are depicted in Table 1. Blood samples were obtained after informed consent in the context of studies performed in accordance with the Declaration of Helsinki Good Clinical Guidelines and the Declaration of Istanbul, and approved by the local medical ethics committee.
Cells
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll‐Paque (LUMC Pharmacy, Leiden, the Netherlands) gradient centrifugation and stored in liquid nitrogen until further use. PBMC were thawed in the presence of DNAse to prevent clump formation. Cell yield after thawing was comparable between patient groups (mean percentage living cells, AR = 93·5%, stable patients = 90·4%, healthy controls = 90·9%, P = 0·758). B cells were isolated by negative selection using the EasySep Human B cell enrichment kit (Stem Cell Technologies, Grenoble, France), according to the manufacturer’s instructions.
Biopsies
Biopsy cores for clinical indication or according protocol were obtained by percutaneous, ultrasound‐guided biopsy using an 18‐gauge needle. A minimum of two cores were collected from each patient, one core of which was formalin‐fixed, embedded in paraffin and used for histological and immunohistochemical analyses. The second biopsy core was immediately snap‐frozen in liquid nitrogen and stored at –80°C.
Immunohistochemistry
Sequential serial sections (4 μm) of paraffin‐embedded biopsy samples were dried overnight at 37oC on Superfrost+ glass slides. After deparaffinization (xylene, decreasing concentrations of ethanol, water), blocking with 30% hydrogen peroxide and antigen retrieval with 10 mM citrate buffer (pH 6.0), the sections were incubated with antibodies directed against CD20 (clone L26; Dako, Glostrup, Denmark), immunoglobulin (Ig)D (polyclonal; Abcam, Cambridge, UK), CD3 (clone ab828; Abcam) or C4d (polyclonal; Biomedica Immunoassays, Vienna, Austria). Staining protocols have been described previously 19. After rinsing the sections three times with phosphate‐buffered saline (PBS), slides were covered with Vectashield (Vector Laboratories Ltd, Burlingame, CA, USA). Photos were taken with a Leica DM5500 at ×40 magnification. The extent of CD20 positivity was determined by using ImageJ analysis 20, allowing calculation of the percentage of CD20+ surface area of the total biopsy area.
Gene expression analysis
Total RNA from isolated peripheral blood B cells or frozen biopsies was extracted using the NucleoSpin RNA kit (Macherey‐Nagel, Düren, Germany). Biopsy RNA quality was determined using Experion RNA analysis kits with the Experion Automated Electrophoresis Station (Bio‐Rad Laboratories, Veenendaal, the Netherlands). Synthesis of cDNA, primer design and quantitative polymerase chain reaction (qPCR) analysis were performed according to previously described protocols 21. Primer sequences are depicted in Table 2. IgG and IgM gene expression levels were determined as previously described 22. The mRNA expression levels were normalized to the geometric mean signal of the reference genes glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) and β‐actin.
Table 2.
Sequences for primers used in quantitative polymerase chain reaction (qPCR)
| Transcript | Forward | Reverse | Amplicon |
|---|---|---|---|
| β‐actin | ACCACACCTTCTACAATGAG | TAGCACAGCCTGGATAGC | 161 bp |
| GAPDH | ACCCACTCCTCCACCTTTGAC | TCCACCACCCTGTTGCTGTAG | 110 bp |
| CD3ε | CCGCCATCTTAGTAAAGTAACAG | AATACCACCCATTTCTTCATTACC | 131 bp |
| CD19 | GCTGGAAAGTATTATTGTC | TTGAAGATGAAGAATGCC | 177 bp |
| MS4A1 | GGGGCTGTCCAGATTATGAA | CCAGGAGTGATCCGGAAATA | 148 bp |
| IgG1 | CATCTCCAAAGCCAAAGG | ATGTCGCTGGGATAGAAG | 126 bp |
| IgG2‐4 | CATCTCCAAAGCCAAAGG | ATGTCGCTGGGGTAGAAG | 126 bp |
| IgM | CAGGGCACAGACGAACAC | CGGCAATCACTGGAAGAGG | 85 bp |
| CXCL13 | TCAGCAGCCTCTCTCCAG | GACTTGTTCTTCTTCCAGACTATG | 178 bp |
| CXCR5 | CGGCACAGCCATGAACTAC | CAATCTGTCCAGTTCCCAGAA | 82 bp |
| BAFF | CGTTCAGGGTCCAGAAGAAA | AAAGCTGAGAAGCCATGGAA | 115 bp |
GAPDH = glyceraldehyde‐3‐phosphate dehydrogenase; Ig = immunoglobulin; bp = base pairs; CCCL = C‐X‐C motif chemokine ligand; CXCR = C motif chemokine receptor; BAFF = B cell activating factor; bp: base pairs.
Flow cytometry
Flow cytometry was performed on thawed PBMC samples according to standard protocols using the following antibodies (clone): CD19 (SJ25C1), IgM (G20‐127), IgD (IA6‐2), CD3 (UCHT1), CD25 (M‐A251) (all from BD Biosciences, Breda, the Netherlands), CD27 (CLB‐CD27/1, 9F4) (Sanquin, Amsterdam, the Netherlands) and CD38 (HIT2) (eBioscience, San Diego, CA, USA) or relevant isotype controls.
B cell activation
Isolated B cells were cultured at 1 × 105 cells/well in 96‐well round‐bottomed plates (BD Falcon, Breda, the Netherlands) in Iscove’s modified Dulbecco’s medium (IMDM) (gibco Invitrogen, Paisley, UK) containing 10% fetal calf serum (FCS) (gibco Invitrogen) and supplemented with 50 μM 2‐mercaptoethanol (Sigma‐Aldrich, Zwijndrecht, the Netherlands), 2 mM L‐glutamine (gibco Invitrogen), ITS (5 μg/ml insulin, 5 μg/ml transferrin, sodium selenite 5 ng/ml (Sigma Aldrich) and 100 U/ml penicillin with 100 μg/ml streptomycin (gibco Invitrogen). B cells were activated with 500 ng/ml of agonistic anti‐CD40, 25 ng/ml of interleukin (IL)‐10 (both from R&D Systems, Abingdon, UK), 100 ng/ml of IL‐21 (Invitrogen), 600 IU/ml IL‐2 (Proleukin, Amsterdam, the Netherlands) and 2·5 μg/ml of ODN‐2006 CpG (Hycult Biotechnology, Uden, the Netherlands), as described previously 23.
Proliferation assay
B cells were activated as described above for 7 days. For the last 18 h of culture, 1 μCi tritiated thymidine ([3H]‐TdR) (Amersham International, Amersham, UK) was added per well. [3H]‐TdR incorporation was measured using a liquid scintillation counter (Wallac, Turku, Finland).
Immunoglobulin enzyme‐linked immunospot (ELISPOT) and human leucocyte antigen (HLA) antibody detection
To quantify the number of B cells producing IgM and IgG, ELISPOT assays were performed as described previously 24. Presence of HLA antibodies in serum samples was determined by using Lifecodes Lifescreen Deluxe kits (Immucor Transplant Diagnostics, Stamford, CT, USA). In the case of positive reactions, antibody specificity was determined by using LabScreen single antigen beads (One Lambda, Canoga, CA, USA).
Statistics
Clinical parameters were compared using the Mann–Whitney U‐test and Fisher’s exact test, where appropriate. Differences between patient groups were analysed using the Mann–Whitney U‐test, and differences within patient groups at different time‐points were analysed with the Wilcoxon matched‐pairs signed‐rank test with Bonferroni correction for multiple testing. Data were considered statistically significant if P < 0·05.
Results
Significant B cell infiltrates in biopsies of kidneys undergoing T cell‐mediated AR
To determine the level of B cell infiltrates in kidney biopsies during AR, we performed immunohistochemical staining for CD20 and found B cells to be abundantly present in dense clusters in 12 of 16 acute rejection biopsies, scattered throughout the tissue in two biopsies and absent in two biopsies (representative examples depicted in Fig. 1a). There was no difference in CD20 density between TCMR I and TCMR II rejections (P = 0·1033, data not shown). No, or only few, scattered B cells were found in protocol biopsies (median CD20+ staining area 2·82% for AR biopsies versus 0·19% for protocol biopsies, P = 0·0008, Fig. 1b).
Figure 1.
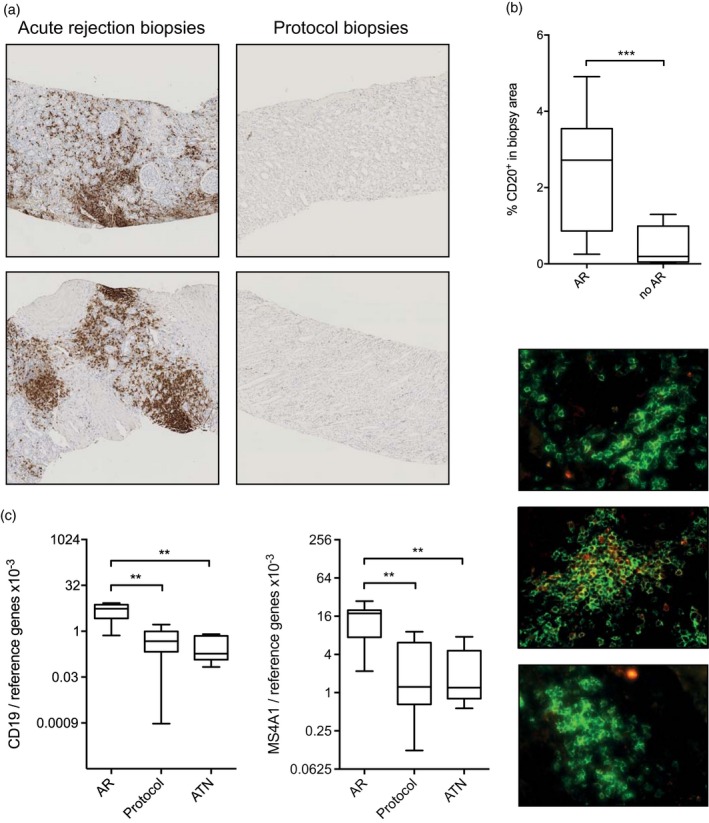
Memory B cell infiltrates are present in grafts undergoing acute rejection (AR). (a) Representative examples of the presence of B cell infiltrates during AR and the absence of B cell infiltrates during stable graft function. (b) Quantification of CD20+ B cell infiltrates in biopsies from grafts undergoing AR and during stable graft function. AR: n = 16, no AR n = 7. (c) Gene expression levels for CD19 and MS4A1 are elevated in biopsies from grafts undergoing AR compared to biopsies during stable graft function and grafts showing acute tubular necrosis (ATN). AR: n = 7, protocol: n = 9, ATN: n = 7. (d) Representative examples of fluorescent stainings of CD20 (green) and IgD (red). Level of significance: *P < 0·05, **P < 0·01, ***P < 0·001.
To confirm our immunohistochemistry data, we assessed CD19 and MS4A1 (encoding for CD20) gene expression levels in the biopsies (frozen biopsy samples available for seven AR patients and nine patients with stable function). For this analysis, we included seven biopsies showing ATN as an additional control group without immune‐mediated graft damage. We observed significantly higher CD19 gene expression levels in the AR group compared to the protocol biopsy group (P = 0·0012) and the ATN group (P = 0·0012, Fig. 1c), confirming our immunohistochemical data. Similar differences were found for MS4A1 (P = 0·0021 and P = 0·0041, respectively). CD20 protein expression and MS4A1 gene expression were correlated (Spearman’s ρ = 0·65, P = 0·04, data not shown). We did not observe any difference in either CD19 or MS4A1 gene expression in TCMR I and TCMR II rejections (P = 0·8571, data not shown). We further validated our immunohistochemical and qPCR findings in a second, independent group of kidney transplant recipients, and found a similar, albeit slightly less pronounced increase in CD20+ staining area and MS4A1 mRNA expression in AR biopsies compared to controls (Supporting information, Fig. S1).
To gain more insight into the quality of the B cell infiltrates during AR we performed simultaneous immunofluorescent staining for CD20 and IgD. Naive B cells are positive for both markers, whereas memory B are positive only for CD20. Representative examples are shown in Fig. 1d. The majority of B cells found in kidney biopsies during AR were CD20 single‐positive–, implying a memory phenotype. Unfortunately, corroboration of the memory phenotype by staining for CD27 was unsuccessful, due to high expression of this marker by surrounding T cells.
Phenotypical changes in the peripheral B cell pool upon AR
As we found B cells abundantly present in the vast majority of biopsies at time of AR, we assessed whether we could find any indirect evidence for the involvement of B cells derived from peripheral blood (flow cytometry gating strategy depicted in Fig. 2a). In both patients with AR and controls there was a slight, but significant increase in the relative level of CD19+ B cells at time of hospital discharge, which normalized upon the final time‐point analysed (Fig. 2b). We then determined the percentages of switched memory, unswitched memory and naive B cells in the peripheral blood at the aforementioned time‐points. Whereas in stable patients the relative levels of naive and memory B cells within the total B cell population remained constant after transplantation at the discharge time‐point, as well as the follow‐up time‐point, we observed a strong decrease in the relative level of memory B cells at time of AR (pre‐transplant versus AR, P = 0·0051, discharge versus AR, P = 0·0006), coinciding with a relative increase in the level of naive B cells (pretransplant versus AR, P = 0·027, discharge versus AR, P = 0·0015, Fig. 2c,d). To corroborate these findings, we also performed analysis on naive and memory B cell compartments using IgD and CD38 (Bm1–Bm5 classification) and IgD with IgM (double‐negative cells representing class‐switched memory B cells), and found similar results (Supporting information, Fig. S2).
Figure 2.
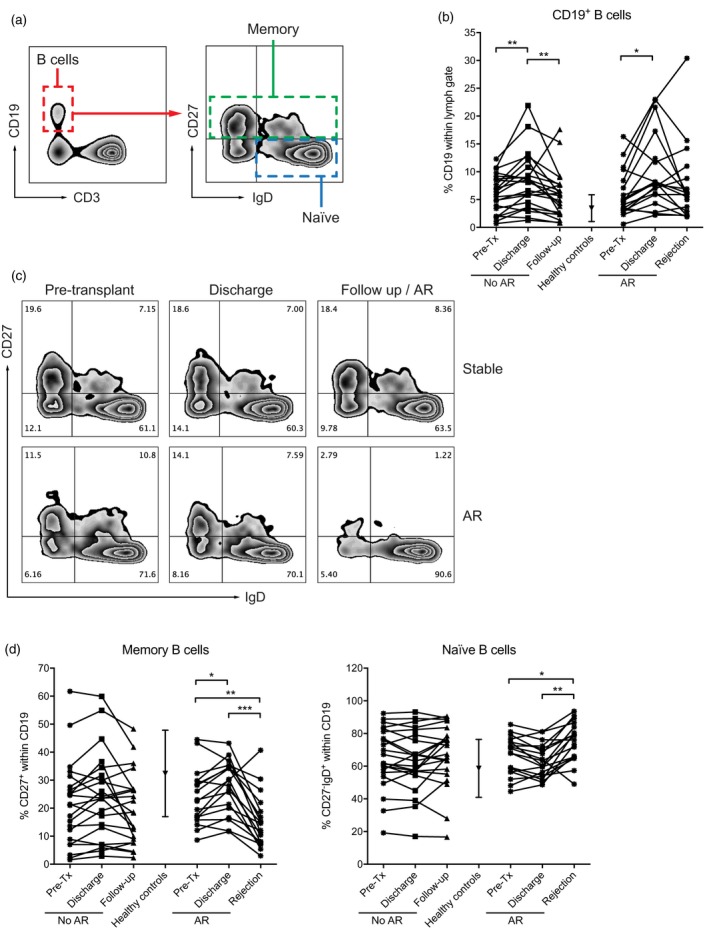
The peripheral blood subset distribution is significantly altered at time of acute rejection (AR). (a) Flow cytometry gating strategy. (a) CD19+ B cells are increased at time of hospital discharge and normalize at time of rejection or follow‐up. (c) Representative examples of CD27 and immunoglobulin (Ig)D staining of a patient with stable graft function and a patient who underwent AR. (d) Memory B cell levels (defined by CD27 positivity) are increased at time of AR, coinciding with a decrease in naive B cells (defined by CD27 negativity and IgD positivity). Level of significance: *P < 0·05, **P < 0·01, ***P < 0·001.
AR is accompanied by a change in ratio of IgM‐ and IgG‐producing B cells
We further validated our findings on peripheral B cell phenotype changes during AR by gene expression analysis on isolated B cells from peripheral blood. In line with the flow cytometry data, we observed a decrease in the level of IgG expression within the total B cell population at time of AR (Fig. 3b) compared to the discharge time‐point (P = 0·0051), leading to an increase in the ratio of IgM over IgG transcripts (P = 0·0243, data not shown). No differences were found in IgM expression levels (Fig. 3a) for patients with AR, or in IgM and IgG expression levels for stable patients (Fig. 3a,b).
Figure 3.
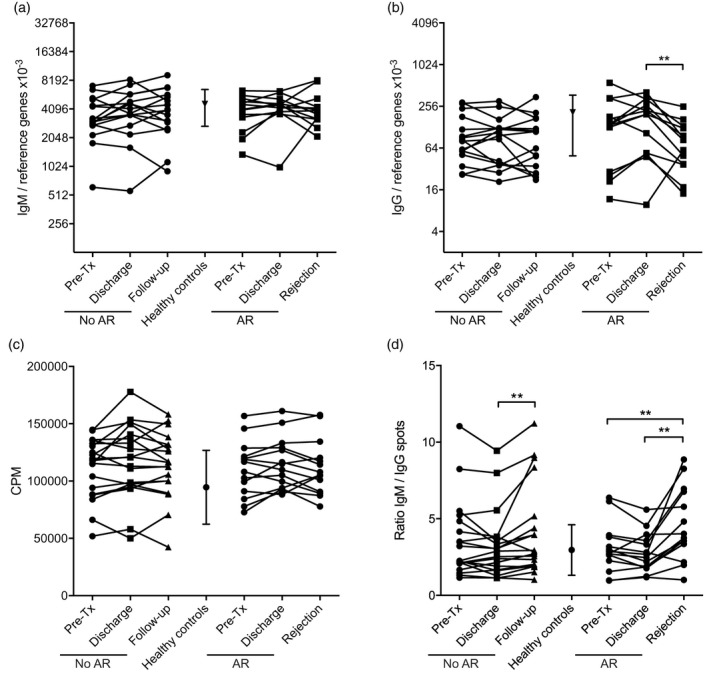
Characterization of peripheral B cells in patients at time of acute rejection (AR) or controls. (a) In all samples immunoglobulin (Ig)M gene expression levels remained stable, whereas (b) IgG gene expression levels were decreased at time of AR. (c) Polyclonal B cell activation did not show a difference in proliferative capacity between patients with AR or stable patients, or within the patient groups in time. (d) Ratio of IgM over IgG producing numbers of B cells upon polyclonal activation as measured by enzyme‐linked immunospot (ELISPOT) revealed that patients with AR and patients with stable graft function show a similar increase in IgM‐producing B cells from the moment of discharge. Level of significance: *P < 0·05, **P < 0·01, ***P < 0·001.
When we polyclonally activated isolated B cells for 6 days using a CD40‐based activation cocktail, B cells from both patient groups at all time‐points showed similar proliferative responses (Fig. 3c). Furthermore, no differences in the level of CD25 expression after activation were visible (data not shown). After activation we observed a significant increase in the ratio of cells producing IgM over those producing IgG at time of AR (pretransplant versus AR, P = 0·0093, discharge versus AR, P = 0·0051, Fig. 3d) by ELISPOT. We also observed an increase in the IgM over IgG spot ratio in the stable patient cohort in the follow‐up sample (discharge versus follow‐up, P = 0·0099), albeit less profound.
Donor‐specific antibodies and C4d staining in biopsies
We next investigated whether the B cells during AR were contributing to DSA production by differentiation towards antibody producing plasma cells. De‐novo DSA were detected in the serum of four of 18 patients at time of AR (22%) and in one of 22 (5%) stable patients at the follow‐up time‐point. Strikingly, all DSA were directed at mismatched HLA‐DQ antigens (one patient also against mismatched DR). Although circulating DSA were more often present in patients in the AR group, C4d positivity was found in none of the biopsies (data not shown).
B cells may interact with T cells in the graft
We analysed CD3 gene expression in AR biopsies, and found high mRNA expression levels compared to protocol biopsies (P = 0·0002) and biopsies showing ATN (P = 0·0012, Fig. 4a, left panel). We found a strong correlation between CD3 and MS4A1 transcripts (Spearman’s ρ = 0·86, P < 0·0001), indicating that the level of infiltrating B cells was proportional to that of T cells (Fig. 4a, right panel). As ectopic lymphoid structures have been shown to develop through local production of several chemokines such as CXCL13, we determined gene expression levels of CXCL13, as well as its ligand C‐X‐C motif chemokine receptor 5 (CXCR5). CXCL13 gene expression was significantly higher in AR biopsies compared to protocol biopsies (P = 0·0021) and biopsies showing ATN (P = 0·0006, Fig. 4b, left panel). Similarly, CXCR5 was elevated in AR biopsies compared to protocol biopsies (P = 0·0003) and biopsies showing ATN (P = 0·0006, Fig. 4b, centre panel). MS4A1 gene expression correlated strongly with CXCR5 (Spearman’s ρ = 0.91, P < 0·0001, data not shown), as well as with CXCL13 (Spearman’s ρ = 0·79, P < 0·0001, Fig. 4c, left panel). B cell activating factor (BAFF), an important B cell survival factor produced by dendritic cells in germinal centres, was significantly more highly expressed in AR biopsies compared to protocol biopsies (P = 0·0003) and biopsies showing ATN (P = 0·0041, Fig. 4b, right panel). The expression levels of BAFF and CXCL13 correlated highly with each other (Spearman’s ρ = 0·78, P < 0·0001, Fig. 4c, right panel).
Figure 4.
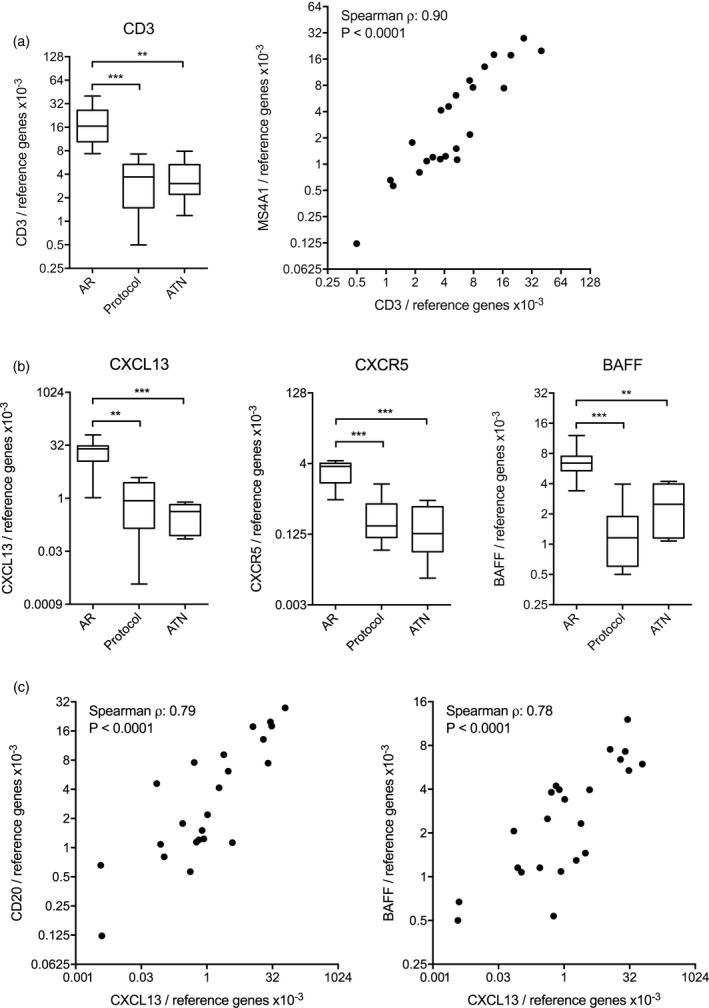
B cell infiltrates are correlated to T cell infiltrates and accompanying chemokines. (a) Gene expression levels for CD3 are elevated in biopsies from grafts undergoing acute rejection (AR) compared to biopsies taken during stable graft function or on indication of acute tubular necrosis (ATN). CD3 gene expression levels strongly correlate to MS4A1 gene expression levels. (b) Gene expression levels for C‐X‐C motif chemokine ligand 13 (CXCL13), C‐X‐C motif chemokine receptor 5 (CXCR5) and B cell activating factor (BAFF) are elevated in biopsies from grafts undergoing AR compared to biopsies during stable graft function and grafts showing ATN. Level of significance: *P < 0·05, **P < 0·01, ***P < 0·001. (c) CXCL13 gene expression levels strongly correlate both to MS4A1 and BAFF gene expression levels.
Discussion
In this study, we have investigated graft infiltrating B cells as well as peripheral blood B cells in kidney transplant recipients at time of AR. We found B cells abundantly present in the graft at time of AR, accompanied by a change in the peripheral blood B cell subset distribution. While these findings stand by themselves, they are suggestive for a role of B cells in acute T cell‐mediated rejection.
Infiltration of B cells at the time of AR has been reported previously, albeit with conflicting data on whether AR episodes with B cell infiltrates are more resistant to steroid treatment and result in inferior graft survival 10, 11, 12, 13, 25, 26, 27, 28. Interestingly, and in concert with a recent study 14, in our cohort the majority of biopsies taken at time of AR showed signs of B cell infiltration. This infiltration is not limited to B cells, as we observed a strong correlation with infiltrating T cells. High expression of CXCL13 and CXCR5 in the graft are suggestive for a chemokine‐induced homing of both CXCR5+ B cells and T cells to the graft 15, 29, 30. Follicular helper T cells (Tfh) are characterized by expression of CXCR5, and pivotal for germinal centre formation. The formation of ectopic lymphoid structures has been described at sites of chronic inflammation during autoimmunity 31, 32, 33, 34, 35 as well as in various forms of renal inflammation 36. Similarly, in both mouse and human organ transplants, chronic rejection has been associated with the formation of ectopic lymphoid structures 37, 38, 39, in which T cells can be primed to become effector and memory T cells 40. In a study using cell distance mapping, Liarski and colleagues provided evidence for cognate interaction between Tfh cells and B cells in inflamed human renal tissue, including renal biopsies taken at time of AR 41. Additionally, we found elevated levels of BAFF gene expression in the graft, which is in concert with data on B cell infiltrates during chronic rejection 39. Interestingly, the combination of CXCL13 and BAFF have been shown to preferentially lead to chemoattraction of memory B cells in in‐vitro studies 42. Formal proof of cognate T and B cell interaction in future studies will be required to further clarify the role of these cells in TCMR. Our data on the peripheral blood B cell subset distribution showed a shift towards a decreased percentage of memory B cells at time of AR. The intragraft B cell infiltrates suggest that B cells may home to the graft by chemokine‐mediated signals. Unfortunately, the limitation of clinical samples did not allow us to establish formally whether these peripheral blood derived memory B cells home to the graft and give rise to the substantial B cell infiltrates we observed. Given the sheer number of B cells in the peripheral blood and the dramatic change in B cell subset distribution, homing to the graft only is unlikely, and additional homing to the secondary lymphoid organs seems a plausible scenario.
Interestingly, in a previous study, van de Berg et al. determined the B cell subset distribution in renal transplant recipients with stable graft function (n = 10) as well as subclinical (n = 10) or clinical AR (n = 10) at 6 months, 3 years and 5 years after transplantation, and found no detectable differences in B cell subsets, defined by CD27 and IgD expression 43. Differences in timing after transplantation and immunosuppressive treatment may underlie these discordant results. While this study and ours both have used thawed PBMC for flow cytometric analysis, freezing and thawing may alter cell subset frequencies, which should be kept in mind when comparing to other studies.
The absence of C4d staining in the biopsies studied here are suggestive for an antibody‐independent role of B cells, as has been suggested previously 10, 11. As antibody‐mediated rejection can occur in the absence of C4d staining in the biopsy 17, we also determined the presence of circulating de‐novo DSA. Partially, DSA may have been involved in the rejection cases studied, as 22% of patients with AR showed DSA against mismatched HLA‐DQ antigens. The prevalence of DSA against HLA‐DQ is not surprising, as this is the most frequent DSA reported after transplantation 44. However, while DSA were found more frequently in the AR group, the majority of patients with AR did not show de‐novo circulating DSA. Moreover, no correlation between the presence of de‐novo DSA and the extent of B cell infiltration in biopsies was observed (data not shown).
There is increasing evidence for antibody‐independent functions of B cells in the setting of organ transplantation, such as antigen presentation and immune regulation. While the current study is limited by its retrospective nature and the inability to formally link the observations in the graft and the peripheral blood, our data contribute to the notion that B cells may be involved in cellular rejection events, and they warrant further research on the interaction of B cells and T cells in these processes.
Disclosures
The authors declare that they have no competing financial interests.
Author contributions
S. H. designed the study, performed experiments, analyzed data and wrote the manuscript. M. V., J. D. H. A., G. M. J. S. S. and M. J. W. performed experiments. E. M. J. G., K. E. G., D. L. R. and M. E. analyzed data. H. W. F. designed the study. M. E. J. R. and F. H. J. C. designed the study and co‐wrote the manuscript.
Supporting information
Fig. S1. (a) Quantification of CD20+ B cell infiltrates in biopsies from grafts undergoing AR and during stable graft function in an independent validation cohort. (b) Gene expression levels for MS4A1 are elevated in biopsies from grafts undergoing AR compared to biopsies during stable graft function in an independent validation cohort.
Fig. S2. Alternative gating for peripheral B cell subsets. (a) Gating strategy Bm1‐Bm5 classification within CD19+ B cells. (b‐d) Early and late Bm5 memory B cells are decreased at time of AR, whereas Bm2 activated naïve B cells are increased. (e) Gating strategy IgM‐IgD‐ class‐switched B cells within CD19+ B cells. (f) Class‐switched B cells are decreased at time of AR. Level of significance: *: P < 0.05, **: P < 0.01.
Table S1. Patient demographics validation cohort.
Acknowledgements
The authors would like to thank Geert Haasnoot for assistance in statistical analysis, Malu Zandbergen (both Leiden University Medical Center) for cutting the biopsy sections and Marcel Kaps and Marian Clahsen‐van Groningen (Erasmus Medical Center, Rotterdam, the Netherlands) for the scanning of immunohistochemistry slides. This work was supported by the National Reference Center for Histocompatibility Testing.
References
- 1. Loupy A, Lefaucheur C, Vernerey D et al Complement‐binding anti‐HLA antibodies and kidney‐allograft survival. N Engl J Med 2013; 369:1215–26. [DOI] [PubMed] [Google Scholar]
- 2. Lund FE. Cytokine‐producing B lymphocytes‐key regulators of immunity. Curr Opin Immunol 2008; 20:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodriguez‐Pinto D. B cells as antigen presenting cells. Cell Immunol 2005; 238:67–75. [DOI] [PubMed] [Google Scholar]
- 4. Yuseff MI, Pierobon P, Reversat A, Lennon‐Dumenil AM. How B cells capture, process and present antigens: a crucial role for cell polarity. Nat Rev Immunol 2013; 13:475–86. [DOI] [PubMed] [Google Scholar]
- 5. Noorchashm H, Reed AJ, Rostami SY et al B cell‐mediated antigen presentation is required for the pathogenesis of acute cardiac allograft rejection. J Immunol 2006; 177:7715–22. [DOI] [PubMed] [Google Scholar]
- 6. Ng YH, Oberbarnscheidt MH, Chandramoorthy HC, Hoffman R, Chalasani G. B cells help alloreactive T cells differentiate into memory T cells. Am J Transplant 2010; 10:1970–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thaunat O, Patey N, Caligiuri G et al Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J Immunol 2010; 185:717–28. [DOI] [PubMed] [Google Scholar]
- 8. Cheng J, Torkamani A, Grover RK et al Ectopic B‐cell clusters that infiltrate transplanted human kidneys are clonal. Proc Natl Acad Sci USA 2011; 108:5560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferdman J, Porcheray F, Gao B et al Expansion and somatic hypermutation of B‐cell clones in rejected human kidney grafts. Transplantation 2014; 98:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarwal M, Chua MS, Kambham N et al Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med 2003; 349:125–38. [DOI] [PubMed] [Google Scholar]
- 11. Zarkhin V, Kambham N, Li L et al Characterization of intra‐graft B cells during renal allograft rejection. Kidney Int 2008; 74:664–73. [DOI] [PubMed] [Google Scholar]
- 12. Hippen BE, DeMattos A, Cook WJ, Kew CE II, Gaston RS. Association of CD20+ infiltrates with poorer clinical outcomes in acute cellular rejection of renal allografts. Am J Transplant 2005; 5:2248–52. [DOI] [PubMed] [Google Scholar]
- 13. Tsai EW, Rianthavorn P, Gjertson DW, Wallace WD, Reed EF, Ettenger RB. CD20+ lymphocytes in renal allografts are associated with poor graft survival in pediatric patients. Transplantation 2006; 82:1769–73. [DOI] [PubMed] [Google Scholar]
- 14. de Leur K, Clahsen‐van Groningen MC, van den Bosch TPP et al Characterization of ectopic lymphoid structures in different types of acute renal allograft rejection. Clin Exp Immunol 2018; 192:224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steinmetz OM, Panzer U, Kneissler U et al BCA‐1/CXCL13 expression is associated with CXCR15‐positive B‐cell cluster formation in acute renal transplant rejection. Kidney Int 2005; 67:1616–21. [DOI] [PubMed] [Google Scholar]
- 16. Ishida H, Furusawa M, Shimizu T, Nozaki T, Tanabe K. Influence of preoperative anti‐HLA antibodies on short‐ and long‐term graft survival in recipients with or without rituximab treatment. Transpl Int 2014; 27:371–82. [DOI] [PubMed] [Google Scholar]
- 17. Loupy A, Haas M, Solez K et al The Banff 2015 Kidney Meeting Report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant 2017; 17:28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roos‐van Groningen MC, Scholten EM, Lelieveld PM et al Molecular comparison of calcineurin inhibitor‐induced fibrogenic responses in protocol renal transplant biopsies. J Am Soc Nephrol 2006; 17:881–8. [DOI] [PubMed] [Google Scholar]
- 19. Schonkeren D, van der Hoorn M‐L, Khedoe P et al Differential distribution and phenotype of decidual macrophages in preeclamptic versus control pregnancies. Am J Pathol 2011; 178:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eikmans M, Rekers NV, Anholts JD, Heidt S, Claas FH. Blood cell mRNAs and microRNAs: optimized protocols for extraction and preservation. Blood 2013; 121:e81–e89. [DOI] [PubMed] [Google Scholar]
- 22. Heidt S, Roelen DL, Eijsink C, Eikmans M, Claas FH, Mulder A. Intravenous immunoglobulin preparations have no direct effect on B cell proliferation and immunoglobulin production. Clin Exp Immunol 2009; 158:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karahan GE, Eikmans M, Anholts JD, Claas FH, Heidt S. Polyclonal B cell activation for accurate analysis of pre‐existing antigen‐specific memory B cells. Clin Exp Immunol 2014; 177:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heidt S, Roelen DL, Eijsink C, van Kooten C, Claas FH, Mulder A. Effects of immunosuppressive drugs on purified human B cells: evidence supporting the use of MMF and rapamycin. Transplantation 2008; 86:1292–300. [DOI] [PubMed] [Google Scholar]
- 25. Jiqiu W, Jinsong C, Dongrui C, Mingchao Z, Shuming J, Zhi‐Hong L. CD20+ B‐cell infiltration is related to the time after transplant and poor prognosis of acute cellular rejection in renal transplant. Exp Clin Transplant 2013; 11:412–7. [DOI] [PubMed] [Google Scholar]
- 26. Doria C, di Francesco F, Ramirez CB et al The presence of B‐cell nodules does not necessarily portend a less favorable outcome to therapy in patients with acute cellular rejection of a renal allograft. Transplant Proc 2006; 38:3441–4. [DOI] [PubMed] [Google Scholar]
- 27. Martins HL, Silva C, Martini D, Noronha IL. Detection of B lymphocytes (CD20+) in renal allograft biopsy specimens. Transplant Proc 2007; 39:432–4. [DOI] [PubMed] [Google Scholar]
- 28. Bagnasco SM, Tsai W, Rahman MH et al CD20‐positive infiltrates in renal allograft biopsies with acute cellular rejection are not associated with worse graft survival. Am J Transplant 2007; 7:1968–73. [DOI] [PubMed] [Google Scholar]
- 29. Steinmetz OM, Lange‐Husken F, Turner JE et al Rituximab removes intrarenal B cell clusters in patients with renal vascular allograft rejection. Transplantation 2007; 84:842–50. [DOI] [PubMed] [Google Scholar]
- 30. de Graav GN, Dieterich M, Hesselink DA et al Follicular T helper cells and humoral reactivity in kidney transplant patients. Clin Exp Immunol 2015; 180:329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schroder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci USA 1996; 93:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen‐driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjogren's syndrome. J Clin Invest 1998; 102:938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Armengol MP, Juan M, Lucas‐Martin A et al Thyroid autoimmune disease: demonstration of thyroid antigen‐specific B cells and recombination‐activating gene expression in chemokine‐containing active intrathyroidal germinal centers. Am J Pathol 2001; 159:861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sims GP, Shiono H, Willcox N, Stott DI. Somatic hypermutation and selection of B cells in thymic germinal centers responding to acetylcholine receptor in myasthenia gravis. J Immunol 2001; 167:1935–44. [DOI] [PubMed] [Google Scholar]
- 35. Corcione A, Casazza S, Ferretti E et al Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci USA 2004; 101:11064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heller F, Lindenmeyer MT, Cohen CD et al The contribution of B cells to renal interstitial inflammation. Am J Pathol 2007; 170:457–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kurkijarvi R, Jalkanen S, Isoniemi H, Salmi M. Vascular adhesion protein‐1 (VAP‐1) mediates lymphocyte–endothelial interactions in chronic kidney rejection. Eur J Immunol 2001; 31:2876–84. [DOI] [PubMed] [Google Scholar]
- 38. Thaunat O, Field AC, Dai J et al Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci USA 2005; 102:14723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thaunat O, Patey N, Gautreau C et al B cell survival in intragraft tertiary lymphoid organs after rituximab therapy. Transplantation 2008; 85:1648–53. [DOI] [PubMed] [Google Scholar]
- 40. Nasr IW, Reel M, Oberbarnscheidt MH et al Tertiary lymphoid tissues generate effector and memory T cells that lead to allograft rejection. Am J Transplant 2007; 7:1071–9. [DOI] [PubMed] [Google Scholar]
- 41. Liarski VM, Kaverina N, Chang A et al Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci Transl Med 2014; 6:230ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Badr G, Borhis G, Lefevre EA et al BAFF enhances chemotaxis of primary human B cells: a particular synergy between BAFF and CXCL13 on memory B cells. Blood 2008; 111:2744–54. [DOI] [PubMed] [Google Scholar]
- 43. van de Berg PJ, Hoevenaars EC, Yong SL et al Circulating lymphocyte subsets in different clinical situations after renal transplantation. Immunology 2012; 136:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. DeVos JM, Gaber AO, Knight RJ et al Donor‐specific HLA‐DQ antibodies may contribute to poor graft outcome after renal transplantation. Kidney Int 2012; 82:598–604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. (a) Quantification of CD20+ B cell infiltrates in biopsies from grafts undergoing AR and during stable graft function in an independent validation cohort. (b) Gene expression levels for MS4A1 are elevated in biopsies from grafts undergoing AR compared to biopsies during stable graft function in an independent validation cohort.
Fig. S2. Alternative gating for peripheral B cell subsets. (a) Gating strategy Bm1‐Bm5 classification within CD19+ B cells. (b‐d) Early and late Bm5 memory B cells are decreased at time of AR, whereas Bm2 activated naïve B cells are increased. (e) Gating strategy IgM‐IgD‐ class‐switched B cells within CD19+ B cells. (f) Class‐switched B cells are decreased at time of AR. Level of significance: *: P < 0.05, **: P < 0.01.
Table S1. Patient demographics validation cohort.


