Summary
Psoriasis (Ps), psoriatic arthritis (PsA) and rheumatoid arthritis (RA) are common diseases dependent on environmental factors that activate the immune system in unknown ways. Mannan is a group of polysaccharides common in the environment; they are potentially pathogenic, because at least some of them induce Ps‐, PsA‐ and RA‐like inflammation in mice. Here, we used positron emission tomography/computed tomography to examine in‐vivo transport and spread of mannan labelled with fluorine‐18 [18F]. The results showed that mannan was transported to joints (knee) and bone marrow (tibia) of mice within 6 h after intraperitoneal injection. The time it took to transport mannan, and its presence in blood, indicated cellular transport of mannan within the circulatory system. In addition, mannan was filtered mainly through the spleen and liver. [18F]fluoromannan was excreted via kidneys, small intestine and, to some extent, the mouth. In conclusion, mannan reaches joints rapidly after injection, which may explain why mannan‐induced inflammatory disease is targeted to these tissues.
Keywords: arthritis, biodistribution, [18F]fluoromannan, psoriatic arthritis, psoriasis
Introduction
Rheumatoid arthritis (RA), psoriasis (Ps) and psoriatic arthritis (PsA) are common inflammatory diseases in humans, triggered by unknown environmental factors in genetically susceptible individuals. These inflammatory diseases can be chronic, systemic and disabling. Ps is characterized by inflammation and hyperproliferation of the skin; in PsA, the joints are also affected. Ps affects approximately 2–3% of the population worldwide, and approximately 25% of these patients develop PsA. RA is a common autoimmune disease that causes pain and inflammatory erosions in the joints. It also affects the cardiovascular system and is associated with other comorbidities; eventually, the disease leads to disability and decreased quality of life 1, 2, 3. Recent studies show that the natural polysaccharide mannan, derived from the baker's yeast Saccharomyces cerevisiae, induces Ps‐ and PsA‐like diseases [mannan‐induced Ps (MIP)] in mice 4. If these mice are pre‐injected with anti‐collagen type II (aCII) antibodies they develop a chronic RA‐like arthritis [known as mannan‐enhanced collagen antibody‐induced arthritis (mCAIA)] 5. Mannan can interact with an array of different pattern recognition receptors, including Toll‐like receptors (TLRs), mannan‐binding lectin and C‐type lectins such as the macrophage mannose receptor (MR, also known as CD206) 6, 7, 8. Both MIP and mCAIA are driven by the innate immune system; i.e. they are not dependent on adaptive immune cells such as B and T cells. MIP and mCAIA are regulated by nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase type 2 (NOX2)‐derived reactive oxygen species (ROS); indeed, mice lacking the neutrophil cytosolic factor 1 (Ncf1) gene develop more severe disease 4, 5, 9. The precise role played by mannans in inducing Ps‐like diseases and (in mice pre‐injected with aCII antibodies) chronic joint‐specific inflammatory disease (i.e. rheumatoid‐like arthritis) is unclear. One possibility is that injected mannan persists within joints and triggers chronic activation of macrophages. However, accumulation of mannan in joints would have to be somehow exacerbated by prior exposure to aCII antibodies, as mannan alone does not have the same effects 4, 5, 10. Another possible explanation could be that mannan is transported to the joints.
One approach to exploring the pathological process underlying MIP, PsA and arthritis in mice is to monitor the biodistribution of mannan within the body. We demonstrated previously that mannan can be labelled with the positron‐emitting radionuclide fluorine‐18 [18F] without affecting its biological properties 11. Here, we examined the biodistribution of [18F]fluoromannan in mice using positron emission tomography/computed tomography (PET/CT) techniques.
Methods
Mice
Female BQ.Ncf1m1J mice, age‐matched within 8–23 weeks between the groups, were used in all experiments. BQ.Ncf1m1J is a mouse strain that is more susceptible to arthritis 9, 12. As previously described 13, mice were housed in the Central Animal Laboratory of University of Turku under specific pathogen‐free conditions and provided with environmental enrichment, standard chow and water ad libitum. All animal experiments were approved by the national Animal Experiment Board in Finland and the Regional State Administrative Agency for Southern Finland (ESAVI/439/04.10.07/2017 and ESAVI/3116/04.10.07/2017), and were carried out in compliance with European Union directives.
In‐vivo administration of [18F]‐labelled mannan
Mannan from S. cerevisiae was purchased from Sigma (St Louis, MO, USA; Cat. no. M7504;) and was labelled with [18F] as described previously 11. The quality control was performed with high‐performance liquid chromatography (HPLC) and thin layer chromatography (TLC). In HPLC analyses, XBridge size‐exclusion column (Waters, Milford, MA, USA; 150 × 7·8 mm, 125 Å, 3·5 μm) was used, the solvent was water and the flow rate was 0·4 ml/min. In TLC analyses, normal phase silica gel TLC plates (Merck, Darmstadt, Germany) were used and the eluent was water in acetonitrile (5% by volume). After development, TLC plates were exposed to Fujifilm BAS‐IP MS 2325 imaging plates, which were subsequently scanned with a Fujifilm BAS‐1800II reader (Fuji, Tokyo, Japan), and evaluated with aida software (Raytest Isotopenmessgeräte GmbH, Straubenhardt, Germany).
Mice were divided into four groups; each mouse in each group received 20 MBq of [18F]‐labelled mannan ([18F]fluoromannan; approximately 2 μg) for PET/CT imaging. The mice in group 1 received [18F]fluoromannan only; mice in group 2 received [18F]fluoromannan plus 2 mg of unlabelled mannan; mice in group 3 received [18F]fluoromannan plus 10 mg of unlabelled mannan (to induce MIP) 4, 10; and mice in group 4 received an intravenous injection of an anti‐CAIA antibody cocktail 5 days prior to imaging plus 2 mg of unlabelled mannan together with [18F]fluoromannan on the day of imaging (to induce mCAIA) 5. All the injections with unlabelled mannan and [18F]fluoromannan were performed intraperitoneally (i.p.); i.p. injection was chosen to facilitate direct comparisons of disease data using the mouse models mCAIA 5 and MIP 4, 10, both disease models being induced by an i.p. injection of mannan. A summary of the four injection groups is presented in Table 1.
Table 1.
Summary of the four injection groups. Group 1 was injected with only labelled mannan, groups 2–4 were supplemented with non‐labelled mannan as indicated. Group 4 also received anti‐collagen type II antibody (aCII antibody), as indicated
| Group | Day –5 | Day 0 (PET/CT imaging day) | Mice per group |
|---|---|---|---|
| 1 | No injection | 2 µg [18F]fluoromannan | 2 |
| 2 | No injection | 2 µg [18F]fluoromannan + 2 mg mannan | 6 |
| 3 | No injection | 2 µg [18F]fluoromannan + 10 mg mannan | 7 |
| 4 | aCII antibody cocktail | 2 µg [18F]fluoromannan + 2 mg mannan | 3 |
PET/CT = positron emission tomography/computerized tomography; [18F]fluoromannan = fluorine‐18.
PET studies with [18F]fluoromannan
Prior to in‐vivo PET/CT imaging, mice were anaesthetized with isoflurane (3–4% for induction and 1–2% isoflurane for maintenance, using air as a carrier gas during induction and oxygen during imaging both at a flow rate of 200–300 ml/h) and a urinary catheter was inserted to minimize accumulation of radioactivity in the urinary bladder. Next, mice received an intraperitoneal injection of 20 ± 1·0 MBq of [18F]fluoromannan. Dynamic PET imaging (Inveon Multimodality PET/CT System; Siemens Medical Solutions, Knoxville, TN, USA) was performed over 6 h. Thereafter, CT was performed for anatomical reference and attenuation correction. PET data were acquired in a list mode and iteratively reconstructed using an ordered subset expectation maximization two‐dimensional (2D) algorithm, followed by maximum a posteriori reconstruction into 10 × 60, 4 × 300, 12 × 600 and 12 × 900 s time‐frames (matrix size = 128 × 128 × 159, pixel size = 0·776 × 0·776 × 0·796 mm). The PET scans were corrected for dead time, decay and photon attenuation, and the image reconstruction algorithm included random and scatter correction. In addition, the PET camera, dose calibrator and gamma counter were cross‐calibrated.
Quantitative PET analyses were performed using Inveon Research Workplace 4.1 software (Siemens Medical Solutions, Malvern, PA, USA). Co‐registration of PET and CT images was automatic, and the PET data were corrected with respect to injected radioactivity dose and radionuclide decay. Regions of interest (ROIs) were defined in the brain, heart, knees, liver, mouth, small intestine, spine, spleen, thymus and tibia, using CT scans for anatomical reference. The mean volume of each ROI (mm3) is presented in Supporting information, Table 1. Standardized uptake values (SUVs)were calculated as a ratio of the mean radioactivity concentration (Bq/ml) of ROI and the injected radioactivity dose was divided by body weight. Unfortunately, the signal from the peritoneum of some mice was so strong that the calculation for SUV in the tibia and knees became unfeasible, and the corresponding tibia and knees in these mice were excluded from the analysis.
Ex‐vivo biodistribution of [18F]fluoromannan
Biodistribution of [18F]fluoromannan was examined ex vivo in a subset of mice (two mice from group 1, two mice from group 3 and two mice from group 4). The rest of the mice were macroscopically studied for disease development (data not shown). At 6 h post‐injection of [18F]fluoromannan, blood was collected by cardiac puncture and mice were euthanized by cervical dislocation. To remove potential signal from blood in the tissues, the mice from groups 3 and 4 were perfused with phosphate‐buffered saline (PBS) before euthanasia. Several tissues were then excised and weighed, and the amount of radioactivity was measured using a gamma counter (Triathler 3″; Hidex Oy, Turku, Finland). Bone marrow was collected by carefully opening and wiping the bone using a cotton swab. Radioactivity measurements were normalized to injected radioactivity dose, radioactive decay and the weight of the tissue sample. Results were expressed as SUVs (all figures) and as the percentage of injected radioactivity dose per gram of tissue (%ID/g) (Supporting information, Table S2).
Statistics
The graphs and statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA), versions 7.0 and 8.0. Student's t‐test was used to analyse the end‐point data (6 h post‐mannan injection) of the in‐vivo study and P < 0·05 was considered significant.
Results
Radiochemistry
[18F]Fluoromannan was obtained in decay‐corrected radiochemical yields of 7·7 ± 2·1% [mean ± standard deviation (s.d.), n = 12] and the radiochemical purity was >95%, as measured by HPLC and TLC. [18F]fluoromannan was stable in PBS (pH 7.4), as tested for up to 4·5 h at room temperature.
Mannan is transported to the skin, joints and bone marrow
Mannan was not (or barely) detectable in the knee joints of mice treated with [18F]fluoromannan (approximately 2 μg, group 1) alone (Figs. 1a and 2a). However, when injected together with 2 or 10 mg of additional unlabelled mannan (groups 2–3), it was detected in knee joints and tibiae after approximately 6 h. Although the differences were not so obvious in PET images (Fig. 1b,c), the quantitative ROI analysis revealed increased uptake in knee and tibia (Fig. 2a). There was no macroscopically visual arthritis in mice from any group at 6 h post‐injection. However, we have previously shown 4, 5, 10 that arthritis appears in the mice at later stages of disease when injected with 10 mg of mannan‐inducing MIP (visual disease starting at approximately days 2–3) or with 2 mg of mannan + aCII antibodies inducing mCAIA (visual disease starting at approximately day 7), with an incidence of at least 80%. Mice injected with 2 mg of mannan alone showed mild disease, with a mean incidence of 40% 5.
Figure 1.
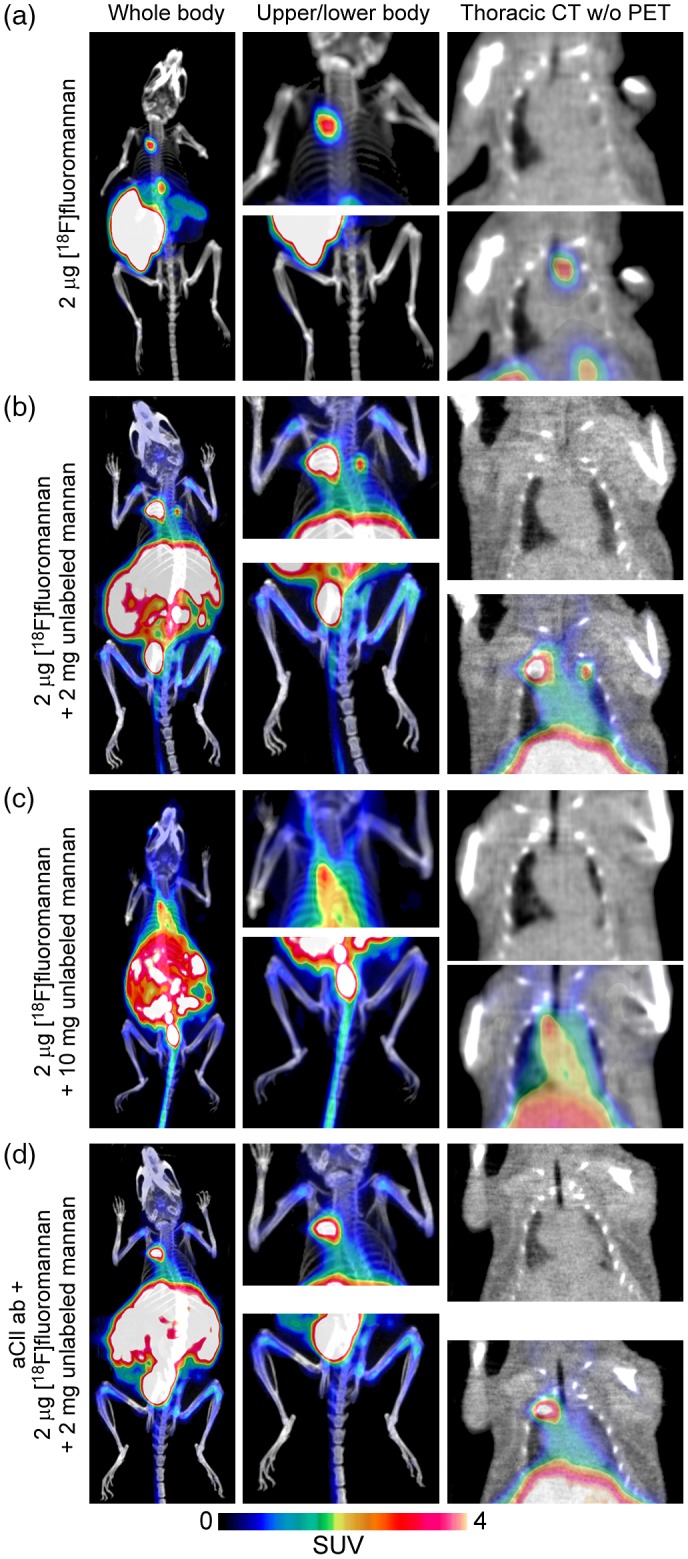
Representative images of positron emission tomography/computerized tomography (PET/CT) scan. Mice were assigned to four groups; all were injected intraperitoneally (i.p.) with 2 μg of fluorine‐18 ([18F])fluoromannan; group 1 received only radiolabelled mannan (a), group 2 also received 2 mg of unlabelled mannan (b), group 3 also received 10 mg of unlabelled mannan (c) and group 4 also received 2 mg of unlabelled mannan 5 days after an injection of an aCII antibody cocktail (d). Representative images are from mouse 3 from cage 6160 (group 1), mouse 1 from cage 4509 (group 2), mouse 30 from cage 6160 (group 3) and mouse 3 cage 4509 (group 4). All PET images are displayed at the same scale [standardized uptake value (SUV) 0–4], colours indicated by the scale bar included. aCII antibody = anti‐collagen type II.
Figure 2.
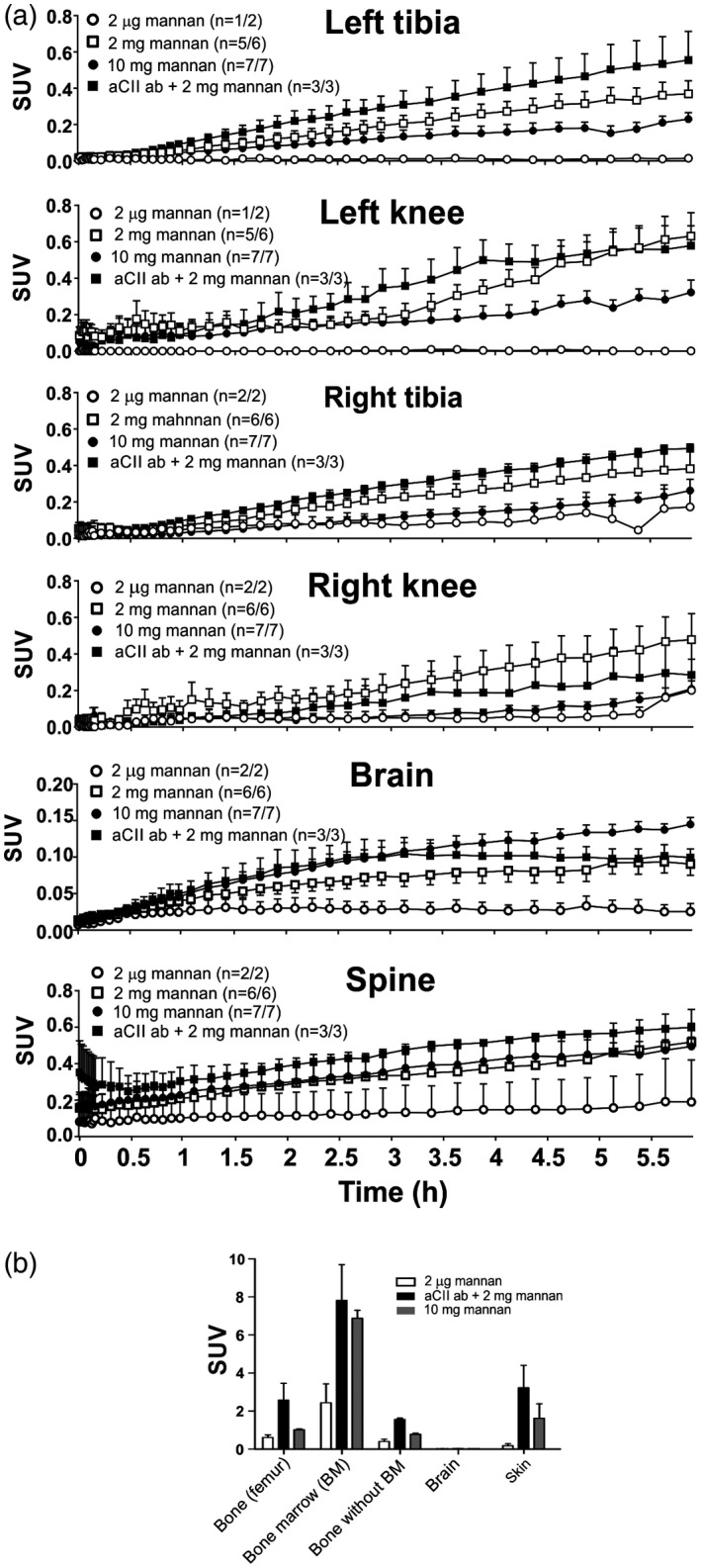
Mannan reaches central nervous system (CNS), bone and joints of the mice. Mice from each of the four groups underwent positron emission tomography/computerized tomography (PET/CT) imaging for 6 h (a). All mice received 2 μg of fluorine‐18 ([18F])fluoromannan. Group 1 received only [18F]fluoromannan (open circle/open box), group 2 also received 2 mg of unlabelled mannan (open square), group 3 also received 10 mg of unlabelled mannan to initiate mannan‐induced psoriasis (closed circles/grey box) and group 4 also received 2 mg of mannan after pretreatment with an anti‐collagen type II (aCII) antibody cocktail to induce collagen antibody‐induced arthritis (CAIA) (closed square/black box). The number of mice in which mannan was detected in/total number of mice is shown in round brackets for each graph. Ex‐vivo measurement of mannan accumulation in different tissues after 6 h of PET/CT imaging (b); n = 2. SUV = standardized uptake value. Values are presented as the mean ± standard error of the mean (s.e.m.).
Ex‐vivo analysis, 6 h after mannan injection, revealed that the main signal was emitted from the bone marrow rather than the bone itself (Fig. 2b and Supporting information, Table S2). This may be because CII is expressed in the bone marrow and is accessible by injected aCII antibodies 14. [18F]Fluoromannan entered both the spine and brain in vivo (Fig. 2a); however, ex‐vivo analysis indicated very low levels in the brain (Fig. 2b and Supporting information, Table S2). Accumulation in the central nervous system (CNS) might be due to normal uptake of [18F]fluoromannan by these tissues. However, there was significantly more mannan in the brain and spine of mice induced with 10 mg of mannan (group 3) and with the combination of aCII antibodies and 2 mg of mannan (group 4) compared to mice induced with 2 µg of mannan (group 1), as evaluated by Student's t‐test, P = 0·0005 (group 3, MIP) and P = 0·023 (mCAIA, group 4) for brain and P = 0·0066 (MIP) and P = 0·0468 (mCAIA) for spine, respectively. Interestingly, we detected no [18F]fluoromannan in the lymph nodes, indicating that transport of mannan to different tissues and organs does not occur via the lymphatic system. However, this does not exclude possible transport to draining lymph nodes after mannan reaches the joints. Ex‐vivo analysis also detected mannan in the skin after 6 h (Fig. 2b and Supporting information, Table S2).
Mannan is exported to the thymus
Interestingly, we detected mannan in the thymus (Fig. 3). Indeed, the low (2 μg) dose resulted in markedly high levels in one mouse, although this was not observed upon ex‐vivo analysis, so interpretation is difficult. However, the overall amount of mannan detected in the thymus was confirmed by ex‐vivo analysis.
Figure 3.
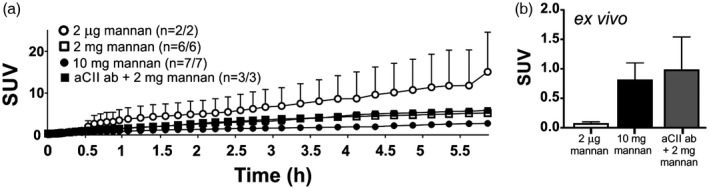
Mannan is transported to the thymus. Following intraperitoneal (i.p.) injection of fluorine‐18 ([18F])fluoromannan, positron emission tomography/computerized tomography (PET/CT) reveals transportation of the radiolabelled moiety to the thymus over time (a). The number of mice showing accumulation of mannan in the thymus/total number of mice is shown in round brackets. In addition, ex‐vivo measurements 6 h after mannan injection show the same result (b); n = 2. Three treatment groups were used: all mice received 2 μg of [18F]fluoromannan; one group received 10 mg of mannan (group 3), the other two groups received 2 mg mannan either after pretreatment with an anti‐collagen type II (aCII) (group 4) antibody cocktail to induce CAIA (black square/box) or without these antibodies (group 2, open square). Two mice received [18F]fluoromannan alone (white box). All mice (except control mice receiving 2 μg of [18F]fluoromannan alone) were perfused prior to ex‐vivo measurement. SUV = standardized uptake value. Values are presented as the mean ± standard error of the mean (s.e.m.).
Mannan is transported in the circulatory system
Independently of dose or pre‐injection with aCII antibodies, our results indicate that mannan reaches the heart (Fig. 4a). Mice injected with 10 or 2 mg of mannan (groups 2 and 3) had significantly more mannan in the heart 6 h post‐injection compared with mice receiving 2 µg of mannan (group 1), as evaluated by Student's t‐test, P = 0·0062 (10 mg of mannan versus 2 µg) and P = 0·0186 (2 mg of mannan versus 2 µg). Ex‐vivo analysis (Fig. 4b) at 6 h post‐injection confirmed the in‐vivo results. Ex‐vivo analysis of the blood after 6 h of PET/CT imaging also confirmed the presence of mannan in the circulatory system (Fig. 4b and Supporting information, Table S2). This suggests elevation of levels of mannan in mice pretreated with aCII antibodies prior to receiving 2 mg of mannan (group 4) compared to other groups, although the number of mice should be observed.
Figure 4.
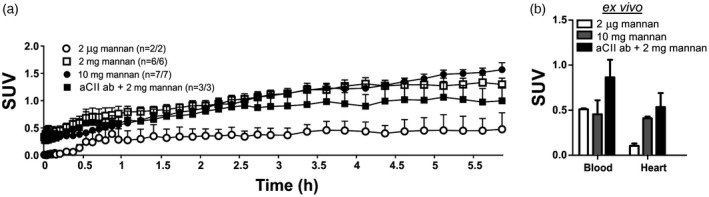
Mannan is most probably transported by cells via the blood circulatory system. Following intraperitoneal injection of fluorine‐18 ([18F])fluoromannan, mice were subjected to positron emission tomography/computerized tomography (PET/CT) imaging. The images show transportation of the radiolabelled polysaccharide through the heart (a). The number of mice showing a mannan signal in the heart/total number of mice is shown in round brackets. This is also visual ex vivo 6 h post‐mannan injection (b); n = 2. Three treatment groups were utilized. All mice received 2 μg of [18F]fluoromannan. One group received [18F]fluoromannan alone (group 1, white circle/box), one group received [18F]fluoromannan plus 10 mg of mannan (group 3, black circle/grey box), one group received 2 mg mannan with (group 4, black square/box) or without (group 2, open square) pretreatment, 5 days prior, with an anti‐collagen type II (aCII) antibody. The number of mice expressing a signal/total amount of mice per group is shown in brackets. All mice (except the control mice receiving 2 μg of [18F]fluoromannan) were perfused with phosphate‐buffered saline (PBS) before ex vivo measurements. aCII ab, anti‐collagen type II antibodies; SUV, standardised uptake value. Values are presented as the mean ± standard error of the mean (s.e.m.).
Mannan is filtered by the spleen and the liver
Both the spleen and the liver played a role in filtering mannan (Fig. 5a). Interestingly, transport of mannan to these organs was faster when lower doses were injected (a finding that was independent of aCII antibodies). Ex‐vivo analysis (Fig. 5b and Supporting information, Table S2) at the end of the 6‐h PET/CT imaging suggested a huge influx of mannan into the spleen and liver. However, the levels of mannan in these organs in mice treated with aCII antibodies were lower than those in mice not treated with the antibodies. In‐vivo studies suggested that a steady stream of mannan entered the small intestine (Fig. 5a), although this was not evident upon ex‐vivo analysis (Fig. 5b and Supporting information, Table S2). There was no clear signal in vivo (unpublished data) or ex vivo (Fig. 5b) in the kidney that could suggest mannan accumulation; however, mannan was detected in the urine of the mouse, indicating renal excretion common for PET tracers (Fig. 5b and Supporting information, Table S2). In addition, higher mannan doses seemed to result in higher levels excreted via the mouth (Fig. 5a). At end‐point (6 h after mannan injection), mice injected with 10 mg of mannan (group 3) had significantly higher levels of mannan in the mouth compared to animals injected with 2 µg of mannan (group 1) and 2 mg of mannan (group 2), as evaluated by Student's t‐test, P = 0·0100 for 10 versus 2 µg of mannan and P = 0·0038 for 2 versus 2 µg of mannan.
Figure 5.
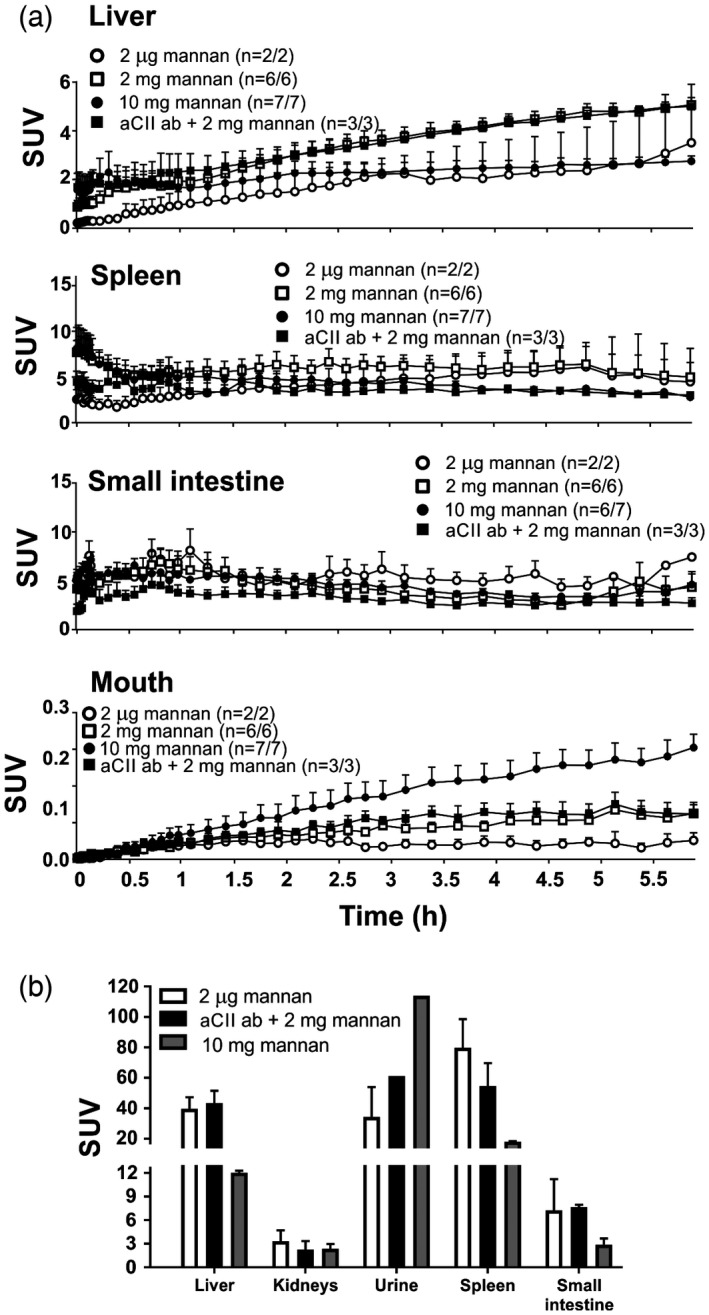
Mannan is most probably filtered through the liver and spleen before excretion via the urine. Following intraperitoneal injection of fluorine‐18 ([18F])fluoromannan, mice were subjected to positron emission tomography/computerized tomography (PET/CT) imaging. (a) [18F]fluoromannan levels in the liver, spleen, small intestine and mouth were measured in vivo at 6 h and (b) ex vivo after the 6 h in‐vivo imaging; n = 2. All mice received 2 μg of [18F]fluoromannan. Group 1 received [18F]fluoromannan alone (white circle/box), group 3 received [18F]fluoromannan plus 10 mg of mannan (black circle/grey box), group 2 received [18F]fluoromannan plus 2 mg of mannan with (group 4, black square/box) or without (group 2, open square) anti‐collagen type II (aCII antibodies) 5 days prior to imaging. The number of mice expressing a signal versus the total number of mice is shown in brackets. All mice except group 1 were perfused using phosphate‐buffered saline (PBS) to remove signal from blood before ex‐vivo measurement. SUV, standardized uptake value. Values are presented as the mean ± standard error of the mean (s.e.m.).
Discussion
Here, we show that mannan reaches a variety of organs and tissues, including bone marrow, knee joints, heart, blood, thymus, spleen and liver, within 6 h of intraperitoneal administration. If labelled mannan is injected together with unlabelled mannan, used at doses that will later trigger inflammation in skin and in joints, the transport of mannan to various tissues will be more efficient. Injection of aCII antibodies is known to trigger a series of pathogenic events, including induction of pain (arthralgia), destabilization of cartilage, infiltration of joints by lymphocytes and proliferation of macrophages 5, 15, 16, 17. Arthritis may develop several days later, particularly if the mouse is also injected with mannan or lipopolysaccaride (minimum 80% prevalence), which is usually associated with a massive infiltration of neutrophils to the joints 4, 5, 10, 18. Importantly, 6 h after mannan injection, both the antibodies 15 and mannan have reached the joints, possibly destabilizing cartilage and inducing pain 16, but not yet causing arthritis. If mannan alone is injected, mice develop a disease characterized by Ps‐like lesions of the skin and PsA 4, 10.
The time taken for mannan to reach the tibia and knee joints suggests cellular transport rather than diffusion. Transporting cells could be macrophages, which have shown to infiltrate the tissues of patients with Ps, PsA or RA 19, 20, 21. Furthermore, macrophages express receptors and complexes proved to play roles in Ps, PsA and rheumatoid‐like diseases. Examples include TLR‐2 and ‐4 22, 23, NOX2 24, 25 and C‐type lectin receptors such as MR 10. Indeed, previous studies show that macrophages play important roles in models of diseases such as MIP 4 and mCAIA 5, which are induced or enhanced by mannan. To investigate whether [18F]fluoromannan has any biological effects, some of the mice were selected for clinical scoring of their arthritis and psoriasis symptoms; as expected, we found that disease developed after injection of [18F]fluoromannan in combination with unlabelled mannan in a manner similar to that after injection of unlabelled mannan only (data not shown). Thus, we confirmed that [18F]‐labelling in itself has no biological impact, as was previously seen using [19F]fluoromannan 11. The length of the imaging was based on the physical half‐life of [18F] radionuclide (t 1/2=1·83 h) 26, as well as our previous experience on detector saturation and sensitivity. We injected the highest dose of radioactivity that did not saturate the PET detectors and gave a reasonable signal for up to 6 h.
Our results are in line with other studies showing that uptake of drug carrying nanoparticles by macrophages is increased when particles are coated with mannose structures; coating particles with mannose also led to greater accumulation of particles in the liver, spleen and kidney 27. These latter results are similar to those presented herein. Furthermore, mannan has previously been shown to be filtered by the spleen and liver in various animals 28, 29. Binding of aCII antibodies to cartilage may contribute to an inflammatory environment in tissues expressing CII; such tissues include bone marrow and cartilaginous joints 30. The results obtained after injection of aCII antibodies and mannan suggest that pre‐exposure to aCII antibodies increases disease severity and facilitates development of chronicity. Indeed, aCII antibodies are likely to form immune complexes within joints, which then activate the innate immune system. Ongoing inflammation attracts more immune cells to the area, thereby exacerbating the inflammation. If not down‐regulated, this chronic inflammatory environment can trigger autoimmunity. Mannan broadly activates the innate immune system, which includes macrophages, dendritic cells, complement and neutrophils. Thus, cells infiltrating inflamed joints and bones will be pre‐activated and proinflammatory. Some, most probably antigen‐presenting cells such as macrophages, will express mannan; however, all will contribute to local inflammation. The presence of mannan in the inflamed area will further exacerbate the disease and, in the absence of immune regulators such as NOX2 and/or MR, the disease will develop into a chronic relapsing form of arthritis 5, 10.
Humans are exposed to mannan through eating, drinking and breathing; it is present, for example, in beer, plants, yeast and bacteria. Thus, humans are constantly challenged by this structure, which can trigger inflammation in genetically susceptible individuals. In view of the finding that mannan triggers inflammatory disease in mice 4, 5, it is important to investigate how and where this occurs in the individual. This is particularly important because some studies have used mannan as an immunostimulant to enhance drug delivery 27. The finding that MR down‐regulates both MIP and mCAIA 10 indicates that not all mannan‐recognizing receptors are immune‐stimulating; indeed, mannan can also trigger immunosuppressive signalling pathways. Thus, modelling these pathways may lead to the development of new therapeutic options for inflammatory diseases.
Limitations
The Inveon small animal PET provides spatial resolution of approximately 1·6 mm with [18F] 31. Unfortunately, some of the structures we were targeting are quite small, as described in Supporting information, Table 1. This might have resulted in spillover and partial‐volume effects, which can confound the accuracy of the in‐vivo quantitative PET data. Only female mice were studied, because the urinary bladder catheterization of females is easier than that of males. The number of animals in ex‐vivo measurements is small and the weight of some tissues is low, which may cause uncertainty in the data.
Disclosures
The authors declare no competing financial interests.
Author contributions
Acquisition of data and analysis: C. H., R. S., X. G. L. and H. L. Study conception and design of the work: C. H., R. S., X. G. L., H. L., A. R. and R. H. Drafting the manuscript: C. H. Revising the manuscript: R. S., X. G. L., H. L., A. R. and R. H. The work was supervised by A. R. and R. H. All authors ensured the accuracy of the work.
Supporting information
Table S1. The volume of ROIs in PET data analysis. The weight of the small intestine, spleen and liver resembles the amount used for ex vivo analysation and not the full organ. N/A = not available.
Table S2. Ex vivo radioactivity concentration in excised organs or tissues at 6 hours after intraperitoneal [18F]fluoromannan injection, n = 2 and both values are shown.
Acknowledgements
This study was funded by the Academy of Finland; the Sigrid Jusélius Foundation; the Jane and Aatos Erkko Foundation; the National Doctoral Programme in Informational and Structural Biology; the Drug Research Doctoral Programme of University of Turku Graduate School; State Research Funding from Turku University Hospital; the Turku University Foundation; the King Gustaf V 80 Years Foundation; the Finnish Cultural Foundation; the Varsinais‐Suomi Regional Fund; the Swedish Foundation for Strategic Research; the KA Wallenberg Foundation; and the Swedish Research Council. The funding bodies played no role in study design; data collection, analysis and interpretation; the writing of the article; or the decision to submit the article for publication. We thank Outi Sareila and Academy Professor Sirpa Jalkanen for scientific discussion, Riina Larmo for animal husbandry and Aake Honkaniemi for assistance in PET/CT imaging. We also would like to thank Timo Kattelus for help with the figures. The study was conducted within the Finnish Centre of Excellence in Cardiovascular and Metabolic Diseases supported by the Academy of Finland, University of Turku, Turku University Hospital and Åbo Akademi University.
References
- 1. Gerlag DM, Norris JM, Tak PP. Towards prevention of autoantibody‐positive rheumatoid arthritis: from lifestyle modification to preventive treatment. Rheumatology (Oxf) 2016; 55:607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Michael PS, Boehncke WH. Psoriasis. N Engl J Med 2005; 352:1899–912. [DOI] [PubMed] [Google Scholar]
- 3. Kopp T, Riedl E, Bangert C et al Clinical improvement in psoriasis with specific targeting of interleukin‐23. Nature 2015; 521:222–6. [DOI] [PubMed] [Google Scholar]
- 4. Khmaladze I, Kelkka T, Guerard S et al Mannan induces ROS‐regulated, IL‐17A‐dependent psoriasis arthritis‐like disease in mice. Proc Natl Acad Sci USA 2014; 111:E3669–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hagert C, Sareila O, Kelkka T et al Chronic active arthritis driven by macrophages without involvement of T cells. Arthritis Rheumatol (Hoboken, NJ) 2018; 70:1343–53. [DOI] [PubMed] [Google Scholar]
- 6. Tada H, Nemoto E, Shimauchi H et al Saccharomyces cerevisiae‐ and Candida albicans‐derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14‐ and Toll‐like receptor 4‐dependent manner. Microbiol Immunol 2002; 46:503–12. [DOI] [PubMed] [Google Scholar]
- 7. Sheng KC, Pouniotis DS, Wright MD et al Mannan derivatives induce phenotypic and functional maturation of mouse dendritic cells. Immunology 2006; 118:372–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taylor PR, Gordon S, Martinez‐Pomares L. The mannose receptor: linking homeostasis and immunity through sugar recognition. Trends Immunol 2005; 26:104–10. [DOI] [PubMed] [Google Scholar]
- 9. Hultqvist M, Olofsson P, Holmberg J, Bäckström BT, Tordsson J, Holmdahl R. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci USA 2004; 101:12646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hagert C, Sareila O, Kelkka T, Jalkanen S, Holmdahl R. The macrophage mannose receptor regulate mannan‐induced psoriasis, psoriatic arthritis, and rheumatoid arthritis‐like disease models. Front Immunol 2018; 9:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li XG, Hagert C, Siitonen R et al (18)F‐labeling of mannan for inflammation research with positron emission tomography. ACS Med Chem Lett 2016; 7:826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sareila O, Jaakkola N, Olofsson P, Kelkka T, Holmdahl R. Identification of a region in p47phox/NCF1 crucial for phagocytic NADPH oxidase (NOX2) activation. J Leukoc Biol 2013; 93:427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sareila O, Hagert C, Rantakari P, Poutanen M, Holmdahl R. Direct comparison of a natural loss‐of‐function single nucleotide polymorphism with a targeted deletion in the Ncf1 gene reveals different phenotypes. PLOS ONE 2015; 10:e0141974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holmdahl R, Mo JA, Jonsson R, Karlstrom K, Scheynius A. Multiple epitopes on cartilage type II collagen are accessible for antibody binding in vivo . Autoimmunity 1991; 10:27–34. [DOI] [PubMed] [Google Scholar]
- 15. Nandakumar KS, Holmdahl R. Efficient promotion of collagen antibody induced arthritis (CAIA) using four monoclonal antibodies specific for the major epitopes recognized in both collagen induced arthritis and rheumatoid arthritis. J Immunol Methods 2005; 304:126–36. [DOI] [PubMed] [Google Scholar]
- 16. Bas DB, Su J, Sandor K et al Collagen antibody‐induced arthritis evokes persistent pain with spinal glial involvement and transient prostaglandin dependency. Arthritis Rheum 2012; 64:3886–96. [DOI] [PubMed] [Google Scholar]
- 17. Croxford AM, Whittingham S, McNaughton D, Nandakumar KS, Holmdahl R, Rowley MJ. Type II collagen‐specific antibodies induce cartilage damage in mice independent of inflammation. Arthritis Rheum 2013; 65:650–9. [DOI] [PubMed] [Google Scholar]
- 18. Nandakumar KS, Svensson L, Holmdahl R. Collagen type II‐specific monoclonal antibody‐induced arthritis in mice: description of the disease and the influence of age, sex, and genes. Am J Pathol 2003; 163:1827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Koning HD, Rodijk‐Olthuis D, van Vlijmen‐Willems IMJJ et al A comprehensive analysis of pattern recognition receptors in normal and inflamed human epidermis: upregulation of dectin‐1 in psoriasis. J Invest Dermatol 2010; 30:2611–20. [DOI] [PubMed] [Google Scholar]
- 20. Wollenberg A, Mommaas M, Oppel T, Schottdorf EM, Günther S, Moderer M. Expression and function of the mannose receptor CD206 on epidermal dendritic cells in inflammatory skin diseases. J Invest Dermatol 2002; 118:327–34. [DOI] [PubMed] [Google Scholar]
- 21. Heftdal LD, Stengaard‐Pedersen K, Ornbjerg LM et al Soluble CD206 plasma levels in rheumatoid arthritis reflect decrease in disease activity. Scand J Clin Lab Invest 2017; 77:385–9. [DOI] [PubMed] [Google Scholar]
- 22. Abdollahi‐Roodsaz S, Joosten LA, Helsen MM et al Shift from Toll‐like receptor 2 (TLR‐2) toward TLR‐4 dependency in the erosive stage of chronic streptococcal cell wall arthritis coincident with TLR‐4‐mediated interleukin‐17 production. Arthritis Rheum 2008; 58:3753–64. [DOI] [PubMed] [Google Scholar]
- 23. Kelkka T, Hultqvist M, Nandakumar KS, Holmdahl R. Enhancement of antibody‐induced arthritis via Toll‐like receptor 2 stimulation is regulated by granulocyte reactive oxygen species. Am J Pathol 2012; 181:141–50. [DOI] [PubMed] [Google Scholar]
- 24. Olofsson P, Holmberg J, Tordsson J, Lu S, Akerstrom B, Holmdahl R. Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat Genet 2003; 33:25–32. [DOI] [PubMed] [Google Scholar]
- 25. Olsson LM, Nerstedt A, Lindqvist AK et al Copy number variation of the gene NCF1 is associated with rheumatoid arthritis. Antioxid Redox Signal 2012; 16:71–8. [DOI] [PubMed] [Google Scholar]
- 26. Garcia‐Torano E, Medina VP, Ibarra MR. The half‐life of 18F. Appl Radiat Isot 2010; 68:1561–5; discussion 5. [DOI] [PubMed] [Google Scholar]
- 27. Jain SK, Gupta Y, Jain A, Saxena AR, Khare P, Jain A. Mannosylated gelatin nanoparticles bearing an anti‐HIV drug didanosine for site‐specific delivery. Nanomed Nanotechnol Biol Med 2008; 4:41–8. [DOI] [PubMed] [Google Scholar]
- 28. Kappe R, Muller J. Rapid clearance of Candida albicans mannan antigens by liver and spleen in contrast to prolonged circulation of Cryptococcus neoformans antigens. J Clin Microbiol 1991; 29:1665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Artursson P, Johansson D, Sjoholm I. Receptor‐mediated uptake of starch and mannan microparticles by macrophages: relative contribution of receptors for complement, immunoglobulins and carbohydrates. Biomaterials 1988; 9:241–6. [DOI] [PubMed] [Google Scholar]
- 30. Holmdahl R, Andersson M, Goldschmidt TJ, Gustafsson K, Jansson L, Mo JA. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol Rev 1990; 118:193–232. [DOI] [PubMed] [Google Scholar]
- 31. Goertzen AL, Bao Q, Bergeron M et al NEMA NU 4–2008 comparison of preclinical PET imaging systems. J Nucl Med 2012; 53:1300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The volume of ROIs in PET data analysis. The weight of the small intestine, spleen and liver resembles the amount used for ex vivo analysation and not the full organ. N/A = not available.
Table S2. Ex vivo radioactivity concentration in excised organs or tissues at 6 hours after intraperitoneal [18F]fluoromannan injection, n = 2 and both values are shown.


