Abstract
In contrast to the successful modeling of early-onset disorders using patient-specific cells, modeling of late-onset neurodegenerative diseases such as Parkinson's disease remains a challenge. This might be related to the often ignored fact that current induced pluripotent stem cell (iPSC) differentiation protocols yield cells that typically show the behavior of fetal stage cells. Acknowledging aging as a contributing factor in late-onset neurodegenerative disorders represents an important step on the road towards faithfully recreating these diseases in vitro. Here, we summarize progress in the field and review the strategies and challenges for triggering late-onset disease phenotypes.
KEY WORDS: Disease modeling, Aging, Rejuvenation
Summary: This opinion piece discusses the difficulties involved in using stem cells to study diseases of the elderly, and how these might be overcome.
Introduction
Age is the primary risk factor for many human pathologies, including neurodegenerative disorders, which represent a major cause of mortality and disability among elderly people. The global aging trend in most modern societies, owing to the continuous increase in longevity and a decrease in fertility, highlights the importance of developing better models for these diseases. Although genetically engineered mice can model some aspects of human brain aging, these animals generally do not recapitulate the full neuropathological or clinical phenotypes seen in humans. Moreover, mice do not spontaneously develop neurodegenerative disorders, suggesting that such diseases are largely human specific (Jucker, 2010). An additional impediment to studying human diseases of the nervous system is the limited accessibility of human brain tissue to direct observation or manipulation.
Given these challenges, human induced pluripotent stem cell (iPSC) technology appears to be an ideal alternative platform for recreating human disease in vitro. There has been considerable success in modeling early developmental disorders of the nervous system such as spinal muscular atrophy, familial dysautonomia or primary herpes simplex encephalitis (Ebert et al., 2009; Lee et al., 2009; Lafaille et al., 2012), among many others. By contrast, the modeling of late-onset disorders has often failed to faithfully recapitulate the late-stage features of the disease phenotypes, such as macromolecular protein aggregates (plaques) or the progressive deterioration of neuronal structures resulting in cell death (Srikanth and Young-Pearse, 2014). The difficulties in modeling late-onset disease phenotypes may be related to the reset of donor age in human pluripotent stem cells (hPSCs) following reprogramming (Suhr et al., 2010; Lapasset et al., 2011; Mahmoudi and Brunet, 2012; Frobel et al., 2014). The resulting hPSCs give rise to cells that typically show the behavior of fetal stage cells following in vitro differentiation. We have recently proposed that aging should be incorporated as an independent factor for the in vitro modeling of late-onset diseases. In a first such attempt, we hijacked the molecular mechanisms responsible for Hutchinson–Gilford progeria syndrome (HGPS), a premature aging disorder, to model late-onset degenerative features of Parkinson's disease (PD) in an iPSC-derived lineage (Miller et al., 2013).
In this Spotlight article we summarize different models and strategies for triggering late-onset diseases, with a special focus on PD, and discuss the future prospects for accelerating aging as an integral part of iPSC-based disease modeling.
Aging, reprogramming and beyond
Reprogramming of somatic cells into iPSCs (Takahashi and Yamanaka, 2006), with embryonic stem cell-like properties, can be conceptually compared to the reset that occurs during fertilization in vivo. It is thought that factors in the egg are crucial for reprogramming the ‘old' genome of the male gamete to eventually create a ‘young' embryo. In fact, several studies have shown that, in addition to the acquisition of pluripotent properties, reprogramming resets aspects of cellular and molecular aging including telomere lengths and mitochondrial fitness (Marion et al., 2009; Suhr et al., 2010). We have recently shown (Miller et al., 2013) that iPSCs derived from old fibroblasts no longer present abnormal nuclear morphologies and age-associated features such as DNA damage foci, increased reactive oxygen species (ROS), reduced levels of a set of nuclear organization proteins and loss of heterochromatin markers. Importantly, upon differentiation of these iPSCs into fibroblast-like cells, the differentiated cells also exhibit a ‘young' phenotype, corroborating that reprogramming has permanently reset the aging status of these cells.
Studying diseases in vitro generally requires the differentiation of patient-specific iPSCs into differentiated, disease-relevant cell types. Differentiation protocols to obtain cell types of interest from human iPSCs typically take weeks to several months but still only generate immature, embryonic-like cell types. For instance, iPSC-derived neurons have been shown to match the first trimester stage of human development (Mariani et al., 2012). In the case of midbrain dopamine neurons, a cell type that requires approximately 30 days for cell fate specification, full maturation requires periods of several months to generate functional neurons capable of rescuing dopamine deficits in animal models of PD upon transplantation (Kriks et al., 2011). The process of maturation is distinct from aging and is defined as the gain of full functionality of a given cell or tissue. Maturation is typically completed by early adulthood. By contrast, aging is defined as the time-driven loss of physiological proficiency that determines the functional decline from adulthood to death.
Current iPSC differentiation protocols yield cells that are both ‘immature' and ‘young'. In the case of maturation, obtaining fully functional cells within a reasonable time frame remains an important issue for disease modeling, drug screening and, eventually, any downstream translational applications. However, even after full maturation is achieved, cells are phenotypically still young. The ‘young' identity of iPSC-derived cells represents a scientific challenge for modeling diseases that appear only late in life.
Late-onset disease modeling: insights from Parkinson's disease
The establishment of reliable models for age-related neurodegenerative diseases, such as PD, is crucial for the development and testing of novel therapeutic strategies. PD, the second most common neurodegenerative disease, is characterized at the cellular level by the loss of dopamine (DA) neurons within the substantia nigra. Surviving DA neurons progressively accumulate α-synuclein, eventually resulting in the formation of Lewy bodies. The main symptoms associated with PD are resting tremor, rigidity and slowness of voluntary movements (Goedert et al., 2013).
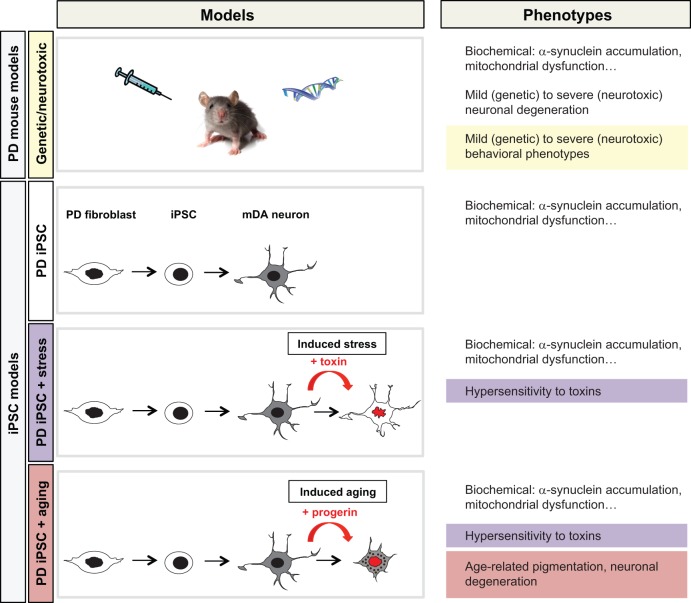
Traditionally, PD has been modeled in animals. There are two main categories of animal models: neurotoxic models, via injection of compounds that selectively kill DA neurons; and genetic models, which target PD-related genes (Fig. 1). Animal models can be very useful, as they allow the disease to be explored over time at the organismal level and may enable behavioral studies to monitor disease symptoms. Some genetic mouse models of PD show α-synuclein aggregation, mitochondrial dysfunction or mild deficits in dopamine transmission or behavioral impairments (Lee et al., 2012b; Le et al., 2014). However, there is no animal model that fully captures the molecular mechanisms, pathophysiology, progressive nature and clinical symptoms of the human disease. In particular, genetic PD mouse models generally do not recapitulate the degeneration of DA neurons within a reasonable time window – perhaps due to the substantial difference in lifespan between human and mouse. By contrast, neurotoxic models can induce an acute degeneration of DA neurons but are far from reproducing the physiological development of the disease (Fig. 1). In addition to these scientific challenges of modeling PD in the mouse, generating transgenic mice is also a slow and expensive process (Merkle and Eggan, 2013; Le et al., 2014).
Fig. 1.
Late-onset disease modeling: cues from Parkinson's disease. Schematic illustration of mouse and iPSC models of PD and strategies used to trigger PD phenotypes. These strategies include, in the case of mouse models, the manipulation of PD-related genes (genetic) or toxin-induced neuronal loss using compounds, such as MPTP or 6-hydroxydopamine, that specifically target mDA neurons (neurotoxic). Current genetic models in the mouse or other model organisms allow for mechanistic studies on molecular and biochemical changes associated with a given PD-related gene. However, few of these genetic models show evidence of neuronal degeneration and, even less so, of degeneration in mDA neurons, a cell type particularly and characteristically affected in the human disease. However, a key feature of PD animal models is the ability to study behavioral phenotypes (highlighted in yellow), a feature particularly prominent in neurotoxic models of disease. The iPSC models are based on the study of genetic susceptibility in patient-specific PD iPSC-derived lineages. Most monogenic PD iPSC models can recreate some of the biochemical changes associated with the specific genetic defect. However, in order to measure disease-related phenotypes such as hypersensitivity to toxins (highlighted in purple) or age-associated features (highlighted in red), additional extrinsic factors are required. These include stress paradigms such as exposure to CCCP, rotenone or hydrogen peroxide or novel ‘aging'-inducing tools to trigger late-onset disease phenotypes.
Considering the lack of an accurate animal model, hPSC lines derived from affected patients appear to provide the desired tool to model a wide range of human diseases – particularly since the technology enables differentiation to potentially any cell type, and hence analysis in the disease-relevant target cell of interest. Indeed, such iPSC-based disease models are rapidly developing into a key platform for drug discovery and preclinical testing of candidate compounds (Lee et al., 2009, 2012a; Merkle and Eggan, 2013; Wainger et al., 2014). In the case of PD, recent studies have demonstrated that neurons derived from patient-specific iPSCs could successfully reproduce several disease-related phenotypes such as increased α-synuclein levels, mitochondrial dysfunction and hypersensitivity to toxins such as compounds that trigger mitochondrial stress or ROS (Byers et al., 2011; Devine et al., 2011; Seibler et al., 2011; Soldner et al., 2011; Cooper et al., 2012; Imaizumi et al., 2012; Liu et al., 2012; Sánchez-Danés et al., 2012; Miller et al., 2013; Reinhardt et al., 2013; Su and Qi, 2013; Sanders et al., 2014; Woodard et al., 2014). Most of these models showed evidence of biochemical changes that are directly dependent on the disease-specific genetic defects (Fig. 1). For example, patient-specific iPSC-derived neurons with an α-synuclein gene (SNCA) triplication show increased accumulation of α-synuclein, which is the main component of the Lewy bodies (Byers et al., 2011). PD iPSC-derived DA neurons mutant for the mitochondrial kinase PINK1 show changes in mitochondrial function (Seibler et al., 2011). iPSC-derived midbrain dopamine (mDA) neurons that carry the G2019S mutation in the leucine-rich repeat kinase 2 (LRRK2) gene showed evidence of reduced neurite length (Reinhardt et al., 2013). However, age-associated features such as neuromelanin accumulation and age-dependent disease phenotypes including neuronal degeneration or the accumulation of Lewy body-like structures could not be observed in most of these models (Fig. 1). The difficulty of detecting late-onset disease phenotypes in iPSC-based models is even more challenging in sporadic disease with unknown contribution of genetic versus environmental factors.
Given that age is the key PD risk factor not taken into account in current PD iPSC models, the next logical step would be to develop strategies to induce ‘age' in iPSC-derived neurons. Such induced aging paradigms may convert cells from a default fetal stage towards a more aged-like status capable of exhibiting late-onset features of the disease that only occur in ‘old' PD individuals.
Strategies for triggering late-onset diseases
The etiology of neurodegenerative diseases is thought to be multifactorial, with both genetic and environmental factors contributing to their onset and progression. A late onset indicates that the genetic mutation provides susceptibility but is not sufficient to trigger the disease. Interestingly, the severity of a mutation can also be directly correlated with an earlier disease onset, as in the case of the dominant autosomal form of PD associated with the SNCA locus. SNCA gene dosage is known to influence disease onset and progression in patients, with triplications causing earlier onset and more rapid progression than disease alleles associated with lower increases in SNCA expression (Byers et al., 2011). In any case, aging is required for the development of any late-onset disease. During the aging process there are a number of cellular alterations, such as the accumulation of misfolded proteins, mitochondrial dysfunction and increased levels of ROS, that may contribute to the development or potentiate the symptoms of aging-related diseases (Keating, 2008). Additional external factors, including lifestyle or exposure to pesticides and other chemicals (Chin-Chan et al., 2015), are linked with disease risk, while most likely triggering some of the age-associated cellular changes. In an effort to mimic the stress-induced changes that can occur in cells during normal aging, several iPSC-derived models of late-onset diseases use exposure to toxins (Fig. 1), including hydrogen peroxide, which induces the production of ROS, or compounds that trigger mitochondrial stress such as CCCP or rotenone (Byers et al., 2011; Cooper et al., 2012).
Current theories of aging can be classified into programmed versus damage- or error-induced aging. The concept of programmed aging implies regulation by intrinsic mechanisms, such as changes in gene expression, that affect the systems responsible for tissue homeostasis and repair. By contrast, the concept of damage- or error-induced aging argues for the main culprit being cumulative damage such as ROS, cross-linked macromolecules, DNA damage, and altered energy machines (Jin, 2010). Neither of these two theories can alone fully explain the phenomenon of aging, as there is still no consensus on the specific molecular and biochemical mechanisms of organismal aging. Exposing iPSC-derived models to toxins is an attempt to reproduce aging under the scope of the ‘error theories'. The alternative approach to recreate aging is by altering specific genes or functional pathways involved in aging, hence considering aging under the ‘programmed theories' point of view. The specific pathways to be altered could include genetic or pharmacological strategies to shorten telomeres, the induction of heterochromatin loss, prompting mitochondrial dysfunction, the induction of cellular senescence or triggering the accumulation of damaged or inappropriately folded proteins (López-Otín et al., 2013). A key question is whether any of these manipulations triggers a broad ‘age-inducing' response or is limited to inducing the specific hallmark associated with their function.
Human progeroid syndromes (in which patients display premature aging) such as HGPS, dyskeratosis congenita, ataxia telangiectasia, Werner syndrome (WS), Bloom or Cockayne syndrome can provide additional clues about the mechanisms that control aging. For example, a recent publication links WS, a premature aging disorder associated with telomere shortening and defects in DNA repair, to a defect in heterochromatin maintenance, suggesting heterochromatin loss as a potential driver of aging (Zhang et al., 2015). The various pathways triggering progeroid syndromes might be particularly suitable to trigger premature aging-like cellular phenotypes. Interestingly, several iPSC models of progeroid syndromes have demonstrated a reset of the age-associated phenotypes upon reprogramming but that cells rapidly reacquire the aging-related cellular features upon differentiation (Agarwal et al., 2010; Batista et al., 2011; Liu et al., 2011; Zhang et al., 2011; Andrade et al., 2012). These results suggest that the molecular mechanisms underlying progeroid syndromes could potentially be harnessed in iPSC-based models as age-inducing tools.
Taking one additional step forward, we have recently presented a strategy to genetically trigger aging-like features in iPSC derivatives towards the goal of modeling of a late-onset disease based on the premature aging syndrome HGPS (Miller et al., 2013). In HGPS patients, progerin, which is a mutant form of the nuclear scaffold protein lamin A, accumulates in the nuclear membrane and in turn triggers changes in nuclear morphology, chromatin organization, heterochromatin, DNA damage response, cell cycle, gene transcription, and telomere maintenance (Dechat et al., 2009). Using a model of PD, we engineered the overexpression of progerin in iPSC-derived mDA neurons, the cell predominantly affected in PD. Progerin expression in neurons induced both general aging-associated phenotypes, such as accumulation of DNA damage and ROS, as well as features more specific to neuronal aging. We observed that progerin-treated mDA neurons present shorter dendrites, a transcriptome compatible with a neurodegenerative process, and the accumulation of neuromelanin, an mDA neuron-specific, age-related pigment. Moreover, progerin overexpression in PD iPSC-derived neurons could synergize with the genetic vulnerability of these cells to trigger relevant phenotypes such as the loss of tyrosine hydroxylase expression and the formation of inclusion bodies, mimicking disease progression (Miller et al., 2013) (Fig. 1). Interestingly, neurons are thought to express only low levels of progerin compared with other cell types such as fibroblasts. Furthermore, a recent study reported that the ectopic expression of progerin in the mouse brain induces structural nuclear abnormalities without significant alterations in gene expression or mouse behavior (Baek et al., 2015). Therefore, it remains to be determined whether progerin expression levels, neuronal subtype identity or mouse versus human origin can explain the presence versus absence of age-related neuronal phenotypes. More importantly, it remains an open question to what extent the aging phenotype induced by progerin mimics physiological or pathological aging. In this regard, alternative strategies, the alteration of which might be more closely associated to physiological aging, such as telomere shortening or heterochromatin loss, should be tested for their ability to induce age-related phenotypes in iPSC-based late-onset disease models.
Conclusions and perspectives
The dream of rejuvenation is as old as humankind. The cellular rejuvenation response observed during reprogramming of somatic cells to iPSCs represents a proof of concept that many aspects of cellular aging are reversible. In fact, the iPSC paradigm could provide a new platform to study mechanisms of both aging and rejuvenation. The ultimate goal of such studies is the identification of drug targets that can slow down or potentially reverse aging phenotypes. Such studies could have important implications for the treatment of age-associated pathologies and might yield novel classes of cosmetic products or strategies that lead to actual extension of human lifespan. Although considered controversial by some, there are clear benefits to such goals, and most would see relief from aging phenotypes as a desirable outcome. However, what if we accelerate the clock and trigger aging instead of reversing or slowing the process? Beyond the famous quote “Live fast, die young and leave a good-looking corpse” (from the 1947 book ‘Knock on Any Door’ by Willard Motley) there seems to be no obvious benefit in learning how to induce aging. Our Spotlight article argues that there is at least one tangible benefit from such an effort: the modeling of late-onset neurodegenerative diseases using human iPSCs (Fig. 1).
Here, we propose that cellular aging strategies can and should be added as an important parameter in order to obtain age-appropriate cell types from iPSCs. However, a fundamental challenge for the concept of induced aging is the assumption that a small set or, ideally, one key driver of aging will allow us to fast-forward cellular life. Although this assumption appears to be correct in the case of human progeroid syndromes, normal aging might elude such a simplistic notion. An obvious alternative could involve the derivation of the desired cell type from affected individuals without erasing cellular age, either by finding strategies that decouple cell fate reprogramming from the rejuvenation process or by direct reprogramming techniques that bypass the iPSC state. We are uncovering only the first few pieces of a much larger puzzle that is yet to be explored on our journey of using iPSCs in human disease modeling and drug discovery. An important parallel track that has emerged on this road is the question of cellular rejuvenation and aging. The future will reveal whether these two tracks can merge to form a larger highway that will ultimately lead us to solutions for some of the most devastating of human disorders.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
E.V. was supported by a New York State Stem Cell Science (NYSTEM) postdoctoral fellowship. The work of the authors described in this article was supported by grants from the Starr Foundation, the NYSTEM, the National Institute of Neurological Disorders and Stroke/National Institutes of Health and the National Cancer Institute/National Institutes of Health.
References
- Agarwal S., Loh Y.-H., McLoughlin E. M., Huang J., Park I.-H., Miller J. D., Huo H., Okuka M., Dos Reis R. M., Loewer S. et al. (2010). Telomere elongation in induced pluripotent stem cells from dyskeratosis congenita patients. Nature 464, 292-296. 10.1038/nature08792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade L. N., Nathanson J. L., Yeo G. W., Menck C. F. M. and Muotri A. R. (2012). Evidence for premature aging due to oxidative stress in iPSCs from Cockayne syndrome. Hum. Mol. Genet. 21, 3825-3834. 10.1093/hmg/dds211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek J.-H., Schmidt E., Viceconte N., Strandgren C., Pernold K., Richard T. J. C., Van Leeuwen F. W., Dantuma N. P., Damberg P., Hultenby K. et al. (2015). Expression of progerin in aging mouse brains reveals structural nuclear abnormalities without detectible significant alterations in gene expression, hippocampal stem cells or behavior. Hum. Mol. Genet. 24, 1305-1321. 10.1093/hmg/ddu541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista L. F. Z., Pech M. F., Zhong F. L., Nguyen H. N., Xie K. T., Zaug A. J., Crary S. M., Choi J., Sebastiano V., Cherry A. et al. (2011). Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature 474, 399-402. 10.1038/nature10084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B., Cord B., Nguyen H. N., Schüle B., Fenno L., Lee P. C., Deisseroth K., Langston J. W., Pera R. R. and Palmer T. D. (2011). SNCA triplication Parkinson's patient's iPSC-derived DA neurons accumulate alpha-synuclein and are susceptible to oxidative stress. PLoS ONE 6, e26159 10.1371/journal.pone.0026159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-Chan M., Navarro-Yepes J. and Quintanilla-Vega B. (2015). Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front. Cell Neurosci. 9, 124 10.3389/fncel.2015.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper O., Seo H., Andrabi S., Guardia-Laguarta C., Graziotto J., Sundberg M., McLean J. R., Carrillo-Reid L., Xie Z., Osborn T. et al. (2012). Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson's disease. Sci. Transl. Med. 4, 141ra90 10.1126/scitranslmed.3003985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechat T., Adam S. A. and Goldman R. D. (2009). Nuclear lamins and chromatin: when structure meets function. Adv. Enzyme Regul. 49, 157-166. 10.1016/j.advenzreg.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine M. J., Ryten M., Vodicka P., Thomson A. J., Burdon T., Houlden H., Cavaleri F., Nagano M., Drummond N. J., Taanman J.-W. et al. (2011). Parkinson's disease induced pluripotent stem cells with triplication of the alpha-synuclein locus. Nat. Commun. 2, 440 10.1038/ncomms1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert A. D., Yu J., Rose F. F. Jr, Mattis V. B., Lorson C. L., Thomson J. A. and Svendsen C. N. (2009). Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457, 277-280. 10.1038/nature07677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frobel J., Hemeda H., Lenz M., Abagnale G., Joussen S., Denecke B., Saric T., Zenke M. and Wagner W. (2014). Epigenetic rejuvenation of mesenchymal stromal cells derived from induced pluripotent stem cells. Stem Cell Rep. 3, 414-422. 10.1016/j.stemcr.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Del Tredici K. and Braak H. (2013). 100 years of Lewy pathology. Nat. Rev. Neurol. 9, 13-24. 10.1038/nrneurol.2012.242 [DOI] [PubMed] [Google Scholar]
- Imaizumi Y., Okada Y., Akamatsu W., Koike M., Kuzumaki N., Hayakawa H., Nihira T., Kobayashi T., Ohyama M., Sato S. et al. (2012). Mitochondrial dysfunction associated with increased oxidative stress and alpha-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol. Brain 5, 35 10.1186/1756-6606-5-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K. (2010). Modern biological theories of aging. Aging Dis. 1, 72-74. [PMC free article] [PubMed] [Google Scholar]
- Jucker M. (2010). The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat. Med. 16, 1210-1214. 10.1038/nm.2224 [DOI] [PubMed] [Google Scholar]
- Keating D. J. (2008). Mitochondrial dysfunction, oxidative stress, regulation of exocytosis and their relevance to neurodegenerative diseases. J. Neurochem. 104, 298-305. [DOI] [PubMed] [Google Scholar]
- Kriks S., Shim J.-W., Piao J., Ganat Y. M., Wakeman D. R., Xie Z., Carrillo-Reid L., Auyeung G., Antonacci C., Buch A. et al. (2011). Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480, 547-551. 10.1038/nature10648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaille F. G., Pessach I. M., Zhang S.-Y., Ciancanelli M. J., Herman M., Abhyankar A., Ying S.-W., Keros S., Goldstein P. A., Mostoslavsky G. et al. (2012). Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 491, 769-773. 10.1038/nature11583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapasset L., Milhavet O., Prieur A., Besnard E., Babled A., Ait-Hamou N., Leschik J., Pellestor F., Ramirez J.-M., De Vos J. et al. (2011). Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 25, 2248-2253. 10.1101/gad.173922.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le W., Sayana P. and Jankovic J. (2014). Animal models of Parkinson's disease: a gateway to therapeutics? Neurotherapeutics 11, 92-110. 10.1007/s13311-013-0234-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Papapetrou E. P., Kim H., Chambers S. M., Tomishima M. J., Fasano C. A., Ganat Y. M., Menon J., Shimizu F., Viale A. et al. (2009). Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461, 402-406. 10.1038/nature08320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Ramirez C. N., Kim H., Zeltner N., Liu B., Radu C., Bhinder B., Kim Y. J., Choi I. Y., Mukherjee-Clavin B. et al. (2012a). Large-scale screening using familial dysautonomia induced pluripotent stem cells identifies compounds that rescue IKBKAP expression. Nat. Biotechnol. 30, 1244-1248. 10.1038/nbt.2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Dawson V. L. and Dawson T. M. (2012b). Animal models of Parkinson's disease: vertebrate genetics. Cold Spring Harb. Perspect. Med. 2, pii: a009324 10.1101/cshperspect.a009324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G.-H., Barkho B. Z., Ruiz S., Diep D., Qu J., Yang S.-L., Panopoulos A. D., Suzuki K., Kurian L., Walsh C. et al. (2011). Recapitulation of premature ageing with iPSCs from Hutchinson-Gilford progeria syndrome. Nature 472, 221-225. 10.1038/nature09879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G.-H., Qu J., Suzuki K., Nivet E., Li M., Montserrat N., Yi F., Xu X., Ruiz S., Zhang W. et al. (2012). Progressive degeneration of human neural stem cells caused by pathogenic LRRK2. Nature 491, 603-607. 10.1038/nature11557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C., Blasco M. A., Partridge L., Serrano M. and Kroemer G. (2013). The hallmarks of aging. Cell 153, 1194-1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi S. and Brunet A. (2012). Aging and reprogramming: a two-way street. Curr. Opin. Cell Biol. 24, 744-756. 10.1016/j.ceb.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J., Simonini M. V., Palejev D., Tomasini L., Coppola G., Szekely A. M., Horvath T. L. and Vaccarino F. M. (2012). Modeling human cortical development in vitro using induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 109, 12770-12775. 10.1073/pnas.1202944109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion R. M., Strati K., Li H., Tejera A., Schoeftner S., Ortega S., Serrano M. and Blasco M. A. (2009). Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell 4, 141-154. 10.1016/j.stem.2008.12.010 [DOI] [PubMed] [Google Scholar]
- Merkle F. T. and Eggan K. (2013). Modeling human disease with pluripotent stem cells: from genome association to function. Cell Stem Cell 12, 656-668. 10.1016/j.stem.2013.05.016 [DOI] [PubMed] [Google Scholar]
- Miller J. D., Ganat Y. M., Kishinevsky S., Bowman R. L., Liu B., Tu E. Y., Mandal P. K., Vera E., Shim J.-W., Kriks S. et al. (2013). Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell 13, 691-705. 10.1016/j.stem.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt P., Schmid B., Burbulla L. F., Schöndorf D. C., Wagner L., Glatza M., Höing S., Hargus G., Heck S. A., Dhingra A. et al. (2013). Genetic correction of a LRRK2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell 12, 354-367. 10.1016/j.stem.2013.01.008 [DOI] [PubMed] [Google Scholar]
- Sánchez-Danés A., Richaud-Patin Y., Carballo-Carbajal I., Jiménez-Delgado S., Caig C., Mora S., Di Guglielmo C., Ezquerra M., Patel B., Giralt A. et al. (2012). Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson's disease. EMBO Mol. Med. 4, 380-395. 10.1002/emmm.201200215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L. H., Laganière J., Cooper O., Mak S. K., Vu B. J., Huang Y. A., Paschon D. E., Vangipuram M., Sundararajan R., Urnov F. D. et al. (2014). LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson's disease patients: reversal by gene correction. Neurobiol. Dis. 62, 381-386. 10.1016/j.nbd.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibler P., Graziotto J., Jeong H., Simunovic F., Klein C. and Krainc D. (2011). Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J. Neurosci. 31, 5970-5976. 10.1523/JNEUROSCI.4441-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldner F., Laganière J., Cheng A. W., Hockemeyer D., Gao Q., Alagappan R., Khurana V., Golbe L. I., Myers R. H., Lindquist S. et al. (2011). Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell 146, 318-331. 10.1016/j.cell.2011.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth P. and Young-Pearse T. L. (2014). Stem cells on the brain: modeling neurodevelopmental and neurodegenerative diseases using human induced pluripotent stem cells. J. Neurogenet. 28, 5-29. 10.3109/01677063.2014.881358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y.-C. and Qi X. (2013). Inhibition of excessive mitochondrial fission reduced aberrant autophagy and neuronal damage caused by LRRK2 G2019S mutation. Hum. Mol. Genet. 22, 4545-4561. 10.1093/hmg/ddt301 [DOI] [PubMed] [Google Scholar]
- Suhr S. T., Chang E. A., Tjong J., Alcasid N., Perkins G. A., Goissis M. D., Ellisman M. H., Perez G. I. and Cibelli J. B. (2010). Mitochondrial rejuvenation after induced pluripotency. PLoS ONE 5, e14095 10.1371/journal.pone.0014095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K. and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663-676. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Wainger B. J., Kiskinis E., Mellin C., Wiskow O., Han S. S. W., Sandoe J., Perez N. P., Williams L. A., Lee S., Boulting G. et al. (2014). Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 7, 1-11. 10.1016/j.celrep.2014.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard C. M., Campos B. A., Kuo S.-H., Nirenberg M. J., Nestor M. W., Zimmer M., Mosharov E. V., Sulzer D., Zhou H., Paull D. et al. (2014). iPSC-derived dopamine neurons reveal differences between monozygotic twins discordant for Parkinson's disease. Cell Rep. 9, 1173-1182. 10.1016/j.celrep.2014.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Lian Q., Zhu G., Zhou F., Sui L., Tan C., Mutalif R. A., Navasankari R., Zhang Y., Tse H.-F. et al. (2011). A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell 8, 31-45. 10.1016/j.stem.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Zhang W., Li J., Suzuki K., Qu J., Wang P., Zhou J., Liu X., Ren R., Xu X., Ocampo A. et al. (2015). A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science 348, 1160-1163. 10.1126/science.aaa1356 [DOI] [PMC free article] [PubMed] [Google Scholar]