Abstract
Semaphorins are a large family of axon guidance molecules that are known primarily as ligands for plexins and neuropilins. Although class-6 semaphorins are transmembrane proteins, they have been implicated as ligands in different aspects of neural development, including neural crest cell migration, axon guidance and cerebellar development. However, the specific spatial and temporal expression of semaphorin 6B (Sema6B) in chick commissural neurons suggested a receptor role in axon guidance at the spinal cord midline. Indeed, in the absence of Sema6B, post-crossing commissural axons lacked an instructive signal directing them rostrally along the contralateral floorplate border, resulting in stalling at the exit site or even caudal turns. Truncated Sema6B lacking the intracellular domain was unable to rescue the loss-of-function phenotype, confirming a receptor function of Sema6B. In support of this, we demonstrate that Sema6B binds to floorplate-derived plexin A2 (PlxnA2) for navigation at the midline, whereas a cis-interaction between PlxnA2 and Sema6B on pre-crossing commissural axons may regulate the responsiveness of axons to floorplate-derived cues.
Keywords: Spinal cord development, In ovo RNAi, Cis-interaction, PlexinA2, Chick
INTRODUCTION
During development, a large number of neurons must connect with their target cells to establish functional neural circuits. A well-orchestrated set of axon guidance cues and their receptors on growth cones directs axonal navigation through the pre-existing tissue. On their journey, axons contact one or several intermediate targets before they reach their final destination. The floorplate at the CNS midline is one such intermediate target, where commissural axons cross to the contralateral side and grow along the rostrocaudal axis (Nawabi and Castellani, 2011; Chédotal, 2011). Owing to their stereotypic trajectory, dI1 commissural axons are a well-studied model for axon guidance. A number of long-range guidance cues directing these axons ventrally toward the floorplate have been identified (Kennedy et al., 1994; Augsburger et al., 1999; Charron et al., 2003; Islam et al., 2009). Midline crossing is regulated by a shift from attraction to repulsion (Stoeckli and Landmesser, 1995; Stoeckli et al., 1997; Long et al., 2004; Sabatier et al., 2004; Mambetisaeva et al., 2005; Chen et al., 2008). Axonal repulsion is induced by the upregulation of Robo1 on the growth cone surface, which allows for the detection of Slit, a negative floorplate-derived signal (Philipp et al., 2012). Axonal navigation at the floorplate exit site is subsequently directed by F-spondin (Burstyn-Cohen et al., 1999), SynCAMs/Nectin-like molecules (Niederkofler et al., 2010), MDGA2 (Joset et al., 2011), and the morphogens Wnts (Lyuksyutova et al., 2003; Domanitskaya et al., 2010) and sonic hedgehog (Shh) (Bourikas et al., 2005; Wilson and Stoeckli, 2013).
Semaphorin-plexin signaling also has been implicated in guiding commissural axons (Zou et al., 2000; Nawabi et al., 2010). GDNF- and NrCAM-mediated inhibition of the protease calpain 1 stabilizes plexin A1 (PlxnA1) on the growth cone (Nawabi et al., 2010; Charoy et al., 2012). The receptor complex neuropilin 2-PlxnA1 triggers sensitivity to the repulsive function of Sema3B, thus expelling axons from the midline area. Interestingly, Shh is also involved in this switch by downregulating protein kinase A (PKA) activity and thus inducing growth cone sensitivity to midline-derived class-3 semaphorins (Parra and Zou, 2010).
In contrast to the well-studied class-3 semaphorins, much less is known about class-6 semaphorins (Pasterkamp and Kolodkin, 2003; Kolodkin and Tessier-Lavigne, 2011; Pasterkamp, 2012). Class-6 semaphorins are a family of four type-I transmembrane proteins that bind directly to class-A plexins (plexinAs) in a neuropilin-independent manner. They have mainly been implicated as ligands for plexinAs. For example, Sema6A and Sema6B are required in the navigation of cardiac neural crest cells. They interact with PlxnA2 for proper cardiac outflow tract formation (Toyofuku et al., 2008). In axon guidance, Sema6A and Sema6B cooperate in the regulation of hippocampal mossy fiber targeting via interactions with PlxnA2 and PlxnA4 (Suto et al., 2007; Tawarayama et al., 2010). Sema6A directs the growth of sensory and sympathetic neurons (Xu et al., 2000; Haklai-Topper et al., 2010), and Sema6A/PlxnA4 signaling regulates the dendritic development of motoneurons (Zhuang et al., 2009). Sema6A also regulates cerebellar granule cell migration by controlling nucleus-centrosome coupling (Kerjan et al., 2005; Renaud et al., 2008). At the optic chiasm, Sema6D interacts with PlxnA1 and NrCAM to promote midline crossing of retinal axons (Kuwajima et al., 2012).
Although class-6 semaphorins act as ligands in all these processes, their intracellular domains, which include Src homology-3 (SH3) and zyxin-like domains, also suggest receptor functions (Eckhardt et al., 1997; Klostermann et al., 2000; Toyofuku et al., 2004a; Kolodkin and Tessier-Lavigne, 2011; Pasterkamp, 2012). This idea is supported by the analysis of corticospinal tract formation in Sema6a knockout mice, in which Sema6A may guide axons in a cell-autonomous manner. However, Sema6A is also expressed in the surrounding area, making it impossible to distinguish between cell-autonomous versus non-autonomous functions (Leighton et al., 2001; Rünker et al., 2008). A receptor function for Sema6A was also suggested by our previous studies on boundary cap cell clustering (Mauti et al., 2007). Boundary cap cell clusters act as gate keepers, allowing axons, but not cell bodies, to cross the CNS/PNS boundary. After knocking down Sema6A in boundary cap cells, motoneurons migrated out of the spinal cord along the ventral roots. This phenotype could be explained by a model in which Sema6A is a receptor on migrating boundary cap cells that recognizes PlxnA1 on motor axons as a stop signal. The Sema6A-PlxnA1 interaction would lead to the accumulation of boundary cap cells and indirectly initiate their clustering at the ventral motor exit point (Mauti et al., 2007).
So far, the only direct evidence for a receptor function of class-6 semaphorins in vivo comes from heart development, where Sema6D was shown to be a ligand and receptor for PlxnA1 (Toyofuku et al., 2004a,b).
Here, we demonstrate in chick that Sema6B is an axon guidance receptor. We show that Sema6B guides post-crossing commissural axons by binding to PlxnA2 expressed by floorplate cells. In the absence of axonal Sema6B or its ligand PlxnA2 in the floorplate, post-crossing commissural axons fail to turn rostrally along the longitudinal axis of the spinal cord.
RESULTS
Sema6b is transiently expressed in dorsal commissural neurons
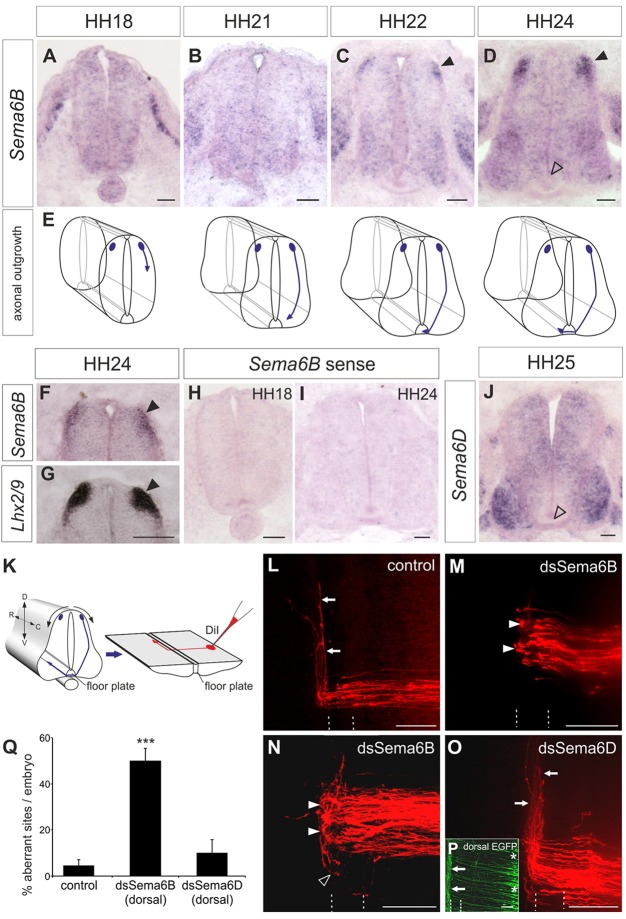
Sema6b mRNA was readily detectable in dI1 commissural neurons of the chicken neural tube during the time window when their axons cross and exit the floorplate (Fig. 1A-F). Importantly, Sema6b mRNA was only detectable at HH22 (Fig. 1C), when most axons have reached the ipsilateral floorplate border. No Sema6b expression was detectable in commissural neurons at HH21, that is, shortly before their axons reach the floorplate (Fig. 1B,E). Highest expression levels were found at HH24, when commissural axons exit the floorplate and turn rostrally along the contralateral floorplate border (Fig. 1D,E). At later stages (not shown), Sema6b was found at lower levels throughout the gray matter, consistent with findings in mouse (Suto et al., 2005). The identity of dI1 neurons was confirmed by labeling adjacent sections with probes for the marker Lhx2/9 (Helms and Johnson, 2003) (Fig. 1F,G).
Fig. 1.
Sema6b expression in dI1 commissural neurons peaks at HH24 and is required for post-crossing commissural axon guidance. (A-E) Expression of Sema6b mRNA in transverse sections of the chicken lumbar spinal cord at the indicated developmental stages (A-D) with schematic representations of the corresponding growth of dI1 axons (E). Sema6b is first detected in dI1 neurons at HH22, when their axons have reached the ipsilateral floorplate border (C, arrowhead). Expression of Sema6b in dI1 neurons is strongest at HH24 (D, arrowheads). (F,G) Probing adjacent sections with Lhx2/9 confirmed that Sema6b is expressed in dI1 neurons (arrowheads). (H,I) No staining is seen with a Sema6b sense probe. (J) Diffuse expression of Sema6d at HH25, with higher levels in motoneurons. Neither Sema6b nor Sema6d is expressed in the floorplate (D,J, open arrowheads). (K) Schematic of an open-book preparation and application of DiI for axonal tracing (red). D, dorsal; V, ventral; R, rostral; C, caudal. (L) Commissural axons cross the midline and extend along the contralateral floorplate border (arrows) in untreated control embryos. (M,N) After silencing Sema6B by injection and electroporation of dsRNA (dsSema6b), axons stall at the contralateral floorplate border (closed arrowheads) or even turn caudally (open arrowhead). (O) Silencing Sema6D by injection and electroporation of dsRNA (dsSema6d) did not affect axon guidance (arrows). (P) Expression of a co-electroporated EGFP reporter confirmed the exclusively dorsal targeting of dsRNA (asterisks). Arrows mark axons of contralaterally projecting commissural neurons. The floorplate is indicated by dashed lines. (Q) Quantification of DiI injection sites with aberrant axonal pathfinding. ***P<0.001; error bars indicate s.e.m. Scale bars: 50 µm in A-D,F-J; 100 µm in L-P.
As we described previously, Sema6a was not expressed in commissural neurons at the lumbar level (Mauti et al., 2007; but see dorsal expression at the brachial level described by Toyofuku et al., 2008). Sema6d was strongly expressed in motor neurons and at lower levels in the spinal cord gray matter (Fig. 1J) (Mauti et al., 2007). No class-6 semaphorins were expressed in the floorplate (Fig. 1) (Mauti et al., 2007). No ortholog of Sema6c is found in the chicken genome.
Loss of Sema6B causes defects in commissural axon guidance
The transient expression of Sema6b in dI1 neurons during the window of axonal midline crossing and turning suggested a role for Sema6B in axonal pathfinding. To test this hypothesis, we used in ovo RNAi to knock down Sema6B in commissural neurons (Fig. 1K-Q). Long dsRNA derived from Sema6b or Sema6d, together with an EGFP reporter plasmid, was injected into the central canal at HH18 and electroporated into the dorsal spinal cord (Fig. 1P). Commissural axon pathfinding was assessed 2 days later by axonal tracing with lipophilic DiI applied to the cell bodies of dI1 neurons (Fig. 1K-O).
In untreated control embryos, commissural axons crossed the floorplate and turned rostrally along the contralateral floorplate border (Fig. 1L,Q; 95.3±2.5% of DiI sites were normal; n=19 embryos, N=164 DiI injection sites). When Sema6B was downregulated, axons failed to turn rostrally and instead stalled or even turned caudally at the floorplate exit site in 50.1±5.4% of the DiI sites (n=22, N=149; Fig. 1M,N,Q). By contrast, loss of Sema6D did not affect the guidance of post-crossing axons (abnormalities at only 10.2±5.7% of injection sites; n=13, N=84; Fig. 1O,Q). Taken together, these experiments indicated that downregulation of Sema6B specifically interfered with commissural axon guidance upon floorplate exit.
Sema6B acts as a receptor in commissural axons
Semaphorins are typically known as ligands for plexin receptors. However, Sema6b was expressed in commissural neurons but not in the floorplate, suggesting a role for the protein as receptor rather than ligand. To test this idea, we asked whether a truncated version of Sema6B lacking the intracellular domain could rescue the axon guidance defects induced by Sema6B knockdown. Here, we used artificial microRNAs (miRNAs) to knock down Sema6B, and then expressed knockdown-resistant forms of Sema6B in commissural neurons (Fig. 2A). The efficiency of the Sema6B miRNA (miS6B) and rescue constructs (Sema6BΔmiR) were verified in vivo and in vitro (supplementary material Fig. S1A-E).
Fig. 2.

The intracellular domain of Sema6B is essential for post-crossing commissural axon guidance. (A) Schematics of miRNA and rescue constructs used in B-H. (B-G″) Open-book analysis of DiI injection sites in embryos co-electroporated with: (B) miLuc and pMES; (C) miS6B and pMES; (D) miS6B and pSema6BΔmiR; (E) miS6B and pSema6BΔCTΔmiR; (F) pSema6BΔmiR alone; or (G) pSema6BΔCTΔmiR alone. Arrows (B,D,F) indicate normal crossing and turning of commissural axons. Post-crossing axons that failed to turn correctly at the contralateral floorplate border are indicated by arrowheads (C,E,G). (B′-G′) Merge of DiI-labeled axons (red) and EGFP (green) used to visualize the expression of pMES constructs. (B″-G″) Enhanced blue fluorescent protein-2 (EBFP2) visualizes the expression of miRNA constructs. The floorplate is indicated by dashed lines. Scale bars: 100 µm. (H) Quantification of DiI injection sites with normal axonal pathfinding. **P<0.01, ***P<0.001; n.s., not significant; error bars indicate s.e.m.
In control embryos expressing a miRNA against Luciferase (miLuc) in the dorsal spinal cord, normal turning of post-crossing commissural axons was observed at 69.5±6.4% of injection sites (n=9, N=69; Fig. 2B,H). However, only 20.8±6.6% of injection sites showed normal axon pathfinding after downregulation of Sema6B with miS6B (n=10, N=81; Fig. 2C,H). In agreement with its temporal expression pattern, loss of Sema6B did not affect commissural axon guidance towards the ventral midline (supplementary material Fig. S3). These results were in line with our previous experiments in which Sema6B was downregulated with long dsRNA (Fig. 1). The turning defects of post-crossing axons observed after silencing of Sema6B could be rescued by the concomitant expression of Sema6BΔmiR (Fig. 2D,H). Normal axonal pathfinding in the rescue condition was observed at 58.2±7.7% of injection sites (n=13, N=91). By contrast, expression of Sema6BΔCTΔmiR, a truncated version of Sema6B lacking the cytoplasmic domain, was unable to rescue commissural axon guidance, as only 21.5±6.2% of injection sites (n=15, N=84) were normal (Fig. 2E,H). Expression of Sema6B alone did not significantly change axon pathfinding (Fig. 2F,H; 47.3±7.4% normal DiI sites; n=13, N=101). By contrast, expression of Sema6BΔCTΔmiR resulted in aberrant post-crossing axon pathfinding (Fig. 2G,H; 25.6±6.8% normal DiI sites; n=9, N=61), suggesting that Sema6BΔCTΔmiR acts in a dominant-negative manner. Taken together, these results demonstrate that the intracellular portion of Sema6B is crucial for correct navigation of commissural axons and that Sema6B functions cell-autonomously as an axon guidance receptor.
PlxnA2 is a potential interaction partner for Sema6B in commissural axon guidance
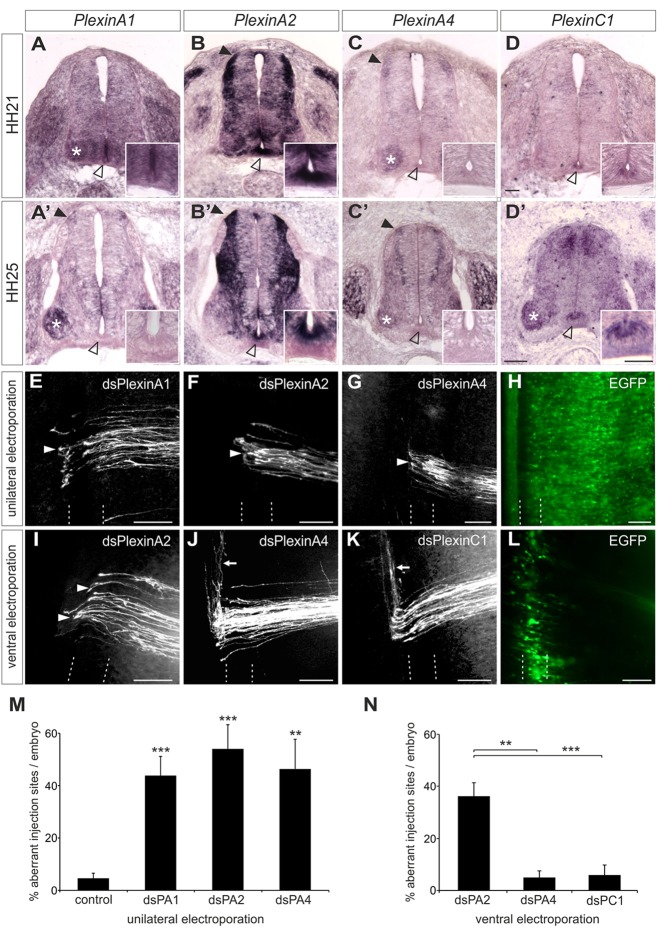
We next sought a potential floorplate-derived ligand for the Sema6B receptor. Based on the known interactions of class-6 semaphorins with plexinAs (Suto et al., 2005; Mauti et al., 2007; Toyofuku et al., 2008), we assessed the expression patterns of plexinAs during commissural axon pathfinding. As we described previously (Mauti et al., 2006), plexins have dynamic and distinct expression patterns in the chicken lumbar spinal cord (Fig. 3). At HH21, just before the first dI1 commissural axons reach the floorplate at lumbar levels of the spinal cord, Plxna1 was strongly expressed in the ventral spinal cord (including the floorplate), and at lower levels throughout the rest of the neural tube (Fig. 3A). By HH25, Plxna1 expression was high in dI1 neurons and in motoneurons (Fig. 3A′), but was no longer expressed in the floorplate (inset in Fig. 3A′). Plxna2 was expressed strongly in dI1 neurons and the floorplate at both HH21 and HH25 (Fig. 3B,B′). Plxna4 was expressed in dI1 neurons, but was not found in the floorplate (Fig. 3C,C′). By contrast, Plxnc1 was found in the floorplate (Fig. 3D,D′). No ortholog of Plxna3 is found in the chicken genome.
Fig. 3.
For correct pathfinding, PlxnA2 is required in both commissural neurons and the floorplate. (A-D′) Expression patterns of plexins analyzed by in situ hybridization at stages HH21 (A-D) and HH25 (A′-D′). Staining in commissural neurons is indicated by closed arrowheads. Motoneurons are marked with asterisks. The floorplate is indicated by open arrowheads and shown at higher magnification in the insets. (E-G) After downregulation of PlxnA1 (E), PlxnA2 (F) or PlxnA4 (G) by unilateral electroporation of dsRNA, axons failed to turn rostrally at the contralateral floorplate border (arrowheads). (H) Only injection sites in the electroporated area (verified by EGFP expression) were included in the analysis. (I-K) Analysis of commissural axon pathfinding after downregulation of PlxnA2 (I), PlxnA4 (J) or PlxnC1 (K) exclusively in the ventral spinal cord. After ventral downregulation of PlxnA2 (I, arrowheads), post-crossing axons failed to turn into the longitudinal axis or even turned caudally. Axon guidance was unaffected after ventral downregulation of PlxnA4 (J, arrow) or PlxnC1 (K, arrow). (L) Successful and exclusive targeting of cells at the ventral midline was verified by EGFP expression. The floorplate is indicated by dashed lines. (M,N) Quantification of injection sites with aberrant axonal pathfinding after (M) unilateral or (N) ventral electroporation of dsRNA. ***P<0.001; **P<0.01; error bars indicate s.e.m. Scale bars: 50 μm in A-D′; 100 µm in E-L.
Consistent with their potential role as interaction partners for Sema6B, unilateral downregulation of plexinAs interfered with commissural axon pathfinding (Fig. 3E-H). The loss of PlxnA1 caused post-crossing defects at 43.8±7.3% of DiI sites (n=16, N=106; Fig. 3E,M). After downregulation of PlxnA2, post-crossing commissural axons failed to turn rostrally at 54.1±9.1% of injection sites (n=15, N=92; Fig. 3F,M). The same phenotype was observed at 46.3±11.3% of injection sites after downregulation of PlxnA4 (n=11, N=66; Fig. 3G,M).
Based on these results, we analyzed the requirement for plexinAs in post-crossing commissural axon guidance more specifically by targeted electroporation of the floorplate (Fig. 3I-L). Because Plxna1 expression disappeared from the floorplate during axonal crossing and turning, and based on biochemical interaction assays that indicated that PlxnA1 did not bind to Sema6B (Toyofuku et al., 2008; and see below), we instead considered PlxnA2 as the most likely binding partner for axonal Sema6B in the floorplate. Indeed, after knocking down PlxnA2 in the ventral spinal cord, we found contralateral stalling phenotypes comparable to those observed in the absence of Sema6B at 36.2±5.2% of injection sites (n=18, N=149; Fig. 3I,N). By contrast, ventral electroporation of Plxna4 dsRNA did not interfere with commissural axon guidance (Fig. 3J,N). As Plxna4 was never found in the floorplate (Fig. 3C,C′), this experiment was a negative control. Aberrant axonal behavior was detected at only 5.0±2.6% of injection sites (n=7, N=56). Downregulation of PlxnC1, which is expressed in the floorplate but interacts with class-7 semaphorins (Pasterkamp, 2012), did not interfere with commissural axon navigation: aberrant behavior was detected at only 5.8±4.1% of injection sites (n=12, N=75; Fig. 3K,N). Together, the targeted electroporation experiments supported a specific axon guidance role of floorplate-derived PlxnA2.
The intracellular domain of PlxnA2 is dispensable for its axon guidance function in the floorplate
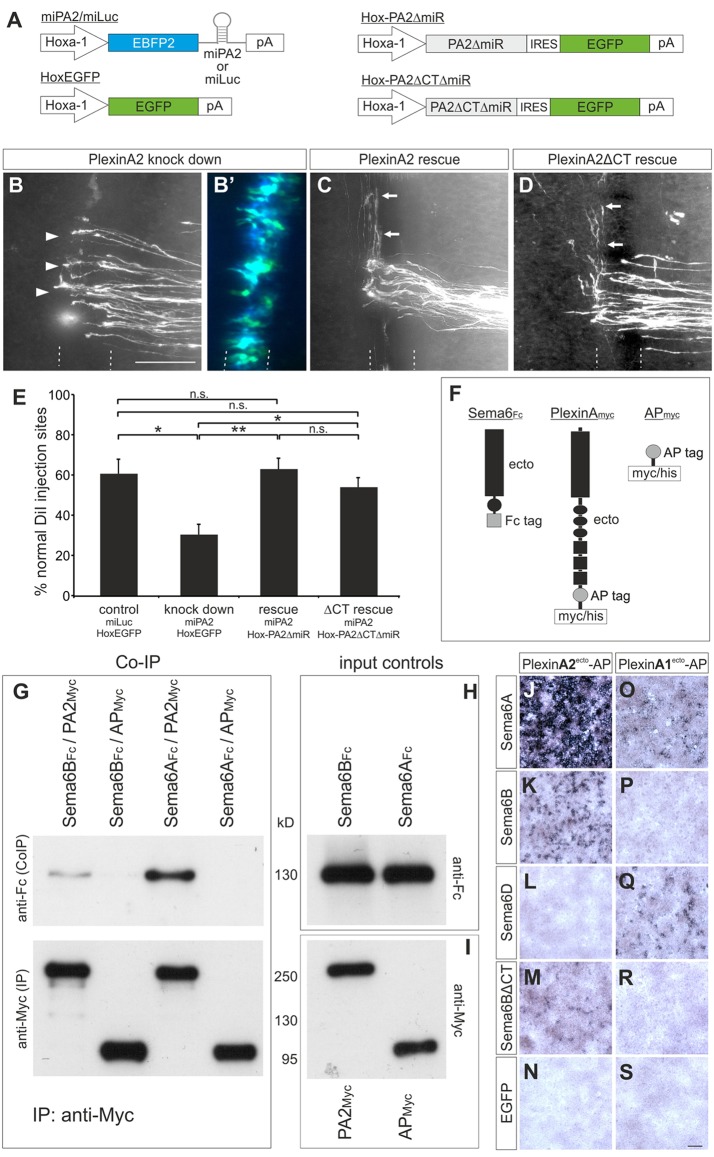
If PlxnA2 was acting as a floorplate-derived ligand for Sema6B during commissural axon guidance, then the cytoplasmic domain of PlxnA2 could be dispensable for its axon guidance activity in the floorplate. To test this idea, we downregulated PlxnA2 specifically in the floorplate using an miRNA (miPA2), then attempted to rescue the phenotype by expressing miRNA-resistant constructs encoding either the full-length protein (PlexinA2ΔmiR) or a truncated version containing the extracellular and the transmembrane domains but lacking the cytoplasmic tail (PlexinA2ΔCTΔmiR). The miRNA and rescue constructs were verified in vivo and in vitro (supplementary material Fig. S2). All constructs were under the control of a Hoxa1 enhancer in order to drive specific expression in the floorplate (Fig. 4A) (Wilson and Stoeckli, 2011).
Fig. 4.
The extracellular domain of PlxnA2 mediates its axon guidance activity in the floorplate and binds to Sema6B. (A) Schematics of the constructs used in B-E. Hoxa1 drives floorplate-specific expression. (B-D) Open-book analysis of embryos co-electroporated with: (B) miPA2 and Hox-EGFP; (C) miPA2 and Hox-PA2ΔmiR; or (D) miPA2 and Hox-PA2ΔCTΔmiR. (B′) The successful and exclusive targeting of floorplate cells was confirmed by EGFP and EBFP2 fluorescence from the co-electroporated constructs. Post-crossing axons that failed to turn correctly at the contralateral floorplate border are indicated by arrowheads (B). Arrows (C,D) indicate normal crossing and turning of commissural axons. (E) Quantification of injection sites with normal axon pathfinding after floorplate-specific manipulations of PlxnA2. *P<0.05, **P<0.01; n.s., not significant; error bars indicate s.e.m. (F) Schematics of the proteins used for co-IPs. (G) Sema6BFc or Sema6AFc ectodomains were incubated with either PA2Myc ectodomains or APMyc, and immunoprecipitated with anti-myc agarose beads. Co-IP of Sema6B and Sema6A was detected with anti-Fc antibodies on western blots (upper panel). Blots stained with anti-myc antibodies, to demonstrate the successful immunoprecipitation, are shown below. As controls, the input proteins from conditioned media were analyzed on blots developed with (H) anti-Fc antibodies to detect Sema6BFc and Sema6AFc ectodomains and (I) anti-myc antibodies to detect PA2Myc and APMyc. (J-S) HEK293T cells were transfected with chicken Sema6A (J,O), Sema6B (K,P), Sema6D (L,Q), Sema6BΔCT (M,R) or EGFP (N,S) and incubated with conditioned medium containing AP-tagged ectodomains of PlxnA2 (PlexinA2ecto-AP, J-N) or PlxnA1 (PlexinA1ecto-AP, O-S). Scale bars: 100 μm.
As expected, the miRNA-based knockdown of PlxnA2 specifically in the floorplate caused axon pathfinding errors. Whereas the control group injected and electroporated with miLuc and the EGFP plasmid showed normal axon guidance at 60.9±8.5% of the injection sites per embryo (n=12 embryos, N=107 injection sites), downregulation of PlxnA2 specifically in the floorplate with miPA2 reduced normal axon guidance to only 30.6±6.1% of the injection sites (n=14, N=97). Axon guidance was rescued by co-injection and electroporation of knockdown-resistant full-length PlxnA2 (PlexinA2ΔmiR; normal axon guidance at 63.2±5.5% of the injection sites per embryo; n=12, N=79) and truncated PlxnA2 (PlexinA2ΔCTΔmiR; normal axon guidance at 54.2±5.1% of the injection sites per embryo; n=17, N=119). The integrity of the floorplate was not affected in these experiments, as the expression of neither markers (Hnf3β and Nkx2.2) nor other axon guidance molecules (Shh and Slit2) was perturbed (supplementary material Fig. S4).
Together, these results suggest that PlxnA2 acts directly as an axon guidance molecule in the floorplate. The extracellular domain of PlxnA2, and not its intracellular domain, is crucial for this function. This finding was consistent with our hypothesis that PlxnA2 is a floorplate-derived ligand for Sema6B during commissural axon guidance.
Sema6B interacts with PlxnA2
To support the above hypothesis, we next confirmed a physical interaction between Sema6B and PlxnA2 in co-immunoprecipitation (co-IP) and binding assays. The co-IP of Sema6A with PlxnA2 was used as positive control, as this interaction has been described previously (Suto et al., 2005, 2007; Toyofuku et al., 2008; Janssen et al., 2010; Nogi et al., 2010). Immunoprecipitation of myc-tagged PlxnA2 ectodomains from conditioned medium was found to pull down both Sema6B-Fc and Sema6A-Fc ectodomains (Fig. 4F-I).
We confirmed the interaction between Sema6B and PlxnA2 by cell-binding assays. HEK293 cells expressing different class-6 semaphorins were incubated with PlxnA2 ectodomains fused to alkaline phosphatase (PlexinA2ecto-AP; Fig. 4J-N). As expected, binding of PlexinA2ecto-AP to Sema6A-expressing cells was very strong (Fig. 4J). PlexinA2ecto-AP binding to Sema6B-expressing cells was clearly detectable, but weaker than to Sema6A-expressing cells (Fig. 4K). PlexinA2ecto-AP also bound to Sema6BΔCT (Fig. 4M). However, no binding of PlexinA2ecto-AP was found to cells transfected with Sema6D (Fig. 4L) or a control EGFP plasmid (Fig. 4N). By contrast, PlexinA1ecto-AP bound to cells expressing Sema6A (Fig. 4O) and Sema6D (Fig. 4Q) but not to cells expressing Sema6B (Fig. 4P,R). Thus, we could exclude PlxnA1 as a Sema6B interaction partner. Taken together, our results confirmed a previously reported interaction between Sema6B and PlxnA2 (Toyofuku et al., 2008), and strongly supported our hypothesis that PlxnA2 is a floorplate-derived ligand for Sema6B during commissural axon guidance.
Sema6B mediates an outgrowth response of commissural neurons to PlxnA2
We next investigated whether the response of commissural axons to PlxnA2 substrate was altered by a lack of Sema6B (Fig. 5). On coverslips coated with Albumax, Laminin or concentrated conditioned medium from cells expressing either AP alone or PlexinA2ecto-AP, we plated dissociated commissural neurons from embryos that were electroporated with a vector expressing EBFP2 and miS6B to knock down Sema6B. Because electroporation is not 100% efficient, the pools of commissural neurons obtained comprised both wild-type and miRNA-expressing (EBFP2-positive) neurons from the same embryos. Thus, the wild-type neurons provided an internal control. Cultures were allowed to grow for 48 h before fixation and immunolabeling for the axonal marker axonin 1 (contactin 2).
Fig. 5.

The commissural axon response to PlxnA2 is Sema6B dependent. (A-F) Examples of axon outgrowth of commissural neurons obtained from embryos electroporated with βactin-EBFP2-miS6B on Laminin (A,B), AP only (C,D) and PlexinA2ecto-AP (E,F). Axons were co-immunolabeled for axonin 1 and EBFP2. Co-labeled neurons appear yellow in A,C,E. Scale bar: 50 μm. (G) Quantification of axon lengths on different substrates after electroporation of βactin-EBFP2-miS6B. WT, wild type; Alb, Albumax; AP, concentrated conditioned medium from AP-expressing cells; Lam, Laminin; PA2-AP, concentrated conditioned medium from PlexinA2ecto-AP-expressing cells. ***P<0.001; error bars indicate s.e.m.
As expected, we observed modest outgrowth of commissural axons on Albumax (Fig. 5G; data not shown), whereas the Laminin and AP substrates encouraged slightly longer axons (Fig. 5A-D,G). By far the longest axons were found on PlxnA2-coated coverslips (Fig. 5E-G). However, the expression of miS6B (but not miLuc) significantly dampened this outgrowth response to PlxnA2 (Fig. 5E-H). These results indicate that PlxnA2 promotes the outgrowth of commissural axons in a pathway that is mediated by Sema6B.
Axonal PlxnA2 contributes to post-crossing commissural axon guidance
Our functional analyses suggested that PlxnA2 is a floorplate-derived ligand for Sema6B during commissural axon guidance. However, Plxna2 was expressed not only in the floorplate but also in commissural neurons (Fig. 3B). Because PlxnA1 has previously been identified as an axonally expressed receptor with important roles in commissural axon guidance (Nawabi et al., 2010; Charoy et al., 2012), we hypothesized that other plexinAs might function similarly. To test this idea, we knocked down PlxnA2 and PlxnA4 only in the dorsal spinal cord by targeted electroporation of long dsRNA. In line with results from unilateral electroporations, knockdown of PlxnA2 in commissural neurons perturbed axon guidance at 44.3±8.1% of injection sites (n=15 embryos, N=115 injection sites; Fig. 6A,D). Similarly, dorsal downregulation of PlxnA4 interfered with post-crossing axon guidance at 38.9±6.0% of injection sites (n=23, N=153; Fig. 6D). Based on these results, both PlxnA2 and PlxnA4 could act as guidance receptors on commissural axons after crossing the midline. Furthermore, these findings suggested the possibility of axonal cis-interactions with Sema6B.
Fig. 6.
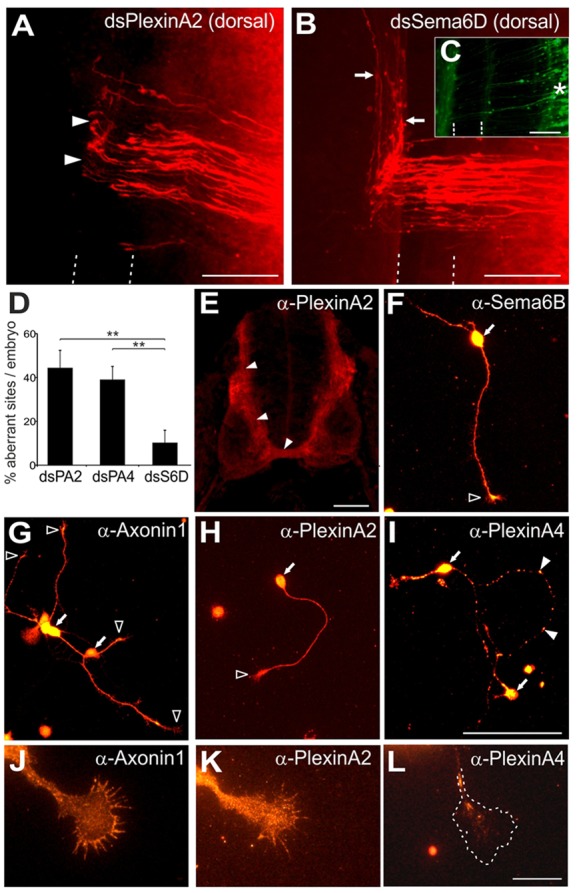
Axonal PlxnA2 is required for the pathfinding of post-crossing axons. (A) Downregulation of PlxnA2 in the dorsal spinal cord led to aberrant commissural axon guidance at the contralateral floorplate border (arrowheads). (B) Commissural axon guidance was normal (arrows) when dsSema6D was electroporated dorsally. (C) Electroporation of only the dorsal spinal cord was verified by EGFP expression (asterisk). (D) Quantifications of DiI injection sites with aberrant axon pathfinding. **P<0.01; error bars indicate s.e.m. The dsSema6D value is taken from Fig. 1. (E) Staining of transverse sections of HH25 spinal cords localized PlxnA2 immunoreactivity to the pre-crossing segment and commissure (arrowheads). (F) Sema6B was expressed all along dissociated commissural axons and on growth cones (open arrowhead), as was axonin 1 (G,J), a marker for commissural neurons. PlxnA2 (H,K) was also expressed on axons and growth cones, in contrast to PlxnA4 (I) which was found in a punctate pattern along axons (arrowheads). No PlxnA4 was found on growth cones (L). Arrows (F-I) indicate neuronal soma. Scale bars: 100 μm in A-C,E; 50 μm in F-I; 20 μm in J-L.
To investigate this idea, we examined the localization patterns of Sema6B and plexinAs in more detail. In vivo, the expression of PlxnA2 was strongest on pre-crossing and commissural segments (Fig. 6E). Unfortunately, staining of Sema6B on sections was not possible with the available antibody, but our earlier in situ results (Fig. 1) suggested that Sema6b was only expressed in pre-crossing axons shortly before they contacted the floorplate. In cultured commissural neurons obtained from HH25 embryos, we found expression of Sema6B all along the axons and on growth cones (Fig. 6F). PlxnA2 (Fig. 6H,K) was expressed similarly. By contrast, PlxnA4 was found in a punctate pattern along the axons (Fig. 6I), suggesting an intracellular, vesicular localization. Additionally, only very low levels of PlxnA4 were found in growth cones (Fig. 6L), making the formation of a cis-complex with Sema6B on the growth cone or axonal surface unlikely.
Taken together, the co-expression of PlxnA2 and Sema6B in similar subcellular locations, together with their ability to interact with each other (Fig. 4; supplementary material Fig. S5), suggested a possible cis-interaction between these molecules on commissural axons as they enter and cross the floorplate.
PlxnA2 misexpression in commissural neurons leads to axon stalling in the dorsal neural tube
As knockdown of PlxnA2 in commissural neurons impaired axon guidance (Fig. 6), we tested whether it was required cell-autonomously for growth cone turning (Fig. 7; supplementary material Fig. S2). We used the previously described rescue constructs encoding either full-length PlxnA2 or PlexinA2ΔCT (supplementary material Fig. S2). This time, the miRNA and rescue constructs were driven by a dI1 neuron-specific enhancer of Math1 (Atoh1) (Fig. 7A; supplementary material Fig. S2) (Helms and Johnson, 2003; Wilson and Stoeckli, 2011). However, the expression of neither PlexinA2ΔmiR nor PlexinA2ΔCTΔmiR rescued the axon guidance phenotypes.
Fig. 7.
Overexpression of full-length PlxnA2 prevents ventral growth of dI1 axons. (A) Schematics of the constructs used in B-I. Math1 drives dI1 neuron-specific expression. Axon growth was assessed by (B,F) anti-HA immunolabeling and (C,G) EGFP fluorescence. (B-E′) The dorsal spinal cord (D,H) and floorplate (E,I) are shown at higher magnification. The expression of full-length PlxnA2 impaired the ventral growth of commissural axons (arrowheads in D). Only EGFP-positive, but no HA-positive, axons were found at the midline (asterisk in E,E′). Note that not all EGFP-positive neurons/axons showed PlxnA2 expression (arrowheads in D′,E′), which indicates an inverse relationship between PlxnA2 protein levels in commissural neurons and the ventral projection of their axons. (F-I′) PlexinA2ΔCT misexpression did not impair the ventral growth of commissural axons (arrowheads in H,H′). HA-positive axons grew ventrally and crossed the midline (arrowheads in I,I′). Scale bars: 100 µm.
We found an explanation for these unexpected findings when we carefully analyzed commissural axons expressing PlxnA2 constructs. Misexpression of full-length PlxnA2 specifically in dI1 neurons resulted in premature axonal stalling in the dorsal spinal cord in all embryos analyzed (n=7), thus preventing the analysis of axonal pathfinding at the ventral midline (Fig. 7B-E′). As PlxnA2 is expressed in pre-crossing axons (Fig. 6E), its levels have to be tightly regulated. Obviously, too much PlxnA2 renders axons sensitive to repellent class-3 semaphorins from the ventral spinal cord, whereas too little PlxnA2 prevents the proper pathfinding of post-crossing axons. Although the overexpression of PlexinA2ΔCT did not affect the ventral axonal trajectory (in all embryos analyzed, n=7; Fig. 7F-I′), it nevertheless failed to rescue the post-crossing axon guidance defects (supplementary material Fig. S2D,E).
Taken together, these findings suggest that: (1) the PlxnA2 intracellular domain transduces axonal signals that are required for post-crossing axon pathfinding; and (2) PlxnA2 intracellular signaling is normally inhibited in pre-crossing axons in the ventral spinal cord before floorplate contact to allow growth cone entry into the floorplate. In the dorsal spinal cord, endogenous levels of PlxnA2 are low, as are the levels of repulsive class-3 semaphorins. Therefore, axons extend ventrally. However, when PlxnA2 levels were increased experimentally (Fig. 7B-E), axons were hypersensitive to class-3 semaphorins and failed to extend into the ventral spinal cord.
In summary, our in vivo analyses suggest a complex mode of interaction between Sema6B and PlxnA2. As PlxnA2 is required both as a ligand, when expressed on floorplate cells, and as a receptor or co-receptor, when expressed on commissural axons, we suggest the following model (Fig. 8). In the dorsal spinal cord PlxnA2 expressed on pre-crossing commissural axons is not activated by the low levels of repulsive class-3 semaphorins derived from the ventral spinal cord, and axons extend readily towards the floorplate (Fig. 8A). However, in the vicinity of the floorplate, where levels of class-3 semaphorins are high, PlxnA2 on pre-crossing commissural axons is prevented from mediating a repulsive signal by cis-interaction with Sema6B, which is now expressed on pre-crossing axons of dI1 neurons (Fig. 8B). Upon floorplate contact (Fig. 8C), the cis-complex between axonal PlxnA2 and Sema6B is replaced by a trans-interaction between Sema6B and floorplate PlxnA2. The change from Sema6B-PlxnA2 cis- to trans-interaction mediates a Sema6B-dependent turning signal. Axonal PlxnA2 is available for cis-interaction with neuropilin, resulting in a repulsive signal on post-crossing axons (Nawabi et al., 2010; Parra and Zou, 2010; Zou et al., 2000).
Fig. 8.

Model of PlxnA2-Sema6B interactions in commissural axon guidance. (A) In the dorsal spinal cord, pre-crossing axons do not yet express Sema6B. However, axons are not affected by the low levels of repulsive signals (blue diamond) and readily grow towards the ventral midline. Note that we have drawn PlxnA2 (green) arbitrarily as dimer or monomer based on reports on the crystal structures of Sema6A and PlxnA2 (for details see Janssen et al., 2010; Nogi et al., 2010). For simplicity, we omitted neuropilins, which would form complexes with plexins. (B) In the ventral spinal cord, close to the floorplate, where repulsive class-3 semaphorin activity is high (large blue diamond), PlxnA2 (green) is prevented from mediating a repulsive signal due to the cis-interaction with Sema6B (red). (C) At the contralateral floorplate border, post-crossing commissural axons can respond to repulsive class-3 semaphorins, in agreement with published reports (Parra and Zou, 2010; Nawabi et al., 2010), as the cis-interaction of axonal PlxnA2 with Sema6B is replaced by a Sema6B-PlxnA2 trans-interaction. Trans-interactions between floorplate PlxnA2 and growth cone Sema6B result in a turning response and support elongation of post-crossing axons along the contralateral floorplate border.
DISCUSSION
Our analyses of Sema6B function in post-crossing commissural axon guidance provide the first report of a receptor function of class-6 semaphorins in vertebrate axon guidance. Our evidence strongly supports the conclusion that Sema6B on commissural axons is the receptor that binds PlxnA2 expressed at the intermediate target: (1) Sema6B is expressed by commissural neurons, PlxnA2 is expressed by floorplate cells, and a direct interaction between Sema6B and PlxnA2 was shown in binding studies and by co-IP; (2) our loss-of-function studies revealed similar defects in post-crossing commissural axon guidance after silencing Sema6B or PlxnA2; (3) the axon guidance effects of Sema6B were dependent on the presence of its intracellular domain; (4) the extracellular domain of PlxnA2 was sufficient to rescue the effects of PlxnA2 knockdown in the floorplate; and (5) a substrate of PlxnA2 ectodomains enhanced the outgrowth of commissural axons in a Sema6B-dependent manner. Although our in vitro assay (Fig. 5) did not elucidate the guidance activities of PlxnA2 (because the axons were not faced with a choice of substrate), our results suggest that PlxnA2 is a floorplate-derived ligand that affects axonal behavior in a Sema6B-mediated manner.
A receptor function for class-6 semaphorins is not without precedent. In vertebrates, our previous studies suggested a receptor role of Sema6A in boundary cap cell clustering (Mauti et al., 2007). In addition, Sema6D acts as both ligand and receptor in heart development (Toyofuku et al., 2004a,b). In invertebrates, there is direct evidence that transmembrane semaphorins are axon guidance receptors. Sema1a, which is the closest Drosophila homolog of class-6 semaphorins, was shown to act as a receptor in R-cell axon guidance in the visual system (Cafferty et al., 2006; Yu et al., 2010), in the guidance of projection neurons in the olfactory system (Komiyama et al., 2007) and in motor axon defasciculation (Jeong et al., 2012). Sema1a also acts as both a ligand and receptor in synapse formation (Godenschwege et al., 2002; Godenschwege and Murphey, 2009). However, prior to our current study, the direct evidence that class-6 semaphorins were receptors in vertebrate axon guidance was still missing, despite reports of aberrant axonal decussation in Sema6a mutant mice that were suggestive of this function (Leighton et al., 2001; Rünker et al., 2008).
Receptor functions for class-6 semaphorins are in line with their structural characteristics. The C-terminal part of Sema6A contains a zyxin-like domain that represents an Ena/VASP-like protein-binding site (Klostermann et al., 2000). Ena/VASP proteins regulate actin dynamics and therefore influence neuronal polarization and axon growth (Tahirovic and Bradke, 2009). Ena/VASP-mediated actin dynamics also affect cell migration. Phosphorylation of Ena/VASP by Abl kinase, which was shown to be recruited to the cytoplasmic domain of Sema6D in heart development, is consistent with the observed effects on myocardial cell migration (Toyofuku et al., 2004a).
Because PlxnA2 is not only expressed in the floorplate, but also co-expressed with Sema6B in commissural neurons, the interactions between Sema6B and PlxnA2 might be more complicated than a straightforward receptor-ligand interaction between molecules expressed on the growth cone and floorplate, respectively. In fact, a cis-interaction between Sema6B and PlxnA2 might be part of a regulatory mechanism that controls the sensitivity of commissural axons to repulsive guidance cues in the ventral spinal cord and the floorplate, and may additionally contribute to their switch from attraction to repulsion at the midline.
In line with this idea, a regulatory role of Sema6-plexinA cis-interactions on trans-interactions has been demonstrated in the PNS (Haklai-Topper et al., 2010). Dorsal root ganglion (DRG) axons are not repelled by Sema6A due to the attenuation of Sema6A trans-binding to PlxnA4 by a Sema6A-PlxnA4 cis-interaction. Ablation of Sema6A in DRG axons renders them more susceptible to repulsion by Sema6A. In the same study, Sema6B was identified as a modulator of Sema6A trans-interactions with PlxnA4 (Haklai-Topper et al., 2010). Similarly, an attenuation of the repulsive activity of Sema6A by cis-interacting PlxnA2 has been reported in hippocampal mossy fibers (Suto et al., 2007) and in cerebellar granule cell migration (Renaud et al., 2008).
Our results support the existence of cis-interactions between class-6 semaphorins and plexinAs on commissural axons. A cis-interaction between PlxnA2 and Sema6B might prevent PlxnA2 from interacting with repulsive class-3 semaphorins in the vicinity of the floorplate and, thus, regulate the sensitivity of pre-crossing axons to repulsive cues. Premature sensitivity of pre-crossing axons to class-3 semaphorins would prevent growth cone/floorplate contact and, thus, midline crossing. In agreement with this idea, we found that the overexpression of PlxnA2 in commissural neurons prevented their normal ventral growth (Fig. 7), suggesting that the signaling activity of PlxnA2 is precisely regulated in pre-crossing axons to prevent premature repulsion. Furthermore, our data support the idea that Sema6B-PlxnA2 cis-interactions modulate the binding of PlxnA2 in trans (supplementary material Fig. S5). When axons have reached the midline, floorplate-expressed PlxnA2, which is not involved in any cis-interaction with Sema6B, may bind to growth cone-derived Sema6B in trans, thus inducing a growth/turning signal in the axons. Furthermore, axonal PlxnA2 is freed to interact with class-3 semaphorins derived from the midline. Together, these pathways facilitate the post-crossing trajectory (Fig. 8)
At present, it is not known how the turning signals are mediated intracellularly. However, we propose that the axonal PlxnA2 and Sema6B pathways are separated by the Sema6B-PlxnA2 trans-interaction, resulting in distinct Sema6B- and PlxnA2-mediated parallel signaling in post-crossing axons. This scenario would explain why commissural axons acquire responsiveness to class-3 semaphorins only when crossing the floorplate, but not before. Based on its precise temporal and spatial coincidence with axonal midline crossing, the switch from Sema6B-PlxnA2 cis- to trans-interaction constitutes an excellent regulatory mechanism that contributes to and strengthens the previously described mechanisms: the Shh-induced regulation of PKA activity (Parra and Zou, 2010) and the GDNF-mediated and NrCAM-dependent inhibition of calpain, which in turn stabilizes PlxnA1 on the growth cone (Nawabi et al., 2010; Charoy et al., 2012).
MATERIALS AND METHODS
Plasmids and miRNAs
Primers for cloning and mutagenesis are listed in supplementary material Table S1; for cloning details see supplementary material methods. miRNA target sequences are listed in supplementary material Table S2. Full-length Sema6B (GenBank accession number KJ201030) and a truncated form maintaining the transmembrane region and the five adjacent C-terminal amino acids, but lacking the rest of the intracellular domain, were cloned into pMES (Wilson and Stoeckli, 2011) for in vivo studies and into pcDNA3.1 (Invitrogen) for in vitro studies. Site-directed mutagenesis (Zheng et al., 2004; Wilson and Stoeckli, 2011) was used to introduce six silent mutations into the miS6B target site of Sema6b to make it resistant to knockdown (supplementary material Fig. S1). The same strategy was used to synthesize a knockdown-resistant version of Plxna2 (supplementary material Fig. S2). Constructs for the expression of miRNAs against Sema6b and Plxna2 were synthesized as described (Wilson and Stoeckli, 2011). GenScript Target Finder was used to predict miRNA target sequences.
In situ probes, dsRNA and immunohistochemistry
Expressed sequence tags (ChESTs) were used to generate dsRNA and in situ probes (supplementary material Table S3). In situ hybridization and dsRNA synthesis were performed as described (Mauti et al., 2006; Pekarik et al., 2003). Antibodies used for expression analyses on 20 µm thick cryosections, commissural neurons or HEK293 cells are listed in supplementary material Table S4. Commissural explants were dissected from HH25-26 embryos and cultured on LabTek slides (Nunc, Thermoscientific) coated with poly-lysine (10 μg/ml) and Laminin (10 μg/ml) as described previously (Niederkofler et al., 2010).
In ovo and in vitro RNAi
After 3 days of incubation at 39°C, fertilized eggs (Hisex) were windowed for injection and electroporation, as described previously (Wilson and Stoeckli, 2011, 2012). Embryos were staged according to Hamburger and Hamilton (1992). All experiments including animals were carried out in accordance with Swiss law on animal experimentation and approved by the cantonal veterinary office of Zurich. Supplementary material Table S5 lists the concentrations and electroporation conditions used. Electrodes were positioned to target one half or the spinal cord (Fig. 3H), or exclusively either dorsal commissural neurons (Fig. 1P) or the ventral midline area (Fig. 3L) (Bourikas et al., 2005; Niederkofler et al., 2010).
The specificity and efficiency of plexin downregulation by the same dsRNA sequences was shown previously (Mauti et al., 2007). The efficiency of gene silencing by miRNAs (supplementary material Figs S1 and S2) was assessed by in situ hybridization (Wilson and Stoeckli, 2013) and by in vitro screening (Wilson and Stoeckli, 2011). For quantitative analysis of knockdown, the ratio between the signal intensities (grayscale values) of Sema6B or PlxnA2 and the corresponding fluorescent protein from the co-transfected miRNA constructs was calculated using ImageJ (NIH) and subsequently normalized to the miLuc control condition.
Phenotype analysis
Low thoracic and lumbar levels of HH25-26 spinal cords were dissected as open-book preparations to label dI1 commissural neurons with Fast-DiI (5 mg/ml in ethanol; D-7756, Life Technologies) as described previously (Perrin and Stoeckli, 2000). An injection site was considered to exhibit a stalling phenotype when more than 50% of the growth cones stalled at the floorplate exit site. Even single axons turning caudally were considered as a ‘caudal turning phenotype', as caudal turns were only very rarely seen in control embryos. Caudal turning was always seen in combination with axonal stalling at the exit site. Therefore, we did not separate these phenotypes for quantification. No defects in the growth of commissural axons towards the floorplate were observed after Sema6B or PlxnA2 knockdown (supplementary material Fig. S3).
Protein-protein binding experiments
PlxnA1 (aa 31-1040) and PlxnA2 (aa 39-689) ectodomains without the signal peptide were inserted into the pAPtag5 vector (GenHunter) between an Igκ-chain signal sequence and alkaline phosphatase (AP), followed by a myc/6×his tag. For pAPtag5-PlexinA1 we digested with SfiI and HindIII, and for pAPtag5-PlexinA2ecto with BglII and BspEI. To obtain pAPtag5-Sema6Becto-Fc (aa 1-590) and pAPtag5-Sema6Aecto-Fc (aa 1-604) fusion constructs, we digested pAPtag5 with BglII and BspEI to insert a human Fc tag with a 3′ termination codon. Sema6A and Sema6B ectodomains were cloned 5′ of the Fc tag using NheI and BglII. Transfection and production of fusion proteins were performed according to published protocols (Flanagan et al., 2000). To assess binding of soluble Plexinecto-AP fusion proteins, HEK293T cells were transfected with pcDNA3.1-Sema6A-myc/his, pCAGGs-Sema6B-ha or pCAGGs-Sema6D-ha for expression of full-length class-6 semaphorins. Live cells were incubated for 1 hour with Plexinecto-AP fusion proteins at 4°C before fixation and staining (Flanagan et al., 2000).
For co-IPs, the soluble fusion proteins described above were loaded on Handee spin columns (Thermoscientific) and incubated for 2 hours at 4°C with 10 µl anti-c-Myc agarose, according to the manufacturer's instructions (ProFound IP-Kit; Thermoscientific, 23620). Immunoprecipitates were analyzed on western blots using the antibodies listed in supplementary material Table S4.
In vitro axon growth assay
Chick embryos at HH17-18 were electroporated with constructs encoding EBFP2 and miLuc or miS6B, as described above. At HH25, dissociated commissural neurons from the electroporated side were collected (pooled from three embryos in each condition) and grown on coverslips pre-coated with poly-lysine, which were incubated for 45 min at 37°C with Albumax (50 μg/ml), Laminin (10 μg/ml), AP only or PlexinA2ecto-AP (both 50× concentrated conditioned medium). Cultures were grown for 48 h before fixation and immunolabeling (Niederkofler et al., 2010) (supplementary material Table S4).
For quantification, at least 20 images per condition were taken from random positions on each coverslip. Each image typically contained both wild-type and miRNA-expressing neurons, which were distinguishable by the expression of EBFP2. At least 34 axons were measured per condition (ImageJ) using a Wacom DTU-1931 tablet and pen tool.
Statistics
For open-books, multiple comparisons by one-way ANOVA followed by Tukey (homoscedasticity) or Tamhane's T2 (heteroscedasticity) post-hoc tests were used to calculate P-values, using Statistics-20 Software (SPSS). For the in vitro assays we performed two-sample t-tests for independent samples using the VassarStats website (http://vassarstats.net/). P<0.05 was regarded as significant.
Acknowledgements
We thank Tiziana Flego and Dr Beat Kunz for excellent technical support.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
I.A., N.H.W. and T.B. performed the experiments; I.A., N.H.W., O.M., M.G. and E.T.S. developed the concepts and approach; S.S. provided unpublished constructs; I.A., N.H.W. and E.T.S. performed the data analysis and prepared the manuscript.
Funding
This work was supported by a grant from the Swiss National Science Foundation to E.T.S. and by fellowships of the Roche Research Foundation and the Neuroscience Center Zurich (ZNZ) to I.A. Work in the lab of S.S. was supported by the National Institutes of Health [NS046336].
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.112185/-/DC1
References
- Augsburger A., Schuchardt A., Hoskins S., Dodd J. and Butler S. (1999). BMPs as mediators of roof plate repulsion of commissural neurons. Neuron 24, 127-141. 10.1016/S0896-6273(00)80827-2 [DOI] [PubMed] [Google Scholar]
- Bourikas D., Pekarik V., Baeriswyl T., Grunditz A., Sadhu R., Nardó M. and Stoeckli E. T. (2005). Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat. Neurosci. 8, 297-304. 10.1038/nn1396 [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen T., Tzarfaty V., Frumkin A., Feinstein Y., Stoeckli E. and Klar A. (1999). F-Spondin is required for accurate pathfinding of commissural axons at the floor plate. Neuron 23, 233-246. 10.1016/S0896-6273(00)80776-X [DOI] [PubMed] [Google Scholar]
- Cafferty P., Yu L., Long H. and Rao Y. (2006). Semaphorin-1a functions as a guidance receptor in the Drosophila visual system. J. Neurosci. 26, 3999-4003. 10.1523/JNEUROSCI.3845-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoy C., Nawabi H., Reynaud F., Derrington E., Bozon M., Wright K., Falk J., Helmbacher F., Kindbeiter K. and Castellani V. (2012). Gdnf activates midline repulsion by Semaphorin3B via NCAM during commissural axon guidance. Neuron 75, 1051-1066. 10.1016/j.neuron.2012.08.021 [DOI] [PubMed] [Google Scholar]
- Charron F., Stein E., Jeong J., McMahon A. P. and Tessier-Lavigne M. (2003). The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell 113, 11-23. 10.1016/S0092-8674(03)00199-5 [DOI] [PubMed] [Google Scholar]
- Chédotal A. (2011). Further tales of the midline. Curr. Opin. Neurobiol. 21, 68-75. 10.1016/j.conb.2010.07.008 [DOI] [PubMed] [Google Scholar]
- Chen Z., Gore B. B., Long H., Ma L. and Tessier-Lavigne M. (2008). Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron 58, 325-332. 10.1016/j.neuron.2008.02.016 [DOI] [PubMed] [Google Scholar]
- Domanitskaya E., Wacker A., Mauti O., Baeriswyl T., Esteve P., Bovolenta P. and Stoeckli E. T. (2010). Sonic hedgehog guides post-crossing commissural axons both directly and indirectly by regulating Wnt activity. J. Neurosci. 30, 11167-11176. 10.1523/JNEUROSCI.1488-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt F., Behar O., Calautti E., Yonezawa K., Nishimoto I. and Fishman M. C. (1997). A novel transmembrane semaphorin can bind c-src. Mol. Cell. Neurosci. 9, 409-419. 10.1006/mcne.1997.0644 [DOI] [PubMed] [Google Scholar]
- Flanagan J. G., Cheng H.-J., Feldheim D. A., Hattori M., Lu Q. and Vanderhaeghen P. (2000). Alkaline phosphatase fusions of ligands or receptors as in situ probes for staining of cells, tissues, and embryos. Methods Enzymol. 327, 19-35. 10.1016/S0076-6879(00)27264-9 [DOI] [PubMed] [Google Scholar]
- Godenschwege T. A. and Murphey R. K. (2009). Genetic interaction of Neuroglian and Semaphorin1a during guidance and synapse formation. J. Neurogenet. 23, 147-155. 10.1080/01677060802441380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godenschwege T. A., Hu H., Shan-Crofts X., Goodman C. S. and Murphey R. K. (2002). Bi-directional signaling by Semaphorin 1a during central synapse formation in Drosophila. Nat. Neurosci. 5, 1294-1301. 10.1038/nn976 [DOI] [PubMed] [Google Scholar]
- Haklai-Topper L., Mlechkovich G., Savariego D., Gokhman I. and Yaron A. (2010). Cis interaction between Semaphorin6A and Plexin-A4 modulates the repulsive response to Sema6A. EMBO J. 29, 2635-2645. 10.1038/emboj.2010.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V. and Hamilton H. L. (1992). A series of normal stages in the development of the chick embryo. Dev. Dyn. 195, 231-272. 10.1002/aja.1001950404 [DOI] [PubMed] [Google Scholar]
- Helms A. W. and Johnson J. E. (2003). Specification of dorsal spinal cord interneurons. Curr. Opin. Neurobiol. 13, 42-49. 10.1016/S0959-4388(03)00010-2 [DOI] [PubMed] [Google Scholar]
- Islam S. M., Shinmyo Y., Okafuji T., Su Y., Naser I. B., Ahmed G., Zhang S., Chen S., Ohta K., Kiyonari H. et al. (2009). Draxin, a repulsive guidance protein for spinal cord and forebrain commissures. Science 323, 388-393. 10.1126/science.1165187 [DOI] [PubMed] [Google Scholar]
- Janssen B. J. C., Robinson R. A., Pérez-Brangulí F., Bell C. H., Mitchell K. J., Siebold C. and Jones E. Y. (2010). Structural basis of semaphorin-plexin signalling. Nature 467, 1118-1122. 10.1038/nature09468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S., Juhaszova K. and Kolodkin A. L. (2012). The Control of semaphorin-1a-mediated reverse signaling by opposing pebble and RhoGAPp190 functions in drosophila. Neuron 76, 721-734. 10.1016/j.neuron.2012.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joset P., Wacker A., Babey R., Ingold E. A., Andermatt I., Stoeckli E. T. and Gesemann M. (2011). Rostral growth of commissural axons requires the cell adhesion molecule MDGA2. Neural Dev. 6, 22 10.1186/1749-8104-6-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy T. E., Serafini T., de la Torre J. R. and Tessier-Lavigne M. (1994). Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78, 425-435. 10.1016/0092-8674(94)90421-9 [DOI] [PubMed] [Google Scholar]
- Kerjan G., Dolan J., Haumaitre C., Schneider-Maunoury S., Fujisawa H., Mitchell K. J. and Chédotal A. (2005). The transmembrane semaphorin Sema6A controls cerebellar granule cell migration. Nat. Neurosci. 8, 1516-1524. 10.1038/nn1555 [DOI] [PubMed] [Google Scholar]
- Klostermann A., Lutz B., Gertler F. and Behl C. (2000). The orthologous human and murine semaphorin 6A-1 proteins (SEMA6A-1/Sema6A-1) bind to the enabled/vasodilator-stimulated phosphoprotein-like protein (EVL) via a novel carboxyl-terminal zyxin-like domain. J. Biol. Chem. 275, 39647-39653. 10.1074/jbc.M006316200 [DOI] [PubMed] [Google Scholar]
- Kolodkin A. L. and Tessier-Lavigne M. (2011). Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb. Perspect. Biol. 3, a001727 10.1101/cshperspect.a001727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T., Sweeney L. B., Schuldiner O., Garcia K. C. and Luo L. (2007). Graded expression of semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell 128, 399-410. 10.1016/j.cell.2006.12.028 [DOI] [PubMed] [Google Scholar]
- Kuwajima T., Yoshida Y., Takegahara N., Petros T. J., Kumanogoh A., Jessell T. M., Sakurai T. and Mason C. (2012). Optic chiasm presentation of Semaphorin6D in the context of Plexin-A1 and Nr-CAM promotes retinal axon midline crossing. Neuron 74, 676-690. 10.1016/j.neuron.2012.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton P. A., Mitchell K. J., Goodrich L. V., Lu X., Pinson K., Scherz P., Skarnes W. C. and Tessier-Lavigne M. (2001). Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature 410, 174-179. 10.1038/35065539 [DOI] [PubMed] [Google Scholar]
- Long H., Sabatier C., Ma L., Plump A., Yuan W., Ornitz D. M., Tamada A., Murakami F., Goodman C. S. and Tessier-Lavigne M. (2004). Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron 42, 213-223. 10.1016/S0896-6273(04)00179-5 [DOI] [PubMed] [Google Scholar]
- Lyuksyutova A. I., Lu C.-C., Milanesio N., King L. A., Guo N., Wang Y., Nathans J., Tessier-Lavigne M. and Zou Y. (2003). Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science 302, 1984-1988. 10.1126/science.1089610 [DOI] [PubMed] [Google Scholar]
- Mambetisaeva E. T., Andrews W., Camurri L., Annan A. and Sundaresan V. (2005). Robo family of proteins exhibit differential expression in mouse spinal cord and Robo-Slit interaction is required for midline crossing in vertebrate spinal cord. Dev. Dyn. 233, 41-51. 10.1002/dvdy.20324 [DOI] [PubMed] [Google Scholar]
- Mauti O., Sadhu R., Gemayel J., Gesemann M. and Stoeckli E. T. (2006). Expression patterns of plexins and neuropilins are consistent with cooperative and separate functions during neural development. BMC Dev. Biol. 6, 32 10.1186/1471-213X-6-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauti O., Domanitskaya E., Andermatt I., Sadhu R. and Stoeckli E. T. (2007). Semaphorin6A acts as a gate keeper between the central and the peripheral nervous system. Neural Dev. 2, 28 10.1186/1749-8104-2-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawabi H. and Castellani V. (2011). Axonal commissures in the central nervous system: how to cross the midline? Cell. Mol. Life Sci. 68, 2539-2553. 10.1007/s00018-011-0691-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawabi H., Briancon-Marjollet A., Clark C., Sanyas I., Takamatsu H., Okuno T., Kumanogoh A., Bozon M., Takeshima K., Yoshida Y. et al. (2010). A midline switch of receptor processing regulates commissural axon guidance in vertebrates. Genes Dev. 24, 396-410. 10.1101/gad.542510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkofler V., Baeriswyl T., Ott R. and Stoeckli E. T. (2010). Nectin-like molecules/SynCAMs are required for post-crossing commissural axon guidance. Development 137, 427-435. 10.1242/dev.042515 [DOI] [PubMed] [Google Scholar]
- Nogi T., Yasui N., Mihara E., Matsunaga Y., Noda M., Yamashita N., Toyofuku T., Uchiyama S., Goshima Y., Kumanogoh A. et al. (2010). Structural basis for semaphorin signalling through the plexin receptor. Nature 467, 1123-1127. 10.1038/nature09473 [DOI] [PubMed] [Google Scholar]
- Parra L. M. and Zou Y. (2010). Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nat. Neurosci. 13, 29-35. 10.1038/nn.2457 [DOI] [PubMed] [Google Scholar]
- Pasterkamp R. J. (2012). Getting neural circuits into shape with semaphorins. Nat. Rev. Neurosci. 13, 605-618. 10.1038/nrn3302 [DOI] [PubMed] [Google Scholar]
- Pasterkamp R. J. and Kolodkin A. L. (2003). Semaphorin junction: making tracks toward neural connectivity. Curr. Opin. Neurobiol. 13, 79-89. 10.1016/S0959-4388(03)00003-5 [DOI] [PubMed] [Google Scholar]
- Pekarik V., Bourikas D., Miglino N., Joset P., Preiswerk S. and Stoeckli E. T. (2003). Screening for gene function in chicken embryo using RNAi and electroporation. Nat. Biotechnol. 21, 93-96. 10.1038/nbt770 [DOI] [PubMed] [Google Scholar]
- Perrin F. E. and Stoeckli E. T. (2000). Use of lipophilic dyes in studies of axonal pathfinding in vivo. Microsc. Res. Tech. 48, 25-31. [DOI] [PubMed] [Google Scholar]
- Philipp M., Niederkofler V., Debrunner M., Alther T., Kunz B. and Stoeckli E. T. (2012). RabGDI controls axonal midline crossing by regulating Robo1 surface expression. Neural Dev. 7, 36 10.1186/1749-8104-7-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J., Kerjan G., Sumita I., Zagar Y., Georget V., Kim D., Fouquet C., Suda K., Sanbo M., Suto F. et al. (2008). Plexin-A2 and its ligand, Sema6A, control nucleus-centrosome coupling in migrating granule cells. Nat. Neurosci. 11, 440-449. 10.1038/nn2064 [DOI] [PubMed] [Google Scholar]
- Rünker A. E., Little G. E., Suto F., Fujisawa H. and Mitchell K. J. (2008). Semaphorin-6A controls guidance of corticospinal tract axons at multiple choice points. Neural Dev. 3, 34 10.1186/1749-8104-3-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier C., Plump A. S., Ma L., Brose K., Tamada A., Murakami F., Lee E. Y.-H. P. and Tessier-Lavigne M. (2004). The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell 117, 157-169. 10.1016/S0092-8674(04)00303-4 [DOI] [PubMed] [Google Scholar]
- Stoeckli E. T. and Landmesser L. T. (1995). Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron 14, 1165-1179. 10.1016/0896-6273(95)90264-3 [DOI] [PubMed] [Google Scholar]
- Stoeckli E. T., Sonderegger P., Pollerberg G. E. and Landmesser L. T. (1997). Interference with axonin-1 and NrCAM interactions unmasks a floor-plate activity inhibitory for commissural axons. Neuron 18, 209-221. 10.1016/S0896-6273(00)80262-7 [DOI] [PubMed] [Google Scholar]
- Suto F., Ito K., Uemura M., Shimizu M., Shinkawa Y., Sanbo M., Shinoda T., Tsuboi M., Takashima S., Yagi T. et al. (2005). Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J. Neurosci. 25, 3628-3637. 10.1523/JNEUROSCI.4480-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto F., Tsuboi M., Kamiya H., Mizuno H., Kiyama Y., Komai S., Shimizu M., Sanbo M., Yagi T., Hiromi Y. et al. (2007). Interactions between plexin-A2, plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron 53, 535-547. 10.1016/j.neuron.2007.01.028 [DOI] [PubMed] [Google Scholar]
- Tahirovic S. and Bradke F. (2009). Neuronal polarity. Cold Spring Harb. Perspect. Biol. 1, a001644 10.1101/cshperspect.a001644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawarayama H., Yoshida Y., Suto F., Mitchell K. J. and Fujisawa H. (2010). Roles of semaphorin-6B and plexin-A2 in lamina-restricted projection of hippocampal mossy fibers. J. Neurosci. 30, 7049-7060. 10.1523/JNEUROSCI.0073-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T., Zhang H., Kumanogoh A., Takegahara N., Yabuki M., Harada K., Hori M. and Kikutani H. (2004a). Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat. Cell Biol. 6, 1204-1211. 10.1038/ncb1193 [DOI] [PubMed] [Google Scholar]
- Toyofuku T., Zhang H., Kumanogoh A., Takegahara N., Suto F., Kamei J., Aoki K., Yabuki M., Hori M., Fujisawa H. et al. (2004b). Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 18, 435-447. 10.1101/gad.1167304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T., Yoshida J., Sugimoto T., Yamamoto M., Makino N., Takamatsu H., Takegahara N., Suto F., Hori M., Fujisawa H. et al. (2008). Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev. Biol. 321, 251-262. 10.1016/j.ydbio.2008.06.028 [DOI] [PubMed] [Google Scholar]
- Wilson N. H. and Stoeckli E. T. (2011). Cell type specific, traceable gene silencing for functional gene analysis during vertebrate neural development. Nucleic Acids Res. 39, e133 10.1093/nar/gkr628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N. H. and Stoeckli E. T. (2012). In ovo electroporation of miRNA-based plasmids in the developing neural tube and assessment of phenotypes by DiI injection in open-book preparations. J. Vis. Exp. e4384 10.3791/4384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N. H. and Stoeckli E. T. (2013). Sonic hedgehog regulates its own receptor on postcrossing commissural axons in a glypican1-dependent manner. Neuron 79, 478-491. 10.1016/j.neuron.2013.05.025 [DOI] [PubMed] [Google Scholar]
- Xu X. M., Fisher D. A., Zhou L., White F. A., Ng S., Snider W. D. and Luo Y. (2000). The transmembrane protein semaphorin 6A repels embryonic sympathetic axons. J. Neurosci. 20, 2638-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Zhou Y., Cheng S. and Rao Y. (2010). Plexin a-semaphorin-1a reverse signaling regulates photoreceptor axon guidance in Drosophila. J. Neurosci. 30, 12151-12156. 10.1523/JNEUROSCI.1494-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Baumann U. and Reymond J.-L. (2004). An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 32, e115 10.1093/nar/gnh110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang B., Su Y. S. and Sockanathan S. (2009). FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane Semaphorin6A and PlexinA4 signaling. Neuron 61, 359-372. 10.1016/j.neuron.2008.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Stoeckli E., Chen H. and Tessier-Lavigne M. (2000). Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell 102, 363-375. 10.1016/S0092-8674(00)00041-6 [DOI] [PubMed] [Google Scholar]