Abstract
Testicular teratomas result from anomalies in embryonic germ cell development. In 129 inbred mice, teratoma initiation coincides with germ cell sex-specific differentiation and the mitotic-meiotic switch: XX and XY germ cells repress pluripotency, XX germ cells initiate meiosis, and XY germ cells activate male-specific differentiation and mitotic arrest. Here, we report that expression of Nanos2, a gene that is crucial to male sex specification, is delayed in teratoma-susceptible germ cells. Decreased expression of Nanos2 was found to be due, in part, to the Nanos2 allele present in 129 mice. In teratoma-susceptible germ cells, diminished expression of genes downstream of Nanos2 disrupted processes that were crucial to male germ cell differentiation. Deficiency for Nanos2 increased teratoma incidence in 129 mice and induced developmental abnormalities associated with tumor initiation in teratoma-resistant germ cells. Finally, in the absence of commitment to the male germ cell fate, we discovered that a subpopulation of teratoma-susceptible germ cells transition into embryonal carcinoma (EC) cells with primed pluripotent features. We conclude that delayed male germ cell sex-specification facilitates the transformation of germ cells with naïve pluripotent features into primed pluripotent EC cells.
KEY WORDS: Teratomas, Germ cells, Nanos2, Pluripotency, Development
Highlighted Article: Delayed male germ cell sex specification facilitates the transformation of germ cells with naïve pluripotent properties into primed pluripotent-like embryonal carcinoma cells, the tumor stem cells of testicular germ cell tumors.
INTRODUCTION
In both mice and humans, defects during embryonic male germ cell development can lead to the formation of testicular germ cell tumors (TGCTs) (Stevens, 1967b; Looijenga et al., 2003; Almstrup et al., 2004; Oosterhuis and Looijenga, 2005). The 129 family of inbred mice, which has a TGCT incidence of 1-10% depending on the substrain, is an important experimental model system for studying the embryonic origins and complex genetic susceptibility of human TGCTs (Heaney and Nadeau, 2008). TGCTs in mice are first evident at embryonic day (E)15.5 as foci of highly proliferative and pluripotent tumor stem cells (embryonal carcinoma cells or EC cells), which differentiate into various cell lineages derived from all three embryonic germ layers and form teratomas in adults (Stevens and Hummel, 1957; Stevens, 1962). Importantly, the developmental origins and genetic risk factors of TGCTs in mice have proven to be highly similar to human type I pediatric testicular teratomas and adolescent/adult type II testicular non-seminomas (Litchfield et al., 2016; Lanza and Heaney, 2017).
In mice, germ cells are first specified during embryogenesis as primordial germ cells (PGCs) that arise from the proximal epiblast around E6.0 (Lawson et al., 1999; McLaren, 2000; Surani, 2001). Upon specification, PGCs undergo an almost complete global demethylation and maintain an expression profile with similarities to pluripotent cells in culture (i.e. murine embryonic stem cells), including expression of core pluripotency factors [Nanog, Pou5f1 (Oct4) and Sox2] (Matsui et al., 1991; Resnick et al., 1992; Yeom et al., 1996; McLaren, 1999; Pesce and Schöler, 2000; Seki et al., 2005; Yamaguchi et al., 2005). After PGCs populate the gonadal ridge at about E11.5, expression of the naïve pluripotency and germ cell lineage marker DAZL is induced in an anterior-to-posterior wave (Tesar et al., 2007; Gill et al., 2011; Xu et al., 2011; Factor et al., 2014; Hu et al., 2015; Welling et al., 2015). Subsequently, germ cells become competent to interpret meiotic and sex differentiation signals from supporting somatic cells. In response to these signals, germ cells downregulate expression of the core pluripotency factors and undergo sex-specific differentiation from approximately E13.5 to E15.5 (Spiller and Bowles, 2015). Concomitantly, XX germ cells (oogonia) enter meiosis and XY germ cells (gonocytes) enter mitotic arrest (the mitotic-meiotic switch) (McLaren, 1984).
Paracrine and autocrine signals, including the TGFβ superfamily ligands activin and NODAL, act to establish male germ cell identity, initiate mitotic arrest and protect against meiotic induction in XY germ cells through activation of the male-specific transcriptional program (i.e. Nanos2) (Spiller and Bowles, 2015). NANOS2, an RNA-binding protein expressed specifically in XY germ cells starting at E12.5-E13.5, is indispensable for male germ cell development (Suzuki et al., 2007). NANOS2 is crucial for suppressing meiosis in XY germ cells by binding and sequestering meiosis-associated transcripts (Suzuki and Saga, 2008; Suzuki et al., 2010, 2016). Independent of its role in meiotic inhibition, NANOS2 maintains mitotic arrest, indirectly inhibits expression of pluripotency factors and activates male-specific differentiation, including de novo re-methylation of the genome and silencing of retrotransposons (Saba et al., 2014). Thus, NANOS2 plays essential roles in transitioning XY germ cells from a naïve pluripotent-like state towards a lineage-committed, unipotent state.
In mice, testicular teratoma initiation coincides with the critical time period in germ cell development during which male sex-specification and mitotic arrest occur (Stevens, 1966, 1967a; Noguchi and Stevens, 1982; Matin et al., 1998). We have previously shown that a subpopulation of teratoma-susceptible germ cells delay entry into mitotic arrest, continue to express core pluripotency factors, and misexpress genes normally only expressed in pre-meiotic XX germ cells (Heaney et al., 2012). From E13.5 to E15.5, aberrant proliferation, retention of pluripotency and expression of pre-meiotic genes become restricted to a continually smaller sub-population of germ cells, ultimately being maintained in the few cells predisposed to transformation into EC cells (Heaney et al., 2012). Given the coincidental timing of sex-specific differentiation and tumor initiation, we hypothesized that a delay or block in the male sex-specification program disrupts the lineage restriction of XY germ cells, retaining features of pluripotency and leaving them susceptible to transformation into EC cells.
In the present study, we examine the contribution of male germ cell sex-specification to teratoma susceptibility and the pluripotent state of germ cells and EC cells during tumor initiation. We demonstrate that expression of germ cell intrinsic factors that are crucial to specification of the male lineage, including Nanos2 and several of its downstream effectors, is delayed in teratoma-susceptible XY germ cells. This delay results in developmental phenotypes indicative of disrupted male germ cell differentiation and increased teratoma risk. Crucially, Nanos2 deficiency significantly increased teratoma incidence on the 129 background. Finally, we investigated the transformation of XY germ cells, delayed in male germ cell sex specification, into pluripotent EC cells. We provide evidence that a subpopulation of teratoma-susceptible germ cells acquires features of primed pluripotency and downregulates features of naïve pluripotency during the transformation process. Based on these findings, we propose that a delay in male germ cell sex-specification in teratoma-susceptible mice facilitates transformation of XY germ cells with naïve pluripotent properties into primed pluripotent EC cells.
RESULTS
Male germ cell sex-specification is delayed in teratoma-susceptible mice
To test whether developmental abnormalities associated with teratoma susceptibility are caused by defects in male germ cell sex specification, we examined expression of male sex-specification genes in germ cells of the teratoma-resistant FVB/NJ (FVB) mouse strain and two teratoma-susceptible strains, 129/SvImJ (129) and 129-Chr19MOLF/Ei (M19). 129 inbred mice have a low risk of developing teratomas (1-10%), whereas M19 mice, in which both copies of chromosome 19 are derived from the MOLF/Ei strain, have a high risk of developing teratomas (∼80% of males affected) (Matin et al., 1999). Using these strains, we can investigate germ cell abnormalities associated with increasing teratoma risk and further define the pool of germ cells capable of transformation into EC cells. Additionally, because most 129 and M19 germ cells develop normally, the fate of teratoma-susceptible germ cells that do not transform can also be studied.
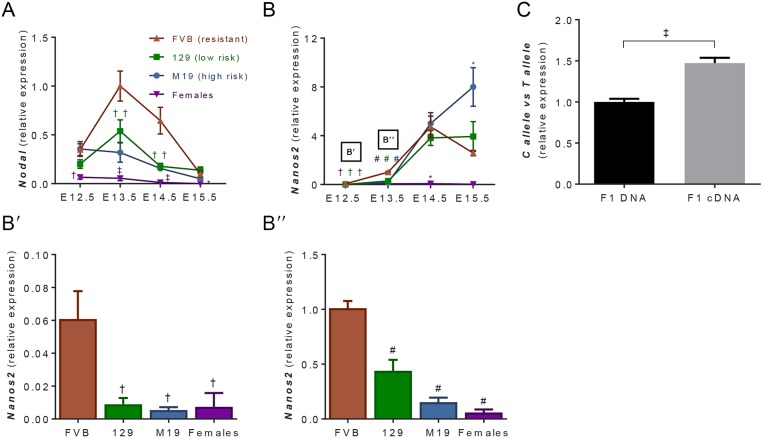
First, we assessed perturbations in male germ cell sex-specification by measuring expression of members of the NODAL signaling pathway in germ cells isolated by fluorescence-activated cell sorting (FACS) from FVB, 129 and M19 embryos harboring a germ cell-specific GFP transgene driven by the Oct4 promoter with the proximal enhancer deleted (Oct4ΔPE::GFP) (Yoshimizu et al., 1999). NODAL signaling, which is restricted to XY germ cells after E12.5, transiently maintains pluripotency and protects against meiotic entry in the XY germ cell population (Souquet et al., 2012; Spiller et al., 2012). Similar to previous reports, we found that, in the teratoma-resistant FVB strain, expression of the autocrine factor Nodal was specific to XY germ cells, peaked at E13.5 and then decreased at E15.5 (Spiller et al., 2012; Miles et al., 2013) (Fig. 1A). In contrast, germ cell expression of Nodal was significantly decreased in 129 and M19 compared with FVB at E13.5 and E14.5. Intriguingly, male germ cell expression of the NODAL co-receptor Cripto (Tdgf1) was unchanged between FVB and 129 germ cells, but was significantly increased in M19 germ cells at E12.5 and E13.5 (Fig. S1A). Despite the increased expression of the Cripto co-receptor in M19 germ cells, downstream targets of NODAL signaling, Lefty1 and Lefty2, had decreased expression in both 129 and M19 germ cells at E13.5 and E14.5 (Fig. S1B,C). Therefore, we conclude that NODAL signaling is disrupted in teratoma-susceptible germ cells.
Fig. 1.
Expression of male germ cell sex-specification genes is delayed in teratoma-susceptible germ cells. (A-B″) Oct4ΔPE::GFP transgenic FVB, 129 and M19 germ cells from E12.5-E15.5 gonads were FACS enriched and analyzed by qPCR (n=3-11) for (A) Nodal and (B-B″) Nanos2 expression. Female germ cell expression data from all strains were pooled. Gene expression at all embryonic time-points was plotted relative to expression in E13.5 FVB germ cells. (C) Oct4ΔPE::GFP-positive germ cells from E13.5 F1[FVB×129] embryos were FACS enriched and analyzed by qPCR (n=5-7) for differential allelic expression of Nanos2 using SYBR primers specific to the FVB ‘C’ allele and 129 ‘T’ allele. Gene expression was plotted relative to E13.5 F1 DNA. Data are mean±s.e.m. *P<0.05, †P<0.01, ‡P<0.001 and #P<0.0001.
Next, we measured expression of the essential male sex determination gene Nanos2 in germ cells from FVB, 129 and M19 strains. The expression pattern of Nanos2 in FVB germ cells was consistent with previous reports that Nanos2 was specific to XY germ cells and peaked at E14.5 (Suzuki and Saga, 2008). However, Nanos2 expression was significantly decreased in 129 and M19 compared to FVB at E12.5 and E13.5 (Fig. 1B). At E14.5, Nanos2 expression in 129 and M19 germ cells increased to levels observed in FVB germ cells. Interestingly, at E15.5 Nanos2 expression in M19 germ cells was significantly increased compared to FVB germ cells, suggesting that there is a compensatory program responsible for establishing the male germ cell fate in teratoma-susceptible germ cells that do not transform into EC cells. Together, these data indicate that teratoma-susceptible germ cells delay expression of genes that are crucial to male sex-specification.
Expression of somatic cell-derived signaling factors appears unchanged between teratoma-resistant and susceptible strains
To determine whether delayed male germ cell sex specification is due to defective male somatic cells signaling, we measured somatic cell expression of signals that are crucial to male germ cell sex specification in FVB, 129 and M19 Oct4ΔPE::GFP-negative somatic cells that were enriched from embryonic urogenital ridges using FACS. These male somatic signals include Fgf9, activin A (Inhba), activin B (Inhbb) and the enzymes that synthesize PGD2 (Hpgds and Ptgds) (Fig. S1D-H). Importantly, we observed no gene expression differences in any of these key male somatic cell genes between teratoma-resistant and susceptible strains from E12.5 to E15.5. Additionally, we observed no differences in male somatic cell expression of Wnt4 (Fig. S1I), a somatic signal that is restricted to the ovary during sex determination and antagonizes male differentiation.
Finally, we tested for an imbalance in the metabolism of retinoic acid (RA), a somatic signal that initiates meiosis in the embryonic ovary and is catabolized by CYP26B1 in the embryonic testis (Bowles and Koopman, 2007). We measured expression of the RA-catabolizing enzyme gene Cyp26b1 as well as genes encoding the RA synthesizing enzymes Aldh1a1 and Aldh1a2 in Oct4ΔPE::GFP-negative somatic cells. We observed no differences in expression of these enzymes between teratoma-resistant and susceptible strains from E12.5 to E15.5 (Fig. S1J-L). Thus, RA metabolism appeared to be unaltered in teratoma-susceptible testes. Together, these data suggest that alterations in male somatic cell signals are not responsible for downstream changes in male germ cell sex specification in teratoma-susceptible germ cells. However, unbiased whole-transcriptome experiments on sorted somatic cells from isolated gonads may reveal differences not detected via our candidate approach.
129. Nanos2 allele variant leads to decreased Nanos2 expression in germ cells
Next, we investigated whether the delay in male germ cell sex specification is due to 129 genetic variants in either Nodal or Nanos2. Analysis of annotated single nucleotide polymorphisms (SNPs) at the Nodal and Nanos2 loci in 129S1/SvImJ and several other teratoma-resistant inbred strains of mice failed to identify coding or potential regulatory SNP variants specific to the 129 strain (Tables S1 and S2). However, analyses of annotated SNPs within the Nanos2 locus combined with Sanger sequencing did reveal two SNP variants (rs32114982 and rs46240014) in the Nanos2 3′ untranslated region (UTR) and one synonymous SNP variant (rs31939358a) in the coding sequence that define a Nanos2 allele variant shared by 129 and C3H/HeJ (C3H) mice. Crucially, the Nanos2 3′UTR has been implicated in both its expression and function (Tsuda et al., 2006), suggesting that variants in this region could have a functional effect on the expression of Nanos2.
To test whether the Nanos2 allele variant shared by 129 and C3H mice influences Nanos2 expression, we FACS-enriched germ cells from E13.5 F1 hybrid embryos generated by reciprocal crosses between FVB and 129 as well as FVB and C3H inbred mice. The F1 hybrid provides an ideal experimental system for testing allelic contribution in a shared testis microenvironment. We assessed the expression of the FVB-derived allele versus the 129- or C3H-derived allele by exploiting the variants at rs32114982 and primers specific to the FVB ‘C’ allele versus 129 or C3H ‘T’ allele (Fig. S2B). We performed quantitative PCR (qPCR) using allele-specific primers on F1 hybrid cDNA and DNA as a control, which has equivalent contribution from each allele. This analysis revealed that there was 1.5-fold higher expression of the FVB ‘C’ allele versus the 129/C3H ‘T’ allele in F1[FVB×129] hybrids (Fig. 1C). Preferential expression of the ‘C’ allele was replicated in F1[FVB×C3H] embryos and was independent of the parent of origin (Fig. S3C,D). Together, this evidence suggests that the 129 Nanos2 allele variant contributes to the decreased expression of Nanos2 in teratoma-susceptible germ cells.
Features of male germ cell sex-specific differentiation are delayed in teratoma-susceptible mice
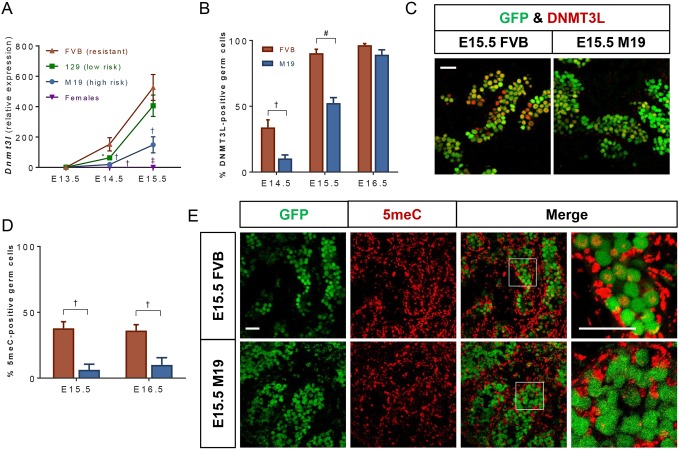
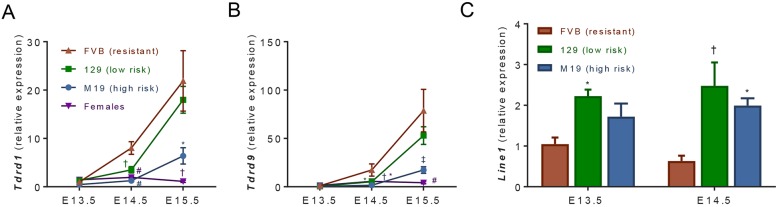
Nanos2 expression in germ cells is crucial for commitment to the male germ cell fate by directly and indirectly inducing expression of male-specific genes that promote de novo methylation of the genome (e.g. Dnmt3l) and transposon silencing (e.g. Tdrd1 and Tdrd9) (Suzuki and Saga, 2008; Saba et al., 2014). To determine whether delayed Nanos2 expression leads to diminished expression of these downstream targets, we measured gene expression in germ cells from E13.5-E15.5 FVB, 129 and M19 testes. As previously reported, expression of Dnmt3l (Fig. 2A), as well as Tdrd1 and Tdrd9 (Fig. 3A,B), was specific to XY germ cells and increased from E13.5 until E15.5 in all strains (Chuma et al., 2003; Bourc'his and Bestor, 2004; Shoji et al., 2009). However, at E14.5, expression of these genes was significantly decreased in both 129 and M19 germ cells compared with FVB germ cells. At E15.5, expression increased in the low teratoma risk 129 germ cells to levels similar to those in FVB germ cells, but remained significantly decreased in high teratoma risk M19 germ cells. Results of the Dnmt3l qPCR analyses were further confirmed by immunostaining in FVB and M19 testes (Fig. 2B,C, Fig. S3). From E14.5 to E16.5, the percentage of DNMT3L-positive germ cells increased in both strains. However, consistent with RNA expression levels, the incidence of DNMT3L staining was significantly lower in teratoma susceptible testes at E14.5 and E15.5. By E16.5, most M19 germ cells expressed DNMT3L, suggesting that teratoma-susceptible germ cells that have not transformed by this time-point commit appropriately to the male germ cell fate and develop normally.
Fig. 2.
DNMT3L expression and de novo re-methylation of the germ cell genome is delayed in teratoma-susceptible strains. (A) Oct4ΔPE::GFP transgenic FVB, 129 and M19 germ cells from E13.5-E15.5 were FACS-enriched and analyzed by qPCR (n=4-8) for Dnmt3l expression. Female germ cell expression data from all strains were pooled. Gene expression at all embryonic time-points was plotted relative to expression in E13.5 FVB germ cells. Data are mean±s.e.m. *P<0.05, †P<0.01 and ‡P<0.001. (B-E) Oct4ΔPE::GFP transgenic FVB and M19 embryonic testes were sectioned and immunostained for (C) DNMT3L or (E) 5-methyl cytosine (5-meC). Oct4ΔPE::GFP-positive germ cells, which are positive and negative for DNMT3L or 5-meC, were counted. Data are plotted as the average percentage of germ cells positive for (B) DNMT3L or (D) 5-meC ±s.e.m. (n=3-7). †P<0.01 and #P<0.0001. (C,E) Confocal microscopy images of representative E15.5 Oct4ΔPE::GFP transgenic FVB and M19 gonads immunostained for (C) DNMT3L or (E) 5-meC. Boxed areas are shown at higher magnification on the right. Scale bars: 50 μm.
Fig. 3.
Expression of genes involved in retrotransposon silencing and the Line-1 class of retrotransposons is diminished in teratoma-susceptible germ cells. (A-C) Oct4ΔPE::GFP-positive germ cells were FACS enriched from E13.5-E15.5 FVB, 129 and M19 gonads, and expression of (A) Tdrd1, (B) Tdrd9 and (C) Line-1 was analyzed by qPCR (n=4-8). Female germ cell expression data from all strains were pooled. Gene expression at all time-points was plotted relative to expression in E13.5 FVB male germ cells. Data are mean±s.e.m. *P<0.05; †P<0.01; ‡P<0.001 and #P<0.0001.
Genome re-methylation and transposon silencing are promoted by the downstream targets of Nanos2. Upon entering the gonad, both XX and XY germ cells undergo almost complete genome demethylation. After E13.5, XY germ cells undergo progressive de novo genome re-methylation due to the methyltransferase activity of DNMT3A/B paired with the male-specific modulatory protein DNMT3L. To determine whether decreased DNMT3L expression has an effect on genome re-methylation in teratoma-susceptible germ cells, we immunostained FVB and M19 testes for 5-methylcytosine (5meC; Fig. 2D,E). At both E15.5 and E16.5, the percentage of germ cells positive for 5meC staining was significantly decreased in M19 versus FVB, indicating that genome re-methylation is impaired in teratoma-susceptible germ cells. We next assessed whether decreased expression of Tdrd1 and Tdrd9, which participate in piRNA-mediated suppression of retrotransposon expression and activity (Shoji et al., 2009; Bamezai et al., 2012) leads to increased transposon expression. We measured expression of Line-1, the most abundant class of autonomous retrotransposon (Kazazian, 2004; Malki et al., 2014), in germ cells from E13.5 and E14.5 FVB, 129 and M19 testes. At both time-points, Line-1 expression was increased in teratoma-susceptible germ cells when compared with FVB germ cells, indicating that transposon silencing was compromised in 129 and M19 germ cells (Fig. 3C). Thus, these data reveal that key features of male differentiation are delayed in teratoma-susceptible mice.
Nanos2 partial deficiency promotes teratoma-susceptible phenotypes on a resistant background
Because Nanos2 is delayed in teratoma-susceptible strains, we next investigated whether decreased expression of Nanos2 induced teratoma-susceptible phenotypes in teratoma-resistant, C57BL6 (B6), germ cells. Previous studies in teratoma-resistant mice demonstrated that Nanos2 deficiency ultimately induces widespread meiotic initiation and germ cell death (Tsuda et al., 2003; Suzuki and Saga, 2008). Thus, we investigated these phenotypes in mice heterozygous for a CRISPR/Cas9-generated Nanos2 knockout allele. Although, previous studies indicate that heterozygosity for a Nanos2 deletion does not lead to a meiotic initiation defect (Suzuki and Saga, 2008), the influence of Nanos2 partial deficiency on male germ cell sex-specification has not been investigated.
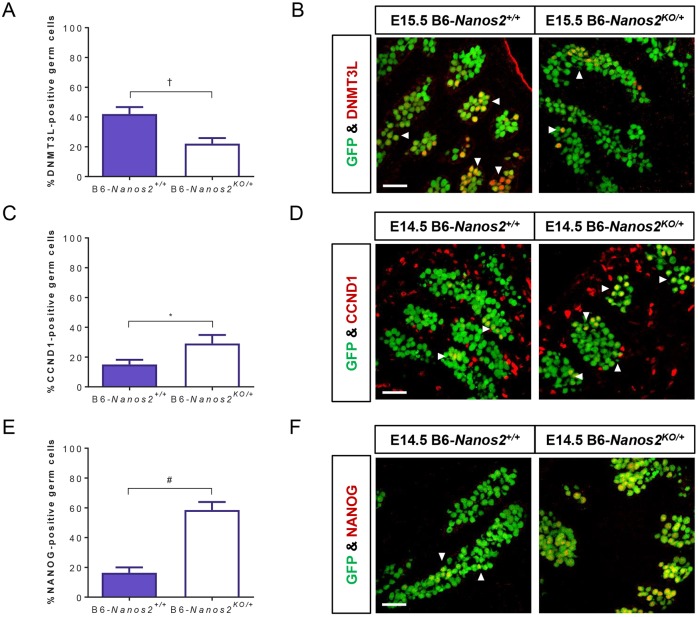
First, we determined whether heterozygosity for the Nanos2 knockout allele affected initiation of the male differentiation program by immunostaining Oct4ΔPE::GFP transgenic E15.5 B6-Nanos2KO/+ and Nanos2+/+ embryonic testes for DNMT3L (Fig. 4A,B, Fig. S4A). Nanos2 partial deficiency significantly decreased the percentage of germ cells positive for DNMT3L by nearly 50%. Next, we immunolabeled E14.5 B6-Nanos2KO/+ and Nanos2+/+ testes for CCND1 to test whether Nanos2 partial deficiency induced germ cell expression of a pre-meiotic oogonia marker known to be aberrantly expressed in teratoma-susceptible strains (Fig. 4C,D, Fig. S4B) (Heaney et al., 2012). Nanos2 partial deficiency caused a twofold increase in the percentage of germ cells positive for CCND1. It has been previously reported that Nanos2-null mice have increased expression of pluripotency genes, including Nanog, in XY germ cells prior to aberrant entry into meiosis (Saba et al., 2014). To determine whether Nanos2 partial deficiency also permits retention of pluripotency, we immunostained E14.5 B6-Nanos2KO/+ and Nanos2+/+ testes for NANOG (Fig. 4E,F, Fig. S4C). Nanos2 partial deficiency induced a nearly fourfold increase in the percentage of germ cells expressing NANOG. Collectively, these results reveal that partial deficiency of Nanos2 is sufficient to promote aberrant developmental phenotypes associated with teratoma-susceptible germ cells on a teratoma-resistant background.
Fig. 4.
Partial deficiency of Nanos2 promotes teratoma-susceptible phenotypes in tumor-resistant germ cells. (A-F) Oct4ΔPE::GFP transgenic E14.5 or E15.5 B6-Nanos2+/+ and B6-Nanos2KO/+ testes were sectioned and immunostained for (A,B) DNMT3L, (C,D) CCND1 and (E,F) Nanog. (A,C,E) Oct4ΔPE::GFP-positive germ cells, which are positive and negative for DNMT3L, CCND1 or NANOG, were counted. Data are plotted as the average percentage of germ cells positive for (A) DNMT3L, (C) CCND1 or (E) NANOG ±s.e.m. (n=5-9). *P<0.05; †P<0.01; #P<0.0001. (B,D,F) Representative confocal microscopy images of E14.5 or E15.5 Oct4ΔPE::GFP transgenic B6-Nanos2+/+ and B6-Nanos2KO/+ gonads immunostained for (B) DNMT3L, (D) CCND1 or (F) NANOG. Small groups of DNMT3L-, CCND1- or NANOG-positive germ cells are indicated (arrowheads). Scale bars: 50 μm.
Nanos2 deficiency increases teratoma incidence
We next investigated whether Nanos2 deficiency promotes teratoma susceptibility in the 129 strain. Given that 129 germ cells are enriched for anti-apoptotic and pro-proliferative pathways (Cook et al., 2011), we hypothesized that in this context Nanos2 deficiency would increase teratoma incidence.
Intercrosses between 129 mice heterozygous for a CRISPR/Cas9-generated Nanos2 knockout allele (Nanos2KO/+) generated experimental mice heterozygous (Nanos2KO/+) or homozygous for the Nanos2 deletion and control wild-type littermates (Nanos2+/+) for surveys of tumor incidence. 129-Nanos2+/+ male offspring derived from the 129-Nanos2KO/+ intercross had a teratoma incidence of 3%, which is consistent with previously published data for the 129 strain (Matin et al., 1999). Importantly, 129-Nanos2KO/KO mice had a teratoma incidence of 16%, which represented a significant increase when compared with wild-type controls (P<0.04, Table 1). Importantly, no germ cells were detected in adult testes from 129-Nanos2KO/KO mice examined by histology (Fig. S5A), which is consistent with what has previously been observed on a teratoma-resistant background (Tsuda et al., 2003). Histopathology verified that the tumors that formed in 129-Nanos2KO/KO mice were teratomas (Fig. S5A). Interestingly, tumor surveys revealed that 8% of 129-Nanos2KO/+ males had a teratoma (Fig. S5B). Although the increase in teratoma incidence in 129-Nanos2KO/+ mice compared with wild-type 129 controls was not significant with the samples sizes analyzed (P=0.285, Table 1), it suggests that Nanos2 may have a gene dose effect on teratoma susceptibility. Based on the results of our tumor survey, we conclude that decreased Nanos2 expression significantly contributes to teratoma incidence.
Table 1.
Nanos2 deficiency increases teratoma risk on the 129 background
Embryonal carcinoma cells acquire features of primed pluripotent cells
In the absence of male differentiation, we questioned what is involved in the transformation of germ cells into pluripotent EC cells. Over two decades of research involving the Oct4ΔPE::GFP transgene indicate that germ cells and EC cells exist in states suggestive of naïve and primed pluripotency, respectively (Yeom et al., 1996; Pesce et al., 1998; Yoshimizu et al., 1999; Heaney et al., 2012). The naïve pluripotent state represents the pre-implantation inner cell mass, cells that are at an epigenetic ground state, whereas the primed pluripotent state represents the post-implantation epiblast, cells that are poised to transition to a somatic regulatory program (Weinberger et al., 2016). Expression of the core pluripotency factor, Oct4, is controlled differentially between its distal enhancer, which drives expression in naïve pluripotent cells, and proximal enhancer, which drives expression in primed pluripotent cells (Yeom et al., 1996). Expression of the Oct4ΔPE::GFP transgene is controlled by the distal enhancer, thus driving expression in germ cells, which are known to maintain a naïve pluripotent expression profile (Yamaguchi et al., 2005; Western et al., 2010). However, previous studies indicate that Oct4ΔPE::GFP and Oct4ΔPE::LacZ transgenes, which lack the proximal enhancer, have diminished expression in mouse EC cells both in vitro and in vivo (Yeom et al., 1996; Pesce et al., 1998; Yoshimizu et al., 1999; Heaney et al., 2012). Thus, we hypothesized that teratoma initiation involves the transition of germ cells with features of naïve pluripotent cells into primed pluripotent EC cells.
To test whether germ cell transformation involves a switch in pluripotent state, we first investigated whether germ cells, in fact, express markers consistent with naïve pluripotency. Several markers of naïve pluripotency are known to be expressed in embryonic germ cell cells [e.g. Dazl, Dppa3 (Stella) and Dnmt3l] (Bortvin et al., 2004; Bourc'his and Bestor, 2004; Saiti and Lacham-Kaplan, 2007; Gill et al., 2011; Factor et al., 2014). Thus, we first investigated whether germ cells express other markers considered to be more specific to naïve pluripotent cells such as mouse embryonic stem (ES) cells [i.e. Zfp42 (Rex1) and Esrrb] (Leitch et al., 2010; Coronado et al., 2013; Factor et al., 2014; Kalkan et al., 2017). We compared expression of Rex1 and Esrrb between germ and somatic cells from E13.5 FVB and M19 testes, as well as 129 ES cells (Fig. S6). Rex1 and Esrrb were expressed 8- and 10-fold higher, respectively, in ES cells when compared with germ cells of both strains. However, expression in germ cells was 528- and 362-fold higher, respectively, when compared with somatic cells. Together with previously published data (Bourc'his and Bestor, 2004; Yamaguchi et al., 2005; Saiti and Lacham-Kaplan, 2007; Western et al., 2010), these results indicate that germ cells of both teratoma-susceptible and resistant strains, prior to sex specification, express several genes indicative of a naïve pluripotent state.
Next, we investigated whether EC cell foci express the primed pluripotency marker OTX2, which is required for naïve pluripotent cells to transition into primed pluripotent cells and for the stabilization of the primed state (Acampora et al., 2013; Weinberger et al., 2016). The transcription factor OTX2 is downstream of TGFβ superfamily signaling pathway, including NODAL and activin, which are both active in the embryonic testis. In fact, OTX2 is transiently induced at low levels in germ cells from E12.5 to E14.5 by NODAL/activin signaling (Wu et al., 2015). We first immunostained sections from E15.5, E16.5 and E18.5 M19 testes for OTX2. From a nascent focus at E15.5 to a well-established focus at E18.5, EC cells robustly expressed the primed pluripotency marker OTX2 (Fig. 5A, Figs S7 and S8). EC cell foci were initially identified by weak expression of the Oct4ΔPE::GFP transgene and subsequently verified as bona fide pluripotent EC cells by immunostaining serial sections for NANOG and E-cadherin (Fig. S8).
Fig. 5.
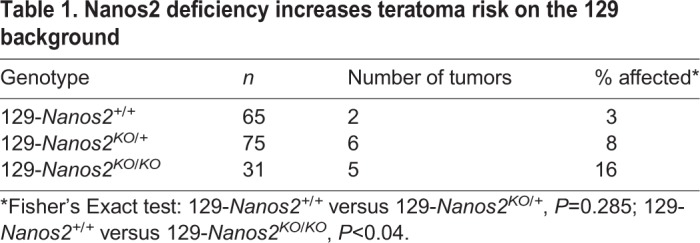
EC cells acquire features of primed pluripotency and downregulate features of naïve pluripotency. (A,B) Representative confocal microscopy images of E15.5, E16.5 and E18.5 Oct4ΔPE::GFP transgenic M19 gonads sectioned and immunostained for OTX2 and (A) DAZL or (B) DNMT3L. EC cell foci are outlined. Scale bars: 25 μm. See also Figs S7 and S10.
To investigate whether EC cells repress markers of naïve pluripotency as they transition from nascent to well-established foci, we co-immunolabeled sections from E15.5, E16.5 and E18.5 M19 testes for OTX2 and DAZL, the germ cell lineage and naïve pluripotency marker (Fig. 5 and Fig. S7) (Tesar et al., 2007; Gill et al., 2011; Xu et al., 2011; Factor et al., 2014; Hu et al., 2015; Welling et al., 2015). From E15.5 to E18.5, germ cells adjacent to the EC cell foci continued to express DAZL, with a gradual decrease in intensity over time. By comparison, expression of DAZL diminished in EC cell foci from E15.5 to E18.5. Importantly, despite the loss of DAZL expression, EC cells retained expression of the pluripotency marker NANOG (Fig. S8). Co-immunolabeling experiments with a second naïve pluripotency marker (stella, DPPA3) revealed a similar pattern of expression (Fig. S9) (Factor et al., 2014).
We next assessed EC cell foci expression of an additional naïve pluripotency marker, DNMT3L (Factor et al., 2014). Intriguingly, in vitro, DNMT3L status changes dynamically as cells are cultured in various media conditions that cultivate a hyper-naïve, naïve or primed pluripotent state (Leitch et al., 2013a; Weinberger et al., 2016). As cells transition from a hyper-naïve true epigenetic ground state to a naïve pluripotent state, expression of DNMT3L is induced. Then, as cells transition from a naïve state to a primed state, DNMT3L expression is lost. We hypothesized that DNMT3L expression status could be used to observe the transition from germ cells with naïve pluripotent properties to primed pluripotent-like EC cells. Above, we provide evidence indicating that the number of germ cells expressing DNMT3L is decreased in teratoma-susceptible strains at both E14.5 and E15.5, indicating a subpopulation of germ cells retains features that are consistent with hyper-naïve pluripotent cells (Fig. 2B,C). We next co-immunolabeled sections from E15.5, E16.5 and E18.5 M19 testes for OTX2 and DNMT3L (Fig. 5B, Fig. S10). Curiously, although not all adjacent germ cells expressed DNMT3L, nascent EC cell foci at E15.5 exhibited strong DNMT3L expression. However, by E16.5, when nearly all germ cells were strongly DNMT3L positive, DNMT3L expression was diminished or absent in EC cells. DNMT3L expression was completely lost in EC cells by E18.5.
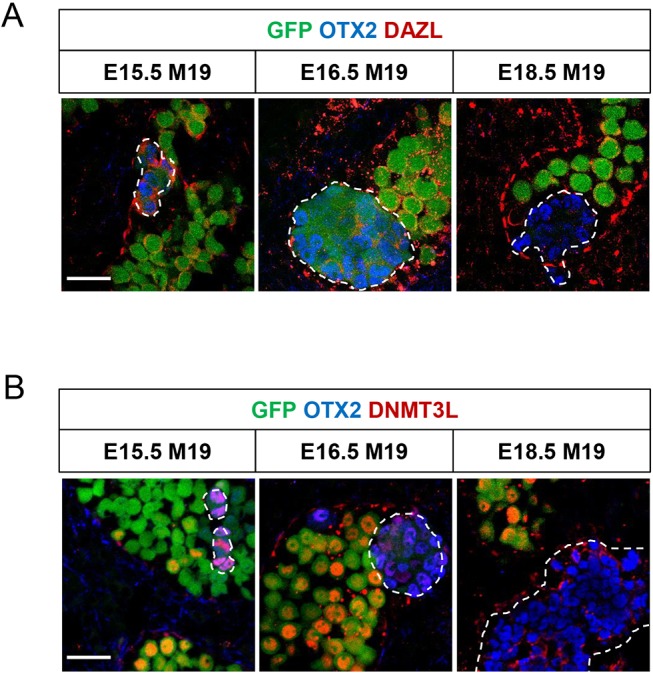
Because nascent EC cell foci express markers of both naïve and primed pluripotency, we questioned at what stage the transition towards a primed-like state begins. Given the strong expression of OTX2 in EC cells, we hypothesized that OTX2 is expressed in teratoma-susceptible germ cells prior to EC cell transition. We co-immunolabeled E15.5 M19 testes for OTX2 and the proliferation marker KI67, which distinguishes cells in any stage of the active cell cycle from those arrested in G0. Importantly, 30% of OTX2-positive germ cells were proliferating, whereas fewer than 1% of OTX2-negative germ cells were proliferating (Fig. 6). Additionally, we observed that the intensity of OTX2 staining in teratoma-susceptible germ cells at E15.5 and EC cells was much more robust than that of germ cells at E13.5, when OTX2 is normally and transiently expressed (Fig. S11) (Wu et al., 2015). Therefore, OTX2 expression is increased in teratoma-susceptible germ cells and EC cells, persists longer or is re-acquired in teratoma-susceptible germ cells, and is predictive of the pool of aberrantly proliferating germ cells that are capable of transitioning into EC cells. In summary, our analysis of naïve and primed pluripotency markers in teratoma-susceptible germ cells and EC cells indicate that, as teratoma-susceptible germ cells transition into EC cells, they move along the pluripotent state continuum and gradually adopt a primarily primed pluripotent profile.
Fig. 6.
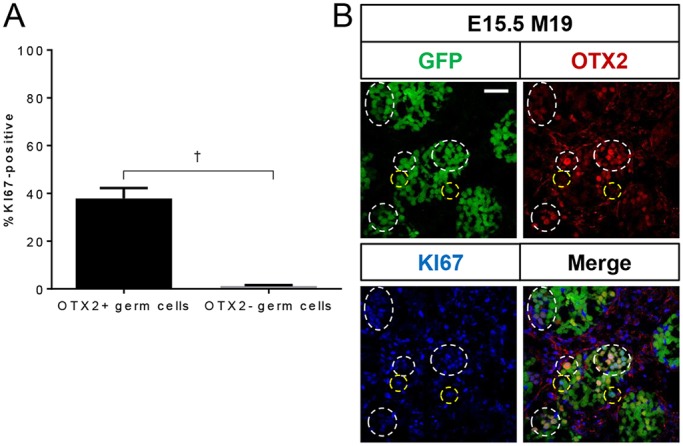
OTX2 expression associates with aberrant germ cell proliferation. Oct4ΔPE::GFP transgenic E15.5 M19 testes were sectioned and immunostained for OTX2 and KI67. Oct4ΔPE::GFP-positive germ cells, single positive for OTX2 or KI67, and double positive for both OTX2 and KI67 were counted. (A) Data are plotted as the average percentage of OTX2-positive and -negative germ cells positive for KI67 ±s.e.m. (n=4). (B) Confocal microscopy images of E15.5 Oct4ΔPE::GFP transgenic M19 gonads immunostained for OTX2 and KI67. KI67-positive, OTX2-positive germ cells (white outlines) and KI67-positive, OTX2-negative germ cells (yellow outlines) are indicated. Scale bar: 50 μm.
TGFβ superfamily signaling is active in EC cells
Primed pluripotent cells are in part defined by their long-term dependence on TGFβ superfamily signals, such as activin and NODAL (Weinberger et al., 2016). Both of these signals are normally active in the embryonic testis (Spiller et al., 2012; Miles et al., 2013). Thus, we hypothesized that TGFβ superfamily signaling is active in teratoma-susceptible germ cells and EC cells, and induces or maintains expression of primed markers such as OTX2. To test this, we immunolabeled adjacent sections from E18.5 M19 testes for phospho-SMAD2, which is activated by TGFβ superfamily signaling, or NANOG and E-cadherin, markers of EC cell foci (Fig. 7, Fig. S12). Not only was phospho-SMAD2 detected in germ cells, we also observed it in EC cell foci. These results suggest that TGFβ superfamily signaling is active throughout the teratoma-susceptible testis and that only a subpopulation of germ cells are uniquely poised to aberrantly respond to these signals and to form EC cell foci.
Fig. 7.
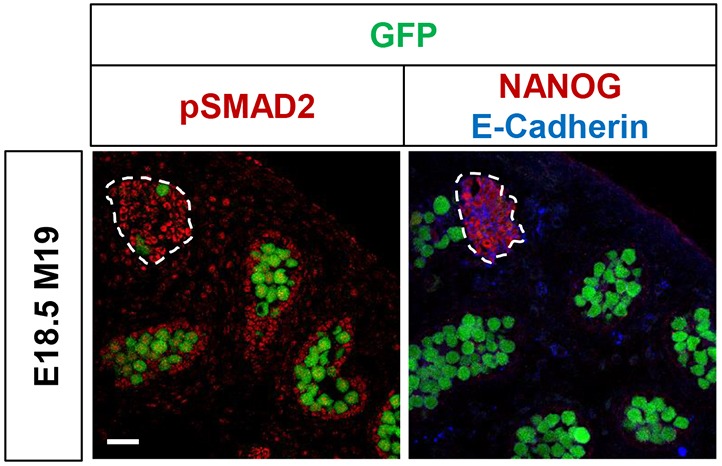
TGFβ signaling pathways are active in EC cells. Representative confocal microscopy images of serial sections from an Oct4ΔPE::GFP transgenic E18.5 M19 testis immunostained for phospho-SMAD2 (pSMAD2) or NANOG and E-cadherin. EC cell foci are outlined. Scale bar: 50 μm. See also Fig. S12.
DISCUSSION
It has long been hypothesized that TGCTs arise in both mice and humans from embryonic germ cells that continue to proliferate and reacquire or retain pluripotent potential (Stevens, 1967a; Jørgensen et al., 2015). However, the underlying developmental defects that induce or permit maintenance of these tumorigenic phenotypes have yet to be elucidated. By employing the 129 and M19 strains, in which both normal germ development and EC cell transformation occur, we reveal that male germ cell sex-specification is delayed in teratoma-susceptible germ cells. Specifically, Nanos2 expression was found to be decreased in teratoma-susceptible germ cells due, in part, to an allele variant present in 129 mice. Although the delay in Nanos2 expression precedes a delay in Nodal expression, Nodal may perpetuate the decrease in Nanos2 expression and increase the severity of the defect in the M19 strain. As a consequence of delayed Nanos2 expression, the expression of several downstream targets of Nanos2 is reduced or delayed in teratoma-susceptible germ cells, resulting in phenotypes that are indicative of delayed male germ cell sex specification and increased teratoma risk. As observed with other developmental phenotypes associated with teratoma initiation in mice (Heaney et al., 2012), the degree to which male germ cell differentiation is delayed increases with TGCT incidence of the background (129 versus M19).
Based on our tumor surveys, Nanos2 deficiency promotes testicular teratocarcinogenesis. Although the increase in tumor incidence in 129-Nanos2KO/KO mice may seem modest, it parallels the change in incidence observed due to a Kitl mutation (Steel) in 129 mice (Heaney et al., 2008). Importantly, genome-wide association studies (GWAS) have identified KITLG as a candidate TGCT risk gene in humans (Kanetsky et al., 2009) and have revealed that KITLG containing susceptibility loci have the most significant risk associations ever detected by a cancer GWAS (Welter et al., 2014). Therefore, a modest but significant increase in tumor incidence in mice has proven to be relevant to understanding genetic risk factors for TGCTs in humans. We suspect that the tumor incidence is not higher in 129-Nanos2KO/KO mice because most germ cells undergo meiotic catastrophe, which is evidenced by germ cell deficiency in adult 129-Nanos2KO/KO animals, as an alternative fate to transformation. Pairing Nanos2 deficiency with Stra8 deficiency, to suppress meiosis (Anderson et al., 2008), or Bax deficiency, to repress apoptosis (Cook et al., 2009), would likely push more teratoma-susceptible germ cells toward transformation into EC cells. In 129-Nanos2KO/+ animals, tumor incidence appears to be increased when compared with wild-type littermates. Although the change in incidence was not significant with the samples size analyzed, it suggests that Nanos2 has a gene dose effect on teratoma susceptibility. We propose that decreasing Nanos2 expression creates a larger susceptibility pool of germ cells that fail lineage restriction and from which transformation events are more likely to arise.
In addition to providing evidence that Nanos2 contributes to teratoma incidence, we determined that teratoma-resistant germ cells partially deficient for Nanos2 had diminished expression of male-specific genes required for differentiation, retained expression of pluripotency factors, and misexpressed pre-meiotic oogonia markers. Together, these results indicate that decreased Nanos2 expression cultivates teratoma-susceptibility phenotypes (Heaney et al., 2012). Importantly, Nanos2 deficiency is not sufficient to induce teratomas on teratoma-resistant backgrounds (Saba et al., 2014). Embryonic germ cells from teratoma-resistant backgrounds are enriched for cell death and G2/M checkpoint pathways (Cook et al., 2009, 2011). Therefore, it is likely that errant germ cells are eliminated before they can transform. Thus, consistent with a polygenic trait, other variants in the 129 genome, in addition to those found in Nanos2, are required for tumor initiation.
Although NANOS2 has not been identified as a putative human TGCT risk gene by GWAS, variants in several genes involved in germ cell development and sex differentiation are associated with TGCT risk in humans (Litchfield et al., 2016). These genes can be grouped into those expressed during migration (e.g. PRDM14, KIT) and those expressed upon colonization of the gonad (e.g. DAZL, DMRT1, ZFPM1). Genetic defects at various times in germ cell development likely interact to disrupt commitment to the male germ cell fate. The enrichment for these pathways in human GWAS suggests, much like we observed in teratoma-susceptible mice, failed germ cell lineage restriction contributes to tumor initiation in humans. Intriguingly, the germ cell lineage marker Dazl, which was identified as a candidate TGCT risk gene in humans (Ruark et al., 2013), is required for the induction of Nanos2 expression in B6 mice (Gill et al., 2011). Therefore, altered function of DAZL in humans may facilitate TGCT initiation through delays in male germ cell sex specification, as we observed in teratoma-susceptible mouse strains. We propose that in both mice and humans, subpopulations of XY germ cells fail to properly commit to a unipotent male germ cell fate, leaving them susceptible to transformation.
In the absence of male germ cell lineage commitment, we and others have previously demonstrated that teratoma-susceptible germ cells retain features of pluripotency prior to transformation (Cook et al., 2009, 2011; Heaney et al., 2012). It has long been known that PGC specification from the proximal epiblasts involves the reactivation of the core components of pluripotency (Oct4, Sox2 and Nanog) and that expression of these factors normally persists until sex-specific differentiation (Saitou and Miyauchi, 2016). Importantly, our expression data shown here (Fig. S6) indicate that this pluripotent-like state is most similar to that of naïve pluripotent cells. We show that prior to sex-specific differentiation, germ cells robustly express not only naïve pluripotency markers that are implicated in normal germ cell development (e.g. Dazl, Stella, Dnmt3l) (Bortvin et al., 2004; Bourc'his and Bestor, 2004; Saiti and Lacham-Kaplan, 2007; Gill et al., 2011; Factor et al., 2014), but those directly associated with the naïve state (e.g. Rex1, Esrrb). Our findings are consistent with other features, including a hypomethylated genome, expression of Kit and robust expression of the Oct4ΔPE::GFP transgene, which indicate that embryonic germ cells, prior to sex-specific differentiation, possess features of naïve pluripotency (Matsui et al., 1992; Yeom et al., 1996; Yoshimizu et al., 1999; Weinberger et al., 2016).
Importantly, although embryonic germ cells harbor features of naïve pluripotency prior to sex-specific differentiation, they lack one characteristic of this cell type, i.e. the capacity to form chimeras following injection into the inner cell mass of blastocysts (Le Bin et al., 2014). They are, however, easily reprogrammed in vitro into naïve pluripotent embryonic germ (EG) cell lines with chimera-forming capacity (Matsui et al., 1992). Based on these observations, it has been proposed that teratoma-susceptible germ cells must undergo a similar reprogramming process in vivo to form EC cells (Leitch et al., 2013b). Interestingly, a key differentiating feature between PGCs and bona fide naïve pluripotent cells is that only the former express Prdm1 (Blimp1), which is necessary for repressing the somatic program during germ cell specification (Saitou and Miyauchi, 2016). In fact, downregulation of Prdm1 is an essential step in establishing EG cells in culture as it permits the expression of Klf4 and Myc (Durcova-Hills et al., 2008). Curiously, germ cells lose Prdm1 expression as sex-specific differentiation begins (Saitou and Miyauchi, 2016). In teratoma-susceptible germ cells, if Prdm1 expression is downregulated as expected but male sex-specific differentiation is delayed, this may permit acquisition of a naïve pluripotent state and facilitate transformation into pluripotent EC cells.
In contrast to embryonic germ cells, EC cells acquire features of primed pluripotency, including robust expression of the primed marker OTX2 (Fig. 5A), KIT-negative expression status, a switch to the use of the proximal enhancer to drive Oct4, and inefficient contribution to chimeras and their germline (Yeom et al., 1996; Gardner, 1998; Solter, 2006; Tesar et al., 2007; Schemmer et al., 2013; Weinberger et al., 2016). Curiously, there is a strong parallel between the paracrine and autocrine signals that normally drive male germ cell sex specification and those required for maintenance of primed pluripotency in culture (e.g. mouse epiblast stem cells and human ES cells) (Spiller and Bowles, 2015; Weinberger et al., 2016). However, despite the shared need of these cells for NODAL/activin, their responses are very different; XY germ cells differentiate into unipotent male germ cells, whereas stem cells in culture transition to and maintain a primed pluripotent state.
The dynamics of OTX2 expression in primed cells in culture and in the embryonic testis provide insight about how these cells differentially integrate NODAL/activin signaling and the role of primed pluripotency in EC cell transformation. In culture, NODAL/activin signaling cultivates the primed pluripotent state by sustaining robust expression of OTX2 (Acampora et al., 2013). However, in XY germ cells, NODAL/activin signaling transiently induces low levels of OTX2 expression before Nanos2 expression reaches its peak (Suzuki and Saga, 2008; Wu et al., 2015). Interestingly, OTX2 is not required for male germ cell sex specification (Wu et al., 2015). We speculate that, with OTX2 expression, the male germline enters a transient primed pluripotent-like state prior to terminal differentiation. We suggest that, at this juncture, NANOS2 and other male sex-specification signals act as a road sign to direct these cells towards male germ cell lineage commitment rather than an extended primed pluripotent-like period. Intriguingly, we provide evidence here that EC cells have activated TGFβ superfamily signaling and exhibit strong and sustained expression of OTX2. Delayed Nanos2 expression may permit some teratoma-susceptible germ cells to robustly express OTX2 outside of its normal transient window in response to NODAL/activin signaling. We propose these germ cells fail to exit the normally transitory, primed pluripotent-like state, leading to their transformation into EC cells.
Mirroring what we observed in the mouse, NODAL/activin signaling is activated in the tumor stem cells of TGCTs in humans. A recent study determined expression of NODAL increases in mixed non-seminoma tumors in proportion to pluripotent EC cell content (Spiller et al., 2012). Additionally, the priming marker downstream of TGFβ superfamily signaling, OTX2, is expressed ninefold higher in human EC cells compared with normal testis tissue (Sperger et al., 2003). These studies provide further evidence that in both mouse and human, NODAL/activin signaling and the acquisition of primed pluripotent features play crucial roles in tumor initiation.
Intriguingly, identification of PRDM14 as a candidate human TGCT risk gene lends credence to the notion that germ cell transition from a naïve- to primed-like state is involved in TGCT initiation in humans (Ruark et al., 2013). In mice, Prdm14 acts in concert with Prdm1 and Tfap2c to specify PGCs from the proximal epiblast by inducing DNA demethylation and histone remodeling, repressing the somatic cell program and establishing an expression profile similar to naïve pluripotent cells in culture (Saitou and Yamaji, 2010; Yamaji et al., 2013). In vitro, Prdm14 plays a critical role in establishing and maintaining the naïve state in mouse ES cells through these same mechanisms (Yamaji et al., 2013; Weinberger et al., 2016). If Prdm14 ensures proper germ cell specification in part by safeguarding or establishing characteristics consistent with naïve pluripotency, then its altered function may push germ cells to transition to primed pluripotent tumor stem cells.
MATERIALS AND METHODS
Mice
129S1/SvImJ (129, JR#002448), FVB/NJ (FVB, JR#001800), C57BL/6J (B6, JR#000664) and C3H/HeJ (C3H, JR#000659) mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Chromosome substitution strain 129-Chr19MOLF/Ei (M19) mice were obtained from our research colony (Matin et al., 1999). Mice harboring the Nanos2em2Bay (Nanos2KO) knockout allele were obtained from the International Phenotyping Consortium. The Nanos2KO allele was generated on a C57BL/6NJ background using CRISPR/Cas9 genome editing in zygotes, as previously described (Szafranski et al., 2017), with a pair of guide RNAs (chr7:18987609-18987631, 5′-ACCTACCGCCCTTTGACATG; chr7:18987970-18987992, 5′-CTGAGTTTCGCCCACTGCGT; GRCm38/mm10) that facilitated the deletion of the entire open reading frame in the single exon of Nanos2. Animals were subsequently backcrossed to the C57BL/6J strain. An independent Nanos2KO was generated and maintained on the 129/SvImJ strain using CRISPR/Cas9 and the same guide RNAs. Both alleles were verified by Sanger sequencing. PCR genotyping for both alleles was performed using primers 5′-GCTCCACAAAACTCCTGCTC and 5′-GGATCCTGAAGGCACAGAAA, which produce a wild-type product of 629 bp and a null allele product of 245 bp. The germ cell-specific Oct4ΔPE::GFP transgene was previously backcrossed onto the 129, FVB, B6 and M19 backgrounds to establish congenic lines (Yoshimizu et al., 1999). All protocols were approved by the BCM Institutional Animal Care and Use Committee.
SNP analysis
The Mouse Genome Informatics Strain, SNP and Polymorphism analysis tool (http://www.informatics.jax.org/) was used to search for strain-specific genetic variants in the Nodal and Nanos2 loci (Blake et al., 2017). 129S1/SvImJ was used as the reference strain and compared with selected testicular teratoma-resistant inbred strains C57BL/6J, FVB/NJ, A/J, C3H/HeJ and BALB/cJ. A SNP query was performed to identify SNPs within the open reading frame in functional classes coding-nonsynonymous, intron, mRNA-UTR, splice-site and noncoding-transcript polymorphisms. The UCSC Genome Browser (GRCm38/mm10) was used to confirm or determine the SNP variant in the C57BL/6J strain. Furthermore, SNP variants in Nanos2 for each strain were confirmed or determined by Sanger sequencing.
Timed matings and gonad dissections
For immunofluorescence and fluorescence-activated cell sorting (FACS), wild-type females were bred to males homozygous for the Oct4ΔPE::GFP transgene to produce FVB, 129, M19 or B6 transgenic embryos heterozygous for Oct4ΔPE::GFP. Crosses between B6 females and B6-Nanos2KO/+ males homozygous for Oct4ΔPE::GFP were used to produce B6-Nanos2+/+ and B6-Nanos2KO/+ embryos heterozygous for Oct4ΔPE::GFP. For allele expression analyses, FVB mice homozygous for Oct4ΔPE::GFP and 129 or C3H mice were bred in reciprocal crosses to generate F1 hybrid embryos heterozygous for Oct4ΔPE::GFP. Embryonic day 0.5 (E0.5) was assumed to be noon of the day the vaginal plug was observed. Pregnant females were euthanized by carbon dioxide followed by cervical dislocation and gonads were removed from embryos in ice-cold 1×PBS. Embryos older than E14.5 were decapitated prior to dissection. PCR genotyping for Sry identified the sex of E12.5 embryos (Heaney et al., 2009). Gonad morphology identified the sex of E13.5 to E18.5 embryos. Tissues were collected for PCR genotyping for the Nanos2 knockout allele described above.
129. ES cell line culture
The feeder-free cell line E14TG2a (derived from strain 129P2/OlaHsd) was cultured in GMEM (G5154, Millipore) supplemented with 2 mM glutamine (25030081, ThermoFisher), 1 mM sodium pyruvate (11360070, ThermoFisher), 1× non-essential amino acids (11140, Life Technologies), 10% fetal bovine serum (10437, Life Technologies), 1:1000 dilution of β-mercaptoethanol stock solution (21985023, ThermoFisher) and 500-1000 units per ml of LIF (ESG1107, Millipore Sigma) at 37°C and 5% CO2.
Fluorescence-activated cell sorting and quantitative real-time PCR expression analysis
Fluorescence-activated cell sorting (FACS) of GFP+ and GFP− germ and somatic cells, respectively, from embryonic gonads and their paired mesonephroi, RNA preparation, reverse transcription, quantitative real-time PCR analysis and normalization of gene expression were performed as previously described (Lanza et al., 2016). One-way ANOVA with the Bonferroni post-test for pair-wise comparisons were used to detect significant differences in expression between germ cells or somatic cells from FVB (control), 129 and M19 gonads. Primer sequences can be found in Table S4.
Allele expression analysis
SYBR primers were designed to be specific to the FVB ‘C’ Nanos2 allele and the 129/C3H ‘T’ Nanos2 allele. The annealing temperature was optimized by performing RT-PCR at various annealing temperatures to ensure that the ‘C’ primers only led to priming in the FVB sample and the ‘T’ primers only led to priming in 129 or C3H samples. With an annealing temperature of 64°C, these primers discerned appropriately between the strains: forward-C allele, TGCCAGAGACTGATCCTGATC; forward-T allele, TGCCAGAGACTGATCCTGATT; reverse, TCCTGATGGACATGGCCATG. qPCR was performed using these primers on cDNA and DNA from FACS-enriched germ cells from E13.5 F1[FVB×129/C3H]. DNA from these samples served as a 1:1 control, because it has equal contribution from each allele. Expression was normalized to the expression from the ‘T’ allele. Unpaired-t-tests were used to detect significant differences in expression between cDNA and DNA isolated from the F1 hybrids.
Immunohistochemistry
Gonads were collected from embryos and processed for sectioning and immunofluorescence as previously described (Heaney et al., 2009). For 5-methyl cytosine staining, sections were treated with 3.5 N HCl for 30 min to denature DNA and incubated in MOM blocking solution (Vector Laboratories) for 1 h to block non-specific staining. Sections were incubated with primary antibodies (Table S3) overnight at 4°C. For secondary detection by confocal microscopy, sections were incubated with a 1:500 dilution of the goat anti-rabbit AlexaFluor 555 (A-21428, Life Technologies), goat anti-rat AlexaFluor 647 (A-21247, Life Technologies), donkey anti-goat AlexaFluor 555 (ab150130, Abcam), donkey anti-rabbit AlexaFluor 647 (ab150075, Abcam), donkey anti-rabbit AlexaFluor 555 (ab150074, Abcam) and/or donkey anti-goat AlexaFluor 647 (ab150131, Abcam) antibodies in blocking solution for 2 h at room temperature. Nuclei were counter-stained with DAPI. Confocal images of sections were taken with a Nikon A1-Rs inverted Laser Scanning Microscope.
Cell counts
Oct4ΔPE::GFP-positive germ cells positive or negative for CCND1, NANOG, DNMT3L, 5-methylcytosine, OTX2 or KI67 immunostaining were counted as previously described (Heaney et al., 2009). Unpaired t-tests were used to detect significant differences in the percentage of CCND1, NANOG, DNMT3L or 5-methylcytosine-positive germ cells between FVB and M19 testes or B6-Nanos2+/+ and B6-Nanos2KO/+ testes. Paired t-tests were used to detect significant differences between the percentage of OTX2-positive germ cells versus OTX2-negative germ cells that were KI67 positive in the sections from M19 testes.
Tumor surveys
Crosses between 129 mice heterozygous for Nanos2KO were used to produce wild-type (129-Nanos2+/+), heterozygous knockout (129-Nanos2KO/+) and homozygous knockout (129-Nanos2KO/KO) male offspring to survey for teratomas. Males, 4-8 weeks of age, were necropsied prior to genotyping and testes were visually and histologically examined for tumors, which are readily detected at this age (Matin et al., 1999; Lam et al., 2004). Fisher's exact tests were used to assess statistical differences between the number of teratoma-affected 129 wild-type (129-Nanos2+/+) control and heterozygous knockout (129-Nanos2KO/+) or homozygous knockout (129-Nanos2KO/KO) experimental progeny.
Histology
Tissues were fixed in 10% buffered formalin, embedded in paraffin wax, sectioned (6 µm) and stained with Hematoxylin and Eosin. Bright-field images were captured using a Zeiss Axioplan2 microscope.
Acknowledgements
We thank the Baylor College of Medicine Integrated Microscopy and Cytometry and Cell Sorting Advanced Technologies Cores for assisting with confocal microscopy and FACS, respectively.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: E.P.D., J.D.H.; Methodology: E.P.D., J.D.H.; Formal analysis: E.P.D., D.G.L.; Investigation: E.P.D., D.G.L., N.J.W., S.M.B.; Resources: I.S.; Writing - original draft: E.P.D., J.D.H.; Writing - review & editing: E.P.D., D.G.L., N.J.W., J.D.H.; Visualization: E.P.D.; Supervision: J.D.H.; Project administration: J.D.H.; Funding acquisition: E.P.D., J.D.H.
Funding
This work was supported by Cancer Prevention and Research Institute of Texas (CPRIT) (RP150081 to J.D.H. and RP140102 to J.M.R.) and the Baylor Research Advocates for Student Scientists (BRASS) Scholarship Program (E.P.D.). Resources accessed through all cores were supported by a National Institutes of Health-National Cancer Institute grant (CA125123) to the Dan L. Duncan Cancer Center. Additionally, National Institutes of Health grants (HD007495 and DK56338) and a grant from the John S. Dunn Gulf Coast Consortium for Chemical Genomics supported resources accessed through the Integrated Microscopy Core.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.156612.supplemental
References
- Acampora D., Di Giovannantonio L. G. and Simeone A. (2013). Otx2 is an intrinsic determinant of the embryonic stem cell state and is required for transition to a stable epiblast stem cell condition. Development 140, 43-55. 10.1242/dev.085290 [DOI] [PubMed] [Google Scholar]
- Almstrup K., Hoei-Hansen C. E., Wirkner U., Blake J., Schwager C., Ansorge W., Nielsen J. E., Skakkebaek N. E., Rajpert-De Meyts E. and Leffers H. (2004). Embryonic stem cell-like features of testicular carcinoma in situ revealed by genome-wide gene expression profiling. Cancer Res. 64, 4736-4743. 10.1158/0008-5472.CAN-04-0679 [DOI] [PubMed] [Google Scholar]
- Anderson E. L., Baltus A. E., Roepers-Gajadien H. L., Hassold T. J., de Rooij D. G., van Pelt A. M. M. and Page D. C. (2008). Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Natl. Acad. Sci. USA 105, 14976-14980. 10.1073/pnas.0807297105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamezai S., Rawat V. P. S. and Buske C. (2012). Concise Review: The Piwi-piRNA Axis: Pivotal Beyond Transposon Silencing. Stem Cells 30, 2603-2611. 10.1002/stem.1237 [DOI] [PubMed] [Google Scholar]
- Blake J. A., Eppig J. T., Kadin J. A., Richardson J. E., Smith C. L. and Bult C. J. (2017). Mouse Genome Database (MGD)-2017: community knowledge resource for the laboratory mouse. Nucleic Acids Res. 45, D723-D729. 10.1093/nar/gkw1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortvin A., Goodheart M., Liao M. and Page D. C. (2004). Dppa3/Pgc7/stella is a maternal factor and is not required for germ cell specification in mice. BMC Dev. Biol. 4, 2 10.1186/1471-213X-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourc'his D. and Bestor T. H. (2004). Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature 431, 96-99. 10.1038/nature02886 [DOI] [PubMed] [Google Scholar]
- Bowles J. and Koopman P. (2007). Retinoic acid, meiosis and germ cell fate in mammals. Development 134, 3401-3411. 10.1242/dev.001107 [DOI] [PubMed] [Google Scholar]
- Chuma S., Hiyoshi M., Yamamoto A., Hosokawa M., Takamune K. and Nakatsuji N. (2003). Mouse Tudor Repeat-1 (MTR-1) is a novel component of chromatoid bodies/nuages in male germ cells and forms a complex with snRNPs. Mech. Dev. 120, 979-990. 10.1016/S0925-4773(03)00181-3 [DOI] [PubMed] [Google Scholar]
- Cook M. S., Coveney D., Batchvarov I., Nadeau J. H. and Capel B. (2009). BAX-mediated cell death affects early germ cell loss and incidence of testicular teratomas in Dnd1(Ter/Ter) mice. Dev. Biol. 328, 377-383. 10.1016/j.ydbio.2009.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. S., Munger S. C., Nadeau J. H. and Capel B. (2011). Regulation of male germ cell cycle arrest and differentiation by DND1 is modulated by genetic background. Development 138, 23-32. 10.1242/dev.057000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado D., Godet M., Bourillot P.-Y., Tapponnier Y., Bernat A., Petit M., Afanassieff M., Markossian S., Malashicheva A. and Iacone R. (2013). A short G1 phase is an intrinsic determinant of naïve embryonic stem cell pluripotency. Stem Cell Res. 10, 118-131. 10.1016/j.scr.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Durcova-Hills G., Tang F., Doody G., Tooze R. and Surani M. A. (2008). Reprogramming Primordial Germ Cells into Pluripotent Stem Cells. PloS ONE 3, e3531 10.1371/journal.pone.0003531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor D. C., Corradin O., Zentner G. E., Saiakhova A., Song L., Chenoweth J. G., McKay R. D., Crawford G. E., Scacheri P. C. and Tesar P. J. (2014). Epigenomic comparison reveals activation of ‘seed’ enhancers during transition from naive to primed pluripotency. Cell Stem Cell 14, 854-863. 10.1016/j.stem.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R. L. (1998). Contributions of blastocyst micromanipulation to the study of mammalian development. BioEssays 20, 168-180. [DOI] [PubMed] [Google Scholar]
- Gill M. E., Hu Y.-C., Lin Y. and Page D. C. (2011). Licensing of gametogenesis, dependent on RNA binding protein DAZL, as a gateway to sexual differentiation of fetal germ cells. Proc. Natl. Acad. Sci. USA 108, 7443-7448. 10.1073/pnas.1104501108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney J. D. and Nadeau J. H. (2008). Testicular germ cell tumors in mice: new ways to study a genetically complex trait. Methods Mol. Biol. 450, 211-231. 10.1007/978-1-60327-214-8_15 [DOI] [PubMed] [Google Scholar]
- Heaney J. D., Lam M.-Y. J., Michelson M. V. and Nadeau J. H. (2008). Loss of the transmembrane but not the soluble kit ligand isoform increases testicular germ cell tumor susceptibility in mice. Cancer Res. 68, 5193-5197. 10.1158/0008-5472.CAN-08-0779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney J. D., Michelson M. V., Youngren K. K., Lam M. Y. and Nadeau J. H. (2009). Deletion of eIF2beta suppresses testicular cancer incidence and causes recessive lethality in agouti-yellow mice. Hum. Mol. Genet. 18, 1395-1404. 10.1093/hmg/ddp045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney J. D., Anderson E. L., Michelson M. V., Zechel J. L., Conrad P. A., Page D. C. and Nadeau J. H. (2012). Germ cell pluripotency, premature differentiation and susceptibility to testicular teratomas in mice. Development 139, 1577-1586. 10.1242/dev.076851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.-C., Nicholls P. K., Soh Y. Q. S., Daniele J. R., Junker J. P., van Oudenaarden A. and Page D. C. (2015). Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling. PLoS Genet. 11, e1005019 10.1371/journal.pgen.1005019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen A., Johansen M. L., Juul A., Skakkebaek N. E., Main K. M. and Rajpert-De Meyts E. (2015). Pathogenesis of germ cell neoplasia in testicular dysgenesis and disorders of sex development. Semin. Cell Dev. Biol. 45, 124-137. 10.1016/j.semcdb.2015.09.013 [DOI] [PubMed] [Google Scholar]
- Kalkan T., Olova N., Roode M., Mulas C., Lee H. J., Nett I., Marks H., Walker R., Stunnenberg H. G. and Lilley K. S. (2017). Tracking the embryonic stem cell transition from ground state pluripotency. Development 144, 1221-1234. 10.1242/dev.142711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsky P. A., Mitra N., Vardhanabhuti S., Li M., Vaughn D. J., Letrero R., Ciosek S. L., Doody D. R., Smith L. M., Weaver J. et al. (2009). Common variation in KITLG and at 5q31.3 predisposes to testicular germ cell cancer. Nat. Genet. 41, 811-815. 10.1038/ng.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H. (2004). Mobile Elements: Drivers of Genome Evolution. Science 303, 1626-1632. 10.1126/science.1089670 [DOI] [PubMed] [Google Scholar]
- Lam M.-Y. J., Youngren K. K. and Nadeau J. H. (2004). Enhancers and suppressors of testicular cancer susceptibility in single- and double-mutant mice. Genetics 166, 925-933. 10.1534/genetics.166.2.925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza D. G. and Heaney J. D. (2017). Testicular germ cell tumors and teratomas. In The Biology of Mammalian Spermatogonia (ed. J. Oatley and M. Griswold), pp. 225-267. New York, NY: Springer. [Google Scholar]
- Lanza D. G., Dawson E. P., Rao P. and Heaney J. D. (2016). Misexpression of cyclin D1 in embryonic germ cells promotes testicular teratoma initiation. Cell Cycle 15, 919-930. 10.1080/15384101.2016.1149272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson K. A., Dunn N. R., Roelen B. A. J., Zeinstra L. M., Davis A. M., Wright C. V. E., Korving J. P. W. F. M. and Hogan B. L. M. (1999). Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 13, 424-436. 10.1101/gad.13.4.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bin G. C., Muñoz-Descalzo S., Kurowski A., Leitch H., Lou X., Mansfield W., Etienne-Dumeau C., Grabole N., Mulas C. and Niwa H. (2014). Oct4 is required for lineage priming in the developing inner cell mass of the mouse blastocyst. Development 141, 1001-1010. 10.1242/dev.096875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch H. G., Blair K., Mansfield W., Ayetey H., Humphreys P., Nichols J., Surani M. A. and Smith A. (2010). Embryonic germ cells from mice and rats exhibit properties consistent with a generic pluripotent ground state. Development 137, 2279-2287. 10.1242/dev.050427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch H. G., McEwen K. R., Turp A., Encheva V., Carroll T., Grabole N., Mansfield W., Nashun B., Knezovich J. G., Smith A. et al. (2013a). Naïve pluripotency is associated with global DNA hypomethylation. Nat. Struct. Mol. Biol. 20, 311-316. 10.1038/nsmb.2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch H. G., Nichols J., Humphreys P., Mulas C., Martello G., Lee C., Jones K., Surani M. A. and Smith A. (2013b). Rebuilding Pluripotency from Primordial Germ Cells. Stem Cell Reports 1, 66-78. 10.1016/j.stemcr.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield K., Levy M., Huddart R. A., Shipley J. and Turnbull C. (2016). The genomic landscape of testicular germ cell tumours: from susceptibility to treatment. Nat. Rev. Urol 13, 409-419. 10.1038/nrurol.2016.107 [DOI] [PubMed] [Google Scholar]
- Looijenga L. H., Stoop H., de Leeuw H. P., de Gouveia Brazao C. A., Gillis A. J., van Roozendaal K. E., van Zoelen E. J., Weber R. F., Wolffenbuttel K. P., van Dekken H. et al. (2003). POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 63, 2244-2250. [PubMed] [Google Scholar]
- Malki S., van der Heijden G. W., O'Donnell K. A., Martin S. L. and Bortvin A. (2014). A Role for Retrotransposon LINE-1 in Fetal Oocyte Attrition in Mice. Dev. Cell 29, 521-533. 10.1016/j.devcel.2014.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Collin G. B., Varnum D. S. and Nadeau J. H. (1998). Testicular teratocarcinogenesis in mice–a review. APMIS 106, 174-182. 10.1111/j.1699-0463.1998.tb01333.x [DOI] [PubMed] [Google Scholar]
- Matin A., Collin G. B., Asada Y., Varnum D. and Nadeau J. H. (1999). Susceptibility to testicular germ-cell tumours in a 129.MOLF-Chr 19 chromosome substitution strain. Nat. Genet. 23, 237-240. 10.1038/13874 [DOI] [PubMed] [Google Scholar]
- Matsui Y., Toksoz D., Nishikawa S., Nishikawa S.-I., Williams D., Zsebo K. and Hogan B. L. M. (1991). Effect of Steel factor and leukaemia inhibitory factor on murine primordial germ cells in culture. Nature 353, 750-752. 10.1038/353750a0 [DOI] [PubMed] [Google Scholar]
- Matsui Y., Zsebo K. and Hogan B. L. M. (1992). Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell 70, 841-847. 10.1016/0092-8674(92)90317-6 [DOI] [PubMed] [Google Scholar]
- McLaren A. (1984). Meiosis and differentiation of mouse germ cells. Symp. Soc. Exp. Biol. 38, 7-23. [PubMed] [Google Scholar]
- McLaren A. (1999). Signaling for germ cells. Genes Dev. 13, 373-376. 10.1101/gad.13.4.373 [DOI] [PubMed] [Google Scholar]
- McLaren A. (2000). Germ and somatic cell lineages in the developing gonad. Mol. Cell Endocrinol. 163, 3-9. 10.1016/S0303-7207(99)00234-8 [DOI] [PubMed] [Google Scholar]
- Miles D. C., Wakeling S. I., Stringer J. M., van den Bergen J. A., Wilhelm D., Sinclair A. H. and Western P. S. (2013). Signaling through the TGF Beta-Activin Receptors ALK4/5/7 Regulates Testis Formation and Male Germ Cell Development. PloS ONE 8, e54606 10.1371/journal.pone.0054606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T. and Stevens L. C. (1982). Primordial germ cell proliferation in fetal testes in mouse strains with high and low incidences of congenital testicular teratomas. J. Natl. Cancer Inst. 69, 907-913. [PubMed] [Google Scholar]
- Oosterhuis J. W. and Looijenga L. H. (2005). Testicular germ-cell tumours in a broader perspective. Nat. Rev. Cancer 5, 210-222. 10.1038/nrc1568 [DOI] [PubMed] [Google Scholar]
- Pesce M. and Schöler H. R. (2000). Oct-4: control of totipotency and germline determination. Mol. Reprod. Dev. 55, 452-457. [DOI] [PubMed] [Google Scholar]
- Pesce M., Wang X., Wolgemuth D. J. and Scholer H. (1998). Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech. Dev. 71, 89-98. [DOI] [PubMed] [Google Scholar]
- Resnick J. L., Bixler L. S., Cheng L. and Donovan P. J. (1992). Long-term proliferation of mouse primordial germ cells in culture. Nature 359, 550-551. 10.1038/359550a0 [DOI] [PubMed] [Google Scholar]
- Ruark E., Seal S., McDonald H., Zhang F., Elliot A., Lau K., Perdeaux E., Rapley E., Eeles R., Peto J. et al. (2013). Identification of nine new susceptibility loci for testicular cancer, including variants near DAZL and PRDM14. Nat. Genet. 45, 686-689. 10.1038/ng.2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba R., Kato Y. and Saga Y. (2014). NANOS2 promotes male germ cell development independent of meiosis suppression. Dev. Biol. 385, 32-40. 10.1016/j.ydbio.2013.10.018 [DOI] [PubMed] [Google Scholar]
- Saiti D. and Lacham-Kaplan O. (2007). Mouse germ cell development in-vivo and in-vitro. Biomark. Insights 2, 241 10.1177/117727190700200024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M. and Miyauchi H. (2016). Gametogenesis from Pluripotent Stem Cells. Cell Stem Cell 18, 721-735. 10.1016/j.stem.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Saitou M. and Yamaji M. (2010). Germ cell specification in mice: signaling, transcription regulation, and epigenetic consequences. Reproduction 139, 931-942. 10.1530/REP-10-0043 [DOI] [PubMed] [Google Scholar]
- Schemmer J., Araúzo-Bravo M. J., Haas N., Schäfer S., Weber S. N., Becker A., Eckert D., Zimmer A., Nettersheim D. and Schorle H. (2013). Transcription factor TFAP2C regulates major programs required for murine fetal germ cell maintenance and haploinsufficiency predisposes to teratomas in male mice. PloS ONE 8, e71113 10.1371/journal.pone.0071113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki Y., Hayashi K., Itoh K., Mizugaki M., Saitou M. and Matsui Y. (2005). Extensive and orderly reprogramming of genome-wide chromatin modifications associated with specification and early development of germ cells in mice. Dev. Biol. 278, 440-458. 10.1016/j.ydbio.2004.11.025 [DOI] [PubMed] [Google Scholar]
- Shoji M., Tanaka T., Hosokawa M., Reuter M., Stark A., Kato Y., Kondoh G., Okawa K., Chujo T., Suzuki T. et al. (2009). The TDRD9-MIWI2 Complex Is Essential for piRNA-Mediated Retrotransposon Silencing in the Mouse Male Germline. Dev. Cell 17, 775-787. 10.1016/j.devcel.2009.10.012 [DOI] [PubMed] [Google Scholar]
- Solter D. (2006). From teratocarcinomas to embryonic stem cells and beyond: a history of embryonic stem cell research. Nat. Rev. Genet. 7, 319 10.1038/nrg1827 [DOI] [PubMed] [Google Scholar]
- Souquet B., Tourpin S., Messiaen S., Moison D., Habert R. and Livera G. (2012). Nodal signaling regulates the entry into meiosis in fetal germ cells. Endocrinology 153, 2466-2473. 10.1210/en.2011-2056 [DOI] [PubMed] [Google Scholar]
- Sperger J. M., Chen X., Draper J. S., Antosiewicz J. E., Chon C. H., Jones S. B., Brooks J. D., Andrews P. W., Brown P. O. and Thomson J. A. (2003). Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc. Natl. Acad. Sci. USA 100, 13350-13355. 10.1073/pnas.2235735100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller C. M. and Bowles J. (2015). Sex determination in mammalian germ cells. Asian J. Androl. 17, 427-432. 10.4103/1008-682X.150037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller C. M., Feng C.-W., Jackson A., Gillis A. J. M., Rolland A. D., Looijenga L. H. J., Koopman P. and Bowles J. (2012). Endogenous Nodal signaling regulates germ cell potency during mammalian testis development. Development 139, 4123-4132. 10.1242/dev.083006 [DOI] [PubMed] [Google Scholar]
- Stevens L. C. (1962). Testicular teratomas in fetal mice. J. Natl. Cancer Inst. 28, 247-267. [PubMed] [Google Scholar]
- Stevens L. C. (1966). Development of resistance to teratocarcinogenesis by primordial germ cells in mice. J. Natl. Cancer Inst. 37, 859-867. [PubMed] [Google Scholar]
- Stevens L. C. (1967a). The biology of teratomas. Adv. Morph. 6, 1-31. 10.1016/B978-1-4831-9953-5.50005-6 [DOI] [PubMed] [Google Scholar]
- Stevens L. C. (1967b). Origin of testicular teratomas from primordial germ cells in mice. J. Natl. Cancer Inst. 38, 549-552. [PubMed] [Google Scholar]
- Stevens L. and Hummel K. (1957). A description of spontaneous congenital testicular teratomas in strain 129 mice. J. Natl. Cancer Inst. 18, 719-747. [PubMed] [Google Scholar]
- Surani M. A. (2001). Reprogramming of genome function through epigenetic inheritance. Nature 414, 122-128. 10.1038/35102186 [DOI] [PubMed] [Google Scholar]
- Suzuki A. and Saga Y. (2008). Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev. 22, 430-435. 10.1101/gad.1612708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Tsuda M. and Saga Y. (2007). Functional redundancy among Nanos proteins and a distinct role of Nanos2 during male germ cell development. Development 134, 77-83. 10.1242/dev.02697 [DOI] [PubMed] [Google Scholar]
- Suzuki A., Igarashi K., Aisaki K., Kanno J. and Saga Y. (2010). NANOS2 interacts with the CCR4-NOT deadenylation complex and leads to suppression of specific RNAs. Proc. Natl. Acad. Sci. USA 107, 3594-3599. 10.1073/pnas.0908664107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Niimi Y., Shinmyozu K., Zhou Z., Kiso M. and Saga Y. (2016). Dead end1 is an essential partner of NANOS2 for selective binding of target RNAs in male germ cell development. EMBO Rep. 17, 37-46. 10.15252/embr.201540828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafranski P., Karolak J. A., Lanza D., Gajęcka M., Heaney J. and Stankiewicz P. (2017). CRISPR/Cas9-mediated deletion of lncRNA Gm26878 in the distant Foxf1 enhancer region. Mamm. Genome 28, 275-282. 10.1007/s00335-017-9686-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar P. J., Chenoweth J. G., Brook F. A., Davies T. J., Evans E. P., Mack D. L., Gardner R. L. and McKay R. D. G. (2007). New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196-199. 10.1038/nature05972 [DOI] [PubMed] [Google Scholar]
- Tsuda M., Sasaoka Y., Kiso M., Abe K., Haraguchi S., Kobayashi S. and Saga Y. (2003). Conserved role of nanos proteins in germ cell development. Science 301, 1239-1241. 10.1126/science.1085222 [DOI] [PubMed] [Google Scholar]
- Tsuda M., Kiso M. and Saga Y. (2006). Implication of nanos2-3'UTR in the expression and function of nanos2. Mech. Dev. 123, 440-449. 10.1016/j.mod.2006.04.002 [DOI] [PubMed] [Google Scholar]
- Weinberger L., Ayyash M., Novershtern N. and Hanna J. H. (2016). Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat. Rev. Mol. Cell Biol. 17, 155-169. 10.1038/nrm.2015.28 [DOI] [PubMed] [Google Scholar]
- Welling M., Chen H.-H., Muñoz J., Musheev M. U., Kester L., Junker J. P., Mischerikow N., Arbab M., Kuijk E. and Silberstein L. (2015). DAZL regulates Tet1 translation in murine embryonic stem cells. EMBO Rep. 16, 791-802. 10.15252/embr.201540538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter D., MacArthur J., Morales J., Burdett T., Hall P., Junkins H., Klemm A., Flicek P., Manolio T., Hindorff L. et al. (2014). The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 42, D1001-D1006. 10.1093/nar/gkt1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western P. S., van den Bergen J. A., Miles D. C. and Sinclair A. H. (2010). Male fetal germ cell differentiation involves complex repression of the regulatory network controlling pluripotency. FASEB J. 24, 3026-3035. 10.1096/fj.09-151555 [DOI] [PubMed] [Google Scholar]
- Wu Q., Fukuda K., Weinstein M., Graff J. M. and Saga Y. (2015). SMAD2 and p38 signaling pathways act in concert to determine XY primordial germ cell fate in mice. Development 142, 575-586. 10.1242/dev.119446 [DOI] [PubMed] [Google Scholar]
- Xu X., Pantakani D. V. K., Lührig S., Tan X., Khromov T., Nolte J., Dressel R., Zechner U. and Engel W. (2011). Stage-Specific Germ-Cell Marker Genes Are Expressed in All Mouse Pluripotent Cell Types and Emerge Early during Induced Pluripotency. PloS ONE 6, e22413 10.1371/journal.pone.0022413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Kimura H., Tada M., Nakatsuji N. and Tada T. (2005). Nanog expression in mouse germ cell development. Gene Expr. Patterns 5, 639-646. 10.1016/j.modgep.2005.03.001 [DOI] [PubMed] [Google Scholar]
- Yamaji M., Ueda J., Hayashi K., Ohta H., Yabuta Y., Kurimoto K., Nakato R., Yamada Y., Shirahige K. and Saitou M. (2013). PRDM14 ensures naive pluripotency through dual regulation of signaling and epigenetic pathways in mouse embryonic stem cells. Cell Stem Cell 12, 368-382. 10.1016/j.stem.2012.12.012 [DOI] [PubMed] [Google Scholar]
- Yeom Y. I., Fuhrmann G., Ovitt C. E., Brehm A., Ohbo K., Gross M., Hubner K. and Scholer H. R. (1996). Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development 122, 881-894. [DOI] [PubMed] [Google Scholar]
- Yoshimizu T., Sugiyama N., De Felice M., Yeom Y. I., Ohbo K., Masuko K., Obinata M., Abe K., Scholer H. R. and Matsui Y. (1999). Germline-specific expression of the Oct-4/green fluorescent protein (GFP) transgene in mice. Dev. Growth Differ. 41, 675-684. 10.1046/j.1440-169x.1999.00474.x [DOI] [PubMed] [Google Scholar]