Abstract
Antisocial behavior (AB), including violence, criminality, and substance abuse, is often linked to deficits in emotion processing, reward-related learning, and inhibitory control, as well as their associated neural networks. To better understand these deficits, the structural connections between brain regions implicated in AB can be examined using diffusion tensor imaging (DTI), which assesses white matter microstructure. Prior studies have identified differences in white matter microstructure of the uncinate fasciculus (UF), primarily within offender samples. However, few studies have looked beyond the UF or determined whether these relationships are present dimensionally across the range of AB and callous-unemotional (CU) traits. In the current study, we examined associations between AB and white matter microstructure from major fiber tracts, including the UF. Further, we explored whether these associations were specific to individuals high on CU traits. Within a relatively large community sample of young adult men from low-income, urban families (N = 178), we found no direct relations between dimensional, self-report measures of either AB or CU traits and white matter microstructure. However, we found significant associations between AB and white matter microstructure of several tracts only for those with high co-occurring levels of CU traits. In general, these associations did not differ according to race, socioeconomic status, or comorbid psychiatric symptoms. The current results suggest a unique neural profile of severe AB in combination with CU traits, characterized by widespread differences in white matter microstructure, which differs from either AB or CU traits in isolation and is not specific to hypothesized tracts (i.e., the UF).
Keywords: Antisocial behavior, Callous-unemotional traits, Diffusion tensor imaging, Income, Race
Highlights
-
•
Antisocial Behavior (AB; aggression, rule breaking) is a major public health concern
-
•
AB and Callous-unemotional (CU) traits may emerge from disrupted neural connections
-
•
AB was associated with white matter microstructure only at high levels of CU traits
-
•
Associations did not differ according to race, income, or comorbid psychopathology
-
•
Results suggest a unique neural profile for severe AB when combined with CU traits
1. Introduction
Antisocial behavior (AB) is characterized by a variety of harmful acts, including violence, aggression, criminality, and substance abuse. AB confers significant social and financial costs for individuals and society as a whole, with the cost of crime in the United States estimated at $3.2 trillion each year (Anderson, 2012). As such, AB is a major health concern, particularly given lifetime prevalence rates of 4.3% for the clinical diagnosis of AB (Antisocial Personality Disorder), and lifetime prevalence rates of subclinical, but significant AB of 20.3% (Goldstein et al., 2017). Accordingly, research has focused on identifying risk factors that are linked to AB to inform prevention and treatment efforts.
1.1. Neural correlates of antisocial behavior
Recent research has focused on identifying the neural mechanisms leading to AB. fMRI studies have examined functioning in brain areas involved in processes that are disrupted in AB, including emotion processing and regulation, reward approach, and inhibitory control (Raine, 2018; Waller et al., 2015a). These studies have suggested that AB is associated with differences in amygdala reactivity during emotion processing (e.g., Coccaro et al., 2007; Hyde et al., 2014; Hyde et al., 2016) and orbito-frontal/ventral-medial prefrontal cortex (OFC/vmPFC) reactivity during reward-based and reinforcement learning (Blair, 2017; Raine, 2018). Moreover, AB has been related to reduced functional connectivity between the same regions (e.g., amygdala-vmPFC) during socio-emotional processing (e.g., Contreras-Rodríguez et al., 2015; Decety et al., 2013; Marsh et al., 2011; Waller et al., 2018) and at rest (e.g., Motzkin et al., 2011; Philippi et al., 2015). These findings suggest that abnormalities in both activation of and connectivity between the amygdala and prefrontal regions may contribute to the various socioemotional, reward, and impulse-control deficits associated with AB (Raine, 2018). Indeed, in a meta-analysis of 43 studies, AB was associated with reduced function and structure of prefrontal regions (e.g., orbito-frontal cortex, anterior cingulate and dorsolateral PFC) (Yang and Raine, 2009). Interestingly, associations with amygdala reactivity have been less consistent, with evidence for both reduced and increased amygdala structure and function within AB (Raine, 2018). These results have informed theories positing that disruption in the amygdala and PFC and their connections are key to the etiology of severe AB (Blair, 2007). Thus, building on functional activation and connectivity studies, one important line of research has begun to examine how the structural connections between these regions may be related to AB.
1.2. White matter structure and antisocial behavior
1.2.1. Antisocial behavior and the uncinate fasciculus
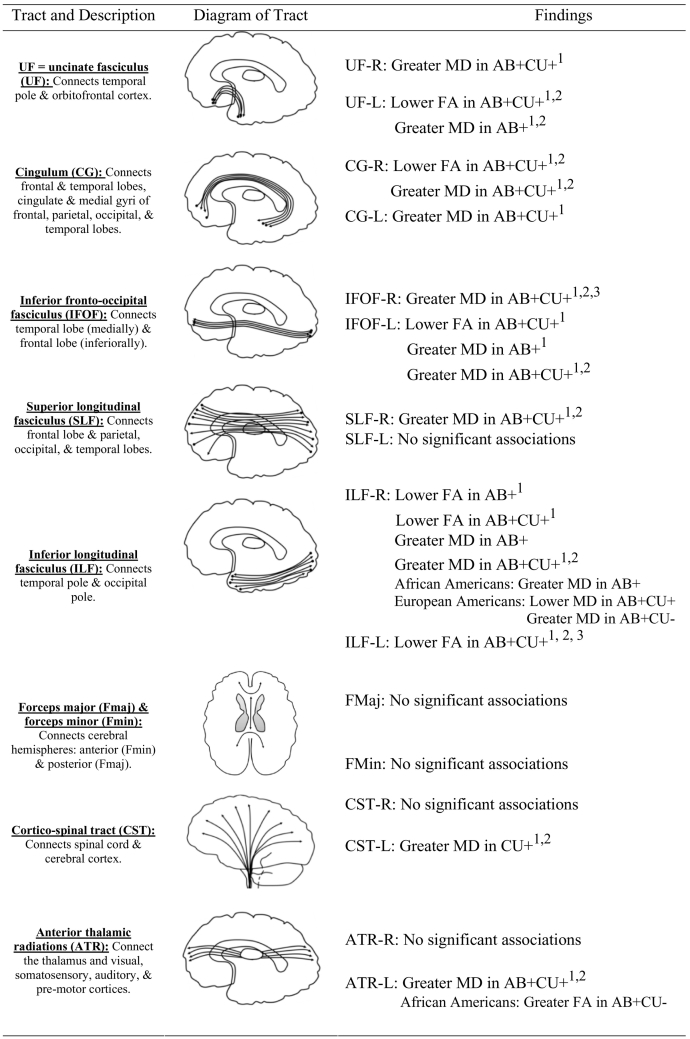
To examine the structural connections in the brain, the microstructural properties of white matter tracts can be examined using non-invasive diffusion tension imaging (DTI). DTI measures the restriction of water molecule movement within each voxel in the brain (i.e., water diffusion; Beaulieu, 2002) and thus has been interpreted as indexing the “integrity” of white matter tracts (Soares et al., 2013). Due to the focus on areas of the temporal lobe (the amygdala) and medial prefrontal cortex (vmPFC) in neuroetiologic theories of AB (Blair, 2007), existing DTI studies of AB have focused primarily on the uncinated fasciculus (UF), a major fiber tract connecting these areas of the brain (Leng et al., 2016). Consistent with this focus, a recent systematic review of existing studies of AB using DTI approaches (n = 10 adult studies; Waller et al., 2017a) found substantial evidence for disrupted white matter microstructure in the UF being associated with AB across different populations (i.e., prison, community). Although the associations were less consistent, the review also identified several youth studies that found associations between AB and white matter microstructure in the UF (e.g., Breeden et al., 2015; Sarkar et al., 2013; Zhang et al., 2014). Importantly, this review found that most studies specifically targeted the UF via a region of interest approach, with little attention to whether white matter may be disrupted in other tracts. Notably, in studies that examine other white matter tracts both within the review (e.g., Hoppenbrouwers et al., 2013; Lindner et al., 2016) and in more recent studies (Bolhuis et al., 2019; Jiang et al., 2017), there is evidence of widespread white matter disruption in AB (i.e., disruption across multiple tracts). For example, AB has been associated with disruptions in white matter of association fibers, which include the UF and connect the lower portions of the brain and spinal cord (e.g., cingulum CG; inferior fronto-occipital fasiculus IFOF; Bolhuis et al., 2019; Hoppenbrouwers et al., 2013; Jiang et al., 2017; Lindner et al., 2016; Mori et al., 2005). Additional tracts implicated in AB include commissural tracts, which connect the two brain hemispheres (e.g., forceps minor and major; Lindner et al., 2016), and projection tracts, which connect the cortex with lower portions of the brain and spinal cord (e.g., anterior thalamic radiation, ATR; Hoppenbrouwers et al., 2013; corticospinal tract, CST; Pape et al., 2015). However, as so few studies have examined multiple tracts, additional research is needed to examine the association between AB and white matter at a more global level to determine disruption of white matter and if evident, its specificity (i.e., the UF versus widespread disruptions).
1.2.2. Sample type and consideration of callous-unemotional traits
Additionally, the sample types commonly used within the DTI and AB literature add complexity to associations between AB and white matter microstructure. The recent systematic review found that 70% of the adult studies (n = 10 studies total) were offender or clinical populations within primarily smaller sample sizes (Ns = 18–147; 70% of Ns < 50 participants). However, previous research suggests that AB is dimensional in nature (Kreuger et al., 2007; Olson-Ayala and Patrick, 2018), highlighting the need to test these relationships across the distribution of AB, particularly in community samples with levels of AB that vary from low to clinical. Moreover, in forensic samples, AB may be confounded with callous-unemotional (CU) or psychopathic traits (Frick and Myers, 2017; Patrick, 2007). CU traits are a core affective and interpersonal component of psychopathic traits and refer to a lack of empathy, shallow affect, and remorselessness (Frick et al., 2014; Hare and Neumann, 2008). High levels of co-occurring CU traits are associated with more persistent and severe trajectories of AB (Frick et al., 2014). As such, white-matter microstructure abnormalities may be uniquely associated with more severe AB marked by co-occurring CU traits, as opposed to AB more broadly. However, the review noted that many prior studies did not account for co-occurring CU or psychopathic traits (Waller et al., 2017a).
Importantly, neuroimaging studies have begun to show that associations between neural correlates and AB differ depending on the presence of psychopathic or CU traits. Task-based fMRI studies have found that AB without co-occurring CU traits is associated with hypersensitivity to threat (e.g., increased amygdala reactivity to threat), whereas AB with co-occurring psychopathic or CU traits is associated with diminished responsivity to interpersonal distress (e.g., decreased amygdala reactivity to distress or harm) (Hyde et al., 2013; Viding and McCrory, 2018). Moreover, studies examining connectivity during resting-state MRI (e.g., Philippi et al., 2015) and DTI (e.g., Breeden et al., 2015; Motzkin et al., 2011; Vermeij et al., 2018) have also found that the level of psychopathic or CU traits moderated the relationship between AB and brain structure and function. Associations between AB and white matter microstructure may therefore be moderated by co-occurring psychopathic or CU traits such that only individuals with significant levels of both AB and CU traits demonstrate widespread white matter abnormalities. Thus, it is still unclear whether previous findings are the result of an interactive effect between AB and CU traits, and whether these associations are also present within adult community samples with a full dimensional range of AB and CU traits.
1.2.3. Psychiatric and demographic confounds
In addition to CU traits, there are other demographic factors and psychiatric comorbidities that could impact white matter microstructure, which have rarely been explored in prior studies of AB. For example, age and other psychiatric illnesses have been linked to differences in white matter microstructure (e.g., Baker et al., 2013; Daniels et al., 2013; Westlye et al., 2009; White et al., 2008), but few studies have examined these questions in a high-risk community sample that include a range of psychopathology that can be accounted for in analyses. Further, most previous studies have focused on middle-class European American samples. Unfortunately, studies have found that African Americans are disproportionately exposed to low-income, dangerous neighborhoods (Leventhal & Brooks-Gunn, 2000). Increased risk of exposure to environmental stressors (i.e., discrimination, poor nutrition, exposure to toxicants, material hardship) that are associated with lower socioeconomic status have been shown to impact brain development (Mays et al., 2007; Raine, 2002). Thus, different socioeconomic backgrounds could result in different neurodevelopmental trajectories toward AB (Chiang et al., 2011; Raine, 2018; Shaw and Shelleby, 2014). Moreover, studies that have examined behavioral phenotypes (e.g., fear-potentiated startle; Baskin-Sommers et al., 2011) and functional brain correlates of AB (e.g., amygdala reactivity to distress and threat; Hyde et al., 2016) have found that many well-established findings do not replicate in samples of African Americans. Indeed, the sole study of AB and DTI to examine race as a moderator found racial differences in white matter microstructure (Decety et al., 2015). Taken together, additional research is therefore necessary in larger, racially and economically diverse community samples across the full dimension of AB, outside of forensic settings, while also examining the specificity of associations across tracts and accounting for the co-occurrence of CU traits, demographic factors, and psychiatric comorbidities.
1.3. Current study
The current study examined associations between AB and fractional anisotropy (FA) and mean diffusivity (MD) values derived from DTI from major white matter tracts previously implicated in AB (Waller et al., 2017a). FA, the degree to which water in an individual voxel diffuses coherently along a single orientation, has most commonly been used in previous studies of AB and DTI (Beaulieu, 2002; Chang et al., 2017). However, changes in MD, the average diffusion in a voxel regardless of orientation, may further clarify the association between AB and white matter abnormalities. In particular, FA is sensitive to factors contributing to directionally coherent diffusion (e.g., density, caliber, and myelination of parallel axons), whereas MD is sensitive to factors contributing to directionally coherent diffusion (e.g., presence or absence of neural tissue) (Beaulieu, 2002; Chang et al., 2017). We extracted FA and MD values using streamline tractography, which segment fiber pathways at the individual subject level by using the primary direction of diffusion in each voxel (Soares et al., 2013). Streamline tractography is thought to be more sensitive than other approaches (e.g., voxel-based methods; Lebel et al., 2017) because it better accounts for inter-subject variability in tract features (i.e., tract shape, location; Lebel et al., 2017).
We utilized a low-income male sample, considered to be at heightened risk for AB based on gender, familial socioeconomic status, and urbanicity (Hyde et al., 2016). Thus, we examined our hypotheses in a community sample enriched for higher levels of AB, providing a wide range of variability in AB and CU traits (Beck and Shaw, 2005; Gard et al., 2017; Hyde et al., 2016; Waller et al., 2017b). Our primary goal was to test whether AB was associated with greater rates of diffusion (i.e., greater FA and lower MD) within white matter tracts and whether these associations were stronger at high levels of CU traits. Our second goal was to establish the specificity of effects, by examining the impact of potential demographic and psychiatric confounds, including by testing moderation by race, and controlling for income and co-morbid psychopathology.
2. Methods
2.1. Participants
178 participants were drawn from the Pitt Mother & Child Project, an ongoing longitudinal study of 310 low-income, ethnically diverse boys and their families (Shaw et al., 2012). Participants were recruited from Allegheny County Women, Infants, and Children Nutritional Supplement Clinics in 1991 and 1992 when the boys were 6 to 17 months of age (Shaw et al., 2003; Shaw et al., 2012). Within the full sample, the mean per capita income was $241 per month ($2892 per year) with a mean Hollingshead socioeconomic status score of 24.5, indicative of a working class to impoverished sample. Children and mothers were seen almost yearly from 1.5 to 20 years of age in the laboratory and/or home with assessments that included questionnaires and psychiatric interviews, and at 20 years of age, a magnetic resonance imaging (MRI) scanning session (Shaw et al., 2012). The MRI component introduced several sources of data loss (Supplemental Table 1) resulting in 185 men with available DTI data. Of the 185 participants, one participant was excluded because of a diagnosis of autism spectrum disorder. Assessments included questionnaires Additionally, six participants were missing data for a variable of interest (i.e., antisocial behavior). Of the included participants (n = 178), most participants self-reported their race as either European American (n = 93, 52.2%) or African American (n = 69, 38.8%), and a small proportion (n = 16, 9%) of participants self-reported as “other”. The participants were scanned in a narrow age range (Range = 19–21 years old, M = 19.52 years, SD = 0.55) and varied in self-reported yearly income, though with a relatively low mean income (Range: 0 - $120,000, M = $15,103, SD = $19,414). Consistent with a community sample enriched for adversity, a proportion of participants met criteria for other psychiatric disorders (1.1% generalized anxiety disorder; 1.1% post-traumatic stress disorder; 6% specific phobia; 5.4% social phobia; 2.2% panic disorder; 1.6% bipolar disorder; 13% major depressive disorder; 13% substance use disorder). Additionally, some participants (14.1%, n = 26) reported that they were currently taking medications, although fewer than 4% reported taking medications specifically to treat psychopathology. At the age 20 assessment, within the current sample, 15.7% of participants (n = 28) had been charged with a non-violent crime, 10.1% of participants (n = 18) had been charged with a violent crime, and 12.9% of participants (n = 23) had been charged with a violent crime before the age of 18. Thus, in contrast to prior studies of AB and DTI in criminal populations, the current sample reflects a community sample with enrichment of and substantial range of AB.
2.2. Measures
The measures used in the current study were all administered at the age 20 assessment.
2.2.1. Self-reported antisocial behavior
To assess AB, the Self-Report of Delinquency Questionnaire (SRD; Elliott et al., 1985) was administered containing 53 age-appropriate items (e.g., vandalism, stealing, physical aggression, drug use; Hyde et al., 2016). These items were summed to form a dimensional measure of AB and demonstrated good internal consistency (α = 0.88).
2.2.2. Callous-unemotional traits
CU traits were assessed using a measure that combined the CU traits from the Antisocial Process Screening Device (APSD; Frick and Hare, 2001; e.g., ‘concern about the feelings of others’, ‘feel guilty after wrongdoing’) and 10 inversely-coded prosocial items from the self-report version of the Child and Adolescent Disposition Scale that assessed concern for others, helping, and sharing (CADS; Lahey et al., 2010; e.g., ‘help others when they get hurt’, ‘share things with others’). The measure was developed to address widely replicated problems of low internal consistency of the APSD CU scale alone (Kotler and McMahon, 2010), which was indeed marginal in this sample (α = 0.61). In a prior study also using this sample at age 20, we showed that the items from the APSD CU scale and CADS items formed a single construct, which demonstrated acceptable internal consistency (α = 0.83; Waller et al., 2015b).
2.3. DTI preprocessing
DTI images were acquired using a research-dedicated Siemens 3-T Tim Trio. Diffusion sensitizing gradients were applied along 61 diffusion-weighted directions (b = 1000 s/mm2). Seven non-diffusion weighted volumes (b = 0 s/mm2) were also collected. Images were collected with the following parameters: repetition time = 8400 ms, echo time = 91 ms, flip angle = 90° degrees, slice thickness = 2 mm, 64 slices. The raw diffusion-weighted images were denoised (Veraart et al., 2016) and then corrected for motion, eddy-current, and signal dropout artifacts using eddy in FSL (version 5.0.11; Andersson and Sotiropoulos, 2016). Intra-volume motion was corrected using slice-to-volume correction (Andersson et al., 2017). Slices with an average intensity at least four standard deviations lower than predicted by eddy's Gaussian process model were considered outliers, and were replaced with model predictions (Andersson et al., 2016). Across participants, 0.02% to 3.68% of slices were replaced (median = 0.37%). Simulations suggest that up to 10% of slices can be replaced in this manner without producing deleterious effects (Andersson et al., 2016). Detailed visual inspection of the ten images with the most slices replaced confirmed that no abnormal artifacts had been introduced.
2.4. DTI tractography
After pre-processing, DWI images were analyzed using mrDiffusion, part of the open-source mrVista package (https://white.stanford.edu/software/). Whole brain fiber tracking was performed on AC–PC aligned tensor maps using a deterministic streamline tracking algorithm (Basser et al., 2000). Automated path tracing proceeded until the FA fell below 0.15 or until the minimum angle between the current and previous path segments was >30°. The mrDiffusion package was then used to conduct automated fiber classification of major fiber tracts included in the Johns Hopkins University White Matter Tractography Atlas (Mori et al., 2005) (see Thomason et al., 2010). Mean FA and MD values were computed for the major fiber tracts outlined in the white matter atlas that have also been previously examined in DTI studies of AB (i.e., the UF, cingulum CG, inferior fronto-occipital fasciculus IFOF, superior longitudinal fasciculus SLF, inferior longitudinal fasciculus ILF, forceps major, forceps minor, anterior thalamic radiation, and corticospinal tract). Of note, in the current study we do not include radial diffusivity (RD) and axial diffusivity (AD), which are additional indices of white matter microstructure and have been thought to contribute to FA values (Winklewski et al., 2018). AD and RD produce biased results due to their estimation method (e.g., utilization of a sorting procedure that does not take into account all three eigenvalues of the diffusion tensor), which is highly susceptible to noise and can result in inflated AD values and deflated RD values (Basser and Pierpaoli, 2011; Pierpaoli and Basser, 1996; Wheeler-Kingshott and Cercignani, 2009). FA and MD are not susceptible to the same biases due to differences in their estimation (e.g., incorporate all three eigenvalues) and have therefore been proposed as more reliable measures of white matter microstructure (Basser and Pierpaoli, 2011; Pierpaoli and Basser, 1996); as such, we only include FA and MD in our analyses.
2.5. Statistical analyses
All analyses were conducted in MPlus version 7.3 (Muthén and Muthén, 2014) using robust maximum likelihood estimation to account for non-normality and missing data (MLR; Yuan and Bentler, 2000). All models were fully saturated. We began by computing bivariate associations among AB, CU traits, and resulting FA and MD of all tracts. Next, we examined a series of three models to isolate the specificity of the association between AB and CU traits and FA and MD values. To test our primary hypotheses regarding the association between structural connectivity and AB being contingent on level of CU traits, all models included main and interactive effects of AB and CU traits as independent variables and estimates for either FA and MD of each fiber tract as dependent variables (with both left and right tracts as correlated dependent variables), controlling for participant age and race (see Supplemental Fig. 1). Models included the white matter indices of left and right tracts of each fiber, which are typically highly correlated (and were highly correlated in this sample), to reduce the number of comparisons when possible. By including both hemispheres in a single path model, we are able to model the covariance between the two measures to decrease multiple testing concerns. Age was included as a covariate in all models based on previous work demonstrating significant alterations in white matter tracts over development, particularly during early adulthood (Westlye et al., 2009). To explore any significant interactions between AB and CU traits, we used an online tool to examine simple slopes at the mean and 1 SD below (low) and 1 SD above (high) the mean of CU traits (Aiken et al., 1991), as well as regions of significance (Preacher et al., 2006). When interaction terms were significant bilaterally, we probed the interaction for the tract with the larger effect size. Note that though all our analyses were planned with specific hypotheses, we additionally highlight and focus on associations that meet a strict conservative threshold to account for the eighteen models (i.e., nine tracts, including both right and left tracts within the same model) that were tested for FA and MD (i.e., Bonferroni-correction 0.05/18 = p < .003) (Table 1; Table 2; Table 3Table 4 ). Additionally, as a second, less conservative approach that is instead not reliant on p-values nor distributional assumptions to determine significance, we also provide 95% confidence intervals from models conducted using bootstrapping (5000 draws; Supplemental Table 4). To test whether associations with structural connectivity were different among African American versus European American participants, also we computed models that included two- and three-way interaction terms between AB, CU traits, and participant race, which we present in Supplemental Materials. To understand the nature of any significant three-way interaction terms, we used the GROUPING command in Mplus version 7.3 (Muthén and Muthén, 2014) to examine associations within African American and European American participants separately.
Table 1.
Regression analyses of fractional anisotropy of white matter tracts, antisocial behavior, and callous-unemotional traits in a community sample of young adult men.
| Model |
Tract |
Model 1: no covariates |
Model 2: with income |
Model 3: with income & co-morbid psychopathology |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AB | CU | AB x CU | AB | CU | AB x CU | AB | CU | AB x CU | ||
| 1 | UF-R | −0.02 | 0.08 | −0.12+ | −0.02 | 0.08 | −0.12+ | 0.04 | 0.08 | −0.10+ |
| UF-L | −0.11 | 0.02 | −0.13⁎ | −0.11 | 0.02 | −0.13⁎ | −0.10 | 0.02 | −0.13⁎ | |
| 2 | CG-R | −0.07 | −0.00 | −0.19⁎ | −0.07 | −0.00 | −0.19⁎ | −0.03 | 0.01 | −0.18⁎ |
| CG-L | −0.03 | 0.01 | −0.05 | −0.02 | 0.00 | −0.04 | −0.00 | 0.00 | −0.04 | |
| 3 | IFOF-R | −0.13+ | 0.06 | −0.12+ | −0.13+ | 0.06 | −0.12+ | −0.15+ | 0.09 | −0.12+ |
| IFOF-L | −0.10 | 0.12+ | −0.14⁎ | −0.10 | 0.12+ | −0.14⁎ | −0.08 | 0.11 | −0.14⁎ | |
| 4 | SLF-R | −0.07 | −0.01 | −0.09 | −0.08 | −0.01 | −0.08 | −0.06 | −0.01 | −0.08 |
| SLF-L | −0.08 | 0.05 | −0.09 | −0.08 | 0.05 | −0.09 | −0.11 | 0.04 | −0.09 | |
| 5 | ILF-R | −0.16⁎ | 0.06 | −0.12⁎ | −0.16⁎ | 0.06 | −0.12⁎ | −0.12 | 0.08 | −0.11+ |
| ILF-L | −0.12 | 0.05 | −0.18⁎⁎ | −0.13+ | 0.06 | −0.18⁎⁎ | −0.11 | 0.08 | −0.19⁎⁎ | |
| 6 | FMaj | 0.03 | 0.08 | 0.04 | 0.02 | 0.08 | 0.04 | 0.05 | 0.08 | 0.05 |
| 7 | FMin | −0.04 | 0.04 | 0.03 | −0.02 | 0.04 | 0.03 | 0.00 | 0.05 | 0.03 |
| 8 | CST-R | −0.10 | 0.03 | −0.09 | −0.10 | 0.03 | −0.09 | −0.16⁎ | 0.04 | −0.11 |
| CST-L | −0.10 | −0.05 | −0.02 | −0.09 | −0.05 | −0.02 | −0.12 | −0.02 | −0.02 | |
| 9 | ATR-R | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.01 | 0.02 |
| ATR-L | −0.09 | −0.09 | −0.14 | −0.07 | −0.01 | −0.14 | −0.13 | 0.04 | −0.16+ | |
AB = antisocial behavior; CU = callous-unemotional traits. UF-R = right uncinate fasciculus; UF-L = left uncinate fasciculus; CG-R = right cingulate gyrus; CG-L = left cingulate gyrus; IFOF-R = right inferior occipital fasciculus; IFOF-L = left inferior occipital fasciculus; SLF-R = right superior occipital fasciculus; SLF-L = left superior occipital fasciculus; ILF-R = right inferior longitudinal fasciculus; ILF-L = left inferior longitudinal fasciculus; FMaj = forceps major; FMin = forceps minor; CST-R = right corticospinal tract; CST-L = left corticospinal tract; ATR-R = right anterior thalamic radiation; ATR-L = left anterior thalamic radiation. All models controlled for participant age and race. All models included scores on AB, CU traits, and an interaction term of CU traits x AB. For bilateral tracts, each model included both right and left tracts (i.e., both ATR-L and ATR-R were included in one model). Model 2: beta weights of models that also included yearly income as a covariate. Model 3: beta weights of models that also included scores of substance dependence symptoms, social phobia symptoms, depressive symptoms, anxiety symptoms, and post-traumatic stress disorder symptoms.
p < .10.
p < .05.
p < .01.
Table 2.
Regression analyses of mean diffusivity of white matter tracts, antisocial behavior, and callous-unemotional traits in a community sample of young adult men.
| Model |
Tract |
Model 1: no covariates |
Model 2: with income |
Model 3: with income & co-morbid psychopathology |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AB | CU | AB x CU | AB | CU | AB x CU | AB | CU | AB x CU | ||
| 1 | UF-R | 0.06 | 0.05 | 0.12⁎ | 0.04 | 0.05 | 0.12⁎ | 0.00 | 0.06 | 0.10+ |
| UF-L | 0.15⁎ | −0.05 | 0.04 | 0.14⁎ | −0.04 | 0.04 | 0.15⁎ | −0.05 | 0.03 | |
| 2 | CG-R | 0.09 | 0.08 | 0.18⁎⁎ | 0.07 | 0.09 | 0.17⁎ | 0.02 | 0.10 | 0.16⁎ |
| CG-L | 0.04 | 0.08 | 0.13⁎ | 0.02 | 0.08 | 0.13⁎ | 0.00 | 0.09 | 0.11+ | |
| 3 | IFOF-R | 0.07 | −0.01 | 0.21⁎⁎,a | 0.06 | −0.00 | 0.21⁎⁎,a | 0.02 | −0.00 | 0.20⁎⁎,a |
| IFOF-L | 0.14⁎ | −0.08 | 0.18⁎⁎ | 0.13+ | −0.08 | 0.18⁎⁎ | 0.10 | −0.07 | 0.17⁎⁎ | |
| 4 | SLF-R | 0.11+ | 0.02 | 0.14⁎ | 0.10 | 0.03 | 0.14⁎ | 0.10 | 0.05 | 0.13⁎ |
| SLF-L | 0.13+ | −0.04 | 0.07 | 0.11 | −0.03 | 0.06 | 0.10 | −0.03 | 0.05 | |
| 5 | ILF-R | 0.15⁎ | −0.02 | 0.22⁎⁎ | 0.14+ | −0.01 | 0.22⁎⁎ | 0.07 | −0.00 | 0.20⁎⁎ |
| ILF-L | 0.09 | 0.04 | 0.11 | 0.08 | 0.04 | 0.11 | 0.06 | 0.04 | 0.10 | |
| 6 | FMaj | −0.01 | −0.00 | 0.01 | −0.01 | −0.01 | 0.02 | −0.07 | −0.00 | 0.00 |
| 7 | FMin | 0.03 | −0.03 | 0.10 | −0.01 | −0.03 | 0.10 | −0.03 | −0.02 | 0.09 |
| 8 | CST-R | 0.05 | 0.06 | 0.11+ | 0.05 | 0.06 | 0.11 | 0.04 | 0.07 | 0.10 |
| CST-L | 0.04 | 0.16⁎ | 0.13+ | 0.02 | 0.17⁎ | 0.13+ | −0.03 | 0.18⁎⁎ | 0.11+ | |
| 9 | ATR-R | 0.06 | 0.01 | 0.13+ | 0.04 | 0.02 | 0.12 | −0.01 | 0.03 | 0.10 |
| ATR-L | 0.07 | 0.01 | 0.23⁎ | 0.06 | 0.01 | 0.23⁎ | 0.08 | 0.01 | 0.22⁎⁎ | |
Association significant at conservative Bonferroni correction standard accounting for multiple comparisons (0.05 / 18 models = 0.003). AB = antisocial behavior; CU = callous-unemotional traits. UF-R = right uncinate fasciculus; UF-L = left uncinate fasciculus; CG-R = right cingulate gyrus; CG-L = left cingulate gyrus; IFOF-R = right inferior occipital fasciculus; IFOF-L = left inferior occipital fasciculus; SLF-R = right superior occipital fasciculus; SLF-L = left superior occipital fasciculus; ILF-R = right inferior longitudinal fasciculus; ILF-L = left inferior longitudinal fasciculus; FMaj = forceps major; FMin = forceps minor; CST-R = right corticospinal tract; CST-L = left corticospinal tract; ATR-R = right anterior thalamic radiation; ATR-L = left anterior thalamic radiation. All models controlled for participant age and race. All models included scores on AB, CU traits, and an interaction term of CU traits x AB. For bilateral tracts, each model included both right and left tracts (i.e., both ATR-L and ATR-R were included in one model). Model 2: beta weights of models that also included yearly income as a covariate. Model 3: beta weights of models that also included scores of substance dependence symptoms, social phobia symptoms, depressive symptoms, anxiety symptoms, and post-traumatic stress disorder symptoms.
p < .10.
p < .05.
p < .01.
Table 3.
Simple slopes and regions of significance for significant antisocial behavior x callous-unemotional traits interactions in relation to fractional anisotropy and mean diffusivity of white matter tracts.
| Tract |
Low CU traits |
Mean CU traits |
High CU traits |
CU traits region of |
% Sample ≥ RoS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | t | p | B | SE | t | p | B | SE | t | p | Significance (RoS) | ||
| UF-L: FA | 0.02 | 0.10 | 0.18 | 0.86a | −0.11 | 0.08 | −1.45 | 0.15 | −0.24 | 0.10 | 2.43 | 0.016 | 0.36 | 31.5% |
| UF-R: MD | −0.06 | 0.09 | 0.66 | 0.51 | 0.06 | 0.07 | 0.81 | 0.42 | 0.18 | 0.09 | 1.94 | 0.055 | 1.07 | 13.5% |
| CG-R: FA | 0.12 | 0.12 | 1.03 | 0.30 | −0.07 | 0.08 | 0.89 | 0.37 | −0.26 | 0.12 | 2.21 | 0.029 | 0.62 | 24.7% |
| CG-R: MDa | −0.09 | 0.10 | −0.92 | 0.36 | 0.09 | 0.07 | 1.23 | 0.22 | 0.26 | 0.10 | 2.69 | 0.008 | 0.34 | 33.1% |
| IFOF-L: FA | 0.04 | 0.10 | 0.45 | 0.65 | −0.10 | 0.07 | 1.43 | 0.16 | −0.24 | 0.10 | −2.52 | 0.013 | 0.31 | 33.7% |
| IFOF-R: MDa | −0.14 | 0.10 | −1.49 | 0.14 | 0.07 | 0.07 | 0.95 | 0.35 | 0.28 | 0.10 | 2.94 | 0.004 | 0.39 | 30.3% |
| SLF-L: MD | −0.01 | 0.09 | −0.08 | 0.93 | 0.13 | 0.07 | 1.89 | 0.06 | 0.27 | 0.08 | 2.89 | 0.004 | 0.05 | 43.8% |
| ILF-L: FAa | 0.06 | 0.10 | 0.59 | 0.55 | −0.12 | 0.07 | −1.73 | 0.09 | −0.30 | 0.10 | −3.11 | 0.002 | 0.10 | 42.1% |
| ILF-R: MD | −0.07 | 0.11 | 0.64 | 0.52 | 0.15 | 0.08 | 2.03 | 0.04 | 0.37 | 0.11 | 3.47 | 0.001 | −0.02 | 46.1% |
| ATR-L: MD | −0.16 | 0.12 | −1.30 | 0.20 | 0.07 | 0.08 | 0.94 | 0.35 | 0.30 | 0.12 | 2.49 | 0.014 | 0.44 | 29.2% |
Interaction term was significant bilaterally. When interaction terms were significant bilaterally, we probed the interaction for the tract with the larger effect size. AB = antisocial behavior; CU = callous-unemotional traits. UF-R = right uncinate fasciculus; UF-L = left uncinate fasciculus; CG-R = right cingulate gyrus; IFOF-R = right inferior occipital fasciculus; IFOF-L = left inferior occipital fasciculus; SLF-L = left superior occipital fasciculus; ILF-R = right inferior longitudinal fasciculus; ILF-L = left inferior longitudinal fasciculus; ATR-L = left anterior thalamic radiation. All models controlled for participant age and race. Models were ran using centered variables of AB (Range − 9.80–37.20; M 0.14; SD 8.19; Uncentered Range: 0–47; Uncentered M 9.94) and CU traits (Range − 1.84–3.24; M − 0.016; SD 0.96; Uncentered Range − 1.90–3.18; Uncentered M − 0.07). Region of significance (RoS) indicate the centered value at which the simple slopes are significantly different from zero (i.e., UF-L FA: at centered values of CU traits >0.36, the simple slope is significantly different from zero). The last column indicates the percentage of the sample that exceeds the threshold of CU traits at which the simple slope is significantly different from zero.
Table 4.
Summary of associations between indices of structural connectivity, antisocial behavior, and callous-unemotional traits in a community sample of young adult men.
Note.1 = association significant after controlling for income; 2 = association significant after controlling for income & co-morbid psychopathology; 3 = association significant at conservative Bonferroni correction standard accounting for multiple comparisons (0.05 / 18 models = 0.003). AB = antisocial behavior; CU traits = callous-unemotional traits. UF-R = right uncinate fasciculus; UF-L = left uncinate fasciculus; CG-R = right cingulate gyrus; CG-L = left cingulate gyrus; IFOF-R = right inferior occipital fasciculus; IFOF-L = left inferior occipital fasciculus; SLF-R = right superior occipital fasciculus; SLF-L = left superior occipital fasciculus; ILF-R = right inferior longitudinal fasciculus; ILF-L = left inferior longitudinal fasciculus; FMaj = forceps major; FMin = forceps minor; CST-R = right corticospinal tract; CST-L = left corticospinal tract; ATR-R = right anterior thalamic radiation; ATR-L = left anterior thalamic radiation; FA = Fractional anisotropy; MD = Mean diffusivity. All models controlled for participant age and race. All models included scores on AB, CU traits, and an interaction term of CU traits x AB (AB+CU traits). For bilateral tracts, each model included both right and left tracts (i.e., both ATR-L and ATR-R were included in one model). Significant interactions with race (i.e., AB x Race, CU traits x Race or AB x CU traits x Race) are included in the findings column.
Although we focus our presentation of the results on the age-only covariates model, we computed additional models to determine whether the inclusion of other demographic and psychiatric covariates changed the pattern of findings. Specifically, we computed a model that included age, race, and self-reported yearly income as covariates. Additionally, we computed a model that included covariates of age, race, income and other forms of psychopathology that have been linked to differences in brain white matter as covariates. These included lifetime symptom counts from the Structured Clinical Interview for the DSM-IV (SCID; First et al., 1996) for major depressive disorder, generalized anxiety disorder, social anxiety disorder, post-traumatic stress disorder, and substance use disorders. Substance use disorder symptom counts were established from a combination of alcohol abuse symptoms, alcohol dependence symptoms, substance abuse symptoms, and substance dependence symptoms (see Hyde et al., 2016).
3. Results
3.1. Are associations between AB and DTI moderated by CU traits?
Bivariate correlations between main study variables are presented in Supplemental Table 2 (FA) and Supplemental Table 3 (MD). Overall and surprisingly, there were no significant bivariate associations between AB or CU traits and FA or MD values for any of the nine white matter tracts. Next, we explored main and interactive relationships of AB and CU traits with FA and MD values for each tract in Mplus version 7.3 (see Table 1, FA; Table 2, MD; Supplemental Fig. 1). There were significant interactions between AB and CU traits in relation to FA values for four of the nine tracts tested (i.e., left UF, right CG, left IFOF, bilateral ILF). Specifically, at high levels of CU traits (Bs = −0.30 – -0.24; ps = 0.001–0.029), but not low or mean levels of CU traits, lower FA values were significantly related to higher AB (Table 3). Across the tracts, region of significance analyses revealed that between 24.7 and 42.1% of the sample reached the level of CU traits at which AB was significantly associated with FA (Table 3).
Next, there was a significant interaction between AB and CU traits in relation to MD values for six of the nine tracts (i.e., right UF, bilateral CG, bilateral IFOF, right SLF, right ILF, left ATR). Specifically, at high levels of CU traits (Bs = 0.18–0.37; ps = 0.001–0.014), but not low or mean levels of CU traits, greater MD values were significantly related to higher AB (Table 3). The association specifically with the MD of the right IFOF was particularly robust, as it remained significant at the conservative Bonferroni threshold, correcting for multiple comparisons (Table 2). Notably, the association between AB and the MD of the right UF at high levels of CU traits did not reach significance (B = 0.18, SE 0.09, p = .055; Table 3). Region of significance analyses revealed that between 13.5 and 46.1% of the sample had levels of CU traits at which AB was significantly associated with FA values (Table 3). There were no significant interactions in relation to either the MD or FA of three tracts: the forceps major, forceps minor, and CST.
To examine if the lack of direct association with AB was related to its cross-sectional measurement, we also examined whether developmental history of AB (e.g., early versus late starting) (Moffitt, 2018) was related to white matter microstructure. When comparing trajectory groups created from repeated self-reports of AB within this sample (age 10, 11, 12, 16, 17; resulting in an early starting/high group, a late starting group, and a low AB group; see Hyde et al., 2015; Hyde et al., 2016; Shaw et al., 2012), we again found little evidence for a direct association between AB and white matter or between age of onset of AB and white matter (i.e., only one finding; FA of the left SLF was significantly lower in the early starting/high group compared to the low group).
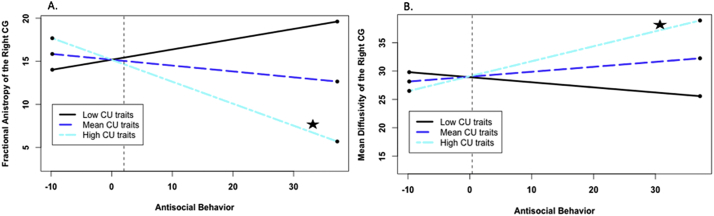
Overall, whereas there were no bivariate associations between either AB or CU traits and indices of white matter integrity, there was substantial evidence that AB was related to FA and MD at high levels of CU traits. That is, the association between AB and white matter integrity was moderated by CU traits for most white matter tracts, and the shape of these interactions was strikingly similar across tracts (i.e., only high AB+CU traits significantly associated with lower FA; only high AB+CU traits significantly associated with greater MD; see Fig. 1 for an example). Finally, the association between the interaction between AB and CU traits and MD of the right IFOF was particularly robust (i.e., significant after Bonferroni correction).
Fig. 1.
Callous-unemotional (CU) traits as a moderator of associations between antisocial behavior (AB) and microstructure of the CG. A. CU traits as a moderator of the association between AB and fractional anistropy of the right CG. Note. CG = cingulate. Simple slopes plotted at mean levels, 1 SD above the mean, and 1 SD below the mean for CU traits, as recommended by Aiken et al. (1991) and using an online computational tool (Preacher et al., 2006). Star next to line indicates significant slope. At high levels of CU traits, but not low or mean levels of CU traits, lower FA values of the right CG were significantly related to higher AB. AB scores presented have been centered. The dashed line indicates the level of AB at which the association is significant (AB centered score > 2.03; AB uncentered score > 11.97; 33.1% of the sample). B. CU traits as a moderator of the association between antisocial behavior and mean diffusivity of the right CG. Note. CG = cingulate. Simple slopes plotted at mean levels, 1 SD above the mean, and 1 SD below the mean for CU traits, as recommended by Aiken et al. (1991) and using an online computational tool (Preacher et al., 2006).Star next to line indicates significant slope. At high levels of CU traits, but not low or mean levels of CU traits, greater MD values were significantly related to higher AB. AB scores presented have been centered. The dashed line indicates the level of AB at which the association is significant (AB centered score > 0.38; AB uncentered score > 10.32; 38.2% of the sample).
3.2. Are associations due to demographic or psychiatric confounds?
In general, associations between AB and lower FA at high levels of CU traits were significant after controlling for income and co-morbid psychopathology (i.e., left UF, right CG, left IFOF, left ILF). The one exception was that, although AB was significantly associated with lower FA in the right ILF at higher levels of CU traits after controlling for income, the association was no longer significant after controlling for co-morbid psychopathology (Table 1; Supplemental Table 7). When looking at associations with each symptom count, greater FA in the right ILF was associated with greater anxiety symptoms (Supplemental Table 7). However, the effect size of the association between the interaction term (AB+CU traits) and FA in the right ILF was similar to the model that did not account for psychopathology.
Similarly, most associations between AB and greater MD at high levels of CU traits remained significant after accounting for income and co-morbid psychopathology (i.e., right CG, bilateral IFOF, right SLF, right ILF, left ATR). The two exceptions were the right UF and left CG. Although AB was significantly associated with greater MD in both the right UF and left CG at higher levels of CU traits after controlling for income, the associations were no longer significant after controlling for co-morbid psychopathology (Table 2; Supplemental Table 8). Of note, the effect sizes of associations between the interaction term (AB+CU traits) and MD of the right UF and left CG were similar to the models that did not account for psychopathology. Further, MD of either the right UF or left CG was not associated with any of the individual symptom counts of psychopathology. Taken together, in general the associations between AB and white matter microstructure at high levels of CU traits were not accounted for by other demographic or psychiatric confounds.
3.3. Do associations differ according to race?
For the most part, associations between AB and white matter microstructure were similar across race, with four exceptions (3 significant interactions in relation to MD out of nine models; one significant interaction in relation to FA out of nine models; Supplemental Table 5). First, within the ILF, AB was associated with greater MD values of the right ILF for African Americans only (Supplemental Table 6; Supplemental Fig. 2). Second, AB was related to lower MD values of the right ILF at high levels of CU traits, but greater MD values at low levels of CU traits for European Americans only (Supplemental Table 6; Supplemental Fig. 3). However, the level of AB at which the slope became significant was extremely high (AB uncentered score > 70.43) and far exceeded the maximum within this sample (AB maximum uncentered = 47). Third, we found a significant interaction between AB and race related to greater MD in the forceps major, but the simple slopes were not significant for either European Americans or African Americans (Supplemental Table 6). Finally, there was a significant interaction between AB and CU traits in relation to FA of the left ATR within African Americans only. Specifically, AB was related to greater FA of the left ATR at low levels of CU traits, but was not significantly associated at mean or high levels of CU traits (Supplemental Table 6; Supplemental Fig. 4). Thus, we found limited moderation of associations between AB+CU traits and white matter microstructure by race, specifically within the ILF and ATR. However, most associations that we found were not moderated by race.
4. Discussion
The current study found significant associations between AB and white matter microstructure that were unique to individuals with high co-occurring levels of CU traits, and were widespread across the brain. Importantly, we did not find any bivariate associations between AB or CU traits and indices of white matter microstructure. However, the interaction between AB and CU traits was significantly related to lower FA and greater MD values in numerous tracts that connect nodes within and between large-scale neural networks (i.e., default mode network, central executive network, salience network) that are postulated to underlie the affective and inhibitory impairments found in AB (Freeman et al., 2015; Hamilton et al., 2015; Tang et al., 2013). In particular, greater MD values of the right IFOF were significantly associated with the interaction between AB and CU traits even after a conservative threshold, correcting for multiple comparisons. Moreover, most of the associations among AB, CU traits, and white matter microstructure were not accounted for by additional demographic factors (i.e., race, income) or psychiatric comorbidities. Building on the literature reporting associations between AB and white matter microstructure, we found strong evidence to suggest that CU traits are a critical factor in these associations.
4.1. AB+CU traits are associated with widespread abnormalities in White matter microstructure
In contrast to previous findings and theory that suggest altered structure would be specific to the UF (Blair, 2007; Craig et al., 2009), we found altered white matter microstructure in several tracts, across multiple large-scale functional networks (Kollias, 2009). Widespread alterations of white matter microstructure have also been linked to AB in studies of adults (e.g., Hoppenbrouwers et al., 2013; Jiang et al., 2017; Lindner et al., 2017; Lindner et al., 2016; Sundram et al., 2012) and youth (e.g., Bolhuis et al., 2019; Decety et al., 2015; Haney-Caron et al., 2014; Pape et al., 2015; Passamonti et al., 2012; Sarkar et al., 2016; Zhang et al., 2014) that have used whole-brain approaches. In particular, we identified associations between AB+CU traits and white matter microstructure in numerous association pathways (i.e., UF, CG, IFOF, ILF, SLF) that connect nodes across the default mode network, the salience network, and the central executive network (Kollias, 2009; Menon, 2011; Mori et al., 2005). Notably, the association with the right IFOF appeared the most robust, as this finding was after Bonferroni correction, an extremely conservative threshold used to account for multiple comparisons. Similarly, AB has previously been related to abnormal activation and functional connectivity of multiple brain regions that are implicated in a variety of cognitive functions (e.g., emotion processing, reward processing, inhibitory control; Raine, 2018; Waller et al., 2015a). Conceptualizing behavioral deficits characteristic of AB as stemming from network-level dysfunction may therefore be a useful framework to integrate findings across regions and modalities.
Consistent with previous research, we found that AB+CU traits were associated with lower FA in the left UF and greater MD in the right UF. The UF reciprocally connects structures in the frontal lobe to structures in the temporal lobe, including the amygdala (Leng et al., 2016). Moreover, we found white matter microstructure abnormalities in the CG (right CG: lower FA and greater MD; left CG: greater MD), IFOF (left IFOF: lower FA; bilateral IFOF: greater MD), ILF (bilateral ILF: lower FA; right ILF: greater MD), SLF (right SLF: greater MD), and ATR (left ATR: greater MD). The CG connects frontal regions with the precuneus, posterior cingulate cortex, hippocampus, and parahippocampus (Wakana et al., 2004). The IFOF connects the frontal and occipital lobes, whereas the ILF connects the temporal and occipital lobes to the frontal lobe, and the SLF connects temporal, parietal and frontal regions (Wakana et al., 2004). The ATR is a major projection from the thalamus, with connections between limbic and frontal regions. White matter microstructure of these tracts has been linked to socioemotional processes relevant to AB, including emotion regulation, emotion recognition, and empathy (e.g., Coad et al., 2017; Parkinson and Wheatley, 2014; Philippi et al., 2009; Pisner et al., 2017). Given that the association with the MD of the IFOF was the most robust (i.e., remained significant after Bonferroni correction), it appears that microstructure of this tract in particular may be relevant for AB.
Notably, these tracts represent the physical connections between regions of the default mode network, the salience network, and executive control networks, which act together in the integration of salient emotional and cognitive information to facilitate goal-orientated behavior (Menon, 2011). Within the current study, abnormalities in the white matter microstructure of these tracts may reflect structural disruptions in the connectivity of the three critical large-scale networks. Abnormal white matter microstructure of pathways within the salience, central executive, and default mode networks could result in a disconnection between affective and cognitive processes, such that affective information (e.g., interpreting other's emotional states) is not integrated with decision-making and behavior (Hamilton et al., 2015). Thus, widespread white matter microstructure abnormalities in these three large-scale networks may be specific markers for individuals with high AB and high CU traits who demonstrate remorselessness and lack of empathy in addition to reward-seeking, rule-breaking, and aggression.
In contrast to some previous studies of DTI and AB, we did not identify any associations with the forceps (major or minor) or the CST (bilaterally). However, the single previous study in adults that had found an association with the forceps minor was within a female clinical sample, with lower FA of the forceps minor differentiating women with conduct disorder from a clinical comparison group (i.e., women matched on clinical co-morbidities) (Lindner et al., 2016). Additionally, only three previous adult studies identified associations with subcomponents of the CST (e.g., corona radiata, internal capsule; Jiang et al., 2017; Lindner et al., 2016; Sundram et al., 2012), and used different approaches to parse apart tracts (e.g., tract-based spatial statistics; Linder et al., 2016, Jiang et al., 2017; voxel-based approach; Sundram et al., 2012). Based on the limited work using whole-brain approaches overall, more research is necessary to determine whether our null findings for the forceps and CST are due to measurement or sample differences.
4.2. Specificity of associations
In general, associations between AB+CU traits and white matter microstructure were not accounted for by race, income, or comorbid psychiatric symptoms, which add to the robustness of our findings. We did identify significant interactive effects with race in relation to the MD of the right ILF and FA of the left ATR. AB was associated with greater MD of the right ILF in African Americans only. Further, in European Americans only, AB+CU traits were associated with greater MD of the right ILF, whereas AB-CU traits was associated with lower MD of the right ILF. Additionally, AB-CU traits was associated with greater FA values of the left ATR in African Americans only. In the only other study that has examined race as a moderator, Decety et al. (2015) instead identified AB x race interactions in relation to the microstructure of other tracts (i.e., CST, SLF, IFOF) in the left, but not right, ILF in a sample of adolescents. However, the authors did not measure CU traits, which may contribute to the discrepancies in results. Based on the exploratory nature of this aim and the lack of previous research in this area, these findings require further replication to better understand the meaning of racial differences in white matter microstructure. Further, in this sample race might be a proxy for additional factors, such as minority-group status, the effects of stress from discrimination, or poverty, and thus it is important to consider our results in a multilayered context (Shaw and Shelleby, 2014). Importantly, however, most associations were similar across race, suggesting that in general, widespread abnormalities in white matter microstructure within AB+CU traits is not race-specific. Moreover, accounting for income did not alter any associations. Accounting for psychiatric symptoms only impacted three associations out of eighteen tested (which continued to show a similar in effect size to models without these symptoms). Notably, previously identified neural correlates of AB (e.g., disrupted amygdala-PFC connectivity) have also been associated with depression and other psychiatric disorders (Buckholtz and Meyer-Lindenberg, 2012), suggesting these neural correlates are not unique markers of AB. Interestingly, however, in the current study we continued to find associations between white matter microstructure and AB in the presence of high CU traits even when accounting for comorbid symptomatology. Thus, the white matter microstructure abnormalities identified in this study were indeed specific to AB+CU traits, even after accounting for demographic and psychiatric confounds.
4.3. Moderation and sample issues
Results from previous studies that have primarily assessed male forensic samples may also reflect associations with AB+CU traits, as offender samples are typically characterized by both higher rates of antisocial behavior and psychopathic/CU traits (Frick and Myers, 2017; Patrick, 2007). Notably, only two studies have parsed apart associations unique to AB versus CU/psychopathic traits in adult samples, both of which were offender populations (Motzkin et al., 2011; Vermeij et al., 2018). Motzkin et al. (2011) found that psychopathic male inmates had significantly lower FA in the right UF compared to non-psychopathic male inmates, and did not find differences in other comparisons tracts (e.g., ILF/IFOF, SLF, superior frontal occipital fasciculus). However, the authors did not extract MD values and did not examine associations with the affective features of psychopathy specifically, which are the features akin to CU traits. More recently, Vermeij et al. (2018) found that affective features of psychopathy specifically were associated with lower FA in the bilateral ILF, bilateral IFOF, and right UF within offenders with co-morbid impulsive control problems only, but not across the entire sample of offenders. In the current study, we did not identify any significant bivariate correlations between white matter microstructure and AB or CU traits, whereas AB+CU traits were associated with microstructural abnormalities of the UF in addition to several other tracts (i.e., CG, IFOF, ILF, SLF, ATR).
After probing significant interactions to determine the region of significance, we found that significant associations between white matter microstructure and AB were only present in participants that had elevated levels of both AB and CU traits, similar to a recent case-control study comparing AB+CU+ to AB+CU- groups (e.g., Puzzo et al., 2018). Depending on the tract, participants had to endorse between 11 and 21 items on our measure of AB to meet the severity threshold for AB (i.e., had to endorse engaging in at least 11 antisocial behaviors) to find significant results. These endorsement rates were close to the mean in the current sample (Range: 0–47; M: 9.94). In terms of the region of significance for CU traits, less than a third of our participants met the severity threshold for the CU traits factor score at which AB and white matter microstructure were associated. Thus, it is unsurprising that we did not identify main effects of either AB or CU traits, given that these effects did not emerge until participants reached the mean of AB and were in the top third of the sample on CU traits. As our sample was from the community but effectively enriched on AB, limited ranges of AB and CU traits could explain null findings in previous studies of community samples that did not explore interactive effects. At the same time, these findings support the use of enriched community samples in which there were a substantial number of participants “high enough” on AB and CU traits to observe these relationships, while also being able to examine these relationships dimensionally across those in the community.
4.4. Strengths and limitations
The findings of the present study were strengthened by several factors, including a large, racially diverse community sample within a narrow age range and robust assessment of other potentially confounding factors, including socioeconomic status and co-morbid psychopathology, as well as use of a whole-brain approach and extracting both FA and MD values. In addition, we used a strict Bonferroni correction across multiple comparisons, and this approach highlights that the associations with the right IFOF are the most robust. Nevertheless, our findings should be considered in the context of several limitations. First, the indices of FA and MD are relatively nonspecific markers of white matter microstructure, such that we are unable to determine the specific factors (e.g., myelination, axon density, axon diameter, branching of tracts) that influence differences (Beaulieu, 2002; Jones et al., 2013). Moreover, a general limitation of DTI research and the interpretation of FA and MD arises from the fitting of a tensor model. We reconstructed fiber pathways using deterministic tractography, which can be limited in terms of the accuracy with which directions and pathways of fibers is determined, particularly for brain regions containing differently oriented fiber bundles within the same voxel (Jones et al., 2013; Soares et al., 2013). Crossing fibers impact FA more than MD (Soares et al., 2013), which could explain the inconsistencies between our FA and MD findings. However, deterministic tractography can better account for tract variability between subjects, including specific tract location and shape (Lebel et al., 2017), thus making this a more ideal approach in our diverse sample. Moreover, the use of deterministic tractography allowed us to directly compare our findings to previous research in adults (Beyer et al., 2014; Craig et al., 2009; Motzkin et al., 2011; Sethi et al., 2015; Sobhani et al., 2015). Finally, while previous studies have also examined associations between AB and other indices of white matter microstructure (i.e., RD and AD), we chose not to include these analyses because of previous research that has suggested these indices are highly susceptible to noise (Pierpaoli and Basser, 1996) and cannot be reliably interpreted (Jones et al., 2013; Wheeler-Kingshott and Cercignani, 2009), as well as to limit comparisons.
Second, our analytic approach involved multiple comparisons, although we sought to limit the number of models by combining bilateral tracts when possible. Importantly, we did have findings that were significant at a conservative threshold (i.e., Bonferroni correction) in the right IFOF, which strongly supports the associations between AB+CU traits and abnormalities in this tract. This suggests that, despite the problematic nature of multiple comparisons, it is nevertheless important to expand beyond the single ROI approach that has been traditionally utilized in the field. As an additional consideration for future work, associations varied in terms of laterality for both MD and FA. While previous studies have identified structural asymmetries of white matter, the functional consequence of lateralization of white matter tracts is still being explored (de Schotten et al., 2011), thus limiting our ability to interpret these findings. Third, within this study we examined cross-sectional associations between AB and white matter microstructure, and thus the directionality of the associations is unclear. Further research is needed to clarify whether white matter abnormalities emerge as a result of AB across development, or whether white matter abnormalities lead to the emergence of AB. Fourth, within the current study we did not have strong data on medication usage, preventing us from accounting for individual differences in medication type and usage in analyses. Fifth, associations may differ according to the measurement of AB. For instance, the Self-Report of Delinquency (SRD) captures versatility in AB (i.e., includes a variety of antisocial acts) and frequency (though only on a 0, 1, 2 scale), whereas other measures may better reflect severity of specific antisocial acts or a history of antisocial behaviors (e.g., clinical symptoms of Antisocial Personality Disorder). The SRD does not contain clinical or forensic cut-offs, so it is difficult with our current measurement of AB to comment on our finding's clinical/forensic utility. Finally, our results may not generalize to different populations, including women, additional racial or ethnic categories (e.g., Hispanic), youth/adolescents, or clinical populations, or those higher in socioeconomic status and/or living in rural or suburban communities.
5. Conclusion
In the current study, we found significant associations between AB and white matter microstructure that were widespread across the brain and were dependent on high co-occurring levels of CU traits in a racially diverse community sample of young adult men. Our findings challenge the current focus of the field, almost exclusively on the UF. Moreover, AB was only associated with differences in white matter at high levels of CU traits, suggesting that relationships between AB and brain structure and function may not be linear and may only be present in those with more extreme levels of AB or in those with a specific type of AB (i.e., with CU traits). AB+CU traits were associated with abnormal white matter microstructure primarily in association pathways (i.e., UF, CG, IFOF, SLF, ILF), and thalamic pathways (i.e., ATR), and these associations in general did not differ according to race, socioeconomic status, or comorbid psychiatric symptoms. Taken together, these findings suggest that AB+CU traits is characterized by impairment in broad neural networks involved in fear conditioning, reward processing, and inhibitory control, consistent with recent theories of AB (Hamilton et al., 2015).
Funding
This research was supported by Grant R01 MH050907 from the National Institutes of Health to D.S.S., Grant R01 DA02622 to D.S.S. and E.E.F. H. L. D. was supported by a National Science Foundation Graduate Research Fellowship. R.W. was supported by Grant T32 AA007477, J.P. was supported by NIH NIDCD R00DC013828.
Acknowledgments
Acknowledgments
The authors would like to thank the staff and study families of the Pittsburgh Mother and Child Project for making this research possible.
Conflict of interest statement
No conflicts declared.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101836.
Appendix A. Supplementary data
Supplementary material
References
- Aiken L.S., West S.G., Reno R.R. Sage; 1991. Multiple Regression: Testing and Interpreting Interactions. [Google Scholar]
- Anderson D.A. The cost of crime. Foundations and Trends® in Microeconomics. 2012;7(3):209–265. [Google Scholar]
- Andersson J.L., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L., Graham M.S., Zsoldos E., Sotiropoulos S.N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage. 2016;141:556–572. doi: 10.1016/j.neuroimage.2016.06.058. [DOI] [PubMed] [Google Scholar]
- Andersson J.L., Graham M.S., Drobnjak I., Zhang H., Filippini N., Bastiani M. Towards a comprehensive framework for movement and distortion correction of diffusion MR images: within volume movement. NeuroImage. 2017;152:450–466. doi: 10.1016/j.neuroimage.2017.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S.T., Yücel M., Fornito A., Allen N.B., Lubman D.I. A systematic review of diffusion weighted MRI studies of white matter microstructure in adolescent substance users. Neurosci. Biobehav. Rev. 2013;37(8):1713–1723. doi: 10.1016/j.neubiorev.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers A.R., Newman J.P., Sathasivam N., Curtin J.J. Evaluating the generalizability of a fear deficit in psychopathic African American offenders. J. Abnorm. Psychol. 2011;120(1):71–78. doi: 10.1037/a0021225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. 2011;213(2):560–570. doi: 10.1016/j.jmr.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Pajevic S., Pierpaoli C., Duda J., Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 2000;44(4):625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system–a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beck J.E., Shaw D.S. The influence of perinatal complications and environmental adversity on boys' antisocial behavior. J. Child Psychol. Psychiatry. 2005;46(1):35–46. doi: 10.1111/j.1469-7610.2004.00336.x. [DOI] [PubMed] [Google Scholar]
- Beyer F., Münte T.F., Wiechert J., Heldmann M., Krämer U.M. Trait aggressiveness is not related to structural connectivity between orbitofrontal cortex and amygdala. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J.R. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn. Sci. 2007;11(9):387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Blair R.J.R. Emotion-based learning systems and the development of morality. Cognition. 2017;167:38–45. doi: 10.1016/j.cognition.2017.03.013. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis K., Muetzel R.L., Stringaris A., Hudziak J.J., Jaddoe V.W., Hillegers M.H.…Tiemeier H. Structural brain connectivity in childhood disruptive behavior problems: a multidimensional approach. Biol. Psychiatry. 2019;85(4):336–344. doi: 10.1016/j.biopsych.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Breeden A., Cardinale E.M., Lozier L., VanMeter J., Marsh A.A. Callous-unemotional traits drive reduced white-matter integrity in youths with conduct problems. Psychol. Med. 2015;45(14):3033–3046. doi: 10.1017/S0033291715000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz J.W., Meyer-Lindenberg A. Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74(6):990–1004. doi: 10.1016/j.neuron.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Chang E.H., Argyelan M., Aggarwal M., Chandon T.-S.S., Karlsgodt K.H., Mori S., Malhotra A.K. The role of myelination in measures of white matter integrity: combination of diffusion tensor imaging and two-photon microscopy of CLARITY intact brains. NeuroImage. 2017;147:253–261. doi: 10.1016/j.neuroimage.2016.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang M.-C., McMahon K.L., de Zubicaray G.I., Martin N.G., Hickie I., Toga A.W.…Thompson P.M. Genetics of white matter development: a DTI study of 705 twins and their siblings aged 12 to 29. NeuroImage. 2011;54(3):2308–2317. doi: 10.1016/j.neuroimage.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coad B.M., Postans M., Hodgetts C.J., Muhlert N., Graham K.S., Lawrence A.D. Structural connections support emotional connections: Uncinate fasciculus microstructure is related to the ability to decode facial emotion expressions. Neuropsychologia. 2017 doi: 10.1016/j.neuropsychologia.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro E.F., McCloskey M.S., Fitzgerald D.A., Phan K.L. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol. Psychiatry. 2007;62(2):168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Contreras-Rodríguez O., Pujol J., Batalla I., Harrison B.J., Soriano-Mas C., Deus J., Hernández-Ribas R. Functional connectivity bias in the prefrontal cortex of psychopaths. Biol. Psychiatry. 2015;78(9):647–655. doi: 10.1016/j.biopsych.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Craig M.C., Catani M., Deeley Q., Latham R., Daly E., Kanaan R., Murphy D.G. Altered connections on the road to psychopathy. Mol. Psychiatry. 2009;14(10):946. doi: 10.1038/mp.2009.40. [DOI] [PubMed] [Google Scholar]
- Daniels J.K., Lamke J.P., Gaebler M., Walter H., Scheel M. White matter integrity and its relationship to PTSD and childhood trauma—a systematic review and meta-analysis. Depress. Anxiety. 2013;30(3):207–216. doi: 10.1002/da.22044. [DOI] [PubMed] [Google Scholar]
- de Schotten M.T., Bizzi A., Dell'Acqua F., Allin M., Walshe M., Murray R.…Catani M. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. NeuroImage. 2011;54(1):49–59. doi: 10.1016/j.neuroimage.2010.07.055. [DOI] [PubMed] [Google Scholar]
- Decety J., Chen C., Harenski C., Kiehl K.A. An fMRI study of affective perspective taking in individuals with psychopathy: imagining another in pain does not evoke empathy. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J., Yoder K.J., Lahey B.B. Sex differences in abnormal white matter development associated with conduct disorder in children. Psychiatry Res. Neuroimaging. 2015;233(2):269–277. doi: 10.1016/j.pscychresns.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D.S., Huizinga D., Ageton S.S. Sage Publications; 1985. Explaining Delinquency and Drug Use. [Google Scholar]
- First M.B., Gibbon M., Spitzer R.L., Williams J.B. Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. User's Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders—Research Version. [Google Scholar]
- Freeman S.M., Clewett D.V., Bennett C.M., Kiehl K.A., Gazzaniga M.S., Miller M.B. The posteromedial region of the default mode network shows attenuated task-induced deactivation in psychopathic prisoners. Neuropsychology. 2015;29(3):493. doi: 10.1037/neu0000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick P.J., Hare R.D. Multi-Health Systems; Toronto: 2001. Antisocial Process Screening Device: APSD. [Google Scholar]
- Frick P.J., Myers T.D. The Wiley Handbook of Disruptive and Impulse-Control Disorders. 2017. Conduct disorder and callous-unemotional traits; pp. 37–54. [Google Scholar]
- Frick P.J., Ray J.V., Thornton L.C., Kahn R.E. Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychol. Bull. 2014;140(1):1–57. doi: 10.1037/a0033076. [DOI] [PubMed] [Google Scholar]
- Gard A.M., Waller R., Shaw D.S., Forbes E.E., Hariri A.R., Hyde L.W. The long reach of early adversity: parenting, stress, and neural pathways to antisocial behavior in adulthood. Biol. Psychiatry. 2017;2(7):582–590. doi: 10.1016/j.bpsc.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R.B., Chou S.P., Saha T.D., Smith S.M., Jung J., Zhang H.…Grant B.F. The epidemiology of antisocial Behavioral syndromes in adulthood: results from the National Epidemiologic Survey on alcohol and related conditions-III. J. Clin. Psych. 2017;78(1):90–98. doi: 10.4088/JCP.15m10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R.K., Hiatt Racer K., Newman J.P. Impaired integration in psychopathy: a unified theory of psychopathic dysfunction. Psychol. Rev. 2015;122(4):770–791. doi: 10.1037/a0039703. [DOI] [PubMed] [Google Scholar]
- Haney-Caron E., Caprihan A., Stevens M.C. DTI-measured white matter abnormalities in adolescents with conduct disorder. J. Psychiatr. Res. 2014;48(1):111–120. doi: 10.1016/j.jpsychires.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare R.D., Neumann C.S. Psychopathy as a clinical and empirical construct. Annu. Rev. Clin. Psychol. 2008;4:217–246. doi: 10.1146/annurev.clinpsy.3.022806.091452. [DOI] [PubMed] [Google Scholar]
- Hoppenbrouwers S.S., Nazeri A., de Jesus D.R., Stirpe T., Felsky D., Schutter D.J.…Voineskos A.N. White matter deficits in psychopathic offenders and correlation with factor structure. PLoS One. 2013;8(8):e72375. doi: 10.1371/journal.pone.0072375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Shaw D.S., Hariri A.R. Understanding youth antisocial behavior using neuroscience through a developmental psychopathology lens: review, integration, and directions for research. Dev. Rev. 2013;33(3):168–223. doi: 10.1016/j.dr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Byrd A.L., Votruba-Drzal E., Hariri A.R., Manuck S.B. Amygdala reactivity and negative emotionality: divergent correlates of antisocial personality and psychopathy traits in a community sample. J. Abnorm. Psychol. 2014;123(1):214–224. doi: 10.1037/a0035467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Burt S.A., Shaw D.S., Donnellan M.B., Forbes E.E. Early starting, aggressive, and/or callous–unemotional? Examining the overlap and predictive utility of antisocial behavior subtypes. J. Abnorm. Psychol. 2015;124(2):329. doi: 10.1037/abn0000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Shaw D.S., Murray L., Gard A., Hariri A.R., Forbes E.E. Dissecting the role of amygdala reactivity in antisocial behavior in a sample of young, low-income, urban men. Clin. Psychol. Sci. 2016;4(3):527–544. doi: 10.1177/2167702615614511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Shi F., Liu H., Li G., Ding Z., Shen H.…Wang W. Reduced white matter integrity in antisocial personality disorder: a diffusion tensor imaging study. Sci. Rep. 2017;7:43002. doi: 10.1038/srep43002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K., Knösche T.R., Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kollias S. SAGE Publications Sage UK; London, England: 2009. Parcelation of the White Matter using DTI: Insights into the Functional Connectivity of the Brain. [Google Scholar]
- Kotler J.S., McMahon R.J. Handbook of Child and Adolescent Psychopathy. 2010. Assessment of child and adolescent psychopathy; pp. 79–109. [Google Scholar]
- Kreuger R., Markon K., Patrick C.J., Benning S., Kramer M. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing Spectrum. J. Abnorm. Psychol. 2007;116(4):645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey B.B., Rathouz P.J., Applegate B., Tackett J.L., Waldman I.D. Psychometrics of a self-report version of the child and adolescent dispositions scale. J. Clin. Child Adolesc. Psychol. 2010;39(3):351–361. doi: 10.1080/15374411003691784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Treit S., Beaulieu C. A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR Biomed. 2017:e3778. doi: 10.1002/nbm.3778. [DOI] [PubMed] [Google Scholar]
- Leventhal T., Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol. Bull. 2000;126(2):309. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- Leng B., Han S., Bao Y., Zhang H., Wang Y., Wu Y., Wang Y. The uncinate fasciculus as observed using diffusion spectrum imaging in the human brain. Neuroradiology. 2016;58(6):595–606. doi: 10.1007/s00234-016-1650-9. [DOI] [PubMed] [Google Scholar]
- Lindner P., Savic I., Sitnikov R., Budhiraja M., Liu Y., Jokinen J.…Hodgins S. Conduct disorder in females is associated with reduced corpus callosum structural integrity independent of comorbid disorders and exposure to maltreatment. Transl. Psychiatry. 2016;6(1):e714. doi: 10.1038/tp.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner P., Budhiraja M., Westerman J., Savic I., Jokinen J., Tiihonen J., Hodgins S. White matter correlates of psychopathic traits in a female community sample. Soc. Cogn. Affect. Neurosci. 2017;12(9):1500–1510. doi: 10.1093/scan/nsx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh A.A., Finger E.C., Fowler K.A., Jurkowitz I.T., Schechter J.C., Henry H.Y.…Blair R.J.R. Reduced amygdala–orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Res. Neuroimaging. 2011;194(3):279–286. doi: 10.1016/j.pscychresns.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays V.M., Cochran S.D., Barnes N.W. Race, race-based discrimination, and health outcomes among African Americans. Annu. Rev. Psychol. 2007;58:201–225. doi: 10.1146/annurev.psych.57.102904.190212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Moffitt T.E. Developmental Theories of Crime and Delinquency. Routledge; 2018. Adolescence-limited and life-course-persistent offending: A complementary pair of developmental theories; pp. 11–54. [Google Scholar]
- Mori S., Wakana S., Van Zijl P.C., Nagae-Poetscher L. Elsevier; 2005. MRI Atlas of Human White Matter. [DOI] [PubMed] [Google Scholar]
- Motzkin J.C., Newman J.P., Kiehl K.A., Koenigs M. Reduced prefrontal connectivity in psychopathy. J. Neurosci. 2011;31(48):17348–17357. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L.K., Muthén B.O. Muthén & Muthén; Los Angeles, CA: 2014. Mplus 7.3. [Google Scholar]
- Olson-Ayala L.A., Patrick C.J. Handbook of Personality Disorders: Theory, Research, and Treatment. vol. 444. 2018. Clinical aspects of antisocial personality disorder and psychopathy. [Google Scholar]
- Pape L.E., Cohn M.D., Caan M.W., van Wingen G., van den Brink W., Veltman D.J., Popma A. Psychopathic traits in adolescents are associated with higher structural connectivity. Psychiatry Res. Neuroimaging. 2015;233(3):474–480. doi: 10.1016/j.pscychresns.2015.07.023. [DOI] [PubMed] [Google Scholar]
- Parkinson C., Wheatley T. Relating anatomical and social connectivity: White matter microstructure predicts emotional empathy. Cereb. Cortex. 2014;24(3):614–625. doi: 10.1093/cercor/bhs347. [DOI] [PubMed] [Google Scholar]
- Passamonti L., Fairchild G., Fornito A., Goodyer I.M., Nimmo-Smith I., Hagan C.C., Calder A.J. Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick C.J. Antisocial personality disorder and psychopathy. In: O'Donohue W., Fowler K.A., Lilienfeld S.O., editors. Personality Disorders: Toward the DSM-V. Sage Publications, Inc; Thousand Oaks, CA US: 2007. pp. 109–166. [Google Scholar]
- Philippi C.L., Mehta S., Grabowski T., Adolphs R., Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. J. Neurosci. 2009;29(48):15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi C.L., Pujara M.S., Motzkin J.C., Newman J., Kiehl K.A., Koenigs M. Altered resting-state functional connectivity in cortical networks in psychopathy. J. Neurosci. 2015;35(15):6068–6078. doi: 10.1523/JNEUROSCI.5010-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C., Basser P.J. Toward a quantitative assessment of diffusion anisotropy. Magn. Reson. Med. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Pisner D.A., Smith R., Alkozei A., Klimova A., Killgore W.D.S. Highways of the emotional intellect: white matter microstructural correlates of an ability-based measure of emotional intelligence. Soc. Neurosci. 2017;12(3):253–267. doi: 10.1080/17470919.2016.1176600. [DOI] [PubMed] [Google Scholar]
- Preacher K.J., Curran P.J., Bauer D.J. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J. Educ. Behav. Stat. 2006;31(4):437–448. [Google Scholar]
- Puzzo I., Seunarine K., Sully K., Darekar A., Clark C., Sonuga-Barke E.J., Fairchild G. Altered white-matter microstructure in conduct disorder is specifically associated with elevated callous-unemotional traits. J. Abnorm. Child Psychol. 2018;46(7):1451–1466. doi: 10.1007/s10802-017-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: a review. J. Abnorm. Child Psychol. 2002;30(4):311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Raine A. Antisocial personality as a neurodevelopmental disorder. Annu. Rev. Clin. Psychol. 2018;(0) doi: 10.1146/annurev-clinpsy-050817-084819. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Craig M., Catani M., Dell'Acqua F., Fahy T., Deeley Q., Murphy D.G. Frontotemporal white-matter microstructural abnormalities in adolescents with conduct disorder: a diffusion tensor imaging study. Psychol. Med. 2013;43(2):401–411. doi: 10.1017/S003329171200116X. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Dell'Acqua F., Walsh S.F., Blackwood N., Scott S., Craig M.C.…Murphy D.G. A whole-brain investigation of white matter microstructure in adolescents with conduct disorder. PLoS One. 2016;11(6):e0155475. doi: 10.1371/journal.pone.0155475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi A., Gregory S., Dell'Acqua F., Thomas E.P., Simmons A., Murphy D.G., Craig M.C. Emotional detachment in psychopathy: involvement of dorsal default-mode connections. Cortex. 2015;62:11–19. doi: 10.1016/j.cortex.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Shaw D.S., Shelleby E.C. Early-starting conduct problems: intersection of conduct problems and poverty. Annu. Rev. Clin. Psychol. 2014;10:503–528. doi: 10.1146/annurev-clinpsy-032813-153650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D.S., Gilliom M., Ingoldsby E.M., Nagin D.S. Trajectories leading to school-age conduct problems. Dev. Psychol. 2003;39(2):189. doi: 10.1037//0012-1649.39.2.189. [DOI] [PubMed] [Google Scholar]
- Shaw D.S., Hyde L.W., Brennan L.M. Early predictors of boys' antisocial trajectories. Dev. Psychopathol. 2012;24(3):871–888. doi: 10.1017/S0954579412000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J., Marques P., Alves V., Sousa N. A hitchhiker's guide to diffusion tensor imaging. Front. Neurosci. 2013;7:31. doi: 10.3389/fnins.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani M., Baker L., Martins B., Tuvblad C., Aziz-Zadeh L. Psychopathic traits modulate microstructural integrity of right uncinate fasciculus in a community population. NeuroImage. 2015;8:32–38. doi: 10.1016/j.nicl.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundram F., Deeley Q., Sarkar S., Daly E., Latham R., Craig M., Barker G.J. White matter microstructural abnormalities in the frontal lobe of adults with antisocial personality disorder. Cortex. 2012;48(2):216–229. doi: 10.1016/j.cortex.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Tang Y., Jiang W., Liao J., Wang W., Luo A. Identifying individuals with antisocial personality disorder using resting-state FMRI. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0060652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Dougherty R.F., Colich N.L., Perry L.M., Rykhlevskaia E.I., Louro H.M.…Glover G.H. COMT genotype affects prefrontal white matter pathways in children and adolescents. NeuroImage. 2010;53(3):926–934. doi: 10.1016/j.neuroimage.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart J., Novikov D.S., Christiaens D., Ades-Aron B., Sijbers J., Fieremans E. Denoising of diffusion MRI using random matrix theory. NeuroImage. 2016;142:394–406. doi: 10.1016/j.neuroimage.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeij A., Kempes M.M., Cima M.J., Mars R.B., Brazil I.A. Affective traits of psychopathy are linked to white-matter abnormalities in impulsive male offenders. Neuropsychology. 2018;32(6):735–745. doi: 10.1037/neu0000448. [DOI] [PubMed] [Google Scholar]
- Viding E., McCrory E. Understanding the development of psychopathy: progress and challenges. Psychol. Med. 2018;48(4):566–577. doi: 10.1017/S0033291717002847. [DOI] [PubMed] [Google Scholar]
- Wakana S., Jiang H., Nagae-Poetscher L.M., Van Zijl P.C., Mori S. Fiber tract–based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Waller R., Murray L., Dotterer H.L., Hyde L.W. 2015. Neuroimaging Approaches to Understanding Youth Antisocial Behavior. [Google Scholar]
- Waller R., Shaw D.S., Forbes E.E., Hyde L.W. Understanding early contextual and parental risk factors for the development of limited prosocial emotions. J. Abnorm. Child Psychol. 2015;43(6):1025–1039. doi: 10.1007/s10802-014-9965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R., Dotterer H.L., Murray L., Maxwell A.M., Hyde L.W. White-matter tract abnormalities and antisocial behavior: a systematic review of diffusion tensor imaging studies across development. NeuroImage. 2017;14:201–215. doi: 10.1016/j.nicl.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R., Shaw D.S., Hyde L.W. Observed fearlessness and positive parenting interact to predict childhood callous-unemotional behaviors among low-income boys. J. Child Psychol. Psychiatry. 2017;58(3):282–291. doi: 10.1111/jcpp.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R., Gard A.M., Shaw D.S., Forbes E.E., Neumann C.S., Hyde L.W. Weakened functional connectivity between the amygdala and the ventromedial prefrontal cortex is longitudinally related to psychopathic traits in low-income males during early adulthood. Clin. Psychol. Sci. 2018 doi: 10.1177/2167702618810231. (2167702618810231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye L.T., Walhovd K.B., Dale A.M., Bjørnerud A., Due-Tønnessen P., Engvig A.…Fjell A.M. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb. Cortex. 2009;20(9):2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Wheeler-Kingshott C.A., Cercignani M. About “axial” and “radial” diffusivities. Magn. Reson. Med. 2009;61(5):1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- White T., Nelson M., Lim K.O. Diffusion tensor imaging in psychiatric disorders. Top. Magn. Reson. Imaging. 2008;19(2):97–109. doi: 10.1097/RMR.0b013e3181809f1e. [DOI] [PubMed] [Google Scholar]
- Winklewski P.J., Sabisz A., Naumczyk P., Jodzio K., Szurowska E., Szarmach A. Understanding the physiopathology behind axial and radial diffusivity changes—what do we know? Front. Neurol. 2018;9:92. doi: 10.3389/fneur.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Res. Neuroimaging. 2009;174(2):81–88. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K.-H., Bentler P.M. 5. Three likelihood-based methods for mean and covariance structure analysis with nonnormal missing data. Sociol. Methodol. 2000;30(1):165–200. [Google Scholar]
- Zhang J., Gao J., Shi H., Huang B., Wang X., Situ W.…Yao S. Sex differences of uncinate fasciculus structural connectivity in individuals with conduct disorder. Biomed. Res. Int. 2014;2014:9. doi: 10.1155/2014/673165. Article ID 673165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material