Graphical abstract
Keywords: Andrographis paniculata, Standardized first true leaf, Acute oral toxicity
Highlights
-
•
Standardized A. paniculata extract contains high 14-deoxyandrographolide.
-
•
The LD50 of standardized A. paniculata extract is more than 5000 mg/kg body weight.
-
•
Standardized A. paniculata extract shows no acute adverse effects.
Abstract
Andrographis paniculata is widely used in traditional herbal medicines for the treatment of common cold, fever and diarrhea, in many regions of Scandinavia and Asia, including Thailand. The pharmacological activities of A. paniculata are mainly attributed to active diterpenoids including 14-deoxyandrographolide, which is uniquely high in first true leaf ethanolic extract (FTLEE) of A. paniculata. In this study, the acute toxicity of the standardized FTLEE of A. paniculata was examined according to the OECD test guideline No. 420. Mice were divided into four groups of each sex and orally received the standardized FTLEE of A. paniculata (0, 300, 2000, or 5000 mg/kg BW). Post-treatment, body weight, signs of toxicity, and/or mortality were observed for 14 days. At Day 15, animals were euthanized, internal organs were observed grossly, and blood samples collected were subjected to hematology and clinical biochemistry analyses. The results showed that all treated animals survived and no apparent adverse effects were observed during the duration of the study. Gross necropsy observation revealed no lesion in any organ of all the standardized FTLEE-treated mice. Although significant alterations in BUN, lymphocytes, neutrophils, hematocrit and hemoglobin were observed, these alterations were not treatment-related toxic effects. Therefore, we concluded that a single oral administration of the standardized FTLEE of A. paniculata with an upper fixed dose of 5000 mg/kg BW has no significant acute toxicological effects.
1. Introduction
Andrographis paniculata (Burm.f.) Nees, has been widely used as a traditional herbal medicine in Asian and Scandinavian countries to treat fever, diarrhea, and the common cold [1]. A. paniculata possesses various beneficial pharmacological activities, including immune-modulatory, antioxidant, antiinflammatory, anticancer, antidiabetic, hepatoprotective, antimalarial, antihypertensive, and antimicrobial [[2], [3], [4], [5], [6]].
A. paniculata contains multiple bioactive diterpenoids, including andrographolide (AP), 14-deoxy-11, 12-didehydroandrographolide (14-deoxy-11,12-didehydro-AP), neoandrographolide (neo-AP), and 14-deoxyandrographolide (14-deoxy-AP) [3,5,6]. Our previous study showed that the differences in growth stages and cultivation environments influenced the contents of active diterpenoids in different plant organs of A. paniculata [7]. Interestingly, our recent study showed that the standardized FTLEE, which contained high 14-deoxy-AP but low AP content, inhibited in vitro viability of cholangiocarcinoma and hepatocarcinoma cells approximately four fold better than mature leaf stage extract, which contained low 14-deoxy-AP but high AP content [8]. Therefore, the therapeutic benefit of the standardized FTLEE may have a potential to be developed as an alternative or adjuvant treatment for cholangiocarcinoma and hepatocellular carcinoma. Hence, safety evaluation must be done to ensure the safe use of this medicinal plant extract. Although the standardized extract of A. paniculata, containing high AP content, was reported to have no toxicity [9], there is currently no toxicological data available for A. paniculata extract having high 14-deoxy-AP content. In addition, there is no report on the adverse effect of 14-deoxy-AP on hematology and clinical biochemistry. In order to confirm the safety margin as well as extrapolating the dosage for further toxicity studies and clinical trial, the acute oral toxicity study of the standardized FTLEE of A. paniculata should be conducted.
OECD test guideline No. 420 (Acute Oral Toxicity-Fixed Dose Procedure), a standard guideline for acute toxicity test, was selected in this study because it provides a range estimate of the LD50 and allows the substance toxicity to be classified according to the Globally Harmonized System (GHS) [10]. The present study evaluated acute oral toxicity of the standardized FTLEE of A. paniculata containing high content of 14-deoxy-AP in mice. Body weight, signs of toxicity, and mortality were monitored for 14 days. At the end of the study, hematology and clinical biochemistry analyses, and macroscopic examination of the internal organs were also performed for overall health status evaluation and identification of organ specific toxic effects.
2. Materials and methods
2.1. Plant material and extract preparation
The authentication of A. paniculata was performed by Dr. Wongsatit Chuakul. The voucher specimen was submitted to the Pharmaceutical Botany Mahidol Herbarium, Department of Pharmaceutical Botany, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand (PBM 3760). The seeds of A. paniculata were cultivated using peat moss soil (pH 5.5; N-P-K 12-14-24; 0.8 kg/m3) (Chia Tai, Bangkok, Thailand) in uncontrolled conditions at Chulabhorn Research Institute’s greenhouse.
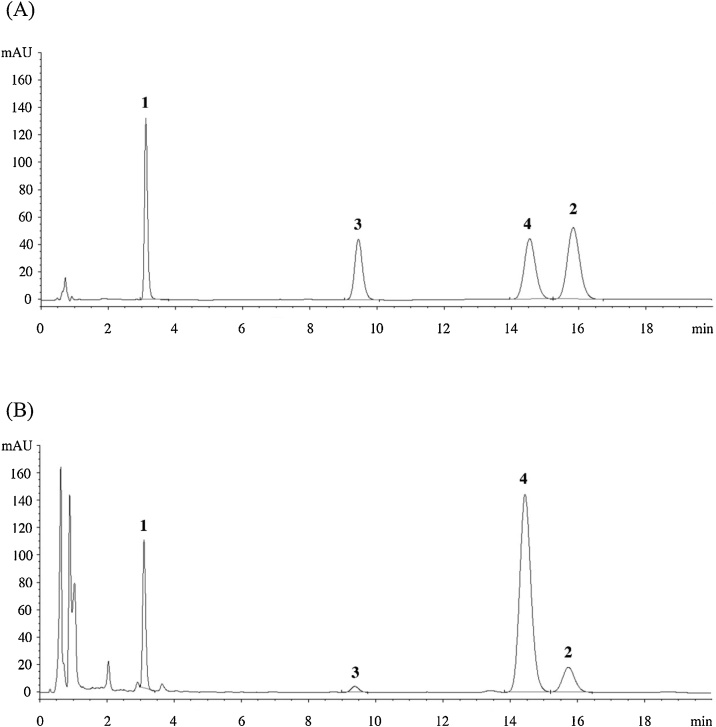
First true leaf stage of plants (approximately 2 weeks after germination) were harvested and separated into aerial parts and roots. The harvested aerial parts were washed, dried at 35–45 °C then blended to powder. First true leaf ethanolic extract (FTLEE) was prepared by extracting 400 g of powdered dried aerial parts with 5.0 L of ethanol. The remaining powdered residues were re-extracted with 5.0 L of ethanol, combined with the first extract, then concentrated and dried by a rotary evaporator at < 50 °C. The % yield of the extract is 12% per dried weight. The contents of four diterpenoids of interest (AP, 14-deoxy-11,12-didehydro-AP, neo-AP, and 14-deoxy-AP) in the standardized FTLEE obtained were measured using HPLC-DAD (Agilent Technologies, Germany) on a reverse-phase column (Zorbax SB-C18; 4.6 × 75 mm, 3.5 μm) connected to a cartridge guard column (Agilent Technologies, USA) [7]. HPLC chromatogram of the standardized FTLEE is shown in Fig. 1. The contents of AP, 14-deoxy-11,12-didehydro-AP, neo-AP, and 14-deoxy-AP in the standardized FTLEE were 69.65, 30.48, 8.51, and 274.78 mg/g dry weight, respectively. Compared to other diterpenoids, 14-deoxy-AP is the major bioactive compound present in the standardized FTLEE. All doses of the standardized FTLEE were suspended in 1% methylcellulose (MC) before administration.
Fig. 1.
Representative chromatograms of standards four major active diterpenoids in the standard diterpenoids (A) (1: andrographolide, 2: 14-deoxy-11, 12-didehydroandrographolide, 3: neoandrographolide, and 4: 14-deoxyandrographolide) (1, 2, 3, and 4 with 99%, 96%, 99%, and 99% purity, respectively) compared with the standardized ethanolic extract of Andrographis paniculata first true leaf (B).
2.2. Animals
Due to limited information on the adverse effects of the standardized FTLEE, both sexes of animal were utilized in this study to examine potential gender discrepancy in response to the standardized FTLEE. Male and female Swiss albino mice (8 weeks old) were obtained from the National Laboratory Animal Center, Salaya, Nakorn-Pathom, Thailand. The mice were acclimatized for 5 days and housed under controlled conditions (24 ± 1 °C, 55 ± 10% humidity, and a 12:12 h light: dark cycle) at the Chulabhorn Research Institute Laboratory Animal Center - an AAALAC International-accredited facility. All animals were fed with food and water ad libitum. The animal study was approved by the Institutional Animal Care and Use Committee (IACUC) at Chulabhorn Research Institute (PN 2015-02). The care and use of animals were in accordance with National Guidelines and Chulabhorn Research Institute-Institutional Animal Care and Use Committee. Our study was conducted according to the OECD test guideline No. 420 (Acute Oral Toxicity-Fixed Dose Procedure), a widely accepted standard guideline for acute toxicity testing. This guideline has been demonstrated to use fewer animals and cause less suffering to animal, whilst still providing a reliable acute toxicity data compared to the traditional acute toxicity study method which used death as an endpoint. With respect to the OECD guideline No. 420, animals were given a single dose of test substance and toxic effects were observed for 14 days. The long recovery period of 14 days was intended for the observation of any potential ‘delayed’ acute toxicity to the single dose administration.
2.3. Acute oral toxicity
2.3.1. Pilot study
Six male and six female mice were randomly assigned into three independent blocks of experiment (2 mice of each sex per block; N = 4). Each animal received a single oral administration of either control (1% MC) or the standardized FTLEE. Prior to the dosing, all animals were fasted for 3–4 h and weighed. In accordance with OECD guideline No. 420, 300 mg/kg BW should be a starting dose for acute toxicity study when toxicological data of test substance is not available or limited.
In the first block of experiment (N = 4), one animal of each sex were administered with a single oral gavage of either control or 300 mg/kg BW of the standardized FTLEE. The signs of toxicity, including changes in skin and fur, eyes, respiration, and behavior pattern, and/or mortality in all animals were observed periodically during the first 24 h (0.5, 1, 6, and 24 h). When no apparent signs of toxicity or mortalities were observed within 24 h post-treatment of the first block, the procedures in the first block of experiment were repeated in the second block animals using 2000 mg/kg BW of the standardized FTLEE. Repeating the same procedures, when no apparent signs of toxicity or mortalities were observed within 24 h post-treatment of the second block, elevated dose of 5000 mg/kg BW of the standardized FTLEE was administered to the third block animals. Post-treatment, animals were weighed weekly. The signs of toxicity and/or mortality were observed once daily for 14 days. At Day 15, all animals were euthanized with carbon dioxide inhalation and undergone gross necropsy.
2.3.2. Main study
Forty mice, 20 male and 20 female mice, were randomly divided into 4 groups (5 mice of each sex per group; N = 10): one control group (1% MC) and three treated groups (300, 2000, and 5000 mg/kg BW of the standardized FTLEE). Prior to the dosing, all animals were fasted for 3–4 h and weighed. Each group received a single oral gavage of control or the standardized FTLEE (300, 2000, or 5000 mg/kg BW). After treatment, signs of toxicity, including changes in skin and fur, eyes, respiration, and behavior pattern, and/or mortality in all animals were observed periodically during the first 24 h (0.5, 1, 6, and 24 h) then once daily for 14 days. Post-treatment, animals were weighed weekly. At Day 15, all animals were euthanized by carbon dioxide inhalation and undergone gross necropsy. The internal organs were observed grossly. Blood samples were collected by cardiac puncture and transferred into K3-EDTA tubes, then analyzed the hematologic parameters (white blood cell count (WBC), lymphocyte (LY), monocyte (MO), neutrophil (NE), eosinophil (EO), basophil (BA), red blood cell count (RBC), hematocrit (HCT), hemoglobin (HGB), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelets (PLT)) using a hematology analyzer (Sysmex XT-2000iV, Japan). Serum samples were analyzed on a clinical biochemistry analyzer (Roche cobas c 111, Switzerland). The following clinical biochemistry values were measured: alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), and creatinine (CRN).
2.4. Statistical analysis
The statistical analysis of the results was performed using R version 3.3.3 (2017-03-06). All data were expressed as means ± SEM and data normality were analyzed using the Sharpiro-Wilkes test. The statistical differences of data with normal distribution were analyzed by one-way analysis of variance (ANOVA), followed by the Bonferroni multiple comparisons whereas the statistical differences of data without normal distribution were analyzed by the Kruskal-Wallis H test. Statistical significance was defined as p < 0.05. The body weight changes during 14 days of treatment were analyzed with repeated measures ANOVA followed by Tukey’s test. The relationship between the standardized FTLEE doses and toxic effect were analyzed by linear regression.
3. Results and discussion
The pilot study served as a preliminary dose selection experiment. The results from the pilot study showed that no signs of toxicity and mortality were observed in either male or female mice administered control (1% MC) or the standardized FTLEE at doses of 300, 2000, or 5000 mg/kg BW. Therefore, these doses of the standardized FTLEE were used for acute oral toxicity evaluation in the main study.
The results of the main study showed that, after 14 days of the standardized FTLEE (300, 2000, and 5000 mg/kg BW) treatment, no signs of toxicity, body weight changes and mortality were observed (data not shown). Since vital organs, including liver, kidneys, heart, lungs, and spleen, are functionally crucial organs often impaired by toxic substances [11], gross examinations of internal organs were performed to identify potential signs of organ-targeted toxicity. No lesions were found upon macroscopic examination of the internal organs of any animals treated with the standardized FTLEE. Aside the gross appearance of the internal organs, the overall health status of the animals was also evaluated by clinical biochemistry (Table 1) and hematology analyses (Table 2). The results showed that all measurements obtained from the standardized FTLEE treated animals were within physiological ranges [12,13]. These results suggest that the standardized FTLEE has negligible acute toxicity at the doses tested. Increased levels of CRN and BUN are indicative of impaired renal function [14]. No change in BUN was observed in the standardized FTLEE-treated male mice however BUN in 300 mg/kg BW-treated female mice was significantly increased when compared to control (p < 0.01) and 5000 mg/kg BW (p < 0.05) (Table 1). Despite the elevated BUN in female mice, CRN levels were not affected in all animals. These results suggest that the renal function was normal in both standardized FTLEE treated and control groups. Hepatotoxicity causes elevation in the levels of hepatocellular enzymes, ALT and AST, and hepatobiliary enzyme, ALP [15,16]. In the present study, no elevation of AST, ALT, and ALP levels was observed in either standardized FTLEE treated-male or -female mice (Table 1). These results suggest that the standardized FTLEE has no hepatotoxic effect.
Table 1.
Clinical biochemistry parameters.
| Parameters | Standardized FTLEE (mg/kg BW) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Malea |
Femalea |
|||||||
| 0b (N = 5) |
300 (N = 5) |
2000 (N = 5) |
5000 (N = 5) |
0b (N = 5) |
300 (N = 5) |
2000 (N = 5) |
5000 (N = 5) |
|
| AST (U/L) | 90.0 ± 9.81 | 102.3 ± 22.15 | 125.9 ± 10.74 | 127.4 ± 24.72 | 92.2 ± 6.23 | 96.3 ± 12.88 | 98.3 ± 12.69 | 94.8 ± 5.23 |
| ALT (U/L) | 27.3 ± 3.20 | 21.0 ± 1.98 | 24.2 ± 1.91 | 26.1 ± 2.66 | 23.2 ± 1.61 | 19.3 ± 0.72 | 21.3 ± 2.33 | 20.5 ± 1.84 |
| ALP (U/L) | 133.3 ± 6.23 | 125.1 ± 8.76 | 129.3 ± 8.63 | 132.6 ± 5.62 | 137.6 ± 9.82 | 140.4 ± 12.59 | 138.6 ± 9.47 | 130.3 ± 3.64 |
| CRN (mg/dL) | 0.13 ± 0.01 | 0.11 ± 0.01 | 0.13 ± 0.01 | 0.11 ± 0.01 | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.14 ± 0.00 |
| BUN (mg/dL) | 22.8 ± 1.10 | 23.2 ± 0.76 | 24.8 ± 1.17 | 24.3 ± 1.73 | 17.9 ± 0.74 | 22.6 ± 1.15** | 19.9 ± 0.88 | 18.6 ± 0.62# |
alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine (CRN).
Significantly different from control group was designated as ** (p < 0.01).
Significantly different from 300 mg/kg BW was designated as # (p < 0.05).
Data are expressed as means ± SEM.
Control, 1% methylcellulose.
Table 2.
Hematologic parameters.
| Parameters | Standardized FTLEE (mg/kg BW) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Malea |
Femalea |
|||||||
| 0b (N = 5) |
300 (N = 4) |
2000 (N = 3) |
5000 (N = 3) |
0b (N = 4) |
300 (N = 5) |
2000 (N = 4) |
5000 (N = 4) |
|
| WBC (×106 cells/mL) | 6.11 ± 0.44 | 5.92 ± 0.40 | 5.40 ± 1.12 | 4.46 ± 1.71 | 6.53 ± 1.03 | 9.72 ± 0.67 | 6.99 ± 1.17 | 7.58 ± 0.73 |
| LY (×106 cells/mL) | 3.88 ± 0.32 | 4.34 ± 0.28 | 3.73 ± 0.89 | 3.30 ± 1.41 | 4.51 ± 0.51 | 6.97 ± 0.72 | 5.37 ± 0.95 | 5.80 ± 0.90 |
| NE (×106 cells/mL) | 1.72 ± 0.09 | 1.10 ± 0.07* | 1.19 ± 0.14 | 0.83 ± 0.25** | 1.49 ± 0.49 | 2.05 ± 0.19 | 1.07 ± 0.23 | 1.25 ± 0.32 |
| EO (×106 cells/mL) | 0.19 ± 0.02 | 0.16 ± 0.02 | 0.16 ± 0.08 | 0.18 ± 0.07 | 0.19 ± 0.04 | 0.30 ± 0.04 | 0.29 ± 0.11 | 0.21 ± 0.01 |
| BA (×106 cells/mL) | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| MO (×106 cells/mL) | 0.30 ± 0.04 | 0.30 ± 0.05 | 0.31 ± 0.06 | 0.15 ± 0.06 | 0.34 ± 0.16 | 0.39 ± 0.02 | 0.25 ± 0.05 | 0.31 ± 0.02 |
| LY (%) | 63.4 ± 1.22 | 73.4 ± 0.33* | 68.17 ± 1.99 | 72.4 ± 4.37 | 70.9 ± 4.78 | 71.1 ± 3.23 | 76.5 ± 2.09 | 75.3 ± 6.17 |
| NE (%) | 28.3 ± 0.90 | 18.7 ± 0.32** | 22.93 ± 2.43 | 20.4 ± 3.20* | 21.5 ± 3.65 | 21.5 ± 2.50 | 15.1 ± 0.74 | 17.5 ± 5.74 |
| EO (%) | 3.2 ± 0.31 | 2.7 ± 0.27 | 2.73 ± 0.81 | 3.8 ± 0.76 | 2.9 ± 0.55 | 3.2 ± 0.47 | 4.8 ± 2.22 | 2.9 ± 0.35 |
| BA (%) | 0.2 ± 0.07 | 0.3 ± 0.09 | 0.1 ± 0.10 | 0.1 ± 0.06 | 0.2 ± 0.05 | 0.1 ± 0.02 | 0.2 ± 0.03 | 0.1 ± 0.03 |
| MO (%) | 4.9 ± 0.50 | 5.0 ± 0.49 | 6.07 ± 1.19 | 3.4 ± 0.82 | 4.6 ± 1.50 | 4.1 ± 0.51 | 3.6 ± 0.40 | 4.2 ± 0.37 |
| RBC (×109 cells/mL) | 9.70 ± 0.22 | 10.08 ± 0.18 | 9.98 ± 0.20 | 9.83 ± 0.31 | 10.09 ± 0.17 | 10.37 ± 0.15 | 10.26 ± 0.03 | 9.91 ± 0.16 |
| HCT (%) | 44.00 ± 1.02 | 47.13 ± 0.44 | 46.93 ± 0.42 | 45.13 ± 1.85 | 45.4 ± 0.87 | 49.1 ± 0.64* | 47.6 ± 0.88 | 46.7 ± 0.67 |
| HGB (g/dL) | 15.1 ± 0.38 | 15.8 ± 0.15 | 15.8 ± 0.23 | 15.6 ± 0.72 | 15.6 ± 0.27 | 16.8 ± 0.35 | 16.3 ± 0.27 | 15.8 ± 0.19 |
| MCV (fL) | 45.3 ± 0.66 | 46.1 ± 0.76 | 46.3 ± 0.47 | 45.8 ± 0.84 | 44.7 ± 0.52 | 46.4 ± 0.52 | 45.2 ± 0.63 | 46.2 ± 0.99 |
| MCH (pg) | 15.6 ± 0.22 | 15.4 ± 0.27 | 15.6 ± 0.07 | 15.8 ± 0.27 | 15.4 ± 0.23 | 15.9 ± 0.19 | 15.5 ± 0.23 | 15.6 ± 0.18 |
| MCHC (g/dL) | 34.4 ± 0.50 | 33.5 ± 0.25 | 33.7 ± 0.24 | 34.5 ± 0.30 | 34.4 ± 0.30 | 34.3 ± 0.46 | 34.3 ± 0.22 | 33.8 ± 0.43 |
| PLT (×106 cells/mL) | 1006 ± 86.51 | 1094 ± 50.39 | 1005 ± 65.78 | 952 ± 17.62 | 956 ± 63.97 | 1049 ± 55.24 | 902 ± 100.75 | 976 ± 54.99 |
white blood cell count (WBC), lymphocyte (LY), monocyte (MO), neutrophil (NE), eosinophil (EO), basophil (BA), red blood cell count (RBC), hematocrit (HCT), hemoglobin (HGB), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelets (PLT).
Significantly different from control group was designated as * (p < 0.05) and ** (p < 0.01).
Data are expressed as means ± SEM.
Control, 1% methylcellulose.
Hematologic parameters are sensitive biomarkers often associated to physiological responses to toxic substances [17]. In this study, hematology analysis revealed no change in total WBC of any treated animals. However, %LY was significantly increased in male mice treated with 300 mg/kg BW of the standardized FTLEE (p < 0.05), whereas %NE was significantly decreased in male mice treated with 300 and 5000 mg/kg BW of the standardized FTLEE (p < 0.01 and p < 0.05, respectively) (Table 2). In addition, male mice treated with 300 and 5000 mg/kg BW of the standardized FTLEE showed significant decrease in total NE count (p < 0.01 and p < 0.05, respectively) (Table 2). Similar changes in NE and LY have been associated with toxicity induced WBC change [18]. However, since the standardized FTLEE treated male mice did not exhibit any change in total WBC, these changes may not reflect toxicity. Red cell mass alteration is determined by RBC, HGB concentration, and HCT. The reduction of red cell mass affects oxygen delivery to the peripheral tissue [19]. Our result revealed that HCT significantly increased in 300 mg/kg BW-treated female mice (p < 0.05) (Table 2). However, compared to control, this elevated HCT did not exceeded 10% and no changes in total RBC were observed. These suggest that the standardized FTLEE does not affect the oxygen delivery to the peripheral tissue.
It should be noted that all statistically significant changes observed in this study were within physiological range. Furthermore, no treatment-related toxic effects were observed as there was no linear correlation between the standardized FTLEE doses and toxic effect (0.1 ≤ R ≤ 0.6). Our results suggest that the standardized FTLEE has no acute oral toxicity, and that the oral LD50 is higher than 5000 mg/kg BW in both male and female mice. GHS toxicity classification classifies substance with oral LD50 in the range of 2000–5000 mg/kg BW as relatively low toxicity. By this classification, the present study has demonstrated that the standardized FTLEE has very low acute toxicity. However, it should be noted that the limitations of this study are the absence of pre-14 day hematological and biochemical data which may answer whether FTLEE has toxic effects prior to complete recovery after 14 days, and the lack of microscopic examination of tissues which provides the information whether there are microscopic tissue lesions associated with FTLEE treatment.
A. paniculata is the well-known medicinal plant, and the safety of using A. paniculata as a traditional medicine is supported by the results of the present study. Moreover, the report demonstrating that A. paniculata has no anaphylactic reaction which is a serious and potential deadly adverse effect [20] reinforces the safety of A. paniculata used. Based on a variety of pharmacological actions on A. paniculata, the possible application of standardized FTLEE of A. paniculata can be diverse including as an alternative or adjuvant treatment for cholangiocarcinoma and hepatocellular carcinoma. One of the major drawbacks of medicinal plant extract is that it generally has low bioavailability and efficacy due to its solubility and absorption problems however these can be overcame by new preparation technology such as nanoparticle which helps increasing solubility and absorption, and minimizing toxicity of medicinal plant extract [21].
4. Conclusion
Acute oral toxicity study of the standardized FTLEE in both male and female mice revealed that there were no adverse effects observed and the LD50 value after a single oral administration was greater than 5000 mg/kg BW. The present study provides the information for the dose selection of long-term toxicity study. Further subchronic and chronic toxicological studies and/or clinical trial should be performed to assure the safe usage of the standardized FTLEE.
Conflict of interest
None.
Acknowledgements
We would like to express our thanks to Mr. Supachai Ritruechai, Mrs. Nujorn Srisamut and Ms. Jittra Saehun for technical assistance. This project was supported by a research grant from Chulabhorn Research Institute (grant number: PH 2015-02).
References
- 1.Mishra S.K., Sangwan N.S., Sangwan R.S. Andrographis paniculata (Kalmegh): a review. Pharmacogn. Rev. 2007;1(2):283–298. [Google Scholar]
- 2.Akbar S. Andrographis paniculata: a review of pharmacological activities and clinical effects. Altern. Med. Rev. 2011;16(1):66–77. [PubMed] [Google Scholar]
- 3.Chao W.W., Lin B.F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian) Chin. Med. 2010;5(17) doi: 10.1186/1749-8546-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dey Y.N., Kumari S., Ota S., Srikanth N. Phytopharmacological review of Andrographis paniculata (Burm.f) Wall. ex Nees. Int. J. Nutr. Pharmacol. Neurol. Dis. 2013;3(1):3–10. [Google Scholar]
- 5.Jarukamjorn K., Nemoto N. Pharmacological aspects of Andrographis paniculata on health and its major diterpenoid constitutent andrographolide. J. Health. Sci. 2008;54(4):370–381. [Google Scholar]
- 6.Niranjan A., Tewari S.K., Lehri A. Biological activities of Kalmegh (Andrographis paniculata Nees) and its active principles-a review. Indian J. Nat. Prod. Resour. 2010;1(2):125–135. [Google Scholar]
- 7.Pholphana N., Rangkadilok N., Saehun J., Ritruechai S., Satayavivad J. Changes in the contents of four active diterpenoids at different growth stages in Andrographis paniculata (Burm.f.) Nees (Chuanxinlian) Chin. Med. 2013;8(2) doi: 10.1186/1749-8546-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suriyo T., Pholphana N., Rangkadilok N., Thiantanawat A., Watcharasit P., Satayavivad J. Andrographis paniculata extracts and major constituent diterpenoids inhibit growth of intrahepatic cholangiocarcinoma cells by inducing cell cycle arrest and apoptosis. Planta Med. 2014;80(7):533–543. doi: 10.1055/s-0034-1368399. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekaran C.V., Thiyagarajan P., Sundarajan K., Goudar K.S., Deepak M., Murali B., Allan J.J., Agarwal A. Evaluation of the genotoxic potential and acute oral toxicity of standardized extract of Andrographis paniculata (KalmColdTM) Food Chem. Toxicol. 2009;47(8):1892–1902. doi: 10.1016/j.fct.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Organization of Economic Co-operation and Development (OECD) OECD. (Paris, France: Organization of Economic Co-operation and Development); 2001. The OECD Guideline for Testing of Chemical: 420 Acute Oral Toxicity-Fixed Dose Procudure; pp. 1–14. [Google Scholar]
- 11.Auletta C.S. Acute, subacute and chronic toxicology. In: Derelanko M.J., Hollinger M.A., editors. CRC Handbook of Toxicology. CRC Press; New York: 1995. pp. 51–104. [Google Scholar]
- 12.Bolliger P.A., Everds N. Hematology of the laboratory rodents. In: Weiss D.J., Wardrop K.J., editors. Schalm’s Veterinary Hematology. sixth edition. Blackwell Publishing; Iowa: 2010. pp. 852–862. [Google Scholar]
- 13.Danneman P.J., Suckow M.A., Brayton C.F. second edition. CRC Press; Boca Raton: 2013. The Laboratory Mouse; pp. 1–17. [Google Scholar]
- 14.Brouwers B., Pruniau V.P.E.G., Cauwelier E.J.G., Schuit F., Lerut E., Ectors N., Declercq J., Creemers J.W. Phlorizin pretreatment reduces acute renal toxicity in a mouse model for diabetic nephropathy. J. Biol. Chem. 2013;288(38):27200–27207. doi: 10.1074/jbc.M113.469486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everds N.E. Evaluation of clinical pathology data: correlating changes with other study data. Toxicol. Pathol. 2015;43(1):90–97. doi: 10.1177/0192623314555340. [DOI] [PubMed] [Google Scholar]
- 16.Mohammadian M., Mianabadi M., Zargari M., Karimpour A., Khalafi M., Amiri F.T. Effects of olive oil supplementation on sodium arsenate-induced hepatotoxicity in mice. Int. J. Prev. Med. 2018;9(59) doi: 10.4103/ijpvm.IJPVM_165_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloom J.C., Schade A.E., Brandt J.T. Toxic responses of the blood. In: Klaassen C.D., editor. Casarett and Doull’s Toxicology: the Basic Science of Poisons. eighth edition. McGraw-Hill Education; New York: 2013. pp. 527–558. [Google Scholar]
- 18.Weiss D.J. Leukocyte response to toxic injury. Toxicol. Pathol. 1993;21(2):135–140. doi: 10.1177/019262339302100204. [DOI] [PubMed] [Google Scholar]
- 19.Hall R.L., Everds N.E. Principles of clinical pathology for toxicology studies. In: Hayes A.W., editor. Principles and Methods of Toxicology. fifth edition. Informa Healthcare USA; New York: 2008. pp. 1317–1358. [Google Scholar]
- 20.Richard E.J., Murugan S., Bethapudi B., Illuri R., Mundkinajeddu D., Velusami C.C. Is Andrographis paniculata extract and andrographolide anaphylactic? Toxicol. Rep. 2017;4:431–437. doi: 10.1016/j.toxrep.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh A., Dar M.Y., Joshi B., Sharma B., Shrivastava S., Shukla S. Phytofabrication of silver nanoparticles: novel drug to overcome hepatocellular ailments. Toxicol. Rep. 2018;5:333–342. doi: 10.1016/j.toxrep.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]