Graphical abstract
Keywords: CCl4, Zingiber officinale, Liver injury, Histopathology, Female Wistar rats
Highlights
-
•
CCl4 induced degeneration of reticular fibre and increased collagen fibre deposition.
-
•
MEZOR treatment restored liver function and antioxidant homeostasis.
-
•
MEZOR’s pharmacological activities are conferred by inherent phytochemicals.
-
•
MEZOR acts via antioxidant, membrane stabilizing and tissue regenerative mechanisms.
Abstract
The world-wide increasing incidence of liver injury has attracted scientific interest in the exploration of better treatment or adjuvant treatment therapies. This study investigated the effects of methanol extract of Zingiber officinale (Roscoe) rhizome (MEZOR) in a Wistar rat model of carbon tetrachloride (CCl4)-induced liver injury. The study recruited thirty female Wistar rats that received graded doses of MEZOR (determined by its LD50) by oral gavage through an oral canula, for 4 consecutive weeks following 1 week oral administration of CCl4 (0.7 ml/kg in olive oil; 1:1, v/v) while livolin forte® (5.2 mg/kg p.o.) was used as a standard. CCl4 induced deleterious hepatic effects as revealed by the liver function biomarkers (AST, ALT, ALP and total protein), antioxidant indicators (GSH and CAT) and histopathological effects, demonstrated by H & E, Gordon and Sweet, Masson’s trichrome, PAS staining techniques as well as by quantificational analyses of the liver micrographs, using image–J. MEZOR treatment was associated with a dose-dependent and significant mitigation of the aforementioned parameters (p < 0.05). This study concluded that MEZOR is a potential therapeutic choice in the adjuvant treatment of subjects with chemically-induced liver injury.
1. Introduction
The liver is a vital organ that is burdened with the regulation of physiological processes like detoxification of biologically harmful substances, secretion, storage and metabolism [1,2]. Man can be exposed to drugs, pesticides and other myriad of chemicals either by way of life or job demands [3]. The cumulative effect of these exposures may result in liver disease or injury, causing deteriorating health conditions which sometimes lead to terminal illness due to the biological accumulation of toxic metabolites [4].
Carbon tetrachloride (CCl4) is a hepatotoxin that is widely used is scientific research to experimentally evaluate the potential of an agent’s protective or curative effects on the liver [[5], [6], [7]]. In modern science, developing toxicological methods that aim at providing scientific information on the deleterious effect of chemical agents (such as CCl4) have become current research trends with beneficial outcomes [8]. Although acute doses of CCl4 have been associated with liver injury that can result in liver failure if left unchecked, high doses of CCl4 have been reported in literature to cause nonspecific toxicity [9], including respiratory failure and depression of the central nervous system [9,10]. Nevertheless, a report in literature also showed that oxidative stress was induced following a single acute hepatotoxic dose of CCl4 [9]. The basic mechanism by which CCl4 induces liver injury is by the generation of free radicals with consequent deleterious alterations of the antioxidant system [7,10]. In an attempt to curb the increasing incidence of chemically-induced liver injury, researchers are exploring the intervention of some plant products with antioxidant potentials [11,12]; a scientific exploration that is producing beneficial outcomes. We, therefore, hypothesized that the intervention of a potent antioxidant may biologically mitigate the deleterious effects of CCl4, thus providing more suitable alternatives for the adjuvant therapy of chemically-induced liver injury.
Zingiber officinale, also known as “Ginger”, is a spice of food that has been recognized as a nutraceutic by the American Diabetic Association [2,13]. Basically, nutraceutics are functional foods that provide important health benefits that include prevention and treatment of disease conditions [2,13,14]. According to literatures, ginger rhizomes are rich in anti-oxidant activity due to the presence of potent polyphenols like 6-gingerol and shogaols [2,15]. Zingiber officinale plant is reputed to have a wide range of pharmacological activities some of which include anti-oxidant [18], anti-inflammatory [19], antitumor [20], anti-diabetic [21], anti-microbial [22], neuro-protective [23], and gastro-protective [20] potentials.
Generally, synthetic medicines are less readily available and relatively less affordable when compared with their plant-derived (nutraceutics) alternatives. Besides, inspirations for novel drug development can be provided by plant-derived medicines [16,17]. Although there are experimental evidences showing that ginger, as a nutraceutical agent, expresses liver-protecting therapeutic effects in male Wistar rats of chemically-induced liver injury [2,15,24,25], there is dearth of literature on the critical assessment of the histopathological effects of any of its extract as well as its effects in a female Wistar rat model of CCl4-induced hepatotoxicity, hence this study.
2. Materials and methods
2.1. Plant material, drug, chemicals and biochemical kits
Fresh rhizomes of Zingiber officinale were purchased from a commercial supplier at Sabo market of Ile-Ife, Osun State, Nigeria and authenticated by a Taxonomist (Mr. G.A. Ademoriyo) at the Department of Botany, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria.
Livolin forte® was purchased from Mega Life Science (Pty. Ltd., Australia; Batch number 107050), methanol was supplied by Crescent Chemical Co. Inc., New York (USA) while carbon tetrachloride was procured from Hopkins and Williams (England). Propylene glycol was supplied by Biovision Inc. (USA) while the assay kits for biochemical analyses were purchased from Randox Laboratory Ltd. (UK).
2.2. Extraction process
Fresh rhizomes of Zingiber officinale were peeled, weighed and thereafter pulverized with a waring blender (Waring Commercial, Torrington, CT) using 90% methanol. This was, thereafter, subjected to 48 h of constant shaking with the aid of an electric shaker. The resulting mixture was filtered under vacuum using Buchner funnel and Whatman number 2 Filter Paper (Whatman Plc, Middlesex, UK). The filtrate was concentrated with a rotary evaporator (Hahn Shin Scientific, HS-2005-N) and freeze-dried in a Lyophilizer (Ilshin Lab. Co. Ltd, Seoul, Republic of Korea). The resulting yield, which was methanol extract of Zingiber officinale rhizome (MEZOR), was weighed and kept in a desiccator until when needed.
The percentage yield of MEZOR was calculated as follows [18];
2.3. Phytochemical screening of MEZOR
Using standard protocols, phytochemical screening of the methanol extract of Zingiber officinale rhizomes has been described by existing literatures. The consistency of result obtained is depicted in Table 2 as described by Bhargava and coworkers [26] as well as Riaz and co-workers [27].
Table 2.
Phytochemical Screening of MEZOR.
| Phytochemical Constituent | Status |
|---|---|
| Alkaloids | + |
| Flavonoids | + |
| Tannins | + |
| Saponins | + |
| Cardiac glycosides | + |
| Terpenoids | + |
| Steroids | – |
+ = present; and - = absent.
2.4. Determination of oral lethal dose (LD50) of MEZOR
The oral LD50 of MEZOR was determined by Lorke’s method [28] as modified by Imafidon and co-workers [29]. The modification is the use of 8 rats in the second phase of study, rather than 4 rats as proposed by Lorke. In the first phase of study, 9 rats were divided into 3 groups of 3 rats each and were administered MEZOR at graded doses of 10, 100 and 1000 mg/kg, by oral gavage with the aid of an oral canula. The rats were observed for 24 h after which the first phase of study was terminated. In the second phase, 8 rats were divided into 4 groups of 2 rats each and were administered MEZOR at 750, 1500, 3000 and 6000 mg/kg, orally. They were also observed for 24 h after which the oral LD50 of the extract was determined by the formula below;
| LD50 = √a x b |
| Where a = least dose that killed a rat; and b = highest dose that did not kill any rat. |
2.5. Preparing solutions of MEZOR, Livolin Forte® and administration of CCl4
The choice of the adopted therapeutic doses was guided by the result of the oral LD50 of the extract. This was taken to be less than 10% of the oral LD50. Hence, doses of 100, 200 and 400 mg/kg of MEZOR, adopted for this study, where prepared as follows;
One gram (1 g) of MEZOR was dissolved in 20 ml of distilled water to prepare a stock solution of 100 mg/kg of MEZOR. Stock solutions of 200 and 400 mg/kg were prepared by dissolving 2 g and 4 g of MEZOR each in 20 ml of distilled water, respectively. The rats, therefore, received 0.2 ml/ 100 g of MEZOR, by oral gavage using an oral canula throughout the study period. Left-overs were stored in a deep-freezer after use and discarded after 48 h. Fresh samples were prepared every 48 h.
Propylene glycol was administered at 0.2 ml/100 g while livolin forte was administered at 5.2 mg/kg, which is the therapeutic dose of the drug in humans [3]. A capsule (376 mg) was dissolved in 20 ml of propylene glycol so that 0.04 ml of the resulting solution (equivalent of 0.75 mg of Livolin Forte) was administered to a 150 g rat, orally.
2.6. Experimental protocol
All experimental protocols were in strict compliance with the guidelines for animal research, as detailed in the NIH Guidelines for the Care and Use of Laboratory Animals [30] and approved by local institutional Research Committee. Thirty (30) female Wistar rats of about 2 months of age, weighing 120–130 g, were used for this study. They were purchased from the Animal Holding Unit of the College of Health Sciences, OAU, Ile-Ife, Osun State, Nigeria where the study was carried out. They were housed in plastic cages under natural light and dark cycle and allowed access to standard laboratory rat chow (ACE Feeds PLC, Osogbo – Nigeria) and water ad libitum.
The rats were divided into six groups of five rats each as follows; Group 1 (control) received propylene glycol by oral gavage through an oral canula at 0.2 ml/ 100 g throughout the study period (5 weeks). Group 2 (toxic) received CCl4, dissolved in olive oil (1:1 v/v) at 0.7 ml/kg for 7 alternating days. Group 3 (standard) were pre-treated as group 2 after which they received oral administration of Livolin forte® at 5.2 mg/kg for 4 consecutive weeks. Groups 4, 5 and 6 were each pre-treated as group 2 and thereafter received oral graded doses of the extract at 100, 200 and 400 mg/kg for 4 consecutive weeks (Table 4). At the end of the study, the rats were euthanized and their blood samples were collected into separate EDTA bottles by cardiac puncture.
Table 4.
Experimental Protocol and Dose Regimen.
| Groups (n = 5 per group) | 7 Days Oral Administration (CCl4 = 0.7 ml/kg in olive oil; 1:1, v/v) | 4 Weeks Oral Treatment |
|---|---|---|
| Group 1 | PG | PG* |
| Group 2 | CCl4* | – |
| Group 3 | CCl4 | Livolin Forte (5.2 mg/kg)* |
| Group 4 | CCl4 | 100 mg/kg of MEZOR* |
| Group 5 | CCl4 | 200 mg/kg of MEZOR* |
| Group 6 | CCl4 | 400 mg/kg of MEZOR* |
n = number of rat; CCl4 = carbon tetrachloride; PG = propylene glycol; MEZOR = methanol extract of Zingiber officinale (Roscoe) rhizome; * = point at which rats were sacrificed.
Blood samples were centrifuged at 4000 rpm for 15 min at -4 °C using a cold centrifuge (Centurium Scientific, Model 8881) to obtain the plasma. About 1 g of the liver of each rat was excised and kept in a cooler for the preparation of tissue homogenates while the other portion of each liver was fixed in 10% formal-saline solution for histopathological examinations.
2.7. Measurement of body and organ weight
Weekly body weight was assessed using Hanson digital weighing balance (Hanson, China) while organ weight was determined using Camry sensitive weighing balance (Camry, China). The percentage weight change (PWC) as well as relative liver weight (RLW) was determined using the formulae below [4,31,32];
2.8. Assessment of liver function biomarkers
Aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were assayed in the plasma of each rat, using Randox standard laboratory kits as described in the protocols provided. However, total protein was assayed in the liver homogenate using biuret method as described by Tietz [33].
2.9. Assessment of oxidative stress indicators
With the aid of an electric homogenizer (S1601001), 10% homogenate in phosphate buffer (100 mM) was prepared with the tissues at pH of 7.4. The homogenates were centrifuged at 3000 rpm for 20 min and the supernatants were collected for the assessment of the following indicators of oxidative stress;
Reduced glutathione (GSH) was assayed by the method of Beutler and co-workers [34] while the activity of catalase (CAT) was determined by the method of Sinha [35].
2.10. Histopathological examination
The liver of each rat was fixed in 10% formal-saline solution. Thereafter, they were dehydrated in graded alcohol and embedded in paraffin wax. Sections taken (7–8 μm thick) were stained using Hematoxylin–Eosin (H & E) technique (for general histoarchitectural appraisal); Periodic Acid Schiff (PAS) technique (for histochemical study); Gordon and Sweets’ silver staining technique (to appraise reticular fibre formation); as well as Masson’s Trichrome staining technique (for the assessment of liver collagen fibres).
Photomicrographs of each slide were taken with the aid of a Leica DM 750 microscope, interfaced with Leica ICC50 digital camera at objectives of x40. The representative micrographs were transported to “Image J” software for quantificational analyses.
2.11. Statistical analysis
Data obtained were expressed as mean ± Standard Error of Mean using one-way analysis of variance and thereafter subjected to Neuman Keuls’ post-hoc test. Student’s t-test was used to determine differences between two variables and, generally, the level of significance was set at p < 0.05. Data were analysed using Graph Pad Prism 5.03 (Graph Pad Software Inc., CA, USA).
3. Results
3.1. Percentage yield (%), phytochemical screening and oral LD50 of MEZOR
The percentage yield of MEZOR, after two different extraction processes, is as presented in Table 1.
Table 1.
Percentage Yield of MEZOR (%).
| Extraction Processes | Weight of peeled ginger rhizome (g) | Yield of MEZOR (g) | Percentage Yield (%) |
|---|---|---|---|
| 1 st Extraction | 1600 | 24.69 | 1.50 |
| 2nd Extraction | 1600 | 32.16 | 2.00 |
Result shows that the percentage yield of MEZOR is 1.75 ± 0.25% (where n = 2).
The phytochemical constituents of the extract are as presented in Table 2.
The oral lethal dose (LD50) of MEZOR is as presented in Table 3.
Table 3.
Acute Oral Toxicity Test (LD50) of MEZOR.
| 1ST PHASE | ||
|---|---|---|
| No of rats | Dose (mg/kg) | Mortality |
| 3 | 10 | 0/3 |
| 3 | 100 | 0/3 |
| 3 | 1000 | 0/3 |
| 2ND PHASE | ||
|---|---|---|
| No of rats | Dose (mg/kg) | Mortality |
| 2 | 750 | 0/2 |
| 2 | 1500 | 0/2 |
| 2 | 3000 | 0/2 |
| 2 | 6000 | 0/2 |
Least dose that killed a rat = nil.
Highest dose that did not kill any rat = 6000 mg/kg.
Therefore, oral LD50 of MEZOR is greater than 6000 mg/kg in Wistar rats.
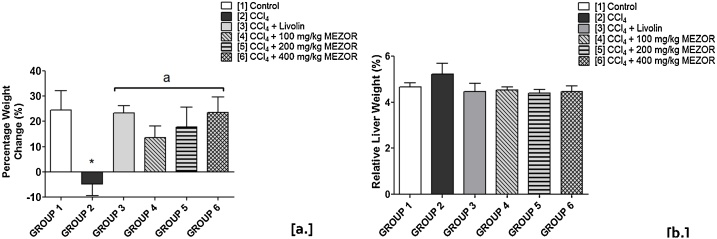
3.2. Effects of MEZOR on percentage weight change (%) and relative liver weight (%) of Wistar rats exposed to CCl4 toxicity
Following CCl4 administration, PWC was significantly lowered in the toxic group (-4.90 ± 4.48) when compared with the control group (24.43 ± 7.81) (p = 0.0115; t = 3.260). The MEZOR-treated groups 4, 5 and 6 (13.57 ± 4.65; 17.75 ± 7.95; and 23.49 ± 6.18 respectively) showed significantly higher PWC when compared with the toxic group (−4.90 ± 4.48) (p = 0.0224; F = 4.216) (Fig. 1a).
Fig. 1.
Effects of MEZOR on [a.] Percentage Weight Change (%), and [b.] Relative Liver Weight (%) of Female Wistar Rats Exposed to CCl4 Toxicity.
Each bar represents mean ± standard error of mean at p < 0.05.
* = significant difference when compared with the control group; and a = significant difference when compared with the toxic group.
Administration of CCl4 was found to be associated with an insignificantly higher RLW in the toxic group (5.22 ± 0.47) when compared with the control group (4.66 ± 0.18) (p = 0.3009; t = 1.106). The MEZOR-treated groups 4, 5 and 6 (4.54 ± 0.12; 4.40 ± 0.15; and 4.48 ± 0.24 respectively) showed a lower but insignificant RLW when compared with the toxic group (5.22 ± 0.47) (p = 0.1813; F = 1.836) (Fig. 1b).
3.3. Effects of MEZOR on plasma AST (U/L), ALT (U/L) and ALP (U/L) levels in Wistar rats with CCl4-induced liver injury
AST level was significantly elevated in the toxic group when compared with the control (p < 0.05). The MEZOR-treated groups showed a significantly lower level of AST when compared with the toxic group (p < 0.05) (Table 5).
Table 5.
Effects of MEZOR on the Plasma Levels of AST, ALT and ALP of Female Wistar Rats with CCl4-induced Liver Injury.
| Group | AST (U/I) | ALT (U/I) | ALP (U/I) |
|---|---|---|---|
| [1] Control | 63.57 ± 13.22 | 48.89 ± 1.03 | 13.89 ± 0.45 |
| [2] CCl4 | 127.42 ± 3.13* | 94.81 ± 3.56* | 26.68 ± 0.53* |
| [3] CCl4 + Livolin | 88.39 ± 1.03a | 56.86 ± 1.49*a | 15.95 ± 0.61a |
| [4] CCl4 + 100 mg/kg MEZOR | 113.34 ± 1.71ab | 75.16 ± 1.22*ab | 21.62 ± 0.46*ab |
| [5] CCl4 + 200 mg/kg MEZOR | 92.31 ± 0.89a | 61.48 ± 0.59*ab | 16.87 ± 0.61*ab |
| [6] CCl4 + 400 mg/kg MEZOR | 91.55 ± 1.13a | 53.12 ± 0.29*ab | 15.24 ± 0.80a |
Each value represents mean ± Standard Error of Mean at p < 0.05 [[1], [2], [3], [4], [5], [6]] = Groups 1–6.
* = significant difference when compared with the control group.
a = significant difference when compared with the toxic group.
b = significant difference when compared with the standard group.
ALT level was also significantly higher in the toxic group when compared with the control (p < 0.05). The MEZOR-treated groups 4, 5 and 6 showed significantly lowered AST level when compared with the toxic group (p < 0.05) (Table 5).
Also, the ALP level in the toxic group was significantly higher when compared with the control (p < 0.05). However, the MEZOR-treated groups 4, 5 and 6 showed significantly lowered ALP level when compared with the toxic group (p < 0.05) (Table 5).
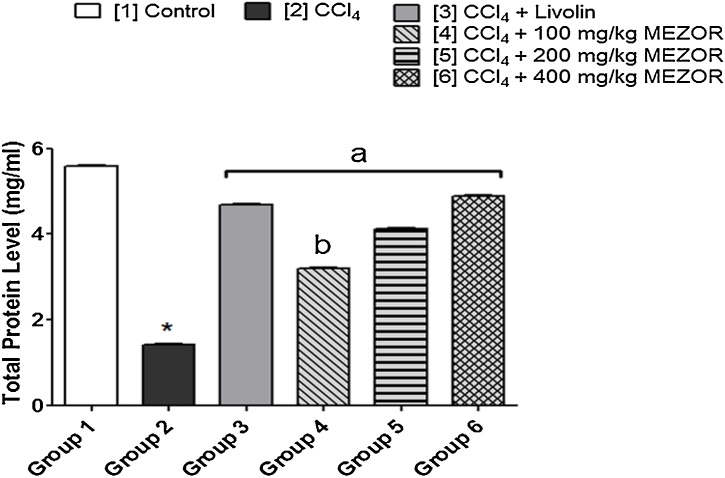
3.4. Effects of MEZOR on hepatic total protein (mg/ml) levels in Wistar rats with CCl4-induced liver injury
Carbon tetrachloride administration was associated with a significantly lowered hepatic total protein level in the toxic group when compared with both the control and standard groups (p < 0.05). The MEZOR-treated groups 4, 5 and 6 showed a significantly elevated level of hepatic total protein when compared with the toxic group (p < 0.05) (Fig. 5).
Fig. 5.
Effects of MEZOR on Total Protein Level in Female Wistar Rats with CCl4-induced Liver Injury.
Each bar represents mean ± standard error of mean at p < 0.05.
* = significant difference when compared with the control group.
a = significant difference when compared with the toxic group; and b = significant difference when compared with the standard group.
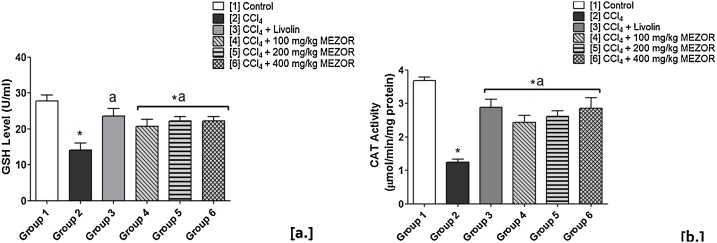
3.5. Effects of MEZOR on hepatic GSH (U/ml) and CAT (μmol/min/mg protein) activities in Wistar rats with CCl4-induced liver injury
The toxic group showed significantly lowered hepatic GSH level when compared with the control group (p < 0.05). There was a significantly higher GSH level in the MEZOR-treated groups 4, 5 and 6 when compared with the toxic group (p < 0.05) (Fig. 6).
Fig. 6.
Effects of MEZOR on [a.] GSH Level, and [b.] CAT Activity in Female Wistar Rats with CCl4-induced Liver Injury.
Each bar represents mean ± standard error of mean at p < 0.05.
* = significant difference when compared with the control group.
a = significant difference when compared with the toxic group; and b = significant difference when compared with the standard group.
The activity of CAT was significantly lowered in the toxic group when compared with the control group (p < 0.05). The MEZOR-treated groups 4, 5 and 6 recorded a significantly elevated activity of CAT when compared with the toxic group (p < 0.05) (Fig. 6).
3.6. Histological effects of MEZOR on the liver of Wistar rats exposed to CCl4 toxicity
3.6.1. Effects on the general histoarchitecture and number of hepatocytes (haematoxylin – eosin staining technique)
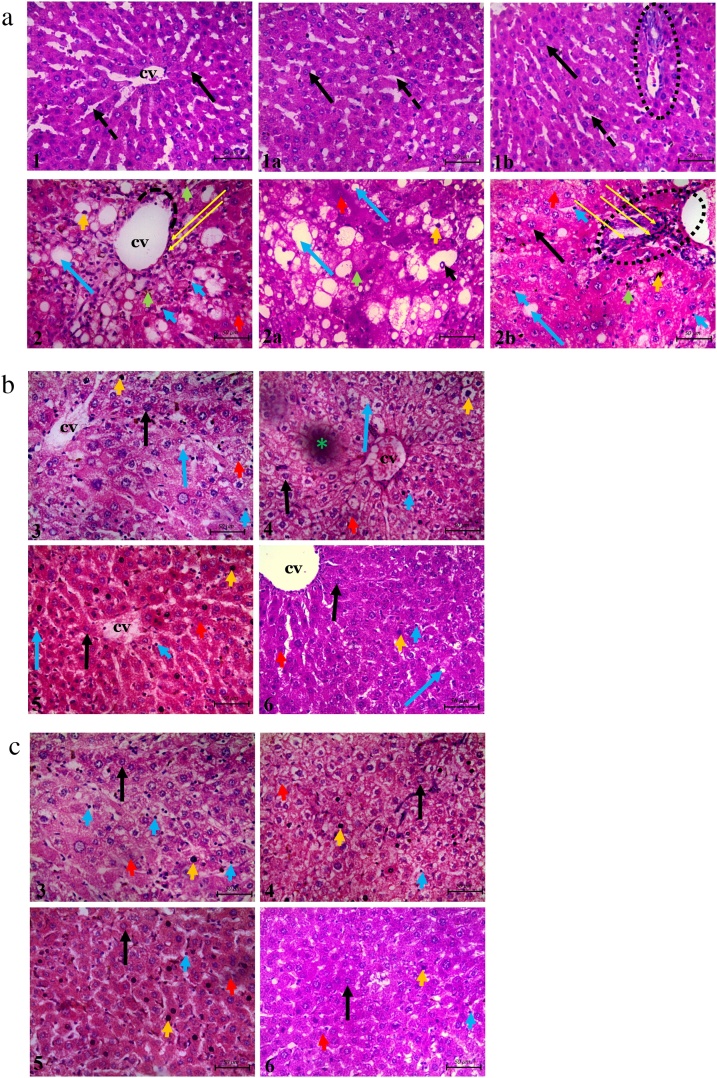
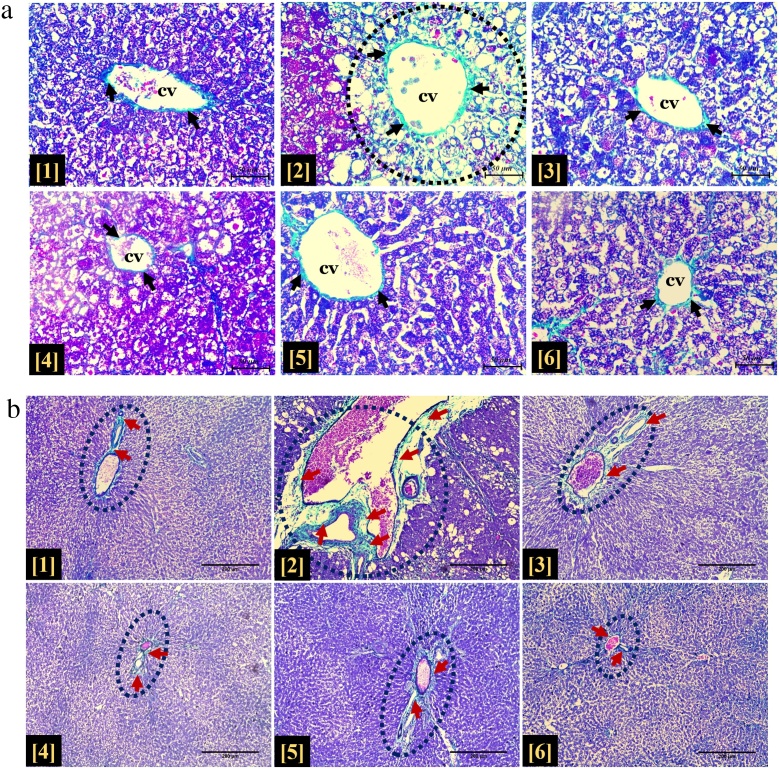
In the toxic group, microscopic examination using H & E staining showed evidence of necrotic hepatocytes and intra-cytoplasmic vacuolation at each zone of the hepatic lobule. This was in contrast to that of the control group. Similar evidence of apparently intact liver histoarchitecture was demonstrated in the MEZOR-treated groups 4, 5 and 6 when compared with both the toxic and control groups (Fig. 7).
Fig. 7.
Histological Effects of MEZOR in Female Wistar Rats with CCl4-induced Liver Injury; General Histoarchitecture Using H & E Staining Technique. Fig. 7a Light micrographs of the control and toxic groups showing the centrilobular zones Scale bar =50 μm. Fig. 7b Light micrographs of the standard (Livolin) and MEZOR-treated groups showing the centrilobular zones. Scale bar =50 μm. Fig. 7c Light micrographs of the standard (Livolin) and MEZOR-treated groups showing the intermediate zones. Scale bar =50 μm.
*1 and 2 = Groups 1 and 2.
Plates 1a and 2a = intermediate zones of liver histoarchitecture.
Plates 1b and 2b = portal areas of liver histoarchitecture.
*3 to 6 = Groups 3–6.
Scale bar =50 μm.
Long black arrow = apparently normal hepatocytes; CV = centrilobular vein; black dashed arrow = hepatic sinusoid; short red arrow = necrotic hepatocytes with nuclei karyolysis; short orange arrow = necrotic hepatocyte with heperchromatic nuclei; short green arrow = necrotic hepatocyte with fragmented nuclei; short blue arrow = massive proliferation of Kupffer cells at the luminal surface of the hepatic sinusoids; short black arrow = intranuclear vacuolation; long blue arrow = hepatic hepatocyte with intracytoplasmic vacuolation; black dotted circle = portal space; long yellow arrow = infiltration of the centrilobular zone by inflammatory cells; blach dotted arc = area of discontinuation of the simple squamous epithelial lining of the central vein; short blue arrow = massive proliferation of kupffer cells at the luminal surface of the hepatic sinusoids; green asterisk = artifact.
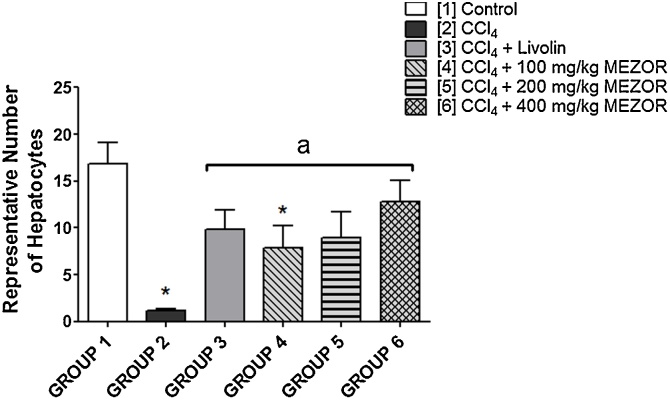
Quantification analysis of the hepatocytes showed a significantly lowered number of hepatocytes in the toxic group (1.17 ± 0.17) when compared with the control group (16.83 ± 2.26) (p < 0.0001; t = 6.92). The MEZOR-treated groups 4, 5 and 6 (7.83 ± 2.41; 9.00 ± 2.76; and 12.83 ± 2.21 respectively) had a significantly increased number of hepatocytes when compared with the toxic group (1.167 ± 0.17) (p = 0.0085; F = 5.140) (Fig. 2).
Fig. 2.
Effects of MEZOR on the Number of Hepatocyte Counts of Female Wistar Rats with CCl4-induced Liver Injury.
Each bar represents mean ± standard error of mean at p < 0.05.
* = significant difference when compared with the control group; and a = significant difference when compared with the toxic group.
3.6.2. Effects on reticular fibres formation and percentage area of reticular fibre (Gordon and sweet’s silver staining technique)
Administration of CCl4 was associated with degenerated and discontinuous reticular fibre in the toxic group, as observed by Gordon and Sweet’s silver staining technique. This was in contrast to that of the control. The MEZOR-treated groups showed similar features like that of the control (Fig. 8).
Fig. 8.
Histological effects of MEZOR on reticular fibre formation in Female Wistar Rats with CCl4-induced Liver Injury.
Gordon and Sweet’s silver stain (Magnification x 400; Scale bar =50 μm).
Long black arrow = apparently normal hepatocyte; yellow arrow = dark stained apparently intact reticular fibres surrounding individual hepatocyte; deep blue arrow = reticular fibre spanning across the perisinusoidal space; red arrow = reticular fibre spanning across the wall of the centrilobular vein; short black arrow = discontinuous reticular fibres surrounding the centrilobular vein; red dotted circle and light blue arrow = intracytoplasmic vacuolation; CV = centrilobular vein.
The quantification analysis of the percentage area of reticular fibre formation (PRF) showed a significantly lowered PRF in the toxic group (8.70 ± 0.69) when compared with that of the control (32.35 ± 0.92) (p < 0.0001; t = 20.58). However, the PRF of the MEZOR-treated groups 4, 5 and 6 increased significantly when compared with the toxic group (8.70 ± 0.69) (p > 0.0001; F = 45.41) (Fig. 3).
Fig. 3.
Effects of MEZOR on the Percentage Area of Reticular Fibre (%) of Female Wistar Rats with CCl4-induced Liver Injury.
Each bar represents mean ± standard error of mean at p < 0.05.
* = significant difference when compared with the control group.
a = significant difference when compared with the toxic group; and b = significant difference when compared with the standard group.
3.6.3. Effects on collagen fibres and percentage area of collagen fibres (Masson’s trichrome staining technique)
Histopathological examination revealed accumulation of collagen fibres at the periportal region with some deposit of collagen fibres surrounding the wall of the centrilobular vein in the toxic group. These features were in contrast to that of the control. The MEZOR-treated groups, as well as the standard, showed similar features as demonstrated by the standard and control groups (Fig. 9).
Fig. 9.
a Histological effects of MEZOR on collagen fibre formation in Female Wistar Rats with CCl4-induced Liver Injury.
Masson’s Trichrome Staining Technique (Magnification x100; Scale bar =200 μm).
Dark blue dotted circle = portal space; short dark red arrow = deposits of light green-stained collagen fibres. Fig. 9b Histological effects of MEZOR on collagen fibre formation in the hepatic centrilobular zone of Female Wistar Rats with CCl4-induced Liver Injury.
Masson’s Trichrome Staining Technique (Magnification x400; Scale bar =50 μm).
CV = centrilobular vein; short black arrow = light green-stained collagen fibres; short black arrow = dilation of centrilobular vein with marked deposits of collagen fibres; dotted circle = parenchymal tissue of the centrilobular zone.
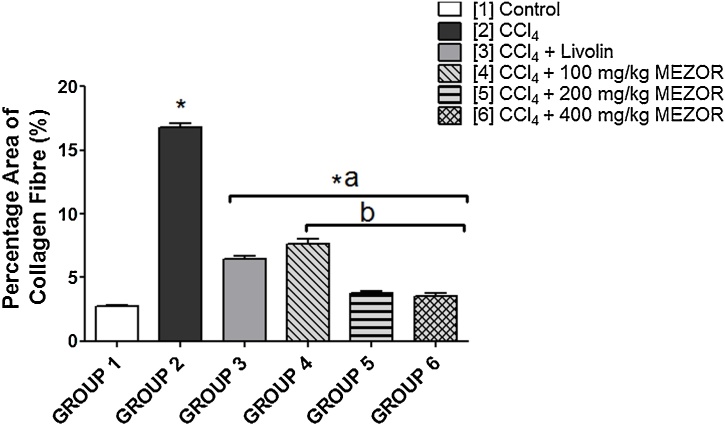
Quantification analysis of the percentage area of collagen fibre (PCF) showed a significant increase in PCF in the toxic group (16.78 ± 0.29) when compared with the control group (2.77 ± 0.04) (p < 0.0001; t = 46.98). The toxic group (16.78 ± 0.29) showed a significantly higher PCF when compared with the MEZOR-treated groups 4, 5 and 6 (7.63 ± 0.40; 3.81 ± 0.12; and 3.54 ± 0.20 respectively) (p < 0.0001) (Fig. 4).
Fig. 4.
Effects of MEZOR on the Percentage Area of Collagen Fibre (%) of Female Wistar Rats with CCl4-induced Liver Injury.
Each bar represents mean ± standard error of mean at p < 0.05.
* = significant difference when compared with the control group.
a = significant difference when compared with the toxic group; and b = significant difference when compared with the standard group.
3.6.4. Histochemical effects (Periodic Acid-Schiff staining technique)
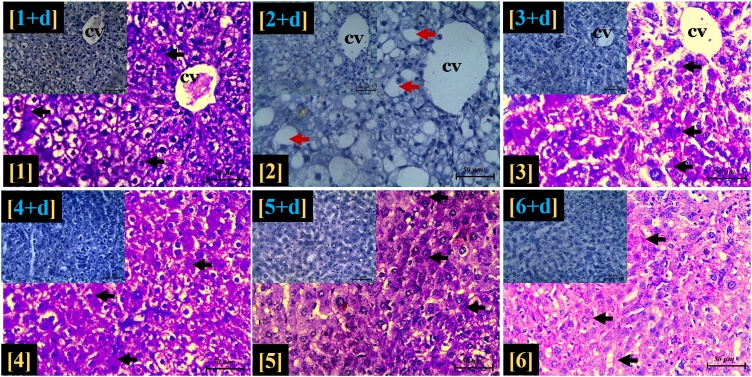
Histochemical assessments revealed a PAS-negative reaction in the toxic group, following exposure to CCl4 toxicity. This feature was in contrast to that of the control group. The evidence of PAS-positive materials was also observed in the standard group as well as the MEZOR-treated groups 4, 5 and 6 (Fig. 10).
Fig. 10.
Histological effects of MEZOR on the hepatic glycogen content of Female Wistar Rats with CCl4-induced Liver Injury.
Period Acid-Schiff (PAS) stain (magnification = ×400; Scale bar =50 μm).
Black arrow = intracytoplasmic PAS-positive substance (glycogen); CV = centrilobular vein; red arrow = intracytoplasmic vacuolation; insets = light micrographs with PAS stain and diastase (PAS + D) showing digestion of hepatic lobule glycogen content by diastase.
4. Discussion
Since weight gain or lose is determined by a balance between food intake and energy expenditure [29,36], the significantly lowered percentage weight change (PWC) that was associated with CCl4 administration can be attributed to a reduction in food intake that was observed during the study period. A secondary contributory factor to the observed reduction in food consumption may be the anaesthetic-mimicking effects of CCl4, which resulted in sluggishness of all body reflexes as well as lowered response to stimuli [37,38]. These attributes, according to our observational study, culminated in the reduction of both food consumption and body weight. This study, therefore, demonstrated the potential anti-analgesic as well as appetite-stimulating effects of MEZOR (in a dose-dependent manner), as the aforementioned conditions were significantly reversed with a resultant increase in PWC. These ameliorative effects can be attributed to the important phytochemical constituents, such as flavonoids and tannins, which are present in the extract. This study supports the findings of Atta et al who attributed the hepatoprotective effects of Zingiber officinale to the presence of flavonoids, tannins and unsaturated sterols [25].
Inflammatory response to liver injury and or a reduction in liver proteins can cause rapid mitosis of liver cells, resulting in liver growth to a larger size [39]. This phenomenon may be a possible explanation for the CCl4-induced increase in relative liver weight. The significant reductions in hepatic total protein level, with micrographic evidence of inflammatory response to CCl4 administration, were significantly attenuated by MEZOR treatment. These effects suggest a dose-dependent anti-inflammatory effect of the extract as well as its potential to re-integrate hepatic total protein to homeostatic levels. A similar finding of the anti-inflammatory effects of Zingiber officinale (but on male Wistar rats) has been reported by Young et al [19].
The mitigating effects of MEZOR on CCl4-induced deleterious alteration of liver function biomarkers are suggestive of its potential to restore normal liver functions via cytosolic and mitochondrial effects. This is because, by location, ALT is solely cytoplasmic while AST is both cytosolic (20%) and mitochondrial (80%) [40,41]. The implications of these mitigating effects include potentiation of reduced leakage of hepatic enzymes into the circulation by the extract through membrane-stabilizing and membrane protecting mechanisms. The synthesis as well as release of ALP from cell surfaces is enhanced by cholestasis (bile flow obstruction) [42,43]. This makes ALP a cholestatic index [44]. Since MEZOR treatment was associated with a significantly lowered plasma ALP level when compared with the toxic control, it is indicative of the extract’s potential to inhibit and or reverse the biological events that promotes cholestasis. This is, however, a subject for further study and verification.
The significant depletion of hepatic GSH and CAT levels can be attributed to their excessive use by the liver against CCl4-generated reacting oxygen species (ROS). It is an established fact that the basic mechanism of CCl4-induced liver injury is generation of free radicals as well as deleterious disruption of the antioxidant system [7]. However, existing literatures have implicated flavonoids to possess hepato-protective potentials due to their ability to scavenge free radicals, including hydroxyl, peroxyl and superoxide radicals and can form complexes with catalytic metal ions to render them inactive [15,45,46]. These flavonoids constitute part of the important phytochemicals of the extract (MEZOR). Therefore, the apparent restoration of normal antioxidant system by the extract clearly demonstrates its dose-dependent antioxidant capacity – the highest dose (400 mg/kg) producing the highest effects.
Reticular fibres are the mesh-like framework for organs, such as the liver, which provides most of the supporting connective tissue of the organ [47]. According to a report by Wen and co-workers [48], degradation or collapse of reticular fibres can be considered as a pathological characteristic of initiation and or progression of hepatic fibrosis. This suggests that MEZOR treatment potentially inhibits the initiation and or progression of hepatic fibrosis via tissue regenerative mechanism(s), as buttressed by H & E stain.
Excessive collagen accumulation is the most commonly associated characteristic of liver fibrosis and can result in hardening, with a consequent functional impairment, of the liver [49]. The micrographic evidence of reduced collagen fibre formation, following MEZOR treatment, further supports the pharmacological potential of the extract in mitigating the progression of liver pathophysiology to fibrosis.
The histochemical demonstration of decreased hepatic glycogen content indicates insulin-inhibiting and glucagon-promoting activities of CCl4. Ginger has been demonstrated in literatures to possess anti-hyperglycemic property via inhibition of oxidative stress and inflammatory processes as well as inducing increased insulin sensitivity [50,51]. This study, providing support for the anti-hyperglycemic potential of the extract, clearly demonstrated an increase in PAS-positive materials following MEZOR treatment. This effect indicated a possible MEZOR- associated restoration of hepatic glycogen homeostasis at tissue level.
This study recommends that liver assessment using electron microscopy and immuno-histochemical assay are worthy of investigation in order to further elucidate the extract`s mechanism of action. It is also recommended that the consumption of Ginger (to be used as spice in food) should be encouraged, as this study demonstrated its potential therapeutic effects on chemically-induced liver injury in a dose-dependent manner; the highest dose being the best effective dose. According to the US FDA document, ginger has been listed as one of the nutraceutics that are Generally Recognized as Safe (GRAS) [2,14]. This conclusion was made after a dose of 0.5–1.0 g of ginger powder ingested for a chronic period (3 months to 2.5 years, 2–3 times daily) was reported not to have any deleterious biological effects [2,14]. The adopted highest dose for this study (400 mg or 0.4 g, once daily) is lower than the aforementioned lower limit (0.5 g, 2–3 times daily) that produced no deleterious biological effects following chronic consumption. This study apparently provides a supporting evidence and or validation of the fact that between 0.2 – 0.5 g of ginger extract ingested 1–3 times daily in humans may sufficiently produce beneficial therapeutic effects in conditions of chemically-induced liver injury. This is, however, subject to further verification using human trials.
5. Conclusion
This study concluded that methanol extract of Zingiber officinale (Roscoe) rhizome mitigated CCl4-induced hepatotoxicity in female Wistar rats through anti-oxidant, membrane-stabilizing and tissue regenerative potentials. These pharmacological activities were conferred by the presence of important phytochemicals that were inherent in the extract. The extract, therefore, represents a potential therapeutic choice in adjuvant treatment or management of subjects with chemically-induced liver injury.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The authors wish to acknowledge the members of staff of the Animal Holding Unit of the College of Health Sciences, as well as those of the Central Science Laboratory, Obafemi Awolowo University, Ile-Ife, Osun State, for their kind support and technical assistance.
References
- 1.Shanmugasundaram P., Venkataraman S. Hepatoprotective and antioxidant effects of Hygrophila auriculata(K. Schum) Heine acanthaceae root extract. J. Ethnopharmacol. 2006;104:124–128. doi: 10.1016/j.jep.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 2.Motawi T.K., Hamed M.A., Shabana M.H., Hashem R.M., ABoul-Naser A.F. Zingiber officinale acts as a nutraceutic agent against liver fibrosis. Nutr. Metab. 2011;8:40–51. doi: 10.1186/1743-7075-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olukiran S.O., Akomolafe R.O., Bamitale K.D., Ajayi A.O., Okonji R.E., Bejide R.A. Protective and curative effects of Livolin forte® on carbon tetrachloride-induced liver damage in Wistar rats. J. Exp. Integr. Med. 2013;4(1):57–65. [Google Scholar]
- 4.Imafidon C.E., Olaoluwa S.O., Ogundipe D.J., Eluwole A.O., Adekunle I.A., Oke G.O. Acetonic extract of Vernonia amygdalina (Del.) attenuates Cd-induced liver injury: potential application in adjuvant heavy metal therapy. Toxicol. Rep. 2018;5:324–332. doi: 10.1016/j.toxrep.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H., Joon-Ki K., Jun-Ho C., Joo-Yeon J., Woo-Yong O., Dong C.K., Hee S.L., Yeong S.K., Sam S., Seung-Ho L., Sun-Mee L. Hepatoprotective effect of pinoresinol on carbon tetrachloride–induced hepatic damage in mice. J. Pharmacol. Sci. 2010;112:105–112. doi: 10.1254/jphs.09234fp. [DOI] [PubMed] [Google Scholar]
- 6.Muthu G.P., Balasubramanian R., Raju S.K., John W.E., Ekambaram P.K., Mani R.K., Kunchu K., Mohanraj P., Nia R., Paper D.H., Essien E.E., Iyadi K.C., Bassey A.I.L., Antai A.B., Franz G. Evaluation of the anti-oxidant and anti-angiogenic effects of sphenocentrum jollyanum pierre. Afr. J. Biomed. Res. 2005;8:47–50. [Google Scholar]
- 7.Vinoth K., Sivaraj A., Elumalai E.K., Senthil Kumar B. Carbon tetrachloride-induced hepatotoxicity in rats – protective role of aqueous leaf extracts of Coccinia grandis. Int. J. Pharm. Technol. Res. 2009;1(4):1612–1615. [Google Scholar]
- 8.Nadezhda V.T., Svetlana I.S. Model of vitamin and mineral deficiency for toxicological research: apoptosis activity under conditions of CCl4 intoxication. Toxicol Rep. 2019;6:151–154. doi: 10.1016/j.toxrep.2018.12.005. ISSN 2214-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritesh K.R., Suganya A., Dileepkumar H.V., Rajashekar Y., Shivanandappa T. A single acute hepatotoxic dose of CCl4 causes oxidative stress in the rat brain. Toxicol. Rep. 2015;2:891–895. doi: 10.1016/j.toxrep.2015.05.012. ISSN 2214-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Recknagel R.O., Glende E.A., Dolak J.A., Waller R.L. Mechanisms of carbon tetrachloride toxicity. Pharmacol. Ther. 1989;43:139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 11.Yavar M., Mohammad M., Lotfollah R. Hepatoprotective effect of methanolic Tanacetum parthenium extract on CCl4-induced liver damage in rats. Toxicol. Rep. 2017;4:455–462. doi: 10.1016/j.toxrep.2017.08.003. ISSN 2214-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elgawish R.A.R., Abdelrahman H.G., Abdelrazek H.M.A. Green tea extract attenuates CCl4-induced hepatic injury in male hamsters via inhibition of lipid peroxidation and p53-mediated apoptosis. Toxicol. Rep. 2015;2:1149–1156. doi: 10.1016/j.toxrep.2015.08.001. ISSN 2214-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloch A., Thomson C.A. Position of the American Diabetic Association: phytochemicals and functional foods. J. Am. Diet Assoc. 1995;95:406–493. doi: 10.1016/s0002-8223(95)00130-1. [DOI] [PubMed] [Google Scholar]
- 14.Langner E., Greifenberg S., Gruenwald J. Ginger: history and use. Adv. Ther. 1998;15:25–30. [PubMed] [Google Scholar]
- 15.Stoilova I., Krastanov A., Stoyanova A., Denev P., Gargova S. Antioxidant activity of a ginger extract (Zingiber officinale) Food Chem. 2007;102:764–770. [Google Scholar]
- 16.Iwu M.M., Duncan A.R., Okunji C.O. New antimicrobials of plant origin. In: Janick J., editor. Prospective on New Crops and New Uses. ASHS Press; Alexan-dria: 1999. [Google Scholar]
- 17.Olaleye R.O., Akomolafe R.O., Imafidon C.E., Ogundipe D.J., Olukiran S.O., Oladele A.A. Treatment with methanolic extract of Ocimum gratissimum (Linn.) leaf reversibly normalizes urine protein-creatinine ratio in Wistar rat model of gentamicin-induced kidney injury. Int. J. Med. Biomed. Res. 2016;5(3):155–171. [Google Scholar]
- 18.Adekunle I.A., Imafidon C.E., Oladele A.A., Ayoka A.O. Ginger polyphenols attenuate cyclosporine-induced disturbances in kidney function: potential application in adjuvant transplant therapy. Pathophysics. 2018;25:101–115. doi: 10.1016/j.pathophys.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Young H.Y., Luo Y.L., Cheng H.Y., Hsieh W.C., Liao J.C., Peng W.H. Analgesic and anti-inflammatory activities of [6]-gingerol. J. Ethnopharmacol. 2005;96:207–310. doi: 10.1016/j.jep.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Rahmani A.H., Fahad M.A., Salah M.A. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int. J. Physiol. Pathophysiol. Pharmacol. 2014;6(2):125–136. [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Amin Z.M., Thomson M., Al-Qattan K.K., Peltonen-Shalaby R., Ali M. Anti-diabetic and hypolidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br. J. Nutr. 2006;96:660–666. doi: 10.1079/bjn20061849. [DOI] [PubMed] [Google Scholar]
- 22.Azu N., Onyeagba R. Antimicrobial properties of extracts of Allium cepa (onions) and Zingiber officinale (ginger) On Escherichia coli, Salmonella typhi and Bacillus subtilis. Intern. J. Trop. Med. 2007;3:1–10. [Google Scholar]
- 23.Ha S.K., Moon E., Ju M.S., Kim D.H., Ryu J.H., Oh M.S., Kim S.Y. 6- shogaol, a ginger product, modulates neuroinflammation: a new approach to neuroprotection. Neuropharm. 2012;63:211–223. doi: 10.1016/j.neuropharm.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Yemitan O.K., Izegbu M.C. Protective effects of Zingiber officinale (Zingiberaceae) against carbontetrachloride and acetaminophen-induced hepatotoxicity in rats. Phytother. Res. 2006;20:997–1002. doi: 10.1002/ptr.1957. [DOI] [PubMed] [Google Scholar]
- 25.Atta A.H., Elkoly T.A., Mouneir S.M., Kamel G., Alwabel N.A., Shaimaa Z. Hepatoprotective effect of methanol extracts of Zingiber officinale and Cichorium intybus. Indian J. Pharm. Sci. 2010;72:564–570. doi: 10.4103/0250-474X.78521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhargava S., Dhabhai K., Batra A., Sharma A., Malhotra A. Zingiber officinale: chemical and phytochemical screening and evaluation of its antimicrobial activities. J. Chem. Pharmaceut. Res. 2012;4:360–364. [Google Scholar]
- 27.Riaz H., Almas B., Syed A., Zia M.K., Hamad Y., Ayesha T. Antimicrobial property and phytochemical study of ginger found in local area of Punjab, Pakistan. Intern. Curr. Pharmaceut. J. 2015;4(7):405–409. [Google Scholar]
- 28.Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 29.Imafidon C.E., Akomolafe R.O., Abubakar S.A., Ogundipe D.J., Olaoluwa O.S., Oladele A.A. Polyphenol-rich extract of Vernonia amygdalina (Del.) Leaves ameliorated cadmium-induced alterations in feeding pattern and urine volume of male wistar rats. J. Intercul. Ethnopharm. 2015;4(4):284–292. doi: 10.5455/jice.20151107021034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.8th edition. 2011. Guide for the Care and Use of Laboratory Animals.https://grants.nih.gov/grants//Guide-for-the-Care-and-use-of-laboratory-animals.pdf (Accessed 5 October 2015) [Google Scholar]
- 31.Ayoka A.O., Ademoye A.K., Imafidon C.E., Ojo O.E., Oladele A.A. Aqueous extract of Allium sativum (Linn.) bulbs ameliorated pituitary-testicular injury and dysfunction in Wistar rats with Pb-induced reproductive disturbances. Open Access Maced. J. Med. Sci. 2016;4(2):200–212. doi: 10.3889/oamjms.2016.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imafidon C.E., Olatoye T.R., Bamidele F.S., Ojo O.E., Ademoye K.A. Cadmium-induced testicular toxicity, oxidative stress and histopathology in Wistar rats: sustained effects of polyphenol-rich extract of Vernonia amygdalina (Del.) leaf. J. Interdiscipl. Histopathol. 2016;4(3):54–62. [Google Scholar]
- 33.Tietz A. Saunders; 2007. Tietz Fundamentals of Clinical Chemistry. eBook ISBN: 9781437719406. [Google Scholar]
- 34.Beutler E., Duron O., Kelly B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 35.Sinha A. Colorimetric assay of catalase. Anal. Biochem. 1971;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 36.Katherine A.S., Niamh M.M., Steve R.B. Hypothalamic regulation of apetite. Expert Rev. Endocrinol. Metab. 2008;3(2008):577–592. doi: 10.1586/17446651.3.5.577. [DOI] [PubMed] [Google Scholar]
- 37.Mir A., Farida A., Naveeda R., Hina I., Hussain M., Wahedi1 J., Miri P., Bae J., Lee D.S. Antibacterial activity of [10] -gingerol and [12] -gingerol isolated from ginger rhizome against periodontal bacteria. Phytothery. Res. 2008;22:1446–1449. doi: 10.1002/ptr.2473. [DOI] [PubMed] [Google Scholar]
- 38.Sherlock S.T., Dooley J. 8th Ed. Vol. 11–12. 1989. Oxford, Black Well; pp. 584–617. (Diseases of the Liver Biliary System). [Google Scholar]
- 39.Guyton A.C., Hall J.E. 10th ed. Published by W.B. Saunders Company; Philadelphia, Pennsylvania: 2001. Textbook of Medical Physiology, Harcourt International Edition; pp. 749–805. [Google Scholar]
- 40.Mauro P., Renze B., Wouter W. 4th ed. Elsevier; 2006. Enzymes: Tietz Text Book of Clinical Chemistry and Molecular Diagnostics; pp. 604–616. 2006. [Google Scholar]
- 41.Thapa B.R., Anuj W. Liver function tests and their interpretation. Indian. J. Pediatr. 2007;74:663–671. doi: 10.1007/s12098-007-0118-7. [DOI] [PubMed] [Google Scholar]
- 42.Giannini E.G., Roberto T., Vincenzo S. Liver enzyme alteration: a guide for clinicians. Can. Med. Assoc. J. 2005;172(3):367–379. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moss D.W. Physiochemical and pathophysiological factors in the release of membrane-bound alkaline phosphatase from cells. Clin. Chim. Acta. 1997;257(1):133–140. doi: 10.1016/s0009-8981(96)06438-8. [DOI] [PubMed] [Google Scholar]
- 44.Nouf R., Laila F., Iman S., Azza M.M., Nawal R., Nayira A. Assessment of the potential role of silymarin alone or in combination with Vitamin E and/ or Curcumin on the carbon tetrachloride induced liver injury in rat. Braz. Arch. Boil. Technol. 2015;58(6):833–842. [Google Scholar]
- 45.Milda E.E. Spices and herbs: natural sources of antioxidants – a mini review. J. Funct. Foods. 2015;18:811–819. [Google Scholar]
- 46.Shirin A.P.R., Jamuna P. Chemical composition and antioxidant properties of ginger root (Zingiber officinale. J. Med. Plants Res. 1996;4(24):2674–2679. [Google Scholar]
- 47.Junqueira L.C., Carneiro J. 10th ed. 2010. Basic Histology. Lange International Edition; pp. 332–343. [Google Scholar]
- 48.Wen S., Feng S., Tang S., Gao J., Zhang L., Tong H., Yan Z., Fang D. Collapsed reticular network and its possible mechanism during the initiation and/ or progression of hepatic fibrosis. Sci. Rep. 2016;6:35426. doi: 10.1038/srep35426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Motawi T.K., Hamed M.A., Shabana M.H., Hashen R.M., Naser A.F.A. Zingiber officinale acts as a nutraceutica agent against liver fibrosis. Nutr. Metab. 2011 doi: 10.1186/1743-7075-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shanmugam K.R., Mallikarjuna K., Kesireddy N., Reddy K.S. Neuroprotective effect of ginger on anti-oxidant enzymes in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2011;49:893–897. doi: 10.1016/j.fct.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 51.Vats V., Grover J.K., Rathi S.S. Evaluation of anti-hyperglycemic and hypoglycemic effect of trigonellafoenum graecumlinn, Ocimum sanctum Linn and Pterocarpus marsupium Linn in Normal and alloxanized diabetic rats. J. Ethnopharmacol. 2002;79:95–100. doi: 10.1016/s0378-8741(01)00374-9. [DOI] [PubMed] [Google Scholar]