ABSTRACT
We aimed to determine how increases in intracellular H+ and inorganic phosphate (Pi) to levels observed during anoxic submergence affect contractility in ventricular muscle of the anoxia-tolerant Western painted turtle, Chrysemys picta bellii. Skinned multicellular preparations were exposed to six treatments with physiologically relevant levels of pH (7.4, 7.0, 6.6) and Pi (3 and 8 mmol l−1). Each preparation was tested in a range of calcium concentrations (pCa 9.0–4.5) to determine the pCa–tension relationship for each treatment. Acidosis significantly decreased contractility by decreasing Ca2+ sensitivity (pCa50) and tension development (P<0.001). Increasing [Pi] also decreased contractility by decreasing tension development at every pH level (P<0.001) but, alone, did not affect Ca2+ sensitivity (P=0.689). Simultaneous increases in [H+] and [Pi] interacted to attenuate the decreased tension development and Ca2+ sensitivity (P<0.001), possibly reflecting a decreased sensitivity to Pi when it is present as the dihydrogen phosphate form, which increases as pH decreases. Compared with that of mammals, the ventricle of turtles exhibits higher Ca2+ sensitivity, which is consistent with previous studies of ectothermic vertebrates.
KEY WORDS: Acidosis, Calcium, Contractility, Force development, Inorganic phosphate, Reptile
Summary: Myofilament contractility of turtle ventricle is more sensitive to physiological levels of acidosis than to increased inorganic phosphate. The myofilament calcium sensitivities of ventricles from turtles and other ectotherms are similar.
INTRODUCTION
The western painted turtle, Chrysemys picta bellii, is the most anoxia-tolerant tetrapod known, having been shown to survive submergence in anoxic water for more than 170 days at 3°C (Ultsch and Jackson, 1982). During anoxia, the turtle heart maintains a reduced level of cardiac function (Farrell et al., 1994; Herbert and Jackson 1985; Hicks and Farrell, 2000; Hicks and Wang, 1998; Jackson, 1987), in contrast to the mammalian heart, which shows rapid and complete cardiac failure (Boutilier, 2001; Gesser and Jorgensen, 1982; Orchard and Kentish, 1990). Functional suppression in turtles is achieved by minimizing heart rate and contractility, allowing for continued function under conditions unfavorable for high rates of ATP synthesis (Shi et al., 1999; Stecyk et al., 2004; Wasser et al., 1990a,b). Avoiding complete circulatory arrest, however, is necessary to ensure adequate convective transport of glucose from the liver to the periphery and transport of lactic acid to the shell for buffering (Jackson, 1997; Jackson and Heisler, 1982; Jackson et al., 1996; Keiver and Hochachka, 1991; Keiver et al., 1992).
The mechanism of anoxia-induced contractile suppression in turtles is thought to be primarily intrinsic, especially at cooler temperatures, and to involve changes in the sarcoplasm that directly affect myofilament function (Jackson et al., 1995; Stecyk et al., 2009). For example, during anoxia, intracellular lactic acidosis results in a large decrease in intracellular pH (pHi) of the turtle heart (Jackson, 2002; Jackson and Heisler, 1983; Warren and Jackson, 2007). Although the effects of acidosis on ventricular function have been characterized at both the organ and tissue level in painted turtles (Jackson et al., 1995; Shi and Jackson, 1997; Wasser et al., 1990b), the direct effects of pHi on myofilament function have yet to be characterized.
Even less well understood is how myofilament function is affected by intracellular inorganic phosphate (Pi), which increases during anoxia (Stecyk et al., 2009; Wasser et al., 1990b). In painted turtle hearts, intracellular [Pi] increases by 65% during 4 h of anoxia, suggesting rapid [Pi] increases may also have a role in immediately decreasing contractility (Wasser et al., 1990b). Atrial myofilaments from the relatively anoxia-tolerant slider turtle, Trachemys scripta, showed a 47% decrease in maximal force development as well as reduced Ca2+ sensitivity with a 6 mmol l−1 increase in Pi, indicating that [Pi] may play a role in suppressing ventricular function during anoxia in turtles (Jensen and Gesser, 1999). Furthermore, in mammals, skinned ventricular myocytes showed decreased contractile force and calcium sensitivity when exposed to increasing [Pi] (Kentish, 1986). In the heart of slider turtles, intracellular [Pi] increases during anoxia from 3.2 to 7.7 µmol g−1 at 21°C and from 4.1 to 8.8 µmol g−1 at 5°C, suggesting that elevated Pi may account for some of the functional suppression observed during anoxia (Stecyk et al., 2009). The direct effect that this physiologically relevant increase in [Pi] might have on myofilament function in turtle ventricle has yet to be determined.
In the present study, we investigated how physiologically relevant changes in pH and Pi affect intracellular cardiac mechanics in the ventricle of the anoxia-tolerant painted turtle. By skinning multicellular preparations, we controlled intracellular conditions, allowing for isolated measurements of myofilament tension development. We simultaneously varied pH (6.6, 7.0 and 7.4) and Pi concentrations (3 and 8 mmol l−1) across levels known to exist in the hearts of anoxic turtles while quantifying their effects on myofilament calcium sensitivity (pCa50) and force production. We also compared our results with similar studies in other vertebrates to determine how these properties vary across different taxa.
MATERIALS AND METHODS
Animals
Western painted turtles, Chrysemys picta bellii (Gray 1831) (both sexes, 174–211 g, purchased from Niles Biological, Sacramento, CA, USA) were housed in fiberglass aquaria filled with dechlorinated, St Louis (MO, USA) municipal water (18–22°C) with access to basking platforms bathed with 10 W UV and 90 W incandescent heating lights that followed a weekly updated, natural, St Louis photoperiod. They were fed ReptoMin® three times a week ad libitum, and liver once per week. All housing and animal procedures were approved by the Saint Louis University Institutional Animal Care and Use Committee protocol 2198.
List of abbreviations.
- [Ca2+50]
free Ca2+ concentration required to produce 50% of maximum Ca2+ activated tension
- E–C coupling
excitation–contraction coupling
- l0
isometric length
- n
Hill constant
- Pact
Ca2+ activated tension
- Pi
intracellular inorganic phosphate
- Ppas
passive tension
- Ptotal
steady-state isometric tension
- pCa50
calcium sensitivity (−log10[Ca2+50])
- pHi
intracellular pH
- SR
sarcoplasmic reticulum
Muscle preparation
All muscle preparations were made following previously published protocols (Campbell et al., 2003; Fitzsimons et al., 2001) from halved ventricles previously flash frozen between liquid nitrogen-cooled tongs and stored at −80°C. Each frozen tissue was mechanically disrupted using a Polytron blender in 10 ml of ice-cooled digestion solution that contained the following (in mmol l−1): 100 KCl, 20 imidazole, 2 EGTA, 4 ATP, 7 MgCl2, 0.04 leupeptin and 0.5 phenylmethanesulfonyl fluoride (PMSF). The resulting homogenate was centrifuged at 650–1360 g for 1 min to form a pellet and the supernatant discarded. The pellet was washed by suspension in 10 ml of additional digestion solution, followed by additional centrifugation at 650–1360 g for 1 min until pellet formation. After the supernatant was discarded, the pellet was resuspended in digestion solution containing 1% Triton X-100 and placed on a shaker for 20 min at 8°C. If mechanical disruption yielded muscle fragments larger than approximately 1 mm in length, incubation in Triton X-100 was continued for an additional 10 min to ensure adequate membrane permeabilization. Following incubation, the muscle preparations were centrifuged at 650–1360 g for 1 min to form a pellet, the supernatant discarded, and the pellet resuspended in 10 ml relax solution with a composition identical to that of the digestion solution except it did not contain leupeptin or PMSF. The preparation was washed at least 2–4 additional times, depending on the presence of visible detergent bubbles, and then stored on ice in 10 ml of relax solution until experimentation.
Experimental solutions
The compositions of the pCa solutions used in this study were similar to those from previous studies and are given in Tables S1 and S2 (Campbell and Holbrook, 2007; Campbell and Moss, 2000, 2002; Campbell et al., 2003; Fitzsimons et al., 2001). Six treatments reflected biologically relevant pH levels (7.4, 7.0, 6.6) and Pi levels (3 and 8 mmol l−1 added Pi). Maxchelator 2.50 software was used to determine pCa (−log10[Ca2+]) solution compositions as previously described (Campbell and Holbrook, 2007; Campbell et al., 2003), while the following were held constant (in mmol l−1): 20 imidazole, 14.5 creatine phosphate, 7 EGTA, 1 Mg2+ free and 4 MgATP bound. KCl was used to maintain 180 mmol l−1 ionic strength across batches. The added Pi levels (3 or 8 mmol l−1) augmented the basal contaminant Pi which has been estimated at ∼0.7 mmol l−1 (Campbell and Holbrook, 2007).
Solutions were prepared at 22°C, and the appropriate pH was reached using 4 mol l−1 KOH. For each treatment, 13 different solutions were made to cover a range in Ca2+ (1–32,000 nmol l−1). To obtain intermediate calcium concentrations, solutions with pCa 4.5 and 9.0 were mixed according to proportions determined using Maxchelator 2.50 software. Creatine phosphate was obtained from Santa Cruz Biotechnology (Dallas, TX, USA), ATP was obtained from Roche Life Science (Indianapolis, IN, USA) and CaCl2 was obtained from Thermo-Fisher Scientific (Waltham, MA, USA). All other reagents were obtained from Sigma-Aldrich (St Louis, MO, USA).
Experiments
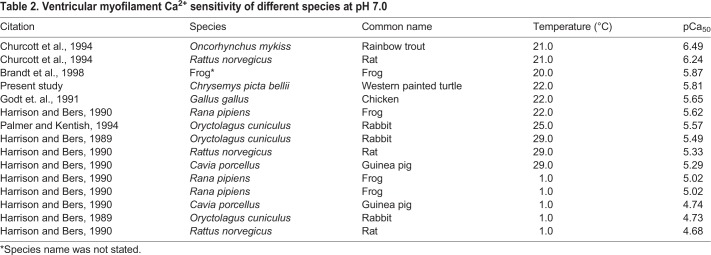
Skinned multicellular preparations were held between a force transducer (resonant frequency, 600 Hz; model 403, Aurora Scientific, Aurora, ON, Canada) and a motor (Series 602X Servo System, Cambridge Technology Inc., Lexington, MA, USA). Muscle preparations that were approximately 200 µm in length and 10–40 µm in width were chosen for experimentation. Ideally, unicellular preparations would be used to isolate force production specific to intracellular changes; however, force production could not be isolated from experimental noise in these smaller preparations. Pins attached to the force transducer and the motor were coated with 100% clear silicone sealant and subsequently positioned over and pressed into the muscle preparation for 10–15 min, allowing the sealant to set. Once the preparation was attached, the pins were raised, suspending the preparation in relax solution between the force transducer and motor (Fig. 1). The preparation was moved into storing solution (pCa 9.0, pH 7.0, Pi 3 mmol l−1), where it was stretched to a sarcomere length of 2.2±0.0034 µm (mean±s.e.m., N=24) and held isometrically (isometric length l0, 146±7.28 µm, N=4) at 22°C. Cross-sectional area (CSA) was estimated (2.39±0.246 nm2), assuming preparations were cylindrically shaped and preparation width was an accurate representation of diameter.
Fig. 1.
Loaded multicellular preparations from turtle ventricle. A multicellular preparation attached between a motor and a force transducer and stored in pCa 9.0, pH 7.0, 3 mmol l−1 Pi. The preparation has been stretched to a sarcomere length of 2.2 µm.
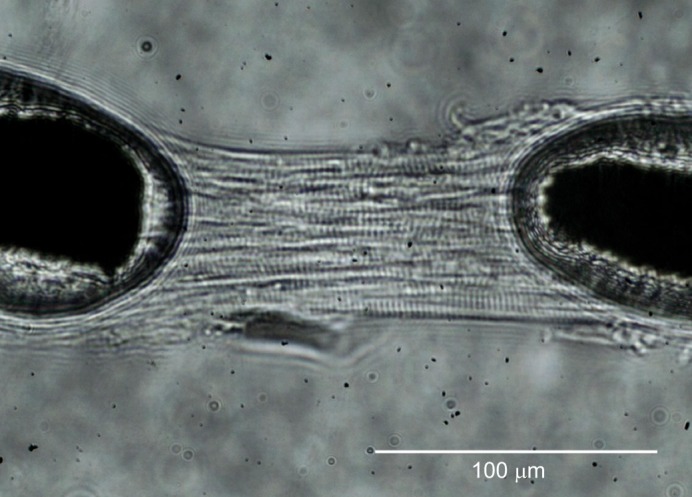
SLControl 4.5.2 software (www.uky.edu/~kscamp3/SLControl/) was used to perform all experimental stretches and shortenings of the muscle preparations (Campbell and Moss, 2003). Prior to activation, passive tension development (1.92±0.20 kN m−2, N=12), was measured in relax solution (pCa 9.0, pH 7.0, Pi 3 mmol l−1). Preparations underwent two triangular, 0.02 l0 stretches from l0 (2000 ms duration each), followed by a 0.1 l0 shortening/re-stretch step for 20 ms (Fig. 2). Each preparation was then immersed in a control activation solution of pCa 4.5, pH 7.0, Pi 3 mmol l−1, after which tension development was allowed to stabilize to determine control, steady-state isometric tension (41.3±3.8 kN m−2, N=12). Each preparation was pseudo-randomly transferred between various pCa solutions (pCa 9.0–4.5) to obtain a Ca2+ activation curve. To prevent run-down of the muscle, preparations were not introduced to all pCa solutions, and activation was halted after the Ca2+ activation curve was characterized. A pCa–isometric tension relationship was determined for each treatment (pH 7.4, 7.0 or 6.6 combined with an added Pi of either 3 or 8 mmol l−1). To monitor run-down, preparations were transferred to the control activation solution between every two or three experimental activations. When a preparation began to exhibit run-down, reflected by a decrease in control, steady-state isometric force by greater than 15%, a new preparation from the same ventricular tissue was used to obtain pCa–isometric tension relationships for any remaining treatments.
Fig. 2.
Mechanical response to experimental stretching and shortening during activation and inactivation. At pH 7.0, 3 mmol l−1 Pi, a multicellular preparation underwent two 0.02 isometric length (l0) stretches (2000 ms) followed by a 0.1 l0 shortening/re-stretching step (20 ms) at pCa 4.5 (activation) and pCa 9.0 (inactivation). Length changes were separated by 1 s.
Statistical analysis
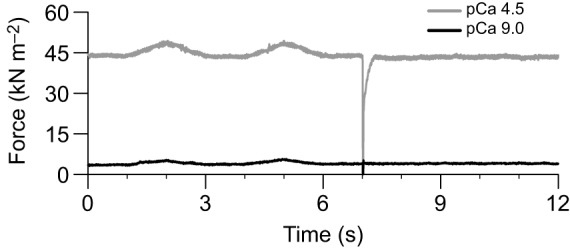
Tension development curves using the Hill equation were determined to characterize calcium binding affinity to the contractile apparatus:
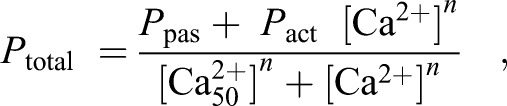 |
(1) |
where Ptotal is the steady-state isometric tension, Ppas is passive tension, Pact is Ca2+-activated tension, n is the Hill constant and [Ca2+50] is the free Ca2+ concentration needed to produce 50% of maximum Ca2+ activated tension. [Ca2+50] values are reported in this work as pCa50 values where pCa50 =−log10[Ca2+50]. Relative tension values were calculated by normalizing to the maximum tension measured for the preparation at pH 7.0 and low Pi (3 mmol l−1) in order to remove the influence of differences in absolute force between multicellular preparations.
Model I, two-way ANOVAs were used to determine the effect of pH and Pi on the curve parameters pCa50, n, Ppas and Pact. When ANOVA revealed a significant treatment effect, post hoc analysis was performed using Holm-corrected pairwise t-tests to determine differences between treatments. All statistical computations were completed using R package version 3.2.4.
RESULTS
Effects of acidosis and Pi on pCa–tension relationships
Pi- and H+-induced changes in the pCa–tension relationship were determined by analyzing the changes in pCa–tension curve parameters (Fig. 3, Table 1). For both Pi levels, as pH decreased, the pCa–tension curve shifted to the right and plateaued at lower tension values. Higher [Pi] (Fig. 3B) resulted in curves plateauing at lower tension values than those at lower [Pi] (Fig. 3A). These changes resulted from significant differences in pCa50 and in Pact while acidosis and Pi had no effect on the Hill constant (n) or on passive tension (Ppas). The effects of pH and Pi accumulation on Pact and pCa50 were further investigated (see below).
Fig. 3.
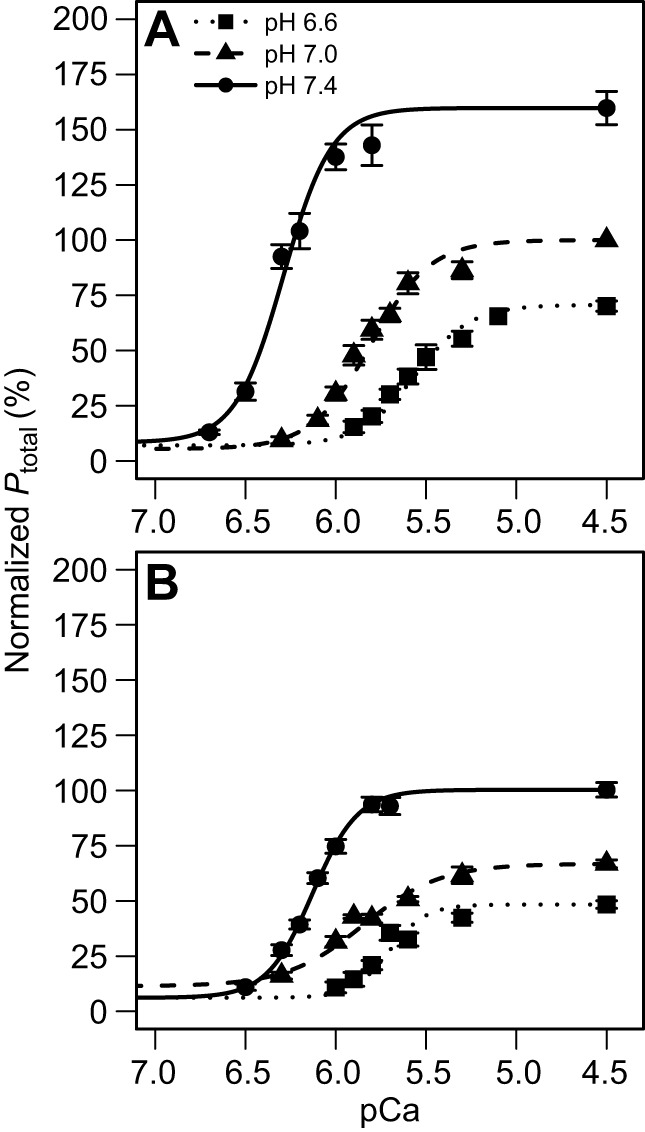
pCa–tension curves from turtle ventricular preparations. Averaged pCa-normalized tension curves at 3 mmol l−1 Pi (A) and 8 mmol l−1 Pi (B). Steady-state isometric tension (Ptotal) values are expressed as a percentage of tension developed by the same muscle preparation when exposed to pH 7, 3 mmol l−1 Pi, at pCa 4.5. Each curve represents the mean of the 12 curves generated from each preparation. The individual data points on the graphs represent mean±s.e.m. values where 4–12 measurements were obtained at the same pCa for each of the 12 preparations. Even though 12 curves were constructed for each treatment, measurements were not obtained from every preparation at all of the pCa levels.
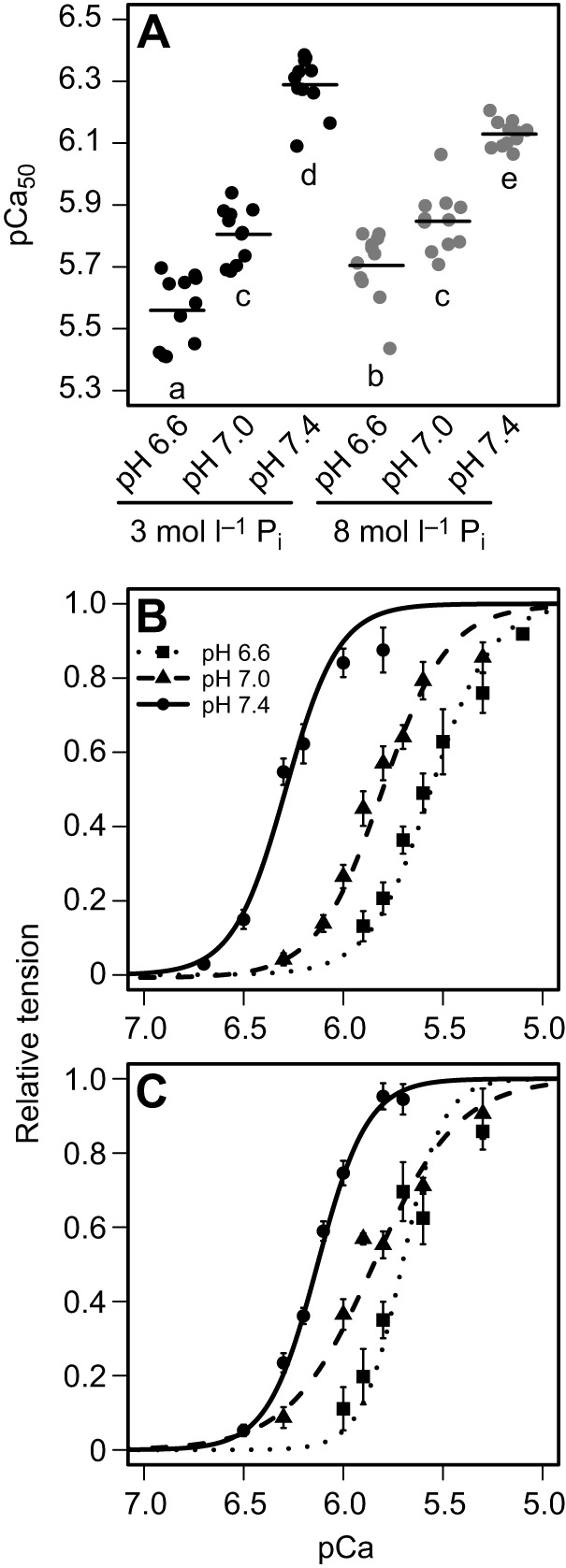
Table 1.
Force–pCa relationship curve parameters
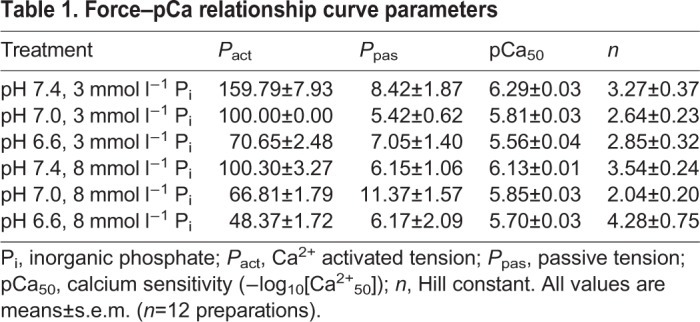
Effects of acidosis and Pi on pCa50
Skinned ventricular myocytes exposed to pH 6.6, 7.0 and 7.4 exhibited pH-induced changes in pCa50 (P<0.001). At pH 7.4, pCa50 was 6.29±0.03 at low Pi and decreased to 6.13±0.01 at high Pi (Fig. 4A). When pH was decreased from 7.4 to 7.0, pCa50 significantly decreased to 5.80±0.03 at low Pi and to 5.85±0.03 at high Pi (P<0.001 at both Pi levels). From pH 7.0 to 6.6, pCa50 decreased further to 5.56±0.03 (P<0.001) and to 5.70±0.03 (P=0.002), at low and high Pi, respectively. Taken together, these data show that acidosis decreases pCa50 and decreases myofilament Ca2+ sensitivity (Fig. 4B,C).
Fig. 4.
Effects of increased H+ and Pi on pCa50. (A) pCa50 values (−log10[Ca2+50], a measure of calcium sensitivity) are depicted for all pH levels at 3 and 8 mmol l−1 Pi, where significant differences are indicated by different letters (P<0.05, n=12). (B,C) Averaged pCa-normalized tension curves (N=12 preparations) for pH 6.6, 7.0 and 7.4 at 3 mmol l−1 Pi (B) and 8 mmol l−1 Pi (C). Each curve on the graphs represents the mean of the 12 curves generated from each of the 12 preparations. The individual data points on the graph represent mean±s.e.m. values where 4–12 measurements were obtained at the same pCa level for each of the 12 preparations. Even though 12 curves were constructed for each treatment, measurements were not obtained from every preparation at all of the pCa levels.
We also detected a significant interaction between Pi and pH on pCa50 (P<0.001), which showed variation in the effects of Pi on pCa50 across pH (Fig. 4A). At pH 7.4, low Pi resulted in a significantly higher pCa50 at high Pi (P<0.001). At pH 7.0, there was no significant difference between the two Pi levels (P=0.298). At pH 6.6, pCa50 was again significantly different across Pi levels (P=0.002), but in this case, low Pi resulted in a lower pCa50 at high Pi, the opposite of the observation at pH 7.4.
Effects of acidosis and Pi on Pact
Skinned ventricular preparations exposed to pH 6.6, 7.0 and 7.4 showed pH-induced changes in maximum tension development (P<0.001). Between pH 7.4 and 7.0, Pact showed a 36% and 41% decrease at low and high Pi, respectively. A further decrease down to pH 6.6 caused additional Pact decreases of 33% and 24% at low and high Pi, respectively. Overall, the decrease in pH from 7.4 to 6.6 produced a 58% decrease in Pact at low Pi and a 55% decrease at high Pi (Figs. 5 and 6).
Fig. 5.
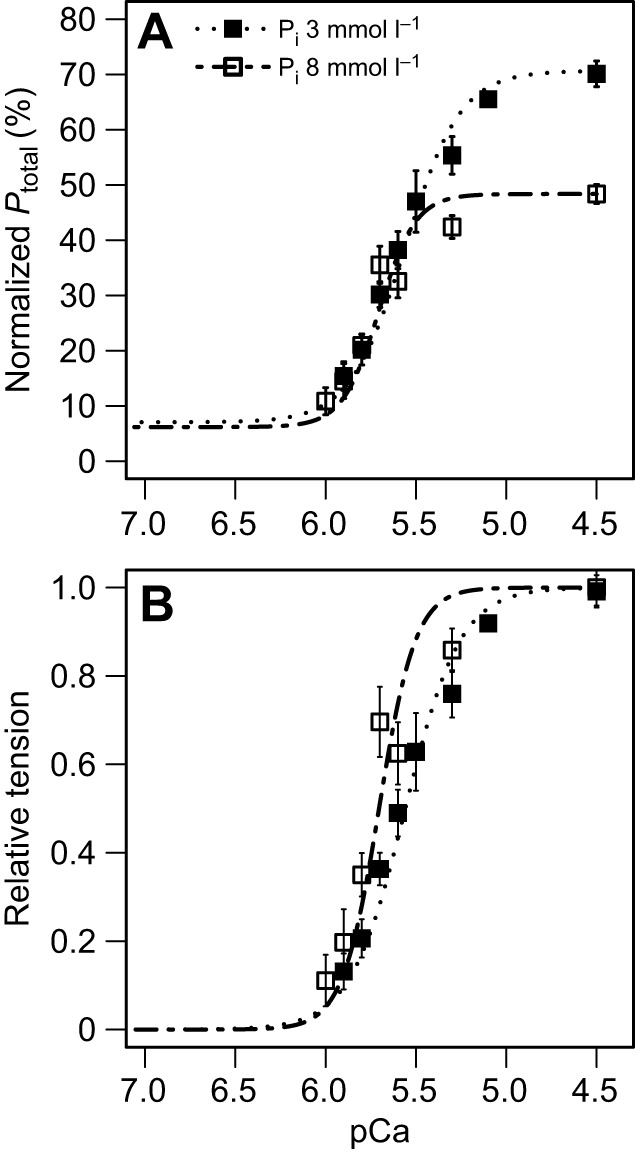
Effect of Pi at pH 6.6. All values are means±s.e.m. from 12 preparations. (A) Averaged pCa-normalized tension curves at 3 and 8 mmol l−1 Pi. Ptotal values are expressed as a percentage of tension developed by the same muscle preparation when exposed to pH 7, 3 mmol l−1 Pi, at pCa 4.5. (B) Relative tension curves for pH 6.6 are depicted at 3 and 8 mmol l−1 Pi.
Fig. 6.
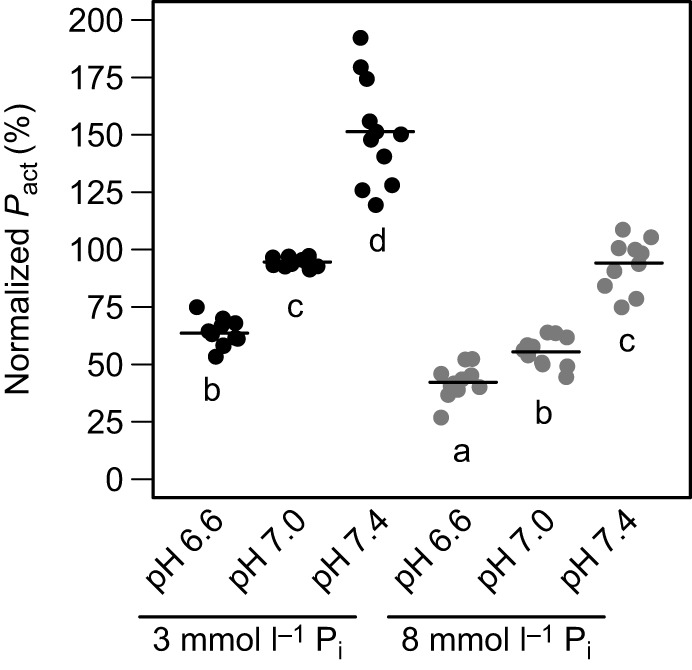
Effects of H+ and Pi elevation on active tension at pCa 4.5. Ca2+- activated tension (Pact) values are expressed as a percentage of tension developed by the same muscle preparation when exposed to pH 7, 3 mmol l−1 Pi, at pCa 4.5. Significant differences are indicated by different letters (P<0.05, n=12 preparations).
Skinned ventricular preparations were also introduced to high or low [Pi] to determine whether maximum tension development was affected. Increasing [Pi] by 5 mmol l−1 significantly decreased Pact at all pH levels (P<0.001). A higher [Pi] resulted in Pact decreases of 38%, 41% and 34% at pH 7.4, 7.0 and 6.6, respectively. Furthermore, we exposed skinned ventricular preparations to [H+] and [Pi] increases simultaneously to determine whether H+ and Pi interacted to modify Pact. The interaction between [H+] and [Pi] significantly attenuated further decreases in Pact (P<0.001).
DISCUSSION
The present study is the first to characterize the contractility of ventricular myofilaments in any reptile, thereby furthering our understanding of how ventricular contractility varies across vertebrate species. We have also elucidated the likely roles of physiologically relevant changes in pH and Pi on the contractile properties of ventricular myofilaments in mediating the intrinsic functional suppression during anoxia in the most anoxia-tolerant tetrapod, the painted turtle. Contractile properties were characterized by comparing pCa–tension relationship differences resulting from changes in tension development (Pact) and calcium sensitivity (pCa50). Pact decreased in the presence of higher H+ or higher Pi. A decrease in pCa50 was only observed in the presence of higher H+. The interaction between Pi and pH decreased Pact and pCa50 to a lesser degree compared with acidosis alone.
Effect of acidosis and Pi on tension development (Pact)
Unsurprisingly, acidosis had a significant negative effect on Pact, supporting previous suggestions that acidosis is a primary factor mediating functional suppression in vivo during anoxia (Shi and Jackson, 1997). The pH effects were quantitatively similar to previous measurements in rats at the same temperature (Fig. 7A). In turtles at both Pi levels, increasing pH from 7.0 to 7.4 increased tension by ∼65% while decreasing pH from 7.0 to 6.6 reduced tension by ∼28% (Fig. 7A). Rat ventricular myofilaments showed a 60% increase in Pact when pH was increased from 7.0 to 7.4–7.5 while a decrease in pH from 7.0 to 6.5–6.6 decreased Pact by 25–40% (Fabiato and Fabiato, 1978; Kentish, 1991; Smith and Steele, 1994; Solaro et al., 1988).
Fig. 7.
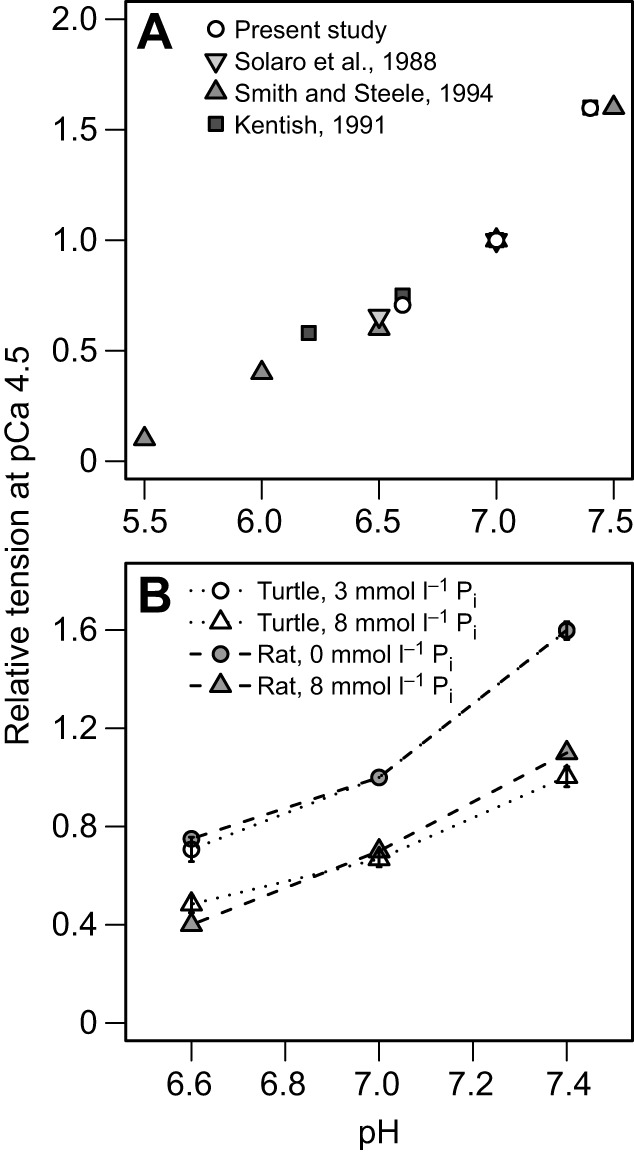
Effects of H+ and Pi on maximum tension development in skinned myocardium from mammals and turtle. (A) Comparison of the effect of acidosis on relative tension development between turtle and rat ventricle (Solaro et al., 1988; Smith and Steele, 1994; Kentish, 1991). (B) The effect of acidosis on relative tension at low and high Pi levels (5 mmol l−1 increase) in both rat and turtle ventricle. All values are normalized to pH 7.0, pCa 4.5 at low [Pi].
Although turtle and rat exhibit similar responses to pH changes at room temperature, the physiological temperatures at which they experience pH changes are different. Mammalian muscle experiences intracellular acidosis during exercise at 37°C, and studies performed at 30°C indicated that Pact is less sensitive to acidosis than at lower temperatures (Knuth et al., 2006; Nelson et al., 2014; Nelson and Fitts, 2014; Orchard et al., 1991; Orchard and Kentish, 1990; Pate et al., 1995). This has led to the general belief that acidosis may only have minor in vivo effects on Pact in mammalian muscle at the physiologically relevant temperature of 37°C (Debold, 2012). In contrast, turtles experience a broad range of body temperatures, from 3°C while overwintering to more than 30°C in summer. Anoxia-induced intracellular acidosis is most likely to occur during winter, so the more pronounced pH-dependent changes in mammalian Pact observed at lower temperatures suggest that acidosis may be especially important in reducing Pact and, therefore, suppressing contractility of cardiac myofilaments in vivo in the anoxic turtle. Clearly, additional studies at temperatures colder than 20°C are needed to confirm this hypothesis.
Increasing [Pi] also decreased Pact in turtle ventricular myofilaments. We observed a ∼40% decrease in Pact across all pH levels with a 5 mmol l−1 increase in Pi (Fig. 3), a response that is similar to that observed in mammalian striated muscle (Nelson and Fitts, 2014; Palmer and Kentish, 1994). It is hypothesized that increasing intracellular [Pi] drives a reversal in the cross-bridge cycle, which shifts cross-bridges from a state of high tension development to a state of lower tension development where a greater proportion of myosin heads are bound to Pi (Palmer and Kentish, 1994; Takagi et al., 2004). This is likely the mechanistic cause of the Pi-induced Pact decrement observed in this study.
Although increased Pi decreased Pact in turtle, the physiologically relevant Pi increase did not decrease Pact by the same magnitude as the physiologically relevant decrease in pH, indicating acidosis is probably the more important factor during anoxia. For example, at pH 7.4, increasing Pi by 5 mmol l−1 only decreased tension by 38%, while the physiologically relevant decrease in pH at 3 mmol l−1 Pi decreased it by 58%. In turtles, studies of whole ventricle and ventricular strips have suggested that acidosis contributes to cardiac functional suppression during anoxia (Jackson, 1987; Overgaard et al., 2005; Wasser et al., 1991); however, other work has suggested that the changing levels of energetic components, such as increasing [Pi], may account for the cardiac functional suppression observed during anoxia (Stecyk et al., 2009). This is the first study to compare the effects of intracellular pH and Pi changes on ventricular myofilament function in turtles, and the large decrease in tension development due to acidosis further supports the notion that acidosis plays a more significant role in functional suppression of the turtle heart during anoxia.
The statistical interaction between [Pi] and pH indicates that when both Pi and H+ are at high concentrations, the decrease in Pact may be slightly attenuated in turtles (Fig. 6), which differs from mammalian striated muscle, where no such attenuation occurs (Eisner et al., 1987; Kentish, 1991; Wilkie, 1986; Wilson et al., 1988). In a previous study of rat ventricle, relative tension was measured at pH 6.6, 7.0 and 7.4 at two levels of Pi differing by 5 mmol l−1 (Kentish, 1991), the results of which we have plotted alongside the results from our present study in Fig. 7B. For this comparison, maximum tension values were normalized to pH 7.0 of the lower Pi concentration for the two studies (0 and 3 mmol l−1 for rat and turtle, respectively). Although no statistical comparisons can be made, the effect of increased Pi on pH-dependent Pact appears strikingly similar between the two species, despite the unique attenuation effect observed in turtle preparations. Further studies at identical [Pi] levels are required to make comparisons across species regarding the Pi–pH interaction effect on Pact.
Effect of acidosis and Pi on calcium sensitivity
A second mechanism by which pH and Pi can alter cardiac contractility is by changing the myofilament sensitivity to Ca2+. This can be assessed by measuring the tension–pCa relationship and thus the pCa50 under different conditions. We found a clear right-shift in the tension–pCa relationship curve (increased pCa50) as [H+] increased (Fig. 4), indicating a decrease in the Ca2+ sensitivity of turtle ventricular myofilaments with acidosis, an affect long-known to occur in mammals (Fabiato and Fabiato, 1978; Orchard et al., 1991; Orchard and Kentish, 1990; Solaro et al., 1988). In contrast, increasing Pi from 3 to 8 mmol l−1 at pH 7.0 had no statistically significant effect on Ca2+ sensitivity in the present study (Fig. 4A). This differs somewhat from rats, which showed significant decreases in Ca2+ sensitivity with much larger Pi increases (20 mmol l−1) (Palmer and Kentish, 1994). Turtle atrial trabeculae also exhibited decreased Ca2+ sensitivity with increasing Pi concentration (Jensen and Gesser, 1999). Although we cannot rule out the possibility that higher levels of Pi might also have decreased Ca2+ sensitivity, we are able to conclude that physiologically relevant increases in Pi are insufficient to cause any change in myofilament Ca2+ sensitivity and that in vivo contractile suppression in anoxic turtles is at least partly related to decreases in pCa50 by acidosis.
Just as there were interactive effects of Pi and pH on Pact, we also found interactive effects on pCa50 (Fig. 4A). At pH 7.4, increasing Pi by 5 mmol l−1 decreased pCa50, while at pH 6.6, the 5 mmol l−1 Pi increase rescued pCa50. Although there was no statistical effect of Pi or pH on the Hill constant (n), closer examination of the relative tension curves at pH 6.6 indicates that there may be a difference in n at pH 6.6, which in turn may impact pCa50 (Fig. 5B). Additionally, the pH–Pi interaction may impact myofilament cooperativity, which could cause a change in myofilament tension kinetics that determine pCa50. Regardless, the results suggest that intracellular acidosis is the primary cause of decreased myofilament Ca2+ sensitivity in this preparation and probably accounts for contractile suppression, in vivo, in the anoxic turtle ventricle.
Calcium sensitivity across species
Our study provides further evidence that high calcium sensitivity is a general property of the ectothermic myofilament apparatus in ventricular tissue (Table 2). Comparison of the properties of the myofilaments from turtle ventricle with previously published values for mammalian ventricle reveals that turtles have a higher calcium sensitivity. At pH 7.0, mammalian ventricular pCa50 ranges from 5.29 to 5.57, while turtle pCa50 was 5.81 (Bers, 1991; Harrison and Bers, 1989, 1990; Palmer and Kentish, 1994). Only at pH 6.6, 3 mmol l−1 Pi, did turtle ventricular myofilaments exhibit a pCa50 within the range of mammals, where pCa50 decreased to 5.56. Similar to turtle cardiomyocytes, frog ventricular myocytes exhibited a pCa50 of 5.87 at pH 7.0, 20°C, indicating a higher Ca2+ sensitivity than in mammals (Brandt et al., 1998). Studies of frog and rainbow trout ventricular myofilaments have also shown higher sensitivity to Ca2+ than mammalian ventricular myofilaments, regardless of temperature (Churcott et al., 1994; Harrison and Bers, 1990). It is reasonable to suspect that differences in pCa50 across species are caused by variation in the isoforms of troponin C (TnC) and troponin I (TnI), which accounts for tissue-specific pCa50 differences in mammals (Debold, 2012).
Table 2.
Ventricular myofilament Ca2+ sensitivity of different species at pH 7.0
The higher ventricular myofilament calcium sensitivity of ectotherms makes sense when considering the differing roles of the sarcoplasmic reticulum (SR) in excitation–contraction (E–C) coupling across vertebrates and their relative heart rates. Mammals have a well-developed, highly organized SR that allows for large calcium transients during E–C coupling (Barcenas-Ruiz and Wier, 1987; DuBell and Houser, 1989) and their high heart rates necessitate a lower pCa to insure rapid relaxation and return to diastole. In contrast, turtle ventricular myocardium relies comparatively less on the SR for Ca2+ during contraction (Galli et al., 2006a,b), as does that of frogs (Fabiato, 1982; Morad et al., 1981) and fish. (Hove-Madsen et al., 1999; Shiels and Galli, 2014; Tibbits et al., 1991; Vornanen, 1989; Vornanen and Haverinen, 2013). It has recently been proposed that the SR in ectotherms may be more important during times of physiological stress (Cros et al., 2014) or in supporting the contractile requirements of more aerobic ectothermic species, such as varanid lizards (Galli et al., 2009; Warren et al., 2010). Interestingly, the chicken ventricle shows a pCa50 of 5.65 at 22°C, which occupies an intermediate calcium sensitivity between mammals and ectothermic vertebrates (Godt et al., 1991), although birds do utilize the SR as a calcium source during contraction (Gwathmey and Morgan, 1991).
Calcium sensitivity within the heart
Our study suggests that cardiac myofilament Ca2+ sensitivity varies not only across species but also within the turtle heart. Turtle atrial trabeculae exhibited less sensitivity to calcium with a pCa50 of 5.67 (pH 7.35–7.45) (Jensen and Gesser, 1999), compared with that for a pCa50 of 6.29 (pH 7.4) observed in this study. Atrial tissue also exhibits a reduced Ca2+ sensitivity in both pigs and humans (Morano et al., 1988). This is not true of all vertebrates, however, as frog atrial and ventricular tissues have similar Ca2+ sensitivities (Brandt et al., 1998).
Conclusions
We have shown that acidosis has profound negative effects on both tension development and Ca2+ sensitivity in skinned ventricular cardiomyocytes from turtles, while increased [Pi] has smaller effects only on tension development, and none on Ca2+ sensitivity. By extending these functional data to the tissue and organ level in the anoxic painted turtle, we can conclude that the intrinsic functional suppression in the ventricular myocardium is primarily from intracellular acidosis. This agrees with previous studies at the organ and tissue level that support a larger role for acidosis in functional suppression of cardiac function in anoxic turtles (Jackson, 1987; Overgaard et al., 2005; Wasser et al., 1991). It is noteworthy that during 12 weeks of anoxia, plasma-free Ca2+ levels gradually increase to 25 mequiv l−1 and total intracellular Ca2+ in heart increases from 1.5 to 5.7 mmol kg−1 (Jackson and Heisler, 1982, 1983). Additional studies measuring intracellular Ca2+ transients during anoxia and acidosis are needed to determine whether these extracellular and intracellular calcium changes are functionally important.
We have also provided further evidence that the ventricles from ectothermic vertebrates have a higher myofilament Ca2+ sensitivity compared with those of mammals. Although turtle Ca2+ sensitivity is intrinsically different from that of mammals, the effect of acidosis on Ca2+ sensitivity is not qualitatively different across species. Likewise, the effects of decreased pH and increased [Pi] on decreased tension development in turtles were not different from those measured in mammals. However, a decrease in Ca2+ sensitivity due to increased [Pi] previously observed in mammals was not observed in our studies of turtle. This suggests that Pi plays a larger role in mammals in decreasing both tension development and Ca2+ sensitivity, while acidosis is the main contributing factor in turtles.
Acknowledgements
We thank Dr Charles Chung for his skillful experimental assistance, Dr Edward Debold for his useful discussion, and two anonymous reviewers for their constructive critiques and suggestions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: C.E.F., D.E.W.; Methodology: C.E.F., K.S.C., D.E.W.; Formal analysis: C.E.F., K.S.C.; Investigation: C.E.F., K.S.C., D.E.W.; Writing - original draft: C.E.F., D.E.W.; Writing - review & editing: C.E.F., K.S.C., D.E.W.; Supervision: D.E.W.; Project administration: D.E.W.; Funding acquisition: D.E.W.
Funding
This work was supported by National Science Foundation CAREER grant 1253939 awarded to D.E.W.
Supplementary information
Supplementary information available online at http://jeb.biologists.org/lookup/doi/10.1242/jeb.164137.supplemental
References
- Barcenas-Ruiz L. and Wier W. G. (1987). Voltage dependence of intracellular [Ca2+]i transients in guinea pig ventricular myocytes. Circ. Res. 61, 148-154. 10.1161/01.RES.61.1.148 [DOI] [PubMed] [Google Scholar]
- Bers D. (1991). Excitation-Contraction Coupling and Cardiac Contractile Force. Netherlands: Springer. [Google Scholar]
- Boutilier R. G. (2001). Mechanisms of cell survival in hypoxia and hypothermia. J. Exp. Biol. 204, 3171-3181. [DOI] [PubMed] [Google Scholar]
- Brandt P. W., Colomo F., Piroddi N., Poggesi C. and Tesi C. (1998). Force regulation by Ca2+ in skinned single cardiac myocytes of frog. Biophys. J. 74, 1994-2004. 10.1016/S0006-3495(98)77906-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. S. and Holbrook A. M. (2007). The rate of tension recovery in cardiac muscle correlates with the relative residual tension prevailing after restretch. Am. J. Physiol. Heart Circ. Physiol. 292, H2020-H2022. 10.1152/ajpheart.00714.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. S. and Moss R. L. (2000). A thixotropic effect in contracting rabbit psoas muscle: prior movement reduces the initial tension response to stretch. J. Physiol. 525, 531-548. 10.1111/j.1469-7793.2000.00531.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. S. and Moss R. L. (2002). History-dependent mechanical properties of permeabilized rat soleus muscle fibers. Biophys. J. 82, 929-943. 10.1016/S0006-3495(02)75454-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. S. and Moss R. L. (2003). SLControl: PC-based data acquisition and analysis for muscle mechanics. Am. J. Physiol. Heart Circ. Physiol. 285, H2857-H2864. 10.1152/ajpheart.00295.2003 [DOI] [PubMed] [Google Scholar]
- Campbell K. S., Patel J. R. and Moss R. L. (2003). Cycling cross-bridges increase myocardial stiffness at submaximal levels of Ca2+ activation. Biophys. J. 84, 3807-3815. 10.1016/S0006-3495(03)75108-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churcott C. S., Moyes C. D., Bressler B. H., Baldwin K. M. and Tibbits G. F. (1994). Temperature and pH effects on Ca2+ sensitivity of cardiac myofibrils: a comparison of trout with mammals. Am. J. Physiol. 267, R62-R70. [DOI] [PubMed] [Google Scholar]
- Cros C., Salle L., Warren D. E., Shiels H. A. and Brette F. (2014). The calcium stored in the sarcoplasmic reticulum acts as a safety mechanism in rainbow trout heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R1493-R1501. 10.1152/ajpregu.00127.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debold E. P. (2012). Recent insights into muscle fatigue at the cross-bridge level. Front Physiol. 3, 151 10.3389/fphys.2012.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBell W. H. and Houser S. R. (1989). Voltage and beat dependence of Ca2+ transient in feline ventricular myocytes. Am. J. Physiol. 257, H746-H759. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Elliott A. C. and Smith G. L. (1987). The contribution of intracellular acidosis to the decline of developed pressure in ferret hearts exposed to cyanide. J. Physiol. 391, 99-108. 10.1113/jphysiol.1987.sp016728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. (1982). Calcium release in skinned cardiac cells: variations with species, tissues, and development. Fed. Proc. 41, 2238-2244. [PubMed] [Google Scholar]
- Fabiato A. and Fabiato F. (1978). Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J. Physiol. 276, 233-255. 10.1113/jphysiol.1978.sp012231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell A., Franklin C., Arthur P., Thorarensen H. and Cousins K. (1994). Mechanical performance of an in situ perfused heart from the turtle Chrysemys scripta during normoxia and anoxia at 5 C and 15 C. J. Exp. Biol. 191, 207-229. [DOI] [PubMed] [Google Scholar]
- Fitzsimons D. P., Patel J. R. and Moss R. L. (2001). Cross-bridge interaction kinetics in rat myocardium are accelerated by strong binding of myosin to the thin filament. J. Physiol. 530, 263-272. 10.1111/j.1469-7793.2001.0263l.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G. L. J., Gesser H., Taylor E. W., Shiels H. A. and Wang T. (2006a). The role of the sarcoplasmic reticulum in the generation of high heart rates and blood pressures in reptiles. J. Exp. Biol. 209, 1956-1963. 10.1242/jeb.02228 [DOI] [PubMed] [Google Scholar]
- Galli G. L. J., Taylor E. W. and Shiels H. A. (2006b). Calcium flux in turtle ventricular myocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1781-R1789. 10.1152/ajpregu.00421.2006 [DOI] [PubMed] [Google Scholar]
- Galli G. L. J., Warren D. E. and Shiels H. A. (2009). Ca2+ cycling in cardiomyocytes from a high-performance reptile, the varanid lizard (Varanus exanthematicus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1636-R1644. 10.1152/ajpregu.00381.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesser H. and Jorgensen E. (1982). pHi, contractility and Ca-balance under hypercapnic acidosis in the myocardium of different vertebrate species. J. Exp. Biol. 96, 405-412. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Fogaca R. T. H. and Nosek T. M. (1991). Changes in force and calcium sensitivity in the developing avian heart. Can. J. Physiol. Pharmacol. 69, 1692-1697. 10.1139/y91-251 [DOI] [PubMed] [Google Scholar]
- Gwathmey J. K. and Morgan J. P. (1991). Calcium handling in myocardium from amphibian, avian, and mammalian species: the search for two components. J. Comp. Physiol. B 161, 19-25. 10.1007/BF00258742 [DOI] [PubMed] [Google Scholar]
- Harrison S. M. and Bers D. M. (1989). Influence of temperature on the calcium sensitivity of the myofilaments of skinned ventricular muscle from the rabbit. J. Gen. Physiol. 93, 411-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. M. and Bers D. M. (1990). Temperature dependence of myofilament Ca sensitivity of rat, guinea pig, and frog ventricular muscle. Am. J. Physiol. 258, C274-C281. [DOI] [PubMed] [Google Scholar]
- Herbert C. V. and Jackson D. C. (1985). Temperature effects on the responses to prolonged submergence in the turtle Chrysemys picta bellii. II. Metabolic rate, blood acid-base and ionic changes, and cardiovascular function in aerated and anoxic water. Physiol. Zool. 58, 670-681. [Google Scholar]
- Hicks J. M. and Farrell A. P. (2000). The cardiovascular responses of the red-eared slider (Trachemys scripta) acclimated to either 22 or 5 degrees C. I. Effects of anoxic exposure on in vivo cardiac performance. J. Exp. Biol. 203, 3765-3774. [DOI] [PubMed] [Google Scholar]
- Hicks J. W. and Wang T. (1998). Cardiovascular regulation during anoxia in the turtle: an in vivo study. Physiol. Zool. 71, 1-14. 10.1086/515892 [DOI] [PubMed] [Google Scholar]
- Hove-Madsen L., Llach A. and Tort L. (1999). Quantification of calcium release from the sarcoplasmic reticulum in rainbow trout atrial myocytes. Pflugers Arch. 438, 545-552. 10.1007/s004249900082 [DOI] [PubMed] [Google Scholar]
- Jackson D. C. (1987). Cardiovascular function in turtles during anoxia and acidosis: In vivo and in vitro studies. Am. Zool. 27, 49-58. 10.1093/icb/27.1.49 [DOI] [Google Scholar]
- Jackson D. (1997). Lactate accumulation in the shell of the turtle Chrysemys picta bellii during anoxia at 3 deg C and 10 deg C. J. Exp. Biol. 200, 2295-2300. [DOI] [PubMed] [Google Scholar]
- Jackson D. C. (2002). Hibernating without oxygen: physiological adaptations of the painted turtle. J. Physiol. 543, 731-737. 10.1113/jphysiol.2002.024729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. C. and Heisler N. (1982). Plasma ion balance of submerged anoxic turtles at 3 degrees C: the role of calcium lactate formation. Respir. Physiol. 49, 159-174. 10.1016/0034-5687(82)90071-8 [DOI] [PubMed] [Google Scholar]
- Jackson D. C. and Heisler N. (1983). Intracellular and extracellular acid-base and electrolyte status of submerged anoxic turtles at 3 degrees C. Respir Physiol 53, 187-201. 10.1016/0034-5687(83)90066-X [DOI] [PubMed] [Google Scholar]
- Jackson D. C., Shi H., Singer J. H., Hamm P. H. and Lawler R. G. (1995). Effects of input pressure on in vitro turtle heart during anoxia and acidosis: a 31P-NMR study. Am. J. Physiol. 268, R683-R689. [DOI] [PubMed] [Google Scholar]
- Jackson D. C., Toney V. I. and Okamoto S. (1996). Lactate distribution and metabolism during and after anoxia in the turtle, Chrysemys picta bellii. Am. J. Physiol. 271, R409-R416. [DOI] [PubMed] [Google Scholar]
- Jensen M. A. and Gesser H. (1999). Influence of inorganic phosphate and energy state on force in skinned cardiac muscle from freshwater turtle and rainbow trout. J. Comp. Physiol. B 169, 439-444. 10.1007/s003600050240 [DOI] [PubMed] [Google Scholar]
- Keiver K. M. and Hochachka P. W. (1991). Catecholamine stimulation of hepatic glycogenolysis during anoxia in the turtle Chrysemys picta. Am. J. Physiol. 261, R1341-R1345. [DOI] [PubMed] [Google Scholar]
- Keiver K. M., Weinberg J. and Hochachka P. W. (1992). The effect of anoxic submergence and recovery on circulating levels of catecholamines and corticosterone in the turtle, Chrysemys picta. Gen. Comp. Endocrinol. 85, 308-315. 10.1016/0016-6480(92)90015-C [DOI] [PubMed] [Google Scholar]
- Kentish J. C. (1986). The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J. Physiol. 370, 585-604. 10.1113/jphysiol.1986.sp015952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish J. C. (1991). Combined inhibitory actions of acidosis and phosphate on maximum force production in rat skinned cardiac muscle. Pflugers Arch. 419, 310-318. 10.1007/BF00371112 [DOI] [PubMed] [Google Scholar]
- Knuth S. T., Dave H., Peters J. R. and Fitts R. H. (2006). Low cell pH depresses peak power in rat skeletal muscle fibres at both 30 degrees C and 15 degrees C: implications for muscle fatigue. J. Physiol. 575, 887-899. 10.1113/jphysiol.2006.106732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Sanders C. and Weiss J. (1981). The inotropic actions of adrenaline on frog ventricular muscle: relaxing versus potentiating effects. J. Physiol. 311, 585-604. 10.1113/jphysiol.1981.sp013606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano I., Arndt H., Gartner C. and Ruegg J. C. (1988). Skinned fibers of human atrium and ventricle: myosin isoenzymes and contractility. Circ. Res. 62, 632-639. 10.1161/01.RES.62.3.632 [DOI] [PubMed] [Google Scholar]
- Nelson C. R. and Fitts R. H. (2014). Effects of low cell pH and elevated inorganic phosphate on the pCa-force relationship in single muscle fibers at near-physiological temperatures. Am. J. Physiol. Cell Physiol. 306, C670-C678. 10.1152/ajpcell.00347.2013 [DOI] [PubMed] [Google Scholar]
- Nelson C. R., Debold E. P. and Fitts R. H. (2014). Phosphate and acidosis act synergistically to depress peak power in rat muscle fibers. Am. J. Physiol. Cell Physiol. 307, C939-C950. 10.1152/ajpcell.00206.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard C. H. and Kentish J. C. (1990). Effects of changes of pH on the contractile function of cardiac muscle. Am. J. Physiol. 258, C967-C981. [DOI] [PubMed] [Google Scholar]
- Orchard C. H., Hamilton D. L., Astles P., McCall E. and Jewell B. R. (1991). The effect of acidosis on the relationship between Ca2+ and force in isolated ferret cardiac muscle. J. Physiol. 436, 559-578. 10.1113/jphysiol.1991.sp018567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard J., Wang T., Nielsen O. B. and Gesser H. (2005). Extracellular determinants of cardiac contractility in the cold anoxic turtle. Physiol. Biochem. Zool. 78, 976-995. 10.1086/432853 [DOI] [PubMed] [Google Scholar]
- Palmer S. and Kentish J. C. (1994). The role of troponin C in modulating the Ca2+ sensitivity of mammalian skinned cardiac and skeletal muscle fibres. J. Physiol. 480, 45-60. 10.1113/jphysiol.1994.sp020339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E., Bhimani M., Franks-Skiba K. and Cooke R. (1995). Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J. Physiol. 486, 689-694. 10.1113/jphysiol.1995.sp020844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H. and Jackson D. C. (1997). Effects of anoxia, acidosis and temperature on the contractile properties of turtle cardiac muscle strips. J. Exp. Biol. 200, 1965-1973. [DOI] [PubMed] [Google Scholar]
- Shi H., Hamm P. H., Lawler R. G. and Jackson D. C. (1999). Different effects of simple anoxic lactic acidosis and simulated in vivo anoxic acidosis on turtle heart. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 122, 173-180. 10.1016/S1095-6433(98)10163-0 [DOI] [PubMed] [Google Scholar]
- Shiels H. A. and Galli G. L. (2014). The sarcoplasmic reticulum and the evolution of the vertebrate heart. Physiology (Bethesda) 29, 456-469. 10.1152/physiol.00015.2014 [DOI] [PubMed] [Google Scholar]
- Smith G. L. and Steele D. S. (1994). Effects of pH and inorganic phosphate on rigor tension in chemically skinned rat ventricular trabeculae. J. Physiol. 478, 505-512. 10.1113/jphysiol.1994.sp020269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro R. J., Lee J. A., Kentish J. C. and Allen D. G. (1988). Effects of acidosis on ventricular muscle from adult and neonatal rats. Circ. Res. 63, 779-787. 10.1161/01.RES.63.4.779 [DOI] [PubMed] [Google Scholar]
- Stecyk J. A., Overgaard J., Farrell A. P. and Wang T. (2004). Alpha-adrenergic regulation of systemic peripheral resistance and blood flow distribution in the turtle Trachemys scripta during anoxic submergence at 5 degrees C and 21 degrees C. J. Exp. Biol. 207, 269-283. 10.1242/jeb.00744 [DOI] [PubMed] [Google Scholar]
- Stecyk J. A., Bock C., Overgaard J., Wang T., Farrell A. P. and Portner H. O. (2009). Correlation of cardiac performance with cellular energetic components in the oxygen-deprived turtle heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R756-R768. 10.1152/ajpregu.00102.2009 [DOI] [PubMed] [Google Scholar]
- Takagi Y., Shuman H. and Goldman Y. E. (2004). Coupling between phosphate release and force generation in muscle actomyosin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359, 1913-1920. 10.1098/rstb.2004.1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbits G. F., Hove-Madsen L. and Bers D. M. (1991). Calcium transport and the regulation of cardiac contractility in teleosts: a comparison with higher vertebrates. Can. J. Zool. 69, 2014-2019. 10.1139/z91-281 [DOI] [Google Scholar]
- Ultsch G. R. and Jackson D. C. (1982). Long-term submergence at 3 degrees C of the turtle Chrysemys picta bellii in normoxic and severely hypoxic water. III. Effects of changes in ambient PO2 and subsequent air breathing. J. Exp. Biol. 97, 87-99. [DOI] [PubMed] [Google Scholar]
- Vornanen M. (1989). Basic functional properties of the cardiac muscle of the common shrew (Sorex araneus) and some other small mammals. J. Exp. Biol. 145, 339-351. [DOI] [PubMed] [Google Scholar]
- Vornanen M. and Haverinen J. (2013). A significant role of sarcoplasmic reticulum in cardiac contraction of a basal vertebrate, the river lamprey (Lampetra fluviatilis). Acta Physiol. (Oxf.) 207, 269-279. 10.1111/j.1748-1716.2012.02479.x [DOI] [PubMed] [Google Scholar]
- Warren D. E. and Jackson D. C. (2007). Effects of temperature on anoxic submergence: skeletal buffering, lactate distribution, and glycogen utilization in the turtle, Trachemys scripta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R458-R467. 10.1152/ajpregu.00174.2006 [DOI] [PubMed] [Google Scholar]
- Warren D. E., Galli G. L. J., Patrick S. M. and Shiels H. A. (2010). The cellular force-frequency response in ventricular myocytes from the varanid lizard, Varanus exanthematicus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R567-R574. 10.1152/ajpregu.00650.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser J. S., Freund E. V., Gonzalez L. A. and Jackson D. C. (1990a). Force and acid-base state of turtle cardiac tissue exposed to combined anoxia and acidosis. Am. J. Physiol. 259, R15-R20. [DOI] [PubMed] [Google Scholar]
- Wasser J. S., Inman K. C., Arendt E. A., Lawler R. G. and Jackson D. C. (1990b). 31P-NMR measurements of pHi and high-energy phosphates in isolated turtle hearts during anoxia and acidosis. Am. J. Physiol. 259, R521-R530. [DOI] [PubMed] [Google Scholar]
- Wasser J. S., Warburton S. J. and Jackson D. C. (1991). Extracellular and intracellular acid-base effects of submergence anoxia and nitrogen breathing in turtles. Respir. Physiol. 83, 239-252. 10.1016/0034-5687(91)90032-E [DOI] [PubMed] [Google Scholar]
- Wilkie D. R. (1986). Muscular fatigue: effects of hydrogen ions and inorganic phosphate. Fed. Proc. 45, 2921-2923. [PubMed] [Google Scholar]
- Wilson J. R., McCully K. K., Mancini D. M., Boden B. and Chance B. (1988). Relationship of muscular fatigue to pH and diprotonated Pi in humans: a 31P-NMR study. J. Appl. Physiol. 64, 2333-2339. [DOI] [PubMed] [Google Scholar]