ABSTRACT
Skeletal muscles share many common, highly conserved features of organization at the molecular and myofilament levels, giving skeletal muscle fibers generally similar and characteristic mechanical and energetic properties; properties well described by classical studies of muscle mechanics and energetics. However, skeletal muscles can differ considerably in architectural design (fiber length, pinnation, and connective tissue organization), as well as fiber type, and how they contract in relation to the timing of neuromotor activation and in vivo length change. The in vivo dynamics of muscle contraction is, therefore, crucial to assessing muscle design and the roles that muscles play in animal movement. Architectural differences in muscle–tendon organization combined with differences in the phase of activation and resulting fiber length changes greatly affect the time-varying force and work that muscles produce, as well as the energetic cost of force generation. Intrinsic force–length and force–velocity properties of muscles, together with their architecture, also play important roles in the control of movement, facilitating rapid adjustments to changing motor demands. Such adjustments complement slower, reflex-mediated neural feedback control of motor recruitment. Understanding how individual fiber forces are integrated to whole-muscle forces, which are transmitted to the skeleton for producing and controlling locomotor movement, is therefore essential for assessing muscle design in relation to the dynamics of movement.
KEY WORDS: In vivo contraction, Fascicle strain, Muscle-tendon force, Muscle activation, Muscle-tendon architecture
Summary: The in vivo dynamics of muscle contractile function reflect the interplay of muscle–tendon architecture and neural activation timing relative to the forces and work that muscles produce to power locomotor movement.
Introduction
At the level of myofibril and sarcomere organization, the underlying machinery of vertebrate striated muscle is highly conserved in terms of the proteins that serve as molecular motors to produce force and change length, the regulatory proteins that control binding sites for cross-bridge formation, and the protein channels that regulate the release and uptake of Ca2+, which underlies neuromuscular excitation–contraction coupling. In addition to expressing different myosin isoforms and different regulatory proteins, skeletal muscles also differ in terms of their fiber length and organization, the connective tissues that link the fibers to the skeleton for force transmission, and the biochemical pathways supporting ATP supply for Ca2+ cycling and to power cross-bridge formation and release that are linked to the properties of myosin as a molecular motor. These latter properties are commonly defined in terms of muscle fiber types. Although important, the features and properties of muscle fiber types will not be a focus of my review. Instead, I examine how the architectural properties of muscle–tendon units (MTUs) relate to key functional properties including the production and control of movement linked to muscle strain (normalized length change), the cost of force generation, and the ability to conserve elastic energy.
In addition to architectural differences of MTUs, the timing of neuromuscular activation and force development relative to length change strongly influences the force and work output of a muscle (Josephson, 1999). Many aspects of locomotion involve cyclical movements of the limbs (as well as the trunk) for weight support and propulsion. Because of this, muscles must be turned on and turned off at specific phases of reciprocating limb movement. Consequently, the time-varying pattern of muscle activation in relation to the load against which a muscle contracts (Marsh, 1999) determines a muscle's resulting in vivo force–length (F–L) behavior and work output. Whereas intrinsic (quasi-static) force–velocity (F–V) and F–L properties of skeletal muscle are also well described and reflect generally similar features of sarcomere organization and cross-bridge dynamics (e.g. Lieber, 1992; McMahon, 1984), the temporal dynamics of muscle activation and force development relative to length change must be considered to understand how muscles power and control movements of the whole animal. In this sense, locomotion is very much an emergent property of muscle contractile dynamics. Nevertheless, the F–V and F–L properties of muscle can provide rapid, intrinsic contractile responses when locomotion is perturbed (Loeb et al., 1999; Rack et al., 1983) that reinforce subsequent neural feedback via reflexes to help stabilize an animal's movement, particularly at faster running speeds (Daley et al., 2009).
To study the contractile dynamics of muscles, a muscle's in vivo activation, length change, and, ideally, force generation must be measured in the context of the locomotor behaviors of interest. Here, I will review work that researchers in my laboratory, and others, have carried out to study the in vivo dynamics of muscle function during terrestrial and aerial locomotion. The in vivo function of muscles will be related to architectural features to examine how MTU architecture affects the contractile behavior of a muscle's fibers over a range of locomotion conditions. These studies allow us to explore whether a proximo-distal gradient of muscle architecture and function exists within the limbs of terrestrial vertebrates (principally studied in mammals and birds). Our working hypothesis is that proximal muscles, which generally tend to be parallel and long-fibered with little or no free tendon, function to modulate the majority of limb work; whereas distal muscles that are pinnate, short-fibered and often transmit forces via a long free tendon favor economical force generation and tendon elastic energy savings.
Muscle–tendon architecture in relation to function
Muscles vary considerably in terms of their fiber architecture and the means by which they transmit force to the skeleton (Gans and de Vree, 1987). These differences have important implications for how MTUs function in relation the physical requirements for effective and economical locomotion. Longer-fibered muscles with little or no external tendon or aponeurosis favor contractions that control and produce large movements, but are limited in the force that can be generated and likely cost greater energy to produce force (Fig. 1). Pinnate, short-fibered muscles produce larger forces and lower the cost of force generation (reduced ATP consumed/force generated), but are limited in the range of length change that can be produced or controlled. By attaching to an aponeurosis or long tendon, pinnate MTUs also favor elastic energy savings.
List of symbols and abbreviations.
- BF
biceps femoris
- DDF
deep digital flexor
- DF-IV
digital flexor IV
- EMG
electromyography
- F–L
force–length
- F–V
force–velocity
- ILPO
iliotibialis lateralis pars postacetabularis
- LG
lateral gastrocnemius
- L
fascicle length
- Lr
resting fascicle length
- MG
medial gastrocnemius
- MTU
muscle–tendon unit
- Pect
pectoralis
- PL
plantaris
- PLong
peroneus longus
- SDF
superficial digital flexor
- Supra
supracoracoideus
- TrLAT
lateral head of triceps
- TrLONG
long head of triceps
- VL
vastus lateralis
Fig. 1.
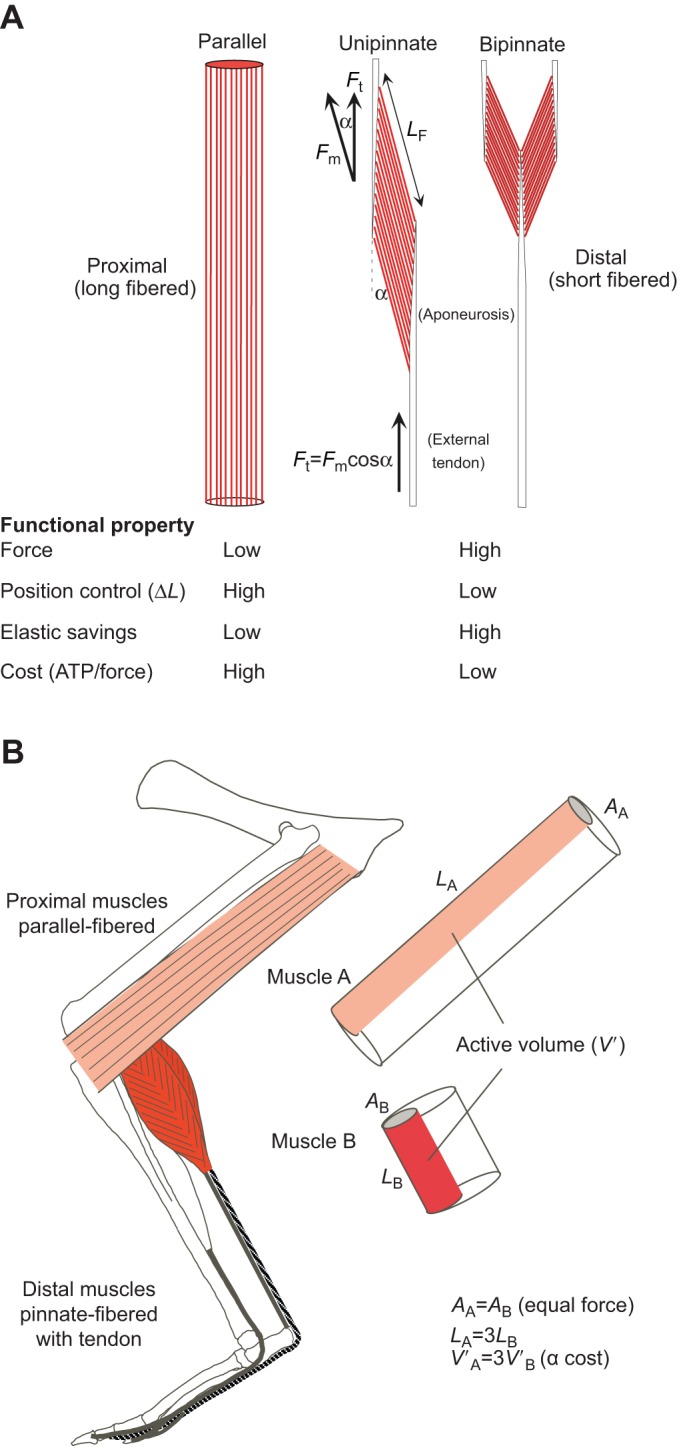
Muscle architecture in relation to functional properties and active muscle volume. (A) Comparison of parallel- versus pinnate-fibered muscle–tendon architecture in relation to functional properties. ΔL, change in length; α, pennation angle; Ft, tendon force; Fm, muscle force; LF, fascicle length. (B) Influence of muscle–tendon architecture on the cost of force generation (Biewener and Roberts, 2000). Cost of force generation is related to active muscle volume (V′), defined as the volume of muscle activated to generate a given force. Given that skeletal muscles generally produce similar peak isometric stresses (∼200–300 kPa), muscle force generation is generally proportional to the cross-sectional area (A) of activated fibers. Consequently, longer-fibered (LF) muscles require a larger volume of activated muscle to generate a given force; as shown, a threefold difference in LF (LA=3LB), where LA and LB equal the fascicle (or fiber) lengths of muscle A and muscle B, results in proximal muscle A consuming roughly threefold more energy to produce a given force compared with distal muscle B (∼33% recruitment of the muscles is depicted to produce a given force).
The ability to produce and control movements depends on fiber length, as well as tendon length and stiffness. Long fibers can produce or control larger changes in overall length for a given strain of individual fibers and sarcomeres in series along the fiber (i.e. change in actin–myosin filament overlap). In the absence of a free tendon, which contributes to the series elasticity of an MTU, length changes of the muscle's fibers more effectively control or produce motions of the skeleton at a joint. Although recent work shows that the passive elasticity of a free tendon or aponeurosis may protect a muscle's fibers from potential damage when rapidly stretched (Konow et al., 2012; Roberts and Azizi, 2010) and can also modulate the F–V effects of the muscle's fibers to produce force with greater efficiency (Lichtwark and Wilson, 2005; Roberts, 2002), passive elasticity of connective tissue in series with a muscle's fibers limits the ability of the fibers to control movement at a skeletal attachment site. Extreme examples are the very short (6–12 mm) pinnate fibered superficial (SDF) and deep digital flexor (DDF) and plantaris (PL) muscles of horses and other large ungulates, which attach to the distal phalanges via long (∼600 mm) tendons (Alexander, 2002; Biewener, 1998b; Wilson et al., 2001). The >60-fold length of the muscle's tendon means that a 2–3% strain of the tendon, commonly experienced during locomotion in a variety of animals (Alexander, 2002; Biewener, 1998b; Biewener and Baudinette, 1995; Fukunaga et al., 2001; Lichtwark et al., 2007), represents a 200% strain of the fibers, well beyond their physiological range for effectively generating force. This becomes more of a problem when greater forces and larger tendon strains are produced at faster speeds. In these extreme short-fibered, long-tendon MTUs, the muscles likely act as dampers to absorb potentially damaging impact energy when the limb collides with the ground (Wilson et al., 2001), while the tendons provide elastic energy recovery. The short-fibered architecture of these highly pinnate muscles also greatly reduces the cost of force generation (Fig. 1B; Biewener and Roberts, 2000; Roberts, 2002) during locomotion.
In vivo contractile dynamics of muscle function
The cyclical dynamics of limb movement in locomotion, whether in running or flying, favors muscles that undergo stretch–shorten contraction cycles, in which activation of the muscle during an eccentric (or near isometric) phase of contraction enhances force development, elastic energy storage, and subsequent work output during the shortening phase of contraction (Biewener, 1998a; Komi, 2000). By allowing for increased force generation, eccentric activation of muscles also likely reduces the energy consumed by the muscle as a whole relative to the force it must generate. In this context, studies of in vivo muscle function during terrestrial locomotion are examined before being compared with studies of muscles that power flight.
The majority of in vivo studies of muscle function have focused on distal MTUs because the use of tendon force transducers allows muscle–tendon forces to be related to in vivo patterns of neuromuscular activation by means of indwelling electromyography (EMG) electrodes and fascicle length change by means of implanted piezoelectric sonomicrometry electrodes. In contrast, the forces produced by proximal muscles must be inferred from the timing of myoelectric activity or assessed indirectly by means of inverse dynamics analysis of the joint torques to which the muscle contributes.
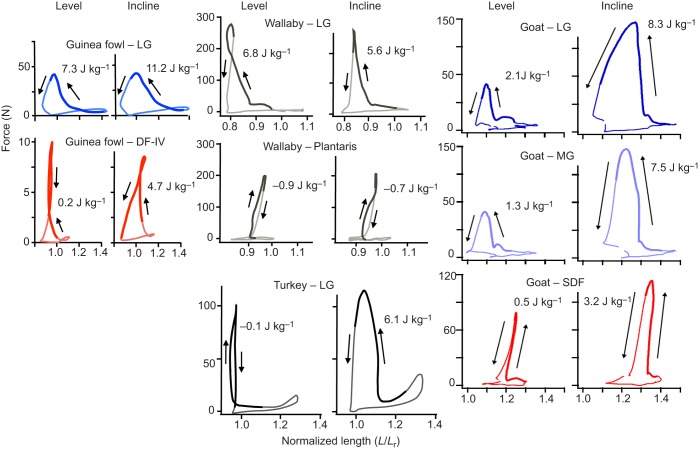
In general, distal MTUs display similar patterns of largely isometric contractile behavior of the muscle fascicles when force is generated during the stance phase of steady level locomotion, with flexion and extension of the ankle or more distal foot joints mediated by stretch and subsequent recoil of the muscle's tendon, providing elastic energy return. Shortening strains of the muscle (defined and reported here as negative strains measured relative to resting fascicle length, Lr) and positive work output (mathematically summed as increments of force times the absolute value of shortening strain) typically occur either early (Biewener et al., 1998b; Roberts et al., 1997) or late (Lichtwark and Wilson, 2006) in force development. Fascicle strains during force generation are limited to as little as −2 to −6% in the PL and lateral gastrocnemius (LG) of hopping wallabies (Biewener et al., 1998b), the LG of running turkeys (Roberts et al., 1997), and the SDF of trotting goats, but can be as high as −15 to −20% in the LG of running guinea fowl and trotting goats (Fig. 2) (Daley and Biewener, 2003; McGuigan et al., 2009). Consequently, the guinea fowl LG produces a significant amount of positive work (11.4±2.1 J kg−1 at 2.0 m s−1) on the level compared with the turkey LG (0.3 J kg−1 at 2.5 m s−1) and the wallaby LG (−1.4 J kg−1, averaged over speeds from 3.0 to 6.0 m s−1), as well as compared with the goat LG (2.11 J kg−1), medial gastrocnemius (MG; 1.27 J kg−1) and SDF (0.49 J kg−1) during trotting at 2.5 m s−1. In contrast to the limited amount of fascicle work generated by most of these muscles, the tendons store and return from 2.5- to 36-fold more elastic strain energy, the most extreme cases being the wallaby LG and PL, and the goat SDF (Biewener et al., 1998b; McGuigan et al., 2009).
Fig. 2.
Comparison of the in vivo force–length behavior and net work done by distal muscle–tendon units under level versus incline conditions. Recordings show muscle forces determined from tendon force measurements, along with normalized fascicle length changes (L/Lr) as determined via sonomicrometry. Data are drawn from the following sources: guinea fowl lateral gastrocnemius (LG) and digital flexor IV (DF-IV) running at 1.3 m s−1 (Daley and Biewener, 2003); wallaby LG and plantaris (PL) hopping at 4.5 m s−1 (Biewener et al., 2004); turkey LG running at 2.5 m s−1 (Biewener and Roberts, 2000; Roberts et al., 1997); and goat medial gastrocnemius (MG), LG, and superficial digital flexor (SDF) trotting at 2.5 m s−1 (McGuigan et al., 2009). Whereas the wallaby LG force–length patterns presented here show positive work during level and incline hopping, with 10–12% shortening strains during active force development, LG shortening strains recorded across four animals averaged −1.0±4.6% for level versus 0.6±4.5% for incline hopping, with net work averaging −8.4±8.4 J kg−1 for level versus −6.8±7.5 J kg−1 for incline hopping (Biewener et al., 2004).
When moving up an incline or down a decline, certain distal muscles adjust their in vivo length-change behavior to adjust net work output, but others do not, or exhibit limited changes in work. The turkey LG (Fig. 2) and peroneus longus (PLong) adjust work output generally in proportion to their mass (Gabaldón et al., 2004), shortening more on an incline versus lengthening more and absorbing energy on a decline, with little change in peak force. Although the work output of the goat MG and LG increases during incline walking or trotting (Fig. 2) and decreases when moving down a decline, the shifts in mass-specific muscle work are a fraction (∼45%) of the amount estimated to be required for the hind limb muscles as a whole (McGuigan et al., 2009). Although some of the change in muscle work results from changes in shortening or lengthening strain, distal goat muscles also generate greater force on an incline than at the same speed on the level. The goat SDF and the wallaby LG and PL (Fig. 2), however, show little change in in vivo F–L and work behavior when locomotion shifts from level to incline or decline grades (Biewener et al., 2004; McGuigan et al., 2009). Consequently, in contrast to the turkey LG and PLong (Gabaldón et al., 2004), the limited shifts in work output by the goat and wallaby MTUs, as well as the guinea fowl LG and digital flexor IV (DF-IV) (Daley and Biewener, 2003), indicate that the majority of net work is performed by more proximal hind limb muscles in these species.
It is worth noting that the mallard LG (Biewener and Corning, 2001) is an exception to the general pattern observed for distal MTUs. In contrast to the LG of more cursorial ground birds and mammals, the mallard LG generates significant work (13.1 J kg−1) during over-ground walking and running through substantial (−37%) fascicle shortening, with little tendon elastic energy recovery (<5% of muscle work). This behavior was also observed during swimming, suggesting that design and work performance of the mallard LG reflects the interacting requirements of swimming and terrestrial locomotion in this species.
A proximo-distal gradient in muscle architecture, contractile behavior and work output?
Assessing proximal muscle work across locomotion conditions is challenged by the inability to measure muscle force directly. Consequently, studies of proximal muscle function depend on recordings of EMG timing in relation to fascicle strain and joint torque patterns. In general, proximal muscles undergo larger strains when generating force, as inferred from EMG or from joint torques determined through inverse dynamics. Fascicle strains of proximal hind limb muscles also change their length trajectory according to locomotion grade, with increased fascicle shortening commonly observed on an incline and reduced shortening and/or increased lengthening observed on a decline compared with level gait.
Whereas the distal LG and PL of tammar wallabies exhibit negligible change in F–L behavior for level versus incline hopping and generate little net work, the more proximal biceps femoris (BF; primarily hip extensor) and vastus lateralis (VL; knee extensor) muscles contract with reduced fascicle lengthening and increased shortening when active during incline hopping compared with level hopping (McGowan et al., 2007). As a result, net BF fascicle shortening is increased and net VL lengthening is decreased during incline hopping. Estimates of muscle work based on inverse dynamics are consistent with required changes in work: both BF and VL shift from net negative work (−17.2 J kg−1, combined for both muscles) on the level to positive work (19.9 J kg−1) on an incline (Fig. 3). Because the mass-specific negative work during level hopping and positive work during incline hopping are less than expected for these conditions (relative to hind limb muscle work as a whole), other hip and knee extensors must produce a more substantial fraction of the positive work required for level and incline hopping.
Fig. 3.
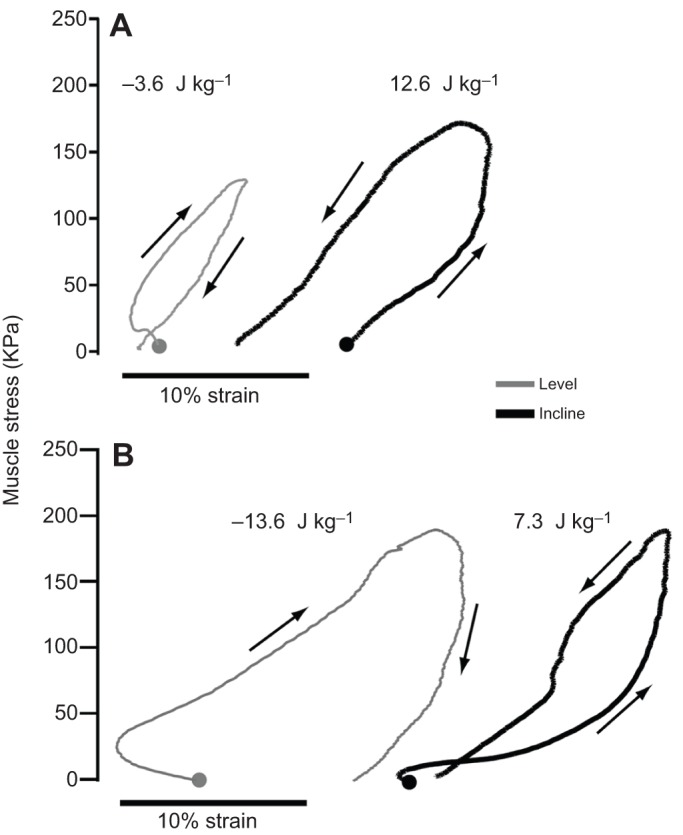
Summary statement. In vivo work loops estimated for the wallaby (A) biceps femoris and (B) vastus lateralis muscles during hopping at 4.2 m s−1 on a level versus at an incline. Muscle stress was estimated from inverse dynamics of joint torques to obtain muscle force and muscle fiber cross-sectional area, with fascicle strains recorded via sonomicrometry (McGowan et al., 2007).
Measurements of fascicle strains in the BF and VL of goats and rats (Gillis and Biewener, 2001, 2002) show similar patterns relative to EMG timing as for wallabies, but the muscles contract over larger in vivo strain ranges. In goats, BF shortening strains range from −22 to −32% during stance for level locomotion across gaits, whereas the VL undergoes a stretch–shorten contraction cycle, with net shortening strains ranging from −5 to −14% across gaits. In rats, BF shortening strains recorded during stance range from −18% during level walking and trotting to −9% during galloping. Changes in grade result in either increased (incline) or reduced (decline) rat BF shortening strain. In contrast, the rat VL undergoes primarily lengthening strains (+8 to +15%) followed by limited shortening during stance, indicating that the rat VL absorbs rather than produces energy during level locomotion, paralleling the pattern observed for the wallaby VL (McGowan et al., 2007). The rat VL also shifts its strain behavior across locomotor grades, reducing net lengthening on an incline and increasing lengthening to absorb energy on a decline.
In vivo study of triceps fascicle strain in the goat forelimb relative to EMG and joint kinematics (Carroll and Biewener, 2009) reveals that the mono-articular lateral head (TrLAT) undergoes stretch–shorten contractile patterns (+6.8% stretch, followed by −10.6% shortening) consistent with elbow flexion and extension during the stance phase of walking, trotting, and galloping. In contrast, the biarticular long head of triceps (TrLONG) uniformly shortens (−16.4%) over stance across speed and gait. These patterns parallel the stretch–shorten behavior of the mono-articular goat hind limb VL (McGuigan et al., 2009), which undergoes stretch–shorten contractile behavior consistent with knee flexion and extension, compared with fascicle shortening by the biarticular goat hind limb BF. Similar patterns are also observed in the dog forelimb biarticular TrLONG, which shortens when active early in stance during level trotting (−15.0%) and galloping (−16.3%; Gregersen et al., 1998). Thus, counter to biomechanical analyses of humans (Lichtwark and Wilson, 2006; Prilutsky and Zatsiorsky, 1994; van Ingen Schenau, 1990) and in vivo measurements of cat (Prilutsky et al., 1996), turkey, wallaby, and goat distal hind limb muscles (discussed above), indicating that biarticular muscles primarily transfer energy between adjacent joints rather than performing net work, the fascicle strain and activation patterns of goat and dog TrLONG indicate that this forelimb biarticular muscle contributes significant positive work during level locomotion. In contrast, the mono-articular goat TrLAT likely absorbs and then produces energy during stance.
A different pattern emerges from in vivo studies of fascicle strain in relation to EMG of the horse forelimb TrLAT and hind limb VL (Hoyt et al., 2005; Wickler et al., 2005), in which both mono-articular muscles were found to shorten when activated during the stance phase of level walking and trotting, rather than exhibiting expected stretch–shorten strain patterns. Both muscles also significantly exhibited increased shortening when trotting up a 10 deg incline versus on the level (TrLAT level, −10.6% versus incline, −18.0%; VL level, −8.1% versus incline, −18.5%), consistent with contributing increased work for incline locomotion.
In vivo study of the iliotibialis lateralis pars postacetabularis (ILPO) in guinea fowl (Carr et al., 2011) shows that this large biarticular parallel-fibered hip and knee extensor undergoes consistent stretch–shortening behavior when activated during stance, with fascicle strains increasing across speed and gait (maximum recorded: +6% followed by −14% at 3.0 m s−1). Muscle shortening is mainly associated with hip extension, likely contributing positive work during locomotion. Architecturally, the guinea fowl ILPO is a broad biarticular muscle with short anterior fascicles and much longer posterior fascicles. The short anterior fascicles insert into an aponeurosis that connects to the patellar tendon complex at the knee, whereas the muscle's long posterior fascicles insert directly into the knee tendon complex. Despite large differences in fascicle length across the muscle's breadth, similar fascicle strains occurred in both regions of the muscle. This results from differences in fascicle moment arms at the hip joint: long posterior fascicles operate with a large hip extensor moment arm, whereas short anterior fascicles have a short moment arm. Consequently, similar fascicle strains across the breadth of the muscle contribute to similar ranges of hip extension.
It is apparent that proximal muscles show considerable variation in the range and nature of in vivo fascicle strain across locomotion conditions and species in comparison with distal muscle patterns that have been observed. This variation clearly stems, in part, from differences in specific anatomical features of the proximal muscles that have been studied: whether the muscles are parallel-fibered or pinnate, and biarticular or mono-articular (e.g. biceps femoris versus vastus lateralis), as well as whether a biarticular muscle extends and flexes adjacent joints (e.g. goat BF and TrLONG) or extends both joints (e.g. guinea fowl ILPO). Nevertheless, despite the diverse contractile patterns observed, proximal limb muscles generally operate over large fascicle strain ranges, consistent with playing a major role in modulating work output of the limb as a whole in response to changing locomotor demands. It is also important to bear in mind that even when proximal muscles contract over more limited strains, their greater fiber lengths result in substantially larger muscle length changes and work output compared with short-fibered distal muscles.
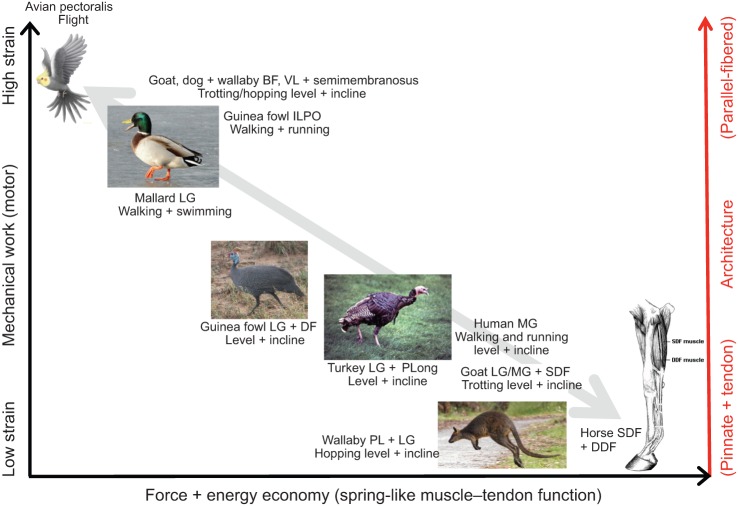
Based on the comparative results for in vivo contractile patterns observed to date for different muscles, we can begin to map out where different muscles reside in relation to functional axes comparing work output versus force economy and elastic savings, relative to fiber architecture (Fig. 4). Qualitatively, an inverse relationship appears to exist between work output versus force economy and tendon elastic savings. High work output and low force economy/elastic savings are observed in longer-fibered muscles that operate over larger shortening strains, whereas low work output but high force economy and the capacity for elastic savings exist for short-fibered muscles that operate over more limited ranges of fascicle strain. Even though in vivo experimental data do not exist for horse digital flexor muscles, the extreme architecture of these distal MTUs demonstrates a design for limited work output, but economical force generation and recovery of elastic energy from long tendons (Biewener, 1998a; Wilson et al., 2001). Experimental studies of human MG during walking and running based on ultrasound imaging in relation to joint moment patterns (Lichtwark et al., 2007) and recent musculoskeletal modeling analysis (Arnold et al., 2013) reinforce this pattern: proximal human muscles contract over larger lengths and perform the majority of lower extremity work compared with distal MTUs that contract with more limited length change, favoring economical force generation and tendon energy recovery.
Fig. 4.
Qualitative comparison of muscle work in relation to force and energy economy and muscle–tendon architecture based on in vivo results obtained from a variety of animal muscles and locomotor conditions. In general, an inverse relationship exists for muscle work relative to force and energy economy, with long parallel-fibered muscles favoring greater work and shorter pinnate-fibered muscles favoring spring-like muscle–tendon function and low cost of force generation. Results for horse distal limb muscles are inferred from muscle–tendon architecture in relation to joint mechanics (Biewener, 1998b; McGuigan and Wilson, 2003). Other data are drawn from the following sources: wallaby plantaris (PL) and lateral gastrocnemius (LG) (Biewener et al., 1998b, 2004); goat LG, medial gastrocnemius (MG), and superficial digital flexor (SDF) (McGuigan et al., 2009); turkey LG and peroneus longus (PLong) (Gabaldón et al., 2004; Roberts et al., 1997); guinea fowl LG and digital flexor IV (DF-IV) (Daley and Biewener, 2003); mallard LG (Biewener and Corning, 2001); goat biceps femoris (BF) and vastus lateralis (VL) (Gillis et al., 2005); dog VL and semimembranosus (Gregersen et al., 1998); wallaby BF and VL (McGowan et al., 2007); and cockatiel pectoralis (Hedrick et al., 2003).
Whereas the cost of force generation (based on active muscle volume) is strongly dependent on muscle architecture, muscle work is not, owing to the fact that work per unit volume of muscle is generally the same across muscles of different architecture (i.e. for a given muscle volume, any increase in force resulting from increased fiber area is offset by reduced fiber length and overall shortening capacity) (Hill, 1950). Therefore, the presence of a proximo-distal gradient in muscle function within an animal's limb in terms of work performance (proximal) versus force economy and elastic energy savings (distal) reflects the relative distribution of muscle mass within a limb, in addition to architectural differences in proximal versus distal muscles and tendons. Modulation of limb work by proximal muscles likely reflects selective pressure on concentrating muscle mass proximally and reducing distal muscle mass to lower the inertial costs of swinging the limbs. Indeed, in a recent study of muscle work relative to the cost of force generation, Holt et al. (2014) argue that shorter-fibered pinnate muscles with long tendons may reflect selection for reduced cost of force generation and inertial cost of limb movement, rather than for increased tendon elastic energy savings per se. This intriguing hypothesis based on muscle ergometry and heat measurements of energy cost merits further consideration and study.
Muscle contractile dynamics underlying avian flight
In comparison to the distal MTUs of terrestrial animals, the contractile behavior of muscles that power bird flight are consistent with the need to generate force while contracting over large shortening strains (Fig. 5A). Avian flight muscles, therefore, parallel the contractile behavior of proximal limb muscles during steady or incline locomotion, as well as during human cycling (e.g. Davies and Sandstrom, 1989; Ericson, 1988) and frog jumping (e.g. Peplowski and Marsh, 1997). This reflects the aerodynamic power demand for flapping flight, which requires that the wing be moved over a large distance to generate the necessary lift for weight support and overcoming drag. The principal muscle that generates the mechanical work (per wingbeat cycle) and power output for flight is the pectoralis (Pect). Across different avian groups, the (left and right) Pect muscles generally constitute 16–22% of the bird's total body mass and 65–70% of flight muscle mass. The Pect therefore contributes the large majority of work for powered flight, and measurements of the in vivo contractile strain and force of the pectoralis provide a fairly direct estimate of the mechanical power costs of flight over a range of speeds (determined from recording of birds trained to fly in a wind tunnel; Askew and Ellerby, 2007; Dial et al., 1997; Hedrick et al., 2003; Tobalske et al., 2003).
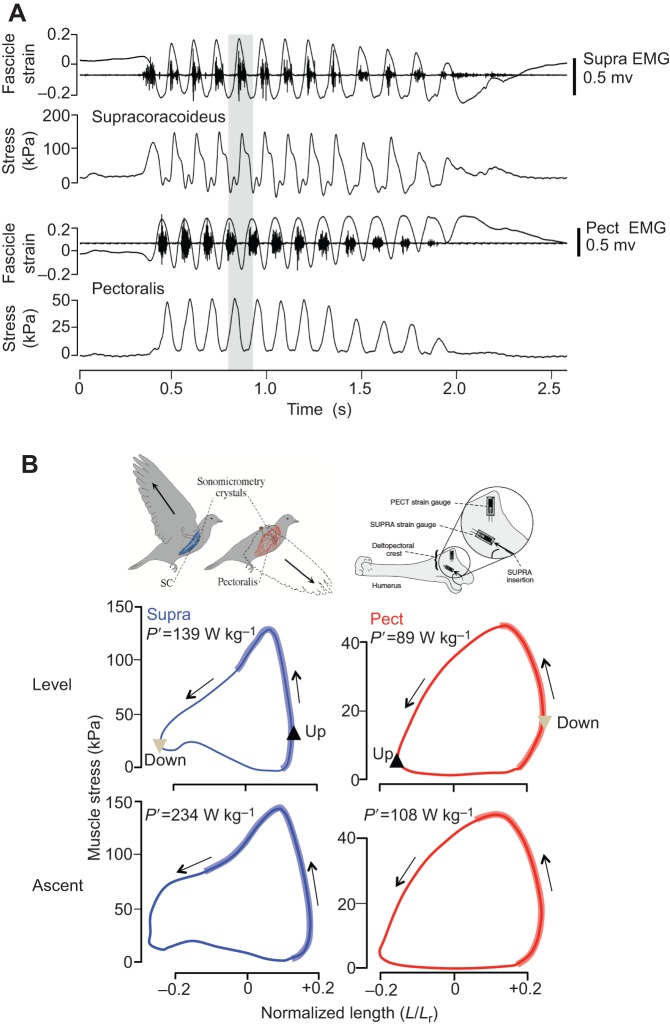
Fig. 5.
In vivo recordings of the pigeon supracoracoideus and pectoralis during free flight. (A) In vivo fascicle length, muscle force and activation (electromyography [EMG]) of the pigeon supracoracoideus (Supra; upstroke muscle) and pectoralis (Pect; downstroke muscle) during perch take-off and slow level flight to a landing perch. (B) In vivo stress versus normalized length work loops of the Supra and Pect corresponding to the fourth wingbeat cycle shown in grey in A. Insets in B show the muscle anatomy and the location of strain gauges bonded to the proximal humerus in locations where the Pect inserts ventrally on the deltopectoral crest and the tendon of the Supra passes dorsally over the shoulder to insert on the dorsal aspect of the humerus. Pull calibrations were used to calibrate muscle force from voltage recordings of bone strain (see Tobalske and Biewener, 2008 for further details). P′, muscle mass-specific power output.
These studies, together with those of free flight (Biewener et al., 1998a; Williamson et al., 2001), reveal that the Pect of cockatiels, doves, magpies, budgerigars, zebra finches, pigeons, and mallards contract with shortening strains of −30 to −40% (depending on flight condition, speed, and species) during the downstroke, when the muscle produces force. Such large shortening strains follow initial passive stretch of the muscle to +20 to +25% beyond Lr at the end of upstroke, with subsequent active shortening to approximately −15 to −20% Lr at the end of downstroke. The Pect is activated late in upstroke, enabling force development under nearly isometric (and in some cases, eccentric) conditions. Activation of the Pect ends close to the timing of peak force generation early in the shortening phase of its strain cycle. Shortening–deactivation of the muscle (Josephson, 1999) likely enables the Pect to relax prior to being passively stretched during the upstroke. As a result, the muscle's in vivo F–L behavior describes a broad counterclockwise work loop (Fig. 5B), the area inside representing the net positive work done by the muscle during each contraction cycle. The architecture of the Pect is consistent with the demand for large shortening strains, as the muscle has a broad origin (from ribs, sternal keel and clavicle) with generally long fascicles attaching either directly or via an internal aponeurosis to the proximal ventral surface of the humerus. Modulation of muscle work and power output across speed is achieved mainly through variation in muscle force, and to a lesser extent fascicle strain and wingbeat frequency (Hedrick et al., 2003), yielding a U-shaped power curve for steady level flight as a function of speed that is generally consistent with aerodynamic theory (Askew and Ellerby, 2007; Hedrick et al., 2003; Tobalske et al., 2003).
The supracoracoideus (Supra) is the main upstroke flight muscle of birds. Lying deep to the Pect along the keel of the sternum, the Supra is a bipinnate muscle that attaches to the proximal dorsal surface of the humerus via a long tendon that passes medially and over the shoulder, acting as a pulley to elevate the wing (Fig. 5B inset). The pigeon Supra undergoes large strains (−33 to −40% during level, ascending, and descending flight conditions) similar to the Pect, with its overall shortening strain comprising +6 to +15% lengthening and −27% net shortening relative to Lr at the end of upstroke across conditions (Fig. 5) (Tobalske and Biewener, 2008). Although the pigeon Supra has 50% shorter fascicles than the Pect (Supra: 24±2 mm versus Pect: 46±6 mm), Supra fascicles undergo similar strains and contract with similar shortening velocities (6–7L s−1) as the Pect, despite the two muscles producing relatively symmetric opposing shoulder motions during upstroke and downstroke. This results from the fact that the Supra operates with a smaller moment arm (2.57±0.70) for shoulder elevation than the Pect moment arm (8.67±1.53) for shoulder depression, allowing its shorter fascicles to operate with a similar strain range to achieve a similar shoulder angular excursion. Fascicle contractile strains in relation to shoulder motion are also influenced by the greater tendon compliance of the Supra compared with the Pect, which contributes to substantial elastic energy recovery by the Supra tendon (Tobalske and Biewener, 2008).
Despite these similarities in contractile strains and relative fascicle shortening velocities, the Supra generates greater forces for its size, achieving peak stresses (85, 96, and 125 kPa, average for descending, level, and ascending flight, respectively) that exceed those of the Pect (50, 53, and 58 kPa). As a result, the Supra generates a greater mass-specific power output (106, 127, and 194 W kg−1) than the Pect (75, 87, and 105 W kg−1 across descending, level, and ascending flight, respectively). The greater mass-specific muscle work per cycle (Supra: 13.0, 15.3, and 23.4 J kg−1 versus Pect: 8.7, 10.07, and 11.6 J kg−1 for descending, level, and ascending flight, respectively) suggests that a greater fractional volume of the Supra is recruited compared with the Pect across flight conditions. Despite these differences, the timing of muscle activation relative to length change and force development is quite similar (though in opposite phase with respect to the wingbeat cycle) for the two principal flight muscles (Fig. 5), enabling both to develop force under near-isometric conditions. The magnitude of work by these flight muscles parallels the mass-specific work output measured in the mallard LG and guinea fowl LG on an incline, and is in the upper range of mass-specific work (20 J kg−1) that may be expected when muscles contract at shortening velocities that maximize their power output (Alexander, 1992; Woledge et al., 1985).
Influence of activation timing, F–V, and F–L properties on muscle work and running stability
As demonstrated by in situ and in vitro ‘work loop’ experiments (Josephson, 1985, 1999) that measure a muscle's cyclical work performance in relation to stimulation phase and imposed length changes, activation timing is crucial to muscle work performance. When stimulated to produce maximal force during shortening, a muscle's positive (and net) work is maximized for a given shortening strain. Because of electromechanical delays and the time required for force development, activation of a muscle that powers cyclical movement must occur in advance of muscle shortening to maximize its net work performance. This is the pattern observed for both the avian Pect and Supra muscles during flight. However, no significant changes in activation timing are seen across flight speed or flight conditions to modulate muscle work (Hedrick et al., 2003; Tobalske and Biewener, 2008).
Changes in activation timing, however, have been demonstrated for certain limb muscles during terrestrial locomotion that contribute to shifts in work performance with changing grade. In guinea fowl, changes in activation timing (but not EMG magnitude) of the DF-IV are correlated with changes in work output (Daley and Biewener, 2003). In turkeys, changes in the timing of PLong peak force also correspond to changes in work output of the muscle across decline, level, and incline running (Gabaldón et al., 2004). In both muscles, changes in strain amplitude also contribute to adjustments in work output. Although the evidence from in vivo studies to date is rather meager, it seems likely that activation phase relative to force development and resulting muscle length change can play a key role in modulating work across locomotion conditions, as evidenced by its role in in vitro work-loop studies.
The F–V and F–L properties of muscles have also been shown to provide intrinsic feedback control of muscle force and work under in vivo conditions when animals encounter unexpected perturbations or require rapid adjustments to steady level running. These studies have been carried out on guinea fowl (Daley and Biewener, 2011; Daley et al., 2006, 2009), and more recently have compared obstacle negotiation across different-sized avian bipeds (Birn-Jeffery et al., 2014). In vivo recordings of force, EMG, and fascicle strain of the guinea fowl LG show that, when guinea fowl encounter a sudden unexpected drop in substrate height (8.5 cm) while running, the dynamics of the perturbation cause the ankle joint to extend further before foot–ground contact is made, increasing the magnitude and velocity of LG shortening. These F–L and F–V effects likely contribute to the observed reduction in muscle force, as well as shortening deactivation of the muscle. Decreased force and muscle work are appropriate mechanical responses to the limb's more extended configuration when it finally strikes the ground for weight support at a later phase of the perturbed stride (Daley et al., 2009). These changes occur before neural reflexes have time to act and thus reinforce subsequent reflex adjustments to muscle activation.
In contrast to drop perturbations, when guinea fowl encounter obstacles (5 cm height) while running on a treadmill (Daley and Biewener, 2011), the LG shortens less and at a slower velocity during the obstacle negotiation stride, during which the ankle operates over a more flexed range of motion. The muscle's longer length and slower velocity result in more rapid and greater force development. As in drop perturbations, obstacle negotiation strides therefore make use of rapid intrinsic F–L and F–V effects to enhance force development, reinforcing subsequent neural proprioceptive feedback (∼6 ms reflex latency), adjusting how the LG contributes to joint and whole-limb work (Daley and Biewener, 2011). Together, these neuromechanical responses provide effective stabilization when rapid adjustments are required during running.
Conclusions
As has been long recognized, muscle–tendon architecture contributes importantly to the functional role that muscles play in animal locomotion. Understanding muscle–tendon architecture in terms of locomotor movement requires that the contractile dynamics of muscle force and length change be assessed in the context of the time-varying demand for the forces and work that muscles must produce to propel and control the movements of an animal. In this sense, locomotion represents the emergent unifying functional context in which muscle contractile dynamics can be studied and better understood.
In vivo patterns of muscle strain and force generation critically depend on the timing of neuromuscular activation in relation to the cyclical movements of an animal's limbs and the loads against which muscles contract (Marsh, 1999). Stretch–shorten contraction cycles and isometric force development are common features of muscle dynamics that power both terrestrial locomotion and flight, favoring increased shortening work and reduced cost of force generation. When linked to muscle–tendon architecture, these patterns help to reveal how the design of different limb muscles favors work modulation and the control of movement versus MTUs that reduce the cost of force generation and can provide elastic energy return.
Although an understanding of proximal limb muscles is challenged by obtaining direct in vivo measures of muscle force, by combining in vivo techniques to quantify fascicle strain in relation to neuromuscular activation with inverse dynamics analysis of joint torque patterns, the roles of proximal muscles can be elucidated in relation to those of distal MTUs, for which direct measures of muscle–tendon force can be quantified for varying in vivo conditions. It is clear that proximal muscles play diverse roles. Whereas distal biarticular muscles may primarily function to transfer energy, generating force economically with little net work performed, proximal biarticular muscles can contribute to work performed at one or both adjacent joints. Finally, classical F–L and F–V properties along with history-dependent properties, such as stretch–activation and shortening–deactivation, can play important roles in providing rapid, intrinsic adjustments to muscle force and work output that reinforce subsequent neuromotor feedback in the control of movement when steady movement patterns are perturbed. Future studies will benefit by integrating the functional significance of single-fiber properties to features of whole-muscle and connective tissue function, studied in the context of the time-varying dynamics of locomotion across a range of conditions. Use of modeling simulations will also play an increasingly important role for providing an overarching understanding of limb muscle design and use at the whole-animal level.
Acknowledgements
I am indebted to many past students, post-doctoral trainees and collaborators who have shared and contributed importantly to the results from our work that are reviewed here on muscle function in terrestrial locomotion and flight, as well as the constructive critical advice of the two referees. I thank Pedro Ramirez for his superb care and management of the animal program at the Concord Field Station. Thanks also to Stan Lindstedt and Hans Hoppeler for organizing and producing such a broad and well-conceived conference on muscle function that cuts across multiple levels of organization and has broad application to many disciplines.
Footnotes
Competing interests
The author declares no competing or financial interests.
Funding
Support for this work has benefitted from grants from the National Institutes of Health, the National Science Foundation and the Defense Advanced Research Products Agency. Deposited in PMC for release after 12 months.
References
- Alexander R. M. (1992). The work that muscles can do. Nature 357, 360 10.1038/357360a0 [DOI] [PubMed] [Google Scholar]
- Alexander R. M. (2002). Tendon elasticity and muscle function. Comp. Biochem. Physiol. A 33, 1001-1011. 10.1016/S1095-6433(02)00143-5 [DOI] [PubMed] [Google Scholar]
- Arnold E. M., Hamner S. R., Seth A., Millard M. and Delp S. L. (2013). How muscle fiber lengths and velocities affect muscle force generation as humans walk and run at different speeds. J. Exp. Biol. 216, 2150-2160. 10.1242/jeb.075697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew G. N. and Ellerby D. J. (2007). The mechanical power requirements of avian flight. Biol. Lett. 3, 445-448. 10.1098/rsbl.2007.0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewener A. A. (1998a). Muscle function in vivo: the design of muscles used as springs versus muscles used to generate mechanical power. Am. Zool. 38, 703-717. 10.1093/icb/38.4.703 [DOI] [Google Scholar]
- Biewener A. A. (1998b). Muscle-tendon stresses and elastic energy storage during locomotion in the horse. Comp. Biochem. Physiol. B 120, 73-87. 10.1016/S0305-0491(98)00024-8 [DOI] [PubMed] [Google Scholar]
- Biewener A. A. and Baudinette R. V. (1995). In vivo muscle force and elastic energy storage during steady-speed hopping of tammar wallabies (Macropus eugenii). J. Exp. Biol. 198, 1829-1841. [DOI] [PubMed] [Google Scholar]
- Biewener A. A. and Corning W. R. (2001). Dynamics of mallard (Anas platyrynchos) gastrocnemius function during swimming versus terrestrial gait. J. Exp. Biol. 204, 1745-1756. [DOI] [PubMed] [Google Scholar]
- Biewener A. A. and Roberts T. J. (2000). Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exer. Sport Sci. Rev. 28, 99-107. [PubMed] [Google Scholar]
- Biewener A. A., Corning W. R. and Tobalske B. T. (1998a). In vivo pectoralis muscle force–length behavior during level flight in pigeons (Columba livia). J. Exp. Biol. 201, 3293-3307. [DOI] [PubMed] [Google Scholar]
- Biewener A. A., Konieczynski D. D. and Baudinette R. V. (1998b). In vivo muscle force-length behavior during steady-speed hopping in tammar wallabies. J. Exp. Biol. 201, 1681-1694. [DOI] [PubMed] [Google Scholar]
- Biewener A. A., McGowan C., Card G. M. and Baudinette R. V. (2004). Dynamics of leg muscle function in tammar wallabies (M. eugenii) during level versus incline hopping. J. Exp. Biol. 207, 211-223. 10.1242/jeb.00764 [DOI] [PubMed] [Google Scholar]
- Birn-Jeffery A. V., Hubicki C. M., Blum Y., Renjewski D., Hurst J. W. and Daley M. A. (2014). Don't break a leg: running birds from quail to ostrich prioritise leg safety and economy on uneven terrain. J. Exp. Biol. 217, 3786-3796. 10.1242/jeb.102640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J. A., Ellerby D. J. and Marsh R. L. (2011). Function of a large biarticular hip and knee extensor during walking and running in guinea fowl (Numida meleagris). J. Exp. Biol. 214, 3405-3413. 10.1242/jeb.060335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. M. and Biewener A. A. (2009). Mono- versus biarticular muscle function in relation to speed and gait changes: in vivo analysis of the goat triceps brachii. J. Exp. Biol. 212, 3349-3360. 10.1242/jeb.033639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley M. A. and Biewener A. A. (2003). Muscle force-length dynamics during level versus incline locomotion: a comparison of in vivo performance of two guinea fowl ankle extensors. J. Exp. Biol. 206, 2941-2958. 10.1242/jeb.00503 [DOI] [PubMed] [Google Scholar]
- Daley M. A. and Biewener A. A. (2011). Leg muscles that mediate stability: mechanics and control of two distal extensor muscles during obstacle negotiation in the guinea fowl. Philos. Trans. R. Soc. B Biol. Sci. 366, 1580-1591. 10.1098/rstb.2010.0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley M. A., Usherwood J. R., Felix G. and Biewener A. A. (2006). Running over rough terrain: guinea fowl maintain dynamic stability despite a large unexpected change in substrate height. J. Exp. Biol. 209, 171-187. 10.1242/jeb.01986 [DOI] [PubMed] [Google Scholar]
- Daley M. A., Voloshina A. and Biewener A. A. (2009). The role of intrinsic muscle mechanics in the neuromuscular control of stable running in the guinea fowl. J. Physiol. 587, 2693-2707. 10.1113/jphysiol.2009.171017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C. T. M. and Sandstrom E. R. (1989). Maximal mechanical power output and capacity of cyclists and young adults. Eur. J. Appl. Physiol. 58, 838-844. 10.1007/BF02332216 [DOI] [PubMed] [Google Scholar]
- Dial K. P., Biewener A. A., Tobalske B. W. and Warrick D. R. (1997). Mechanical power output of bird flight. Nature 390, 67-70. 10.1038/36330 [DOI] [Google Scholar]
- Ericson M. O. (1988). Mechanical muscular power output and work during ergometer cycling at different work loads and speeds. Eur. J. Appl. Physiol. 57, 382-387. 10.1007/BF00417980 [DOI] [PubMed] [Google Scholar]
- Fukunaga T., Kubo K., Kawakami Y., Fukashiro S., Kanehisa H. and Maganaris C. N. (2001). In vivo behaviour of human muscle tendon during walking. Proc. R. Soc. Lond. B Biol. Sci. 268, 229-233. 10.1098/rspb.2000.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaldón A. M., Nelson F. E. and Roberts T. J. (2004). Mechanical function of two ankle extensors in wild turkeys: shifts from energy production to energy absorption during incline versus decline running. J. Exp. Biol. 207, 2277-2288. 10.1242/jeb.01006 [DOI] [PubMed] [Google Scholar]
- Gans C. and de Vree F. (1987). Functional bases of fiber length and angulation in muscle. J. Morphol. 192, 63-85. 10.1002/jmor.1051920106 [DOI] [PubMed] [Google Scholar]
- Gillis G. B. and Biewener A. A. (2001). Hindlimb muscle function in relation to speed and gait: in vivo strain and activation in a hip and knee extensor of the rat (Rattus norvegicus). J. Exp. Biol. 204, 2717-2731. [DOI] [PubMed] [Google Scholar]
- Gillis G. B. and Biewener A. A. (2002). Effects of surface grade on proximal hindlimb muscle strain and activation during rat locomotion. J. Appl. Physiol. 93, 1731-1743. 10.1152/japplphysiol.00489.2002 [DOI] [PubMed] [Google Scholar]
- Gillis G. B., Flynn J. P., McGuigan P. and Biewener A. A. (2005). Patterns of strain and activation in the thigh muscles of goats across gaits during level locomotion. J. Exp. Biol. 208, 4599-4611. 10.1242/jeb.01940 [DOI] [PubMed] [Google Scholar]
- Gregersen C. S., Silverton N. A. and Carrier D. R. (1998). External work and potential for elastic storage at the limb joints of running dogs. J. Exp. Biol. 201, 3197-3210. [DOI] [PubMed] [Google Scholar]
- Hedrick T. L., Tobalske B. W. and Biewener A. A. (2003). How cockatiels (Nymphicus hollandicus) modulate pectoralis power output across flight speeds. J. Exp. Biol. 206, 1363-1378. 10.1242/jeb.00272 [DOI] [PubMed] [Google Scholar]
- Hill A. V. (1950). The dimensions of animals and their muscular dynamics. Sci. Prog. 38, 209-230. [Google Scholar]
- Holt N. C., Roberts T. J. and Askew G. N. (2014). The energetic benefits of tendon springs in running: is the reduction of muscle work important? J. Exp. Biol. 217, 4365-4371. 10.1242/jeb.112813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt D. F., Wickler S. J., Biewener A. A., Cogger E. A. and De La Paz K. L. (2005). In vivo muscle function vs speed: I. Muscle strain in relation to length change of the muscle-tendon unit. J. Exp. Biol. 208, 1175-1190. 10.1242/jeb.01486 [DOI] [PubMed] [Google Scholar]
- Josephson R. K. (1985). Mechanical power output from striated muscle during cyclical contraction. J. Exp. Biol. 114, 493-512. [Google Scholar]
- Josephson R. K. (1999). Dissecting muscle power output. J. Exp. Biol. 202, 3369-3375. [DOI] [PubMed] [Google Scholar]
- Komi P. V. (2000). Stretch-shortening cycle: a powerful model to study normal and fatigued muscle. J. Biomech. 33, 1197-1206. 10.1016/S0021-9290(00)00064-6 [DOI] [PubMed] [Google Scholar]
- Konow N., Azizi E. and Roberts T. J. (2012). Muscle power attenuation by tendon during energy dissipation. Proc. R. Soc. B Biol. Sci. 279, 1108-1113. 10.1098/rspb.2011.1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtwark G. A. and Wilson A. M. (2005). Effects of series elasticity and activation conditions on muscle power output and efficiency. J. Exp. Biol. 208, 2845-2853. 10.1242/jeb.01710 [DOI] [PubMed] [Google Scholar]
- Lichtwark G. A. and Wilson A. M. (2006). Interactions between the human gastrocnemius muscle and the Achilles tendon during incline, level and decline locomotion. J. Exp. Biol. 209, 4379-4388. 10.1242/jeb.02434 [DOI] [PubMed] [Google Scholar]
- Lichtwark G. A., Bougoulias K. and Wilson A. M. (2007). Muscle fascicle and series elastic element length changes along the length of the human gastrocnemius during walking and running. J. Biomech. 40, 157-164. 10.1016/j.jbiomech.2005.10.035 [DOI] [PubMed] [Google Scholar]
- Lieber R. L. (1992). Skeletal Muscle Structure and Function. Baltimore: Williams and Wilkins. [Google Scholar]
- Loeb G. E., Brown I. E. and Cheng E. J. (1999). A hierarchical foundation for models of sensorimotor control. Exp. Brain Res. 126, 1-18. 10.1007/s002210050712 [DOI] [PubMed] [Google Scholar]
- Marsh R. L. (1999). How muscles deal with real-world loads: the influence of length trajectory on muscle performance. J. Exp. Biol. 202, 3377-3385. [DOI] [PubMed] [Google Scholar]
- McGowan C. P., Baudinette R. V. and Biewener A. A. (2007). Modulation of proximal muscle function during level versus incline hopping in tammar wallabies (Macropus eugenii). J. Exp. Biol. 210, 1255-1265. 10.1242/jeb.02742 [DOI] [PubMed] [Google Scholar]
- McGuigan M. P. and Wilson A. M. (2003). The effect of gait and digital flexor muscle activation on limb compliance in the forelimb of the horse Equus caballus. J. Exp. Biol. 206, 1325-1336. 10.1242/jeb.00254 [DOI] [PubMed] [Google Scholar]
- McGuigan M. P., Yoo E., Lee D. V. and Biewener A. A. (2009). Dynamics of goat distal hind limb muscle–tendon function in response to locomotor grade. J. Exp. Biol. 212, 2092-2104. 10.1242/jeb.028076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon T. A. (1984). Muscles, Reflexes, and Locomotion. Princeton, NJ: Princeton University Press. [Google Scholar]
- Peplowski M. M. and Marsh R. L. (1997). Work and power output in the hindlimb muscles of Cuban tree frogs Osteopilus septentrionalis during jumping. J. Exp. Biol. 200, 2861-2870. [DOI] [PubMed] [Google Scholar]
- Prilutsky B. I. and Zatsiorsky V. M. (1994). Tendon action of two-joint muscles: transfer of mechanical energy between joints during jumping, landing, and running. J. Biomech. 27, 25-34. 10.1016/0021-9290(94)90029-9 [DOI] [PubMed] [Google Scholar]
- Prilutsky B. I., Herzog W. and Leonard T. (1996). Transfer of mechanical energy between ankle and knee joints by gastrocnemius and plantaris muscles during cat locomotion. J. Biomech. 29, 391-403. 10.1016/0021-9290(95)00054-2 [DOI] [PubMed] [Google Scholar]
- Rack P. M., Ross H. F., Thilmann A. F. and Walters D. K. (1983). Reflex responses of the human ankle: the importance of tendon compliance. J. Physiol. 344, 503-524. 10.1113/jphysiol.1983.sp014954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. J. (2002). The integrated function of muscles and tendons during locomotion. Comp. Biochem. Physiol. A 133, 1087-1099. 10.1016/S1095-6433(02)00244-1 [DOI] [PubMed] [Google Scholar]
- Roberts T. J. and Azizi E. (2010). The series-elastic shock absorber: tendons attenuate muscle power during eccentric actions. J. Appl. Physiol. 109, 396-404. 10.1152/japplphysiol.01272.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. J., Marsh R. L., Weyand P. G. and Taylor C. R. (1997). Muscular force in running turkeys: the economy of minimizing work. Science 275, 1113-1115. 10.1126/science.275.5303.1113 [DOI] [PubMed] [Google Scholar]
- Tobalske B. W. and Biewener A. A. (2008). Contractile properties of the pigeon supracoracoideus during different modes of flight. J. Exp. Biol. 211, 170-179. 10.1242/jeb.007476 [DOI] [PubMed] [Google Scholar]
- Tobalske B. W., Hedrick T. L., Dial K. P. and Biewener A. A. (2003). Comparative power curves in bird flight. Nature 421, 363-366. 10.1038/nature01284 [DOI] [PubMed] [Google Scholar]
- van Ingen Schenau G. J. (1990). On the action of bi-articular muscles, a review. Neth. J. Zool. 40, 521-543. 10.1163/156854290X00073 [DOI] [Google Scholar]
- Wickler S. J., Hoyt D. F., Biewener A. A., Cogger E. A. and De La Paz K. L. (2005). In vivo muscle function vs speed II. Muscle function trotting up an incline. J. Exp. Biol. 208, 1191-1200. 10.1242/jeb.01485 [DOI] [PubMed] [Google Scholar]
- Williamson M. R., Dial K. P. and Biewener A. A. (2001). Pectoralis muscle performance during ascending and slow level flight in mallards (Anas platyrynchos). J. Exp. Biol. 204, 495-507. [DOI] [PubMed] [Google Scholar]
- Wilson A. M., McGuigan M. P., Su A. and van den Bogert A. J. (2001). Horses damp the spring in their step. Nature 414, 895-899. 10.1038/414895a [DOI] [PubMed] [Google Scholar]
- Woledge R. C., Curtin N. A. and Homsher E. (1985). Energetic Aspects of Muscle Contraction. London: Academic Press. [PubMed] [Google Scholar]