Abstract
A 73-year-old female presenting with haemoptysis and dyspnoea was found to have a locally advanced left thyroid mass and vocal cord palsy. A CT scan of the neck and thorax and endoscopy demonstrated invasion into the tracheal lumen. Histopathology of the intraluminal tracheal mass confirmed a papillary thyroid cancer (PTC). The tumour was deemed unresectable due to local extent and patient comorbidities. TKI therapy with lenvatinib was used for 14 months. On serial scanning, a marked reduction in tumour volume from 31 × 59 × 32 mm to 17 × 28 × 22 mm was noted. This subsequently allowed a successful surgical resection with a total thyroidectomy and central neck dissection with no evidence of residual macroscopic disease. Histopathology confirmed a well-differentiated PTC with features of tumour regression. In this case, TKI therapy in a locally advanced unresectable DTC reduced tumour size and infiltration to a degree that surgical resection of macroscopic disease was possible, without requiring airway resection. This raises the possibility that TKIs may have a neoadjuvant role in selected cases of locally advanced DTC to reduce tumour volume and therefore morbidity of subsequent surgical resection.
Keywords: Thyroid neoplasm, Papillary carcinoma, Treatment outcome, Tyrosine kinase inhibitors, Antineoplastic agents
What Is Known about This Topic?
To achieve surgical clearance of disease in locally advanced thyroid cancer, vital structures may need to be sacrificed leading to functional impairment and cosmetic deformity. Historically for patients deemed unsuitable for surgery, limited alternative options were available. Sorafenib and lenvatinib both showed a prolonged progression-free survival advantage in patients with radioactive iodine-refractory differentiated thyroid cancer (DTC).
What Does This Case Report Add?
This case illustrates a patient with locally advanced DTC treated with tyrosine kinase inhibitor (TKI) therapy, which markedly reduced tumour volume and allowed subsequent successful surgical resection without requiring laryngectomy. This raises the possibility that TKIs may have a neoadjuvant role in selected cases of locally advanced DTC to reduce tumour volume and therefore morbidity of subsequent surgical resection.
Introduction
Differentiated thyroid cancer (DTC) is increasing in incidence, with the most marked increase seen in small volume, low-risk lesions [1]. However, an increase in more advanced disease has also been reported [2, 3]. The majority of patients present with disease which is amenable to curative treatment, with resection of all macroscopic disease. Adjuvant therapy can then be recommended dependent on patient and tumour-specific features.
However, a small number of patients present with locally advanced disease which is invading the visceral structures of the central neck. Management of such patients is controversial and dependent on the extent of luminal invasion [4], the burden of disease, and the comorbidities of the patient [5]. Historically few treatment options have been available for these patients other than radical surgery with post-operative adjuvant therapy. Although this approach has been shown to optimise oncological outcome [5], the associated morbidity is significant. Such surgery may be deemed impossible or inappropriate depending on the tumour and patient-related factors. For those patients considered unsuitable for radical surgery, few treatment options are available. Chemotherapy has little evidence of benefit, and external beam radiotherapy [6] at best slows disease progression.
Tyrosine kinase inhibitors (TKIs) are oral multi-targeted drugs which act in part by reducing tumour blood supply and subsequent growth. They disrupt angiogenesis by targeting pathways including those controlled by vascular endothelial growth factor receptor and fibroblast growth factor receptor [7]. TKIs have now been approved for use in patients with advanced radioactive iodine (RAI)-refractory progressive DTC by the United States Food and Drug Administration (US FDA) and in the UK by the National Institute for Health and Care Excellence (NICE) [8]. Sorafenib and lenvatinib both showed promising outcomes in the double-blind multicentre phase III trials – DECISION [9] and SELECT [10, 11]. These trials demonstrated prolonged progression-free survival advantage in patients with RAI-refractory DTC compared with placebo.
Here, we discuss the case of a 73-year-old woman who presented with locally invasive papillary thyroid cancer (PTC) treated with TKI therapy and who subsequently underwent total thyroidectomy and central lymph node dissection. Very few patients treated with TKIs for unresectable local disease have been reported to date; hence, the clinical and pathological response to TKI therapy in this setting is poorly understood.
Case Report
A 73-year-old woman presented in February 2016 with episodes of small-volume haemoptysis and shortness of breath on exertion. A CT scan of the thorax found a retrosternal thyroid with evidence of some airway narrowing. Her symptoms progressed to exertional stridor. She denied any dysphagia or pain and had no history of weight loss. She had evidence of a left vocal cord palsy, but no palpable neck masses.
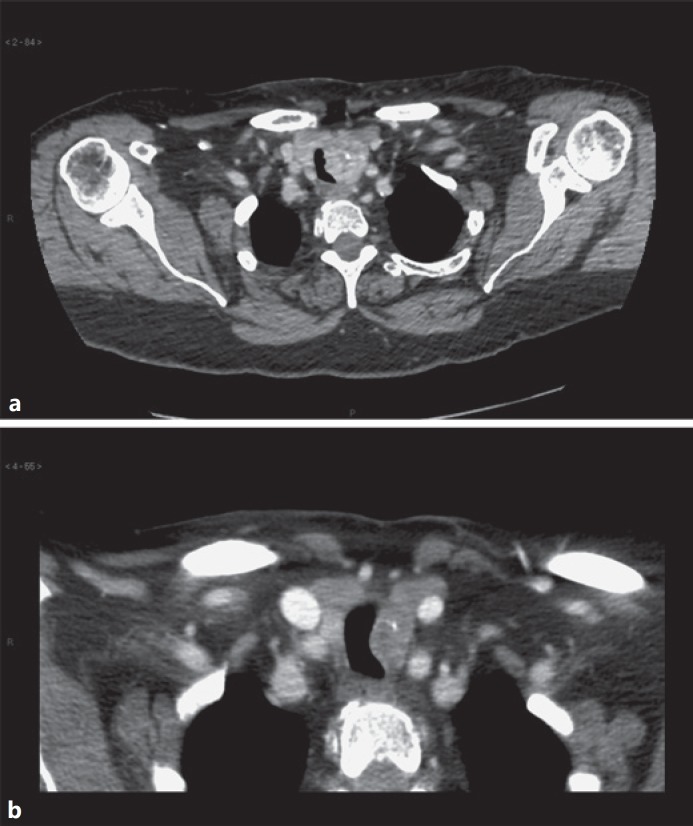
An ultrasound neck scan showed a 23 × 33 mm irregular, poorly defined hypoechoic mass which infiltrated the left lobe of the thyroid, displacing the trachea to the right, and reaching the thyroid capsule. No cervical or supraclavicular lymphadenopathy was noted, and the right thyroid lobe was unremarkable. In view of the degree of tracheal compression, and the patient's worsening stridor, a biopsy was not performed at the time of the ultrasound due to concern about potential haematoma and worsening airway obstruction. A contrast-enhanced CT of the neck confirmed the appearance of a left thyroid lobe mass with evidence of invasion into the trachea, and one suspicious lymph node within the central neck (Fig. 1a). There was no evidence of distant metastatic disease.
Fig. 1.
a CT scan of the neck on presentation showing a left thyroid mass with evidence of tracheal invasion. b CT scan after 14 months of TKI therapy prior to surgical intervention.
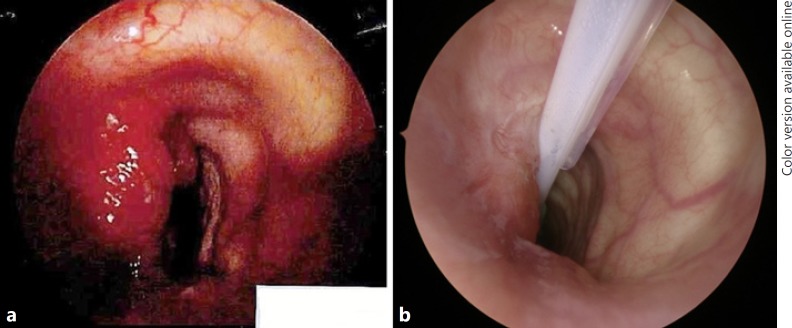
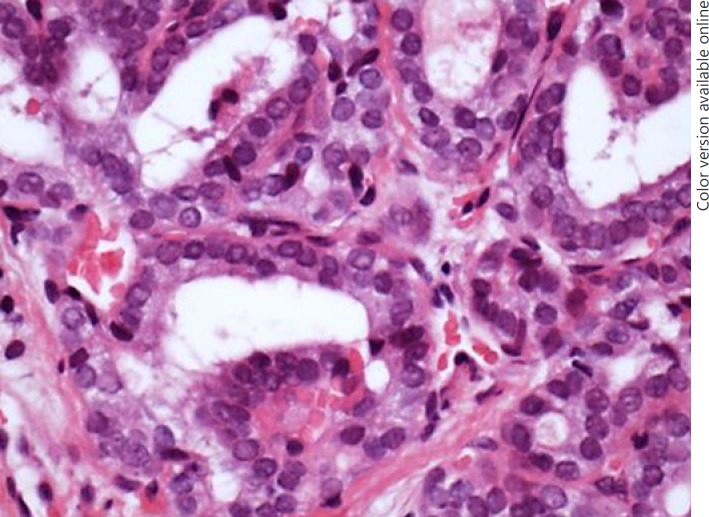
On endoscopic examination, a mass involved the intraluminal tracheal airway immediately below the level of the cricoid for approximately 2 cm (Fig. 2a). The mass was debulked using a laryngeal coblation device. Histological examination showed a follicular variant of PTC (Fig. 3). Her shortness of breath improved post-operatively and the haemoptysis resolved. Due to a combination of concern regarding the position and extent of tracheal resection required which would have made primary anastomosis difficult, comorbidities of the patient, and patient concern about the extent of surgery required, the decision was made not to proceed with a primary surgical resection. It was not possible to offer radioactive iodine treatment whilst the thyroid remained in situ.
Fig. 2.
a Tracheoscopy image before TKI therapy. b Tracheoscopy image at the time of thyroidectomy.
Fig. 3.
Biopsy (×40 magnification) highlighting nuclear features of PTC: ground glass nuclei, grooves, and intranuclear inclusions.
The oncology team discussed off-label TKI therapy with the patient. A plan of neoadjuvant TKI therapy with serial imaging and possible later surgical intervention was agreed.
Treatment with sorafenib 400 mg twice daily was started in August 2016. Due to gastro-intestinal side effects, the dose was halved after the first week. At reassessment 1 month after the start of therapy, a significant QTc prolongation was noted. This was likely to be multifactorial due to electrolyte disturbance from diarrhoea and diuretics as well as due to the sorafenib. Sorafenib was therefore discontinued. Levothyroxine was started for hypothyroidism.
Lenvatinib 24 mg daily was commenced in October 2016 and was well tolerated by the patient. An interval CT scan in December 2016 showed a reduction in the size of the left thyroid mass, with reduced distortion of the adjacent airway. The patient was admitted for a short stay in February 2017 with drug side effects of weight loss, stomatitis, and loose stool. Following a short break from lenvatinib, it was reintroduced at 14 mg daily.
In October 2017, after 14 months of TKI treatment, a further CT scan showed stable disease with a marked reduction in tumour volume compared with pre-treatment from 31 × 59 × 32 mm to 17 × 28 × 22 mm (Fig. 1b). Following discussion in the MDT, an exploration of the central neck was performed in January 2018 aiming to remove the thyroid and nodal disease, without airway resection, to facilitate adjuvant radiation.
Lenvatinib 14 mg daily was stopped 2 weeks prior to surgery. Endoscopic examination of the trachea found some indentation and mucosal hyperaemia but no evidence of frank intraluminal disease (Fig. 2b). A left thyroidectomy was performed. The disease was carefully dissected from the trachea without any mucosal breach, and no oesophageal muscle resection was required. The left recurrent laryngeal nerve was resected en bloc with the tumour. There was no residual macroscopic disease post-resection. A central neck dissection was performed. Completion right thyroid lobectomy with nerve monitoring was performed, with the right recurrent laryngeal nerve being identified and preserved throughout.
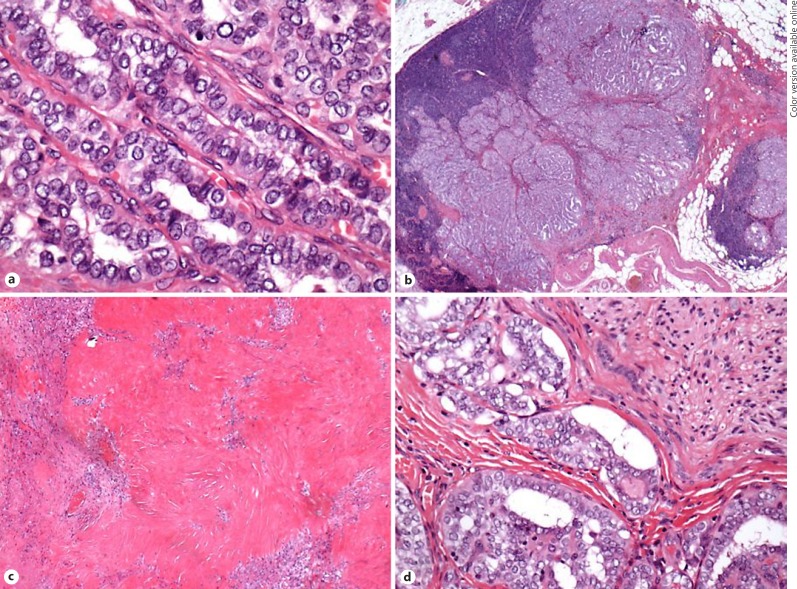
Final staging was pT4a N0 R1. The lymph node seen on CT scan prior to surgery was found to be a direct extension of the thyroid mass and did not appear to be involved due to metastatic spread (Fig. 4b). The tumour itself was at least 14 mm in its maximum dimension and appeared scarred. The cytological features were those of PTC including nuclear clearing, crowding, inclusions, and psammomatoid calcifications (Fig. 4a). Some of the tumour had follicular architecture but the majority had a conventional papillary appearance. The tumour exhibited extra-thyroidal spread into surrounding fat. There was vascular invasion and perineural infiltration of large nerves (Fig. 4d). The background thyroid and the right lobe had a generally atrophic fibrotic appearance, and no evidence of carcinoma was found in the right lobe. In total, 1 of 6 lymph nodes was positive (the positive one being directly involved). The areas of necrosis, scarring, and inflammatory change found on histological examination were thought to represent tumour regression in response to therapy (Fig. 4c). Whilst this is not commonly seen in thyroid cancers, the histological features were similar to tumour regression found following successful administration of chemoradiotherapy in squamous cell carcinoma of the head and neck.
Fig. 4.
Tumour resection specimen (a; ×40 magnification): features of a well-differentiated papillary carcinoma are evident. No features of a poorly differentiated tumour. b Direct spread of tumour into a lymph node (×2 magnification). c Hyalinisation and fibrosis around tumour islands suggesting tumour regression secondary to TKI therapy (×10 magnification). d Perineural invasion (×40 magnification).
The immediate post-operative recovery was uncomplicated, with no airway issues or hypocalcaemia. Post-operative thyroglobulin measurement showed evidence of thyroglobulin antibody interference. She received 3,700 MBq RAI in April 2018, and the post-treatment scan showed uptake in the bed of the thyroid only. The levothyroxine dose has been adjusted aiming for a target TSH of < 0.1 mlU/L. A left vocal cord medialisation procedure was performed in May 2018.
Discussion
Locally advanced thyroid cancer management is controversial and depends on the extent of invasion of critical structures in the neck, including the trachea, oesophagus, and major blood vessels. Overall disease burden and trajectory as well as patient-related factors also play a role in deciding optimum therapy.
To achieve surgical clearance in patients with locally advanced tumours, vital structures may need to be sacrificed, leading to both functional impairment and cosmetic deformity. Patients with tracheal invasion have a worse disease-specific survival rate and this patient group has high rates of distant metastases [12]. If surgical treatment is possible, tumour resection can be performed followed by RAI and external beam radiotherapy [6]. Historically, for patients deemed unsuitable for surgery limited options were available [13].
Only one similar case was found on review of the literature. A group from Japan [14] reported a patient with locally advanced DTC treated with lenvatinib for 22 weeks prior to surgical resection. The primary tumour invaded critical structures in the neck making primary surgery both higher risk and invasive, Therefore, lenvatinib was used to reduce tumour bulk pre-operatively. The patient underwent a total thyroidectomy, modified right neck dissection, resection of the muscular layer of the oesophagus, and a tracheal sleeve resection with reconstruction. The patient was followed up for 10 months post-operatively with no evidence of recurrence or metastases, and they planned post-operative RAI therapy. Both cases raise the possibility that TKIs may have a neoadjuvant role in selected cases of locally advanced DTC to reduce tumour volume and therefore morbidity of subsequent surgical resection.
There is no established form of neoadjuvant therapy described for DTC, although other forms of neoadjuvant treatments have been described in the literature. Pre-operative RAI was described in a case of a locally advanced PTC [15]. Following three treatments of iodine-131, the tumour size had reduced such that surgical resection was possible. A Slovenian review of neoadjuvant chemotherapy (using vinblastine and other regimens) in 16 patients with locally advanced PTC found that tumour size reduced in all patients allowing subsequent resection; however, an R0 resection was only achieved in 2 patients [16].
TKI therapy can cause side effects; nevertheless, in our patient reduced-dose TKI therapy was tolerated and resulted in significant reduction in tumour size, as well as pathological evidence of tumour response. Due to the known anti-angiogenic activity of TKIs it is recommended that they be stopped 7 days prior to a major surgery, and in our case, it was stopped 14 days prior to surgery with no post-operative complications.
Increasing experience with TKI use, including investigation of optimum dosing and administration length, and methods of minimising and managing side effects are likely to improve patient tolerance and outcomes. Further investigation of TKI use in locally advanced DTC is required to better understand the clinical and pathological course, as well as patient and tumour factors affecting response.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
Author Contributions
All authors included in this article made substantial contributions to the design, analysis, and interpretation of the data included, as well as assisted with critical revisions of the writing, and approved the final version for submission for publication. All authors agree to accountability for the accuracy and integrity of the work.
References
- 1.Hughes DT, Haymart MR, Miller BS, Gauger PG, Doherty GM. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid. 2011 Mar;21((3)):231–6. doi: 10.1089/thy.2010.0137. [DOI] [PubMed] [Google Scholar]
- 2.Morris LG, Myssiorek D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg. 2010 Oct;200((4)):454–61. doi: 10.1016/j.amjsurg.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cramer JD, Fu P, Harth KC, Margevicius S, Wilhelm SM. Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results national cancer data registry. Surgery. 2010 Dec;148((6)):1147–52. doi: 10.1016/j.surg.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Shin DH, Mark EJ, Suen HC, Grillo HC. Pathologic staging of papillary carcinoma of the thyroid with airway invasion based on the anatomic manner of extension to the trachea: a clinicopathologic study based on 22 patients who underwent thyroidectomy and airway resection. Hum Pathol. 1993 Aug;24((8)):866–70. doi: 10.1016/0046-8177(93)90136-5. [DOI] [PubMed] [Google Scholar]
- 5.Wang LY, Nixon IJ, Patel SG, Palmer FL, Tuttle RM, Shaha A, et al. Operative management of locally advanced, differentiated thyroid cancer. Surgery. 2016 Sep;160((3)):738–46. doi: 10.1016/j.surg.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shindo ML, Caruana SM, Kandil E, McCaffrey JC, Orloff LA, Porterfield JR, et al. Management of invasive well-differentiated thyroid cancer: an American Head and Neck Society consensus statement. AHNS consensus statement. Head Neck. 2014 Oct;36((10)):1379–90. doi: 10.1002/hed.23619. [DOI] [PubMed] [Google Scholar]
- 7.Brose MS. Sequencing of tyrosine kinase inhibitors in progressive differentiated thyroid cancer. Clin Adv Hematol Oncol. 2016 May;14((5 Suppl 9)):7–12. [PubMed] [Google Scholar]
- 8.National Institute for health and care excellence Clinical Guideline 2018 NICE Lenvatinib and sorafenib for treating differentiated thyroid cancer after radioactive iodine (ID 1059) Available at https://www.nice.org.uk/guidance/indevelopment/gid-ta10101 Accessed May 1, 2018. [Google Scholar]
- 9.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. DECISION investigators Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014 Jul;384((9940)):319–28. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shlumberger M, Tahara M, Wirth LJ, Robinson B, Brose M, Elisei R, et al. A phase 3, mulitcenter, double-blind, placebo-controlled trial of lenvatinib (E7080) in patients with 131-I-refractory differentiated thyroid cancer (SELECT) J Clin Oncol. 2014;32:18. [Google Scholar]
- 11.Cabanillas ME, Habra MA. Lenvatinib: role in thyroid cancer and other solid tumors. Cancer Treat Rev. 2016 Jan;42:47–55. doi: 10.1016/j.ctrv.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Yamada K, Tanaka S, Hiratsuka Y, Kumabe Y, Watanabe Y, Yoshida T, et al. [Prognosis of papillary thyroid carcinoma with local invasion] (Japanese) Nippon Jibiinkoka Gakkai Kaiho. 2015 Feb;118((2)):115–22. doi: 10.3950/jibiinkoka.118.115. [DOI] [PubMed] [Google Scholar]
- 13.Nixon IJ, Whitcher MM, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid. 2012 Sep;22((9)):884–9. doi: 10.1089/thy.2011.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuboi M, Takizawa H, Aoyama M, Tangoku A. Surgical treatment of locally advanced papillary thyroid carcinoma after response to lenvatinib: A case report. Int J Surg Case Rep. 2017;41:89–92. doi: 10.1016/j.ijscr.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shingu K, Kobayashi S, Yokoyama S, Shimizu T, Kasuga Y, Fujimori M, et al. Effectiveness of preoperative radioactive iodine (131I) therapy for locally advanced papillary thyroid cancer: a case report. Thyroid. 1998 Dec;8((12)):1113–6. doi: 10.1089/thy.1998.8.1113. [DOI] [PubMed] [Google Scholar]
- 16.Besic N, Auersperg M, Dremelj M, Vidergar-Kralj B, Gazic B. Neoadjuvant chemotherapy in 16 patients with locally advanced papillary thyroid carcinoma. Thyroid. 2013 Feb;23((2)):178–84. doi: 10.1089/thy.2012.0194. [DOI] [PubMed] [Google Scholar]