Abstract
Background/Aims
Overall, 2–13% of patients with thyroid cancer develop bone metastases (BM). In addition to decreased survival, patients with BM may present skeletal-related events (SRE) that impair the quality of life. Our objectives were to characterize clinical features, treatment approaches, and outcomes of patients with thyroid cancer and BM.
Material and Methods
We identified patients diagnosed with thyroid carcinoma of follicular origin and BM followed at the Instituto Português de Oncologia de Lisboa Francisco Gentil (IPOLFG), Lisbon, Portugal, from 1991 to 2017. SRE were defined as the need for bone irradiation, bone surgery, spinal cord compression, or pathologic fractures.
Results
The final cohort consisted of 86 patients, with a median follow-up time of 54 months (IQR 22.8–82.8), mainly women (67.4%), and a median age of 64 years (IQR 53.6–71.2). BM was the initial presentation of thyroid cancer in 36.0% of the patients. Bone involvement was multiple in 59.3% of the cases. Papillary carcinoma was the most frequent histological type, present in 47.7% of the patients, of which 56.1% presented the follicular variant. SRE were found in 76.7% of the patients. The most frequent SRE was radiotherapy (66.3%). Treatment with bisphosphonates was initiated in 19.8% of the patients. The 5-year specific survival was 60%, whereas the 10-year specific survival decreased to 50%. There were no differences in 5- or 10-year specific survival regarding gender, the occurrence of SRE, or histological type. However, patients with initial radioiodine non-avid lesions had a lower 5- and 10-year specific survival (p = 0.002).
Discussion
The high frequency of patients with SRE was notable. The follicular variant of papillary thyroid cancer was the variant most commonly associated with BM, reflecting a more similar behavior to follicular carcinoma than the classic variant.
Keywords: Bone, Metastases, Thyroid cancer
Introduction
The incidence of thyroid cancer has increased in the last few decades, with an average estimated incidence in 2012 of 20.0 cases per 100,000 in women and 6.3 cases per 100,000 in men [1]. Thyroid cancer is now the most common type of cancer diagnosed in South Korea [2] and is already the third most common cancer in women in Portugal [3]. The prognosis is good with a 5-year survival rate above 85% [1]. However, 1–4% of patients with differentiated thyroid cancer (DTC) present with distant disease at diagnosis, and 7–23% develop metastatic disease during follow-up [4]. These metastases occur mainly in the lungs and bone and decrease the patient's 10-year survival rate by 50% [5]. Overall, bone metastases (BM) occur in 2–13% of patients with DTC [6]. In addition, patients with BM may develop skeletal-related events (SRE) such as pathologic fractures, spinal cord compression, or a need for surgery or radiotherapy. This may impair the patient's quality of life and worsen the prognosis [7]. The bone is a tissue with a high tendency for metastases due to several factors, such as high blood flow in areas of red marrow [8]. In the literature, follicular thyroid carcinoma (FTC) is the histological type most frequently associated with BM [9]. Treatment options for BM include radioiodine therapy (RAIT), surgery, radiotherapy, and bisphosphonates. The American Thyroid Association guidelines state that bisphosphonates should be considered in patients with diffuse and/or symptomatic BM from RAI-refractory DTC, whereas patients with a small number of threatening and/or symptomatic BM are usually treated with local approaches such as radiation therapy and/or surgery [10].
We aimed to describe the clinical characteristics, treatment options, and outcomes of patients with thyroid cancer and BM, with a particular emphasis on SRE.
Material and Methods
We identified patients diagnosed with thyroid cancer and BM followed at the Instituto Português de Oncologia de Lisboa Francisco Gentil (IPOLFG), Lisbon, Portugal, from 1991 to 2017. Exclusion criteria included patients with medullary and anaplastic thyroid cancer, patients with other cancers known to commonly involve bone such as breast, prostate, and multiple myeloma, a follow-up time less than 12 months, and incomplete clinical information. BM were diagnosed by plain radiography, computed tomography (CT), magnetic resonance imaging, positron emission tomography, and RAI uptake, performed for staging purposes or directed by patients' complains. The original histopathology was reviewed at the IPOLFG when the surgery was performed at another hospital. SRE were defined as a need for bone irradiation or bone surgery, spinal cord compression, or pathologic fractures. Cause-specific survival was defined in patients who had a known cause of death from thyroid cancer. This was evaluated from primary treatment to death or the last follow-up date. The clinical data were extracted from medical records. The study was approved by IPOLFG's ethics committee.
Statistical Methods
Normal distribution of the continuous variables was evaluated through histograms. In the case of normal continuous variables, they were expressed as mean and standard deviation. In the cases of non-normal continuous variables, they were expressed as median and interquartile range (IQR). Categorical variables were expressed as absolute frequencies and respective percentages. Comparisons between two groups were performed with the t test for normal continuous variables and with the Mann-Whitney U test for non-normal continuous variables. The χ2 test was used to study categorical variables. The Kaplan-Meier method was used to estimate the survival curves, and the log-rank test was used to assess the differences amongst different groups. Statistical significance was defined at p ≤ 0.05. Statistical analysis was performed using IBM SPSSTM software version 22.
Results
Clinical Features
The final cohort consisted of 86 patients with BM. They were mainly women, 67.4% (n = 58), with a median age at diagnosis of thyroid cancer of 64 years (IQR 53.6–71.2) and a median follow-up time of 54 months (IQR 22.8–82.8). Thyroid surgery was performed in 75 (87.2%) patients, while 11 (12.8%) patients had no thyroid surgery due to high surgical risk, extensive metastatic disease at presentation, or patient's refusal. In total, 78 (90.7%) patients received RAIT. The first RAIT revealed metastatic avidity in 52 (66.6%) patients. The median number of therapies performed was 3 (IQR 2–4), with a total median cumulative activity administered of 440 mCi (IQR 282–886). The reasons to stop RAIT were as follows: death (death = 21), non-avidity (n = 27), and disease progression (n = 30). We were able to evaluate RAIT response in 8 patients: 1 had a partial response, 3 had stable disease, and 4 had progressive disease. In the remaining 44 patients with metastatic lesions avid for RAI, we were unable to evaluate RAIT response because of overlapping treatments and/or lack of appropriate imaging exams to assess that response. Multiple BM were present in 59.3% (n = 51) cases (Table 1). BM were located, in descending order of frequency, in the spine (n = 53; 61.6%), sternum and ribs (n = 32; 37.2%), pelvis (n = 25; 29.1%), skull (n = 16; 18.6%), and limbs (n = 14; 16.3%). The presence of other distant metastases was observed in 44 patients (51.2%), mainly in the lungs (n = 33; 38.4%), but also in the brain (n = 2; 2.3%), both lungs and brain (n = 6; 7%), and in other locations (n = 3; 3.5%). The median time between surgery for thyroid cancer and BM diagnosis was 4.7 months (IQR 1.5–33.1), excluding the 31 (36%) patients in which BM was the initial manifestation of the primary tumor. When BM were not the initial manifestation of the thyroid cancer, they were detected by the post-RAIT scan (n = 17; 30.9%), by other imaging exams performed for staging purposes (n = 14; 25.5%), because of bone pain (n = 13; 23.6%), persistently elevated serum thyroglobulin (Tg) levels (n = 6; 10.9%), bone fractures (n = 1; 1.8%), or unspecified reasons (n = 4; 7.3%). The median serum Tg at diagnosis of BM was 2,774 ng/mL (IQR 364–17,255). Following primary treatment (surgery plus RAIT), Tg levels under levothyroxine suppression were elevated in 73 of 75 patients, and the remaining 2 patients had a serum Tg < 0.2 ng/mL. One was a patient with a poorly differentiated thyroid carcinoma (PDTC), negative anti-Tg antibodies, and BM detected by the whole-body scan at the time of RAIT. The other patient with a papillary thyroid carcinoma (PTC) had strongly positive anti-Tg antibodies during follow-up, and BM were detected preoperatively by CT performed for staging purposes.
Table 1.
General characteristics of patients with thyroid cancer and BM
| Female | 58 (67.4) |
| Median age, years (IQR) | 64 (53.6–71.2) |
| BM at initial presentation | 31 (36.0) |
| Multiple bone involvement | 51 (59.3) |
| Other distant metastases | 44 (51.2) |
| SRE | 66 (76.7) |
| Radiotherapy | 57 (66.3) |
| Bone surgery | 23 (26.7) |
| Spinal compression | 17 (25.7) |
| Pathologic fracture | 16 (24.2) |
| Papillary histotype | 41 (47.7) |
| Follicular variant | 23 (56.1) |
| Classic variant | 7 (17.1) |
| Solid variant | 3 (7.3) |
| Tall-cell variant | 1 (2.4) |
| Follicular and classic variant | 1 (2.4) |
| Missing | 6 (14.6) |
| Follicular histotype | 34 (39.5) |
| Poorly differentiated histotype | 11 (12.8) |
Results are presented as n (%), unless stated otherwise. BM, bone metastases; SRE, skeletal-related events.
Pathological Features
The primary tumor was PTC in 47.7% (n = 41) cases, FTC in 39.5% (n = 34), and PDTC in 12.8% (n = 11). Amongst the variants of PTC, 56.1% (n = 23) were follicular (FVPTC), 17.1% (n = 7) classic (CVPTC), 7.3% (n = 3) solid, 2.4% (n = 1) both FVPTC and CVPTC, and 2.4% (n = 1) tall cell. We were unable to obtain information about 6 patients (14.6%): 3 patients did not undergo thyroid surgery and in 3 patients the pathological report was incomplete. The histopathological features showed that 46.7% (n = 35) had extrathyroidal extension and 76.0% (n = 57) had vascular invasion. The median size of the tumor was 40.0 mm (IQR 20–55) (Table 1).
Skeletal-Related Events
In our study, 76.7% (n = 66) patients developed at least one SRE. Of these, 53% (n = 35) developed more than one SRE. The most common SRE was radiotherapy in 66.3% (n = 57) cases, followed by surgery 26.7% (n = 23), spinal compression 25.7% (n = 17), and bone fracture 24.2% (n = 16). The reasons to perform bone surgery were orthopedic (n = 5) or neurological complications (n = 7), as an initial diagnostic approach (n = 10) or concomitantly to thyroidectomy in 1 patient with sternum metastases. Thirteen of these patients had multiple BM and surgery was performed because of vertebral metastases in 10 patients, femur metastases in 2 patients, and hip metastases in 1 patient. Treatment with bisphosphonates was initiated in 17 (19.8%) patients, and the decision to start such treatment was individualized, usually related to extensive disease and a critical location of BM. The bisphosphonate most frequently used was pamidronic acid (n = 9) followed by zoledronic acid (n = 5) and clodronic acid (n = 3). The presence of SRE was not significantly different according to gender (p = 0.145), age (p = 0.189), histological type (p = 0.221), variant of PTC (p = 0.167), extrathyroidal extension (p = 0.385), BM location (p = 0.313), presence of metastases in other tissues (p = 0.531), or therapy with bisphosphonates (p = 0.454). There were more SRE when the BM was the initial presentation of the thyroid cancer (p = 0.001).
Prognosis
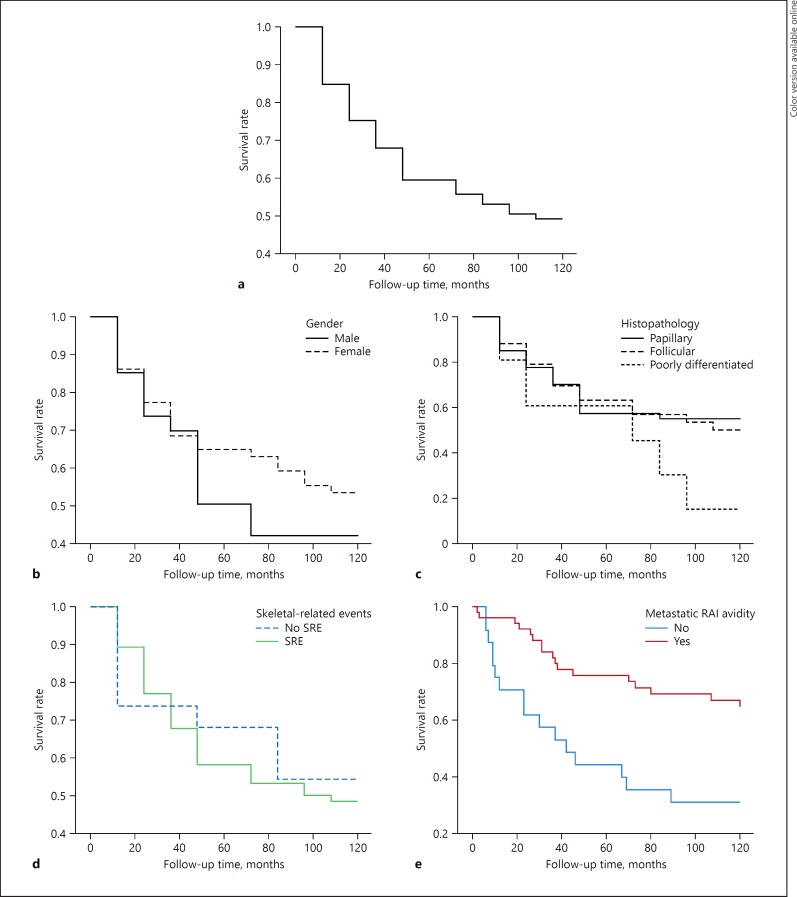
At the end of the study, 32.6% (n = 28) patients were alive, 9.3% (n = 8) had been lost to follow-up, and 58.1% (n = 50) had died. Of these, 8 died of causes unrelated to the disease. Overall, the 5- and 10-year cause-specific survival was 60 and 50% (Fig. 1). Cause-specific survival by age, histological type, presence of SRE, and initial metastatic RAI avidity is shown in Figure 1. The 5-year survival was of 50% for males and 65% for females (p = 0.433), and the 10-year survival dropped to 42% for males and 53% for females (p = 0.429). In relation to histological type, the 5- and 10-year survival rates were, respectively, 58 and 55% for PTC, 63 and 50% for FTC, and 61 and 15% for PDTC (p = 0.415 and p = 0.336, respectively). The 5- and 10-year survival rates were 58 and 49% when at least 1 SRE was present, and 68 and 54% in the absence of bone events (p = 0.664 and p = 0.719, respectively). According to the initial metastatic RAI avidity, the 5- and 10-year survival was 76 and 67% if the lesions were RAI avid and 44 and 31% if the lesions were non-avid (p = 0.002 and p = 0.002, respectively).
Fig. 1.
a Thyroid cancer-specific survival rate for patients with BM. b Thyroid cancer-specific survival rates for patients with BM regarding gender. c Thyroid cancer-specific survival rates for patients with BM regarding histopathology. d Thyroid cancer-specific survival rates for patients with BM regarding presence of SRE. e Thyroid cancer-specific survival rates for patients with BM regarding metastatic RAI avidity.
Discussion
In common with other series, the study population was mainly composed of females and had a median age of > 50 years [11, 13]. Consistent with a recently published systematic review, the patients presented predominantly multiple bone involvement with spine preference [14]. In our cohort, 36% of the patients had BM as the presentation feature of thyroid cancer. In the literature, this percentage ranges from 38.1% [15] up to 62% [16]. The high incidence of BM at presentation stresses the importance to consider thyroid cancer as a possible cause of skeletal metastatic disease when the primary origin is unknown. In our series, PTC was the histological type more commonly found in patients with thyroid carcinoma and BM. This is probably related to the higher prevalence of PTC as primary thyroid cancer [3]. However, the majority of patients with BM in a recent systematic review [12] had a FTC, but most studies did not report the prevalence of BM according to thyroid cancer histotype. PTC is not a homogeneous entity and encompasses several histological variants. The predominant forms are CVPTC which often invades the lymphatic vessels and metastasizes to the lymphatic system, and FVPTC, which resembles FTC pathological behavior, by spreading via blood vessels to distant organs, such as the lungs and bones. FVPTC is also more likely to harbor RAS mutations and PAX8/PPARγ rearrangements, molecular alterations usually found in FTC, but not in CVPTC, in which BRAF mutations and RET/PTC translocations typically predominate [17]. Therefore, it is not surprising that the most frequent PTC variant associated with BM in our series was FVPTC.
The number of patients that developed SRE was high (76.7%), which is similar to the findings of Farooki et al. [7], 78%, but much higher than the results of Choksi et al. [11] that only report SRE in 32% of their cases. The authors of this last study argue that their different results, as compared to Farooki et al., may be due to a higher percentage of patients with FTC in this cohort which was 34 versus 8% in their series. However, in our study, although 39.5% of the patients had a FTC, we did not find different rates in the occurrence of SRE according to histological type. The high percentage of patients with SRE in our series may be the result of a larger number of patients submitted to radiotherapy than in other studies [11, 13]. In addition, in our cohort, the presence of a SRE was not associated with a significant increase in specific mortality at 5- or 10-years, in comparison with patients without SRE which is not consistent with previous reports [11]. Regarding treatment options, we could not confirm, like others [18], the beneficial effects of bisphosphonates described by Vitale et al. [19], probably because the number of patients treated with these drugs in our series was very small. As described in other studies, the prognosis was significantly worse in patients with metastatic lesions non-avid for RAI [20].
It is also important to mention that all patients with BM had evidence of disease (positive imaging and/or detectable Tg levels and/or detectable anti-Tg antibodies) after primary treatment. Therefore, relapse of thyroid cancer with BM in patients with no evidence of disease after surgery and RAIT is quite unusual. Overall, long-term survival is possible in patients presenting with BM, yet it remains a poor prognostic indicator that needs to be diagnosed, evaluated, and treated properly.
The major strength of the study is that the patients were followed at a single center over a long (26 years) period of time. There are some limitations inherent to a retrospective study such as incomplete data for some patients and different imaging modalities used for detection of BM.
Statement of Ethics
The study was approved by the ethics committee of the IPOLFG. Due to the retrospective nature of the study, patient consent was waived.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Jegerlehner S, Bulliard JL, Aujesky D, Rodondi N, Germann S, Konzelmann I, et al. NICER Working Group Overdiagnosis and overtreatment of thyroid cancer: A population-based temporal trend study. PLoS One. 2017 Jun;12((6)):e0179387. doi: 10.1371/journal.pone.0179387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sik Ahn H, Jung Kim H, Gilbert Welch H. Korea's Thyroid-Cancer “Epidemic”— screening and Overdiagnosis. N Engl J Med. 2014;371((19)):1763–5. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 3.RORENO Registo Oncológico Nacional 2010. Instituto Português de Oncologia do Porto Francisco Gentil - EPE, ed, Porto. 2016 Available at www.roreno.com.pt. [Google Scholar]
- 4.Wang LY, Palmer FL, Nixon IJ, Thomas D, Patel SG, Shaha AR, et al. Multi-organ distant metastases confer worse disease-specific survival in differentiated thyroid cancer. Thyroid. 2014 Nov;24((11)):1594–9. doi: 10.1089/thy.2014.0173. [DOI] [PubMed] [Google Scholar]
- 5.Muresan MM, Olivier P, Leclère J, Sirveaux F, Brunaud L, Klein M, et al. Bone metastases from differentiated thyroid carcinoma. Endocr Relat Cancer. 2008 Mar;15((1)):37–49. doi: 10.1677/ERC-07-0229. [DOI] [PubMed] [Google Scholar]
- 6.Wexler JA. Approach to the thyroid cancer patient with bone metastases. J Clin Endocrinol Metab. 2011 Aug;96((8)):2296–307. doi: 10.1210/jc.2010-1996. [DOI] [PubMed] [Google Scholar]
- 7.Farooki A, Leung V, Tala H, Tuttle RM. Skeletal-related events due to bone metastases from differentiated thyroid cancer. J Clin Endocrinol Metab. 2012 Jul;97((7)):2433–9. doi: 10.1210/jc.2012-1169. [DOI] [PubMed] [Google Scholar]
- 8.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004 Apr;350((16)):1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 9.Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998 Jan;338((5)):297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 10.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. 2016;26((1)):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choksi P, Papaleontiou M, Guo C, Worden F, Banerjee M, Haymart M. Skeletal complications and mortality in thyroid cancer: a population based study. J Clin Endocrinol Metab. 2017 Apr;102((4)):1254–60. doi: 10.1210/jc.2016-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu K, Hou SM, Huang TS, Yang RS. Thyroid carcinoma with bone metastases: a prognostic factor study. Clin Med Oncol. 2008;2((2)):129–34. doi: 10.4137/cmo.s333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin JD, Lin SF, Chen ST, Hsueh C, Li CL, Chao TC. Long-term follow-up of papillary and follicular thyroid carcinomas with bone metastasis. PLoS One. 2017 Mar;12((3)):e0173354. doi: 10.1371/journal.pone.0173354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osorio M, Moubayed SP, Su H, Urken ML. Systematic review of site distribution of bone metastases in differentiated thyroid cancer. Head Neck. 2017 Apr;39((4)):812–8. doi: 10.1002/hed.24655. [DOI] [PubMed] [Google Scholar]
- 15.Kallel F, Hamza F, Charfeddine S, Amouri W, Jardak I, Ghorbel A, et al. Clinical features of bone metastasis for differentiated thyroid carcinoma: A study of 21 patients from a Tunisian center. Indian J Endocrinol Metab. 2014 Mar;18((2)):185–90. doi: 10.4103/2230-8210.129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernier M, Leenhardt L, Hoang C, Aurengo A, Mary J, Menegaux F, et al. Survival and Therapeutic Modalities in Patients with Bone Metastases of Differentiated Thyroid Carcinomas. 2001;86((4)):1568–73. doi: 10.1210/jcem.86.4.7390. [DOI] [PubMed] [Google Scholar]
- 17.Castro P, Rebocho AP, Soares RJ, Magalhães J, Roque L, Trovisco V, et al. PAX8-PPARgamma rearrangement is frequently detected in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2006 Jan;91((1)):213–20. doi: 10.1210/jc.2005-1336. [DOI] [PubMed] [Google Scholar]
- 18.Orita Y, Sugitani I, Toda K, Manabe J, Fujimoto Y. Zoledronic acid in the treatment of bone metastases from differentiated thyroid carcinoma. Thyroid. 2011 Jan;21((1)):31–5. doi: 10.1089/thy.2010.0169. [DOI] [PubMed] [Google Scholar]
- 19.Vitale G, Fonderico F, Martignetti A, Caraglia M, Ciccarelli A, Nuzzo V, et al. Pamidronate improves the quality of life and induces clinical remission of bone metastases in patients with thyroid cancer. Br J Cancer. 2001 Jun;84((12)):1586–90. doi: 10.1054/bjoc.2001.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch D, Levy S, Tsvetov G, Gorshtein A, Slutzky-Shraga I, Akirov A, et al. Long-Term Outcomes and Prognostic Factors in Patients With Differentiated Thyroid Cancer and Distant Metastases. Endocr Pract. 2017 Oct;23((10)):1193–200. doi: 10.4158/EP171924.OR. [DOI] [PubMed] [Google Scholar]