Abstract
Infections with Vibrio parahaemolyticus, a gram-negative bacterium found in salt water, are mainly gastrointestinal or cutaneous. The development of sepsis is not uncommon. We report the case of an 85-year-old patient who developed lower limb cellulitis caused by V. parahaemolyticus, originating from leg ulcers and complicated by septicaemia and septic shock, after a sea beach holiday. We discuss the epidemiology, pathogenesis, clinical manifestations and treatment of V. parahaemolyticus infections.
Keywords: Vibrio parahaemolyticus, Cellulitis, Septicaemia, Septic shock, Leg ulcer
Introduction
Infections with Vibrio parahaemolyticus are mainly gastrointestinal [1] or cutaneous [2]. V. parahaemolyticus is a gram-negative, facultative halophile and non-spore-forming bacterium which is found in estuarine, marine and coastal surroundings [3]. Whereas the incidence of gastrointestinal vibriosis has decreased in the last years, the incidence associated with wound infection has increased (42% of all infections with bacteria of the genus Vibrio) [4]. The development of sepsis is not uncommon, especially in patients with an underlying medical illness such as liver disease, diabetes mellitus or alcohol abuse [5].
We present the case of a patient who developed leg cellulitis, septicaemia and a septic shock by V. parahaemolyticus originating from skin ulcers on the left lower limb.
Case Report
An 85-year-old patient with severe valvular and ischaemic-hypertensive cardiopathy, slight chronic renal failure and chronic venous leg ulcers came to our attention for specialist assessment. Two weeks before, he had plunged his legs once in seawater during a summer holiday at the Mediterranean Sea in Italy.
At the first clinical evaluation, the patient did not have any fever or other symptoms except for pain on the distal left lower limb. On clinical examination, we observed an ulcer extending to his left lateral ankle (70 × 50 mm) with a fibrinous, greenish and smelly coating (Fig. 1a). He had other, similar ulcers on his right leg on the pretibial and perimalleolar side. The patient presented strong dermatological signs of chronic venous insufficiency with varicose veins, stasis dermatitis and lipodermatosclerosis. Blood tests showed an elevated CRP at 72 mg/L without leucocytosis. The ankle-brachial pressure index was 0.9. An X-ray confirmed no involvement of the joints and bones on the distal left lower limb.
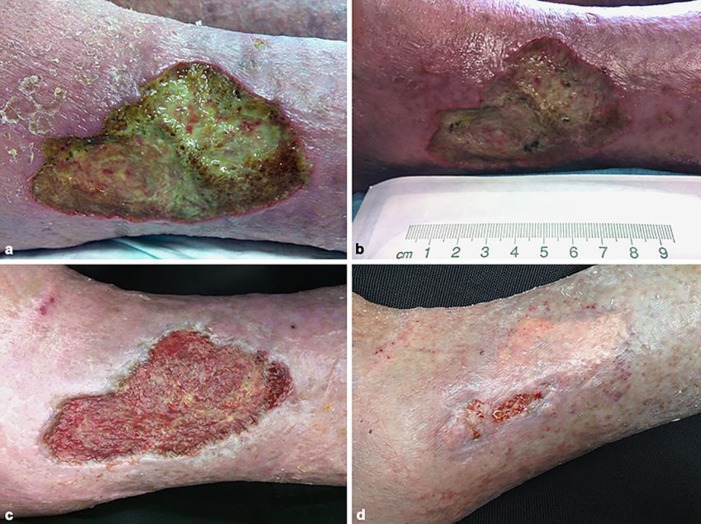
Fig. 1.
aVibrio parahaemolyticus-infected left leg ulcer at the time of admission to our department. bV. parahaemolyticus-infected left leg ulcer at the time of cellulitis and septic shock. c Left leg ulcer after negative-pressure wound therapy. d Left leg ulcer after split-thickness skin grafting.
As we suspected an infection of the wound, we admitted the patient administering intravenous antibiotic therapy with amoxicillin and clavulanic acid 1.2 g intravenously every 8 h. After a few hours, he had developed a fever, associated with a further increase in inflammatory parameters. Within 12 h, he had developed cellulitis in the left leg (Fig. 1b), then hypotension, acute renal failure and a septic shock, for which the antibiotic was changed to parenteral imipenem 500 mg twice daily, and he was treated with intravenous catecholamines at the intensive care unit for 3 days. Blood cultures showed growth of V. parahaemolyticus in 4/4 bottles (Fig. 2). We diagnosed a septic shock induced by V. parahaemolyticus.
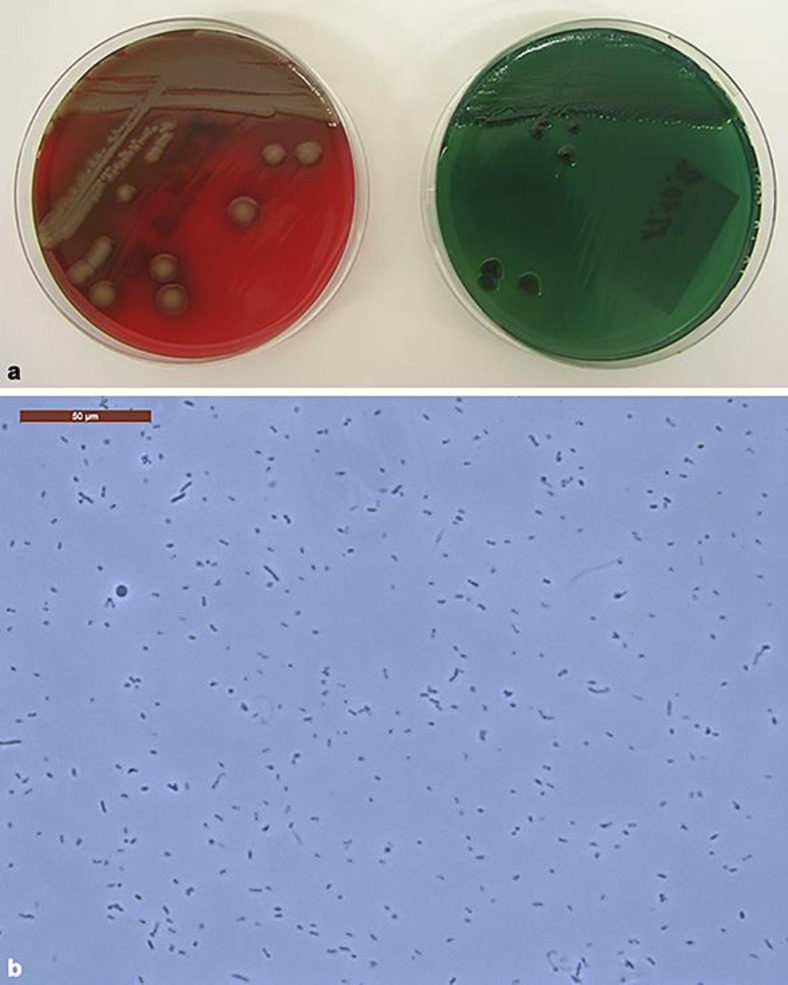
Fig. 2.
a Left: Vibrio parahaemolyticus growth on TCBS (Thiosulfate-Citrate-Bile-Sucrose) Agar; right: V. parahaemolyticus growth on sheep blood agar showing haemolysis. b Native preparation, under the microscope showing the presence of V. parahaemolyticus. Note that this Vibrio strain is not the same as in our patient, but provided by the Institute of Medical Microbiology in Zurich.
The bacteriological smear of the wounds showed growth of V. parahaemolyticus, Pseudomonas aeruginosa, Morganella morganii, Streptococcus mitis and Enterococcus spp. We stopped treatment with imipenem and started a targeted combined antibiotic therapy with oral ciprofloxacin 250 mg twice daily and doxycycline 100 mg daily for 21 days, resulting in normalization of haemodynamics and return to the patient's usual renal parameters.
Further, on laboratory investigations, we found a chronic hepatitis B infection. We could not detect any virulence in the blood. Hepatic function was globally intact, although sonography of the abdomen showed a “cardiac liver” with portal hypertension, which is known in ischaemic, hypertensive and valvular heart disease with right heart failure.
Local therapy of the chronic wounds consisted of daily curettage, application of topic sulphonamide and pressure bandage of the lower limbs. Further, we also tried application of negative pressure wound treatment, which we had to stop because it was not well tolerated by the patient.
After discharge from the hospital, we treated the patient regularly (2–3 times a week) in our outpatient clinic. Four months later, we obtained a satisfactory granulation of the wound ground (Fig. 1c). We proceeded with application of a split-thickness skin graft to the left leg, which resulted in complete wound healing (Fig. 1d).
Discussion
Vibrio spp. include various bacteria. They are usually found in salt water, interacting with zoo- and phytoplankton or marine plants (algae, coral and sponges) and animals (crustaceans, squid and fish) [6]. Despite the fact that the majority of interactions with these species are benign or beneficial, pathogenic Vibrio spp. may cause severe human infectious diseases. They are classified into two groups: cholera and non-cholera infections. Non-cholera Vibrio spp. (V. alginolyticus, V. cincinnatiensis, V. damsela, V. fluvialis, V. furnissii, V. hollisae, V. carchariae, V. mimicus, non-O1 V. cholerae, V. parahaemolyticus and V. vulnificus) are associated with the following clinical presentations: gastroenteritis, skin infection and septicaemia [2, 6, 7]. V. fluvialis, V. furnissii, V. hollisae, V. mimicus and non-O1 V. cholerae cause gastroenteritis almost exclusively, while V. alginolyticus, V. damsela and V. vulnificus especially cause skin infections (42% of all diseases of Vibrio spp. infection). Septicaemia is more frequent among patients affected by non-O1 V. cholerae and V. vulnificus, while it is rare in patients sick from V. alginolyticus, V. fluvialis, V. hollisae, V. mimicus and V. parahaemolyticus infection. It manifests only exceptionally among patients suffering from infection by the other species [2, 6, 7, 8].
First reports on V. parahaemolyticus were published in 1950 by Tsunesaburo Fujino, finding it to be a causative agent of food-borne disease following a large outbreak in Japan which recorded 272 illnesses with 20 deaths after consumption of shirasu, a raw fish [9]. In the past, V. parahaemolyticus infections manifested especially as gastroenteritis. In recent years, an increase in skin infections has been observed. Nowadays, a third of announced Vibrio infections are due to this germ [10, 11].
High levels of V. parahaemolyticus are found in the summer months, when the water in estuary or coastal regions gets warmer. It seems that the increasing seasonal temperatures and decreasing salinity levels favour a greater concentration of Vibrio bacilli. The results of a recent comparative systematic meta-analysis of peer-reviewed articles published between 2003 and 2015 showed that V. parahaemolyticus was more prevalent in oysters (63.4%) than in other seafood. However, the overall prevalence rates were also high in clams (52.9%), fish (51.0%), shrimp (48.3%) and mussels, scallops and periwinkles (28.0%) [11].
The incubation period of V. parahaemolyticus is 2–48 h; gastrointestinal symptoms include nausea, vomiting, watery diarrhoea, abdominal pain and sometimes fever. The duration of illness is 2–8 days. As the vast majority of cases of V. parahaemolyticus food infection are self-limiting, specific treatment is not necessary [7].
Skin infections with V. parahaemolyticus, predominantly cellulitis, occur through exposure of acute or chronic wounds to saline water. Initially, patients almost always report severe pain in the involved body part. Other skin manifestations are erythematous indurated plaques with haemorrhagic bullae, pustules, petechiae, necrosis and ulcers [2, 8]. To the best of our knowledge, necrotizing fasciitis induced by V. parahaemolyticus has been reported rarely in the literature compared to that induced by V. vulnificus [12, 13]. Predisposed to V. parahaemolyticus skin infections are individuals with occupational and recreational exposure to fish and shellfish, chronic liver disease or a liver transplant, diabetes mellitus, chronic kidney disease, alcohol abuse, splenectomy, immune suppression therapy, cancer chemotherapy and AIDS. These conditions may strongly favour the development of V. parahaemolyticus septicaemia in 5% of cases, which is much less frequent than sepsis caused by V. vulnificus (> 30% of cases). Symptoms (high fever, chills, myalgia and pain in the lower extremities) occur with an almost abrupt onset within 7–14 days after contact. The rate of mortality from V. parahaemolyticus infection is low (3% of cases) [2, 8].
In our patient, we found a predisposing factor (chronic hepatitis B virus infection) for the development of septicaemia due to V. parahaemolyticus skin infection. The patient plunged his legs with chronic wounds into warm water close to the sea shore, and after 14 days he had developed septicaemia and septic shock.
The pathogenicity of V. parahaemolyticus depends on the production of thermostable direct haemolysin (V. parahaemolyticus-TDH), which is responsible for β-haemolysis. Most strains of V. parahaemolyticus isolated from the environment or seafood, in contrast to clinical strains, do not produce TDH [14].
V. parahaemolyticus-TDH-related haemolysin is a second group of haemolysins that can be found in certain clinical isolates of V. parahaemolyticus. β-Haemolytic strains are considered to be pathogens [15].
The treatment for patients with wounds infected by this gram-negative bacterium consists of systemic antibiotic therapy with quinolones and tetracyclines, in addition to usual local care such as debridement and application of antiseptic products. Duration of therapy is dictated by clinical response, where patients with mild wound infections who do not have any significant underlying diseases generally respond well to local care and oral antibiotics. Precautionary measures in these cases involve avoidance of fresh fish and shellfish and no exposure of the wounds, particularly the chronic lesions, to saline water [2, 8].
In conclusion, for a differential diagnosis of cellulitis aetiology, even in a landlocked country, we have to consider V. parahaemolyticus among the causative agents, particularly with patients with a history of recent holidays at the seaside in warm regions of the world [8]. We suggest that subjects with chronic wounds, especially on the lower limbs, should not expose themselves to sea water to avoid the risk of infection with V. parahaemolyticus or V. vulnificus, which could have serious health implications.
Statement of Ethics
Informed consent was obtained from the patient.
Disclosure Statement
C.G., F.G. and C.M. declare that they have no conflicts of interest. This study did not receive funding from third parties.
Acknowledgements
The authors would like to thank the Institute of Microbiology (EOC) in Bellinzona for diagnosing the germ, as well as Dr. R. Zbinden, Institute of Medical Microbiology, University of Zurich, Switzerland, for his support in providing bacterial pictures.
References
- 1.Newton AE, Garrett N, Stroika SG, Halpin JL, Turnsek M, Mody RK. Increase in Vibrio parahaemolyticus infections associated with consumption of Atlantic Coast shellfish. Centers for Disease Control and Prevention (CDC)—2013. MMWR Morb Mortal Wkly Rep. 2014;63:335. [PMC free article] [PubMed] [Google Scholar]
- 2.Dechet AM, Yu PA, Koram N, Painter J. Nonfoodborne Vibrio infections: an important cause of morbidity and mortality in the United States, 1997-2006. Clin Infect Dis. 2008 Apr;46((7)):970–6. doi: 10.1086/529148. [DOI] [PubMed] [Google Scholar]
- 3.Ceccarelli D, Hasan NA, Huq A, Colwell RR. Distribution and dynamics of epidemic and pandemic Vibrio parahaemolyticus virulence factors. Front Cell Infect Microbiol. 2013 Dec;3:97. doi: 10.3389/fcimb.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weis KE, Hammond RM, Hutchinson R, Blackmore CG. Vibrio illness in Florida, 1998-2007. Epidemiol Infect. 2011 Apr;139((4)):591–8. doi: 10.1017/S0950268810001354. [DOI] [PubMed] [Google Scholar]
- 5.Daniels NA, MacKinnon L, Bishop R, Altekruse S, Ray B, Hammond RM, et al. Vibrio parahaemolyticus infections in the United States, 1973-1998. J Infect Dis. 2000 May;181((5)):1661–6. doi: 10.1086/315459. [DOI] [PubMed] [Google Scholar]
- 6.Erken M, Lutz C, McDougald D. Interactions of Vibrio spp. with Zooplankton. Microbiol Spectr. 2015 Jun;3((3)) doi: 10.1128/microbiolspec.VE-0003-2014. [DOI] [PubMed] [Google Scholar]
- 7.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011 Jan;17((1)):7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz JH. Skin and soft tissue infections following marine injuries and exposures in travelers. J Travel Med. 2014 May-Jun;21((3)):207–13. doi: 10.1111/jtm.12115. [DOI] [PubMed] [Google Scholar]
- 9.Fujino T, Okuno Y, Nakada D, Aoyama A, Mukai T, Ueho T. On the bacteriological examination of shirasu food poisoning. Med J Osaka Univ. 1953;4:299–304. [Google Scholar]
- 10.Shinoda S. Sixty years from the discovery of Vibrio parahaemolyticus and some recollections. Biocontrol Sci. 2011 Dec;16((4)):129–37. doi: 10.4265/bio.16.129. [DOI] [PubMed] [Google Scholar]
- 11.Odeyemi OA. Incidence and prevalence of Vibrio parahaemolyticus in seafood: a systematic review and meta-analysis. Springerplus. 2016 Apr;5((1)):464. doi: 10.1186/s40064-016-2115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Payinda G. Necrotizing fasciitis due to Vibrio parahaemolyticus. N Z Med J. 2008 Oct;121((1283)):99–101. [PubMed] [Google Scholar]
- 13.Ahmad A, Brumble L, Maniaci M. Vibrio parahaemolyticus Induced Necrotizing Fasciitis: An Atypical Organism Causing an Unusual Presentation. Case Rep Infect Dis. 2013;2013:216854. doi: 10.1155/2013/216854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Letchumanan V, Chan KG, Lee LH. Vibrio parahaemolyticus: a review on the pathogenesis, prevalence, and advance molecular identification techniques. Front Microbiol. 2014 Dec;5:705. doi: 10.3389/fmicb.2014.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson DE, Weinberg L, Ciarkowski J, West P, Colwell RR. Wound infection caused by Kanagawa-negative Vibrio parahaemolyticus. J Clin Microbiol. 1984 Oct;20((4)):811–2. doi: 10.1128/jcm.20.4.811-812.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]