Abstract
Objective
To determine the risk of prolonged opioid use in patients receiving tramadol compared with other short acting opioids.
Design
Observational study of administrative claims data.
Setting
United States commercial and Medicare Advantage insurance claims (OptumLabs Data Warehouse) January 1, 2009 through June 30, 2018.
Participants
Opioid-naive patients undergoing elective surgery.
Main outcome measure
Risk of persistent opioid use after discharge for patients treated with tramadol alone compared with other short acting opioids, using three commonly used definitions of prolonged opioid use from the literature: additional opioid use (defined as at least one opioid fill 90-180 days after surgery); persistent opioid use (any span of opioid use starting in the 180 days after surgery and lasting ≥90 days); and CONSORT definition (an opioid use episode starting in the 180 days after surgery that spans ≥90 days and includes either ≥10 opioid fills or ≥120 days’ supply of opioids).
Results
Of 444 764 patients who met the inclusion criteria, 357 884 filled a discharge prescription for one or more opioids associated with one of 20 included operations. The most commonly prescribed post-surgery opioid was hydrocodone (53.0% of those filling a single opioid), followed by short acting oxycodone (37.5%) and tramadol (4.0%). The unadjusted risk of prolonged opioid use after surgery was 7.1% (n=31 431) with additional opioid use, 1.0% (n=4457) with persistent opioid use, and 0.5% (n=2027) meeting the CONSORT definition. Receipt of tramadol alone was associated with a 6% increase in the risk of additional opioid use relative to people receiving other short acting opioids (incidence rate ratio 95% confidence interval 1.00 to 1.13; risk difference 0.5 percentage points; P=0.049), 47% increase in the adjusted risk of persistent opioid use (1.25 to 1.69; 0.5 percentage points; P<0.001), and 41% increase in the adjusted risk of a CONSORT chronic opioid use episode (1.08 to 1.75; 0.2 percentage points; P=0.013).
Conclusions
People receiving tramadol alone after surgery had similar to somewhat higher risks of prolonged opioid use compared with those receiving other short acting opioids. Federal governing bodies should consider reclassifying tramadol, and providers should use as much caution when prescribing tramadol in the setting of acute pain as for other short acting opioids.
Introduction
Despite increased awareness among the public and the medical community,1 2 the US opioid epidemic continues to result in an economic cost of more than $500bn (£389bn; €445bn),3 and the proportion of people using prescribed opioids has not substantially decreased in recent years.4 In the setting of acute pain, some prescribers have focused on limiting the number of pills prescribed or maximizing the use of multimodal and non-opioid based pain control.5 6 7 In addition to these strategies, tramadol has seen a surge in use in the past few years,8 likely due to its perceived benefits, including what physicians may consider a favorable side effects profile and the widespread assumption that is safer and less addictive than other short acting opioids. As a result, tramadol is now among the most commonly prescribed opioids in the US,4 and it is frequently used by surgeons for the treatment of postoperative acute pain.
Tramadol is a centrally acting synthetic weak μ-opioid receptor agonist and is phenotypically distinct from conventional short acting opioids.9 Although tramadol was first developed in Germany in the late 1970s, it did not receive US Food and Drug Administration (FDA) approval until 1995, first becoming a controlled substance in the US (schedule IV drug) in 2014.10 11 Similarly, tramadol was not classified as a controlled substance in the UK until 2014.12 It remains unscheduled in Canada as of March 2019, although scheduling is being considered.13 Tramadol’s lower affinity for the μ-opioid receptor has given it a reputation for having a more favorable side effect profile, including lower rates of constipation, respiratory depression, overdose, and addiction.14 15 16 17 For these reasons, the US FDA continues to classify tramadol at a lower level than other opioids such as morphine and oxycodone, both schedule II.11 As a result, many studies investigating the risks of opioid use have excluded tramadol,18 19 20 and a recent randomized clinical trial included tramadol in the non-opioid prescribing arm.20 Box 1 summarizes basic information about tramadol.11 12 21 22 23
Box 1. Tramadol fast facts.
Brand names
UK: Invodol, Larapam, Mabron, Maneo, Marol, Maxitram, Oldaram, Tilodol, Tradorec, Tramquel, Tramulief, Zamadol, Zeridame, and Zydol
US: Ultram, Ultram ER (discontinued), and ConZip
Canada: Durela, Ralivia, Tridural, Ultram, and Zytram XL
Primary mechanism of action
Tramadol undergoes demethylation in the liver predominately by CYP2D6 enzymes to the active metabolite O-desmethyltramadol, which is a μ-opioid receptor agonist that results in inhibition of ascending pain pathways
Tramadol also directly inhibits norepinephrine (noradrenaline) and serotonin reuptake inhibitors, which are neurotransmitters involved in the inhibitory pain pathway
Use/off-label use
US: labeled for pain management in people for whom non-opioid drugs are contraindicated or ineffective; off-label use for premature ejaculation and refractory restless legs syndrome
US boxed warnings
Addiction, abuse, and misuse
Opioid analgesic risk evaluation and mitigation strategy (REMS)
Life threatening respiratory depression
Accidental ingestion
Ultra-rapid metabolism of tramadol and other risk factors for life threatening respiratory depression in children
Neonatal opioid withdrawal syndrome
Interactions with drugs affecting cytochrome P450 isoenzymes
Risks from concomitant use with benzodiazepines or other central nervous system depressants
Schedule/control
US: schedule IV since 2014; previously unscheduled
UK: class C, schedule 3 since 2014; previously unscheduled
Canada: unscheduled as of March 2019, although scheduling is being considered
Common adverse effects (>10%)
Dizziness, constipation, nausea, headache, somnolence, flushing, pruritus, vomiting, insomnia, dry mouth
Data to support the reputed safety and lower dependence risk of tramadol are lacking. Recently, a study from the Centers for Disease Control (CDC) unexpectedly found that, for Medicare patients, tramadol was associated with a higher risk of transition from acute to prolonged use at one and three years than other short acting opioids.24 Our aim was to determine the risk of transitioning from acute to prolonged use in opioid-naive patients treated with tramadol for postoperative pain.
Methods
Data source and study population
The study involved a retrospective analysis of claims data from the OptumLabs Data Warehouse (OptumLabs), which includes de-identified claims data for commercial and Medicare Advantage enrollees in a large, private, US health plan. The commercial population includes people who receive healthcare coverage through their or a family member’s employer or who purchase coverage on the individual market. The Medicare Advantage group includes people who qualify for public insurance owing to age (≥65 years) or long term disability; people eligible for Medicare have the choice of enrolling in coverage directly from the government (known as fee-for-service Medicare) or from private companies in a program called Medicare Advantage. As of 2017, 33% of Medicare beneficiaries opted for Medicare Advantage coverage.25
OptumLabs contains longitudinal health information on enrollees, representing a diverse mixture of ages, ethnicities, and geographic regions across the US; see appendix A for a comparison of the OptumLabs population with the broader insured US population. The health plan provides comprehensive insurance coverage for physician, hospital, and prescription drug services. We used data from January 1, 2009 through June 30, 2018, with a last day of surgery being December 31, 2017 to account for required follow-up.
We identified 20 commonly performed surgical procedures including seven common general surgery procedures (laparoscopic cholecystectomy with or without intraoperative cholangiogram, minimally invasive inguinal hernia repair, open inguinal hernia repair, simple mastectomy without reconstruction, breast lumpectomy with or without axillary node biopsy, pancreaticoduodenectomy (Whipple), and parathyroidectomy), six orthopedic operations (carpal tunnel release, knee arthroscopic minesectomy, rotator cuff surgery, total knee replacement, total hip replacement, and lumbar laminotomy or laminectomy via posterior approach), two colorectal procedures (minimally invasive low anterior resection with or without ostomy and partial colectomy with or without ostomy), two urology procedures (minimally invasive partial or total nephrectomy and minimally invasive prostatectomy), and two thoracic procedures (open lung lobectomy and video assisted thoracoscopic lung wedge), as well as minimally invasive hysterectomy. We chose the procedures with the aim of including common inpatient and outpatient procedures across multiple specialties and spanning varying degrees of expected postoperative pain. The array of surgeries allows us to assess the extent to which characteristics of discharge prescription are related to expected pain after surgery.
To reduce confounding as much as possible, we constructed a cohort with minimal clinical complexity. We excluded patients who were taking opioids before surgery—defined as patients who had filled no prescriptions for opioids in the previous six months—as well as those who may be in treatment for opioid use disorder by requiring no buprenorphine or methadone in the 90 days after surgery. In addition, we included only patients with at least six months of continuous enrollment in both medical and prescription coverage before surgery. To limit the clinical complexity of the cohort, we excluded patients having multiple unrelated procedures on the same day (see appendix B), those with an inpatient stay longer than seven days, and those admitted as an inpatient more than one day before surgery was performed. We excluded patients receiving non-cancer surgeries if they had cancer, as well as any patients receiving hospice services. To ensure that we were capturing post-surgery opioid fills, we limited the sample to patients who were discharged home and did not have a stay in a skilled nursing facility within a day of discharge (surgery date for those who were not admitted as inpatients). Finally, we required 90 days of insurance enrollment after surgery to ensure that patients survived surgery when we evaluated continued opioid use. See appendix C for the cohort flow chart.
We summarized discharge prescriptions into one of five mutually exclusive and collectively exhaustive categories: no opioid fill, any long acting opioid (with or without any short acting opioid, including tramadol), tramadol only, a short acting opioid other than tramadol alone (reference group), or tramadol plus another short acting opioid. Throughout, we use the “tramadol only” category to interpret tramadol findings, as people who receive multiple opioids at discharge may be different in important, unobserved ways. We followed patients until they were censored by one of the following events: the end of the study period (June 30, 2018); disenrollment from insurance; or another surgery, as defined by a claim for an anesthesia service (see supplementary methods for a list of CPT (current procedural terminology) codes used).
The analysis of discharge prescriptions included patients with at least 30 days of uncensored follow-up who had an opioid fill of less than 1400 morphine milligram equivalents (MME; excludes top 0.5% of discharge fills). The analyses of chronic opioid use included patients with any post-surgery opioid fill and at least 180 days of uncensored follow-up. All patients in all analyses had at least 90 days of post-surgery insurance coverage, which was used to ensure that patients had survived surgery. Patients included in the main outcome analyses had 180 days with no further surgeries, in addition to having insurance coverage during that time.
Outcomes
We identified all opioid fills for the cohort. See appendix B for the drugs included. We grouped discharge opioid prescriptions for each patient into five mutually exclusive and collectively exhaustive categories: short acting opioids only, excluding tramadol (reference); tramadol only; tramadol and any other short acting opioids (no long acting); any long acting opioids; and no opioids. Using conversion factors from the CDC, we converted active ingredient doses to MME.26 We used a different conversion factor for propoxyphene than is given in the CDC table, using a conversion factor of 0.23 for propoxyphene HCl and 0.15 for propoxyphene napsylate (corrected conversion factor for the napsylate salt based on information in the product monograph).27 We defined a single fill as the total amount of a single drug filled on a single day. For example, if a patient filled prescriptions for 5 mg and 10 mg tablets of oxycodone, we summed the total MME for both formulations and counted it as a single oxycodone fill.
To identify the discharge prescription, we looked for opioid fills between seven days before surgery and seven days after surgery (seven days after discharge for patients who were admitted as inpatients). We selected the earliest fill within that time span as the date of the discharge fill and summed the total MME of all opioids filled on that date.
Definitions of prolonged opioid use
To assess the risk of prolonged opioid use after surgery, we did logistic regression at the individual level on the cohort with at least 180 days of uncensored follow-up time. Given the varying definitions used in the literature, we selected three definitions of prolonged opioid use a priori (box 2).
Box 2. Prolonged opioid use definitions.
Additional opioid use after surgery
This definition used in the surgical literature defines chronic opioid use as at least one opioid fill 90-180 days after surgery28 29 30 31
Persistent opioid use after surgery
This definition identifies any span of opioid use starting in the 180 days after surgery and lasting at least 90 days32 33
CONSORT definition of long term opioid therapy
This definition was developed by the CONsortium to Study Opioid Risks and Trends for studying de facto long term opioid therapy in patients being treated for chronic non-cancer pain.34 35 It has been operationalized here as an opioid use episode starting in the 180 days after surgery that spans at least 90 days and includes either 10 or more opioid fills or 120 or more days’ supply of opioids
We defined a single episode of opioid use as a period of time during which the patient goes no more than 30 days without opioids available. Opioids were considered available from the date of fill until the number of days supplied elapsed.
Patient involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
Statistical analysis
We summarized the total MME dispensed in post-surgery discharge fills by using a box plot to display median, 25th and 75th centiles and Tukey lower and upper adjacent values. This analysis included patients with at least 30 uncensored days of follow-up (that is, no other surgeries in those 30 days) who filled 1-1399 MME of opioids at discharge.
Logistic regression models were adjusted for surgery year, sex, race/ethnicity, type of surgery, beneficiary type (commercially insured, Medicare Advantage aged ≥65, Medicare Advantage disabled), census division, age (categorical), discharge prescription volume (total MME, categorical), binary variables for each Elixhauser comorbidity, and whether the person received any long acting opioids at discharge; Huber-White robust standard errors were specified. Logistic regression results are generally presented as odds ratios. However, as odds ratios are often considered difficult to interpret—most people think in risks rather than odds—we present our findings as risk ratios and differences. After regression, we calculated the adjusted proportion with the outcome among people who received tramadol at discharge and those who did not. We used these proportions to calculate the estimated risk ratio with 95% confidence intervals and the risk difference.
Results
We identified 524 318 patients meeting our inclusion criteria, of whom 444 764 had at least 180 uncensored days of follow-up; 357 884 of those had a discharge prescription for one or more opioids. The most common type of discharge prescription over the entire study period was one or more short acting opioids other than tramadol (74.9%; n=333 289) (fig 1); 3.0% (n=13 519) received tramadol alone, and 1.2% (n=5457) received tramadol with another short acting opioid (table 1; further cohort description is in appendix D). Women were more likely to receive tramadol alone (women represented 62.1% of tramadol alone versus 49.0% of the total cohort).
Fig 1.
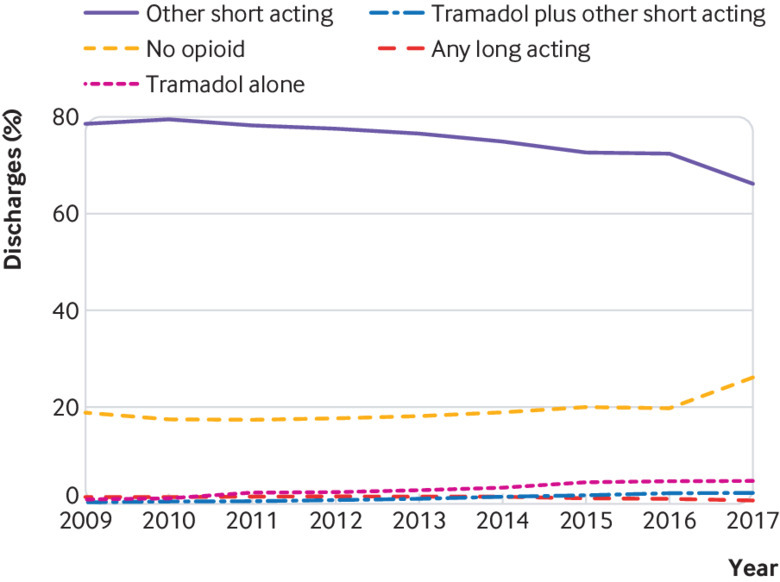
Discharge opioid prescriptions after surgery by type of opioid
Table 1.
Cohort characteristics of all patients with at least 180 days of follow-up. Values are numbers (percentages)
| Characteristics | All patients | No opioid fill | Tramadol only | Other short acting opioid | Tramadol + other short acting opioid | Any long acting opioid |
|---|---|---|---|---|---|---|
| Total | 444 764 (100) | 86 880 (19.5) | 13 519 (3) | 333 289 (74.9) | 5457 (1.2) | 5619 (1.3) |
| Male sex | 226 909 (51.0) | 41 624 (47.9) | 5130 (37.9) | 174 368 (52.3) | 2543 (46.6) | 3244 (57.7) |
| Race/ethnicity: | ||||||
| White | 348 275 (78.3) | 67 365 (77.5) | 10 325 (76.4) | 261 329 (78.4) | 4533 (83.1) | 4723 (84.1) |
| Black | 34 758 (7.8) | 6873 (7.9) | 931 (6.9) | 26 270 (7.9) | 334 (6.1) | 350 (6.2) |
| Hispanic | 37 474 (8.4) | 7535 (8.7) | 1577 (11.7) | 27 801 (8.3) | 316 (5.8) | 245 (4.4) |
| Asian | 10 043 (2.3) | 2030 (2.3) | 245 (1.8) | 7589 (2.3) | 74 (1.4) | 105 (1.9) |
| Unknown/other | 14 214 (3.2) | 3077 (3.5) | 441 (3.3) | 10 300 (3.1) | 200 (3.7) | 196 (3.5) |
| Coverage type: | ||||||
| Commercial | 321 365 (72.3) | 53 479 (61.6) | 8348 (61.8) | 252 186 (75.7) | 3194 (58.5) | 4158 (74.0) |
| Medicare—aged ≥65 | 115 000 (25.9) | 31 441 (36.2) | 4915 (36.4) | 75 138 (22.5) | 2172 (39.8) | 1334 (23.7) |
| Medicare—disabled | 8399 (1.9) | 1960 (2.3) | 256 (1.9) | 5965 (1.8) | 91 (1.7) | 127 (2.3) |
| Surgery: | ||||||
| Carpal tunnel release | 33 730 (7.6) | 9475 (10.9) | 1167 (8.6) | 22 984 (6.9) | 84 (1.5) | 20 (0.4) |
| Knee arthroscopic minesectomy (scope) | 90 064 (20.3) | 15 229 (17.5) | 1869 (13.8) | 71 764 (21.5) | 516 (9.5) | 686 (12.2) |
| Laparoscopic cholecystectomy with or without intraoperative cholangiogram | 105 346 (23.7) | 21 264 (24.5) | 3068 (22.7) | 80 598 (24.2) | 380 (7.0) | 36 (0.6) |
| Laparoscopic inguinal hernia repair | 24 806 (5.6) | 4463 (5.1) | 453 (3.4) | 19 800 (5.9) | 75 (1.4) | 15 (0.3) |
| Laparoscopic low anterior resection with or without stoma | 1132 (0.3) | 368 (0.4) | 48 (0.4) | 710 (0.2.0) | * | * |
| Laparoscopic nephrectomy, partial or total | 1733 (0.4) | 429 (0.5) | 68 (0.5) | 1217 (0.4) | * | * |
| Laparoscopic prostate with or without robot | 8804 (2.0) | 1561 (1.8) | 608 (4.5) | 6601 (2.0) | * | * |
| Laparoscopic/robotic hysterectomy | 3042 (0.7) | 845 (1.0) | 103 (0.8) | 2069 (0.6) | * | * |
| Lumbar laminotomy/ laminectomy (posterior) | 16 695 (3.8) | 3653 (4.2) | 635 (4.7) | 12 159 (3.7) | 126 (2.3) | 122 (2.2) |
| Lumpectomy with or without sentinel lymph node biopsy | 17 181 (3.9) | 4673 (5.4) | 846 (6.3) | 11 593 (3.5) | 57 (1.0) | 12 (0.2) |
| Open lung lobectomy | 767 (0.2) | 153 (0.2) | 31 (0.2) | 553 (0.2) | 13 (0.2) | 17 (0.3) |
| Open inguinal hernia repair | 43 052 (9.7) | 7345 (8.5) | 734 (5.4) | 34 779 (10.4) | 171 (3.1) | 23 (0.4) |
| Parathyroidectomy | 5872 (1.3) | 2532 (2.9) | 156 (1.2) | 3176 (1.0) | * | * |
| Partial colectomy with or without stoma | 1694 (0.4) | 636 (0.7) | 53 (0.4) | 1000 (0.3) | * | * |
| Rotator cuff surgery | 22 587 (5.1) | 2920 (3.4) | 274 (.02) | 17 811 (5.3) | 291 (5.3) | 1291 (23.0) |
| Simple mastectomy without reconstruction | 4317 (1.0) | 1336 (1.5) | 145 (1.1) | 2807 (0.8) | * | * |
| Total knee arthroplasty | 40 250 (9.1) | 5621 (6.5) | 1786 (13.2) | 28 061 (8.4) | 2379 (43.6) | 2403 (42.8) |
| Total hip arthroplasty | 23 068 (5.2) | 4190 (4.8) | 1441 (10.7) | 15 213 (4.6) | 1255 (23.0) | 969 (17.3) |
| Other | 624 (0.1) | 187 (0.2) | 34 (0.3) | 394 (0.1) | * | * |
Cohort characteristics for all patients with at least 30 days of uncensored follow-up are provided in appendix E.
Cell value suppressed to preserve patient de-identification.
Discharge prescription characteristics (cohort with ≥30 days’ follow-up and any opioid fill)
Among patients with any post-surgery opioid prescription fill and at least 30 days of uncensored follow-up, the median amount of opioids dispensed was 225 (interquartile range 150-337.5) MME, the equivalent of 45×5 mg tablets of hydrocodone (30×5 mg tablets of oxycodone). The surgeries with the lowest median discharge fill were carpal tunnel, lumpectomy, and parathyroidectomy, each with 150 MME filled (interquartile ranges: carpal tunnel 135-225 MME, lumpectomy 120-225 MME, parathyroidectomy 125-225 MME). The surgeries with the highest median discharge fill were total hip arthroplasty and total knee arthroplasty, each with 450 MME (interquartile ranges: total hip arthroplasty 300-675MME, total knee arthroplasty 337.5-730 MME) (fig 2). Cohort characteristics are provided in appendix E.
Fig 2.
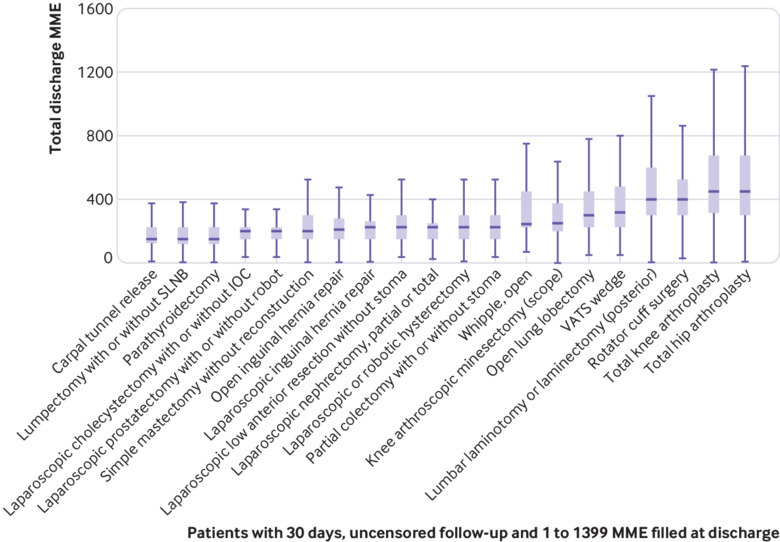
Total amount of opioids prescribed at discharge after surgery in oral morphine milligram equivalents (MME) for each procedure. IOC=intraoperative cholangiogram; SNLB=sentinel lymph node biopsy; VATS=video assisted thoracoscopic
Among patients who received a single commonly prescribed opioid (at least 1% of sample received the drug) at discharge (n=401 864; 95.4% of people with 30 days’ uncensored follow-up and any opioids at discharge), the largest prescriptions were written for propoxyphene (median discharge fill 450 (interquartile range 375-600) MME). The most commonly prescribed opioid after surgery was hydrocodone (n=212 987; 53.0% of patients filling a single common opioid; median fill 200 (150-300) MME), followed by short acting oxycodone (n=150 785; 37.5%; median fill 300 (225-450) MME), tramadol (n=16 059; 4.0%; median fill 150 (150-250) MME), codeine (n=12 377; 3.1%; median fill 135 (90-180) MME), hydromorphone (n=4831; 1.2%; median fill 400 (240-520) MME), and propoxyphene (n=4825; 1.2%). Propoxyphene was available only in the first part of the study period, through November 2010. During the period it was available, propoxyphene was the third most commonly prescribed drug, with 5.8% of discharge prescriptions for a single drug.
Prolonged opioid use
We analyzed three separate measures of prolonged opioid use and calculated adjusted proportions of the sample meeting each measure. Additional use of opioids (defined as one or more opioid fills 90-180 days after surgery) was seen in 7.1% (n=31 431) of the sample with at least 180 days of uncensored follow-up. One per cent of the sample met criteria for persistent opioid use after surgery, which was defined as episodes of opioid use lasting 90 or more days that started in the 180 days after surgery (n=4457; 1.00%). The most stringent criterion—the CONSORT definition requiring an opioid use episode lasting at least 90 calendar days and including either 10 or more opioid fills or 120 or more days’ supply—was present in 0.46% (n=2027) of the sample with at least 180 days of uncensored follow up time (table 2).
Table 2.
Risk of unadjusted persistent opioid use (three definitions) for patients who received short acting opioids excluding tramadol, tramadol only, tramadol and another short acting opioids, any long acting opioids, or no opioids at discharge (cohort with 180 days follow-up). Values are numbers (percentages)
| Definition | All patients (n=444 764) | No opioid fill (n=86 880) | Tramadol only (n=13 519) | Other short acting opioid (n=333 289) | Tramadol + other short acting opioid (n=5457) | Any long acting opioid (n=5619) |
|---|---|---|---|---|---|---|
| Additional opioid use after surgery* | 31 431 (7.07) | 3849 (4.43) | 1066 (7.89) | 25 388 (7.62) | 543 (9.95) | 585 (10.41) |
| Persistent opioid use after surgery† | 4457 (1.00) | 314 (0.36) | 194 (1.44) | 3559 (1.07) | 149 (2.73) | 241 (4.29) |
| CONSORT definition of chronic opioid use‡ | 2027 (0.46) | 175 (0.20) | 78 (0.58) | 1573 (0.47) | 71 (1.30) | 130 (2.31) |
At least one opioid fill 90-180 days after surgery.
Any span of opioid use starting in 180 days after surgery and lasting ≥90 days.
Opioid use episode starting in 180 days after surgery that spans ≥90 days and includes either ≥10 opioid fills or ≥120 days’ supply of opioids.
Among patients meeting the definition of additional opioid use, 72% (n=22 779) had no opioids 31-60 days after surgery, 75% (n=23 630) had no opioids 61-90 days after surgery, and 64% (n=20 258) had no opioids 31-90 days after surgery. For comparison, 11% (n=488) of patients meeting the definition for persistent opioid use (an episode lasting at least 90 days) and 14% (n=281) of those meeting the CONSORT definition had no opioids 31-90 days after surgery.
Association of discharge prescription volume with prolonged use of opioids
Larger discharge prescriptions were associated with a higher risk of prolonged opioid use across all three definitions of prolonged use (table 3). Receipt of 500 or more MME of opioids was associated with nearly five times the risk of prolonged opioid use compared with receipt of 1-199 MME using the CONSORT definition of prolonged use, more than six times the risk of persistent use, and 1.5 times the risk of additional use.
Table 3.
Risk of unadjusted persistent opioid use (three definitions) by amount of opioids prescribed at discharge. Values are numbers (percentages)
| Definition | No opioid fill (n=86 880) | 1-199 MME (n=116 813) | 200-299 MME (n=90 393) | 300-399 MME (n=69 301) | 400-499 MME (n=36 156) | ≥500 MME (n=45 221) |
|---|---|---|---|---|---|---|
| Additional opioid use after surgery* | 3849 (4.43) | 7930 (6.79†) | 6326 (7.00) | 5397 (7.79†) | 3216 (8.89†) | 4713 (10.42†) |
| Persistent opioid use after surgery‡ | 314 (0.36) | 570 (0.49†) | 618 (0.68†) | 837 (1.21†) | 688 (1.90†) | 1430 (3.16†) |
| CONSORT definition of chronic opioid use§ | 175 (0.20) | 317 (0.27†) | 303 (0.34†) | 379 (0.55†) | 266 (0.74†) | 587 (1.30†) |
MME=morphine milligram equivalents.
At least one opioid fill 90-180 days after surgery.
Statistically significantly different risk versus previous dose group (P<0.05; Bonferroni corrected).
Any span of opioid use starting in 180 days after surgery and lasting ≥90 days.
Opioid use episode starting in 180 days after surgery that spans ≥90 days and includes either ≥10 opioid fills or ≥120 days’ supply of opioids.
Association of tramadol at discharge with prolonged use of opioids
Receipt of tramadol at discharge was associated with increased adjusted risk of all three definitions of prolonged opioid use (table 4). Receipt of tramadol alone was associated with a 6% increase in the risk of additional opioid use relative to people receiving other short acting opioids (risk ratio 95% confidence ratio 1.00 to 1.13; risk difference 0.5 percentage points; P=0.049), a 47% increase in the adjusted risk of persistent opioid use (1.25 to 1.69; 0.5 percentage points; P<0.001), and a 41% increase in the adjusted risk of a CONSORT chronic opioid use episode (1.08 to 1.75; 0.2 percentage points; P=0.013).
Table 4.
Adjusted risk ratios* (95% CIs) and P values† for persistent opioid use (three definitions) in patients who received tramadol only, tramadol and another short acting opioid, or any long acting opioids (reference group: short acting opioids excluding tramadol)
| Opioid type | Additional opioid use after surgery‡ | Persistent opioid use after surgery§ | CONSORT definition of opioid dependence¶ |
|---|---|---|---|
| Other short acting | Reference | Reference | Reference |
| Tramadol only | 1.06 (1.00 to 1.13); P=0.049 | 1.47 (1.25 to 1.69); P<0.001 | 1.41 (1.08 to 1.75); P=0.013 |
| Tramadol plus short acting | 1.05 (0.96 to 1.14); P=0.261 | 1.04 (0.86 to 1.21); P=0.685 | 1.40 (1.05 to 1.74); P=0.022 |
| Any long acting | 0.95 (0.87 to 1.03); P=0.218 | 1.18 (1.02 to 1.35); P=0.029 | 1.69 (1.36 to 2.02); P<0.001 |
Risk ratios calculated as ratio of predictive margins after logistic regression including covariates of year, surgery, female sex, beneficiary type, race/ethnicity, census division, age category, categorical measurement of morphine milligram equivalents at discharge, and flags for each of Elixhauser comorbidities; see appendix F for full regression output.
P values from hypothesis test that risk ratio does not equal 1.
At least one opioid fill 90-180 days after surgery.
Any span of opioid use starting in 180 days after surgery and lasting ≥90 days.
Opioid use episode starting in 180 days after surgery that spans ≥90 days and includes either ≥10 opioid fills or ≥120 days’ supply of opioids.
Discussion
Our study suggests that tramadol carries a similar or somewhat greater risk of transitioning from acute to prolonged use compared with other short acting opioids. Although prescribing was relatively infrequent (4% of patients with opioid fills, including those who received tramadol with other short acting opioids), tramadol was the third most frequently prescribed opioid in this study (after hydrocodone and short acting oxycodone), and its use seems to be increasing (fig 1). The low overall prescription rate likely masks variation in tramadol use across health systems; other studies have found that 3% to 25% of patients receive tramadol prescriptions after surgery.36 37 Our findings suggest that from the standpoint of risk of dependency, clinicians prescribing tramadol for acute pain should exercise a level of caution similar to that surrounding the prescribing of other short acting opioids, including those on higher Drug Enforcement Administration schedules.
Larger discharge prescriptions were associated with a higher unadjusted risk of prolonged opioid use across all three definitions of prolonged use (table 3). In the adjusted analyses, doses of 300 MME and larger were associated with higher risk of prolonged use, although with smaller effect sizes than in the unadjusted analysis (odds ratios 1.1 to 1.6, see appendix F). This aligns with CDC data suggesting that the risk of prolonged use increases significantly when patients receive prescriptions for more opioids.24
Although tramadol remains largely unregulated in the developing world, the rates of misuse and awareness of the risks have been increasing outside the US.38 39 40 41 Data to support the avoidance of long acting opioids in the acute setting remain strong,42 43 44 but no clear data are available on the risks versus benefits of other short acting opioids compared with tramadol. Therefore, the choice to prescribe tramadol rather than another short acting opioid remains largely dependent on the provider and scenario.
Comparison with previous studies
Before our work, the strongest study investigating the risk of long term tramadol use was the finding noted in the 2017 CDC report on opioid prescribing. That study found that tramadol was associated with a 13.7% risk of continued use at one year compared with 4.7-8.9% for other short acting opioids.24 Notably, the CDC study defined discontinuation of opioids as at least 180 days without opioid use and included commercially insured people in managed care plans but did not limit the sample to people receiving surgery. Other than the CDC publication, most other studies assessing the risk of long term use for an acute episode of pain either do not include tramadol or do not provide rates of prolonged use by opioid type.
Pharmacologic and neurologic mechanisms for misuse potential
Recent publications have shown an increased rate of complications, emergency department visits, and misuse in patients using tramadol.45 46 Consequently, the pharmacologic and neural mechanisms responsible for the misuse potential of tramadol warrant further consideration. The analgesic effects of tramadol are attributed to two mechanisms of action: μ-opioid receptor agonist and norepinephrine (noradrenaline) reuptake inhibitor. Tramadol undergoes demethylation in the liver to the active metabolite desmetramadol. However, individual phenotypic variation in the quantity and efficiency of the CYP2D6 enzyme influences the bioavailability of that active metabolite, which can range from 3% among people who are poor metabolizers to 86% among those who are extensive metabolizers.47 This is clinically significant because O-desmethyltramadol has a 700 times greater affinity for the μ-opioid receptor than does the parent compound.48 In functional activity assays, O-desmethyltramadol, but not the parent compound, shows high intrinsic activity at the μ-opioid receptor comparable to morphine.49 Thus, the desmetramadol metabolite is responsible for the opioid effects of tramadol. In experimental human studies, the reinforcing effects of supratherapeutic doses of tramadol (400 mg administered as a single oral dose) were comparable to oxycodone.16 These observations are supported by the findings of a functional magnetic resonance imaging study in which reward anticipation was associated with increased neural activity in the nucleus accumbens after tramadol administration.50
Similar to the analgesic effects of tramadol, these studies suggest that the misuse potential of tramadol is due to the effects of O-desmethyltramadol on the μ-opioid receptor, which could be influenced by individualized variations in CYP2D6 expression.14 47 Contrary to the primary effects of O-desmethyltramadol on opioid receptor mediated analgesia, the parent compound, tramadol, is directly responsible for analgesia related to the inhibition of norepinephrine and serotonin. This important mechanism of action is also responsible for the increased risk of serotonin syndrome, which typically occurs with the use of other proserotonergic drugs. Clinically important drug-drug interactions can occur with concomitant use of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors, antipsychotics, triptans, antiparkinsonian drugs, and over the counter drug combinations containing dextromethorphan.51
Prolonged opioid use definitions
The paucity of data to support or refute the risk of tramadol use is due, in part, to the absence of structured approaches for investigating the transition from acute to prolonged use. In previous work, we developed a conceptual model to better understand the mediators and moderators of unintended prolonged opioid use.52 The proposed conceptual framework was composed of three domains including characteristics of the patient, the practice environment, and the opioid prescriber that interact to either facilitate or impede unintended prolonged opioid use.
Our study found that prolonged opioid use as defined by either persistent opioid use (at least 90 days of continuous opioid use in the 180 days after surgery) or the CONSORT definition was relatively rare (≤1%; table 2). However, when we used the less stringent definition found in many studies, which defined prolonged use as at least one opioid fill in the 90-180 days after surgery, this risk of additional use was much higher (7%). This more closely aligns with the published risk in the setting of acute pain that vary from 6% in all patients with acute pain,53 6% after general surgery,28 10% after curative intent surgery for cancer,29 and 15% after lung resection,30 to as high as 24% after orthopedic surgery.54 However, these studies all used less stringent definitions of prolonged opioid use. Studies using claims data and more stringent definitions suggest a significantly lower risk of prolonged use in the setting of acute pain (0.1%).55 We observed that nearly two thirds of patients meeting the additional use definition (one or more opioid fill in 90-180 days after surgery) had no opioids in the 30-90 days after surgery. This pattern suggests that rather than prolonged use of opioids, these patients may be experiencing separate episodes of opioid use separated by one or more months. The additional opioid use definition may therefore measure a separate problem whereby patients who are once exposed to opioids are more likely to receive them for other pain related problems. We did not test this hypothesis, but the fact that 4.4% of the patients who received no opioids at discharge met the prolonged use definition (table 4), whereas 7.7% who did have a fill met this definition, may suggest an avenue for further research.
Limitations of study
The findings of this study are most directly applicable to commercially insured and Medicare Advantage patients in the US undergoing elective surgery of the types we included. Given that our study used claims data, we were also unable to determine actual opioid consumption or capture prescriptions that were not submitted to insurance; however, previous studies suggest that this rate is low.56 Although we excluded patients taking opioids before surgery and those with additional operations after the index operation, we were unable to ascertain the reason patients received additional opioid prescriptions. Lastly, given the available data and scope of the study, we were unable to consider other aspects of the safety profile of tramadol compared with other opioids, including notable benefits such as a potentially lower risk of respiratory depression.57 Further research in this area is warranted before policy changes are implemented.
Conclusions
We found that tramadol, a drug that is scheduled at a lower risk level than other common short acting opioids (schedule IV versus schedule II for hydrocodone and oxycodone), has a similar or somewhat greater risk of prolonged opioid use after surgery. Although all factors related to the safety of a drug must be considered, from the standpoint of opioid dependence, the Drug Enforcement Administration and FDA should consider rescheduling tramadol to a level that better reflects its risks of prolonged use.
What is already known on this topic
Tramadol is a unique short acting opioid that is considered by many physicians to be safer than other short acting opioids
However, data to support the safety and lower risk of prolonged use of tramadol are lacking
What this study adds
Tramadol use was associated with a higher risk of prolonged opioid use in patients with an acute episode of pain compared with other short acting opioids
Providers should use caution when prescribing tramadol in the setting of acute pain
Web extra.
Extra material supplied by authors
Appendices A-F
Contributors: CAT, EBH, WMH, and MMJ conceived and designed this work. MMJ and CAT cleaned and analyzed the data. CAT, EBH, and MMJ interpreted the data, and all authors were responsible for drafting the work and revising it critically for important intellectual content. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. MMJ is the guarantor.
Funding: This study had no external funding. Support was provided by the Mayo Clinic Robert D and Patricia E Kern Center for the Science of Health Care Delivery.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Because it uses pre-existing, de-identified data, this study was determined to be exempt from review by the Mayo Clinic Institutional Review Board.
Data sharing: OptumLabs data are available for research through a virtual data warehouse. The authors are not able to distribute the data.
Transparency statement: The lead author (the manuscript’s guarantor) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1. Thiels CA, Hanson KT, Cima RR, Habermann EB. From Data to Practice: Increasing Awareness of Opioid Prescribing Data Changes Practice. Ann Surg 2018;267:e46-7. 10.1097/SLA.0000000000002623 [DOI] [PubMed] [Google Scholar]
- 2. Blendon RJ, Benson JM. The Public and the Opioid-Abuse Epidemic. N Engl J Med 2018;378:407-11. 10.1056/NEJMp1714529 [DOI] [PubMed] [Google Scholar]
- 3.Council of Economic Advisers. The underestimated cost of the opioid crisis. 2017. https://www.whitehouse.gov/sites/whitehouse.gov/files/images/The%20Underestimated%20Cost%20of%20the%20Opioid%20Crisis.pdf.
- 4. Jeffery MM, Hooten WM, Henk HJ, et al. Trends in opioid use in commercially insured and Medicare Advantage populations in 2007-16: retrospective cohort study. BMJ 2018;362:k2833. 10.1136/bmj.k2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howard R, Waljee J, Brummett C, Englesbe M, Lee J. Reduction in Opioid Prescribing Through Evidence-Based Prescribing Guidelines. JAMA Surg 2018;153:285-7. 10.1001/jamasurg.2017.4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thiels CA, Ubl DS, Yost KJ, et al. Results of a Prospective, Multicenter Initiative Aimed at Developing Opioid-prescribing Guidelines After Surgery. Ann Surg 2018;268:457-68. 10.1097/SLA.0000000000002919 [DOI] [PubMed] [Google Scholar]
- 7. Siddiqui NT, Fischer H, Guerina L, Friedman Z. Effect of a preoperative gabapentin on postoperative analgesia in patients with inflammatory bowel disease following major bowel surgery: a randomized, placebo-controlled trial. Pain Pract 2014;14:132-9. 10.1111/papr.12058 [DOI] [PubMed] [Google Scholar]
- 8. Manchikanti L, Helm S, 2nd, Fellows B, et al. Opioid epidemic in the United States. Pain Physician 2012;15(Suppl):ES9-38. [PubMed] [Google Scholar]
- 9. Power I. An update on analgesics. Br J Anaesth 2011;107:19-24. 10.1093/bja/aer126 [DOI] [PubMed] [Google Scholar]
- 10. Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R. Trends in Tramadol. Anesth Analg 2017;124:44-51. 10.1213/ANE.0000000000001683 [DOI] [PubMed] [Google Scholar]
- 11.Harrigan TM. Schedules of controlled substances: placement of tramadol into schedule IV. 2013. https://www.deadiversion.usdoj.gov/fed_regs/rules/2013/fr1104.htm.
- 12.Monthly Index of Medical Specialities. Tramadol reclassified as a controlled drug. 2019. https://www.mims.co.uk/tramadol-reclassified-controlled-drug/surgery/article/1297952.
- 13.Health Canada. Forward Regulatory Plan 2019-2021: Regulations amending Schedule I to the Controlled Drugs and Substances Act and the Schedule to the Narcotic Control Regulations to add tramadol and related substances. 2019. https://www.canada.ca/en/health-canada/corporate/about-health-canada/legislation-guidelines/acts-regulations/forward-regulatory-plan/plan/tramadol.html.
- 14. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet 2004;43:879-923. 10.2165/00003088-200443130-00004 [DOI] [PubMed] [Google Scholar]
- 15. Radbruch L, Grond S, Lehmann KA. A risk-benefit assessment of tramadol in the management of pain. Drug Saf 1996;15:8-29. 10.2165/00002018-199615010-00002 [DOI] [PubMed] [Google Scholar]
- 16. Babalonis S, Lofwall MR, Nuzzo PA, Siegel AJ, Walsh SL. Abuse liability and reinforcing efficacy of oral tramadol in humans. Drug Alcohol Depend 2013;129:116-24. 10.1016/j.drugalcdep.2012.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benzon H. Practical management of pain. Elsevier/Saunders, 2014. [Google Scholar]
- 18. Dobscha SK, Morasco BJ, Duckart JP, Macey T, Deyo RA. Correlates of prescription opioid initiation and long-term opioid use in veterans with persistent pain. Clin J Pain 2013;29:102-8. 10.1097/AJP.0b013e3182490bdb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lev R, Lee O, Petro S, et al. Who is prescribing controlled medications to patients who die of prescription drug abuse? Am J Emerg Med 2016;34:30-5. 10.1016/j.ajem.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 20. Krebs EE, Gravely A, Nugent S, et al. Effect of Opioid vs Nonopioid Medications on Pain-Related Function in Patients With Chronic Back Pain or Hip or Knee Osteoarthritis Pain: The SPACE Randomized Clinical Trial. JAMA 2018;319:872-82. 10.1001/jama.2018.0899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen Pharmaceuticals. Highlights of prescribing information: Ultram. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020281s041lbl.pdf.
- 22.UK Government. List of most commonly encountered drugs currently controlled under the misuse of drugs legislation. 2017. https://www.gov.uk/government/publications/controlled-drugs-list--2/list-of-most-commonly-encountered-drugs-currently-controlled-under-the-misuse-of-drugs-legislation.
- 23.National Association of Pharmacy Regulatory Authorities (NAPRA). Tramadol or its salts. https://napra.ca/nds/tramadol-or-its-salts.
- 24. Shah A, Hayes CJ, Martin BC. Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use - United States, 2006-2015. MMWR Morb Mortal Wkly Rep 2017;66:265-9. 10.15585/mmwr.mm6610a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jocobson G, Damico A, Neuman T. Medicare Advantage 2017 Spotlight: Enrollment Market Update. 2017. https://www.kff.org/medicare/issue-brief/medicare-advantage-2017-spotlight-enrollment-market-update/
- 26.Agency Medical Directors’ Group. Opioid Dose Calculator v2.01. http://www.agencymeddirectors.wa.gov/Calculator/DoseCalculator.htm.
- 27.Xanodyne Pharmaceuticals. Darvocet-N® 50 and Darvocet-N® 100 (propoxyphene napsylate and acetaminophen tablets, USP). 2009. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/017122s061s062lbl.pdf.
- 28. Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg 2017;152:e170504. 10.1001/jamasurg.2017.0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee JS-J, Hu HM, Edelman AL, et al. New Persistent Opioid Use Among Patients With Cancer After Curative-Intent Surgery. J Clin Oncol 2017;35:4042-9. 10.1200/JCO.2017.74.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brescia AA, Harrington CA, Mazurek AA, et al. Factors Associated With New Persistent Opioid Usage After Lung Resection. Ann Thorac Surg 2019;107:363-8. 10.1016/j.athoracsur.2018.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harbaugh CM, Nalliah RP, Hu HM, Englesbe MJ, Waljee JF, Brummett CM. Persistent Opioid Use After Wisdom Tooth Extraction. JAMA 2018;320:504-6. 10.1001/jama.2018.9023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Delgado MK, Huang Y, Meisel Z, et al. National Variation in Opioid Prescribing and Risk of Prolonged Use for Opioid-Naive Patients Treated in the Emergency Department for Ankle Sprains. Ann Emerg Med 2018;72:389-400.e1. 10.1016/j.annemergmed.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ 2014;348:g1251. 10.1136/bmj.g1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jeffery MM, Hooten WM, Hess EP, et al. Opioid Prescribing for Opioid-Naive Patients in Emergency Departments and Other Settings: Characteristics of Prescriptions and Association With Long-Term Use. Ann Emerg Med 2018;71:326-336.e19. 10.1016/j.annemergmed.2017.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain 2008;24:521-7. 10.1097/AJP.0b013e318169d03b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thiels CA, Anderson SS, Ubl DS, et al. Wide Variation and Overprescription of Opioids After Elective Surgery. Ann Surg 2017;266:564-73. 10.1097/SLA.0000000000002365 [DOI] [PubMed] [Google Scholar]
- 37. Larach DB, Waljee JF, Hu HM, et al. Patterns of Initial Opioid Prescribing to Opioid-Naive Patients. Ann Surg 2018. 10.1097/SLA.0000000000002969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salm-Reifferscheidt L. Tramadol: Africa’s opioid crisis. Lancet 2018;391:1982-3. 10.1016/S0140-6736(18)31073-0 [DOI] [PubMed] [Google Scholar]
- 39. Bassiony MM, Abdelghani M, Salah El-Deen GM, Hassan MS, El-Gohari H, Youssef UM. Opioid Use Disorders Attributed to Tramadol Among Egyptian University Students. J Addict Med 2018;12:150-5. 10.1097/ADM.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 40. Moulis F, Rousseau V, Abadie D, et al. [Serious adverse drug reactions with tramadol reported to the French pharmacovigilance database between 2011 and 2015]. Therapie 2017;72:615-24. 10.1016/j.therap.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 41. Zhang H, Liu Z. The investigation of tramadol dependence with no history of substance abuse: a cross-sectional survey of spontaneously reported cases in Guangzhou City, China. Biomed Res Int 2013;2013:283425. 10.1155/2013/283425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gold LS, Strassels SA, Hansen RN. Health Care Costs and Utilization in Patients Receiving Prescriptions for Long-acting Opioids for Acute Postsurgical Pain. Clin J Pain 2016;32:747-54. 10.1097/AJP.0000000000000322 [DOI] [PubMed] [Google Scholar]
- 43.Thorson D, Biewen P, Bonte B, et al; Institute for Clinical Systems Improvement (ICSI). Acute Pain Assessment and Opioid Prescribing Protocol. 2014. https://crh.arizona.edu/sites/default/files/u35/Opioids.pdf.
- 44. Musclow SL, Bowers T, Vo H, Glube M, Nguyen T. Long-acting morphine following hip or knee replacement: a randomized, double-blind, placebo-controlled trial. Pain Res Manag 2012;17:83-8. 10.1155/2012/704932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bush DM. The CBHSQ Report: Emergency Department Visits for Adverse Reactions Involving the Pain Medication Tramadol. 2015. https://www.samhsa.gov/data/sites/default/files/report_1965/ShortReport-1965.html [PubMed]
- 46. Fournier J-P, Azoulay L, Yin H, Montastruc JL, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med 2015;175:186-93. 10.1001/jamainternmed.2014.6512 [DOI] [PubMed] [Google Scholar]
- 47. Kirchheiner J, Keulen JT, Bauer S, Roots I, Brockmöller J. Effects of the CYP2D6 gene duplication on the pharmacokinetics and pharmacodynamics of tramadol. J Clin Psychopharmacol 2008;28:78-83. 10.1097/JCP.0b013e318160f827 [DOI] [PubMed] [Google Scholar]
- 48. Raffa RB, Buschmann H, Christoph T, et al. Mechanistic and functional differentiation of tapentadol and tramadol. Expert Opin Pharmacother 2012;13:1437-49. 10.1517/14656566.2012.696097 [DOI] [PubMed] [Google Scholar]
- 49. Minami K, Sudo Y, Miyano K, Murphy RS, Uezono Y. µ-Opioid receptor activation by tramadol and O-desmethyltramadol (M1). J Anesth 2015;29:475-9. 10.1007/s00540-014-1946-z [DOI] [PubMed] [Google Scholar]
- 50. Asari Y, Ikeda Y, Tateno A, Okubo Y, Iijima T, Suzuki H. Acute tramadol enhances brain activity associated with reward anticipation in the nucleus accumbens. Psychopharmacology (Berl) 2018;235:2631-42. 10.1007/s00213-018-4955-z [DOI] [PubMed] [Google Scholar]
- 51. Park SH, Wackernah RC, Stimmel GL. Serotonin syndrome: is it a reason to avoid the use of tramadol with antidepressants? J Pharm Pract 2014;27:71-8. 10.1177/0897190013504957 [DOI] [PubMed] [Google Scholar]
- 52. Hooten WM, Brummett CM, Sullivan MD, et al. A Conceptual Framework for Understanding Unintended Prolonged Opioid Use. Mayo Clin Proc 2017;92:1822-30. 10.1016/j.mayocp.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 53. Hooten WM, St Sauver JL, McGree ME, Jacobson DJ, Warner DO. Incidence and Risk Factors for Progression From Short-term to Episodic or Long-term Opioid Prescribing: A Population-Based Study. Mayo Clin Proc 2015;90:850-6. 10.1016/j.mayocp.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jiang X, Orton M, Feng R, et al. Chronic Opioid Usage in Surgical Patients in a Large Academic Center. Ann Surg 2017;265:722-7. 10.1097/SLA.0000000000001780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med 2016;176:1286-93. 10.1001/jamainternmed.2016.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cepeda MS, Fife D, Denarié M, Bradford D, Roy S, Yuan Y. Quantification of missing prescriptions in commercial claims databases: results of a cohort study. Pharmacoepidemiol Drug Saf 2017;26:386-92. 10.1002/pds.4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tarkkila P, Tuominen M, Lindgren L. Comparison of respiratory effects of tramadol and oxycodone. J Clin Anesth 1997;9:582-5. 10.1016/S0952-8180(97)00147-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendices A-F


