Abstract
Bacillus megaterium 314 strain was able to utilize agricultural and industrial wastes for metallo-protease production. Orange peel and wheat bran were found as the most suitable carbon and nitrogen sources, respectively. Optimized production process enhanced the enzyme production by 5.1-folds. Glass and glass-ceramic with different particle sizes based on mica were used as inorganic carrier. Protease enzyme was immobilized by covalent bonding and physical adsorption methods on nanoparticle supports. Enzyme physically adsorbed on glass ceramic (particle size 0.71–1.0 mm) had the highest residual activity and the highest immobilization yield. Glass-ceramic was characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). Immobilized enzyme exhibited activation energy (Ea) and deactivation rate constant at 60 °C (kd) about 1.29 and 1.46-times, respectively lower than free enzyme. Moreover, adsorbed enzyme had higher energy for denaturation (Ed), half-life (t1/2), and decimal reduction time (D). The thermodynamic parameters of irreversible thermal denaturation for the protease enzyme indicate that immobilized enzyme had higher enthalpy (ΔH°), free energy (ΔG°), and entropy (ΔS°) than free one. There was a significant improvement in the maximum reaction velocity Vmax (2.5-fold), Michaelis constant Km (1.9-fold), and catalytic efficiency Vmax/Km (4.7-fold) values after immobilization indicating the efficiency and effectiveness of immobilization approach.
Keywords: Microbiology, Biotechnology, Biochemistry
1. Introduction
Enzymes are powerful biocatalysts enhancing the reaction rates of various chemical and biological processes. Enzymes are well inside the concept of biotechnology. Proteases are among the first enzymes utilized for this kind of application and their utility still strong. However, the methods of utilization of these enzymes have changed by time. In recent years microbial proteases as industrial catalysts offer advantages over the use of conventional chemical catalysts for economically viable, exhibiting high catalytic activity, high degree of substrate specificity, and can be produced in huge amounts etc. [1]. Proteases are a unique class of degradative enzymes which hydrolyze peptide bonds in proteins molecules to peptides and amino acids. Proteases are of immense physiological as well as commercial importance. Enzymes from microbial sources are the largest group of industrial enzymes representing ∼60% of total global sale of enzymes [2]. Over the centuries, microbial proteases have played a major role in the production of traditional fermented foods, and now the industrial enzyme sector, provides the world with biocatalysts for use in many industries. Furthermore, microbial source has the best acceptance by people whose religious beliefs (Halal certification and kosher) and eating habits (vegetarian) versus animal sources which are derived with expensive cost from pigs [3, 4]. In general, proteases production from microorganisms is constitutive or partially inducible in nature. Microorganisms present an excellent source of enzymes due to its advantages as (1) can grow in a shorter period (2) the broad biochemical diversity (3) cheap natural abundance (4) the limited space required for cell cultivation (5) produce a specialized enzyme (6) Possibility of genetic manipulation to generate new enzymes suitable for many applications [5, 6, 7]. Many Bacillus species produce a variety of proteases (extracellular and intracellular) in the production media [8]. The major obstruction for industrial applications of proteases is the high production medium cost (∼40%). Waste from agriculture and industry (wheat bran and peels of fruits and vegetables) are low cost substrates. These wastes can be utilized as substrates by microorganisms because of their rich contents of organic ingredients, which are essential sources of carbon and nitrogen, and many micronutrients that are necessary for the production of metabolites. Recycling of agricultural and industrial residues which are enormously available as carbon and nitrogen sources for enzymes production plays a fundamental role not only in reducing the production charge but also solve the pollution problem [9]. The one variable at a time (OVAT) optimization of the enzyme production was carried to identify the important variables that affect its production. The activity and heat tolerance of enzyme are other major barriers to evaluating the economic feasibility of industrial processes based on enzymes. Generally, high stability of enzyme under harsh conditions is considered an economic advantage due to low enzyme loss [10]. Enzymes could be immobilized before being used as industrial biologics. Enzyme immobilization is the simplest way to solve the solubility problem of protein. Also, immobilization improves the control of the reaction and avoids contamination of product by enzyme. In addition, via immobilization enzyme structural rigidity may be improved, if the spacer arms (using crosslinker as glutaraldehyde) are short enough and the support is rigid [11]. Immobilization improves enzyme properties as activity, reduction of the inhibition by reaction products and metal ions, stability, and specificity to substrates [12]. Immobilization may also permit the prevention of enzyme subunit dissociation of multimeric enzymes [13].
Furthermore, it can reduce the expensive cost of applying them on an industrial scale, because it allows them to be easily separated and reused. In biocatalysis, there is increasing use of immobilized enzymes due to their advantages such as ease of separation and reused, improved product quality and purity, increased enzyme (stability, shelf-life, catalytic efficiency for prolonged period) and reduced chances of contamination [14, 15]. Physical adsorption (PA) is the simplest method of immobilization and has little effect on the conformation of the biocatalyst. In PA method, the enzyme is adsorbed onto the surface of the carrier with H-bond, hydrophobic force and electrostatic interactions [14]. Covalent immobilization of enzymes to supports may become somehow more complex in most cases as the support requires some preliminary activation by crosslinkers [11]. Glutaraldehyde as a cross-linking reagent is molecule that contains two or more reactive ends capable of chemically attaching to specific functional groups on proteins or other molecules. Covalent immobilization is only recommended if the immobilization really provides a significant improvement on the enzyme properties [13].
Due to the high cost of supports there are many searches for cheaper substitutes. Mica glass ceramic appears to be the most attractive because its attractive properties beside it considered as a low-cost carrier [16]. Mica is a natural rock widely distributed in the earth. It occurs in igneous, metamorphic and sedimentary regimes. Mica is a sheet silicate having perfect basal cleavage. The most important micas are muscovite and phlogopite. It is characterized by its layered or platy texture, these sheets are flexible, chemically inert, elastic, dielectric, lightweight, hydrophilic, platy, insulating, and range in opacity from transparent to opaque beside its biocompatibility. Mica is stable when exposed to light, moisture, electricity, and temperatures. Consequently, synthesis of mica glass ceramic attracts great attention from scientists [17, 18]. On the other hands, synthetic fluoroapatite has been used in various forms of biomedical field [19]. Synthesis of glass ceramic contains both of mica and fluoroapatite expected to give advanced properties to be used in biomedical applications, especially when the crystallization procedure adjusted to give nano size crystals. The biocatalytic properties of mica glass-ceramic immobilized proteases have not been reported previously. Moreover, studies on the thermodynamic properties of crude and immobilized proteases are poorly described, especially in the case of immobilization using nanoparticle (from raw material) like the one investigated in this study.
In the present work, we report the optimization of protease production by B. megaterium 314 strain. Crystallization of mica-fluroapatite nano-glass ceramic was utilized as a support for enzyme immobilization. XRD and SEM were employed to characterize phases developed and microstructure respectively. Finally, comparative studies between free and nanoparticle immobilized enzyme was performed (catalytic, kinetics and thermodynamics parameters).
2. Material and methods
2.1. Agricultural and industrial residues
Agricultural and industrial residues (lemon skin, corn cob, orange peel, pomegranate peel, pea peel, strawberry leave, molokihya stem, and banana peel) were collected from local market in Egypt. To remove unwanted dust particles they were washed with distilled water and dried at 50 °C for 24h in an oven. The dried materials were ground in an electric grinder (separated by1cm sieve) and packed in air-tight containers for use in the protease production.
2.2. Bacterial strain and culture conditions
The strain Bacillus megaterium 314 used in this work was obtained from the Culture Collection of the National Research Centre, Egypt. It was maintained at 4 °C and cultured in nutrient agar for 24 h at 37 °C.
2.3. Enzyme production
In the preliminary experiments, three media (M1, M2 and M3) were screened for protease production. M1 [20] was composed as follows (g/l): glucose, 5; peptone, 5; yeast extract, 1; K2HPO4, 1; KH2PO4, 1 and MgSO4.7H2O, 0.5. M2 [21] contained beef, 5; peptone, 10; glucose, 2 and NaCl, 5. M3 [22] was composed of (g/l): peptone, 10; beef extract, 10; yeast extract, 5; glucose, 20; tween-80, 1; C6H17N3O7, 2; C2H3NaO2, 5; MgSO4, 0.1 and K2HPO4, 2. The media were adjusted to pH 7.0 and sterilized in autoclave at 121 °C for 15min. Each flask containing 50 ml of sterile production media was inoculated with 2.0 ml of cell suspension of 24h old slant (OD600∼0.4), followed by incubation at 35 °C on an orbital shaker at 150 rpm for 48h. At the end of incubation period, the culture medium was centrifuged at 10,000× g and 4 °C for 20 min and the supernatant was used to enzyme assay.
2.4. Assay of protease
Protease activity was determined by the method of [23]. The enzyme solution (0.25 ml) was mixed with 0.25 ml of 1.5 % (w/v) casein dissolved in 0.2 M phosphate buffer (pH 6.0) and incubated at 40 °C for 30 min. Then the reaction was stopped by adding 0.5 ml of cold trichloroacetic acid (10 %), and non-hydrolyzed proteins were removed by centrifugation (10,000× g for 20 min at 4 °C). The amount of tyrosine released was determined using the spectroscopic method at 660 nm [24]. All the experiments were carried in triplicate and the results expressed as mean values ±SD (standard deviation).
2.5. Quantitative estimation of protein
The quantitative estimation of protein was conducted by the method Lowry's [24] using bovine serum albumin as the standard.
2.6. Optimization of cultural conditions for protease production
Based on one variable at a time (OVAT) approach different cultural parameters as carbon and nitrogen sources and their concentrations, incubation temperature, initial pH and incubation period were optimized for maximum protease production. Effect of different C-sources on protease production was checked by adding 0.25g of each synthetic C-sources (glucose, sucrose, lactose and starch) to 50ml of the production media. In addition, various agricultural and industrial residues (0.25g/50ml) were examined as natural C-sources (lemon skin, corn cob, orange peel, pomegranate peel, pea peel, strawberry leave, molokihya stem, and banana peel). The optimized C-source was tested again for its optimum concentration in a range of 0.125–1.25g/50ml. Different organic and inorganic N-sources (0.5g/50ml) were checked for maximum protease production including peptone, yeast extract, beef extract, wheat bran, meat, baker's yeast, soy bean, NH4Cl, urea and Na2NO3. The optimized N-source was tested again for its optimum concentration in a range of 0.1–1.5g/50ml. Optimum temperature for protease production was checked in the range of 30 °C–50 °C. The initial pH was checked in the range of 6.0–8.0. Protease production was determined at various incubation periods (24, 48 and 72h).
2.7. Ammonium sulphate precipitation
Cell free supernatant was fractionated using different saturation of ammonium sulphate between 25 and 75 %. After each addition, the enzyme solution was stirred for 2 h at 4 °C. The precipitated protein was collected by centrifugation and resuspended in minimal volume of distilled H2O to get the concentrated enzyme suspension. The precipitate was harvested by centrifugation than dissolved in H2O and dialysed against distilled H2O overnight at 4 °C.
2.8. Glass and glass ceramic preparation
The design of glass ceramic composition was based on crystallization of 80% NaMg3AlSi3O10F2 + 20% Ca5 (PO4)3F. Highly pure chemical substances as Na2CO3, MgCO3, CaCO3, CaF2, quartz; Al2O3, NH4H2PO4 and MgF2 were thoroughly mixed to get ∼100 g batch. After mixing the chemicals well, the batches were melted in a platinum crucible in an electric furnace at 1250–1300 °C for 1h with occasional swirling every 15 min to ensure homogenization. The melts were quenched into deionized water to ascertain its glassy nature. Heat treatment was carried out at 600°C/10h + 750°C/2h to convert the glass onto glass ceramic. Crushing both glass and its counterpart glass ceramic in agate mortar was done to get different grain size (particle size 0.71–1.0 mm).
2.9. Enzyme immobilization
2.9.1. Physical adsorption
Specific weight (0.1 g) of glass (G) and glass-ceramic (G-C) with different particle size (≤0.35, 0.35–0.7, 0.7–1.0 and ≥1.0 mm) were suspended in 0.5 ml of phosphate buffer (0.2 M and pH 6.0) containing enzyme (1247 U) at 4 °C for 24h [25]. The unbound enzyme was removed by washing with the same buffer. The immobilization yield (IY %) and the residual activity (RA %) were calculated as follows:
| IY (%) = I / (A-B) × 100 | (1) |
| RA (%) = (Af/ Ai) × 100 | (2) |
where: I (total activity of immobilized enzyme), A (total activity added for immobilization), B (unbounded enzyme), Af (observed activity), and Ai (initial activity).
2.9.2. Covalent binding
Glass (G) and glass-ceramic (G-C) with different particle size (from ≤0.35 to ≥1.0 mm) were activated by glutaraldehyde (GA). Activation was carried out by treating 0.1 g of particles with 0.5 ml of 1% GA for 24 h at 4 °C to incorporate the new functionality aldehyde group. Subsequently, the activated particles were washed several times with buffer to remove the excess of GA and were suspended in 0.5 ml of 0.2 M phosphate buffer pH 6.0 (1247 U enzyme) at 4 °C for 24h [25].
2.10. Support characterization
2.10.1. X-ray diffraction (XRD)
Glass-ceramics were subjected to powder X-ray diffraction using Ni-filled Cu-Kα radiation for determination of the types and contents of the crystalline phases precipitated in them. X-ray diffraction (XRD) was performed using Bruker D8, an advanced instrument. The reference data for the interpretation of the XRD patterns was obtained from ASTM X-ray diffraction card files.
2.10.2. Scanning electron microscope (SEM)
The morphology of glass-ceramic samples were examined with a scanning electron microscope, SEM model Philips XL30 attached with EDX unit, using an accelerating voltage of 30 K.V., magnification 10x up to 400,000x, and resolution for wavelength (3.5 nm).
2.11. Comparative studies of free and immobilized enzyme
2.11.1. Effect of reaction time on protease activity
The enzyme assay (free and immobilized) was conducted at 40 °C and pH 6.0 for different time intervals from 10 min to 60 min.
2.11.2. Effect of temperature on protease activity
Effect of temperature on free and nanoparticle protease was studied by measuring the activity at different temperature (30–80 °C). The activation energy (Ea) was calculated from the slope of the Arrhenius plot according to the following equation:
| Slope = − Ea/2.303R | (3) |
where: R is the gas constant (8.314 kJ/mol) and T is the absolute temperature (Kelvin). Temperature coefficient value (Q10), the rate of an enzymatic catalysis reaction changes for every 10 °C rise in temperature, was calculated by the equation of [26]:
| ln Q10= (Ea × 10)/RT2 | (4) |
where Ea is the activation energy of the enzyme (J/mol).
2.11.3. Effect of pH on protease activity
Optimal pH for free and nanoparticle enzyme activity was determined by measuring the activity at different pH values ranging from 4.5 to 8.0 in 0.2 M of the phosphate buffer. The relative activities were determined [27].
2.11.4. Determination of kinetic parameters
The Michaelis constant (Km) and the maximum rate (Vmax) of the enzyme were calculated according to [28] at optimum assay conditions and different amounts of substrate. Also, the ratio Vmax/Km (catalytic efficiency) of an enzyme–substrate pair was calculated.
2.11.5. Thermal stability and thermodynamic properties
The thermal inactivation of free and nanoparticle protease was evaluated by incubation at temperatures 40, 50, 60 and 70 °C for defined time intervals (15, 30, 45 and 60 min) in absence of substrate [27]. Then, the samples were cooled to stop heat inactivation and the residual activities were determined. The thermal and thermodynamic constants were calculated as reported by [25, 29]. The deactivation rate constant (kd) was calculated when log of residual activity (%) was plotted as a function of time (min) at the temperatures used for inactivation as following:
| Slope = –kd | (5) |
Decimal reduction time (D-value) at a specific temperature was defined as the time needed to lose 90 % of the initial enzyme activity. The D-value and the half-lives (t1/2) of the enzyme were determined as shown in Eqs. (6) and (7):
| D-value = ln10/kd | (6) |
| t1/2 = ln2/kd | (7) |
The activation energy for denaturation (Ed) was determined from Arrhenius plot of (ln kd) versus (1/T) in Kelvin (K) using the following equation:
| Slope = −Ed/R | (8) |
The change in enthalpy (ΔH°), free energy (ΔG°) and entropy (ΔS°) for thermal denaturation of enzyme were determined as pointed by [10, 29] as follows:
| ΔH° = Ed − RT | (9) |
| ΔG° = −RT ln (Kd. h/Kb.T) | (10) |
| ΔS°= (ΔH° − ΔG°)/T | (11) |
where: Ed is the activation energy for denaturation (KJ/mol)), R is the gas constant (8.314 J/mol/K), T is the absolute temperature (K), kd is the deactivation rate constant (/min), h is the Planck constant (11.04×10−36 J min) and Kb is the Boltzman constant (1.38×10−23 J/K).
2.11.6. Effect of some additives and inhibitors on enzyme activity
The enzyme (free and immobilized) was pre-incubated at 30 °C for 1 h with 10 mM of various additives and inhibitors as sodium dodecyl sulfate (SDS), phenylmethylsulfonyl fluoride (PMSF),-p-Chloromercuribenzoic acid (pCMB), Iodoacetic acid (IAA), Ethylenediaminetetraacetic acid (EDTA), Dimethyl sulfoxide (DMSO) and some metal ions with different concentrations [27]. The reaction without additive was taken as 100% activity (control).
2.12. Reusability
The reusability (operational stability) of nanoparticle protease was assessed by repeated hydrolysis reaction of casein over several cycles. At the end of each cycle, the immobilized enzyme was separated by filtration, was washed with the same buffer several times and was used for new run. The activity of the enzyme in the initial cycle was considered to be 100% and the activity in each cycle was defined as the residual activity (RA %) and was calculated as follows:
| RA (%) = (Af/Ai) × 100 | (12) |
where: Af is the final activity and Ai is the initial activity.
3. Results and discussion
3.1. Screening for suitable production media
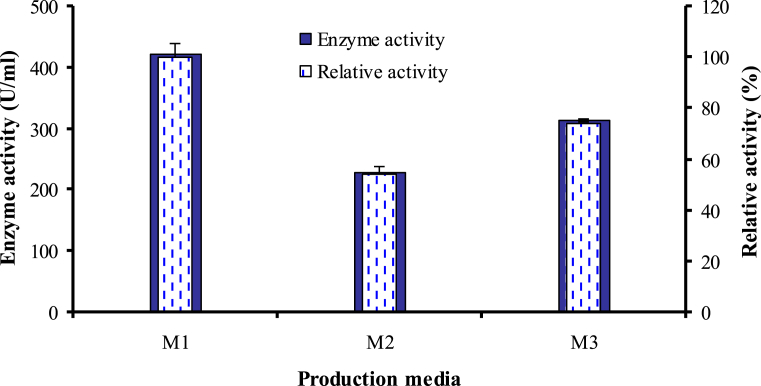
Growth nutrients and conditions promote high yields of microbial enzymes. It was shown that Bacillus megaterium 314 produced caseinase enzyme by growing on all tested media (M1, M2 and M3) with different levels. As illustrated in Fig. 1, caseinase activity ranged from 229.58 to 422.99 (U/ml) refers to the appropriateness of the three media compositions for caseinase production. The highest production obtained using M1 which was 1.84 and 1.34 times higher than M2 and M3. Consequently, M1 was used as basal production medium and was optimized in the following experiments.
Fig. 1.
Production of caseinase by Bacillus megaterium 314 on different media. The reaction was carried out using 1.5 % casein dissolved in 0.2 M phosphate buffer (pH 6.0) for 30 min at 40 °C in a shaking water.
3.2. Optimization of cultural conditions for protease production
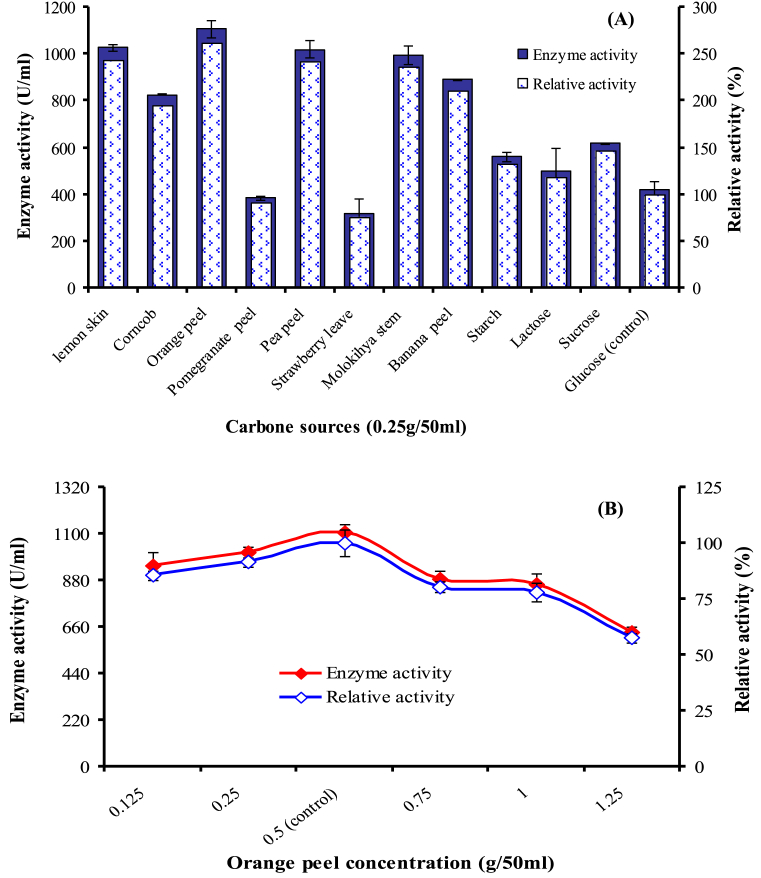
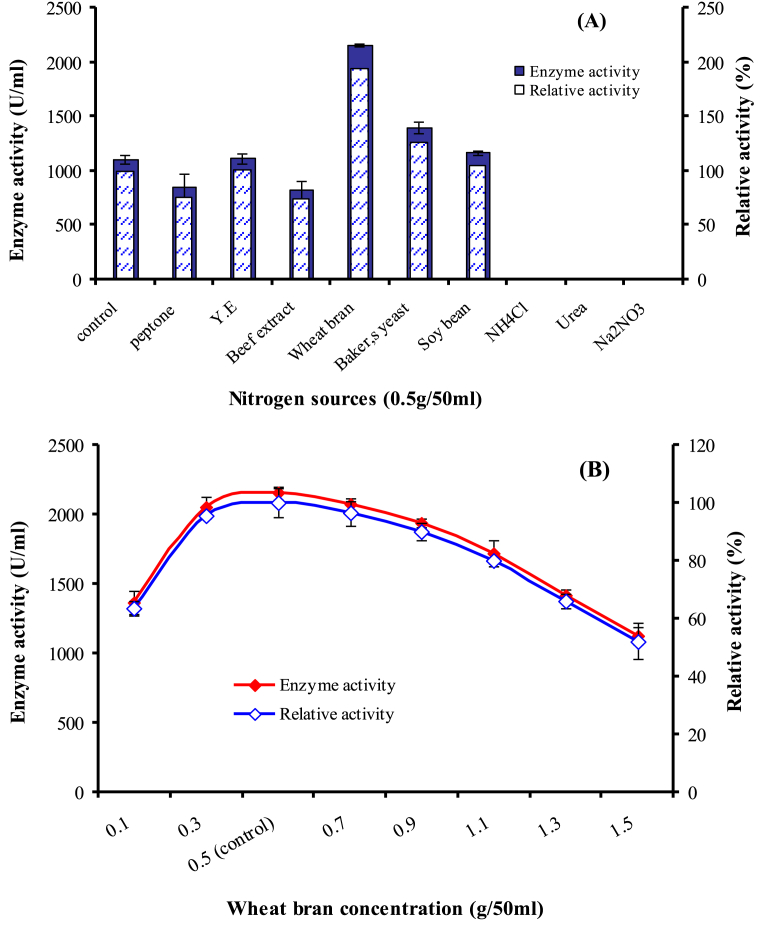
The culture medium represents about 40% of the cost of a fermentation process and plays a significant role in the production of enzymes. However, use of pure carbon and nitrogen sources are not economical, so we can replace them with economically agricultural and industrial wastes [30]. Carbon source plays pivotal role in the enzyme production and different bacteria utilized different C-source for their growth and metabolism [23]. In current study, among all tested C-sources (Fig. 2A) orange peel (OP) was found to be highly influencing the enzyme production by 2.62 times. Also, lemon skin (LS) and pea peel (PP) enhanced enzyme production by 2.42 and 2.41 times, respectively. Results showed that agricultural wastes are suitable C-source for substitution of costly material for the protease production. On the contrary, the synthetic C-sources exerted lower effects on the production. The difference in enzyme production level by different agricultural wastes is typically determined by many factors (as the presence of activator or inhibitor, diffusion of catabolite, surface area, content and sugar composition [31]. Chemical composition of OP (Citrus sinensis) indicates that it contains (%) fiber (7.8), fat (1.9), pectin (7.0), lignin (6.4), total sugar (14.1), protein (5.1), ash (7.4) and moisture (40.7) [32]. In addition, it is cheap and abundantly available in Egypt [33]. Abou El-Hassayeb and Abdel Aziz [8] reported that the addition of other C-sources except starch in the production medium led to the remarkable decreases in protease production by B. subtilis. Concentration of OP in the production medium was also optimized by conducting experiments with different concentrations (Fig. 2B). Maximum protease production was found to be 1106.35 U/ml at 0.5g/50ml. Above or under 0.5g/50ml, protease production decreased to 57.5 and 85.6% at 1.25 and 0.125/50ml, respectively. Moreover, lower concentrations were more suitable than higher concentrations. The present investigation is in contrary to [32, 34] who reported that high concentrations of carbon stimulate enzyme synthesis. Nitrogen sources are biomass sources for microorganisms that play a significant role in the growth and enzyme production [24]. In the current study, substitution of N-source in the medium with wheat bran (WB) and Baker,s yeast (BY) as organic N-sources enhanced the enzyme production by 1.9 and 1.3 times (Fig. 3A). On the other side, all tested inorganic N-sources inhibited protease production. Similarly, Mothe and Sultanpuram [35] found that NH4Cl as inorganic N-source inhibited the enzyme production. Castro et al. [36] reported that the proteases showed the highest activity when soybean meal was used followed by WB. Wheat bran the outer ∼15% of the wheat seed is a good source of nitrogen and carbon due to the presence of protein content (15.5%), carbohydrates (64.5%), fats and other nutrients (4.2%) [10]. There are differences in enzyme production when different levels of WB are added to the media (Fig. 3B). The highest protease production (2151.9 U/ml) was obtained at WB concentration 0.5g/50ml. At higher concentration, enzyme production decreased might be due to the hinder oxygen dissolving in presence of excess of WB and thus limiting the growth of bacteria leading to decrease in enzyme production [37]. On the other hand, at concentration 0.1g WB/50ml the relative activity was about 63.5%, possibly due to reduction in viable cell density and consumption of nutrients. Optimized production condition as temperature, initial pH and incubation period did not enhance protease production. The highest production was obtained at temperature 35 °C and pH 7.0 after 48h (data not shown).
Fig. 2.
Effect of different C-sources (A) and effect of different concentration of OP on caseinase production (B). The production was carried out at pH 7.0 and 35 °C on an orbital shaker at 150 rpm for 48h.
Fig. 3.
Effect of different N-sources (A) and effect of different concentration of WB on caseinase production (B). The production was carried out at pH 7.0 and 35 °C on an orbital shaker at 150 rpm for 48h.
3.3. Enzyme immobilization
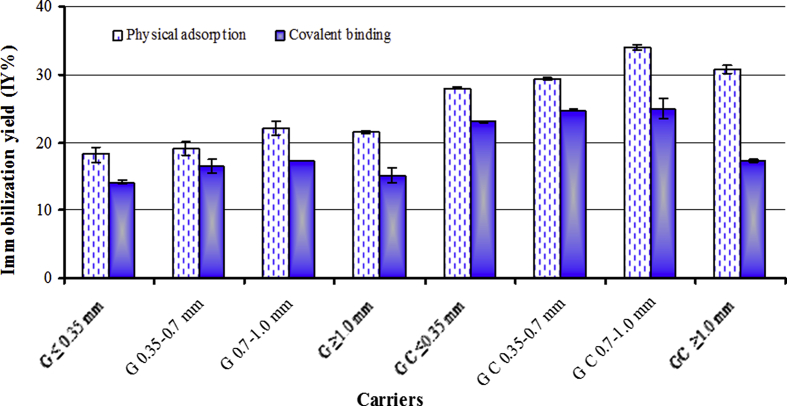
Protease enzyme was immobilized on G and G-C as inorganic supports with different particle sizes (from ≤0.35 to ≥1.0 mm) by covalent binding using glutaraldehyde and physical adsorption methods (Table 1). Usually, inorganic supports are little reactive than the organic. But they are also highly stable consequently, their flowing characteristics and diffusion are good. Among the benefits of inorganic carrier are its stability to thermal treatment, convenient mechanical quality, resistance to organic reagents and microbial contamination attacks [38]. As seen in Fig. 4, G-C is better than G, bigger particle sizes are better than smaller one and physical adsorption is more suitable than covalent binding method. The highest immobilization yield (IY %) was obtained using G-C with particle size 0.7–1.0 mm by physical adsorption (34%) followed by covalent binding (25%). Microstructure in the G-C plays a very significant role in material properties, due to presence of big amount of crystalline state which increase the surface area. Moreover, in the G-C the corners of the crystals and the random edges permit more attachment to the enzymes than the smooth and non-crystalline surface of the amorphous or glassy phase. Among advantage of physical adsorption method as a general immobilization method is that (1) usually no reagents and minimum steps are required, (2) cheap method, (3) easily carried out, and (4) tends to be less destroying to the enzyme than by chemical. Physical adsorption via hydrophobic interactions, electrostatic interactions, Van der Waal's forces, specific/affinity, and hydrogen bonding is the simplest immobilization method [14]. It is based on the functional groups on the surfaces of both enzymes (-NH2, –COOH, –SH and –OH) and carriers [39]. So, it bears the greatest similarity to the situation found in biological membrane in vivo and has been used to model such systems [40]. The free C=O group in activated particles (with GA) reacted with the NH2 group found in the enzyme forming C=N– bond as reported by [41]. Lower IY % for covalent binding of enzyme to the carriers might be due to enzyme denaturation by coupling agent as GA [39].
Table 1.
Immobiization of Bacillus megaterium 314 caseinase by physical adsorption and covalent binding on mica glass and glass-ceramic.
| Carrier | Enzyme added (U/g carrier) [A] |
Unbound enzyme (U/g) [B] |
Immobilized enzyme (U/g) [I] |
Immobilization yield IY (%) = (I/A-B) X100 |
|||
|---|---|---|---|---|---|---|---|
| PA and CB | PA | CB | PA | CB | PA | CB | |
| G ≤ 0.35 | 5 | 2.659 | 2.548 | 0.419 | 0.353 | 18.2 | 14.13 |
| G ≤ 0.35–0.71 | 5 | 2.584 | 1.999 | 0.459 | 0.492 | 19.16 | 16.40 |
| G ≤ 0.71–1.0 | 5 | 2.947 | 2.573 | 0.466 | 0.415 | 22.18 | 17.30 |
| G ≥ 1.0 | 5 | 2.691 | 2.410 | 0.493 | 0.394 | 21.43 | 15.15 |
| G-C ≤ 0.35 | 5 | 2.571 | 2.446 | 0.672 | 0.599 | 28.00 | 23.04 |
| G-C ≤ 0.35–0.71 | 5 | 2.697 | 2.605 | 0.674 | 0.595 | 29.32 | 24.79 |
| G-C ≤ 0.71–1.0 | 5 | 2.859 | 2.781 | 0.713 | 0.550 | 33.97 | 25.02 |
| G-C ≥ 1.0 | 5 | 2.719 | 2.480 | 0.706 | 0.431 | 30.68 | 17.26 |
Where: PA is physical adsorption, CB is covalent binding, G is glass and G-C is glass-ceramic.
Fig. 4.
Immobilization of caseinase enzyme by physical adsorption and covalent binding on glass (G) and glass-ceramic (G–C) particles.
3.4. Carrier characterization
3.4.1. X-ray diffraction (XRD)
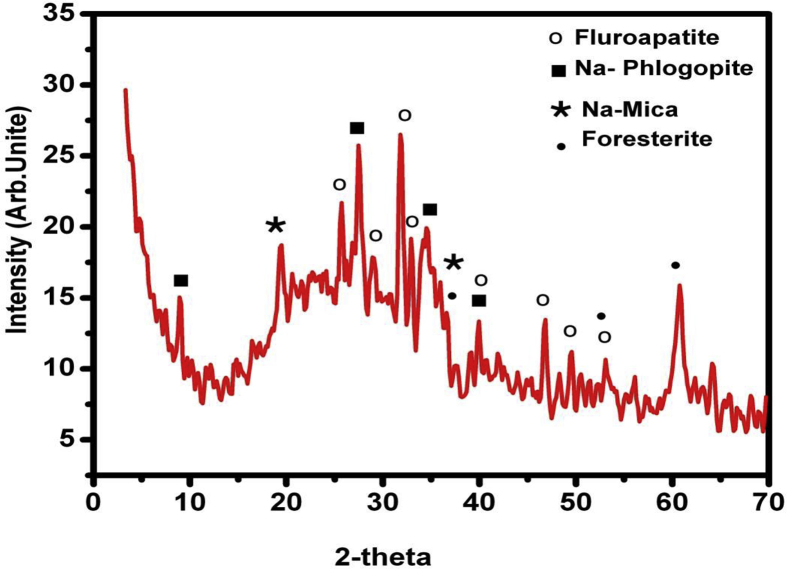
XRD of glass ceramic heat treated at 600°C/10h + 750°C/2h is illustrated in Fig. 5. Bragg's diffraction peaks are well matched with JCPDS card no. 25–0842, 46–0740, 85–1346 and 87–2462 for Na-phlogopite (NaMg3AlSi3O10F2), Na-mica (NaAl3Si3O11), foresterite (Mg2SiO4) beside fluorapatite [Ca5(PO4)3F] in the same order. Crystallite sizes (the average) were calculated from the most intense XRD peaks using Debye-Scherrer equation as follows:
| D = kλ/B cosΘ |
where: D is the particle size, k is the constant, λ for Cu is 1.54 Å, B is the full width at half maximum and 2Θ = 4°. The sizes of crystallite are ∼30 nm (in nano-range) indicating that it is nano particles.
Fig. 5.
XRD of mica-fluroapatite glass-ceramic.
3.4.2. Scanning electron microscope (SEM)
SEM for mica G-C before and after immobilization is depicted in Fig. 6. Homogeneous, uniform bulk crystallization of nanosize particles in compact structure is the main feature of G-C before enzyme immobilization (Fig. 6A). Increasing the magnification revealed crystallization of nanoparicles size <90 nm (Fig. 6B). This texture was changed after enzyme immobilization, where covering the G-C crystals with enzymes is clear (Fig. 6C). This result is revealing the high efficiency of enzyme adsorption process. In this layered G-C, it is considered that the enzyme was retained between mica layers.
Fig. 6.
SEM of mica-fluroapatite glass-ceramic (A and B) before enzyme immobilization at different magnifications and (C) after enzyme immobilization.
3.5. Studies of free and nanoparticles G-C caseinase
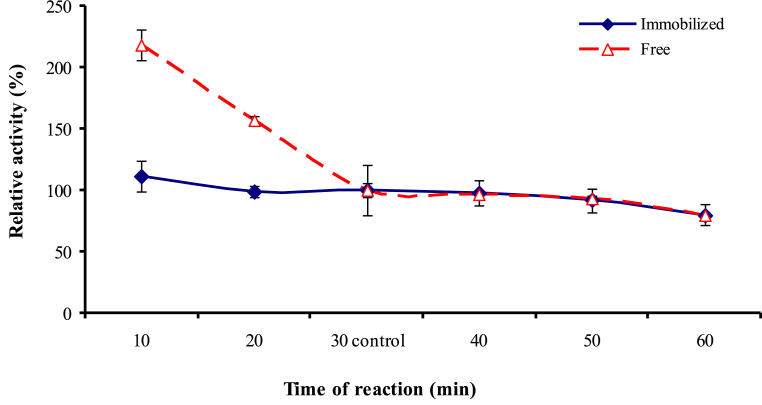
3.5.1. Effect of reaction time on caseinase activity
The results in Fig. 7 showed that maximal activity for free and immobilized caseinase was the same (10 min). This might be due to the adsorption of enzyme molecule on the carrier surface and consequently, the substrate did not need more time to diffuse into and to bind with the active sites of the immobilized caseinase as in free one. Similar observation was obtained by other enzymes by Gómez et al. [42]. In addition, increasing the time reduced the activity of the free caseinase, however, the activity of the immobilized enzyme nearly did not change.
Fig. 7.
Effect of reaction time on caseinase enzyme activity.
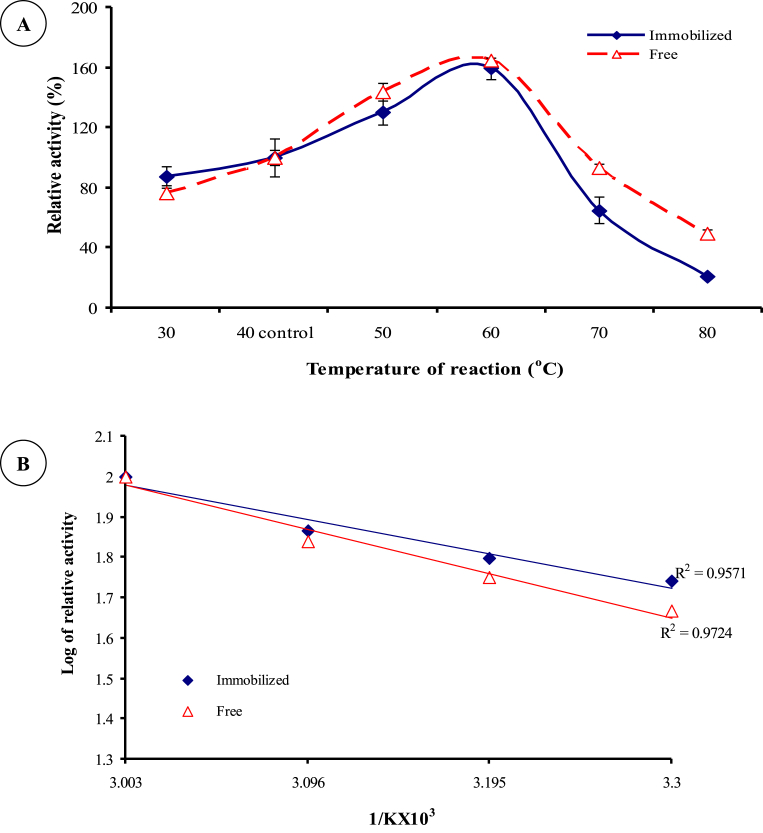
3.5.2. Effect of temperature on protease activity
The optimum temperature for the caseinase adsorbed on G-C was 60 °C, which was also the same as the free caseinase (Fig. 8A). Similar result was observed by [14] in immobilization of protease on polysulfone membrane by physical adsorption. Arrhenius plots of B. megaterium 314 caseinase (Fig. 8B) indicated that the Ea value for immobilized and free caseinase were 16.29 and 20.96 kJ/mol, respectively. These results proved that, adsorption of protease on G-C decreased the Ea needed to make the activated enzyme-substrate complex by about 23%, indicating that its catalytic efficiency was improved. Decreasing the Ea of protease by immobilization was reported by Abdel-Naby et al. [29]. The Ea gave rise to another important kinetic parameter Q10 (temperature coefficient). The Q10 for enzymes generally ranges from 1 to 2 [26]. Deviation from Q10 value indicates that reactions are not temperature dependent (other assay factors participate than temperature) in controlling the reaction rate [43]. As shown in Table 2, the Q10 value for both free and immobilized caseinase ranged from 1.18 to 1.27 at temperature 50, 60 and 70 °C. A similar value (Q10 = 1.0) was recorded for both the native and conjugated alkaline protease [29]. A lower Q10 for the caseinase indicated that the temperature change would not have considerable effect on the tertiary structure of the enzyme protein at up to 70 °C. This result is consistent with that reported by Ghosh et al. [26].
Fig. 8.
Effect of reaction temperature on caseinase activity (A) and Arrhenius plots for the free and G-C caseinase (B).
Table 2.
Kinetic and thermal properties of free and G-C caseinase.
| Property | Free enzyme | Immobilized enzyme |
|---|---|---|
| Optimum reaction temperature (°C) | 60 | 60 |
| Activation energy Ea (Kcal/mol) | 20.96 | 16.29 |
| Deactivation rate constant (Kd/min) | ||
| 60 °C | 8.41 × 10−3 | 5.77 × 10−3 |
| 65 °C | 12.33 × 10−3 | 9.21 × 10−3 |
| 70 °C | 18.65 × 10−3 | 13.10 × 10−3 |
| Half life (t½ min) | ||
| 60 °C | 82.42 | 120.13 |
| 65 °C | 56.22 | 75.26 |
| 70 °C | 37.17 | 52.91 |
| D-value (h) | ||
| 60 °C | 4.56 | 6.65 |
| 65 °C | 3.11 | 4.17 |
| 70 °C | 2.05 | 2.93 |
| Ed (KJ/mol) | 9.0503 | 9.3175 |
| Q10 value at | ||
| 50 °C | 1.27 | 1.21 |
| 60 °C | 1.25 | 1.19 |
| 70 °C | 1.24 | 1.18 |
| V max (U/mg protein) | 476.19 | 1176.47 |
| Km (mg substrate/ml) | 0.36 | 0.19 |
| Vmax/Km (U/mg protein/mg casein/ml) | 1322.75 | 6191.95 |
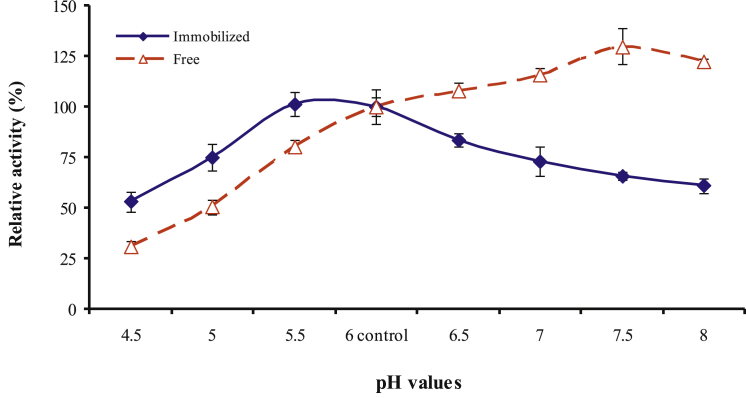
3.5.3. Effect of pH on enzyme activity
As illustrated in Fig. 9, the free protease has an optimum pH of 7.5 whereas that of the immobilized preparation was shifted 2.0 units to more acidic pH value (5.5). Comparatively, at lower pH values the nanoparticle caseinase was more tolerant to the pH changes than the free enzyme. In contrast Sahin, et al. [14] found that immobilization of protease shifted its optimum pH to more alkaline pH value.
Fig. 9.
Effect of reaction pH on caseinase enzyme activity.
3.5.4. Kinetics of casein hydrolysis
Lineweaver–Burk plot was used to present the relation between casein concentration and rate of reaction (data not shown). The results in Table 2 showed that the calculated Km value of the free caseinase (0.36 mg/ml) was higher than that of the immobilized caseinase (0.19 mg/ml). On the other hand, the Vmax of enzyme was increased by 2.5-fold upon immobilization, indicating that adsorption process improves the hydrolysis reaction in aqueous media. Similar observation was obtained by Ibrahim et al. [44]. These effects might be due to the changes of configuration of enzyme and the molecular crowding caused by adsorption [45]. The Km value gives an idea about the affinity of an enzyme to its substrate. Reduction in Km value indicates that the affinity was increased and consequently, the activity of enzyme. The results showed that the affinity of the caseinase to casein was increased 1.9-fold after immobilization. This might be due to that enzyme molecule widened over the surface of G-C particle with a good orientation resulting more available active sites and higher substrate affinity [25]. The catalytic efficiency ratio (Vmax/Km) was taken as the criterion to evaluate substrates specificity [46]. As shown from results, Vmax/Km ratio of G-C caseinase was higher than that of free enzyme by 4.7- time. This may be due to more efficient conformation of immobilized protease within the nanoscale compared to free enzyme [47]. In contrast to this, the ratio Vmax/Km which is measurement of an enzyme–substrate pair for laccase was decreased about 482 times by immobilization [48].
3.5.5. Thermal stability
The main objective of this test was the detection of the rates of thermal inactivation of the free and immobilized protease (Table 3). The results showed positive effect for the immobilization process on the thermal stability of caseinase. According to the results, nanoparticle caseinase is more stable to thermal inactivation than free caseinase. After 45 min at 60 °C the RA (%) for free and G-C caseinase was 38.2 and 56.4%, respectively. The thermal inactivation process of both free and adsorbed enzyme on G-C matches to complies with a simple first order reaction. As shown in Table 2 the deactivation constant rate (kd) value of the nanoparticle caseinase was 1.4-time lower than that of free caseinase at 70 °C. Half-life (t1/2) is the time needed to loss 50% of the starting enzyme activity at a certain temperature. As well known, in many industrial applications t1/2 is an important economic parameter, because the higher of its value, the higher enzyme thermostability. At 60 and 70 °C, the calculated t1/2 of the adsorbed enzyme was 1.5 and 1.4-fold higher than that of the free one. t1/2 values of both enzyme forms decrease with rise in temperature due to enzyme denatured. In addition, the decimal reduction time (D-value) for the G-C caseinase showed higher resistance against thermal inactivation compared with the free form. The results showed that immobilization process improved the thermal stability of enzyme (need more time to denature). The energy needed for the enzyme to be irreversible denatured is the activation energy of denaturation (Ed), so higher Ed values are a powerful indication of higher enzyme thermostability [14]. The Ed was obtained by linear adjustment for the Arrhenius plot equation. The Ed of the nanoparticle caseinase was 9.32 kJ/mol which was 1.1- fold higher than that of the free enzyme, indicating that the immobilization process increased caseinase thermostability. These results are consistent with those reported by Ferreira et al. [12] and Wehaidy et al. [43].
Table 3.
Thermal stability of free and G-C caseinase.
| Temperature (°C) Time (min) |
Residual activity (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 40 |
50 |
60 |
70 |
|||||
| F | I | F | I | F | I | F | I | |
| 15 | 100 ± 2.79 | 100 ± 2.36 | 100 ± 2.57 | 100 ± 1.46 | 100 ± 1.24 | 88.48 ± 1.18 | 5.66 ± 0.74 | 36.40 ± 0.71 |
| 30 | 100 ± 3.92 | 100 ± 2.55 | 100 ± 1.30 | 100 ± 2.10 | 59.45 ± 0.55 | 77.56 ± 1.42 | 4.98 ± 0.59 | 33.40 ± 0.80 |
| 45 | 100 ± 2.69 | 100 ± 1.93 | 98.32 ± 1.1 | 100 ± 2.33 | 38.22 ± 1.54 | 56.42 ± 2.06 | 4.66 ± 0.59 | 22.34 ± 0.87 |
| 60 | 100 ± 2.28 | 100 ± 2.388 | 97.06 ± 2.06 | 100 ± 1.64 | 33.66 ± 1.8 | 46.22 ± 1.26 | 4.40 ± 0.58 | 13.24 ± 0.62 |
3.5.6. Thermodynamics of substrate hydrolysis
The thermodynamic parameters of casein hydrolysis by G-C caseinase showed clear changes (Table 4). The activation enthalpy of denaturation (ΔH°) is a thermodynamic parameter which expresses the total energy amount needed to denature the enzyme. So, positive and large ΔH° values are often associated with high enzyme thermostability [10]. As seen from results, ΔH° for the G-C caseinase was higher than the value of the free enzyme by 1.1-fold at 70 °C. This can be due to the changes of the conformation of the adsorbed enzyme. Similarly, the value of entropy of activation (ΔS°) of the G-C caseinase was slightly higher than that of the free form by about 1.1-fold at 60 °C. Negative values of ΔS° of caseinase enzyme mean that the process under consideration was not spontaneous at all tested temperatures [10]. In addition, ΔG° is significant thermodynamic parameter that combines both ΔH° and ΔS°. It is a precise tool for predicting and assesses the enzymes stability. Negative or smaller value of ΔG° is associated with a more spontaneous process and the enzyme becomes less stable. Negative or smaller value of ΔG° is correlated to more spontaneous process and the enzyme stability becomes less and more easily denaturation [49]. On the other side, an increase in ΔG° detects an increase in resistance to denaturation and increasing enzyme thermostability [10]. The results showed that ΔG° for immobilized enzyme was 1.0-fold higher than that of free enzyme. Ferreira et al. [14] reported that the protein stability is directly related to ΔG° values, where high and positives ΔG° values indicate the non-spontaneity of the heat denaturation reaction of enzyme (higher thermal stability).
Table 4.
Thermodynamic parameters for thermal inactivation of free and G-C caseinase.
| Temperature |
ΔH° (kJ/mol) |
ΔS° (J/mol/K) |
ΔG° (kJ/mol) |
||||
|---|---|---|---|---|---|---|---|
| (°C) | (°K) | F | I | F | I | F | I |
| 60 | 323 | 6.36 | 6.63 | -0.26 | -0.27 | 92.23 | 93.25 |
| 65 | 338 | 6.24 | 6.51 | -0.26 | -0.27 | 95.48 | 96.30 |
| 70 | 343 | 6.19 | 6.47 | -0.26 | -0.26 | 95.76 | 96.77 |
ΔH° = variations in enthalpy; ΔS° = variations in Entropy; ΔG° = variations in free energy.
3.5.7. Effect of various additives and inhibitors
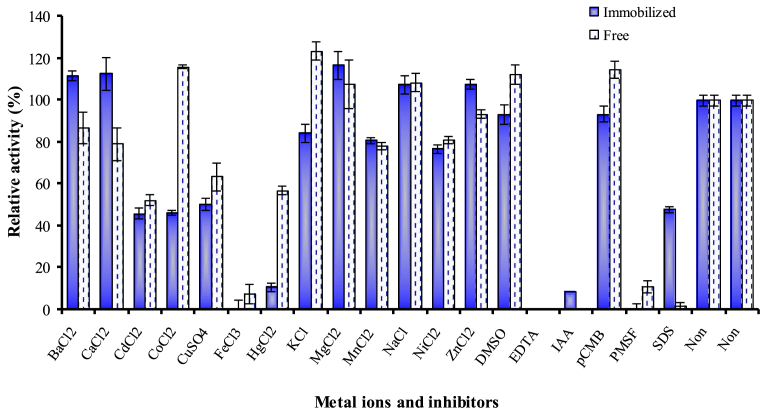
The effects of some additives and inhibitors on caseinase enzyme activity free and G-C enzyme were illustrated in Fig. 10. The free and nanoparticle caseinase were significantly stimulated by Mg+2 and Na+ (108 and 117%) and (108–107.5%), respectively. Both free and nanoparticle enzyme were significantly inactivated by EDTA, IAA and PMSF. EDTA (metallo protease inhibitor) completely inhibited both nanoparticle and free enzyme indicating that it is metallo protease. These findings are in agreement with that reported by Ahmetoglu et al. [50] on the sensitivity of the proteases (metal ion dependent) to some chelating agents such as EDTA.
Fig. 10.
Effect of various additives and inhibitors.
3.6. Reusability of immobilized enzyme
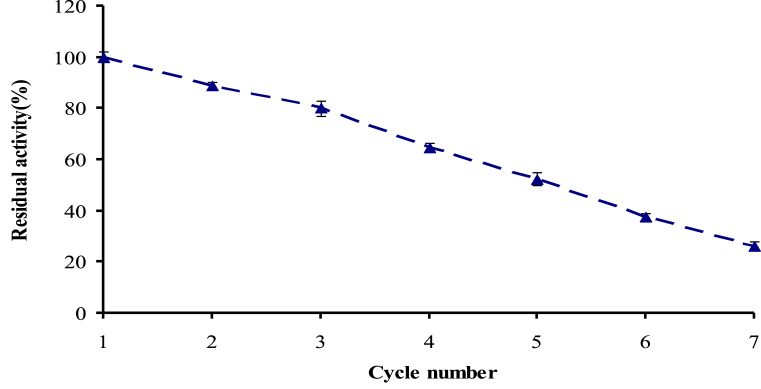
The reusability of G-C caseinase was tested by measuring residual activity (RA%) repeatedly. The G-C caseinase retained 52.3% of its initial activity after 5 times (Fig. 11). This lose in activity might be related to weakened bonds formed between the G-C carrier and the enzyme which simply causes the enzyme to leak [14]. In addition, the loss of a few particles and denaturation of enzyme resulting from continuous use reduce the residual activity [51]. Reusability of the immobilized enzyme is an important factor in reducing the cost of enzyme and evaluating its suitability for industrial processing.
Fig. 11.
Reusability of immobilized enzyme.
4. Conclusions
To meet the industrial demand of proteases, the present study was conducted to enhance the production of B. megaterium 314 proteases (caseinase) by optimizing the cultivation parameters. Enzyme production was enhanced by 5.1-folds after optimization process. Enzyme immobilization is a useful tool to meet cost targets and has many technological advantages. Adsorption of caseinase on G-C particles reduced the Ea needed to form enzyme–substrate complex by 23%. As well as enhancing its catalytic property in aqueous media. Thermodynamic parameters obtained from stability studies (thermal) indicated that the adsorption of caseinase on G-C particles enhances its thermal stability, and consequently its t1/2 and D-value, over the whole of the evaluated temperature. Moreover, kd value of the G-C caseinase was lower than that of free caseinase up to 70 °C. The Ed of the G-C caseinase was 1.0- fold higher than that of the free enzyme meaning that the immobilization process increases the heat resistance of enzyme. Thermodynamic parameters of casein hydrolysis (ΔH°, ΔS°, and ΔG°) by the G-C caseinase showed change which is associated with high enzyme heat resistance. Immobilization process increased the affinity to substrate, improved the hydrolysis reaction and catalytic efficiency. There was a significant improvement in Vmax and Km. Moreover, catalytic efficiency (Vmax/Km) values for the G-C caseinase was 4.7- times higher than that of free one, indicating the efficiency and effectiveness of applied carrier and immobilization approach. G-C caseinase retained 64.7% of its activity after 4 cycles which an important factor for industrial processing. Based on the results, physical adsorption of caseinase on G-C particles was excellent method in the production of an active biocatalyst for applications.
Declarations
Author contribution statement
Samia Ahmed: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Shireen Saleh, Amira Fayad: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Salwa Abdel-Hameed: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Kumar N.S., Sreeja D.P.S., Nair A.S. A review on microbial proteases. Int. J. Adv. Res. 2016;4:2048–2053. [Google Scholar]
- 2.Singh R., Mittal A., Kumar M., Mehta P.K. Microbial proteases in commercial applications. J. of Pharma. Chem. and Biolog Sci. 2016;4:365–374. [Google Scholar]
- 3.Ahmed S.A., Wehaidy H.R., Ibrahim O.A., Abd El Ghani S., El-Hofi M.A. Novel milk-clotting enzyme from Bacillusstearothermophilus as a coagulant in UF-white soft cheese. Biocat and Agricul. Biotech. 2016;7:241–249. [Google Scholar]
- 4.Lemes A.C., Pavon Y., Lazzaroni S., Rozycki S., Brandelli A., Kalil S.J. A new milk-clotting enzyme produced by Bacillus sp. P45 applied in cream cheese development. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2016;66:217–224. [Google Scholar]
- 5.Prasanth M., Nitin M., Shubham J., Saharika S., Ramankannan A., Shanthini T., Ramesh N. Potential of Bacillussubtilis to produce acidic protease under mutagenic condition. Int. J. Pure App. Biosci. 2016;4:126–132. [Google Scholar]
- 6.Aguilar J. G. d.- S., Sato H.H. Microbial proteases: production and application in obtaining protein Hydrolysates. Food Res. Int. 2018;103:253–262. doi: 10.1016/j.foodres.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Si J.-B., Jang E.-J., Charalampopoulos D., Wee Y.-J. Purification and characterization of microbial protease produced extracellularly from Bacillussubtilis FBL-1. Biotechnol. Bioproc. Eng. 2018;23:176–182. [Google Scholar]
- 8.Abou El-Hassayeb H.E., Abdel Aziz S.M.Z. Screening, production and industrial application of protease enzyme from marine bacteria. Int. J. of Curr.Microbio. and Appl. Sci. 2016;5:863–874. [Google Scholar]
- 9.Sathishkumar R., Arun G.A.J. Production, purification and characterization of alkaline protease by ascidian associated Bacillussubtilis GACAS8 using agricultural wastes. Biocat and Agricul. Biotech. 2015;4:214–220. [Google Scholar]
- 10.da Silva O.S., de Oliveira R.L., Silva J. d.- C., Converti A., Porto T.S. Thermodynamic investigation of an alkaline protease from Aspergillus tamarii URM4634: a comparative approach between crude extract and purified enzyme. Int. J. Biol. Macromol. 2018;109:1039–1044. doi: 10.1016/j.ijbiomac.2017.11.081. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Galan C., Berenguer-Murcia Á., Fernandez-Lafuente R., Rodrigues R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011;353:2885–2904. [Google Scholar]
- 12.Ferreira M.M., Santiago F.L.B., da Silva N.A.G., Luiz J.H.H., Fernandéz-Lafuente R., Mendes A.A., Hirata D.B. Different strategies to immobilize lipase from Geotrichumcandidum: kinetic and hermodynamic studies. Process Biochem. 2018 [Google Scholar]
- 13.Tavano O.L., Berenguer-Murcia A., Secundo F., Fernandez-Lafuente R. Biotechnological applications of proteases in food technology. Compr. Rev. Food Sci. Food Saf. 2018;17:412–436. doi: 10.1111/1541-4337.12326. [DOI] [PubMed] [Google Scholar]
- 14.Sahin S., Ozmen I., Kir E. Purification, immobilization, and characterization of protease from local Bacillus subtilis M-11. Asia Pac. J. Chem. Eng. 2015;10:241–247. [Google Scholar]
- 15.Ricardia N.C., de Menezes E.W., Benvenutti E.V., Schöffer J. daN., Hackenhaar C.R., Hertz P.F., Costa T.M.H. Highly stable novel silica/chitosan support for β-galactosidase immobilization for application in dairy technology. Food Chem. 2018;246:343–350. doi: 10.1016/j.foodchem.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Zaidan U.H., Abdul Rahman M.B., Salleh A.B., Othman S.S. Effect of time course, fatty acid chain length and organic solvent on enzymatic synthesis of lactose ester by mica-based immobilized lipases. Aust. J. Basic Appl. Sci. 2015;9:352–358. [Google Scholar]
- 17.Tulyaganov D.U., Agathopoulos S., Fernandes H.R., Ventura J.M., Ferreira J.M.F. Preparation and crystallization of glasses in the system tetrasilicic mica-fluorapatite-diopside. J. Eur. Ceram. Soc. 2004;24:3521–3528. [Google Scholar]
- 18.Abdel-Hameed S.A.M., Ismail N., Youssef H.F., Sadek H.E.H., Marzouk M.A. Preparation and characterization of mica glass-ceramics as hydrogen storage materials. Int. J. of hydr. ener. 2017;42:6829–6839. [Google Scholar]
- 19.Kokubo T. Bioactive glass ceramics: properties and applications. Biomaterials. 1991;12:155–163. doi: 10.1016/0142-9612(91)90194-f. [DOI] [PubMed] [Google Scholar]
- 20.Gaur R., Tiwari S., Singh S. Production and characterization of thermotolerant-organic solvent resistant acidic protease by Pseudomonas aeruginosa RGSS-09 isolated from dairy sludge. Asian J. Biochem. 2015;10:52–66. [Google Scholar]
- 21.Marchand S., Duquenne B., Heyndrickx M., Coudijzer K., De Block J. Destabilization and off-flavors generated by Pseudomonas proteases during or after UHT-processing of milk. Int. J. of Food Contamin. 2017;4:2. [Google Scholar]
- 22.Ahmed S., Divatar M., Gajare S., Shivalee A., Kattimani L. Isolation and screening of Lactobacillus sp. KLSA 22 for lactase production under submerged fermentation. Eur. J. Biomed. Pharm. Sci. 2016;3:574–579. [Google Scholar]
- 23.Asha B., Palaniswamy M. Optimization of alkaline protease production by Bacillus cereus FT 1 isolated from soil. J. Appl. Pharm. Sci. 2018;8:119–127. [Google Scholar]
- 24.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Ahmed S.A., Mostafa F.A., Ouis M.A. Enhancement stability and catalytic activity of immobilized α-amylase using bioactive phospho-silicate glass as a novel inorganic support. Int. J. Biol. Macromol. 2018;112:371–382. doi: 10.1016/j.ijbiomac.2018.01.162. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh P., Das A., Gayen S., Mondal K.C., Ghosh U. Statistical optimization of α-amylase production from Penicilliumnotatum NCIM 923 and kinetics study of the purified enzyme. Acta Biol. Szeged. 2015;59:179–188. [Google Scholar]
- 27.Daoud L., Hmani H., Ben Ali M., Jlidi M., Ben Ali M. An original halo-alkaline protease from Bacillus halodurans strain US193: biochemical characterization and potential use as bio-additive in detergents. J. Polym. Environ. 2018;26:23–32. [Google Scholar]
- 28.Lineweaver H., Burk D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934;56:658–666. [Google Scholar]
- 29.Abdel-Naby M.A., Ahmed S.A., Wehaidy H.R., El-Mahdy S.A. Catalytic, kinetic and thermodynamic properties of stabilized Bacillus stearothermophilus alkaline protease. Int. J. Biol. Macromol. 2017;96:265–271. doi: 10.1016/j.ijbiomac.2016.11.094. [DOI] [PubMed] [Google Scholar]
- 30.Shivakumar S. Production and characterization of an acid protease from a local Aspergillus sp. by Solid substrate fermentation. Arch. Appl. Sci. Res. 2012:188–199. [Google Scholar]
- 31.Ahmed S.A., Saleh S.A.A., Mostafa F.A., Abd El-Aty A.A., Ammar H.A.M. Characterization and valuable applications of xylanase from endophytic fungus Aspergillusterreus KP900973 isolated from Corchorus olitorius. Biocat and Agricul. Biotech. 2016;7:134–144. [Google Scholar]
- 32.Ahmed I., Zia M.A., Hussain M.A., Akram Z., Naveed M.T., Nowrouzid A. Bioprocessing of citrus waste peel for induced pectinase production by Aspergillus niger its purification and characterization. J. Radiat. Res. Appl. Sci. 2016;9:148–154. [Google Scholar]
- 33.Ahmed S.A., Mostafa F.A. Utilization of orange bagasse and molokhia stalk for production of pectinase enzyme. Braz. J. Chem. Eng. 2013;30:449–456. [Google Scholar]
- 34.Irshad M., Anwar Z., Mahmood Z., Aqil T., Mehmmod S., Nawaz H. Bio-processing of agro-industrial waste orange peel for induced production of pectinase by Trichodermaviridi, its purification and characterization. Turkish J. Biochem. 2014;39:9–18. [Google Scholar]
- 35.Mothe T., Sultanpuram V.R. Production, purification and characterization of a thermotolerant alkaline serine protease from a novel species Bacillus caseinilyticus. 3 Biotech. 2016;6:53. doi: 10.1007/s13205-016-0377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castro R.J.S., Soares M.H., Albernaz J.R.M., Sato H.H. Biochemical characterization of solvent, salt, surfactant and oxidizing agent tolerant proteases from Aspergillus niger produced in different agroindustrial wastes. Biocat. Agricul. Biotech. 2016;5:94–98. [Google Scholar]
- 37.Hang F., Liu P., Wang Q., Han J., Wu Z., Gao C., Liu Z., Zhang H., Chen W. High milk-clotting activity expressed by the newly isolated Paenibacillus spp strain BD3526. Molecules. 2016;21:73–86. doi: 10.3390/molecules21010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hettiarachchy N.S., Feliz D.J., Edwards J.S., Horax R. second ed. Woodhead Publishing Series in Food Science, Technology and Nutrition; 2018. “The Use of Immobilized Enzymes to Improve Functionality” Proteins in Food Processing; pp. 569–597. [Google Scholar]
- 39.Misson M., Zhang H., Jin B. Nanobiocatalyst advancements and bioprocessing applications. J. R. Soc. Interface. 2015;12:20140891. doi: 10.1098/rsif.2014.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norouzian D. Enzyme immobilization: the state of art in biotechnology. Iran. J. Biotechnol. 2003;1:197–206. [Google Scholar]
- 41.Yuan Y., Luan X., Rana X., Hassan M.E., Dou D. Covalent immobilization of cellulase in application of biotransformation of ginsenoside Rb1. J. Mol. Catal. B Enzym. 2016;133:S525–S532. [Google Scholar]
- 42.Gómez J.M., Romero M.D., Fernández T.M., García S. Immobilization and enzymatic activity of β-glucosidase on mesoporous SBA-15 silica. J. Porous Mater. 2010;17:657–662. [Google Scholar]
- 43.Wehaidy H.R., Abdel-Naby M.A., Shousha W.G., Elmallah M.I.Y., Shawky M.M. Improving the catalytic, kinetic and thermodynamic properties of Bacillus subtilis KU710517 milk clotting enzyme via conjugation with polyethylene glycol. Int. J. Biol. Macromol. 2018;111:296–301. doi: 10.1016/j.ijbiomac.2017.12.125. [DOI] [PubMed] [Google Scholar]
- 44.Ibrahim A.S.S., Al-Salamah A.A., El-Toni A.M., Almaary K.S., El-Tayeb M.A., Elbadawi Y.B., Antranikian G. Enhancement of alkaline protease activity and stability via covalent immobilization onto hollow core-mesoporous shell silica nanospheres. Int. J. Mol. Sci. 2016;17:1–18. doi: 10.3390/ijms17020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgado Y., Morales-Cruz M., Hernández-Román J., Martínez Y., Griebenow K. Chemical glycosylation of cytochrome c improves physical and chemical protein stability. BMC Biochem. 2014;15:16. doi: 10.1186/1471-2091-15-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro R.J.S., Ohara A., Nishide T.G., Albernaz J.R.M., Soares M.H., Sato H.H. Biochemical characterization of solvent, salt, surfactant and oxidizing agent tolerant proteases from Aspergillus niger produced in different agroindustrial wastes. Biocat and Agricul. Biotech. 2015;4:199–207. [Google Scholar]
- 47.Su R., Shi P., Zhu M., Hong F., Li D. Studies on the properties of graphene oxide-alkaline protease bio-composites. Bioresour. Technol. 2012;115:136–140. doi: 10.1016/j.biortech.2011.12.085. [DOI] [PubMed] [Google Scholar]
- 48.Spinelli D., Fatarella E., Di Michele A., Pogni R. Immobilization of fungal (Trametesversicolor) laccase onto Amberlite IR-120 H beads: optimization and characterization. Proc.Biochem. 2013;48:218–223. [Google Scholar]
- 49.Souza P.M., Aliakbarian B., Filho E.X.F., Magalhães P.O., Junior A.P., Converti A., Perego P. Kinetic and thermodynamic studies of a novel acid protease from Aspergillusfoetidus. Int. J. Biol. Macromol. 2015;81:17–21. doi: 10.1016/j.ijbiomac.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 50.Ahmetoglu N., Bekler F.M., Acer O., Guven R.G., Guven K. Production, purification and characterization of thermostable metalloprotease from newly isolated Bacillus sp. KG5. Euras. J. of Biosci. 2015;9:1–11. [Google Scholar]
- 51.He T., Tian Y.-L., Qi L., Zhang J., Zhang Z.-Q. Improved performance of α-amylase immobilized on poly(glycidyl methacrylate-co-ethylenedimethacrylate) beads. Int. J. Biol. Macromol. 2014;65:492–499. doi: 10.1016/j.ijbiomac.2014.01.066. [DOI] [PubMed] [Google Scholar]