Abstract
Mycobacteriophages express various peptides/proteins to infect Mycobacterium tuberculosis (M. tb). Particular attention has been paid to mycobacteriophage-derived endolysin proteins. We herein characterized a small mycobacteriophage-derived peptide designated AK15 with potent anti-M. tb activity. AK15 adopted cationic amphiphilic α-helical structure, and on the basis of this structure, we designed six isomers with increased hydrophobic moment by rearranging amino acid residues of the helix. We found that one of these isomers, AK15-6, exhibits enhanced anti-mycobacterial efficiency. Both AK15 and AK15-6 directly inhibited M. tb by trehalose 6,6′-dimycolate (TDM) binding and membrane disruption. They both exhibited bactericidal activity, cell selectivity, and synergistic effects with rifampicin, and neither induced drug resistance to M. tb. They efficiently attenuated mycobacterial load in the lungs of M. tb-infected mice. We observed that lysine, arginine, tryptophan, and an α-helix are key structural requirements for their direct anti-mycobacterial action. Of note, they also exhibited immunomodulatory effects, including inhibition of proinflammatory response in TDM-stimulated or M. tb-infected murine bone marrow-derived macrophages (BMDMs) and M.tb-infected mice and induction of only a modest level of cytokine (tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6)) production in murine BMDMs and a T-cell cytokine (interferin-γ (IFN-γ) and TNF-α) response in murine lung and spleen. In summary, characterization of a small mycobacteriophage-derived peptide and its improved isomer revealed that both efficiently restrain M. tb infection via dual mycobactericidal-immunoregulatory activities. Our work provides clues for identifying small mycobacteriophage-derived anti-mycobacterial peptides and improving those that have cationic amphiphilic α-helices.
Keywords: antimicrobial peptide (AMP), peptides, structure-function, amino acid, drug action, drug resistance, α-helix, anti-mycobacterial peptide, hydrophobic moment, mycobacteriophage, antibiotic, endolysin, immune regulation, Mycobacterium tuberculosis
Introduction
Tuberculosis (TB)5 is a serious airborne fatal disease caused by Mycobacterium tuberculosis (M. tb) infection (1). In 2016, TB was the ninth leading cause of death worldwide and the leading cause from a single infectious agent (2). There were an estimated 10.4 million new TB cases, an estimated 1.3 million TB deaths among HIV-negative people, and an additional 374,000 deaths among HIV-positive people (2). Worst of all, there were rapid emergences of drug-resistant TB. Among the new TB cases in 2016, about 600,000 were resistant to rifampicin, the most effective first-line drug, of which 490,000 cases were multidrug-resistant (2). So far, global control of tuberculosis is mainly achieved by the development of effective vaccines, improved diagnostics, and novel and shortened therapy regimens (3). Although several first-line effective anti-M. tb drugs, including rifampin, isoniazid, pyrazinamide, and ethambutol, have displayed critical roles in control of TB, the rapid emergence of drug-resistant M. tb strains makes them invalid (4). Given the severe global TB burden, there is an urgent need to find alternate ways to control M. tb infection.
Mycobacteriophages, the viruses of mycobacteria, have developed unique proteins or peptides to interfere with metabolic processes or cell membranes of mycobacterial hosts (4, 5). It is reasonable to search for potent anti-mycobacterial peptides or proteins from mycobacteriophages (4). In the past decades, thousands of mycobacteriophages have been isolated, and more than 1,400 mycobacteriophage genomes have been completely sequenced (http://phagesdb.org/).6 Based on the genomic information, particular attention has been paid to mycobacteriophage-encoded lytic endolysins, also called enzybiotics, which were considered as potential anti-mycobacterial agents against a number of multidrug-resistant and extensively drug-resistant M. tb strains (6–10). Aside from mycobacteriophage-encoded lytic endolysins, only one mycobacteriophage-derived anti-mycobacterial peptide, PK34, was identified from the genomic information of mycobacteriophage D29 (4). Due to the horizontal genetic exchange, mycobacteriophage genomes are highly genetically diverse (11, 12), providing a staggeringly large number of genes coding for products without functional annotation. Among the numerous mycobacteriophage-derived bioactive agents, we focus on the identification of small mycobacteriophage-derived anti-mycobacterial peptides. In the last few decades, antimicrobial peptides have been considered as potential alternatives for the therapy of bacterial infection with multiple advantages, such as low immunogenicity, selective affinity to negatively charged cell membrane of bacteria, diverse mechanisms of action, no development of drug resistance, and synergistic effects with first-line antibiotics (13–15). These advantages also make them good therapeutic weapons to combat tuberculosis.
In the present study, a small but potent cationic amphiphilic helical peptide derived from mycobacteriophage, designated as AK15, was characterized from the genomic information of mycobacteriophage Che12. In addition, AK15-6, an improved isomer of AK15, was isolated by rearrangement of the amino acid residues to increase the hydrophobic moment of the helix. AK15 and AK15-6 efficiently restrained M. tb infection in vitro and in vivo. Their mechanism of action, cell selectivity, structural requirements, synergistic effects with rifampincin, and induced drug resistance were investigated. The present work indicates that we can identify small but potent anti-mycobacterial peptide from mycobacteriophages, and we can optimize cationic amphiphilic helical anti-mycobacterial peptide by rearrangement of amino acid residues. Collectively, this study provides novel peptide candidates and new clues to find and design small but potent peptide antibiotics for control of M. tb infection.
Results
A small anti-mycobacterial peptide, AK15, was identified from mycobacteriophage
To identify small as well as potent anti-mycobacterial peptide from mycobacteriophage, a series of peptide candidates derived from mycobacteriophage that were 10–30 amino acid residues in length were synthesized for anti-mycobacterial activity assay by minimum inhibitory concentration (MIC) determination (Table S1). Among them, a small peptide named AK15 possessed a potent anti-mycobacterial activity against M. tuberculosis H37Rv (ATCC 27294) with a MIC value of 37.5 μg/ml. AK15 was even active against rifampicin-induced drug-resistant M. tuberculosis H37Rv, clinically isolated M. tb (drug-susceptible or -resistant strains) with MIC values ranging from 18.75 to 75 μg/ml, respectively (Table 1). AK15 is a small peptide with a molecular mass of 2071.55 Da (Table 2). AK15 is derived from a hypothetical protein of mycobacteriophage Che12 with unknown function, which is a small peptide family conserved in many mycobacteriophages, including mycobacteriophage Adzzy, L5, DarthPhader, Alma, Pioneer, and SkiPole. The identification of AK15 provided a small but potent anti-mycobacterial peptide derived from mycobacteriophage.
Table 1.
Anti-mycobacterial activity of AK15 in vitro
| M. tb | MICa |
|---|---|
| μg/ml | |
| M. tuberculosis H37Rv | 37.5 (18.1 μm) |
| M. tuberculosis H37Rv (rifampicin-resistant) | 37.5 (18.1 μm) |
| M. tuberculosis H37Ra | 37.5 (18.1 μm) |
| M. tuberculosis WXY (CI)b | 18.75 (9.1 μm) |
| M. tuberculosis CAS3 (CI) | 75 (36.2 μm) |
| M. tuberculosis FYX (CI, rifampicin-resistant) | 37.5 (18.1 μm) |
a Mean values of three independent experiments performed in duplicates. The MIC values of rifampicin against rifampicin-induced drug-resistant M. tuberculosis H37Rv and clinically isolated drug-resistant M. tuberculosis FYX are 1.5625 and >32 μg/ml, respectively.
b CI, clinically isolated strain.
Table 2.
Amino acid sequence and physiochemical parameters of AK15 and its isomers and their MICs against M. tuberculosis H37Rv in vitro
| Peptide | Amino acid sequence | Mass | Net charge | Hydrophobicity | μHa | MIC |
|
|---|---|---|---|---|---|---|---|
| RIF-susceptible | RIF-resistant | ||||||
| Da | μg/ml | ||||||
| AK15 | AKKKLSRWWLRWWVK | 2,071.55 | +6 | 0.527 | 0.511 | 37.5 | 37.5 |
| AK15-1 | AKKKLVRWWLRWWSK | 2,071.55 | +6 | 0.527 | 0.613 | 37.5 | 37.5 |
| AK15-2 | ASKKLVRWWLRWWKK | 2,071.55 | +6 | 0.527 | 0.722 | 37.5 | 37.5 |
| AK15-3 | ALKKLSRWWKRWWVK | 2,071.55 | +6 | 0.527 | 0.732 | 37.5 | 37.5 |
| AK15-4 | AVKKLSRWWLRWWKK | 2,071.55 | +6 | 0.527 | 0.766 | 37.5 | 37.5 |
| AK15-5 | ALKKLVRWWKRWWSK | 2,071.55 | +6 | 0.527 | 0.830 | 37.5 | 37.5 |
| AK15-6 | AVKKLLRWWSRWWKK | 2,071.55 | +6 | 0.527 | 0.845 | 18. 75 | 18. 75 |
a Hydrophobic moment, determined by HeliQuest (http://heliquest.ipmc.cnrs.fr/). (Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.)
AK15 adopted amphiphilic α-helical confirmation
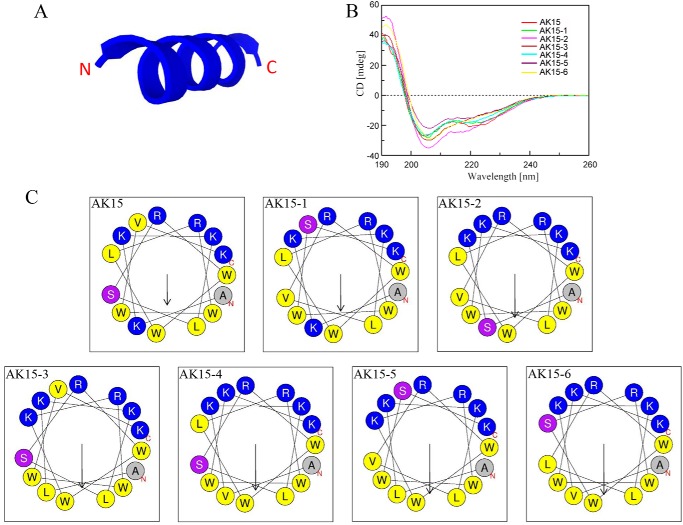
To understand the structural characterization of AK15, its secondary structure was first predicted using a computational framework, PEP-FOLD3. As shown in Fig. 1A, AK15 was predicted to adopt α-helical conformation. To verify the accuracy of the predicted structure, CD spectroscopy was performed to detect its secondary structure in a membrane-mimetic environment (TFE/H2O, 9:1). In accordance with the predicted structure, the CD spectra of AK15 exhibited double minima at 208 and 222 nm, which was indicative of the presence of an α-helical conformation in TFE/H2O solution (Fig. 1B). To estimate the amphipathicity of this helix, a helix-wheel diagram was plotted using HeliQuest. As shown in Fig. 1C, the hydrophobic amino acid residues (shown in yellow and gray) of AK15 are mainly concentrated on one side of the helix, and the hydrophilic amino acid residues (shown in blue and purple) of AK15 are mainly concentrated on the other side, which formed the hydrophobic side and hydrophilic side of the helix, respectively. Collectively, the structural characterization indicated that AK15 is a cationic amphiphilic α-helical peptide.
Figure 1.
AK15 and its isomers adopted amphiphilic α-helical structure. A, secondary structure of AK15 predicted by computational framework PEP-FOLD3. B, CD spectra of AK15 and its isomers (0.2 mg/ml) in a TFE/H2O (9:1) environment. C, AK15 and its isomers adopted amphiphilic helical structures constructed by HeliQuest. The hydrophobic residues are shown in yellow and gray, positively charged hydrophilic residues are shown in blue, and noncharged hydrophilic residues are shown in purple.
Isomers of AK15 with increased hydrophobic moment were designed by rearrangement of amino acid residues
The effects of hydrophobicity, charge, secondary structure, and amphiphilicity on antimicrobial properties for antimicrobial peptides were extensively investigated on Gram-negative bacteria, Gram-positive bacteria, and fungi (15–18). But the effects of these physicochemical parameters on anti-mycobacterial action remain unclear. Recently, several designed α-helical anti-mycobacterial peptides indicated that the cationic and amphiphilic α-helix of these peptides are critical for their interaction with M. tb (15–17). Up to now, no investigation has been conducted on the improvement of cationic α-helical anti-mycobacterial peptides by rearranging amino acid residues. In an attempt to obtain much more potent anti-mycobacterial peptides, we designed six isomers by rearranging the amino acid residues of the helix and plotting by HeliQuest. After the helix-wheel diagram assay, six isomers with increased concentration of hydrophobic amino acid residues on one side and hydrophilic amino acid residues on the other side and the other physicochemical parameters (molecular weight, net charge, hydrophobicity, and α-helical confirmation) unaltered were selected and synthesized for the CD assay (Table 2 and Fig. 1C). CD spectra of these six isomers displayed characteristic double minima at 208 and 222 nm, which confirmed that six isomers kept the same secondary conformation (α-helix) as AK15 after the rearrangement of amino acid residues (Fig. 1B). An in vitro anti-mycobacterial activity assay against M. tuberculosis H37Rv showed that one of them, AK15-6, displayed enhanced anti-mycobacterial activity, as reflected by a decreased MIC value (18.75 μg/ml) relative to AK15 (37.5 μg/ml; Tables 1 and 2).
AK15 and its isomer AK15-6 showed bactericidal activity against M. tb
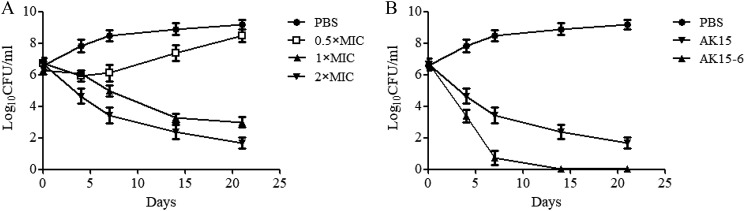
AK15-6 possessed the most effective anti-mycobacterial activity among the six isomers and hence was selected for investigation of mechanism of action together with AK15. As described previously, a ≥3 log decrease in the initial inoculum was defined as bactericidal activity (19). As shown in Fig. 2, compared with the initial inoculum, a 3 log reduction in final cfu counts was observed after incubation of M. tuberculosis H37Rv with AK15 (75 μg/ml) (Fig. 2A), and a 4 log reduction in final cfu counts was observed after the incubation of AK15-6 (75 μg/ml) (Fig. 2B). The results indicated that they both showed bactericidal activity against M. tuberculosis H37Rv at a concentration of 75 μg/ml, and AK15-6 showed an enhanced bactericidal property against M. tb relative to AK15.
Figure 2.
AK15 and its isomer AK15-6 showed bactericidal property against M. tuberculosis H37Rv. A, killing curves of AK15 at different concentrations (0.5 × MIC, 1 × MIC, and 2 × MIC) after incubation for 4, 7, 14, and 21 days. B, comparison of killing curves between AK15 and its isomer AK15-6 against M. tuberculosis H37Rv at a concentration of 75 μg/ml. Error bars, S.E.
AK15 and its isomer AK15-6 were directly bound to M. tb
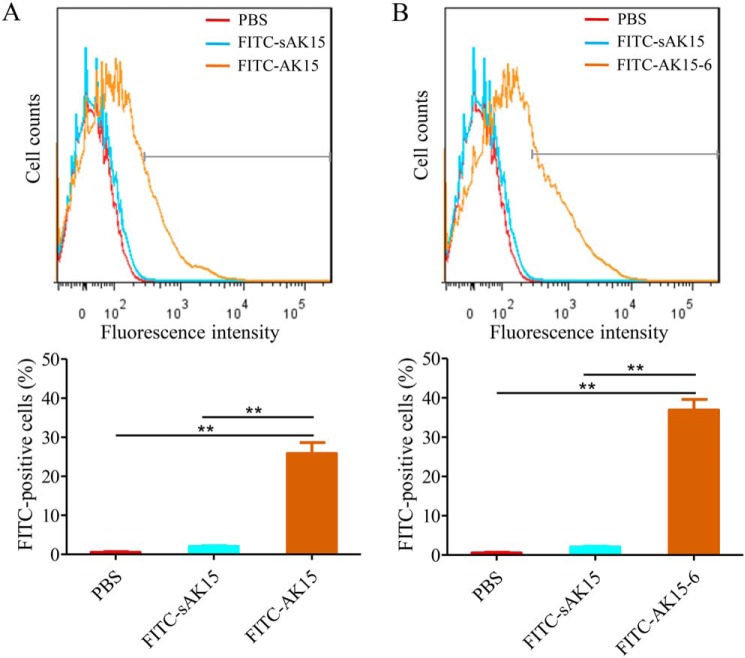
It is well-known that the direct interactions between the positively charged peptides and bacterial anionic phospholipids and acidic polymers drive the initial association and accumulation of antimicrobial peptides onto the membrane, which constitutes the first step of the lytic process of cationic amphiphilic α-helical antimicrobial peptides (16, 20). To address their potential anti-mycobacterial mechanism, the direct interactions between the peptides and M. tb were first investigated by incubation of M. tuberculosis H37Ra with FITC-labeled AK15 or AK15-6. As shown in Fig. 3, the incubation of PBS and scrambled AK15 (sAK15) with M. tb did not induce a significant increase of fluorescence intensity, whereas the exposure of M. tb to FITC-AK15 (1 μg/ml) induced a significant increase of fluorescence intensity, and the percentage of FITC-positive M. tb was increased by 25.3% relative to PBS-exposed M. tb (Fig. 3A). At the same concentration (1 μg/ml), the exposure of M. tb to AK15-6 induced a more remarkable increment of fluorescence intensity, and the fluorescence intensity was increased by 37.7% relative to PBS-exposed M. tb (Fig. 3B). A flow cytometry assay indicated that they both directly bound to M. tb, and AK15-6 showed an increased M. tb-binding ability relative to AK15.
Figure 3.
AK15 (A) and its isomer AK15-6 (B) were directly bound to M. tuberculosis H37Ra cells. M. tuberculosis H37Ra cells were washed twice with PBS and exposed to FITC-labeled AK15 or AK15-6 (1 μg/ml) at 37 °C. PBS and FITC-labeled sAK15 (scrambled AK15) were used as control, respectively. After incubation for 5 min, M. tuberculosis H37Ra cells were washed twice with PBS, assayed on a FACSCalibur flow cytometer, and analyzed by Cell Quest software (BD Immunocytometry). **, p < 0.01. Error bars, S.E.
AK15 and its isomer AK15-6 showed apparent affinity to mycobacterial cord factor
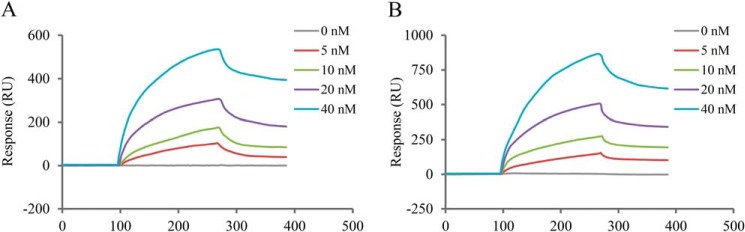
Mycobacterial cord factor, TDM, is the most abundant and toxic glycolipid produced on the mycobacterial cell surface (21). To understand the binding target of AK15 and AK15-6 to M. tb, surface plasmon resonance was performed to investigate whether the peptides can interact with TDM. After immobilizing AK15 or AK16-6 on the chips, TDM as analyte was assayed at four concentrations (5, 10, 20, and 40 nm) in HBS-N buffer. As shown in Fig. 4, the binding of TDM to AK15 (Fig. 4A) and AK15-6 (Fig. 4B) was dose-dependent. The increment in resonance units (RU) by binding of TDM to immobilized AK15 or AK15-6 demonstrated the direct interaction between TDM and AK15 or AK15-6. Kinetic analysis for AK15 or AK15-6 as ligand interacting with TDM as analyte indicated a rapid association of AK15 (KD = 3.19 × 10−11 m) or AK15-6 (KD = 2.03 × 10−11 m) with TDM to form AK15– or AK15-6–TDM complex and slow disassociation, and AK15-6 showed a higher affinity to TDM as compared with AK15.
Figure 4.
AK15 (A) and its isomer AK15-6 (B) showed apparent affinity to mycobacterial cord factor (TDM). Interaction kinetics of peptides and TDM were determined by surface plasma resonance. Peptide was immobilized on a CM5 sensor chip as ligand, and TDM was diluted in a series of concentrations in HBS-N buffer. Responses (resonance units, RU) are recorded for the indicated concentrations of TDM (0, 5, 10, 20, and 40 nm).
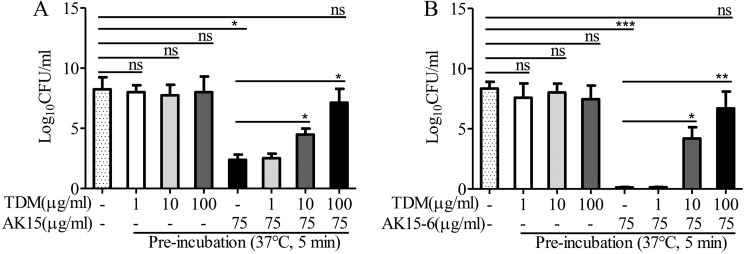
The pre-incubation of AK15 or AK15-6 with TDM blocked the anti-mycobacterial activity of the peptides
To further investigate the interaction between the peptides and TDM, we assessed the anti-mycobacterial activity of the peptides followed by pre-incubation with TDM at 37 °C for 5 min. Compared with vehicle (PBS, DMSO)–treated M. tuberculosis H37Rv, the addition of 1, 10, or 100 μg/ml TDM alone had no significant effect on M. tb replication, and the addition of 75 μg/ml AK15 or AK15-6 alone significantly inhibited M. tb replication, whereas TDM blocked the anti-mycobacterial activity of AK15 or AK15-6 in a dose-dependent manner after the pre-incubation of 1, 10, and 100 μg/ml TDM with 75 μg/ml of AK16 or AK15-6, respectively (Fig. 5), and 100 μg/ml TDM completely blocked the anti-mycobacterial activity of 75 μg/ml of AK15 or AK15-6. The data further indicated that the peptides directly interacted with TDM.
Figure 5.
TDM blocked the anti-mycobacterial activities of AK15 (A) and its isomer AK15-6 (B). Peptides (750 μg/ml), TDM (10, 100, and 1,000 μg/ml), and peptide (750 μg/ml) mixed with 10, 100, or 1000 μg/ml TDM were incubated at 37 °C and shook at 150 rpm for 5 min. Then peptide, TDM, peptide/TDM mixture, or the same volume of vehicle (PBS, DMSO) was incubated with M. tuberculosis H37Rv (∼107 cfu/ml), respectively. The final concentrations of TDM were 1, 10, and 100 μg/ml, and the final concentration of peptides was 75 μg/ml. After incubation at 37 °C for 7 days, the viable mycobacteria were counted. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant. Error bars, S.E.
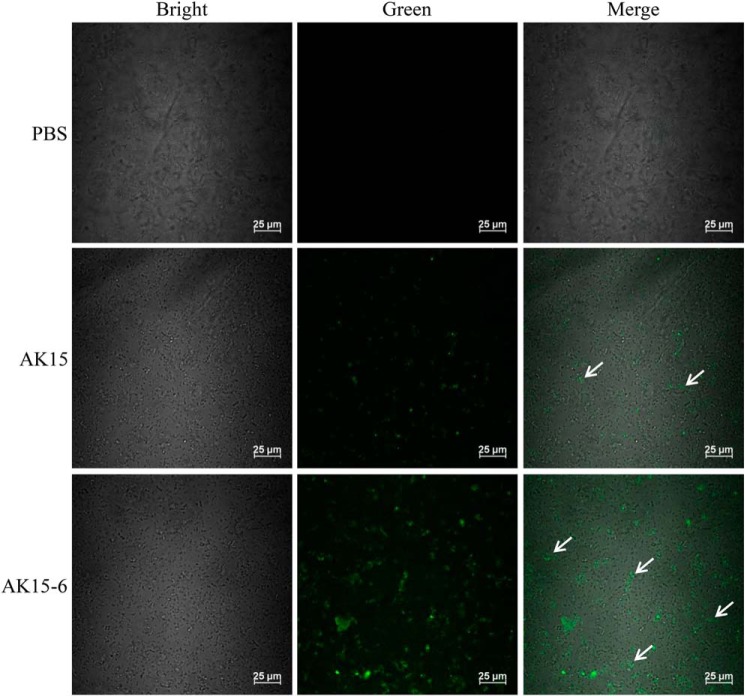
AK15 and its isomer AK15-6 increased the passive diffusion of the fluorescent dye into M. tb
Antimicrobial peptides are usually membrane-active agents that disrupt the cell membrane integrity and result in pore formation after their association and accumulation onto bacterial membrane (15). With direct binding of the peptides to M. tb and apparent affinity of the peptides to TDM, we next evaluated whether AK15 and AK15-6 could disrupt mycobacterial membrane by incubation of M. tuberculosis H37Ra with FITC-labeled dextran probe in the presence or absence of peptide. As shown in Fig. 6, incubation of M. tuberculosis H37Ra with PBS did not result in the uptake of FITC-dextran, whereas the exposure of M. tb to AK15 induced the uptake of fluorescence probe, and the exposure of M. tb to AK15-6 resulted in a greater uptake of the fluorescence probe than AK15 did. The data indicated that the peptides induced the passive diffusion of the fluorescent dye into M. tb. The increase of fluorescence intensity after treatment of AK15-6 as compared with AK15 is indicative of enhanced membrane damage and pore formation, allowing for greater uptake of the fluorescent probe. These results implied a membrane-disrupting mechanism of action of the peptides after direct binding to M. tb.
Figure 6.
AK15 and its isomer AK15-6 increased the passive diffusion of the fluorescent dye into M. tuberculosis H37Ra cells. M. tuberculosis H37Ra suspension (400 μl, ∼5 × 105 cfu/ml) was incubated with an equal volume of PBS solution containing AK15 or AK15-6 (75 μg/ml) and FITC-dextran (150 kDa, 250 μg/ml; Sigma) at 37 °C for 1 h with constant shaking at 200 rpm. The images were acquired using a confocal microscope after mycobacterial cells were washed three times with PBS to remove the free FITC-dextran.
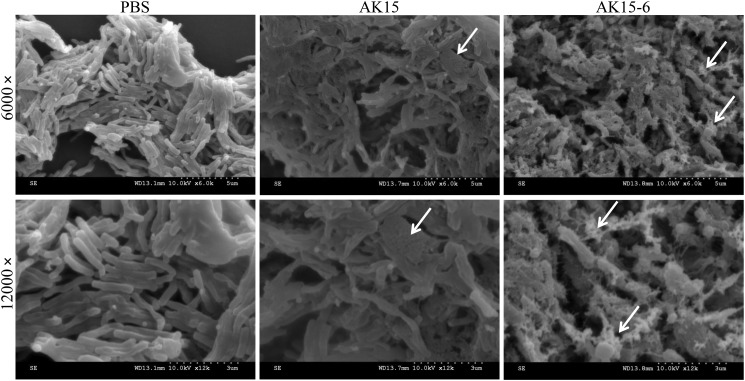
AK15 and its isomer AK15-6 impaired the surface morphology of M. tb
To confirm the membrane-disrupting mechanism of action of the peptides, the surface morphology of M. tuberculosis H37Ra was visualized by scanning EM after exposure to peptides or vehicle (PBS). As shown in Fig. 7, PBS-exposed M. tb exhibited a regular, smooth, and intact surface, whereas AK15-exposed M. tb exhibited an extensively rough and collapsed surface. In the same condition, AK15-6–exposed M. tb showed a greater change of surface morphology with rough and collapsed surface covered with irregular debris. The impaired surface morphology of AK15/AK15-6–exposed M. tb confirmed the membrane-disrupting mechanism of action of the peptides after direct binding to M. tb.
Figure 7.
AK15 and its isomer AK15-6 impaired the surface morphology of M. tuberculosis H37Ra. M. tuberculosis H37Ra cells (∼2 × 107 cfu/ml) were incubated with AK15 or AK15-6 (187.5 μg/ml) or an equal volume of PBS (vehicle) at 37 °C for 4 h. The surface morphology was observed with a Hitachi S-4800 scanning electron microscope. The typical alterations of surface morphology were marked by arrows.
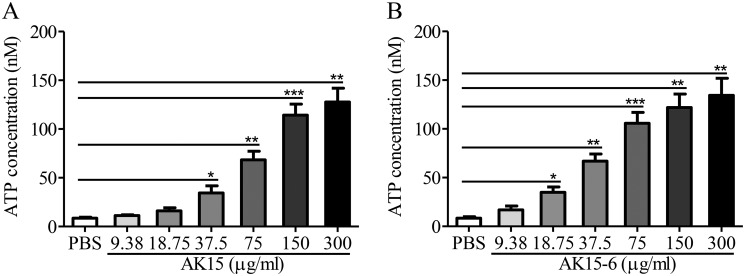
AK15 and its isomer AK15-6 induced the leakage of intracellular ATP from M. tb
As described previously, mycobacterial membrane integrity disrupted by anti-mycobacterial peptides is accompanied by leakage of intracellular contents including ATP (16). To further confirm the membrane-disrupting mechanism, we assessed the extracellular ATP levels after exposure of M. tuberculosis H37Ra to supra- and sub-inhibitory concentrations of AK15 or AK15-6. Compared with PBS-exposed M. tb, AK15 or AK15-6 exposure significantly induced a concentration-dependent release of ATP from mycobacterial cells at concentrations above the respective MIC value (Fig. 8). At a concentration of 75 μg/ml, AK15 and AK15-6 induced 68.4 and 105.7 nm extracellular ATP accumulation released from M. tb, respectively. The data further confirmed the membrane-disrupting mechanism of the peptides, and AK15-6 showed a stronger membrane-disrupting capacity than did AK15.
Figure 8.
AK15 (A) and its isomer AK15-6 (B) induced the leakage of intracellular ATP from M. tb. A series of peptide dilutions and M. tb suspension (A600 = 0.4) was prepared in 10 mm phosphate buffer, and peptide dilutions or PBS were added to an equal volume of M. tb suspension (300 μl). After incubation at 37 °C for 2 h, extracellular ATP concentrations were determined using an ATP assay kit (Beyotime, Shanghai, China) according to the manufacturer's instructions. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Error bars, S.E.
AK15 and its isomer AK15-6 showed cell selectivity to M. tb
With the M. tb (TDM)-binding and membrane-disrupting mechanisms of the peptides, we were interested to determine the relative specificity of AK15 and its isomer AK16-6 on mammalian cells, including their hemolytic activity against human and rabbit red blood cells and cytotoxicity against murine bone marrow-derived macrophages (BMDMs), RAW264.7 cells, and Vero E6 cells. As shown in Table 3, AK15 and its isomer AK15-6 did not show any hemolytic activity and cytotoxicity against all tested mammalian cells at concentrations up to 200 μg/ml.
Table 3.
Hemolysis and cytotoxicity of AK15 and its isomer AK15-6 against mammalian cells
| Peptide | Hemolytic activity |
Cytotoxicity |
|||
|---|---|---|---|---|---|
| Human RBCa | Rabbit RBCa | Murine BMDMs | RAW264.7 | Vero E6 cells | |
| AK15 | NDb | ND | ND | ND | ND |
| AK15-6 | ND | ND | ND | ND | ND |
a RBC, red blood cells.
b ND, no detectable hemolytic activity or cytotoxicity of the peptides against tested mammalian cells at concentration up to 200 μg/ml.
We next evaluated their inhibitory effects on other Gram-negative bacteria, Gram-positive bacteria, and fungi by a MIC assay. As show in Table S2, all of the tested microorganisms were not sensitive to AK15 and its isomer AK15-6 at concentrations below 150 μg/ml. These results indicated that AK15 and its isomer AK15-6 showed cell selectivity to M. tb. As expected, AK15 and AK15-6 showed no significant cell-binding and membrane-permeating capacity to Escherichia coli and Staphylococcus aureus relative to M. tb (Figs. S1 and S2).
Positively charged residues, aromatic residues, and α-helix constitute the key structural requirements of the peptides
As described previously, positively charged residues (arginine and lysine) are essential for the antimicrobial actions of many other antimicrobial peptides (22–24). In addition, aromatic residues (tryptophan and phenylalanine) are key residues for the antimicrobial activity, anti-inflammatory activity, and cell selectivity of these peptides (16, 23, 25). To understand whether positively charged residues and aromatic residues are critical for the anti-mycobacterial activities of mycobacteriophage-derived AK15 and its isomer AK15-6, we substituted the positively charged residues and aromatic residues with alanine, respectively. As shown in Table 4, the substitution of arginine, lysine, or tryptophan with alanine in AK15 and AK15-6 resulted in a significant decrease of anti-mycobacterial activity, as reflected by MIC values increased by 2–8-fold, respectively. In addition, the co-substitution of arginine/lysine and tryptophan with alanine in AK15 and AK15-6 resulted in no detectable anti-mycobacterial activity at concentrations up to 200 μg/ml. Additionally, a scrambled amino acid sequence of the helical AK15 (sAK15) also did not show any anti-mycobacterial activity at concentrations up to 200 μg/ml (Table 4). The data indicated that lysine, arginine, aromatic residues, and an α-helix are key structural requirements for the anti-mycobacterial activity of the peptides, and the positively charged residues and aromatic residues probably play synergistic roles in anti-mycobacterial activity of the peptides, which provides new insights to design small but potent α-helical anti-mycobacterial peptide enriched in positively charged residues (lysine, arginine) and aromatic residues (tryptophan).
Table 4.
Key structural requirement assay for AK15 and its isomer AK15-6
| Peptide | Amino acid sequence | MICa |
||
|---|---|---|---|---|
| H37Rv | WXY | CAS3 | ||
| μg/ml | ||||
| AK15 | AKKKLSRWWLRWWVK | 37.5 | 18.75 | 75 |
| AK15 (R→A) | AKKKLSAWWLAWWVK | 75 | 75 | 150 |
| AK15 (K→A) | AAAALSRWWLRWWVA | 150 | 75 | 150 |
| AK15 (W→A) | AKKKLSRAALRAAVK | 150 | 150 | 150 |
| AK15 (R→A, W→A) | AKKKLSAAALAAAVK | >200 | >200 | >200 |
| AK15 (K→A, W→A) | AAAALSRAALRAAVA | >200 | >200 | >200 |
| AK15-6 | AVKKLLRWWSRWWKK | 18.75 | 9.38 | 18.75 |
| AK15-6 (R→A) | AVKKLLAWWSAWWKK | 75 | 37.5 | 37.5 |
| AK15–6 (K→A) | AVAALLRWWSRWWAA | 75 | 75 | 75 |
| AK15–6 (W→A) | AVKKLLRAASRAAKK | 150 | 75 | 150 |
| AK15-6 (R→A, W→A) | AVKKLLAAASAAAKK | >200 | >200 | >200 |
| AK15–6 (K→A, W→A) | AVAALLRAASRAAAA | >200 | >200 | >200 |
| sAK15 (scrambled AK15) | WSKWKRKAWVRLWLK | >200 | >200 | >200 |
a These concentrations represent mean values of three independent experiments performed in duplicates.
AK15 and its isomer AK15-6 showed no induced drug resistance to M. tb
Microbes are less likely to develop drug resistance against antimicrobial peptides. Hence, they were considered as promising candidates for peptide antibiotic development (24). We herein investigated whether mycobacteriophage-derived peptides could induce drug resistance against M. tuberculosis H37Rv. To simulate drug resistance in vitro, mycobacteria were exposed to sub-therapeutic doses of rifampicin or peptides over 16 passages, slightly modified from a previous method (15). As shown in Table 5, the MIC value of rifampicin against M. tuberculosis H37Rv increased from 0.012 to 1.5625 μg/ml (increased by 128-fold), which indicated that resistance of M. tuberculosis H37Rv against rifampicin did develop with sub-therapeutic treatment. In contrast, resistance of M. tuberculosis H37Rv against AK15 and AK15-6 did not readily develop with sub-therapeutic treatment of the peptides, as shown by the consistent MIC values obtained over 16 passages.
Table 5.
Drug resistance test of the peptides and rifampicin against M. tuberculosis H37Rv
| Stimulation drug | Tested drug | MIC (passage 1)a | MIC (passage 16)a |
|---|---|---|---|
| μg/ml | μg/ml | ||
| PBS | AK15 | 37.5 | 37.5 |
| AK15 | AK15 | 37.5 | 37.5 |
| PBS | AK15-6 | 18.75 | 18.75 |
| AK15-6 | AK15-6 | 18.75 | 18.75 |
| DMSO | Rifampicin | 0.012 | 0.012 |
| Rifampicin | Rifampicin | 0.012 | 1.5625 |
a These concentrations represent mean values of three independent experiments performed in duplicates. Peptide was dissolved in PBS, and rifampicin was dissolved in DMSO.
AK15 and its isomer AK15-6 generated synergistic effects with rifampincin against M. tb
Although antimicrobial peptides exhibit such an advantage without induced drug resistance, their clinical applications are still limited due to drawbacks such as poor stability in vivo (26). To overcome such shortcomings, the combinational usage of antimicrobial peptides and traditional antibiotics might be a promising way to produce synergistic effects between the peptides and antibiotics and a greater antimicrobial effect (27, 28). Given these findings, the interactive effects of mycobacteriophage-derived peptides with rifampicin (the most effective first-line antibiotic in treatment of tuberculosis) were determined by a checkerboard assay. As shown in Table 6, AK15 and its isomer AK15-6 exhibited synergistic effects with rifampicin with an FICI value of 0.5 in rifampicin-susceptible M. tuberculosis H37Rv. Further testing in rifampicin-resistant M. tuberculosis H37Rv (MIC = 1.5625 μg/ml) produced similar results, whereas the AK15– or AK15-6–rifampicin combination generated synergistic effects against rifampicin-resistant M. tuberculosis H37Rv with an FICI of 0.50. Notably, peptide-rifampicin used in combination generated a lower minimum effective concentration for each agent. Synergistic effects of AK15/AK15-6 with rifampincin can likely be attributed to the peptide-mediated permeation of rifampicin from outer membrane to cytoplasmic targets (15, 16, 24). As a result, the combinational usage of anti-mycobacterial peptide and antibiotic may slow the progression of drug-resistant M. tb and even prevent the emergence of drug-resistant M. tb.
Table 6.
Checkerboard assay of rifampicin and AK15 or AK15-6 against M. tuberculosis H37Rv
| M. tuberculosis | Drug combination | MIC |
FIC | FICIa | |
|---|---|---|---|---|---|
| Alone | Combination | ||||
| μg/ml | |||||
| H37Rv (RIF-susceptible) | Rifampicin | 0.012 | 0.003 | 0.25 | 0.5 |
| AK15 | 37.5 | 4.69 | 0.25 | ||
| Rifampicin | 0.012 | 0.003 | 0.25 | 0.5 | |
| AK15-6 | 18.75 | 2.34 | 0.25 | ||
| H37Rv (RIF-resistant) | Rifampicin | 1.5625 | 0.3906 | 0.25 | 0.5 |
| AK15 | 37.5 | 9.38 | 0.25 | ||
| Rifampicin | 1.5625 | 0.3906 | 0.25 | 0.5 | |
| AK15-6 | 18.75 | 4.69 | 0.25 | ||
a The FICI was calculated for each combination using the equation, FICI = FICA + FICB, where FICA = MIC of drug A in combination/MIC of drug A alone, and FICB = MIC of drug B in combination/MIC of drug B alone. FICI of ≤0.5 was interpreted as synergy, 0.5 < FICI ≤ 1.0 as additive, 1.0 < FICI ≤ 4.0 as indifferent, and FICI > 4.0 as antagonism. These concentrations represent mean values of three independent experiments performed in duplicates.
AK15 and its isomer AK15-6 exhibited thermal stability and low toxicity to mice
To translate these promising findings to clinical trials, it is extremely important to evaluate the thermal stability, serum stability, and acute toxicity of the peptides. As shown in Fig. S3A, after incubation at 37 °C for 96 h, the MIC values of the peptides against M. tuberculosis H37Rv just increased by 2–3-fold, which indicated that the peptides exhibited extreme thermal stability in aqueous solution.
We next detected the effect of human serum on the anti-mycobacterial activity of the peptides. As shown in Fig. S3B, the MIC values of the peptides against M. tuberculosis H37Rv gradually increased with the intended incubation time. After incubation of the peptides with human serum for 4 h, the peptides exhibited no detectable anti-mycobacterial activity against M. tuberculosis H37Rv at concentrations up to 200 μg/ml. Serum stability investigation indicated that AK15 and AK15-6 can be degraded by human serum proteins as many other AMPs.
We then evaluated the acute toxicity of the peptides to mice. The acute toxicity (24 h) of AK15 and AK15-6 was tested in female BALB/c mice by i.v. delivery. The highest amount of administered AK15/AK15-6 that does not kill the tested mice is defined as the maximum tolerable dose. The maximum tolerable dose of AK15/AK15-6 by i.v. delivery was between 150 and 175 mg/kg, well above the doses (10 mg/kg) used in mouse models as mentioned below.
AK15 and its isomer AK15-6 restrained TDM-stimulated inflammatory signaling activation and inflammatory cytokine production in murine macrophages
As mentioned above, AK15 and its isomer AK15-6 directly inhibited M. tb replication by binding to M. tb (TDM) and disrupting the mycobacterial membrane. To see whether the peptides could affect TDM-induced inflammatory response, we evaluated their effects on inflammatory signaling activation and inflammatory cytokine production in murine BMDMs stimulated by TDM, which acts as the most immunostimulatory component derived from M. tb.
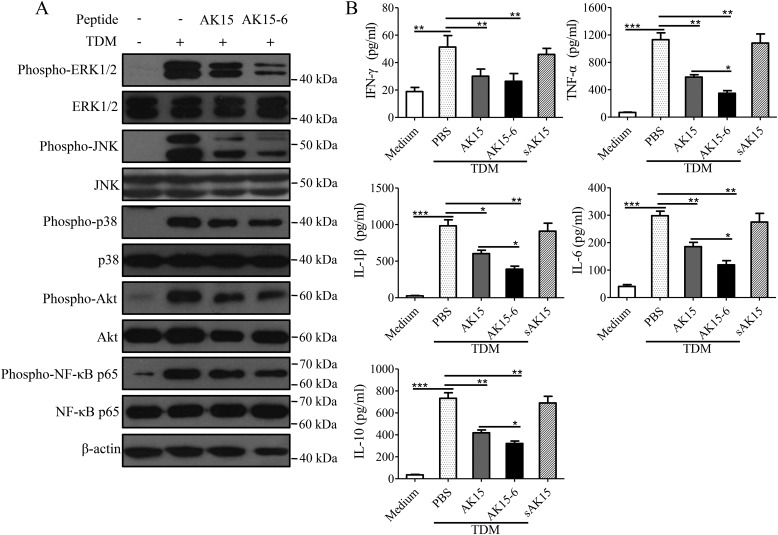
As illustrated in Fig. 9A, TDM stimulation markedly induced the activation (phosphorylation) of mitogen-activated protein kinases (extracellular signal–regulated kinase, c-Jun N-terminal kinase, and p38), protein kinase B (Akt), and NF-κB (p65) signaling, and the addition of AK15 or AK15-6 (20 μg/ml) inhibited TDM-induced inflammatory signaling activation in murine BMDMs.
Figure 9.
AK15 and its isomer AK15-6 restrained TDM-stimulated inflammatory response in murine BMDMs. A, AK15 and AK15-6 inhibited TDM-induced inflammatory signaling activation in murine BMDMs. BMDMs (1 × 106/well) were incubated with TDM (5 μg/ml) in the presence or absence of peptide (20 μg/ml). After incubation for 1 h, cells were lysed for Western blot analysis. B, AK15 and AK15-6 attenuated TDM-induced inflammatory cytokine production in murine BMDMs. BMDMs (2.5 × 105/well) were incubated with TDM (5 μg/ml) in the presence or absence of peptide. After incubation for 24 h, supernatants were harvested for determination of cytokine levels by ELISA. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Error bars, S.E.
As a result, TDM significantly induced inflammatory cytokine production in murine BMDMs, including IFN-γ, TNF-α, IL-1β, IL-6, and IL-10, whereas the peptides significantly attenuated this inflammatory cytokine production (Fig. 9B). As mentioned before, AK15-6 showed a higher binding affinity to TDM than did AK15. AK15-6 herein also showed a higher inhibitory efficiency against TDM-induced inflammatory signaling activation and inflammatory cytokine production in murine BMDMs than did AK15. It is more likely that the inhibitory effects of the peptides on TDM-stimulated inflammatory response in murine BMDMs are attributed to their binding to TDM and neutralizing the immunostimulatory activity of TDM. Similarly, the peptides also significantly attenuated the pro-inflammatory cytokine production in M. tb-infected murine BMDMs (Fig. S4).
AK15 and its isomer AK15-6 efficiently protected mice from M. tb infection
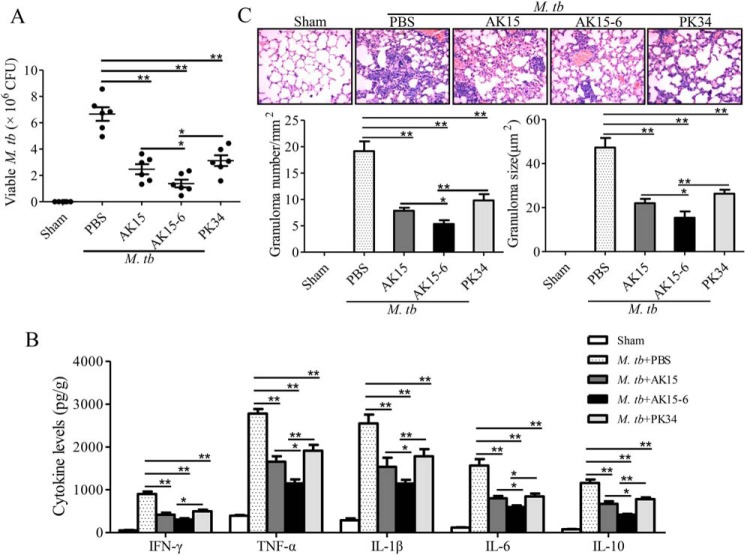
Although several anti-mycobacterial peptides were well-investigated in vitro, most of them lacked an anti-mycobacterial assay in vivo. Given the potent anti-mycobacterial and anti-inflammatory abilities of the peptides with low toxicity to mammalian cells and mice, we next evaluated the in vivo protective effects of AK15 and AK15-6 against M. tuberculosis H37Rv infection in mice. Compared with PBS-treated mice, AK15 or AK15-6 treatment (10 mg/kg) significantly reduced the mycobacterial loads, granulomatous responses (number and size), and inflammatory cytokine production (IFN-γ, TNF-α, IL-1β, IL-6, and IL-10) in lungs of M. tb-infected mice (Fig. 10). In addition, AK15 treatment (10 mg/kg) generated a better protective effect than did PK34, and AK15-6 therapy (10 mg/kg) did show the best protective effect among them, which was consistent with the results observed in vitro. At a dose of 10 mg/kg, AK15-6 inhibited about 79.0% mycobacterial load, 72.2% granuloma number, 67.5% granuloma size, 66.2% IFN-γ, 58.6% TNF-α, 55.1% IL-1β, 61.8% IL-6, and 64.1% IL-10 production in lungs as compared with PBS-treated mice, respectively (Fig. 10). The data indicated that the peptides efficiently restrained M. tb replication and inflammatory responses in vivo but sustained certain levels of pro-inflammatory cytokine production in vivo.
Figure 10.
AK15 and its isomer AK15-6 efficiently protected mice from M. tb infection. A, viable cfu counts in lung. B, protein levels of inflammatory cytokines in lung. C, granulomatous response (granuloma number and granuloma size) in lung. BALB/c mice (20 ± 2 g, n = 6) were intravenously injected with 2 × 106 cfu of M. tuberculosis H37Rv. After M. tb infection, mice were intravenously injected with peptides (10 mg/kg) or an equal volume of PBS (vehicle) once a day from day 1 to day 7. Sham mice received the same volume of vehicle (PBS). Mice were sacrificed by cervical dislocation at 4 weeks postinfection, and the cfu, inflammatory cytokine levels, and granulomatous response were evaluated, respectively. *, p < 0.05; **, p < 0.01. Error bars, S.E.
AK15 and its isomer AK15-6 enhanced modest basal immune responses in BMDMs and mice
In addition to direct antimicrobial activities, many antimicrobial peptides also showed diverse immunomodulatory activities, including induction of proinflammatory cytokines. We next assessed whether the peptides affected the basal immune response of BMDMs and mice by detecting their effects on proinflammatory cytokine production without TDM stimulation and M. tb infection.
As shown in Fig. S5, the incubation of BMDMs with AK15 or AK15-6 (20 μg/ml) elicited a modest level of TNF-α production (p < 0.05) as well as IL-6 production.
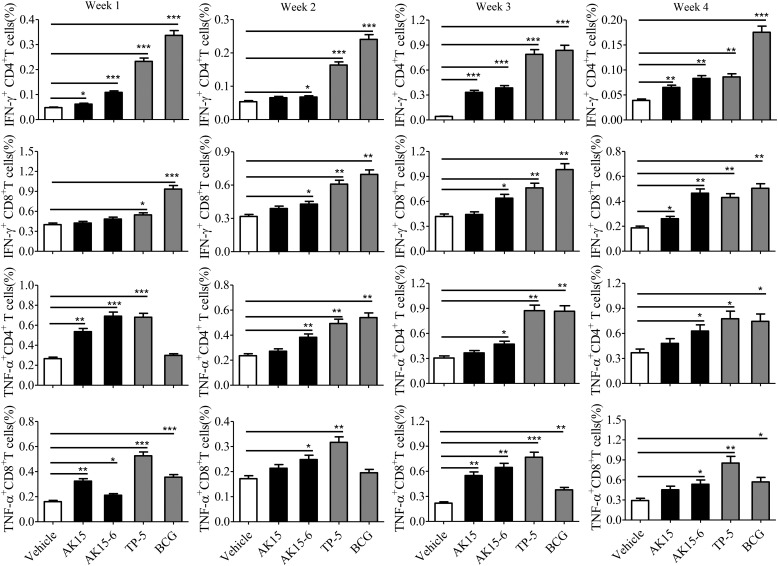
As described previously, the formation of an early granuloma is primarily regulated by IFN-γ and TNF-α released from the infected cells to restrain bacterial spread (29). In addition, the granuloma is mainly composed of CD4+ and CD8+ T cells (29), and IFN–γ+CD3+ T cells have been considered as dominant immunological cells to clear M. tb (30). Thus, we particularly investigated the effects of AK15 and AK15-6 on T-cell cytokine (IFN-γ and TNF-α) response in mice without M. tb infection. As shown in Fig. 11 and Fig. S6, the i.v. injection of AK15 or AK15-6 (10 mg/kg) elicited a modest IFN-γ and TNF-α response by CD4+ and CD8+ T cells in lungs. For example, AK15 and AK15-6 (10 mg/kg) significantly elicited IFN-γ+CD4+, TNF-α+CD4+, and TNF-α+CD8+ T cell response in lungs at week 1 followed by i.v. injection of the peptides as compared with vehicle (PBS)-treated mice. Similarly, the i.v. injection of AK15 or AK15-6 (10 mg/kg) simultaneously elicited a modest IFN-γ and TNF-α response by CD4+ and CD8+ T cells in spleen (Fig. S7). For instance, AK15 and AK15-6 (10 mg/kg) significantly elicited IFN-γ+CD4+, IFN-γ+CD8+, and TNF-α+CD8+ T cell responses in spleen at week 1 followed by i.v. injection of the peptides as compared with vehicle (PBS)-treated mice. The data indicated that AK15 and AK15-6 could modestly elicit the expression of pro-inflammatory cytokines in BMDMs and mice.
Figure 11.
AK15 and its isomer AK15-6 enhanced IFN–γ/TNF-α–secreting CD4+ and CD8+ T cell responses in the lung. Statistical analysis of the frequency of IFN-γ– or TNF-α–secreting CD4+ and CD8+ T cells. BALB/c mice were injected with peptide (10 mg/kg, dissolved in PBS intravenously) once a day at week 1. Control mice received the same volume of vehicle (PBS), M. bovis BCG (106 cfu/mouse), or TP-5 (10 mg/kg). Mice were sacrificed at weeks 1, 2, 3, and 4, respectively. Pulmonary lymphocytes were isolated, intracellularly stained with FITC-IFN-γ and APC-TNF-α antibody, and assayed by flow cytometry. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Error bars, S.E.
Discussion
Due to the limitations of bacillus Calmette–Guérin vaccine and rapid emergence of drug resistance, M. tb results in millions of deaths worldwide each year. We are obligated to look for alternative and/or supplemental ways to combat M. tb infection. The remarkable genetic diversity of mycobacteriophage may provide a promising platform of mycobacteriophage metaproteome to identify potent anti-mycobacterial peptides with potential for the treatment of M. tb infection. In a previous study (4), we identified a mycobacteriophage-derived anti-mycobacterial peptide, PK34, with a MIC value of 50 μg/ml against M. tuberculosis H37Rv (ATCC 27294), which proved that we can identify anti-tubercular peptides from mycobacteriophages. PK34 is composed of 34 amino acids with a molecular mass of 3957.55 Da. It is widely recognized that the large size, which corresponds to a higher production cost for drug development, limits the widespread use of peptide antibiotics (15). In the present study, we focused on the identification of a mycobacteriophage-derived anti-mycobacterial peptide of a small size. To our surprise, a small peptide, AK15, is composed of 15 amino acid residues with a lower molecular mass (2,071.55 Da) as compared with PK34 (3,957.55 Da). AK15 possessed a more potent anti-mycobacterial activity against M. tuberculosis H37Rv (ATCC 27294) with a MIC value of 37.5 μg/ml as compared with PK34 (50 μg/ml). AK15 even stayed active against rifampicin-induced drug-resistant M. tuberculosis H37Rv, which showed a good potential for controlling drug-resistant M. tb infection. The identification of AK15 with potent anti-mycobacterial activities provided a smaller mycobacteriophage-derived peptide candidate for anti-mycobacterial drug development with lower production cost. Combined with PK34 identified previously from mycobacteriophage (4), there are a total of two anti-mycobacterial peptides derived from mycobacteriophages. Although the true roles of these peptides in the mycobacteriophage life cycle are entirely unknown, the membrane-disrupting capacity of these peptides implies that they possibly facilitate the injection of DNA of mycobacteriophage to mycobacterial host or release of mycobacteriophage from mycobacterial host during infection.
Secondary structural analysis indicated that AK15 adopted cationic amphiphilic α-helical structure. The majority of AMPs adopt this arrangement, and their antimicrobial activities are largely attributed to such cationic amphiphilic α-helical structure (22, 31). The interactions between antimicrobial peptides and bacterial membrane are modulated by various physicochemical properties, including peptide hydrophobicity, charge, secondary structure, and amphiphilicity (32, 33). The roles of these physicochemical parameters of peptides in their antimicrobial properties are well-focused on Gram-positive bacteria, Gram-negative bacteria, and fungi (22, 31–34). More recently, an anti-mycobacterial assay of several designed peptides revealed that positive charges, amphiphilicity, and α-helix of such peptides are critical for their interactions with M. tb (15–17). However, there was no information about optimization of anti-mycobacterial peptide through rearranging amino acid residues. In this study, we tried to improve the anti-mycobacterial potency of AK15 by rearranging the amino acid residues of its amphiphilic helix to increase the hydrophobic moment and kept molecular weight, net charge, hydrophobicity, and α-helical confirmation unaltered. After rearrangement, AK15-6, one of the six isomers, exhibited an enhancement of anti-mycobacterial activity. A previous study (35) indicated that hydrophobic moment plot methodology is not a generally reliable predictor of structure–function relationship of α-helical peptide. In accordance with that, the other five isomers of AK15 with increased hydrophobic moment did not show an enhanced anti-mycobacterial potency (Table 2). In addition, we also designed several isomers of AK15 with decreased hydrophobic moment, and not all of them showed a decreased anti-mycobacterial activity (data not shown). As described previously, hydrophobic moment is a measure of the amphiphilicity of a helix (18). A more likely reason is that the increment of concentration of hydrophobic amino acid residues on one side and hydrophilic amino acid residues on the other side resulted in the increment of the hydrophobic moment (Fig. 1 and Table 2), which in turn increased the amphiphilicity and enhanced the interaction of AK15-6 with M. tb.
A large number of antimicrobial peptides showed many drawbacks, including high cytotoxicity against mammalian cells and broad spectrum against microbes, which limited their clinical application. In this study, AK15 and its isomer AK15-6 did not show any cytotoxicity and hemolytic activity toward the tested mammalian cells at concentrations up to 200 μg/ml (Table 3). In addition, AK15 and its isomer AK15-6 did not show any antimicrobial activity against the tested Gram-positive bacteria, Gram-negative bacteria, and fungi at concentrations below 150 μg/ml (Table S2). The results indicated that mycobacteriophage-derived AK15 and its isomer AK15-6 showed cell selectivity to M. tb. Similar results were observed in mycobacteriophage-derived anti-mycobacterial peptide PK34, which also showed cell selectivity to M. tb (4). It is more likely that mycobacteriophages are viruses that specifically infect mycobacterial hosts, and these mycobacteriophage-derived anti-mycobacterial peptides may show specificity to target the highly complex cell wall, cell membrane, or mycolic acids and peptidoglycan-arabinogalactan polymer on the outer membrane of M. tb, such as TDM (4, 21).
Real-time interaction of mycobacteriophage-derived AK15 or AK15-6 with TDM by surface plasmon resonance confirmed that they both showed apparent affinity to TDM, but TDM did not bind to sAK15, which demonstrated the binding specificity of TDM to the peptides (Fig. S8). In addition, the pre-incubation of TDM (100 μg/ml) with AK15 or AK15-6 (75 μg/ml) completely blocked the anti-mycobacterial activities of the peptides. The data indicated that the pre-incubation of TDM with the peptides led to the formation of TDM–AK15 or –AK15-6 complex and blocked the binding of AK15 or AK15-6 to TDM on the outer membrane of M. tb, which in turn blocked the anti-mycobacterial activities of the peptides. The direct interaction of the peptides with TDM demonstrated that TDM comprised a critical binding target of the peptides, and the binding of AK15 or AK15-6 to TDM on the outer membrane of M. tb constituted the first step of the anti-mycobacterial process. The apparent binding affinity of AK15 and AK15-6 to TDM at least partly interpreted the cell selectivity of the peptides to M. tb.
As mentioned above, it is well-known that proinflammatory response is extremely crucial for clearing off M. tb infection. However, AK15 and its isomer AK15-6 exhibited anti-inflammatory response by inhibition of cytokine production in TDM-induced BMDMs and M. tb-infected mice, which seemed to be a contradiction with their anti-mycobacterial activity in vivo. It is most likely that the anti-inflammatory activity of the peptides may be attributed to a consequence of their anti-mycobacterial effect by direct binding to TDM and inactivation of M. tb. In other words, peptide treatment led to a reduction of mycobacterial load, which consequently led to a reduction of granuloma formation and proinflammatory cytokine production in the lungs of M. tb-infected mice.
Recently, cytokines in tuberculosis have been considered as a two-edged sword in TB pathogenesis (29). M. tb can orchestrate a cytokine storm to activate and/or evade host immune responses during infection (29). Thus, the inhibition of a large amount of cytokine production in TDM-stimulated murine BMDMs and M. tb-infected mice by the peptides might be a good aspect for controlling M. tb infection, as reflected by a better outcome after AK15/AK15-6 treatment. In addition to the inhibition of a large amount of cytokine production, AK15 and AK15-6 simultaneously exhibited a modest enhancement of the basal immune response by eliciting a certain level of proinflammatory cytokines. Considering the critical roles of proinflammatory cytokine in clearing off M. tb infection, the induction of certain level of regulatory cytokines by the peptides both in vitro and in vivo may thus complement their anti-mycobacterial activities. We thereby concluded that AK15 and its isomer AK15-6 exhibit immunomodulatory effects against M. tb infection by inhibiting cytokine storm and enhancing host basal immune response.
To compare mycobacteriophage-derived peptides with other published anti-mycobacterial peptides, we selected four well-studied small anti-mycobacterial peptides for an anti-mycobacterial assay in the same conditions as AK15 and AK15-6 and summarized many other small cationic anti-mycobacterial peptides reported in previous papers (37–39). As shown in Table S3, the four selected well-studied small anti-mycobacterial peptides possessed potent anti-M. tb activities against the tested mycobacterial strains with MIC values ranging from 11.2 to 120.4 μm, whereas AK15 and its isomer AK15-6 possessed MIC values ranging from 9.1 to 36.2 μm. The data indicated that AK15 and AK15-6 showed comparable anti-M. tb activities with Pin2[14], Pin2[17], and IDR-HH2. Then we summarized the anti-mycobacterial performance of many other small cationic anti-mycobacterial peptides from previous papers in Table S4. A performance comparison indicated that the well-studied small cationic anti-mycobacterial peptides showed different anti-mycobacterial efficiencies with MIC values ranging from 0.7 to 141 μm, and mycobacteriophage-derived AK15 and its isomer AK15-6 exhibited potent anti-mycobacterial activities as most of the well-studied peptides, with MIC values ranging from 4.53 to 36.2 μm (Table 1). In the future, the anti-mycobacterial potency as well as stability of AK15 and AK15-6 might be further improved in many ways, such as d-enantiomer residue substitution, PEG conjugation, hyaluronic acid nanogel conjugation, chitosan nanogel conjugation, and combinational usage with antibiotics, among many others.
In summary, we characterized a small cationic amphiphilic α-helical anti-mycobacterial peptide (AK15) derived from mycobacteriophage. In addition, an improved isomer named AK15-6 was designed by rearranging the amino acid residues of the helix. AK15 and AK15-6 efficiently restrained mycobacterial replication by binding to TDM and disrupting the mycobacterial membrane. Lysine, arginine, tryptophan, and an α-helix are key structural requirements for their anti-M. tb activities. They showed bactericidal activity, cell selectivity, and synergistic effects with rifampicin, and none induced drug resistance to M. tb. In addition, they simultaneously exhibited immunomodulatory effects by inhibiting the production of a large amount of proinflammatory cytokines and enhancing the basal immune responses of the host. Because of their small size, AK15 and AK15-6 might be good candidates for anti-mycobacterial peptide antibiotic development. Our study provides novel insights to identify small but potent anti-mycobacterial peptide from mycobacteriophage and highlights new clues to design and improve small and potent anti-mycobacterial peptide.
Experimental procedures
Ethics approval
BALB/c mice (20 ± 2 g, female) were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. and housed in a pathogen-free facility in accordance with the Guide for the Care and Use of Medical Laboratory Animals (Ministry of Health, People's Republic of China, 1998), and animal experiment procedures were approved by the Animal Care and Use Committee as well as the Ethical Committee of Soochow University (approval SYXK2017-0043). All surgery was performed under sodium pentobarbital anesthesia with minimum fear, anxiety, and pain.
Collection of venous blood from healthy volunteers was approved by the Ethical Committee of Soochow University. All donors provided informed consent in written form.
Mycobacteria, cells, and peptides
M. tuberculosis H37Rv (ATCC 27294), M. tuberculosis H37Ra, and clinically isolated M. tb strains were provided by the Affiliated Hospital of Zunyi Medical University or Kunming Medical University, and were cultured in a Middlebrook 7H9 medium or enumerated on 7H11 agar supplemented with 10% oleic acid–albumin–dextrose–catalase, 0.50% glycerol, and 0.05% Tween 80.
Murine BMDMs from BALB/c mice were collected (40) and cultured in RPMI 1640 containing l-glutamine, pyruvate, β-mercaptoethanol (all from Sigma), antibiotics (100 units/ml penicillin, 100 μg/ml streptomycin, Gibco), 10% fetal bovine serum, and 20 ng/ml murine recombinant M-CSF (Biolegend) for 7–8 days. Differentiated BMDMs were replated for experiment. Vero E6 cells were gifted by Dr. Chunsheng Dong and cultured in Dulbecco's modified Eagle's medium supplemented with antibiotics and 10% FBS. All cells were cultured in a humidified incubator under 5% CO2 at 37 °C.
Peptides were purchased from Synpeptide Co. Ltd. (Shanghai, China). The crude peptides were purified and analyzed by reverse-phase HPLC and MALDI-TOF MS to confirm that the purity was higher than 98%.
Peptide candidate selection
Up to now, more than 1,400 mycobacteriophage genomes have been completely sequenced (http://phagesdb.org/),6 which provided a promising library to identify anti-mycobacterial peptides (4, 41). By mining mycobacteriophage genomes in the United States National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov/), we selected and synthesized a series of small mycobacteriophage-encoded peptides or their derivatives for anti-mycobacterial assay in vitro. These peptide candidates exhibited a high degree of sequence conservation in different mycobacteriophages and were composed of 10–30 amino acid residues and positively charged.
To improve the anti-mycobacterial potency of the interesting peptide candidate, isomers were designed by rearranging the amino acid residues to increase concentration of hydrophobic amino acid residues on one side, and hydrophilic amino acid residues on the other side by a helix-wheel diagram assay. Isomers with increased hydrophobic moment and unaltered amino acid composition, secondary confirmation, net charge, molecular weight, and hydrophobicity were selected for an anti-mycobacterial activity assay by MIC determination.
Minimum inhibitory concentration assay
MICs of peptides against M. tuberculosis, Gram-positive, Gram-negative and fungi were determined according to previous methods (15, 34). Briefly, a series of 2-fold peptide dilutions was prepared in a 96-well plate (100 μl/well) and added to an equal volume of bacterial cells (∼105 cfu/ml, 100 μl/well) in each well. The plates were then incubated at 37 °C and read after 7 days for M. tuberculosis and 18 h for Gram-negative bacteria, Gram-positive bacteria, and fungi. The MIC was defined as the lowest peptide concentration at which no growth of microorganism was observed visually or measured at 600 nm.
Structural analysis
An online computational framework was used to predict the secondary structure (http://bioserv.rpbs.univ-paris-diderot.fr/services/PEP-FOLD3)6 (42). Physical and chemical parameters of the peptides were analyzed using the software package provided by the Expert Protein Analysis System (ExPASy) proteomics server (http://www.expasy.org).6 The helix-wheel plot was constructed by HeliQuest (http://heliquest.ipmc.cnrs.fr/)6 (31, 45).
The secondary structural confirmation was verified by CD analysis. Peptides (0.2 mg/ml) were dissolved in a membrane-mimetic environment (TFE/H2O, 9:1). CD spectra were collected on a Jasco-810 spectropolarimeter (Jasco, Tokyo, Japan) with a 1-mm path length cell (25 °C, 0.2-nm interval from 190 to 260 nm). For each spectrum, the data from three scans were averaged and smoothed by Jasco-810 software. CD spectra were expressed as the mean residue ellipticity (θ) in degrees·cm2·dmol−1.
Mycobacterial killing kinetic assay
The mycobacterial killing kinetic assay was performed according to a previous report (43). Tubes containing 7H9 broth (10 ml) with peptide at concentrations of 0.5 × MIC, 1 × MIC, and 2 × MIC were inoculated with M. tuberculosis H37Rv (∼107 cfu/ml) and incubated at 37 °C for 4, 7, 14, and 21 days, respectively. At each time point, aliquots were removed and diluted in a series of Difco Middlebrook 7H9 broth for the determination of viable counts. Diluted samples were plated onto Difco Middlebrook 7H11 agar plates, and total mycobacterial counts were determined after incubation at 37 °C for 4 weeks.
Hemolysis and cytotoxicity
Hemolytic activity was evaluated by incubating a series of 2-fold diluted peptides with human and rabbit red blood cells at 37 °C for 30 min. Hemoglobin concentration was measured by monitoring the absorbance at 540 nm. The positive control of 1% Triton X-100 (v/v) was determined as 100% hemolysis, and 0.9% saline was used as negative control (34).
Cytotoxicity was determined by incubating a series of 2-fold diluted peptides with RAW264.7 cells, murine peritoneal macrophages and Vero E6 cells in 96-well plates (2 × 104 cells/well) (34). After incubation with peptides or vehicle (PBS) at 37 °C for 24 h, CCK-8 solution (10 μl/well) was added. After incubation for an additional 2–4 h, absorbance at 450 nm was measured.
Flow cytometry analysis
M. tuberculosis H37Ra, E. coli, and S. aureus cells were washed twice with PBS and exposed to FITC-labeled AK15 or AK15-6 (1 μg/ml) for 5 min at 37 °C. PBS and FITC-labeled sAK15 served as control, respectively. After exposure, bacterial cells were washed three times with PBS, assayed on a FACScalibur flow cytometer, and analyzed by Cell Quest software (BD Immunocytometry).
Surface plasmon resonance
The binding interaction of peptides with mycobacterial cord factor (TDM) was measured using a Biacore 3000 instrument (Biacore, Piscataway, NJ) at 25 °C according to the previous method (4). Peptides were immobilized as ligand on a CM5 sensor chip using an amine-coupling kit (GE Healthcare) according to the manufacturer's instructions. In brief, around 1000 RU of immobilized peptide was obtained. Different dilutions of TDM (5, 10, 20, and 40 nm) in running buffer (HBS-N buffer, GE Healthcare) were injected as analytes for 3 min at a flow rate of 20 μl/min for binding analysis. The surface of the CM5 sensor chip was regenerated with 40 mm NaOH. The values of binding affinity of TDM for the peptides were calculated by BIAevaluation 3.0 software (Biacore) selecting a 1:1 Langmuir binding model for the kinetic calculation.
Effect of TDM on the anti-mycobacterial activity of the peptides
Peptides (750 μg/ml), TDM (10, 100, and 1,000 μg/ml), and peptide (750 μg/ml) mixed with 10, 100, or 1,000 μg/ml of TDM were incubated at 37 °C for 5 min with 150-rpm shaking. Then peptide, TDM, peptide-TDM mixture, or the same volume of vehicle (PBS, DMSO) was inoculated with M. tuberculosis H37Rv (∼107 cfu/ml), respectively. The final concentrations of TDM were 1, 10, and 100 μg/ml, and the final concentration of peptide was 75 μg/ml, respectively. After incubation at 37 °C for 7 days, the viable mycobacteria were counted as mentioned above.
Confocal microscopy
M. tuberculosis H37Ra, E. coli, and S. aureus suspension (400 μl, 5 × 105 cfu/ml) was seeded into an 8-well coverslip chamber. Then an equal volume of PBS solution containing peptide (75 μg/ml) and FITC-dextran (150 kDa, 250 μg/ml; Sigma) were added. After incubation for 1 h at 37 °C with constant shaking at 200 rpm, the bacterial cells were washed three times with PBS to remove the free FITC-dextran. The images were acquired using a Nikon A1 confocal microscope.
ATP bioluminescence assay
Extracellular ATP levels after incubation of M. tuberculosis H37Ra with peptides were detected as described previously (16). Briefly, M. tuberculosis H37Ra was washed and resuspended in 10 mm phosphate buffer (A600 = 0.4). A series of peptide dilutions was prepared with the same buffer and added to an equal volume of M. tb suspension (300 μl). After incubation at 37 °C for 2 h, samples were centrifuged at 5,000 × g for 5 min. The ATP levels in the supernatant (extracellular ATP concentrations) were determined using an ATP assay kit (Beyotime, Shanghai, China) according to the manufacturer's instructions.
Scanning EM
M. tuberculosis H37Ra cells (∼2 × 107 cfu/ml) were incubated with peptides (187.5 μg/ml) or an equal volume of vehicle (PBS) at 37 °C for 4 h. After centrifugation at 1,000 × g for 10 min, mycobacterial cells were prepared for scanning EM analysis as described previously (15). The surface morphology was observed with a Hitachi S-4800 scanning electron microscope following the manufacturer's instruction.
Drug resistance stimulation
Drug resistance was induced in M. tuberculosis H37Rv by repeated treatment with rifampicin, peptide, or the same volume of vehicle (DMSO, PBS) for 16 passages and checked via MIC measurement. For each passage, M. tuberculosis H37Rv was exposed to sub-MIC concentration of rifampicin or peptide (one-eighth of MIC at that particular passage) until growing to the log phase, and the MICs of peptide and rifampicin against M. tuberculosis H37Rv were determined as mentioned above (15).
Checkerboard assay
Synergistic interactions between peptide and rifampicin were detected by a checkerboard assay as described previously (15, 44). Briefly, a series of 2-fold rifampicin or peptide dilutions were prepared, and equal volumes of rifampicin (50 μl/well) and peptide (50 μl/well) were mixed in a 96-well plate. Then M. tuberculosis H37Rv (∼105 cfu/ml, 100 μl/well) was added into each well. After incubation at 37 °C for 7 days, microbial growth was observed visually or recorded spectrophotometrically at 600 nm.
Effects of the peptides on TDM-stimulated inflammatory signaling activation and cytokine production
BMDMs (1 × 106/well) were cultured in RPMI 1640 containing 2% FBS in a 6-well culture plate. TDM (5 μg/ml; Sigma) and peptide (20 μg/ml) were added and incubated for 1 h. After incubation, BMDMs were harvested and lysed with radioimmune precipitation assay lysis buffer (Beyotime, China). Total protein (40 μg) was separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The inflammatory signals were detected as described previously (4).
To detect the effect of the peptides on TDM-stimulated inflammatory cytokine production in macrophages, BMDMs (2.5 × 105/well) were plated into a 24-well culture plate in RPMI 1640 containing 2% FBS. TDM (5 μg/ml; Sigma) and peptide (20 μg/ml) were added and incubated for 24 h. Cytokine levels in the supernatant were measured using mouse cytokine ELISA kits (eBioscience) according to the kit instructions.
Thermal stability and serum stability
Peptide solution was prepared in sterile deionized water (2 mg/ml) and incubated at 37 °C for 0, 6, 12, 24, 48, 72, and 96 h. Thermal stability of the peptide was evaluated by detection of the MIC value of the peptide against M. tuberculosis H37Rv after incubation of the peptide at 37 °C for 0–96 h (22).
To evaluate serum stability of the peptide, peptide solution was prepared in sterile deionized water (10 mg/ml), and peptide solution was mixed with human serum at a volume ratio of 1:4 to reach a final concentration of 2 mg/ml. The mixture was incubated at 37 °C for 0–6 h. Serum stability of the peptide was evaluated by determination of the MIC value of the peptide against M. tuberculosis H37Rv after incubation of the peptide with human serum for different times (22).
In vivo anti-mycobacterial assay
The in vivo protective effects of the peptides were determined in a mouse model of pulmonary tuberculosis using PK34 as a positive control. BALB/c mice (20 ± 2 g, n = 6) were infected by i.v. injection of 2 × 106 cfu of M. tuberculosis H37Rv suspension in 7H9 medium. Mice were administered by i.v. injection of peptides (10 mg/kg, dissolved in PBS) once a day from day 1 to day 7 after M. tb infection. PBS treatment served as a negative control. Sham mice received the same volume of vehicle (PBS). Mice were sacrificed by cervical dislocation at 4 weeks postinfection. The left lungs were taken and homogenized in PBS (1 mg of lung/ml of PBS) on ice. Series of diluted homogenates (10−1, 10−2, 10−3, 10−4, 10−5, and 10−6) were plated onto Difco Middlebrook 7H11 agar plates, and cfu were counted after culture at 37 °C for 4 weeks. For cytokine measurement, homogenates of left lung were centrifuged at 12,000 × g for 15 min. The supernatants were harvested for cytokine determination using ELISA kits (eBioscience) according to the manufacturer's instructions. The right lungs were collected and fixed in 10% formalin solution for 24 h. After dehydration by an increasing concentration of alcohol, tissues were embedded in paraffin and sectioned into a thickness of 5 μm using a histocut (Leica, Germany). Sections were stained with hematoxylin and eosin and observed by light microscopy (Nikon Eclipse TE2000-S, Japan). Granulomatous response was calculated from the photographs using PhotoShop (Adobe Photoshop Element 2.0, Adobe Systems). The granuloma number of each mouse in the whole lung section was counted. The average granuloma size of each mouse was determined by randomly selecting 10 granulomas in three microscopic fields (36).
Effects of the peptides on the basal immune response of BMDMs and mice
BMDMs (2.5 × 105/well, 24-well culture plate) were incubated with peptide (20 μg/ml) in RPMI 1640 medium (2% FBS). After incubation for 24 h, cytokine levels in the supernatant were measured as mentioned above.
To investigate the effects of the peptides on the basal immune response of mice, BALB/c mice (20 ± 2 g, n = 6) were intravenously injected with peptide (10 mg/kg, dissolved in PBS) once a day at week 1. Control mice received the same volume of vehicle (PBS), M. bovis BCG (106 cfu/mouse), or TP-5 (10 mg/kg, thymopentin, RKDVY, a clinically used immunomodulatory peptide). Mice were sacrificed at weeks 1, 2, 3, and 4, respectively. Pulmonary lymphocytes were isolated according to a previous method (30). Briefly, lungs were cut and added with collagenase IV (1 mg/ml; Sigma) and DNase I (5 units/ml; Sigma). After digestion for 1.5 h at 37 °C, the digested lungs were mashed through a 70-μm Falcon strainer. Cells were centrifuged and resuspended in RPMI 1640 medium (2% FBS).
Pulmonary lymphocytes or splenocytes were stimulated with PMA (10 ng/ml; Sigma) and ionomycin (500 ng/ml, Sigma) for 5 h in the presence of brefeldin A (10 μg/ml; BD Pharmingen). All antibody stains were performed on ice, and centrifugation was carried out at 4 °C. About 1 × 106 cells were first stained with surface markers of FITC-CD3, APC-Cy7-CD4, and PerCP-CD8 (BD Pharmingen) for 30 min and then fixed and permeabilized by Cyto Fix/Perm (BD Pharmingen) for 30 min. After intracellular staining with FITC-IFN-γ and APC-TNF-α antibody (BD Biosciences), cells were assayed by flow cytometry as mentioned above.
Statistical analysis
Statistical analyses between two groups were done by Student's t tests using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA). The threshold for significance was 0.05 or better. All experiments were repeated at least three times, and data are presented as mean ± S.E.
Author contributions
Y. Y., Z. L., and X. H. data curation; Y. Y., Z. L., X. H., and J. Y. investigation; Y. Y., Z. L., X. H., J. W., H. Y., Q. Q., and L. W. methodology; J. W., H. Y., and R. L. resources; M. L. project administration; R. L., W. X., and L. W. supervision; R. L., W. X., and L. W. funding acquisition; R. L., W. X., and L. W. writing-review and editing; L. W. writing-original draft.
Supplementary Material
This work was supported by National Natural Science Foundation of China Grants 81402830, 31870868, 81603080, 31670930, 31700656, and 81802023; China Postdoctoral Science Foundation Grant 2015M571815; Ministry of Science and Technology of China Grant 2018ZX10731301-001-006; Natural Science Foundation of Jiangsu Province Grant BK20140362 and Fujian Province Grant 2017J05050; Priority Academic Program Development of Jiangsu Higher Education Institutions; Program for Changjiang Scholars and Innovative Research Team in University Grant PCSIRTIRT1075; and Science and Technology Development of Suzhou Grants SNG2017050 and sys2018017. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Tables S1–S4 and Figs. S1–S8.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.
- TB
- tuberculosis
- FIC
- fractional inhibitory concentration
- FICI
- fractional inhibitory concentration index
- Mt. b
- Mycobacterium tuberculosis
- MIC
- minimum inhibitory concentration
- RU
- resonance units
- sAK15
- scrambled AK15
- i.v.
- intravenous
- TDM
- trehalose 6,6′-dimycolate
- BMDM
- bone marrow-derived macrophage
- IFN
- interferon
- TNF
- tumor necrosis factor
- IL
- interleukin
- FBS
- fetal bovine serum
- TFE
- trifluoroethanol.
References
- 1. Walzl G., Ronacher K., Hanekom W., Scriba T. J., and Zumla A. (2011) Immunological biomarkers of tuberculosis. Nat. Rev. Immunol. 11, 343–354 10.1038/nri2960 [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (2017) Global Tuberculosis Report 2017, World Health Organization, Geneva [Google Scholar]
- 3. Abu-Raddad L. J., Sabatelli L., Achterberg J. T., Sugimoto J. D., Longini I. M. Jr., and Dye C., and Halloran M. E. (2009) Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc. Natl. Acad. Sci. U.S.A. 106, 13980–13985 10.1073/pnas.0901720106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wei L., Wu J., Liu H., Yang H., Rong M., Li D., Zhang P., Han J., and Lai R. (2013) A mycobacteriophage-derived trehalose-6,6′-dimycolate–binding peptide containing both antimycobacterial and anti-inflammatory abilities. FASEB J. 27, 3067–3077 10.1096/fj.13-227454 [DOI] [PubMed] [Google Scholar]
- 5. Rybniker J., Kramme S., and Small P. L. (2006) Host range of 14 mycobacteriophages in Mycobacterium ulcerans and seven other mycobacteria including Mycobacterium tuberculosis—application for identification and susceptibility testing. J. Med. Microbiol. 55, 37–42 10.1099/jmm.0.46238-0 [DOI] [PubMed] [Google Scholar]
- 6. Payne K. M., and Hatfull G. F. (2012) Mycobacteriophage endolysins: diverse and modular enzymes with multiple catalytic activities. PLoS One 7, e34052 10.1371/journal.pone.0034052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pohane A. A., Joshi H., and Jain V. (2014) Molecular dissection of phage endolysin: an interdomain interaction confers host specificity in Lysin A of Mycobacterium phage D29. J. Biol. Chem. 289, 12085–12095 10.1074/jbc.M113.529594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pohane A. A., Patidar N. D., and Jain V. (2015) Modulation of domain–domain interaction and protein function by a charged linker: a case study of mycobacteriophage D29 endolysin. FEBS Lett. 589, 695–701 10.1016/j.febslet.2015.01.036 [DOI] [PubMed] [Google Scholar]
- 9. Lai M. J., Liu C. C., Jiang S. J., Soo P. C., Tu M. H., Lee J. J., Chen Y. H., and Chang K. C. (2015) Antimycobacterial activities of endolysins derived from a mycobacteriophage, BTCU–1. Molecules 20, 19277–19290 10.3390/molecules201019277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gil F., Catalão M. J., Moniz-Pereira J., Leandro P., McNeil M., and Pimentel M. (2008) The lytic cassette of mycobacteriophage Ms6 encodes an enzyme with lipolytic activity. Microbiology 154, 1364–1371 10.1099/mic.0.2007/014621-0 [DOI] [PubMed] [Google Scholar]
- 11. Pedulla M. L., Ford M. E., Houtz J. M., Karthikeyan T., Wadsworth C., Lewis J. A., Jacobs-Sera D., Falbo J., Gross J., Pannunzio N. R., Brucker W., Kumar V., Kandasamy J., Keenan L., Bardarov S., et al. (2003) Origins of highly mosaic mycobacteriophage genomes. Cell 113, 171–182 10.1016/S0092-8674(03)00233-2 [DOI] [PubMed] [Google Scholar]
- 12. Hatfull G. F., Jacobs-Sera D., Lawrence J. G., Pope W. H., Russell D. A., Ko C. C., Weber R. J., Patel M. C., Germane K. L., Edgar R. H., Hoyte N. N., Bowman C. A., Tantoco A. T., Paladin E. C., Myers M. S., et al. (2010) Comparative genomic analysis of 60 Mycobacteriophage genomes: genome clustering, gene acquisition, and gene size. J. Mol. Biol. 397, 119–143 10.1016/j.jmb.2010.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teng T., Liu J., and Wei H. (2015) Anti–mycobacterial peptides: from human to phage. Cell Physiol. Biochem. 35, 452–466 10.1159/000369711 [DOI] [PubMed] [Google Scholar]
- 14. Khusro A., Aarti C., and Agastian P. (2016) Anti–tubercular peptides: a quest of future therapeutic weapon to combat tuberculosis. Asian Pac. J. Trop. Med. 9, 1023–1034 10.1016/j.apjtm.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 15. Khara J. S., Wang Y., Ke X. Y., Liu S., Newton S. M., Langford P. R., Yang Y. Y., and Ee P. L. (2014) Anti-mycobacterial activities of synthetic cationic α-helical peptides and their synergism with rifampicin. Biomaterials 35, 2032–2038 10.1016/j.biomaterials.2013.11.035 [DOI] [PubMed] [Google Scholar]
- 16. Khara J. S., Lim F. K., Wang Y., Ke X. Y., Voo Z. X., Yang Y. Y., Lakshminarayanan R., and Ee P. L. R. (2015) Designing α-helical peptides with enhanced synergism and selectivity against Mycobacterium smegmatis: Discerning the role of hydrophobicity and helicity. Acta Biomater. 28, 99–108 10.1016/j.actbio.2015.09.015 [DOI] [PubMed] [Google Scholar]
- 17. Brennan P. J., and Nikaido H. (1995) The envelope of mycobacteria. Annu. Rev. Biochem. 64, 29–63 10.1146/annurev.bi.64.070195.000333 [DOI] [PubMed] [Google Scholar]
- 18. Eisenberg D., Weiss R. M., and Terwilliger T. C. (1982) The helical hydrophobic moment: a measure of the amphiphilicity of a helix. Nature 299, 371–374 10.1038/299371a0 [DOI] [PubMed] [Google Scholar]
- 19. Haas W., Pillar C. M., Hesje C. K., Sanfilippo C. M., and Morris T. W. (2010) Bactericidal activity of besifloxacin against staphylococci, Streptococcus pneumoniae and Haemophilus influenzae. J. Antimicrob. Chemother. 65, 1441–1447 10.1093/jac/dkq127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oh D., Shin S. Y., Lee S., Kang J. H., Kim S. D., Ryu P. D., Hahm K. S., and Kim Y. (2000) Role of the hinge region and the tryptophan residue in the synthetic antimicrobial peptides, cecropin A(1–8)-magainin 2(1–12) and its analogues, on their antibiotic activities and structures. Biochemistry 39, 11855–11864 10.1021/bi000453g [DOI] [PubMed] [Google Scholar]
- 21. Matsunaga I., and Moody D. B. (2009) Mincle is a long sought receptor for mycobacterial cord factor. J. Exp. Med. 206, 2865–2868 10.1084/jem.20092533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wei L., Gao J., Zhang S., Wu S., Xie Z., Ling G., Kuang Y. Q., Yang Y., Yu H., and Wang Y. (2015) Identification and characterization of the first cathelicidin from sea snakes with potent antimicrobial and anti-inflammatory activity and special mechanism. J. Biol. Chem. 290, 16633–16652 10.1074/jbc.M115.642645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei L., Huang C., Yang H., Li M., Yang J., Qiao X., Mu L., Xiong F., Wu J., and Xu W. (2015) A potent anti-inflammatory peptide from the salivary glands of horsefly. Parasit. Vectors 8, 556 10.1186/s13071-015-1149-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Y., Ke X. Y., Khara J. S., Bahety P., Liu S., Seow S. V., Yang Y. Y., and Ee P. L. (2014) Synthetic modifications of the immunomodulating peptide thymopentin to confer anti-mycobacterial activity. Biomaterials 35, 3102–3109 10.1016/j.biomaterials.2013.12.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee E., Kim J. K., Jeon D., Jeong K. W., Shin A., and Kim Y. (2015) Functional roles of aromatic residues and helices of papiliocin in its antimicrobial and anti-inflammatory activities. Sci. Rep. 5, 12048 10.1038/srep12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anantharaman A., Rizvi M. S., and Sahal D. (2010) Synergy with rifampin and kanamycin enhances potency, kill kinetics, and selectivity of de novo–designed antimicrobial peptides. Antimicrob. Agents Chemother. 54, 1693–1699 10.1128/AAC.01231-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barriere S. L. (1992) Bacterial resistance to β-lactams, and its prevention with combination antimicrobial therapy. Pharmacotherapy 12, 397–402 [PubMed] [Google Scholar]
- 28. Wu Y. L., Scott E. M., Po A. L., and Tariq V. N. (1999) Ability of azlocillin and tobramycin in combination to delay or prevent resistance development in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 44, 389–392 10.1093/jac/44.3.389 [DOI] [PubMed] [Google Scholar]
- 29. Etna M. P., Giacomini E., Severa M., and Coccia E. M. (2014) Pro- and anti-inflammatory cytokines in tuberculosis: a two-edged sword in TB pathogenesis. Semin. Immunol. 26, 543–551 10.1016/j.smim.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 30. Wu M., Zhao H., Li M., Yue Y., Xiong S., and Xu W. (2017) Intranasal vaccination with mannosylated chitosan formulated DNA vaccine enables robust IgA and cellular response induction in the lungs of mice and improves protection against pulmonary mycobacterial challenge. Front. Cell Infect. Microbiol. 7, 445 10.3389/fcimb.2017.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jin L., Bai X., Luan N., Yao H., Zhang Z., Liu W., Chen Y., Yan X., Rong M., Lai R., and Lu Q. (2016) A designed tryptophan- and lysine/arginine-rich antimicrobial peptide with therapeutic potential for clinical antibiotic-resistant Candida albicans vaginitis. J. Med. Chem. 59, 1791–1799 10.1021/acs.jmedchem.5b01264 [DOI] [PubMed] [Google Scholar]
- 32. Dathe M., and Wieprecht T. (1999) Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells. Biochim. Biophys. Acta 1462, 71–87 10.1016/S0005-2736(99)00201-1 [DOI] [PubMed] [Google Scholar]
- 33. Fjell C. D., Hiss J. A., Hancock R. E., and Schneider G. (2011) Designing antimicrobial peptides: form follows function. Nat. Rev. Drug Discov. 11, 37–51 10.1038/nrd3591 [DOI] [PubMed] [Google Scholar]
- 34. Wei L., Yang J., He X., Mo G., Hong J., Yan X., Lin D., and Lai R. (2013) Structure and function of a potent lipopolysaccharide-binding antimicrobial and anti-inflammatory peptide. J. Med. Chem. 56, 3546–3556 10.1021/jm4004158 [DOI] [PubMed] [Google Scholar]
- 35. Phoenix D., and Harris F. (2003) Is use of the hydrophobic moment a sound basis for predicting the structure-function relationships of membrane interactive α-helices? Curr. Protein Pept. Sci. 4, 357–366 [DOI] [PubMed] [Google Scholar]
- 36. Welsh K. J., Hwang S. A., Hunter R. L., Kruzel M. L., and Actor J. K. (2010) Lactoferrin modulation of mycobacterial cord factor trehalose 6–6′-dimycolate induced granulomatous response. Transl. Res. 156, 207–215 10.1016/j.trsl.2010.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gutsmann T. (2016) Interaction between antimicrobial peptides and mycobacteria. Biochim. Biophys. Acta 1858, 1034–1043 10.1016/j.bbamem.2016.01.031 [DOI] [PubMed] [Google Scholar]
- 38. Arranz-Trullén J., Lu L., Pulido D., Bhakta S., and Boix E. (2017) Host antimicrobial peptides: the promise of new treatment strategies against tuberculosis. Front. Immunol. 8, 1499 10.3389/fimmu.2017.01499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abedinzadeh M., Gaeini M., and Sardari S. (2015) Natural antimicrobial peptides against Mycobacterium tuberculosis. J. Antimicrob. Chemother. 70, 1285–1289 10.1093/jac/dku570 [DOI] [PubMed] [Google Scholar]
- 40. Genoula M., Marín Franco J. L., Dupont M., Kviatcovsky D., Milillo A., Schierloh P., Moraña E. J., Poggi S., Palmero D., Mata-Espinosa D., González-Domínguez E., León Contreras J. C., Barrionuevo P., Rearte B., Córdoba Moreno M. O., et al. (2018) Formation of foamy macrophages by tuberculous pleural effusions is triggered by the interleukin–10/signal transducer and activator of transcription 3 axis through ACAT upregulation. Front Immunol. 9, 459 10.3389/fimmu.2018.00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hatfull G. F. (2014) Mycobacteriophages: windows into tuberculosis. PLoS Pathog. 10, e1003953 10.1371/journal.ppat.1003953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lamiable A., Thévenet P., Rey J., Vavrusa M., Derreumaux P., and Tufféry P. (2016) PEP–FOLD3: faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 44, W449–W454 10.1093/nar/gkw329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang F., Sambandan D., Halder R., Wang J., Batt S. M., Weinrick B., Ahmad I., Yang P., Zhang Y., Kim J., Hassani M., Huszar S., Trefzer C., Ma Z., et al. (2013) Identification of a small molecule with activity against drug–resistant and persistent tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 110, E2510–E2517 10.1073/pnas.1309171110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rand K. H., Houck H. J., Brown P., and Bennett D. (1993) Reproducibility of the microdilution checkerboard method for antibiotic synergy. Antimicrob. Agents Chemother. 37, 613–615 10.1128/AAC.37.3.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gautier R., Douguet D., Antonny B., and Drin G. (2008) HELIQUEST: a web server to screen sequences with specific α-helical properties. Bioinformatics 24, 2101–2102 10.1093/bioinformatics/btn392 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.