Abstract
The 78-kDa glucose-regulated protein (GRP78) is a well-established endoplasmic reticulum (ER)-resident chaperone that maintains protein homeostasis and regulates the unfolded protein response. Under conditions of ER stress, GRP78 is also expressed at the cell surface and implicated in tumorigenesis, immunity, and cellular signaling events. The role of cell surface–associated GRP78 (csGRP78) in the pathogenesis of diabetic nephropathy has not yet been defined. Here we explored the role of csGRP78 in regulating high glucose (HG)–induced profibrotic AKT Ser/Thr kinase (AKT) signaling and up-regulation of extracellular matrix proteins. Using primary kidney mesangial cells, we show that HG treatment, but not the osmotic control mannitol, induces csGRP78 expression through an ER stress–dependent mechanism. We found that csGRP78, known to be located on the outer membrane leaflet, interacts with the transmembrane protein integrin β1 and activates focal adhesion kinase and downstream PI3K/AKT signaling. Localization of GRP78 at the cell surface and its interaction with integrin β1 were also required for extracellular matrix protein synthesis in response to HG. Surprisingly, both the N and C termini of csGRP78 were necessary for this profibrotic response. Increased localization of GRP78 at the plasma membrane was also found in the glomerular mesangial area of type 1 diabetic mice in two different models (streptozotocin-induced and Akita). In freshly isolated glomeruli from Akita mice, csGRP78 co-localized with the mesangial cell surface marker α8-integrin. In conclusion, our work reveals a role for csGRP78 in HG-induced profibrotic responses in mesangial cells, informing a potential approach to treating diabetic nephropathy.
Keywords: fibrosis, diabetic nephropathy, integrin, extracellular matrix, chaperone, 78-kDa glucose-regulated protein (GRP78), cell stress, kidney disease
Introduction
Diabetic nephropathy (DN)3 is a major complication of diabetes and the leading cause of kidney failure in North America. It is characterized by glomerulosclerosis (1), with glomerular mesangial cells (MCs) known to play a key role in its pathogenesis through their synthesis of extracellular matrix (ECM) proteins in response to high glucose (HG) (2). Recent research has indicated that the 78-kDa glucose-regulated protein 78 (GRP78) may be translocated to the cell surface and play a pathological role in the development of various diseases such as cancer, inflammatory and immune conditions, and atherosclerosis (3–5). The relevance of cell surface GRP78 (csGRP78) in DN has not been examined previously.
GRP78 was originally described as an ER-resident molecular chaperone that assists with the proper folding of de novo proteins in the ER (5). It is ubiquitously expressed in mammalian cells and acts to control ER stress through regulation of the unfolded protein response (5). In response to agents and/or conditions that elicit ER stress, GRP78 has also been reported to translocate to the cell surface through association with the co-chaperone proteins MTJ-1 and Par-4 (6–8). At the cell surface, GRP78 acts as a signaling receptor for agonists such as α2-macroglobulin and anti-GRP78 autoantibodies. Activation of csGRP78 promotes its association with other cell surface proteins, leading to intracellular signaling events, the nature of which depends on the cell type and interacting partners (9). Expression of csGRP78 has been described in a wide range of cell types, predominantly in association with various cancers, and has been suggested to drive tumorigenesis, immunity, and cellular signaling events (9).
Induction of ER stress and elevated cellular expression of GRP78 are well recognized features of rodent and human DN (10, 11). However, whether cell surface expression of GRP78 is increased and contributes to DN is unknown. Our results show that, in response to hyperglycemia, GRP78 is translocated to the cell surface in MCs. In support of these findings, csGRP78 is also present in the glomeruli of diabetic mice. In terms of its contribution to DN, csGRP78 drives the production of ECM by HG. These data suggest that csGRP78 represents a novel potential therapeutic target for DN.
Results
High glucose induces translocation of GRP78 to the cell surface
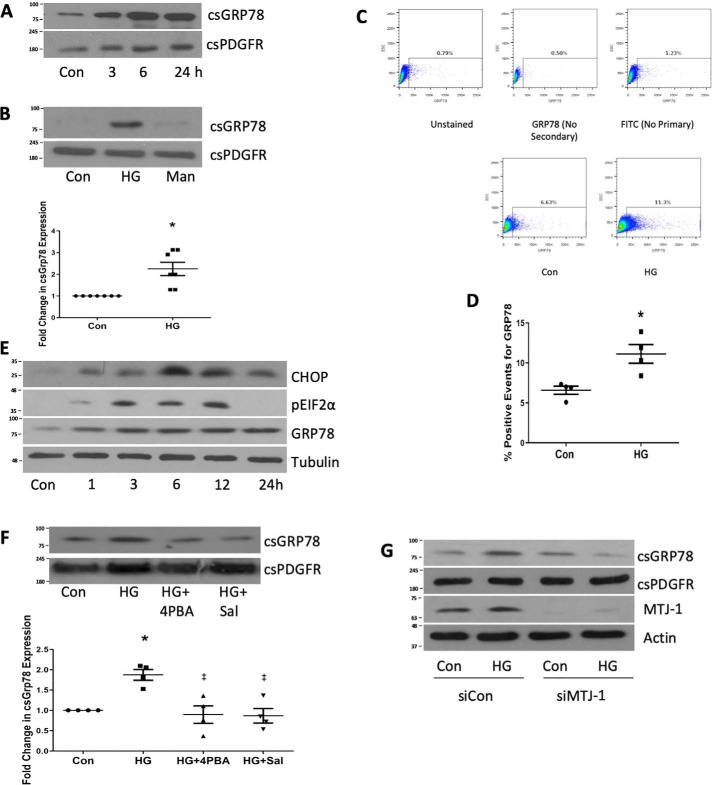
Translocation of GRP78 to the cell surface has been noted under conditions of ER stress in carcinoma, endothelial, liver, and immune cells (9) but has not yet been assessed in diabetic kidneys or MCs. Using a biotinylation/streptavidin pulldown technique, we first sought whether we could identify cell surface expression of GRP78. Fig. 1A shows that HG induced persistent cell surface expression of GRP78, seen as early as 3 h. The cell surface protein platelet-derived growth factor receptor (PDGFR) was used as a loading control. For subsequent experiments, csGRP78 was assessed after 24 h of HG. Fig. 1B shows that only HG, but not the osmotic control mannitol, induced cell surface expression of GRP78. We next used flow cytometry to confirm that HG induces expression of GRP78 at the cell surface (Fig. 1, C and D).
Figure 1.
High glucose promotes the expression of GRP78 at the cell surface. A, MCs were treated with HG for the indicated times, and then csGRP78 was assessed by a biotinylation assay as described under “Experimental procedures.” PDGFR was used as a loading control (Con). B, csGRP78 was assessed by biotinylation after HG or mannitol (Man, 24 h, n = 7). C and D, expression of csGRP78 after HG for 24 h was confirmed by flow cytometry (n = 4). E, markers of ER stress were assessed by immunoblotting after HG treatment for the indicated times (n = 3) CHOP, CCAAT-enhancer-binding protein homologous protein. F, the effects of ER stress inhibitors 4-PBA (2.5 μm) or salubrinal (Sal, 30 μm) on HG-induced csGRP78 expression were assessed by biotinylation (n = 5). G, the effect of MTJ-1 down-regulation using siRNA on HG-induced csGRP78 expression was assessed by biotinylation (n = 3). MTJ-1 down-regulation was assessed by immunoblotting of whole-cell lysate. *, p < 0.05 HG versus control; ‡, p < 0.05 versus HG.
Cell surface translocation of GRP78 has been suggested to occur because of ER stress, although its movement to the surface can occur independently of ER stress (12). Hyperglycemia has been shown to induce ER stress in MCs (13, 14), and markers of ER stress have been identified in both diabetic animals and humans (10, 11). Similar to these studies, we found that HG induced ER stress in MCs, characterized by phosphorylation of eIF2α at early time points (3–12 h), as well as expression of CHOP and GRP78 (Fig. 1E). Furthermore, we found that HG-induced cell surface expression of GRP78 depended on ER stress. Fig. 1F shows that the ER stress inhibitors 4-PBA and salubrinal, which have both been shown previously to inhibit HG-induced ER stress (15, 16), attenuate HG-induced cell surface GRP78 expression. Lastly, the protein DnaJ-like protein 1 (MTJ-1) has been shown to act as a co-chaperone for translocation of GRP78 to the cell surface (7). We found that knockdown of MTJ-1 prevented translocation of GRP78 to the cell surface in response to HG (Fig. 1G). These data demonstrate that, in MCs, HG induces cell surface expression of GRP78 in an ER stress–dependent manner through MTJ-1.
Cell surface GRP78 is required for activation of FAK and Akt in response to high glucose
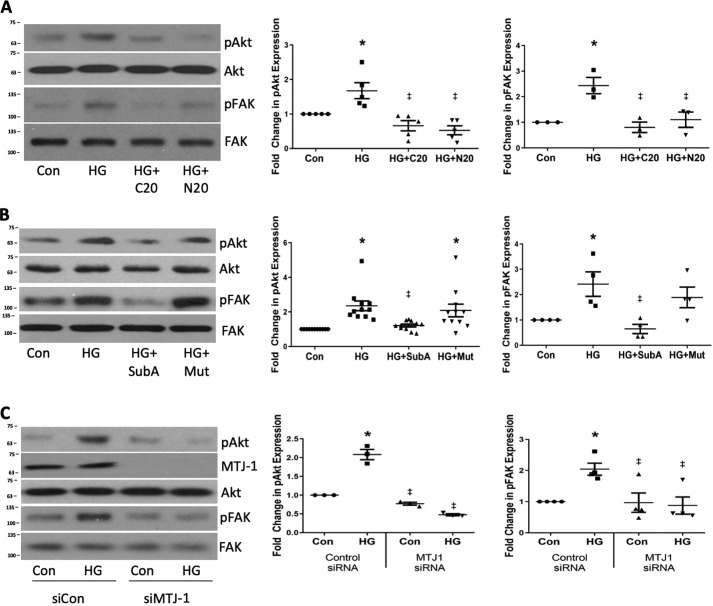
FAK and Akt have been shown to be downstream mediators of csGRP78 signaling in cancer cells (17–19), and we demonstrated previously that they are both important mediators of the HG-induced profibrotic response in MCs (20–22). We thus used three distinct methods to inhibit csGRP78 to determine whether this would attenuate FAK and Akt activation by HG. Antibodies that bind to either the N-terminal or C-terminal portion of GRP78 are commonly used to assess effects on csGRP78 signaling (23, 24). In cancer cells, C terminus–binding antibodies have been reported to inhibit Akt activation and induce apoptosis (23, 25), whereas N terminus–binding antibodies promote Akt activation, survival, and proliferation (26). Surprisingly, antibodies targeting either the N or C terminus of GRP78 attenuated FAK and Akt activation in response to HG, as assessed by their phosphorylation on Tyr-397 and Ser-473, respectively (Fig. 2A). Of note, using a lactate dehydrogenase assay, we did not detect any apoptosis in MCs treated with either antibody (data not shown). The enzyme SubA is a cell-impermeable proteinase that selectively cleaves the C terminus of cell surface GRP78, whereas the SubAA272 mutant lacks proteinase activity and serves as a control for the active enzyme (27). In line with our previous results, SubA, but not the mutant form, attenuated HG-induced FAK and Akt activation (Fig. 2B). Because we and others have shown that MTJ 1 is required for translocation of GRP78 to the cell surface (7), we tested the effects of MTJ-1 knockdown on activation of Akt and FAK. Knockdown of MTJ-1 using siRNA also attenuated HG-induced FAK and Akt activation (Fig. 2C). Together, our data suggest that csGRP78 is an upstream regulator of FAK and Akt activation in response to HG in MCs.
Figure 2.
Cell surface GRP78 mediates HG-induced Akt and FAK activation in response to HG. MCs were treated with HG for 3 h, a time when we previously showed these to be activated upstream of profibrotic effects. FAK and Akt activation were assessed by their phosphorylation on Tyr-397 and Ser-473, respectively. A, MCs were pretreated with antibodies targeting the C (C20, 2 μg/ml) or N terminus (N20, 2 μg/ml) of GRP78 for 1 h. (n = 5). Con, control. B, MCs were pretreated with SubA (25 ng/ml) or an inactive mutant (Mut, 25 ng/ml) for 1 h. (n = 10). C, MTJ-1 was down-regulated using siRNA prior to treatment with HG (n = 3). *, p < 0.05 HG versus control; ‡, p < 0.05 versus HG.
csGRP78 interaction with integrin β1 mediates FAK and Akt activation by HG
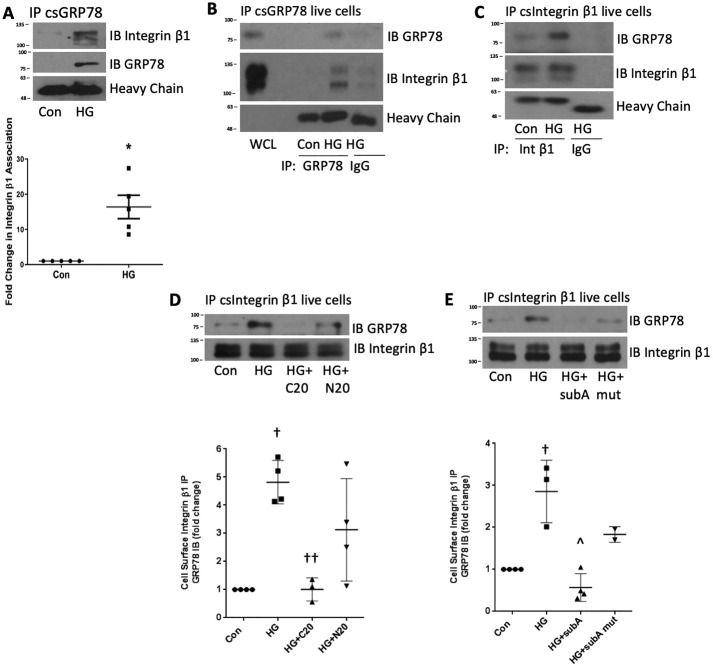
Integrin β1 is a cell surface receptor that mediates cell–matrix adhesion through formation of focal adhesion points (28). In colorectal tumor cells, csGRP78 interaction with integrin β1 promotes FAK activation, cell migration, and invasion (19). Furthermore, HG has been shown to activate integrin β1 in MCs (29). We thus assessed whether integrin β1 mediated FAK and Akt activation downstream of csGRP78 in response to HG. We first determined whether csGRP78 and integrin β1 interacted. Because coimmunoprecipitation following cell surface protein biotinylation is challenging, requiring harsh conditions to release the biotin bond (30), we isolated plasma membrane proteins as described under “Experimental procedures.” From this, we immunoprecipitated GRP78 and assessed for interaction with integrin β1 by immunoblotting. Fig. 3A shows that HG increased association between integrin β1 and GRP78 derived from the plasma membrane fraction. This was associated with an increased amount of GRP78 pulled down from plasma membrane preparations after HG treatment, consistent with increased csGRP78 in response to HG, as seen in Fig. 1.
Figure 3.
HG induces integrin β1 interaction with GRP78 at the cell surface. A, after treatment with HG (24 h), GRP78 was immunoprecipitated (IP) from plasma membrane isolates, and association with integrin β1 was assessed by immunoblotting (IB; n = 5). Con, control. B–E, after HG, either csGRP78 or membrane integrin β1 were immunoprecipitated from live cells, and association was assessed by immunoblotting. Nonspecific IgG was used as a control. WCL, whole cell lysate. D, MCs were cotreated with antibodies targeting the C (C20, 2 μg/ml) or N terminus (N20, 2 μg/ml) of GRP78. E, MCs were pretreated with SubA (25 ng/ml) or an inactive mutant (Mut, 25 ng/ml) for 1 h. *, p < 0.05 versus control; †, p < 0.01 versus control; ††, p < 0.01 versus HG; ∧, p < 0.001 versus HG.
To further confirm an association between csGRP78 and integrin β1, we incubated live cells with the GRP78 antibody after HG treatment to immunoprecipitate csGRP78. Fig. 3B shows increased GRP78/integrin β1 association, which was also confirmed by reverse immunoprecipitation with the integrin β1 antibody (Fig. 3C). Because antibodies targeting either the N or C terminus of GRP78 attenuated FAK and Akt activation, we next determined their effects on HG-induced integrin β1–csGRP78 association. Fig. 3D shows that the C-terminal antibody C20 prevents their association, whereas the N-terminal antibody N20 was only partially effective. Further confirming that the C terminus of GRP78 is required for this association, Fig. 3E shows that SubA also prevents integrin β1–csGRP78 association in response to HG. These findings suggest an important role for the C terminus of csGRP78 in mediating FAK and Akt activation through association with integrin β1, whereas contribution from the N terminus of csGRP78 is independent of this integrin.
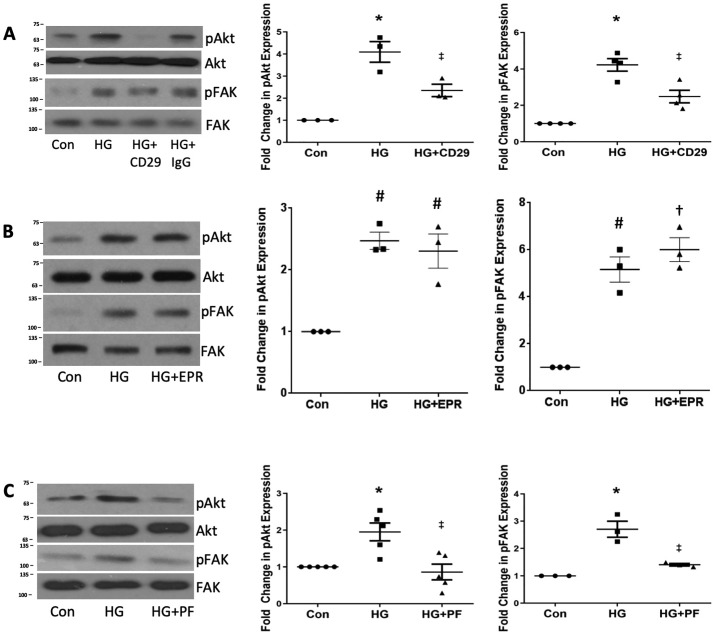
Finally, to confirm that integrin β1 is required for FAK–Akt activation in response to HG, we determined the effect of inhibiting integrin β1 with the CD29 neutralizing antibody. Fig. 4A shows that this attenuated HG-mediated activation of both FAK and Akt. However, the non-neutralizing antibody to integrin β1 had no effect on either Akt or FAK activation by HG (Fig. 4B), suggesting that integrin β1 activity, in addition to its interaction with csGRP78, is required for downstream signaling. Fig. 4C confirms that inhibition of FAK with PF573228 prevents HG-induced Akt activation. These data support an important role for csGRP78 interaction with integrin β1 in downstream activation of FAK and Akt.
Figure 4.
GRP78 interaction with integrin β1 mediates FAK and Akt activation by HG. A–C, MCs were treated with HG for 3 h, and FAK and Akt activation was assessed by their phosphorylation, with MCs pretreated either with an integrin β1-neutralizing antibody (CD29) or nonspecific IgG (both 10 μg/ml) for 1 h (A, n = 3), a non-neutralizing integrin β1 antibody (B, EPR16895 (EPR), 10 μg/ml, 1 h, n = 3), or with the FAK inhibitor PF573228 (PF, 1 μm) for 1 h (C, n = 5). *, p < 0.05 versus control (Con); ‡, p < 0.05 versus HG; #, p < 0.01 versus control; †, p < 0.001 versus control.
csGRP78 mediates the expression of ECM protein in response to HG
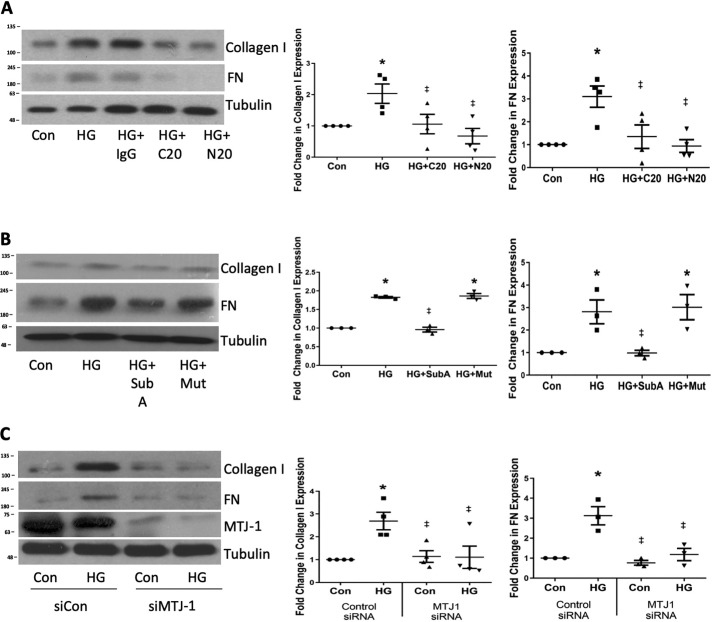
Accumulation of ECM in the glomerulus is a classic pathological hallmark of DN (1). In response to HG, MCs produce ECM proteins present in diabetic glomeruli, including collagens and fibronectin (20, 31). We showed previously that FAK–Akt signaling is required for this profibrotic response (20, 21). We thus assessed the effects of blocking csGRP78 on HG-induced ECM up-regulation. Fig. 5A shows that both collagen I and fibronectin production in response to HG were blocked by pretreatment with either N- or C-terminal targeting csGRP78 antibodies, with no effect seen when using a control IgG (Fig. 5A). Similarly, SubA cleavage of the C terminus of csGRP78 or knockdown of the chaperone protein MTJ-1 prevented HG-induced collagen I and fibronectin up-regulation (Fig. 5, B and C). These results demonstrate an important role for csGRP78 in HG-induced induction of ECM protein synthesis in MCs.
Figure 5.
Cell surface GRP78 mediates the expression of ECM protein in response to HG. MCs were treated with HG for 72 h; this longer HG incubation is required for synthesis of matrix proteins. Collagen I and fibronectin (FN) protein were determined in whole-cell lysate. A, MCs were pretreated with antibodies targeting the C (C20, 2 μg/ml) or N terminus (N20, 2 μg/ml) of GRP78 or an IgG control (Con) antibody for 1 h (n = 4). (B) MCs were pretreated with SubA (Sub, 25 ng/ml) or an inactive mutant (Mut, 25 ng/ml) for 1 h (n = 3). C, MTJ-1 was down-regulated using siRNA (n = 4) *, p < 0.05 versus control; ‡, p < 0.05 versus all HG.
Cell surface GRP78 is up-regulated in kidneys of type I diabetic mice
To assess the in vivo relevance of csGRP78 to DN, we sought to determine whether GRP78 was translocated to the cell surface in diabetic kidneys. We examined kidneys in two models of type I diabetes: induced by streptozotocin in CD-1 mice and in genetic model Akita mice.
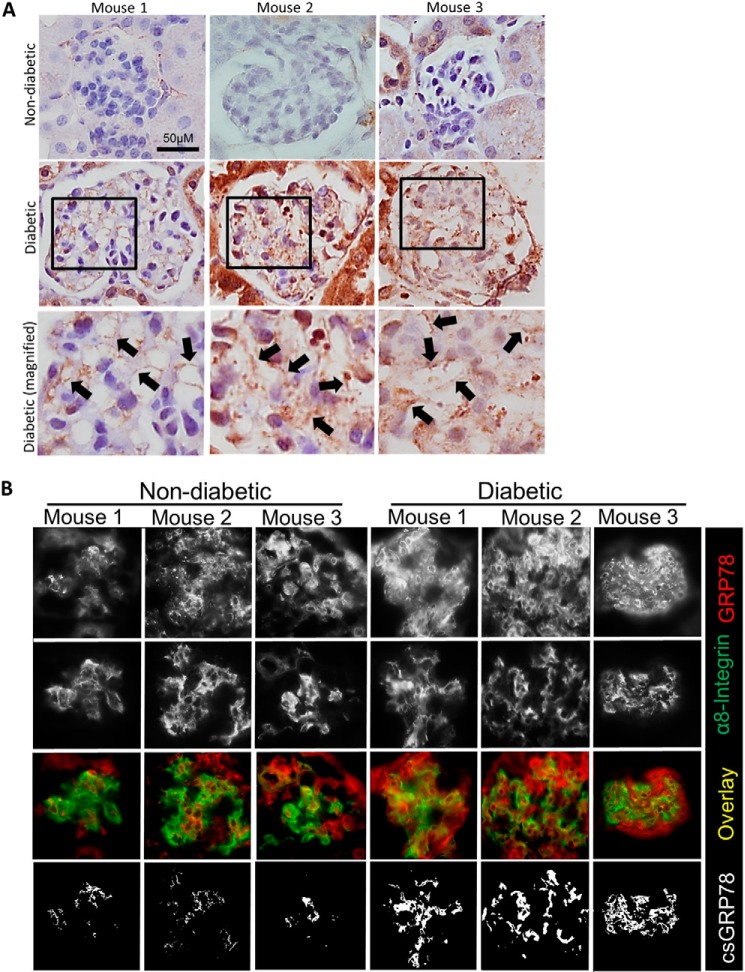
Our previous studies showed that induction of type 1 diabetes in CD-1 mice with streptozotocin produces robust changes in DN (32). After 12 weeks of diabetes in this model, we assessed expression of GRP78 by IHC. Fig. 6A shows an increase in plasma membrane GRP78 in mesangial areas of glomeruli in diabetic kidneys. The staining pattern suggested expression of GRP78 at the plasma membrane, as indicated by black arrows. To provide further evidence of expression of GRP78 at the plasma membrane of MC in diabetic mice, we colocalized the expression of GRP78 with the plasma membrane and cell surface marker α8-integrin by immunofluorescence. Areas showing colocalization between GRP78 and α8-integrin are expressed as white in the mask. We found that there was increased colocalization between GRP78 and α8-integrin in diabetic mice, suggesting that GRP78 is up-regulated at the mesangial cell plasma membrane during pathogenesis of DN (Fig. 6B).
Figure 6.
Plasma membrane GRP78 is seen in glomeruli of type I diabetic CD-1 mice. Kidneys were harvested from vehicle- or streptozotocin-treated CD-1 mice after 12 weeks of diabetes (n = 3/group). A, paraffin-embedded sections were stained for GRP78. Black arrows indicate areas in which GRP78 appears to be localized to the plasma membrane. B, OCT sections were stained for GRP78 (red) or the plasma membrane marker α8-integrin (green). Areas of colocalization between GRP78 and the plasma membrane are indicated by white in the overlay and mask images.
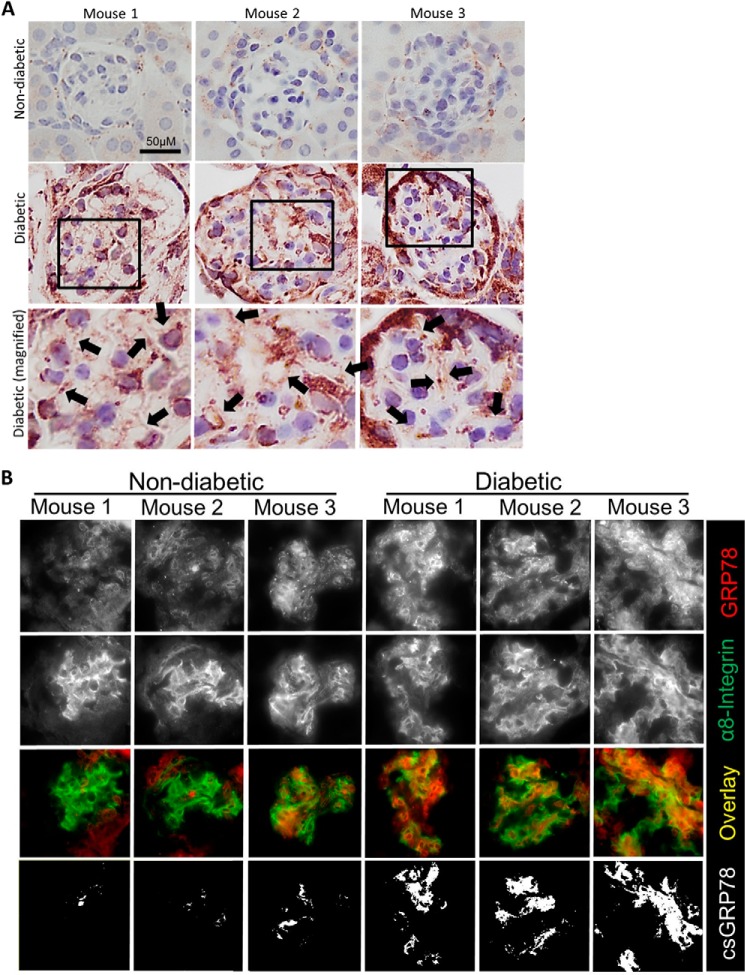
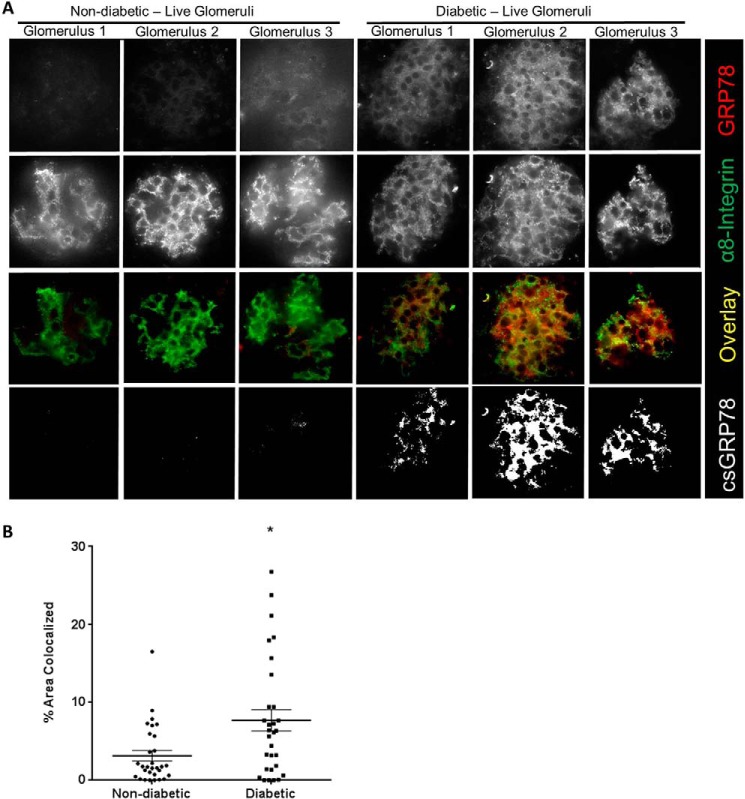
To confirm these findings in CD-1 mice, we next assessed a second model of type 1 diabetes. Akita mice carry a mutation in the Ins2 gene, leading to improper formation of insulin and pancreatic β-cell injury, resulting in type 1 diabetes (34). Similar to CD-1 diabetic mice, Akita mice demonstrated overall increased expression of GRP78 in their glomeruli as well as surrounding tubules, as determined by IHC (Fig. 7A). MC plasma membrane GRP78 expression was assessed next, as above, through colocalization between GRP78 and α8-integrin expression by immunofluorescence. Similar to the CD-1 diabetic mice, plasma membrane GRP78 expression was increased in glomeruli of diabetic mice in MC areas (Fig. 7B). Finally, to further confirm these findings, we stained live kidney tissue obtained from freshly isolated WT and Akita kidneys. Here we again used α8-integrin as a cell surface marker for MCs, which was also used to identify glomeruli (35, 36). Fig. 8 shows that csGRP78 was increased in the mesangium of diabetic glomeruli. Areas of co-staining between GRP78 and α8-integrin were identified as white; quantification is shown in the accompanying graph. Together, our data show that csGRP78 is increased in the mesangium of diabetic kidneys.
Figure 7.
GRP78 is expressed at the plasma membrane in type I diabetic Akita mice. Kidneys were harvested from WT or Akita mice at 30 weeks of age (n = 3/group). A, paraffin-embedded sections were stained for GRP78. Black arrows indicate areas in which GRP78 appears to be localized to the plasma membrane. B, OCT sections were stained for GRP78 (red) or the plasma membrane marker α8-integrin (green). Areas of colocalization between GRP78 and the plasma membrane are indicated by white in the overlay and mask images.
Figure 8.
GRP78 is expressed at the plasma membrane in live type I diabetic Akita mouse glomeruli. A, kidneys from one WT mouse and one diabetic Akita mouse aged 40 weeks were digested and stained for α8-integrin (green) to identify MCs and GRP78 (red). Fixation was performed after primary antibody incubation to enable more specific cell surface staining. Colocalization between α8-integrin and GRP78 is indicated by white in the mask images. B, 30 glomeruli from each mouse were assessed for quantification of colocalization. *, p < 0.05.
Discussion
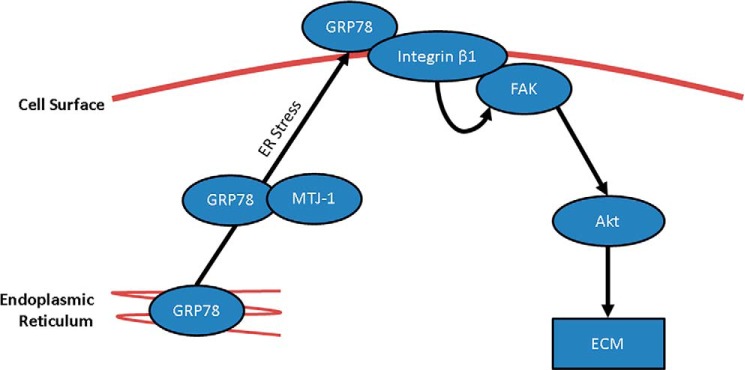
Presentation of GRP78 on the cell surface has been identified in a wide number of cells, including various cancers, endothelial cells, and macrophages, and has been suggested to play an important role in cell signaling, viral entry, and antigen presentation (37). Our study shows that kidney MCs express GRP78 on the cell surface in response to HG as well as in diabetic kidneys. Importantly, csGRP78 mediates HG-induced matrix production through FAK/Akt activation (Fig. 9). These novel findings establish a potentially highly significant role for csGRP78 in the pathogenesis of DN.
Figure 9.
Schematic of cell surface GRP78 signaling in response to high glucose in kidney mesangial cells. In response to high glucose, GRP78 is translocated from the ER to the cell surface through an ER stress–dependent mechanism. This is mediated by the chaperone MTJ-1. At the cell surface, GRP78 interacts with integrin β1, which mediates the phosphorylation and activation of downstream FAK and Akt. This contributes to fibrosis by promoting the expression of ECM proteins, including fibronectin and collagen I.
Previous studies have shown that HG induces the expression of total GRP78 in kidney cells (38), but cell surface expression had not been assessed. Our results demonstrate that HG induces cell surface presentation of GRP78 and that this is mediated by ER stress. ER stress induction is clearly implicated in the development of DN and has been shown to occur because of numerous stimuli, including free fatty acids, oxidative stress, and advanced glycation end products (39). Although an increase in csGRP78 independently of ER stress has also been described (12), an important role for ER stress in the induction of csGRP78 has most commonly been identified (12, 40). Recently, Tsai et al. (40) discovered that ER stress activates the kinase Src, resulting in dispersion of the KDEL receptor in the Golgi and leading to elevated csGRP78. Whether inhibition of ER stress or Src in vivo impacts the presentation of GRP78 on the cell surface needs to be investigated.
Cell surface GRP78 regulation of Akt activation has been shown predominantly in cancer cells but also in endothelial cells (41–43). Binding of anti-GRP78 autoantibodies or the activated antiproteinase α2 macroglobulin (α2M) to the N terminus of GRP78 promotes Akt activation, whereas autoantibody binding to the C terminus is inhibitory (9, 23, 41). Our data also show a major role for csGRP78 in the regulation of HG-induced activation of Akt. In keeping with other studies, our results show that targeting the N-terminal domain of csGRP78 using the N20 antibody attenuates HG-induced Akt activation. Surprisingly, targeting the C-terminal domain using the C20 antibody also attenuates Akt activation to a similar extent. This suggests that, in MCs, the N- and C-terminal domains of csGRP78 both contribute to regulate Akt activation in response to HG. This may represent a requirement for two different stimuli to activate csGRP78, such as an N-terminal autoantibody or α2M and C-terminal interaction with a transmembrane binding partner like integrin β1 (discussed below). Alternatively, inhibitory antibody binding to the C terminus or C terminus cleavage with SubA may affect GRP78 topology at the cell surface, which leads to its inability to initiate intracellular signaling in response to HG. Supporting this hypothesis, Misra et al. (23) have shown that targeting the C-terminal domain of GRP78 attenuates α2M-mediated activation of csGRP78. The detailed mechanism by which N- and C termini of csGRP78 contribute to HG-induced signaling, however, requires further study. Furthermore, whether there is a role for csGRP78 ligands, such as α2M or autoantibodies against GRP78, in HG-induced signaling through csGRP78 also needs to be addressed.
Interaction of csGRP78 with a cell surface–anchored or transmembrane protein such as integrin β1 is likely important in promoting intracellular signaling (9, 19). In colon cancer cells, csGRP78 interaction with integrin β1 leads to activation of the focal adhesion protein FAK (19). Our results similarly show that HG induces interaction between csGRP78 and integrin β1, with the latter an important upstream mediator of glucose-induced FAK as well as Akt activation. FAK is a well-known mediator of the Akt activator PI3K (44), and our data confirm that FAK is required for HG-induced Akt activation in MCs. Taken together, our results support an important role for csGRP78 interaction with integrin β1 in activation of FAK and Akt, which, as we have shown previously, mediates downstream synthesis of matrix proteins (28, 29).
Cell surface GRP78 is primarily expressed under pathologic conditions and has so far been demonstrated in various cancers and atheromatous lesions (3, 45–47). Although a role of cell surface GRP78 in DN has not as yet been established, several studies have suggested that csGRP78 expression may be induced by diabetes. Rondas et al. (48) identified autoantibodies against the posttranslationally citrullinated GRP78 found in pancreatic β-cells of nonobese diabetic mice. Although their pathogenic role in type 1 diabetes remains to be investigated, production of autoantibodies against GRP78 has been positively associated with increased expression of csGRP78 (46). Infection of endothelial cells by the fungus Rhizopus oryzae, commonly responsible for infections in patients with diabetic ketoacidosis, was found to be mediated by csGRP78. Treatment with a GRP78 antibody (in endothelial cells challenged with glucose) and anti-GRP78 serum (in mice with diabetic ketoacidosis) reduced infection and improved survival, respectively (49). Interestingly, the adipokine and serine protease inhibitor vaspin has been shown to act as a ligand for csGRP78 in obese diabetic mouse liver (50). Overexpression of vaspin improves features of the metabolic syndrome in mice fed a high-fat, high-sucrose diet (50). Finally, the GRP78 targeting peptide ADoPep increases weight gain and improves glycemic control in type I diabetic mice (51). These studies suggest that csGRP78 may be induced during diabetes in various tissues, although direct evidence of an increase of csGRP78 in these murine models has not been provided. For the first time, our study shows that GRP78 is found at the plasma membrane in the kidneys of type I diabetic mice, and by IHC and IF, these appeared to localize to mesangial cell areas. Since concerns exist regarding use of IHC and IF when determining cell surface staining because of the effects of fixation and freezing on the integrity of the cell surface in tissue, we verified our IHC and IF results with live-tissue staining on freshly isolated and unfixed kidneys. Furthermore, using a mesangial cell plasma membrane marker (α8 integrin), we confirmed colocalization with csGRP78 in mesangial cells of glomeruli.
A role of csGRP78 in matrix synthesis has not been demonstrated previously. However, in cancer cells, csGPR78 has been shown to regulate signaling by TGFβ1, a profibrotic cytokine known to be a central regulator of matrix synthesis and fibrosis in diabetic nephropathy (52). TGFβ1 exerts important tumor suppressive effects, and therefore csGRP78 inhibits TGFβ1 signaling in cancer cells, primarily through its association with Cripto, a small glycosylphosphatidylinositol-anchored protein and negative regulator of TGFβ1 (53). Tsai et al. (40) have also demonstrated that csGRP78, through its association with another glycosylphosphatidylinositol-anchored protein, CD109, prevents TGFβ1 signaling in HeLa cells by routing its receptor to caveolae for degradation. The role of csGRP78 in the regulation of TGFβ1 in primary cells, however, is likely to differ. The establishment of TGFβ1 as an important mediator of HG-induced matrix up-regulation in MCs (54), coupled with our findings that csGRP78 is required for HG-induced matrix synthesis, strongly suggest that csGRP78 promotes TGFβ1 effects, at least in MCs. Indeed, we showed previously that Akt mediates TGFβ1 synthesis in response to HG (20–22). It is thus likely that csGRP78, through Akt activation, mediates TGFβ1 synthesis in this setting, although this requires further study.
Collectively, our data reveal csGRP78 as a novel regulator of fibrosis in response to HG through its regulation of FAK/Akt signaling. In vivo relevance is supported by the observed increase in csGRP78 in diabetic kidneys. Cell surface GRP78 thus represents a potential novel target for the treatment and/or prevention of DN. Future studies will seek identification of a ligand for csGRP78 that enables its HG-induced signaling, thus expanding opportunities for the development of novel therapeutic agents.
Experimental procedures
Cell culture
Primary MCs were isolated from Sprague-Dawley rats, as described previously (55–59). MCs were cultured in Dulbecco's modified Eagle's medium supplemented with 20% fetal bovine serum, streptomycin (100 μg/ml), and penicillin (100 μg/ml) (Invitrogen) at 37 °C in 95% air and 5% CO2. Cells between passages 8 to 15 were used. Cells were serum-deprived at 80–90% confluence overnight prior to treatment with 24.4 mm glucose (Sigma) to a final concentration of 30 mm glucose for HG treatment. Mannitol (24.4 mm, Sigma) was used as an osmotic control. Prior to treatment with HG, cells were treated with the inhibitors 4-sodium phenylbutyrate (4-PBA, Scandinavian Formulas Inc.), salubrinal (Selleckchem, 1614), integrin β1-neutralizing antibody CD29 (Biolegend, 102209), integrin β1 non-neutralizing antibody (Abcam, EPR16895), GRP78 antibody C20 (Santa Cruz Biotechnology, SC1051), GRP78 antibody N20 (Santa Cruz Biotechnology, SC1050), subtilase cytotoxin A (SubA), the SubA inactive mutant SubAA272, and PF573228 (Tocris, 3239). SubA and SubAA272 were purified as described previously (27). The times and doses of treatments are provided in the figure legends.
Protein extraction
Cells were lysed in buffer containing 50 mm Tris (pH 7.4), 150 mm NaCl, 5 mm EDTA, 1% Triton X-100, 10% glycerol, and protease/phosphatase inhibitors. Cellular debris was cleared from cell lysate by centrifugation at 13,000 rpm for 10 min at 4 °C. Proteins were separated by SDS-PAGE and probed for PDGFR (1:10,000, Santa Cruz Biotechnology, SC432), GRP78 (1:1000, BD Transduction Laboratories, 610979), phosphorylated Akt (pAkt) Ser-473 (1:1000, Cell Signaling Technology, 9271), Akt (1:1000, Cell Signaling Technology, 9272), phosphorylated focal adhesion kinase (pFAK) Tyr-397 (1:1000, Millipore, 07-012), FAK (1:1000, Santa Cruz Biotechnology, SC558), MTJ-1 (1:1000, Santa Cruz Biotechnology, SC104898), tubulin (1:10,000, Sigma, T6074), integrin β1 (1:1000, Abcam, 102209), collagen I (1:1000, Novus Biologicals, NB600-408), and fibronectin (1:5000, BD Transduction Laboratories, 610078).
Cell surface biotinylation
Cells were washed with ice-cold PBS and incubated with EZ-link Sulfo-NHSLC-Biotin (Pierce, 21331, 0.5 mg/ml in PBS) for 20 min. Biotinylation was terminated with 0.1 m glycine in PBS. Cells were lysed, and biotinylated proteins were precipitated with a 50% neutravidin slurry (Fisher, PI29200) overnight. Beads were then washed, and cell surface proteins were analyzed.
Flow cytometry
Following treatment, cells were washed three times with cold PBS and harvested with Accutase (Innovative Cell Technologies, AT104), followed by three further washes (1% FBS/PBS). Cells were incubated with C20 GRP78 antibody (5 μg/106cells) for 1 h at 37 °C, washed with FACS buffer (0.2% BSA/PBS), and then incubated with 1 μg of AF488 anti-goat secondary antibody per 106 cells (Molecular Probes) for 1 h at 37 °C in the dark. Cells were then washed with FACS buffer and resuspended in 1% paraformaldehyde in PBS. Staining was assessed using the LSRII software from BD Biosciences and analyzed using FlowJo software from TreeStar.
RNA interference
Rat MTJ-1 On-Target Plus SMARTpool siRNA and nonspecific control siRNA were obtained from Dharmacon. MCs were plated to 70% confluence and transfected with 50 nm siRNA utilizing GeneEraser siRNA reagent (Stratagene, 204152). After 48 h, they were serum-deprived for 24 h, treated, and harvested for protein.
Immunoprecipitation
After cells were washed with ice-cold PBS, plasma membrane protein was isolated using the MinuteTM plasma membrane protein isolation kit (Invent Biotechnologies, SM005) according to the manufacturer's protocol. 2 μg of the C20 antibody was used to immunoprecipitate GRP78, and antibody–protein complexes were purified using protein G–Sepharose beads. These were washed prior to analysis by immunoblotting. Alternatively, after HG treatment, live cells were exposed to GRP78 antibody or preadsorbed nonspecific IgG for 2 h on ice, followed by washing, cell lysis, and pulldown using protein G beads.
Animals
Animal studies were carried out in accordance with McMaster University and the Canadian Council on Animal Care guidelines. Male CD1 mice (Charles River Laboratories) underwent a left nephrectomy at 9 weeks of age. One week following this, diabetes was induced by a single intraperitoneal injection of streptozotocin (Sigma, S0130) at 200 mg/kg. Control mice were injected with an equal volume of citrate buffer. Blood glucose was tested the following week, and mice with values of more than 17 mm were enrolled in the study. Diabetic mice that developed ketonuria (assessed by dipstick; Bayer Multistix) were implanted with an insulin pellet (LinShin Canada) to maintain body weight and hyperglycemia. CD1 mice were sacrificed following 12 weeks of diabetes. Male diabetic C57BL/6-Ins2Akita/J mice (Jackson Laboratory) and their WT littermates were assessed at 30 or 40 weeks of age.
Imaging
Immunohistochemistry was performed on 4-μm deparaffinized sections using the GRP78 rabbit antibody (1:1000, Abcam, ab21685) at 4 °C overnight after steaming. Pictures were taken at ×1000 magnification. Immunofluorescence (IF) was performed on 6-μm OCT sections. Sections were stained with the GRP78 rabbit antibody (1:20,000, Abcam) together with the α8-integrin antibody (1:500, R&D Systems, BAF4076) overnight, followed by secondary antibody (rabbit AF647 and goat AF488, Molecular Probes; 1:500 for 1 h) Pictures were taken at ×1000 magnification using an Olympus 1X81 microscope. Colocalization between GRP78 and α8 integrin was determined using the colocalization plugin available for ImageJ. Areas of colocalization are presented as white in the mask and overlay images.
Live tissue staining
Kidney samples were diced and digested with collagenase P (1 mg/ml, Roche,11213857001) and DNase (0.1 mg/ml, Roche, 10104159001) in Hank's balanced salt solution for 20 min at 37 °C. Tissue was then passed through a 100-μm sieve followed by a 70-μm sieve (BD Falcon), and glomeruli were captured on a 53-μm sieve (VWR International). These were suspended in wash buffer (1% FBS in HBSS) and incubated with both the GRP78 rabbit antibody (1:40,000, Abcam) and α8-integrin antibody (1:500, R&D Systems) overnight, followed by secondary antibody (rabbit AF647 and goat AF488, Molecular Probes; 1:500) for 2 h in the dark. After washing with FACS buffer, cells were fixed in 4% paraformaldehyde for 30 min and counterstained with 4′,6-diamidino-2-phenylindole for 5 min before mounting in Fluoro-Gel (Electron Microscopy Sciences). Colocalization between GRP78 and α8-integrin was determined using the colocalization plugin available for ImageJ.
Statistical analysis
Statistical analysis was performed using a two-tailed t test for experiments with only two experimental groups. Experiments with more than two groups were analyzed by one-way analysis of variance with Tukey's multiple comparison test for post hoc analysis. p < 0.05 was considered significant. Data are presented as mean ± S.E.
Author contributions
R. V. K., R. C. A., and J. C. K. conceptualization; R. V. K., N. M., and T. W. data curation; R. V. K., R. C. A., and J. C. K. supervision; R. V. K., R. C. A., and J. C. K. funding acquisition; R. V. K., T. W., M. Z., R. L., B. G., E. A., K. A., and J. C. K. investigation; R. V. K., N. M., E. A., K. A., R. C. A., and J. C. K. methodology; R. V. K. and T. W. writing-original draft; R. V. K., A. W. P., R. C. A., and J. C. K. writing-review and editing; N. M. formal analysis; K. A., J. C. P., and A. W. P. resources.
Acknowledgments
We thank Dr. John Paul Oliveria for optimization of the flow cytometry experiments. We also thank the Research Institute of St. Joe's Hamilton for nephrology research support.
This work was supported by Canadian Institutes of Health Research Grant PJT-148628 (to J. C. K.) and Heart and Stroke Foundation of Canada Grant G-15-0009389 (to R. C. A.). The authors declare that they have no conflicts of interest with the contents of this article.
- DN
- diabetic nephropathy
- MC
- mesangial cell
- ECM
- extracellular matrix
- HG
- high glucose
- cs
- cell surface
- ER
- endoplasmic reticulum
- PDGFR
- platelet-derived growth factor receptor
- 4-PBA
- 4-sodium phenylbutyrate
- FAK
- focal adhesion kinase
- IF
- immunofluorescence
- TGF
- transforming growth factor
- SubA
- subtilase cytotoxin A
- IHC
- immunohistochemistry
- OCT
- optimal cutting temperature compound.
References
- 1. Brosius F. C., 3rd (2008) New insights into the mechanisms of fibrosis and sclerosis in diabetic nephropathy. Rev. Endocr. Metab. Disord. 9, 245–254 10.1007/s11154-008-9100-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schlöndorff D., and Banas B. (2009) The mesangial cell revisited: no cell is an island. J. Am. Soc. Nephrol. 20, 1179–1187 10.1681/ASN.2008050549 [DOI] [PubMed] [Google Scholar]
- 3. Crane E. D., Al-Hashimi A. A., Chen J., Lynn E. G., Won K. D., Lhoták Š., Naeim M., Platko K., Lebeau P., Byun J. H., Shayegan B., Krepinsky J. C., Rayner K. J., Marchiò S., Pasqualini R., et al. (2018) Activation of cell-surface GRP78 by anti-GRP78 autoantibodies induces endothelial cell activation and accelerates the development of atherosclerotic lesions. JCI Insight 3, 99363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shimizu F., Schaller K. L., Owens G. P., Cotleur A. C., Kellner D., Takeshita Y., Obermeier B., Kryzer T. J., Sano Y., Kanda T., Lennon V. A., Ransohoff R. M., and Bennett J. L. (2017) Glucose-regulated protein 78 autoantibody associates with blood-brain barrier disruption in neuromyelitis optica. Sci. Transl. Med. 9, eaai9111 10.1126/scitranslmed.aai9111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gonzalez-Gronow M., Selim M. A., Papalas J., and Pizzo S. V. (2009) GRP78: a multifunctional receptor on the cell surface. Antioxid. Redox Signal. 11, 2299–2306 10.1089/ars.2009.2568 [DOI] [PubMed] [Google Scholar]
- 6. Tsai Y. L., Zhang Y., Tseng C. C., Stanciauskas R., Pinaud F., and Lee A. S. (2015) Characterization and mechanism of stress-induced translocation of 78-kilodalton glucose-regulated protein (GRP78) to the cell surface. J. Biol. Chem. 290, 8049–8064 10.1074/jbc.M114.618736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Misra U. K., Gonzalez-Gronow M., Gawdi G., and Pizzo S. V. (2005) The role of MTJ-1 in cell surface translocation of GRP78, a receptor for α2-macroglobulin-dependent signaling. J. Immunol. 174, 2092–2097 10.4049/jimmunol.174.4.2092 [DOI] [PubMed] [Google Scholar]
- 8. Cohen M., Ribaux P., Epiney M., and Irion O. (2013) Role of prostate apoptosis response 4 in translocation of GRP78 from the endoplasmic reticulum to the cell surface of trophoblastic cells. PLoS ONE 8, e80231 10.1371/journal.pone.0080231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ni M., Zhang Y., and Lee A. S. (2011) Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem. J. 434, 181–188 10.1042/BJ20101569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu G., Sun Y., Li Z., Song T., Wang H., Zhang Y., and Ge Z. (2008) Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochem. Biophys. Res. Commun. 370, 651–656 10.1016/j.bbrc.2008.04.031 [DOI] [PubMed] [Google Scholar]
- 11. Sanchez-Niño M. D., Benito-Martin A., and Ortiz A. (2010) New paradigms in cell death in human diabetic nephropathy. Kidney Int. 78, 737–744 10.1038/ki.2010.270 [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y., Liu R., Ni M., Gill P., and Lee A. S. (2010) Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J. Biol. Chem. 285, 15065–15075 10.1074/jbc.M109.087445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yao F., Li Z., Ehara T., Yang L., Wang D., Feng L., Zhang Y., Wang K., Shi Y., Duan H., and Zhang L. (2015) Fatty acid-binding protein 4 mediates apoptosis via endoplasmic reticulum stress in mesangial cells of diabetic nephropathy. Mol. Cell. Endocrinol. 411, 232–242 10.1016/j.mce.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 14. Xu Z., Zhao Y., Zhong P., Wang J., Weng Q., Qian Y., Han J., Zou C., and Liang G. (2017) EGFR inhibition attenuates diabetic nephropathy through decreasing ROS and endoplasmic reticulum stress. Oncotarget 8, 32655–32667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y., Gao X., Chen S., Zhao M., Chen J., Liu R., Cheng S., Qi M., Wang S., and Liu W. (2017) Cyclin-dependent kinase 5 contributes to endoplasmic reticulum stress induced podocyte apoptosis via promoting MEKK1 phosphorylation at Ser280 in diabetic nephropathy. Cell. Signal. 31, 31–40 10.1016/j.cellsig.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 16. Zhu M., Guo M., Fei L., Pan X. Q., and Liu Q. Q. (2014) 4-Phenylbutyric acid attenuates endoplasmic reticulum stress-mediated pancreatic β-cell apoptosis in rats with streptozotocin-induced diabetes. Endocrine 47, 129–137 10.1007/s12020-013-0132-7 [DOI] [PubMed] [Google Scholar]
- 17. Misra U. K., and Pizzo S. V. (2004) Potentiation of signal transduction mitogenesis and cellular proliferation upon binding of receptor-recognized forms of α2-macroglobulin to 1-LN prostate cancer cells. Cell. Signal. 16, 487–496 10.1016/j.cellsig.2003.09.010 [DOI] [PubMed] [Google Scholar]
- 18. Misra U. K., Gonzalez-Gronow M., Gawdi G., Hart J. P., Johnson C. E., and Pizzo S. V. (2002) The role of Grp 78 in α2-macroglobulin-induced signal transduction: evidence from RNA interference that the low density lipoprotein receptor-related protein is associated with, but not necessary for, GRP 78-mediated signal transduction. J. Biol. Chem. 277, 42082–42087 10.1074/jbc.M206174200 [DOI] [PubMed] [Google Scholar]
- 19. Li Z., Zhang L., Zhao Y., Li H., Xiao H., Fu R., Zhao C., Wu H., and Li Z. (2013) Cell-surface GRP78 facilitates colorectal cancer cell migration and invasion. Int. J. Biochem. Cell Biol. 45, 987–994 10.1016/j.biocel.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 20. Wu D., Peng F., Zhang B., Ingram A. J., Gao B., and Krepinsky J. C. (2007) Collagen I induction by high glucose levels is mediated by epidermal growth factor receptor and phosphoinositide 3-kinase/Akt signalling in mesangial cells. Diabetologia 50, 2008–2018 10.1007/s00125-007-0721-1 [DOI] [PubMed] [Google Scholar]
- 21. Li R., Wang T., Walia K., Gao B., and Krepinsky J. C. (2018) Regulation of profibrotic responses by ADAM17 activation in high glucose requires its C-terminus and FAK. J. Cell Sci. 131, jcs208629 10.1242/jcs.208629 [DOI] [PubMed] [Google Scholar]
- 22. Wu D., Peng F., Zhang B., Ingram A. J., Kelly D. J., Gilbert R. E., Gao B., and Krepinsky J. C. (2009) PKC-β1 mediates glucose-induced Akt activation and TGF-β1 upregulation in mesangial cells. J. Am. Soc. Nephrol. 20, 554–566 10.1681/ASN.2008040445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Misra U. K., and Pizzo S. V. (2010) Ligation of cell surface GRP78 with antibody directed against the COOH-terminal domain of GRP78 suppresses Ras/MAPK and PI 3-kinase/AKT signaling while promoting caspase activation in human prostate cancer cells. Cancer Biol. Ther. 9, 142–152 10.4161/cbt.9.2.10422 [DOI] [PubMed] [Google Scholar]
- 24. Misra U. K., Sharma T., and Pizzo S. V. (2005) Ligation of cell surface-associated glucose-regulated protein 78 by receptor-recognized forms of α2-macroglobulin: activation of p21-activated protein kinase-2-dependent signaling in murine peritoneal macrophages. J. Immunol. 175, 2525–2533 10.4049/jimmunol.175.4.2525 [DOI] [PubMed] [Google Scholar]
- 25. Misra U. K., Mowery Y., Kaczowka S., and Pizzo S. V. (2009) Ligation of cancer cell surface GRP78 with antibodies directed against its COOH-terminal domain up-regulates p53 activity and promotes apoptosis. Mol. Cancer Ther. 8, 1350–1362 10.1158/1535-7163.MCT-08-0990 [DOI] [PubMed] [Google Scholar]
- 26. Gonzalez-Gronow M., Cuchacovich M., Llanos C., Urzua C., Gawdi G., and Pizzo S. V. (2006) Prostate cancer cell proliferation in vitro is modulated by antibodies against glucose-regulated protein 78 isolated from patient serum. Cancer Res. 66, 11424–11431 10.1158/0008-5472.CAN-06-1721 [DOI] [PubMed] [Google Scholar]
- 27. Ray R., de Ridder G. G., Eu J. P., Paton A. W., Paton J. C., and Pizzo S. V. (2012) The Escherichia coli subtilase cytotoxin A subunit specifically cleaves cell-surface GRP78 protein and abolishes COOH-terminal-dependent signaling. J. Biol. Chem. 287, 32755–32769 10.1074/jbc.M112.399808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weston B. S., Wahab N. A., and Mason R. M. (2003) CTGF mediates TGF-β-induced fibronectin matrix deposition by upregulating active α5β1 integrin in human mesangial cells. J. Am. Soc. Nephrol. 14, 601–610 10.1097/01.ASN.0000051600.53134.B9 [DOI] [PubMed] [Google Scholar]
- 29. Miller C. G., Pozzi A., Zent R., and Schwarzbauer J. E. (2014) Effects of high glucose on integrin activity and fibronectin matrix assembly by mesangial cells. Mol. Biol. Cell 25, 2342–2350 10.1091/mbc.e14-03-0800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holman D., and Henley J. M. (2007) A novel method for monitoring the cell surface expression of heteromeric protein complexes in dispersed neurons and acute hippocampal slices. J. Neurosci. Methods 160, 302–308 10.1016/j.jneumeth.2006.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ayo S. H., Radnik R. A., Garoni J. A., Glass W. F. 2nd, and Kreisberg J. I. (1990) High glucose causes an increase in extracellular matrix proteins in cultured mesangial cells. Am. J. Pathol. 136, 1339–1348 [PMC free article] [PubMed] [Google Scholar]
- 32. Van Krieken R., Marway M., Parthasarathy P., Mehta N., Ingram A. J., Gao B., and Krepinsky J. C. (2018) Inhibition of SREBP with fatostatin does not attenuate early diabetic nephropathy in male mice. Endocrinology 159, 1479–1495 10.1210/en.2018-00093 [DOI] [PubMed] [Google Scholar]
- 33. Deleted in proof.
- 34. Alpers C. E., and Hudkins K. L. (2011) Mouse models of diabetic nephropathy. Curr. Opin. Nephrol. Hypertens. 20, 278–284 10.1097/MNH.0b013e3283451901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hartner A., Marek I., Cordasic N., Haas C., Schocklmann H., Hulsmann-Volkert G., Plasa I., Rascher W., Hilgers K. F., and Amann K. (2008) Glomerular regeneration is delayed in nephritic α8-integrin-deficient mice: contribution of α8-integrin to the regulation of mesangial cell apoptosis. Am. J. Nephrol. 28, 168–178 10.1159/000110022 [DOI] [PubMed] [Google Scholar]
- 36. Hartner A., Cordasic N., Menendez-Castro C., Volkert G., Yabu J. M., Kupraszewicz-Hutzler M., Rascher W., and Hilgers K. F. (2010) Lack of α8-integrin aggravates podocyte injury in experimental diabetic nephropathy. Am. J. Physiol. Renal Physiol. 299, F1151–1157 10.1152/ajprenal.00058.2010 [DOI] [PubMed] [Google Scholar]
- 37. Quinones Q. J., de Ridder G. G., and Pizzo S. V. (2008) GRP78: a chaperone with diverse roles beyond the endoplasmic reticulum. Histol. Histopathol. 23, 1409–1416 [DOI] [PubMed] [Google Scholar]
- 38. Lindenmeyer M. T., Rastaldi M. P., Ikehata M., Neusser M. A., Kretzler M., Cohen C. D., and Schlöndorff D. (2008) Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J. Am. Soc. Nephrol. 19, 2225–2236 10.1681/ASN.2007121313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fan Y., Lee K., Wang N., and He J. C. (2017) The role of endoplasmic reticulum stress in diabetic nephropathy. Curr. Diab. Rep. 17, 17 10.1007/s11892-017-0842-y [DOI] [PubMed] [Google Scholar]
- 40. Tsai Y. L., Ha D. P., Zhao H., Carlos A. J., Wei S., Pun T. K., Wu K., Zandi E., Kelly K., and Lee A. S. (2018) Endoplasmic reticulum stress activates SRC, relocating chaperones to the cell surface where GRP78/CD109 blocks TGF-β signaling. Proc. Natl. Acad. Sci. U.S.A. 115, E4245–E4254 10.1073/pnas.1714866115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Misra U. K., Deedwania R., and Pizzo S. V. (2006) Activation and cross-talk between Akt, NF-κB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J. Biol. Chem. 281, 13694–13707 10.1074/jbc.M511694200 [DOI] [PubMed] [Google Scholar]
- 42. Raiter A., Weiss C., Bechor Z., Ben-Dor I., Battler A., Kaplan B., and Hardy B. (2010) Activation of GRP78 on endothelial cell membranes by an ADAM15-derived peptide induces angiogenesis. J. Vasc. Res. 47, 399–411 10.1159/000281580 [DOI] [PubMed] [Google Scholar]
- 43. Philippova M., Ivanov D., Joshi M. B., Kyriakakis E., Rupp K., Afonyushkin T., Bochkov V., Erne P., and Resink T. J. (2008) Identification of proteins associating with glycosylphosphatidylinositol-anchored T-cadherin on the surface of vascular endothelial cells: role for Grp78/BiP in T-cadherin-dependent cell survival. Mol. Cell Biol. 28, 4004–4017 10.1128/MCB.00157-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen H. C., and Guan J. L. (1994) Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. U.S.A. 91, 10148–10152 10.1073/pnas.91.21.10148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steiner N., Borjan B., Hajek R., Jöhrer K., Göbel G., Willenbacher W., Kern J., Gunsilius E., and Untergasser G. (2017) Expression and release of glucose-regulated protein-78 (GRP78) in multiple myeloma. Oncotarget 8, 56243–56254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mintz P. J., Kim J., Do K. A., Wang X., Zinner R. G., Cristofanilli M., Arap M. A., Hong W. K., Troncoso P., Logothetis C. J., Pasqualini R., and Arap W. (2003) Fingerprinting the circulating repertoire of antibodies from cancer patients. Nat. Biotechnol. 21, 57–63 10.1038/nbt774 [DOI] [PubMed] [Google Scholar]
- 47. Liu C., Bhattacharjee G., Boisvert W., Dilley R., and Edgington T. (2003) In vivo interrogation of the molecular display of atherosclerotic lesion surfaces. Am. J. Pathol. 163, 1859–1871 10.1016/S0002-9440(10)63545-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rondas D., Crèvecoeur I., D'Hertog W., Ferreira G. B., Staes A., Garg A. D., Eizirik D. L., Agostinis P., Gevaert K., Overbergh L., and Mathieu C. (2015) Citrullinated glucose-regulated protein 78 is an autoantigen in type 1 diabetes. Diabetes 64, 573–586 10.2337/db14-0621 [DOI] [PubMed] [Google Scholar]
- 49. Liu M., Spellberg B., Phan Q. T., Fu Y., Fu Y., Lee A. S., Edwards J. E. Jr, Filler S. G., and Ibrahim A. S. (2010) The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Invest. 120, 1914–1924 10.1172/JCI42164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nakatsuka A., Wada J., Iseda I., Teshigawara S., Higashio K., Murakami K., Kanzaki M., Inoue K., Terami T., Katayama A., Hida K., Eguchi J., Horiguchi C. S., Ogawa D., Matsuki Y., et al. (2012) Vaspin is an adipokine ameliorating ER stress in obesity as a ligand for cell-surface GRP78/MTJ-1 complex. Diabetes 61, 2823–2832 10.2337/db12-0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Raiter A., Tenenbaum A., Yackobovitch-Gavan M., Battler A., and Hardy B. (2016) Peptide binding glucose regulated protein 78 Improves type 1 diabetes by preventing pancreatic β cell apoptosis. Exp. Clin. Endocrinol. Diabetes 124, 239–245 10.1055/s-0035-1569356 [DOI] [PubMed] [Google Scholar]
- 52. Hathaway C. K., Gasim A. M., Grant R., Chang A. S., Kim H. S., Madden V. J., Bagnell C. R. Jr, Jennette J. C., Smithies O., and Kakoki M. (2015) Low TGFβ1 expression prevents and high expression exacerbates diabetic nephropathy in mice. Proc. Natl. Acad. Sci. U.S.A. 112, 5815–5820 10.1073/pnas.1504777112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gray P. C., and Vale W. (2012) Cripto/GRP78 modulation of the TGF-beta pathway in development and oncogenesis. FEBS Lett. 586, 1836–1845 10.1016/j.febslet.2012.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li J. H., Huang X. R., Zhu H. J., Johnson R., and Lan H. Y. (2003) Role of TGF-β signaling in extracellular matrix production under high glucose conditions. Kidney Int. 63, 2010–2019 10.1046/j.1523-1755.2003.00016.x [DOI] [PubMed] [Google Scholar]
- 55. Peng F., Zhang B., Ingram A. J., Gao B., Zhang Y., and Krepinsky J. C. (2010) Mechanical stretch-induced RhoA activation is mediated by the RhoGEF Vav2 in mesangial cells. Cell. Signal. 22, 34–40 10.1016/j.cellsig.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 56. Uttarwar L., Gao B., Ingram A. J., and Krepinsky J. C. (2012) SREBP-1 activation by glucose mediates TGF-β upregulation in mesangial cells. Am. J. Physiol. Renal Physiol. 302, F329–F341 10.1152/ajprenal.00136.2011 [DOI] [PubMed] [Google Scholar]
- 57. Uttarwar L., Peng F., Wu D., Kumar S., Gao B., Ingram A. J., and Krepinsky J. C. (2011) HB-EGF release mediates glucose-induced activation of the epidermal growth factor receptor in mesangial cells. Am. J. Physiol. Renal Physiol. 300, F921–F931 10.1152/ajprenal.00436.2010 [DOI] [PubMed] [Google Scholar]
- 58. Zhang Y., Peng F., Gao B., Ingram A. J., and Krepinsky J. C. (2012) High glucose-induced RhoA activation requires caveolae and PKCβ1-mediated ROS generation. Am. J. Physiol. Renal Physiol. 302, F159–F172 10.1152/ajprenal.00749.2010 [DOI] [PubMed] [Google Scholar]
- 59. Zhang Y., Peng F., Gao B., Ingram A. J., and Krepinsky J. C. (2010) Mechanical strain-induced RhoA activation requires NADPH oxidase-mediated ROS generation in caveolae. Antioxid. Redox Signal. 13, 959–973 10.1089/ars.2009.2908 [DOI] [PubMed] [Google Scholar]