Abstract
The Ca2+/Mn2+ transport ATPases 1a and 2 (SPCA1a/2) are closely related to the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) and are implicated in breast cancer and Hailey–Hailey skin disease. Here, we purified the human SPCA1a/2 isoforms from a yeast recombinant expression system and compared their biochemical properties after reconstitution. We observed that the purified SPCA1a displays a lower Ca2+ affinity and slightly lower Mn2+ affinity than SPCA2. Remarkably, the turnover rates of SPCA1a in the presence of Mn2+ and SPCA2 incubated with Ca2+ and Mn2+ were comparable, whereas the turnover rate of SPCA1a in Ca2+ was 2-fold higher. Moreover, we noted an unusual biphasic activation curve for the SPCA1a ATPase and autophosphorylation activity, not observed with SPCA2. We also found that the biphasic pattern and low apparent ion affinity of SPCA1a critically depends on ATP concentration. We further show that the specific properties of SPCA1a at least partially depend on an N-terminal EF-hand–like motif, which is present only in the SPCA1a isoform and absent in SPCA2. This motif binds Ca2+, and its mutation lowered the Ca2+ turnover rate relative to that of Mn2+, increased substrate affinity, and reduced the level of biphasic activation of SPCA1a. A biochemical analysis indicated that Ca2+ binding to the N-terminal EF-hand–like motif promotes the activity of SPCA1a by facilitating autophosphorylation. We propose that this regulation may be physiologically relevant in cells with a high Ca2+ load, such as mammary gland cells during lactation, or in cells with a low ATP content, such as keratinocytes.
Keywords: Golgi, calcium ATPase, protein purification, membrane transporter reconstitution, calcium transport, manganese, breast cancer, Hailey–Hailey disease, ion homeostasis, proteoliposomes
Introduction
Transient alterations in subcellular Ca2+ concentrations drive numerous physiological processes. The local Ca2+ levels in cells are tightly regulated by membrane transport proteins such as ion channels, exchangers, and active transport ATPases (1, 2). Among them, the Ca2+ transport ATPases utilize the energy released from ATP hydrolysis to pump Ca2+ ions against the concentration gradient, i.e. from the cytosol to the exoplasmic compartments. In humans, three types of Ca2+-ATPases were identified that are localized in distinct membrane compartments: the closely related P2A-type ATPases sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)3 and secretory pathway Ca2+-ATPase (SPCA), sharing 29% sequence identity and over 40% sequence similarities, and the P2B-type ATPase plasma membrane Ca2+-ATPase (PMCA), which is more distantly related (3). All Ca2+-ATPases undergo transient autophosphorylation during the transport cycle.
The SPCA pumps are located in later compartments of the Golgi/secretory pathway and transport both Ca2+ and Mn2+ ions with high affinities, which represents a unique feature among the Ca2+ transport ATPases. SPCA isoforms ensure a constant filling of the Golgi and secretory pathway with Ca2+ and also Mn2+. Both ions are cofactors of many Golgi enzymes that support protein trafficking and post-translational modifications (reviewed in Ref. 3). Excess Mn2+ intake leads to manganism, a Parkinson-like disease that affects miners, welders, steel, and battery workers due to Mn2+ intoxication (4), and SPCA1 may play a role in Mn2+ detoxification in the liver (5).
Most eukaryotes express a single SPCA orthologue, which is called plasma membrane-related Ca2+-ATPase 1 (PMR1). In tetrapods, two isoforms are found, SPCA1 and SPCA2, which share 63% sequence identity and 73% similarity. The human isoform SPCA1 is ubiquitously expressed from the ATP2C1 gene. Haplo-insufficiency of SPCA1 leads to Hailey–Hailey disease, an autosomal dominant skin disorder characterized by loss of cell–cell adhesion (6). The second human isoform, SPCA2, is encoded by the ATP2C2 gene and SPCA2 protein expression is constrained to brain, testis, and actively secreting cells, such as mammary gland epithelial cells, suggesting a more specialized function (7–10).
The two human SPCA isoforms activate the plasma membrane Ca2+ channel Orai1, which is referred to as store-independent Ca2+ entry (SICE) (11, 12). Although store-operated Ca2+ entry (SOCE) relies on the depletion of the endoplasmic reticulum Ca2+ store that triggers STIM1-mediated Orai1 activation, SICE leads to Ca2+ entry through the Orai1 channel independently of STIM1 (11, 12). The incoming Ca2+ in cells is subsequently transferred to the secretory pathway (12, 13). Because both SPCA1 and SPCA2 are up-regulated in the mammary gland during lactation, SICE may promote cellular Ca2+ uptake for subsequent secretion in the milk via the secretory pathway (14, 15). In addition, SICE is chronically activated by SPCA2 in luminal-type breast cancer cells, which causes Ca2+ dyshomeostasis and aberrant cell proliferation (11). SPCA1 is implicated in the basal-type breast cancer (16, 17), whereas up-regulation of both SPCA genes is associated with microcalcifications during breast cancer (16).
Structures of the SERCA1a Ca2+ pump were solved in the main catalytic states providing detailed insights into its Ca2+ transport mechanism and domain architecture, which seems to be highly conserved between Ca2+-ATPases (18, 19). But compared with SERCA1a, the PMCA and SPCA pumps contain longer N- and C-terminal extensions, which presumably act as regulatory elements. The C terminus of PMCA clearly serves as an autoinhibitory domain that is regulated by calmodulin, protein kinases, and lipids (20). The functional role of the N or C termini of SPCA isoforms is also gradually emerging. Truncations of the SPCA2 N and C termini functionally impair SPCA2 (12). Moreover, a motif in the N terminus of SPCA2 controls the binding to Orai1 (11), whereas at the corresponding position of the Saccharomyces cerevisiae orthologue PMR1, a Ca2+-binding EF-hand–like motif is found, which is important for ion selectivity (21). Of interest, the ion selectivity of the yeast PMR1 protein to Ca2+ and Mn2+ depends on this N-terminal element (21), although other residues in the TM region are also important (22).
In this study, we directly compared the functional properties of the purified SPCA1a and SPCA2 protein in reconstituted proteoliposomes and discovered unexpected isoform-specific differences in Ca2+ versus Mn2+ affinities and turnover rates. These differences depend at least in part on a Ca2+-binding EF-hand–like motif in the SPCA1a N terminus that is absent in SPCA2.
Results
Purified SPCA1a and SPCA2 present different Ca2+-, but not Mn2+-dependent activities
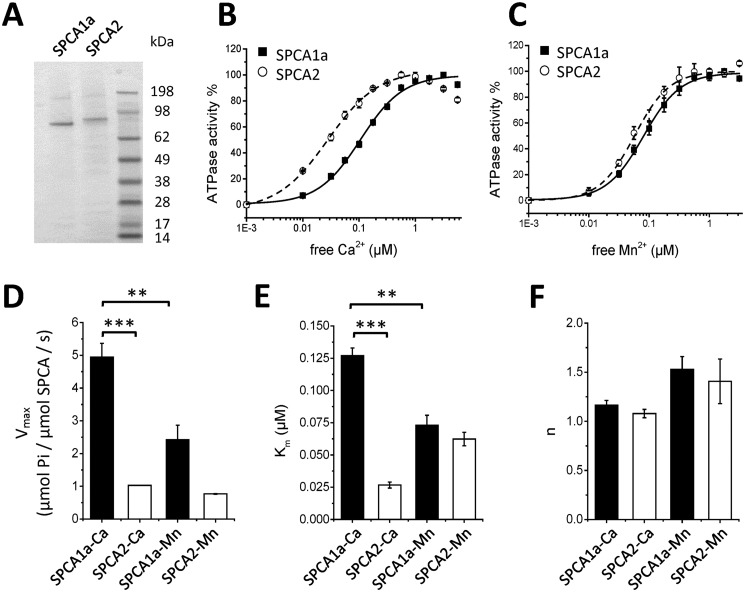
To functionally compare the SPCA1a and SPCA2 isoforms, we purified both isoforms and reconstituted them in proteoliposomes following the same procedure as we described before for SPCA1a (23). In short, the human His-tagged SPCA1a or SPCA2 isoforms were expressed in S. cerevisiae, affinity purified from solubilized membrane fractions, and then incorporated into lipid vesicles (Fig. 1A). The His10-tagged SPCA2 monomer (105.9 kDa) ran at a slightly higher position on SDS-PAGE than the His8-tagged SPCA1a (101.8 kDa), matching their predicted molecular masses. To compare the functional properties of the reconstituted SPCA1a and SPCA2 isoforms, we evaluated the Ca2+- (Fig. 1B) or Mn2+-dependent (Fig. 1C) ATPase activities with a colorimetric method to detect Pi levels (24), and observed remarkable isoform-specific differences. SPCA1a displays a 2-fold higher Ca2+-dependent maximal ATPase activity (Vmax) as compared with Mn2+, whereas SPCA2 presents a similar Vmax for both substrates (Fig. 1D). SPCA1a also exhibits a lower affinity for Ca2+ than for Mn2+, whereas this is opposite for SPCA2 (Fig. 1E). The cooperativity (n) of both enzymes for Ca2+ approximates 1, confirming that one Ca2+ is transported per ATP. The cooperativity of both isoforms toward Mn2+ is slightly, but not significantly higher (Fig. 1F). In conclusion, SPCA1a presents similar properties as SPCA2 in Mn2+ conditions, but a higher Vmax and lower apparent affinity in Ca2+ conditions (Table 1, Fig. 1, B–E).
Figure 1.
ATPase activity of reconstituted SPCA1a and SPCA2. A, a Coomassie-stained SDS-PAGE gel displays purified and reconstituted SPCA1a and SPCA2 proteins. The SeeBlue Plus2 was used as molecular weight marker. Ca2+ (B) and Mn2+ (C)- dependent ATPase activities measured on reconstituted SPCA1a (filled squares) and SPCA2 (empty circles) were normalized to their maximal activities, and fitted with logistic functions. D–F, bar graphs depicting the parameters of the maximal ATPase activity (Vmax, D), apparent affinities (Km, E) and cooperativities (n, F) for Ca2+ and Mn2+ were derived from B and C (n = 3–5).
Table 1.
Summary of parameters obtained from the ATPase assay
| Km | n | Vmax | |
|---|---|---|---|
| μm | μmol Pi/μmol SPCA/s | ||
| SPCA1a-Ca | 0.13 ± 0.01 | 1.17 ± 0.05 | 4.94 ± 0.42 |
| SPCA2-Ca | 0.03 ± 0.00 | 1.08 ± 0.04 | 1.03 ± 0.01 |
| SPCA1a-Mn | 0.07 ± 0.01 | 1.53 ± 0.13 | 2.43 ± 0.44 |
| SPCA2-Mn | 0.06 ± 0.01 | 1.41 ± 0.23 | 0.77 ± 0.01 |
SPCA1a, but not SPCA2, autophosphorylation presents a biphasic activation pattern
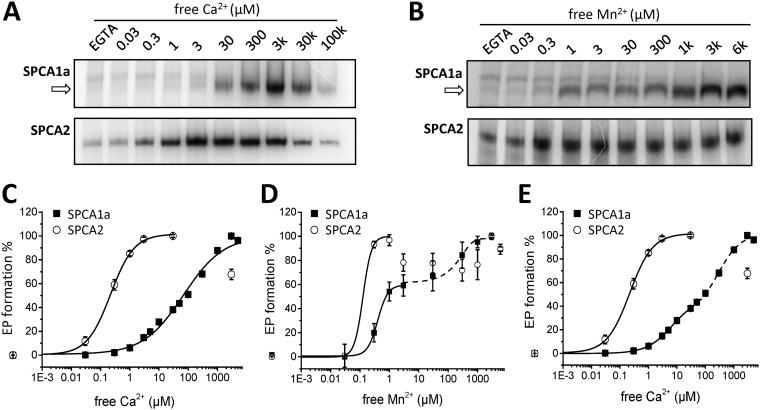
Like other P-type ATPases, SPCA isoforms catalyze a substrate-dependent autophosphorylation reaction on a conserved Asp residue in the phosphorylation domain (3, 25). We here confirm that the reconstituted SPCA isoforms form Ca2+- or Mn2+-dependent phospho-enzyme (EP) intermediates when incubated with radiolabeled ATP (Fig. 2, A and B). Note that a batch-dependent background phosphorylation was sometimes detected in the EGTA conditions, which most likely represents another low abundant phosphorylated protein that runs higher than SPCA1a. Because SPCA2 is slightly larger than SPCA1, this background signal runs at the same height as SPCA2, making it impossible to separate both phosphorylation signals. Because this additional signal is Ca2+- and Mn2+-independent, we subtracted it as background from the Ca2+- or Mn2+-dependent EP levels that represent the SPCA activity.
Figure 2.
Autophosphorylation of reconstituted SPCA1a and SPCA2. A and B, phosphorimages depict the radiolabeled SPCA1a (upper) and SPCA2 (lower) phospho-intermediates (EP) at various Ca2+ (A) or Mn2+ (B) concentrations, separated by gel electrophoresis. The arrows indicate the Ca2+- or Mn2+-sensitive phosphorylated SPCA1a bands. The radioactivity was quantified with ImageJ software and the EGTA signal was subtracted as background. C and D, radioactivity was used as a measure of EP formation, which was plotted in a Ca2+ (C)- or Mn2+ (D)-dependent manner and was fitted with logistic functions. The SPCA1a Mn2+-dependent autophosphorylation curve was fitted with two consecutive logistic functions, revealing a biphasic activation curve. E, re-fitting of the SPCA1a Ca2+-dependent autophosphorylation data in C with two consecutive logistic functions, revealing a biphasic activation pattern (n = 3–7).
Interestingly, we observed a remarkable difference between the Ca2+-dependent autophosphorylation behavior of both isoforms (Fig. 2, A and C). SPCA2 reached maximal autophosphorylation already at low micromolar free Ca2+, whereas the SPCA1a EP level raised further above 3 μm and reached a maximum at 3 mm free Ca2+, i.e. well above the physiological Ca2+ concentrations in the cytosol. Thus, the difference between the apparent Ca2+ affinities of SPCA1a and SPCA2 is much more pronounced in the EP assay (Table 2) than in the ATPase assay (Fig. 1 and Table 1). This most likely relates to the difference in ATP concentration between both assays (5 mm ATP in ATPase assay, versus 5 μm cold and radiolabeled ATP in the autophosphorylation assay). Indeed, increasing the ATP concentration from 5 to 50 μm shifts the SPCA1a EP curve to higher affinity, whereas reducing the ATP to 0.5 μm lowers the Ca2+ affinity (Fig. S1A). Conversely, decreasing the ATP concentration from 5 mm to 500 μm in an enzyme-coupled ATPase assay (which ensures that ATP is not depleted) also lowers the Ca2+ affinity of SPCA1a (Fig. S1B). This highlights that the Ca2+ affinity of SPCA1a is highly sensitive to the available ATP concentration. Moreover, the apparent Ca2+ affinity of SPCA1a autophosphorylation would be considerably higher at physiological ATP concentrations, i.e. more in line with the ATPase assay.
Table 2.
Summary of parameters obtained from the autophosphorylation assay of SPCA1 and SPCA2
| Km | n | Fitting | |
|---|---|---|---|
| μm | |||
| SPCA1a-Ca | 68.5 ± 10.4 | 0.63 ± 0.05 | Monophasic |
| 7.9 ± 0.1 | 0.95 ± 0.03 | Biphasic part I | |
| 294.0 ± 51.0 | 1.14 ± 0.27 | Biphasic part II | |
| SPCA2-Ca | 0.22 ± 0.01 | 1.08 ± 0.07 | Monophasic |
| SPCA1a-Mn | 0.43 ± 0.03 | 1.89 ± 0.32 | Biphasic part I |
| 103.2 ± 52.4 | 0.004 ± 0.001 | Biphasic part II | |
| SPCA2-Mn | 0.13 ± 0.05 | 2.99 ± 1.38 | Monophasic |
A similar EP pattern is observed in Mn2+ conditions (Fig. 2, B and D). SPCA2 already reached a maximal EP level in the presence of 0.3 μm free Mn2+, in line with a very high apparent Mn2+ affinity of SPCA2, whereas SPCA1a displayed a biphasic stimulation by Mn2+. The first part of the curve plateaus at ∼3 μm Mn2+, when ∼60% of the maximal EP level is reached (Fig. 2D). The second part of the curve rises from ∼30 μm Mn2+ onwards, and the maximal EP level is obtained around 1 mm. Like for the ATPase activities, both SPCA1a and SPCA2 present n values higher than 1 in Mn2+-induced phosphorylation, indicating positive cooperativities (Table 2). However, SPCA1a's cooperativity toward Ca2+ is lower than one.
Inspired by the biphasic profile of the Mn2+-dependent EP formation results, we carefully re-analyzed the results of the Ca2+-dependent EP assays and fitted a biphasic instead of a mono-sigmoidal curve in the data presented in Fig. 2C. Fitting with two logistic curves that are separated at 30 μm Ca2+ rendered an SPCA1a cooperativity for both the first and second phases of n = 1 (Fig. 2E, Table 2), suggesting that this may represent a better fit of the data. However, the biphasic pattern is not observed in ATPase measurements at 5 mm ATP, because at high Ca2+ or Mn2+ concentrations (i.e. above 10 μm) the ATPase activity of both isoforms decreases, which is known as back-inhibition of the pump (Fig. S2, A and B). However, once the ATP concentration in the ATPase assay is lowered to 0.5 mm, we found that SPCA1a also displayed a biphasic activation profile in the physiological Ca2+ concentration range (above 1 μm Ca2+) (Fig. S3). We therefore conclude that at low ATP concentration SPCA1a presents an unusual biphasic activation behavior, which may point to an autoregulatory mechanism that controls SPCA1a's activity and substrate affinity and is sensitive to the ATP concentration.
The efficacy of SPCA1a's autophosphorylation is higher with Ca2+ than Mn2+
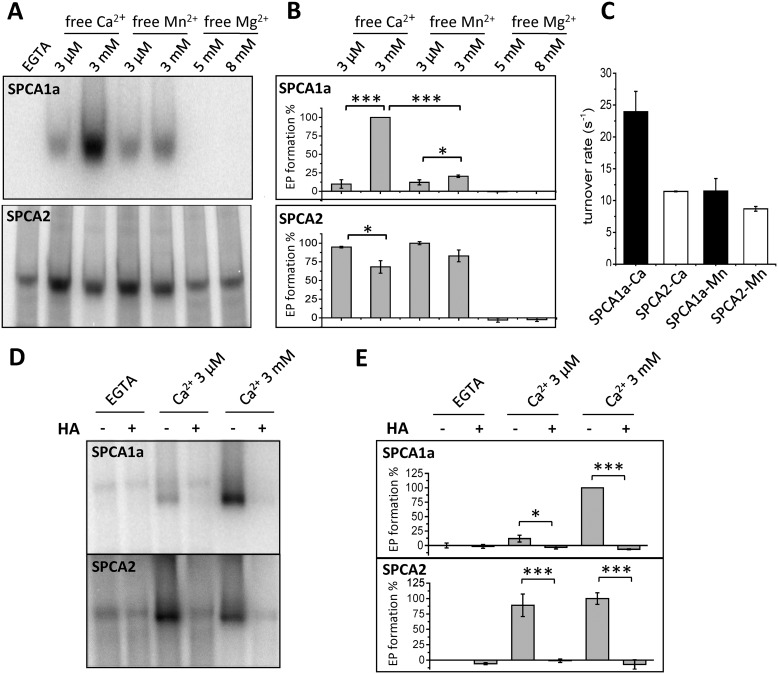
SPCA proteins transport Ca2+ and Mn2+ via the same ion-binding pocket in the transmembrane domain (26). Because binding of Mn2+ or Ca2+ at this binding pocket causes EP formation, we anticipated that saturating Ca2+/Mn2+ concentrations (3 μm for SPCA2 or 3 mm for SPCA1a) should yield similar EP levels for each isoform. To verify this, we directly compared the EP levels of both isoforms at 3 μm and 3 mm Ca2+ or Mn2+. As predicted, we observed that the SPCA2 EP levels were comparable at saturating concentrations of Ca2+ or Mn2+ (3 μm). No stimulation of EP levels was observed at higher Ca2+ or Mn2+ concentrations (Fig. 3, A and B). Although the EP level at 3 μm Ca2+ or Mn2+ was also similar for SPCA1a, the maximal EP level at 3 mm Ca2+ was 4.2-fold higher than at 3 mm Mn2+ (Fig. 3, A and B), which indicates that the autophosphorylation reaction of SPCA1a is more stimulated by Ca2+ than Mn2+. To further test the ion specificity of this EP stimulation, we compared normal (5 mm) versus higher (8 mm) Mg2+ concentrations. Mg2+ is an essential divalent ion that is included in the assay at 5 mm to support the ATP coordination and autophosphorylation reaction. However, Mg2+ ions, in the absence of Ca2+ or Mn2+, did not induce any EP formation of the SPCA isoforms (Fig. 3, A and B), indicating that only Ca2+ is able to maximally activate SPCA1a autophosphorylation.
Figure 3.
Comparison of the Ca2+-, Mn2+-, or Mg2+-induced autophosphorylation in SPCA1a and SPCA2. A, phosphorimages of the radiolabeled SPCA1a (upper) and SPCA2 (lower) phospho-intermediates (EP) separated by gel electrophoresis. Indicated concentrations of Mn2+ or Ca2+ were supplied in the presence of 5 mm MgCl2. In the Mg2+ conditions, only 5 or 8 mm MgCl2 was administered. B, bar graphs depicting the EP levels in various conditions. The radioactivity was quantified with ImageJ software and the EGTA signal was subtracted as background. C, bar graph depicting the turnover rates of both isoforms. Turnover rates were calculated by dividing the maximal ATPase activities over the maximal phosphorylation activities (n = 3). D, representative phosphorimages of SPCA1a or SPCA2 treated with radiolabeled ATP followed by administration of HA, a quencher of aspartyl-phosphate intermediates. Experiments were conducted in the presence of EGTA, 3 μm or 3 mm Ca2+ (n = 3). E, quantification of radioactivity in D was performed using ImageJ software. One-way ANOVA was performed followed by post hoc Bonferroni test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
The maximal EP levels observed at 3 μm Ca2+ for SPCA2 and 3 mm Ca2+ for SPCA1a reflect the active protein fractions, which can be used to determine the maximal turnover rates. The ratio between the maximal ATPase activity (nmol of Pi released/μg of protein/s) and the maximal level of autophosphorylated protein (nmol of protein phosphorylated/μg of protein) is typically used to calculate the maximal turnover rates of Ca2+ transport ATPases (s−1), as described before (27–29). The maximal turnover rate of SPCA1a is almost twice as high with Ca2+ than with Mn2+, or as compared with SPCA2 (Fig. 3C). For SPCA2, the maximal turnover rates are similar with Mn2+ or Ca2+ (Fig. 3C).
Next, we verified that the second activation step of the SPCA1a autophosphorylation represents the catalytic autophosphorylation of the conserved Asp residue. It is possible that the second activation step may be caused by a low abundant kinase that promotes phosphorylation of SPCA1a on a Ser/Thr/Tyr acceptor site. To discriminate between both possibilities, we administered hydroxylamine (HA) to the phosphoenzymes, which specifically quenches acyl phosphates on the Asp, but is not reactive to phospho-Ser/Thr/Tyr residues (30). HA treatment completely abolished the Ca2+-dependent phosphorylation signal of SPCA1a and SPCA2, confirming that the EP levels represent aspartyl phosphorylation at the catalytic autophosphorylation site (Fig. 3, D and E). In contrast, HA treatment did not quench the Ca2+-insensitive background phosphorylation signal, confirming that it occurs independently of the autophosphorylation reaction, most likely on an unrelated trace protein (Fig. 3, D and E). Together, only Ca2+ is able to maximally activate catalytic SPCA1a autophosphorylation, which may point to a Ca2+-dependent regulation mechanism of SPCA1a.
Only in SPCA1a, Ca2+ is able to replace Mg2+ to induce autophosphorylation
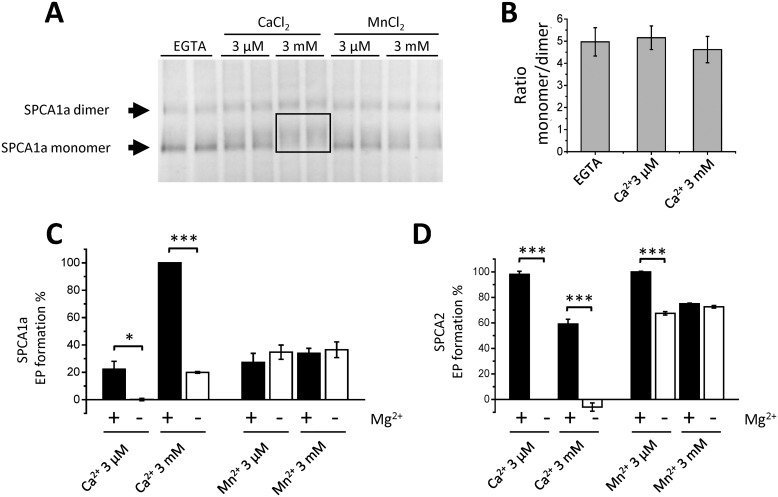
We hypothesized that the strongest stimulation of SPCA1a autophosphorylation by Ca2+ and the two-step activation pattern may be explained by a regulatory Ca2+-binding site that is only present in SPCA1a and not in SPCA2. The presence of such an ion-binding element is supported by the observation that high concentrations of Ca2+ result in a mobility shift of the SPCA1a monomer on native PAGE (Fig. 4A). The sharp SPCA1a monomer band that is observed in the EGTA condition becomes more diffuse and migrates at a higher part of the gel when 3 mm Ca2+ is supplied (box in Fig. 4A). This behavior is less pronounced with 3 μm Ca2+ or 3 μm/3 mm Mn2+ (Fig. 4A). Note that we also observed SPCA1a dimers on native PAGE, which were less influenced by Ca2+ binding (presumably due to the low resolution of the separation of larger protein complexes in gradient gels). The addition of Ca2+ had no effect on the SPCA1a monomer/dimer ratio (Fig. 4B). Furthermore, no mobility shift of the monomer band was observed for SPCA2 and SERCA1a at 3 μm or 3 mm Ca2+, although the monomer/dimer ratio was altered (Fig. S4). Together this indicates that the Ca2+-induced mobility shift of the monomer is an SPCA1a-specific behavior, which may be due to changes in charge, mass, and/or conformation of SPCA1a caused by the binding of Ca2+. Because the mobility shift was less pronounced with Mn2+, this indicates that the regulatory binding site of Ca2+ may differ from the Ca2+/Mn2+-binding site for transport in the transmembrane region.
Figure 4.
Binding of Ca2+ and Mn2+ to SPCA proteins. A, native PAGE of purified SPCA1a protein following incubation with varying concentrations of Ca2+ or Mn2+. Bands were visualized by Coomassie staining. The arrows point to the SPCA1a monomer and dimer bands in the EGTA condition. The monomer protein band appears less focused in the presence of 3 μm Ca2+ and migrates higher as a smear in the 3 mm Ca2+ condition (boxed region). B, quantified monomer/dimer band ratio of the SPCA1a in Native PAGE (n = 7). C and D, bar graphs depicting the quantified EP levels of SPCA1a (C) and SPCA2 (D). Indicated concentrations of Mn2+ or Ca2+ were supplied in the presence (5 mm) or absence (0 mm) of MgCl2 (n = 2). The EP levels were normalized to the maximal level of each isoform. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
As a first possibility for a regulatory divalent cation-binding site, we considered the conserved Mg2+-binding site near the ATP-binding site that is present in all P-type ATPases. It is known that high Mn2+ levels interfere with Mg2+ binding in proteins (31, 32). We therefore reasoned that by competing with Mg2+ at this site, the millimolar concentrations of Ca2+ or Mn2+ may affect the ATP coordination and autophosphorylation reaction of SPCA1a in a different way than SPCA2. We therefore tested if the elevated EP level in SPCA1a may be explained by the competition between Mg2+ and Ca2+ or Mn2+ (Fig. 4, C and D). Both SPCA1a and SPCA2 largely retained high EP levels when 5 mm Mg2+ was replaced by 3 μm or 3 mm Mn2+ (Fig. 4, C and D), indicating that Mn2+ is able to substitute for Mg2+ in both isoforms. In contrast, in the absence of Mg2+, 3 μm or 3 mm Ca2+ is unable to induce EP formation in SPCA2 (Fig. 4D). Remarkably, EP formation is observed for SPCA1a when 3 mm Ca2+ is used, albeit significantly lower (19.3%) than when Mg2+ is present (100.0%) (Fig. 4C).
Thus, SPCA2 can be autophosphorylated with Mg2+ or Mn2+ as ATP coordinating ions, whereas SPCA1a is also able to use Ca2+ for ATP coordination and autophosphorylation. However, Ca2+ is 4-fold less efficient to induce autophosphorylation in the absence of Mg2+. Because the strong stimulatory effect of millimolar Ca2+ on the SPCA1a autophosphorylation depends on the presence of Mg2+ (Fig. 4C), we hypothesized that another SPCA1a-specific region than the Mg2+-binding site may be responsible.
The N terminus of SPCA1a, but not SPCA2, contains a Ca2+-binding site
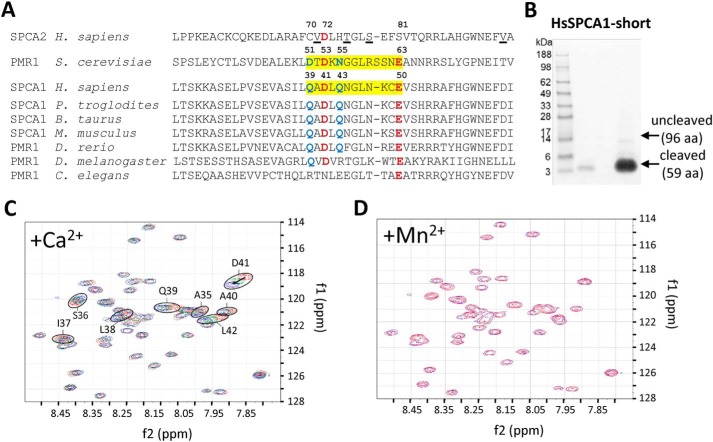
Yeast PMR1 possesses a Ca2+-binding EF-hand–like motif in its N terminus, which determines substrate selectivity and pumping activity (21). This motif is partially conserved in SPCA1a sequences of higher vertebrates (Fig. 5A), but the loop of the EF-hand–like motif of PMR1 comprises one extra amino acid. The critical acidic residues at positions Asp-51, Asp-53, and Glu-63 of the motif in PMR1 are reasonably conserved in SPCA1a (Gln-39, Asp-41, and Glu-50), but less conserved in SPCA2 (Cys-70, Asp-72, and Ser-81) or in invertebrate PMR1 sequences. Based on this alignment, we hypothesized that the N-terminal region of SPCA1a may hold a Ca2+-binding motif that may explain the Ca2+-dependent stimulation of SPCA1a's autophosphorylation activity.
Figure 5.
Binding of Ca2+ to the N-terminal EF-hand–like motif in SPCA1a. A, MUSCLE alignment of part of the N-terminal sequences of yeast PMR1 (NP_011348.1), human SPCA1 (NP_001186110.1), and SPCA2 (B4E2Q0), with various animal SPCA1/PMR1 orthologues (XP_001146246.2; NP_786979.1; NP_001240760.1; XP_003200287.2; NP_730742.1; NP_001021862.1). The regions highlighted in yellow in yeast PMR1 and human SPCA1 correspond to the loop of the EF-hand–like motif. The critical residues that are identical (red) or conserved (blue) between yeast PMR1 and human SPCA1 are indicated with numbering. In SPCA2, the underlined residues interact with Orai1 during store-independent Ca2+ entry (11). B, Coomassie staining of purified N-terminal fragment of SPCA1a (including an N-terminal linker SPMGYRGSM followed by the human SPCA1a sequence Val-20 to Glu-67). C, the heteronuclear single quantum coherence spectra overlay of the SPCA1a N-terminal peptide purified from B in the presence of 0 (red), 4.65 (green), and 9 mm (blue) Ca2+ ions. Encircled residues with the indicated numbers were identified in close vicinity of a Ca2+ ion. D, overlay of heteronuclear single quantum coherence spectra before (blue) and after (red) further addition of Mn2+ after the Ca2+ titration.
We turned to an NMR analysis of the human SPCA1a N terminus to pinpoint residues that may contribute to Ca2+ binding. We first purified a GST-labeled N-terminal SPCA1 fragment and removed the GST tag via thrombin cleavage. This rendered a >95% pure protein fragment corresponding to Val-20 to Glu-67 of SPCA1a (Fig. 5B), which was suitable for NMR analysis. This purified peptide was analyzed in the absence and presence of Ca2+ using NMR spectroscopy (Fig. 5C). Based on the chemical shift changes of backbone amide signals in 15N-HSQC spectra at different titration steps, we were able to unambiguously identify a stretch of residues that are significantly affected at their backbone by Ca2+ binding: Ser-36 to Leu-42 (Fig. 5C). The observed chemical shift changes can be attributed to direct interaction with Ca2+ or local conformational changes upon Ca2+ binding (33). The identified region contains Gln-39 and Asp-41, which are conserved residues in the EF-hand–like motif of PMR1 and SPCA1a (Fig. 5A).
After titrating with CaCl2, we also added MnSO4 as a competition experiment. Strong impact on protein signals upon binding Mn2+ is expected because its unpaired electron induces paramagnetic relaxation effects on NMR active nuclei within a distance of about 15 Å. Therefore, binding of the peptide with Mn2+ would result in line broadening and potentially removing signals from the NMR spectra (34). To avoid this problem and to potentially allow a second titration, the amount of Mn2+ added was an order of magnitude smaller than the added amount of Ca2+ but should still suffice to establish any influence (binding) of Mn2+ to the peptide. The absence of any observable changes in NMR spectra after the addition of MnSO4 indicates that this N-terminal peptide of SPCA1a does not bind Mn2+ (Fig. 5D). Together, our data show that SPCA1a harbors a Ca2+-selective EF-hand–like motif in its N terminus.
The N-terminal EF-hand–like motif provides Ca2+ specific control of SPCA1a activity
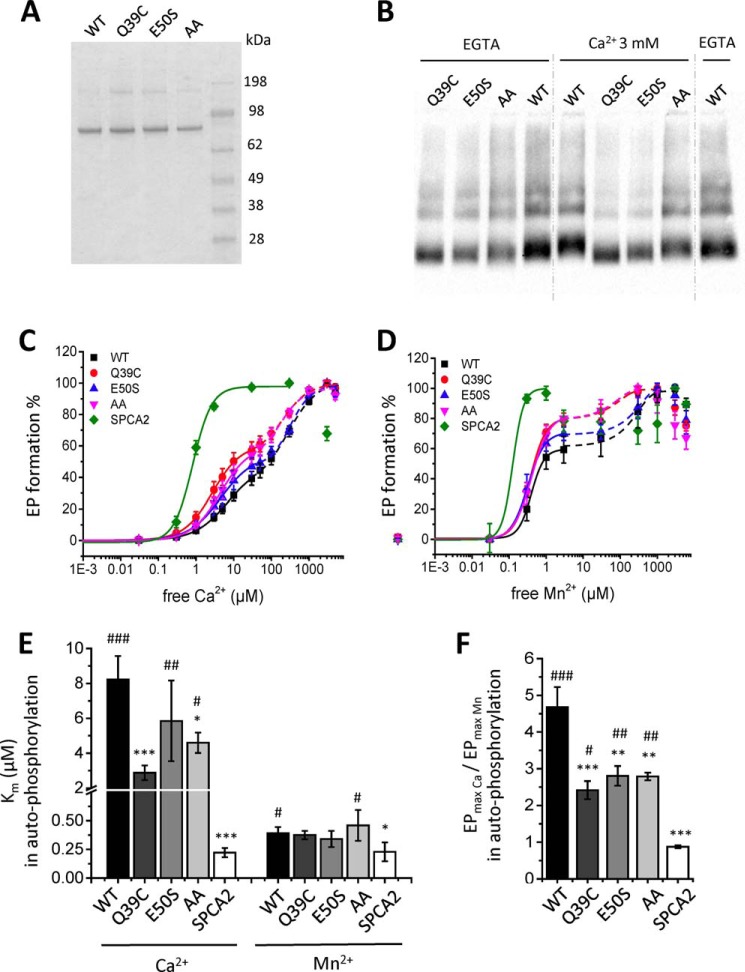
Next, we explored whether the N-terminal EF-hand–like motif of SPCA1a is responsible for the Ca2+-specific activation mechanism of autophosphorylation. We performed site-directed mutagenesis to disturb the motif, and generated two single mutants, Q39C and E50S, in which SPCA1a residues are substituted by the corresponding SPCA2 residues. Because SPCA2 may not bind Ca2+ at the N terminus (Fig. S4), we hypothesize that by introducing SPCA2 elements, we will affect the 3D configuration of the EF-hand–like motif and compromise Ca2+ coordination. Note also that Glu-50 is a highly conserved acidic residue in the canonical EF-hand sequences (35). Finally, we constructed a double mutant in which the two conserved acidic residues between SPCA1 and PMR1 are replaced by alanines (D41A/E50A). The three SPCA1a mutants were purified and reconstituted following the same procedure as for WT (Fig. 6A). Interestingly, the Ca2+-dependent mobility shifts of the mutant proteins are less prominent as compared with the purified WT protein, indicating that Ca2+ binding is hampered by the EF-hand mutations (Fig. 6B).
Figure 6.
Autophosphorylation activities of SPCA1a WT and N-terminal EF-hand–like mutants. A, a Coomassie-stained SDS-PAGE gel of the reconstituted SPCA1a WT and mutants. B, native PAGE of the SPCA1a WT and mutants incubated with or without Ca2+, followed by immunoblotting for detection with a SPCA1-specific antibody (1:50,000 dilution, Frodo, homemade). C and D, Ca2+- and Mn2+-dependent autophosphorylation activities were measured from radiograms for all constructs, and fitted with either one (for SPCA2) or two (for SPCA1a and the mutants) logistic curves (n = 3–7). Bar graphs depict the apparent ion affinities of the first phase (Km, E) and the ratio of the maximal phosphorylation levels of Ca2+ over Mn2+ (F), which were derived from the measurements by fitting of each individual measurement, shown in B and C. One-way ANOVA was performed followed by post hoc Fisher (E) and Bonferroni (F) tests. *, significantly different compared with SPCA1a WT. #, significantly different compared with SPCA2. *, p < 0.05; **, p < 0.01; ***, p < 0.001. AA, double alanine mutant.
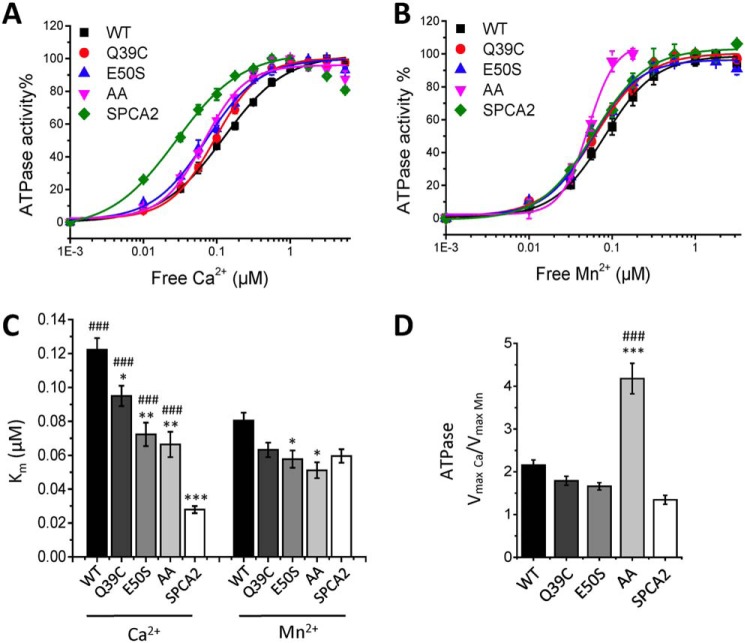
Remarkably, the biphasic pattern of the Ca2+-dependent autophosphorylation at 5 μm ATP is more pronounced for the SPCA1a mutants than for WT (Fig. 6B), which further shows that our earlier formulated proposal for a biphasic fitting of the Ca2+-dependent autophosphorylation of WT is justified (Fig. 2E). The mutants Q39C and AA displayed a significantly higher Ca2+ affinity in the first activation phase of Ca2+-dependent autophosphorylation, whereas the affinity shift for E50S is more modest (Fig. 6, C and E, Table 3). In ATPase assays in the presence of 5 mm ATP, all mutants exhibited a significantly higher apparent affinity for Ca2+, i.e. shifting the Km value closer to SPCA2's Km (Fig. 7, A and C). A more modest effect of the mutations was observed on the apparent Mn2+ affinity (Figs. 6, D and E, and 7, A–C, Table 3). Thus, we show that the EF-hand–like motif mainly regulates the apparent Ca2+ affinity of SPCA1a, but has a lower impact on Mn2+ affinity, which is in line with the selectivity of the EF-hand–like motif for Ca2+ over Mn2+ (Fig. 5, C and D).
Table 3.
The affinity of Ca2+ and Mn2+ obtained from autophosphorylation assay of SPCA1 mutants
| Km, biphasic part Ia | Km, biphasic part IIb | |
|---|---|---|
| μm | ||
| WT-Ca | 7.9 ± 0.1 | 285 |
| Q39C-Ca | 2.8 ± 0.1 | 204 |
| E50S-Ca | 4.6 ± 0.4 | 373 |
| AA-Ca | 4.4 ± 0.1 | 189 |
| WT-Mn | 0.43 ± 0.03 | 242 |
| Q39C-Mn | 0.37 ± 0.02 | 78 |
| E50S-Mn | 0.32 ± 0.01 | 259 |
| AA-Mn | 0.40 ± 0.02 | 73 |
a The Km of biphasic part I is calculated from the overall fitting of n = 3–7 curves via logistic function in Origin Pro9.
b Km of biphasic part II is calculated by enabling “finding x from y” function in Origin Pro 9. The y values are determined with formula, 100 − 0.5 × (100 − A1), A1 is the starting y value of the biphasic part II.
Figure 7.
ATPase activities of SPCA1a WT and N-terminal EF-hand–like mutants. A and B, Ca2+- and Mn2+-dependent ATPase activities were measured on all constructs and fitted with logistic curves. Bar graphs depict the apparent ion affinities (Km, C) and the ratio of maximal ATPase activities with Ca2+ over Mn2+ (D) (n = 3). Statistical analysis was performed using one-way ANOVA followed by a post hoc Bonferroni test. *, significantly different from SPCA1a WT. #, significantly different from SPCA2. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Moreover, the EF-hand–like motif also plays a role in the biphasic activation pattern of SPCA1a (Fig. 6, C and D). Indeed, the relative magnitudes of the second phosphorylation phase were reduced in all three mutants (Fig. 6, C and D). The apparent Ca2+ and Mn2+ affinity of the second phase was higher for the Q39C and AA mutants as compared with E50S and WT (Fig. 6, C and D, Table 3). However, the ratio of the maximal EP levels obtained in Ca2+ over Mn2+ (EPmax(Ca)/EPmax(Mn)) shows that the three mutants became less responsive to Ca2+ relative to Mn2+ (Fig. 6F). In addition, we confirmed in ATPase measurements that the single mutants displayed a lower Vmax(Ca)/Vmax(Mn) ratio, more closely resembling SPCA2 (Fig. 7D). Thus, these results reveal that the EF-hand–like motif contributes to the Ca2+-specific activation of autophosphorylation in SPCA1a, which regulates the maximal Ca2+-dependent ATPase activity.
Also the maximal ATPase activity of the double mutant is severely impaired (Fig. S5). However, the Vmax for Mn2+ reduced more than the Vmax for Ca2+, resulting in a remarkably high Vmax(Ca)/Vmax(Mn) ratio (Fig. 7D). Furthermore, the back-inhibition of the double mutant already takes place at lower Ca2+ or Mn2+ concentrations than WT (Fig. S2, C and D). Thus, disrupting the N-terminal motif by two alanine mutations dramatically impairs the overall activity of SPCA1a. However, the difference between the ATPase and EP results of the double mutant suggests a more complex regulatory mechanism that most likely also involves downstream steps in EP turnover. In conclusion, Ca2+ binding at the EF-hand–like motif in the N terminus of SPCA1a is at least partially responsible for the biphasic stimulation pattern of SPCA1a autophosphorylation, and contributes to the lower Ca2+ affinity and higher maximal activity of SPCA1a versus SPCA2.
Discussion
In the present study, we compared the properties of the Golgi/secretory pathway Ca2+/Mn2+-ATPases SPCA1a and SPCA2 in a purified, reconstituted system. We report isoform-specific differences between the Ca2+- and Mn2+-dependent properties of SPCA1a and SPCA2 that can at least in part be attributed to a Ca2+-binding regulatory motif in the N terminus of SPCA1a, which is absent in SPCA2.
An N-terminal EF-hand–like motif regulates the ion affinity and maximal activity of SPCA1a
Via a diverse set of approaches on purified SPCA1a and N-terminal fragments, we revealed a previously unrecognized Ca2+-dependent regulatory element that is present in SPCA1a, but absent in SPCA2. First, we observed a biphasic curve for SPCA1a autophosphorylation, which points to a regulatory mechanism that controls the activation state in a substrate-dependent manner. Second, we found that Ca2+ stimulates SPCA1a's EP formation more than Mn2+, in line with a Ca2+-specific regulatory site. Third, through NMR experiments, we confined the Ca2+ binding to specific Ca2+-coordinating residues belonging to the EF-hand–like motif in the SPCA1a N terminus. No Mn2+ binding was observed to this site. Fourth, Ca2+ alters the mobility of purified SPCA1a on native PAGE, which is less clear with Mn2+ or with mutations in the EF-hand–like motif. Fifth, via mutagenesis we demonstrated that the EF-hand–like motif contributes to the stronger Ca2+-dependent activation of autophosphorylation, the higher turnover rate and lower apparent Ca2+ affinity of SPCA1a as compared with SPCA2. In contrast, the mutations minimally affect the Mn2+ properties of SPCA1a. Taken together, the N-terminal EF-hand–like motif specifically binds Ca2+ and contributes to the isoform-specific properties of SPCA1a.
Besides the EF-hand–like motif, also the ATP concentration critically influences (i) the apparent Ca2+ affinity, and (ii) biphasic activation behavior of SPCA1a. Indeed, we first observed that the Ca2+ affinity of SPCA1a in the autophosphorylation assay at 5 μm ATP is unusually low, whereas SPCA1a's Ca2+ affinity increases at higher ATP concentrations. Conversely, reducing the ATP concentration in the ATPase assay lowers the apparent Ca2+ affinity of SPCA1a. Second, the biphasic activation pattern of SPCA1a is most clear at low ATP concentrations in both the autophosphorylation (Fig. 2E) and ATPase assay (Fig. S3). Moreover, the ATP concentration determines the Ca2+ sensitivity of the SPCA1a activation. At 0.5 mm ATP, the second activation step takes place between 1 and 10 μm Ca2+ (Fig. S3), i.e. at physiological Ca2+ concentrations. However, at 5 μm ATP, Ca2+ concentrations between 30 μm and 3 mm are required to trigger maximal autophosphorylation, i.e. well above physiological Ca2+ concentrations.
We therefore conclude that at high Ca2+ concentrations, Ca2+ binds to the N-terminal EF-hand–like motif of SPCA1a, and activates it. In contrast, at low Ca2+ concentrations SPCA1a is in a less active state due to the lack of Ca2+ binding to the EF-hand–like motif, which hinders autophosphorylation. Overall, this explains the low apparent Ca2+ affinity of SPCA1a and its higher Ca2+-dependent ATPase activity. We showed that this system is sensitive to the ATP concentration. When the ATP concentration is low, a Ca2+ concentration above 10 μm is required to maximally activate SPCA1a, whereas at more physiological ATP concentrations a Ca2+ level between 1 and 10 μm is sufficient to maximally activate SPCA1a. These observations suggest that ATP binding and N-terminal regulation are mechanistically linked.
It is not entirely clear how the N terminus may regulate the relative substrate affinities or how ATP may control this system. Because the distal N-terminal region is not directly involved in Ca2+ coordination in the membrane domain, it most likely modulates the substrate affinity indirectly. The N terminus may influence the packing of the Ca2+- and or Mn2+-coordinating residues in the ion entry/exit channels in the membrane domain or at the substrate-binding site. The ionic radius of Mn2+ (0.8 Å) is smaller than that of Ca2+ (0.99 Å) (36) and the N terminus may induce a local change at the ion-binding sites that differently affects the ion affinity. Alternatively, the N terminus of SPCA1a may modulate the conformational transitions in the catalytic cycle. A reduced apparent Ca2+ affinity may, for instance, be explained by stabilizing the SPCA1a protein more in the E2 than E1 state.
Both models depend on the interaction of the N terminus with downstream elements of the pump. Such a mode of action was already proposed for the yeast PMR1 protein. PMR1 also contains a Ca2+ binding N-terminal EF-hand–like motif that regulates the substrate affinities and Ca2+/Mn2+ selectivity, and a partial proteolytic digestion analysis indicated that the N-terminal region may interact with the catalytic ATP-binding domain of PMR1 (21). An interaction between the N terminus and the ATP-binding domain may offer a mechanistic explanation of how ATP may modulate the regulation by the N terminus. Future work will focus on identifying the interacting site of the N terminus in SPCA1a, which will further reveal the mechanism of SPCA1a activation.
Post-translational modifications may influence SPCA1a activity in a cellular context
In a previous report from our lab we compared the biochemical properties of SPCA1d and SPCA2 in membrane fractions of HEK-293 overexpressing cells (27). In that study, SPCA1d and SPCA2 displayed a similar apparent affinity in the ATPase assay (Km of 0.041 and 0.037 μm, respectively), whereas the catalytic turnover rate of SPCA1a was lower (27). This is clearly different from the measurements on purified SPCA1a and SPCA2 in this study. Also the biphasic activation pattern of SPCA1a was not observed before. Because the SPCA1a and SPCA1d splice variants only differ in the C terminus and contain the same N terminus, the discrepancies between both studies may not merely reflect differences between splice variants. Instead, several factors may influence the N-terminal activation mechanism in a cellular context, such as post-translational modifications, lipid interactions, or regulatory proteins that are absent in the purified systems. In that respect, we observed remarkable differences in the mobility of SPCA1a in HEK-293 microsomes versus proteoliposomes. The purified SPCA1a from yeast runs as a single monomer and single dimer band (Fig. 1A), whereas we, and others (14, 37, 38), observe that monomers of the SPCA1 splice variants in mammalian membrane fractions migrate as a double band (Fig. S6A). Remarkably, the lower band of SPCA1a in microsomes diminishes after treatment with reducing agent in combination with heating, and the higher band becomes stronger, which is not observed for SPCA1a in proteoliposomes (Fig. S6B). This may point to post-translational modifications of SPCA1a, such as disulfide bridges, which are only present in microsomes. These modifications may influence the biochemical properties, possibly explaining the difference between SPCA1a in proteoliposomes and in microsomes. These intriguing observations may point to an unknown regulatory system of SPCA1a in the cell, but future studies are needed to clarify this point. Analyzing the purified proteins, i.e. in the absence of confounding cellular elements, revealed an N-terminal regulatory element in SPCA1a, which otherwise would have been missed in the microsome context.
Physiological implications of the Ca2+-dependent regulation of SPCA1a
We compared the biochemical properties of purified SPCA1a and SPCA2 proteins in a fixed membrane environment, which eliminated cell-based confounding factors. The two human SPCA isoforms present different affinities and turnover rates toward Ca2+ and Mn2+ ions, their preferred substrates. SPCA1a displayed a 2-fold higher maximal turnover rate with Ca2+ than with Mn2+, whereas SPCA2 presented similar turnover rates for both substrates. In addition, the two isoforms exhibited similar Mn2+ affinities, whereas the apparent Ca2+ affinity of SPCA1a is significantly lower as compared with SPCA2.
These isoform-specific properties may have important physiological implications. Due to its extremely high Ca2+ affinity, but 2-fold lower Mn2+ affinity, SPCA2 may mainly transport Ca2+ ions. The dominant Ca2+ transport function of SPCA2 may be relevant for secretory cells where SPCA2 is predominantly expressed (7, 8). In secretory cell types like mammary gland, SPCA2 is functionally coupled to the Orai1 Ca2+ channel where it transports Ca2+ that comes in from the extracellular environment via Orai1 into the secretory pathway (11, 13). In contrast, SPCA1a is ubiquitously expressed and fulfills a housekeeping function by delivering both Ca2+ and Mn2+ into the Golgi/secretory compartment to support protein folding and maturation (3). The slightly higher affinity of SPCA1a for Mn2+ than Ca2+ suggests that at low ion availability the relative Ca2+ and Mn2+ concentrations will determine which ion will be transported by SPCA1a.
Our biochemical analysis further suggests that depending on the Ca2+ and ATP concentration, the EF-hand–like motif may switch SPCA1a between a high affinity/low capacity Ca2+/Mn2+ pump to a lower affinity, but higher capacity Ca2+ pump. Consequently, SPCA1a presents a higher Ca2+ transport capacity when the cytosolic Ca2+ load increases, which may occur in cell types undergoing Ca2+ signaling or fulfilling secretory functions. Like SPCA2, SPCA1a expression is elevated in mammary gland during lactation (14, 38), and both isoforms may operate together to transfer Ca2+ into the milk (11, 13). Although SPCA2 may be the major activator of Orai1 (11), SPCA1a may present a higher Ca2+ transport capacity than SPCA2. SPCA1a is detected in the subplasma membrane region close to the plasma membrane Ca2+ channel Orai1, where it may participate in SICE (13) and may become fully activated by the local Ca2+ increase (up to 300 μm in microdomains near Ca2+ channels (39)). Our data suggest that SPCA2 may not be further activated by Ca2+, but this does not exclude that the SPCA2 activity may respond to other stimuli such as the direct interaction with Orai1.
Mutations in SPCA1a lead to the skin disease Hailey–Hailey, which is manifested in the keratinocytes. Keratinocytes undergo substantial changes in their intracellular ATP and Ca2+ concentrations, and in this context, SPCA1a regulation by the EF-hand–like motif may take place. Keratinocytes contain merely one-third to one-fourth of the ATP concentration of artery smooth muscle cells (40). These low ATP levels further decrease when extracellular Ca2+ levels are increased (40), which may resemble the situation in the outer layers of the skin epidermis where extracellular Ca2+ progressively increases (41). The local environment with low ATP and high Ca2+ concentrations may affect the activity of SPCA1 via the N-terminal EF-hand–like motif. Moreover, in isolated keratinocytes of Hailey–Hailey disease patients with dysfunctional SPCA1, the ATP concentration is reduced, whereas the ATP levels fail to increase when extracellular Ca2+ is supplied (40). The altered ATP and Ca2+ concentrations in Hailey–Hailey disease may contribute to the dysregulation of SPCA1a activity.
In conclusion, our work on purified SPCA1a and SPCA2 proteins provides a comprehensive analysis of the isoform-specific Ca2+- and Mn2+-dependent activities. We discovered a Ca2+-binding EF-hand–like motif in the N terminus of SPCA1a that is absent in SPCA2. This motif is at least partially responsible for the SPCA1a-specific biochemical properties. Depending on the ATP concentration, the N terminus switches SPCA1a between a high affinity/low capacity state at low Ca2+ concentrations, and a low affinity/high capacity state at high Ca2+ loads. This mechanism may play a role in secreting cells like mammary gland during lactation, or in keratinocytes in the outer layers of the skin epidermis.
Experimental procedures
Plasmids and mutagenesis
The C-terminal His8-tagged human SPCA1a WT or mutants were introduced via Q5 site-directed mutagenesis (New England Biolabs) as previously described (23). The C-terminal His10-tagged human SPCA2 in the pADANS vector was a kind gift from Dr. S. Yamamoto, Kyorin University School of Medicine (42).
Purification and reconstitution of SPCA1a/2
Vectors containing SPCA1a and SPCA2 WT or mutants were transformed into S. cerevisiae strain BY4741a (his3Δ1;leu2Δ0;met15Δ0;ura3Δ0;MATa) as previously described (23). Each yeast clone was inoculated in minimal medium deprived of Leu for selection, then grown in yeast peptone dextrose medium for 30 h before harvesting. Yeast cells were pelleted at 5,000 × g for 5 min, 4 °C, washed with Milli-Q water, flash-frozen, and stored at −20 °C. The membrane fractions of yeast were isolated via differential centrifugations as previously described (23). The purification of His-tagged SPCA1a and SPCA2 proteins was performed by nickel-nitrilotriacetic acid affinity chromatography (23). Reconstitution of SPCA1a and SPCA2 in proteolipsomes composed of 80% phosphatidylcholine and 20% phosphatidic acid (Avanti Polar Lipids) was performed following the protocol in Ref. 23.
SDS protein electrophoresis and protein quantification
Purified or reconstituted SPCA1a or SPCA2 was separated on a NuPAGE 4–12% BisTris polyacrylamide gel (200 V, 35 min, MES buffer) (Thermo Fisher Scientific) and visualized with Imperial Protein Stain (Thermo Fisher Scientific). SeeBlue Plus2 Pre-stained Protein Standard (Thermo Fisher Scientific) was used as a molecular weight marker. The concentration of purified protein was determined by direct comparison to a bovine serum albumin (BSA) standard on the same gel (BSA Standard Ampules, Pierce).
Native PAGE
Purified proteins were incubated for 15 min at room temperature in buffer containing 100 mm MOPS, pH 7.0, 1 mm EGTA, 160 mm KCl, 0.2 mg/ml of dodecyl maltoside and CaCl2 or MnCl2 to reach the final Ca2+ or Mn2+ concentration indicated in the experimental conditions. The samples were then mixed with 4× native PAGE sample buffer supplemented with NativePAGETM 5% G-250 Sample Additive according to the instructions from the manufacturer. The electrophoresis was performed at 150 V for 100 min at room temperature using native PAGE running buffer as the anode buffer, and the cathode buffer was supplemented with 0.02% Coomassie Blue G-250. The native PAGE gel, sample buffer, sample additive and Coomassie Blue G-250 were purchased from Thermo Fisher Scientific. The protein bands were visualized by de-staining or immunoblotting.
ATPase assay
The ATPase activity of reconstituted samples was assayed by a colorimetric assay via the detection of Pi in the presence of 5 mm ATP, as previously described (43). The reactions were performed for 30 min using 300 ng of purified SPCA1a or SPCA2 in a reaction volume of 50 μl. The ATPase measurements with lower ATP concentrations were performed using the NADH-coupled assay in a 96-well plate. A total reaction mix of 250 μl contained 100 ng of purified SPCA1a, 50 mm TES/Tris, pH 7, 100 mm KCl, 7 mm MgCl2, 1 mm EGTA, 0.36 mm NADH, 1 mm phosphoenolpyruvate, 2.4 units of pyruvate kinase, 2.4 units of lactate dehydrogenase and the indicated free Ca2+ concentrations. The reaction was started by addition of the required amount of ATP. After 20 s mixing, the absorbance was read at 340 nm over 10 min in 30-s intervals with a SPECTRAmax PLUS384 Microplate Spectrophotometer (Molecular Devices). The slope of the decreased absorbance over time was used as a measure of the ATPase rate. All products were purchased from Merck.
Autophosphorylation assay
The autophosphorylation reaction was performed as described in Ref. 44 with slight modifications. Briefly, the reaction mixture contains 0.5 μg of purified SPCA1a or SPCA2 and 40 μg of BSA in a buffer containing 160 mm KCl, 17 mm Hepes, pH 7.0, 1 mm DTT, 5 mm MgCl2, 1 mm EGTA and various concentration of CaCl2, MnCl2, or MgCl2 as indicated in the experimental conditions. Unless specified otherwise, all autophosphorylation experiments were performed with 5 mm Mg2+. The reaction was initiated by addition of 0.074 MBq of radioactively labeled [γ-32P]ATP (PerkinElmer Life Sciences) in a final concentration of 5 μm. After a 20-s reaction on ice, 500 μl of stop solution containing 6% TCA, 10 mm phosphoric acid, and 1 mm ATP was added to the reaction mixture for precipitation. The pellet was washed three times with stop solution before detection in a scintillation counter (Packard, 2900TR) or by running on an acidic gel as previously described (44) and then detected with a phosphorimager (Typhoon, FLA9500). For HA treatment an additional washing step with 0.25 m HA was performed.
NMR analysis
A GST-labeled N-terminal fragment of the human SPCA1a (Val-20 to Glu-67) was cloned in the pET41a(+) vector and expressed in BL21 Escherichia coli with 15N-enriched growth medium. After affinity purification and thrombin cleavage to remove GST, a 59-amino acid long peptide (HsSPCA1-short) was obtained that consists of a 9-residue long linker (SPMGYRGSM) and the N-terminal SPCA1a sequence (20VLTSKK-ASELPVSEVASILQADLQNGLNKCVSHRRAFHGWNEFDISEDE69). NMR experiments were conducted with a Bruker Avance 600 MHz (1H) UltrashieldTM Plus with a CP TCI 600 S3 H-C/N-D-05 Z probe (Bruker, Fällanden, Switzerland). All data were processed using the TopSpin software (version 3.2) provided by Bruker and the resulting spectra were analyzed using the computer-aided resonance assignment software. 1H,15N-HSQC spectra (45, 46) were recorded at 5 °C using 12 scans with 4096 data points in the t2 dimension and 200 data points in the t1 dimension. The spectral width for the t1 dimension was 2311 Hz (38 ppm) and for the t2 dimension 9615 Hz (16 ppm). During each titration step, 0.5 μl of a CaCl2 solution (0.5 m) was added. After the Ca2+ titration, 5 μl of a MnSO4 solution (0.1 mm) was added to verify interactions between the SPCA1 N terminus and Mn2+.
Statistical analysis
Values are provided as mean ± S.E. from a n number of independent measurements. Radiograms and gel images are representative for a minimum of n = 3 experiments. Logistic fitting was performed via OriginPro 9 software. Statistical significance was calculated by one-way ANOVA followed by a Bonferroni post hoc test and indicated by asterisks: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Author contributions
J. C., F. W., and P. V. conceptualization; J. C., S. S., and C.-A. M. formal analysis; J. C. validation; J. C., S. S., C.-A. M., F. P., I. V., J. V., E. L., and P. V. investigation; J. C. visualization; J. C., C.-A. M., I. V., J. V., E. L., and P. V. methodology; J. C. and P. V. writing-original draft; J. C., J. E., and P. V. writing-review and editing; E. L. and P. V. resources; E. L., J. E., and P. V. supervision; P. V. funding acquisition; P. V. project administration.
Supplementary Material
This work was supported by Flanders Research Foundation FWO Grants G044212N and G0B1115N (to P. V.) and Inter-University Attraction Poles program P7/13. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S6.
- SERCA
- sarco(endo)plasmic reticulum Ca2+-ATPase
- SPCA
- secretory pathway Ca2+-ATPase
- PMCA
- plasma membrane Ca2+-ATPase
- PMR1
- plasma membrane-related Ca2+-ATPase 1
- SICE
- store-independent Ca2+ entry
- SOCE
- store-operated Ca2+ entry
- HA
- hydroxylamine
- GST
- glutathione S-transferase
- HSQC
- heteronuclear single quantum coherence
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- TES
- 2-{[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino}ethanesulfonic acid
- ANOVA
- analysis of variance
- AA
- double alanine mutant.
References
- 1. Berridge M. J., Bootman M. D., and Roderick H. L. (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529 10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- 2. Monteith G. R., McAndrew D., Faddy H. M., and Roberts-Thomson S. J. (2007) Calcium and cancer: targeting Ca2+ transport. Nat. Rev. Cancer 7, 519–530 10.1038/nrc2171 [DOI] [PubMed] [Google Scholar]
- 3. Vangheluwe P., Sepúlveda M. R., Missiaen L., Raeymaekers L., Wuytack F., and Vanoevelen J. (2009) Intracellular Ca2+- and Mn2+-transport ATPases. Chem. Rev. 109, 4733–4759 10.1021/cr900013m [DOI] [PubMed] [Google Scholar]
- 4. Sepulveda M. R., Dresselaers T., Vangheluwe P., Everaerts W., Himmelreich U., Mata A. M., and Wuytack F. (2012) Evaluation of manganese uptake and toxicity in mouse brain during continuous MnCl2 administration using osmotic pumps. Contrast Media Mol. Imaging 7, 426–434 10.1002/cmmi.1469 [DOI] [PubMed] [Google Scholar]
- 5. Leitch S., Feng M., Muend S., Braiterman L. T., Hubbard A. L., and Rao R. (2011) Vesicular distribution of secretory pathway Ca2+-ATPase isoform 1 and a role in manganese detoxification in liver-derived polarized cells. Biometals 24, 159–170 10.1007/s10534-010-9384-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu Z., Bonifas J. M., Beech J., Bench G., Shigihara T., Ogawa H., Ikeda S., Mauro T., and Epstein E. H. Jr. (2000) Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat. Genet. 24, 61–65 10.1038/71701 [DOI] [PubMed] [Google Scholar]
- 7. Xiang M., Mohamalawari D., and Rao R. (2005) A novel isoform of the secretory pathway Ca2+, Mn2+-ATPase, hSPCA2, has unusual properties and is expressed in the brain. J. Biol. Chem. 280, 11608–11614 10.1074/jbc.M413116200 [DOI] [PubMed] [Google Scholar]
- 8. Vanoevelen J., Dode L., Van Baelen K., Fairclough R. J., Missianen L., Raeymaekers L., and Wuytack F. (2005) The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi-localized pump with high affinity for Ca2+ ions. J. Biol. Chem. 280, 22800–22808 10.1074/jbc.M501026200 [DOI] [PubMed] [Google Scholar]
- 9. Baron S., Struyf S., Wuytack F., Van Damme J., Missiaen L., Raeymaekers L., and Vanoevelen J. (2009) Contribution of intracellular Ca2+ stores to Ca2+ signaling during chemokinesis of human neutrophil granulocytes. Biochim. Biophys. Acta 1793, 1041–1049 10.1016/j.bbamcr.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 10. Faddy H. M., Smart C. E., Xu R., Lee G. Y., Kenny P. A., Feng M., Rao R., Brown M. A., Bissell M. J., Roberts-Thomson S. J., and Monteith G. R. (2008) Localization of plasma membrane and secretory calcium pumps in the mammary gland. Biochem. Biophys. Res. Commun. 369, 977–981 10.1016/j.bbrc.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feng M., Grice D. M., Faddy H. M., Nguyen N., Leitch S., Wang Y., Muend S., Kenny P. A., Sukumar S., Roberts-Thomson J. J., Monteith G. R., and Rao R. (2010) Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell 143, 84–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smaardijk S., Chen J., Wuytack F., and Vangheluwe P. (2017) SPCA2 couples Ca2+ influx via Orai1 to Ca2+ uptake into the Golgi/secretory pathway. Tissue Cell 49, 141–149 10.1016/j.tice.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 13. Smaardijk S., Chen J., Kerselaers S., Voets T., Eggermont J., and Vangheluwe P. (2018) Store-independent coupling between the secretory pathway Ca2+ transport ATPase SPCA1 and Orai1 in Golgi stress and Hailey-Hailey disease. Biochim. Biophys. Acta 1865, 855–862 10.1016/j.bbamcr.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 14. Cross B. M., Hack A., Reinhardt T. A., and Rao R. (2013) SPCA2 regulates Orai1 trafficking and store independent Ca2+ entry in a model of lactation. PLoS ONE 8, e67348 10.1371/journal.pone.0067348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cross B. M., Breitwieser G. E., Reinhardt T. A., and Rao R. (2014) Cellular calcium dynamics in lactation and breast cancer: from physiology to pathology. Am. J. Physiol. Cell Physiol. 306, C515–C526 10.1152/ajpcell.00330.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dang D., Prasad H., and Rao R. (2017) Secretory pathway Ca2+-ATPases promote in vitro microcalcifications in breast cancer cells. Mol. Carcinogen. 56, 2474–2485 10.1002/mc.22695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grice D. M., Vetter I., Faddy H. M., Kenny P. A., Roberts-Thomson S. J., and Monteith G. R. (2010) Golgi calcium pump secretory pathway calcium ATPase 1 (SPCA1) is a key regulator of insulin-like growth factor receptor (IGF1R) processing in the basal-like breast cancer cell line MDA-MB-231. J. Biol. Chem. 285, 37458–37466 10.1074/jbc.M110.163329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sitsel A., De Raeymaecker J., Drachmann N. D., Derua R., Smaardijk S., Andersen J. L., Vandecaetsbeek I., Chen J., De Maeyer M., Waelkens E., Olesen C., Vangheluwe P., and Nissen P. (2019) Structures of the heart specific SERCA2a Ca2+-ATPase. EMBO J. 38, e100020 10.15252/embj.2018100020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gong D., Chi X., Ren K., Huang G., Zhou G., Yan N., Lei J., and Zhou Q. (2018) Structure of the human plasma membrane Ca2+-ATPase 1 in complex with its obligatory subunit neuroplastin. Nat. Commun. 9, 3623 10.1038/s41467-018-06075-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopreiato R., Giacomello M., and Carafoli E. (2014) The plasma membrane calcium pump: new ways to look at an old enzyme. J. Biol. Chem. 289, 10261–10268 10.1074/jbc.O114.555565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei Y., Marchi V., Wang R., and Rao R. (1999) An N-terminal EF hand-like motif modulates ion transport by Pmr1, the yeast Golgi Ca2+/Mn2+-ATPase. Biochemistry 38, 14534–14541 10.1021/bi9911233 [DOI] [PubMed] [Google Scholar]
- 22. Mandal D., Woolf T. B., and Rao R. (2000) Manganese selectivity of pmr1, the yeast secretory pathway ion pump, is defined by residue Gln-783 in transmembrane segment 6: residue Asp-778 is essential for cation transport. J. Biol. Chem. 275, 23933–23938 10.1074/jbc.M002619200 [DOI] [PubMed] [Google Scholar]
- 23. Chen J., De Raeymaecker J., Hovgaard J. B., Sammrdijk S., Vandecaetsbeek I., Wuytack F., Møller J. V., Eggermont J., De Maeyer M., Christensen S. B., and Vangheluwe P. (2017) Structure/activity relationship of thapsigargin inhibition on the purified Golgi/secretory pathway Ca2+/Mn2+-transport ATPase (SPCA1a). J. Biol. Chem. 292, 6938–6951 10.1074/jbc.M117.778431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baginski E. S., Foà P. P., and Zak B. (1967) Microdetermination of inorganic phosphate, phospholipids, and total phosphate in biologic materials. Clin. Chem. 13, 326–332 [PubMed] [Google Scholar]
- 25. Kühlbrandt W. (2004) Biology, structure and mechanism of P-type ATPases. Nat. Rev. Mol. Cell Biol. 5, 282–295 10.1038/nrm1354 [DOI] [PubMed] [Google Scholar]
- 26. Ton V. K., Mandal D., Vahadji C., and Rao R. (2002) Functional expression in yeast of the human secretory pathway Ca2+, Mn2+-ATPase defective in Hailey-Hailey disease. J. Biol. Chem. 277, 6422–6427 10.1074/jbc.M110612200 [DOI] [PubMed] [Google Scholar]
- 27. Dode L., Andersen J. P., Vanoevelen J., Raeymaekers L., Missiaen L., Vilsen B., and Wuytack F. (2006) Dissection of the functional differences between human secretory pathway Ca2+/Mn2+-ATPase (SPCA) 1 and 2 isoenzymes by steady-state and transient kinetic analyses. J. Biol. Chem. 281, 3182–3189 10.1074/jbc.M511547200 [DOI] [PubMed] [Google Scholar]
- 28. Dode L., Andersen J. P., Raeymaekers L., Missiaen L., Vilsen B., and Wuytack F. (2005) Functional comparison between secretory pathway Ca2+/Mn2+-ATPase (SPCA) 1 and sarcoplasmic reticulum Ca2+-ATPase (SERCA) 1 isoforms by steady-state and transient kinetic analyses. J. Biol. Chem. 280, 39124–39134 10.1074/jbc.M506181200 [DOI] [PubMed] [Google Scholar]
- 29. Lytton J., Westlin M., Burk S. E., Shull G. E., and MacLennan D. H. (1992) Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J. Biol. Chem. 267, 14483–14489 [PubMed] [Google Scholar]
- 30. Post R. L., and Kume S. (1973) Evidence for an aspartyl phosphate residue at the active site of sodium and potassium ion transport adenosine triphosphatase. J. Biol. Chem. 248, 6993–7000 [PubMed] [Google Scholar]
- 31. Bolton E. C., Mildvan A. S., and Boeke J. D. (2002) Inhibition of reverse transcription in vivo by elevated manganese ion concentration. Mol. Cell 9, 879–889 10.1016/S1097-2765(02)00495-1 [DOI] [PubMed] [Google Scholar]
- 32. Beckman R. A., Mildvan A. S., and Loeb L. A. (1985) On the fidelity of DNA replication: manganese mutagenesis in vitro. Biochemistry 24, 5810–5817 10.1021/bi00342a019 [DOI] [PubMed] [Google Scholar]
- 33. Drmota Prebil S. D., Slapšak U., Pavšič M., Ilc G., Puž V., de Almeida Ribeiro E., Anrather D., Hartl M., Backman L., Plavec J., Lenarčič B., and Djinović-Carugo K. (2016) Structure and calcium-binding studies of calmodulin-like domain of human non-muscle α-actinin-1. Sci. Rep. 6, 27383 10.1038/srep27383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rule G. S., and Hitchens T. K. (2006) Fundamentals of protein NMR spectroscopy. in Focus on Structural Biology (Kaptein R., ed) pp. 530, Springer, Dordrecht [Google Scholar]
- 35. Zhou Y., Frey T. K., and Yang J. J. (2009) Viral calciomics: interplays between Ca2+ and virus. Cell Calcium 46, 1–17 10.1016/j.ceca.2009.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moody B. J. (1991) Comparative Inorganic Chemistry (Bernard M., ed) 3rd Ed, pp. xiii and 562, Academic Press, Washington, D. C [Google Scholar]
- 37. Sepúlveda M. R., Marcos D., Berrocal M., Raeymaekers L., Mata A. M., and Wuytack F. (2008) Activity and localization of the secretory pathway Ca2+-ATPase isoform 1 (SPCA1) in different areas of the mouse brain during postnatal development. Mol. Cell. Neurosci. 38, 461–473 10.1016/j.mcn.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 38. Reinhardt T. A., Filoteo A. G., Penniston J. T., and Horst R. L. (2000) Ca2+-ATPase protein expression in mammary tissue. Am. J. Physiol. Cell Physiol 279, C1595–1602 10.1152/ajpcell.2000.279.5.C1595 [DOI] [PubMed] [Google Scholar]
- 39. Llinás R., Sugimori M., and Silver R. B. (1992) Microdomains of high calcium concentration in a presynaptic terminal. Science 256, 677–679 10.1126/science.1350109 [DOI] [PubMed] [Google Scholar]
- 40. Aronchik I., Behne M. J., Leypoldt L., Crumrine D., Epstein E., Ikeda S., Mizoguchi M., Bench G., Pozzan T., and Mauro T. (2003) Actin reorganization is abnormal and cellular ATP is decreased in Hailey-Hailey keratinocytes. J. Investig. Dermatol. 121, 681–687 10.1046/j.1523-1747.2003.12472.x [DOI] [PubMed] [Google Scholar]
- 41. Elias P., Ahn S., Brown B., Crumrine D., and Feingold K. R. (2002) Origin of the epidermal calcium gradient: regulation by barrier status and role of active vs passive mechanisms. J. Investig. Dermatol. 119, 1269–1274 10.1046/j.1523-1747.2002.19622.x [DOI] [PubMed] [Google Scholar]
- 42. Yamamoto S., Takehara M., Kabashima Y., Fukutomi T., and Ushimaru M. (2016) Identification of novel inhibitors of human SPCA2. Biochem. Biophys. Res. Commun. 477, 266–270 10.1016/j.bbrc.2016.06.055 [DOI] [PubMed] [Google Scholar]
- 43. Vandecaetsbeek I., Holemans T, Wuytack F., and Vangheluwe P. (2014) High-throughput measurement of the Ca2+-dependent ATPase activity in COS microsomes. Cold Spring Harb. Protoc. 2014, 865–875 [DOI] [PubMed] [Google Scholar]
- 44. Holemans T., Sørensen D. M., van Veen S., Martin S., Hermans D., Kemmer G. C., Van den Haute C., Baekelandt V., Günther Pomorski T., Agostinis P., Wuytack F., Palmgren M., Eggermont J., and Vangheluwe P. (2015) A lipid switch unlocks Parkinson's disease-associated ATP13A2. Proc. Natl. Acad. Sci. U.S.A. 112, 9040–9045 10.1073/pnas.1508220112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Davis A. L., Keeler J., Laue E. D., and Moskau D. (1992) Experiments for recording pure-absorption heteronuclear correlation spectra using pulsed field gradients. J. Magn. Res. 98, 207–216 10.1016/0022-2364(92)90126-R [DOI] [Google Scholar]
- 46. Grzesiek S., and Bax A. (1993) The importance of not saturating H2O in protein NMR: application to sensitivity enhancement and NOE measurements. J. Am. Chem. Soc. 115, 12593–12594 10.1021/ja00079a052 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.