Abstract
The pathology Alzheimer’s disease (AD) is associated with the self-assembly of amyloid-β (Aβ) peptides into β-sheet enriched fibrillar aggregates. A promising treatment strategy is focused on the inhibition of amyloid fibrillization of Aβ peptide. Fullerene C60 is proved to effectively inhibit Aβ fibrillation while the poor water-solubility restricts its use as a biomedicine agent. In this work, we examined the interaction of fullerene C60 and water-soluble fullerenol C60(OH)6/C60(OH)12 (C60 carrying 6/12 hydroxyl groups) with preformed Aβ40/42 protofibrils by multiple molecular dynamics simulations. We found that when binding to the Aβ42 protofibril, C60, C60(OH)6 and C60(OH)12 exhibit distinct binding dynamics, binding sites and peptide interaction. The increased number of hydroxyl groups C60 carries leads to slower binding dynamics and weaker binding strength. Binding free energy analysis demonstrates that the C60/C60(OH)6 molecule primarily binds to the C-terminal residues 31–41, whereas C60(OH)12 favors to bind to N-terminal residues 4–14. The hydrophobic interaction plays a critical role in the interplay between Aβ and all the three nanoparticles, and the π-stacking interaction gets weakened as C60 carries more hydroxyls. In addition, the C60(OH)6 molecule has high affinity to form hydrogen bonds with protein backbones. The binding behaviors of C60/C60(OH)6/C60(OH)12 to the Aβ40 protofibril resemble with those to Aβ42. Our work provides a detailed picture of fullerene/fullerenols binding to Aβ protofibril, and is helpful to understand the underlying inhibitory mechanism.
Keywords: amyloid protofibril, fullerene, binding site, inhibitory mechanism, molecular dynamics simulation
1. Introduction
Amyloids are involved in a broad range of neurodegenerative diseases, including Alzheimer’s, Huntington’s and Parkinson’s diseases [1,2,3]. The major constituents of amyloid plaques are associated with fibrils formed by amyloid-β (Aβ) protein that display a cross-β structure characterized by β-strands perpendicular to and inter-strand hydrogen bonds parallel to the fibril axis [4,5]. The fibrillation occurs through a complex multistep process, involving the formation of soluble oligomers, protofibrils and insoluble mature fibrils [6,7]. Small aggregates (soluble oligomers and protofibrils) in the early stage of aggregation are suggested as primary neurotoxic agents [8,9,10,11]. Therefore, a promising strategy to reduce the small toxic oligomer species is to inhibit Aβ peptide aggregation.
The search for effective inhibitors has become an active area of research. An increasing number of experimental and computational studies have reported that Aβ aggregation can be modulated by nanoparticles [12,13,14], small molecules [15,16], short peptides [17,18], antibodies [19] and metal ions [20]. Their findings provided new clues for the design of inhibitors targeting Aβ formation. In recent years, the fullerenes have gained great attention, not only because of their antioxidant, neuroprotective and antitumor properties [21], but also due to their promising ability of carrying contrast agents, radiopharmaceuticals or drugs [22]. However, the poor solubility of fullerenes in water restricts their potential biomedical applications. One of the common strategies to increase of their solubility is to attach hydroxyl groups to the carbon cage, leading to the formation of hydroxylated fullerene (i.e., fullerenol, C60(OH)n). Fullerenols have high solubility and ability to cross the blood brain barriers [23]. Fullerene derivatives are reported to have remarkable anti-amyloid properties for Alzheimer’s disease and other neurodegenerative diseases [24,25,26,27,28].
Computational studies have investigated the molecular mechanism of fullerenes/fullerenols binding and binding-induced protein remodeling using docking method and molecular dynamics (MD) simulations [29,30,31,32,33]. For example, Li et al. examined the binding affinity of fullerenes in different sizes and found that C60 destroys pentameric Aβ17–42 fibril structure to a greater extent with respect to other fullerenes [29]. They also found that C60(OH)16 is inclined to bind at the central hydrophobic and the hydrophobic C-terminal region of monomer Aβ40 to prevent amyloid fibrillization [30]. Wei et al. identified three primary binding sites of 1,2-(dimethoxymethano) fullerene (DMF) to Aβ1–42 protofibril: the central hydrophobic residues 17–21, the residues 27–31 in turn region and the C-terminal residues 31–41 [31]. They also demonstrated that the fullerene nanoparticles – C60 and C180 exhibit stronger inhibition on Aβ16–22 β-sheet formation [33]. Ding et al. found that different extent of hydroxylation would significantly influence C60(OH)n–protein interactions [32]. These results reveal the binding modes and inhibitory/disruptive mechanisms of fullerenes/fullerenols, which greatly enhances our understanding of fullerenes/fullerenols-protein interactions at atomic level.
Our previous study examined the influence of DMF on Aβ42 dimerization by replica-exchange MD simulations [34]. However, the interactions between Aβ protofibril and fullerenes with different degree of hydroxylation remain elusive. Previous computational study showed that stable Aβ trimer with well-preserved parallel β-strands could act as the smallest seed for Aβ polymerization on self-assembled monolayers [35]. Following the work by Zheng and Wei [35,36], we chose a trimer as Aβ protofibril model in our MD simulations. Here, we investigated the interaction of a C60/C60(OH)6/C60(OH)12 molecule with Aβ42/40 protofibrillar trimer and the resulting protein structural alterations by performing multiple MD simulations. We found that the increased hydroxylation extent of C60 leads to slower binding dynamics and weaker binding strength. Binding sites and free energy analyses demonstrate that the C60/C60(OH)6 molecule primarily binds to the C-terminal hydrophobic region, whereas C60(OH)12 favors to bind to N-terminal residues 4–14. Our simulations revealed the dominant role of hydrophobic interaction in Aβ−nanoparticle interplay. Moreover, the water-soluble C60(OH)6 molecule has high affinity to form hydrogen bonds with protein backbones, which makes it a more efficient inhibitor than C60 and C60(OH)12.
2. Results and Discussion
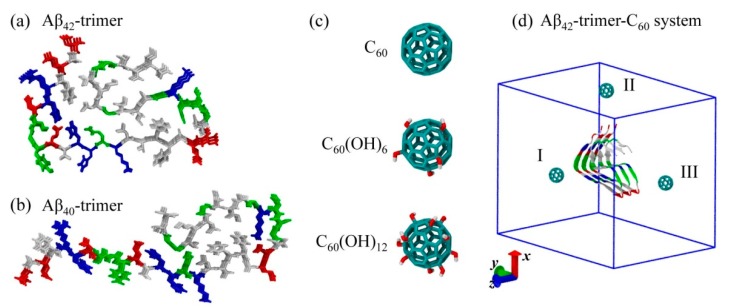
We performed MD simulations to study the binding behavior of C60/C60(OH)6/ C60(OH)12 to Aβ42 protofibrillar trimer (Aβ42-trimer for short) and Aβ40 protofibrillar trimer (Aβ40-trimer for short), respectively. The systems are labeled as Aβ42-trimer-C60, Aβ42-trimer-C60(OH)6, Aβ42-trimer-C60(OH)12, Aβ40-trimer-C60, Aβ40-trimer-C60(OH)6 and Aβ40-trimer-C60(OH)12. The molecular structures of Aβ42-trimer, Aβ40-trimer and C60/C60(OH)6/C60(OH)12 are shown in Figure 1. The initial state of Aβ42-trimer-C60 system is also displayed, and the other systems are constructed similarly. More details are given in Model and Methods section.
Figure 1.
Molecular structures and simulation system setup. (a–c) The structures of Aβ42-trimer, Aβ40-trimer and C60/C60(OH)6/C60(OH)12. (d) The initial conformation of the Aβ42-trimer-C60 system with the C60 molecule placed at three different positions (I-III). Color codes: positively charged residues (blue), negatively charged residues (red), hydrophobic residues (white) and polar residues (green) in Aβ peptides; carbon atoms (cyan), oxygen atoms (red) and hydrogen atoms (white) in fullerene/fullerenol. For clarity, water molecules in the simulation box are not displayed; box vectors are shown, and z-axis is the fibrillar elongation direction.
2.1. Dynamics of the Fullerene/Fullerenol Molecule Binding to Aβ42-Trimer
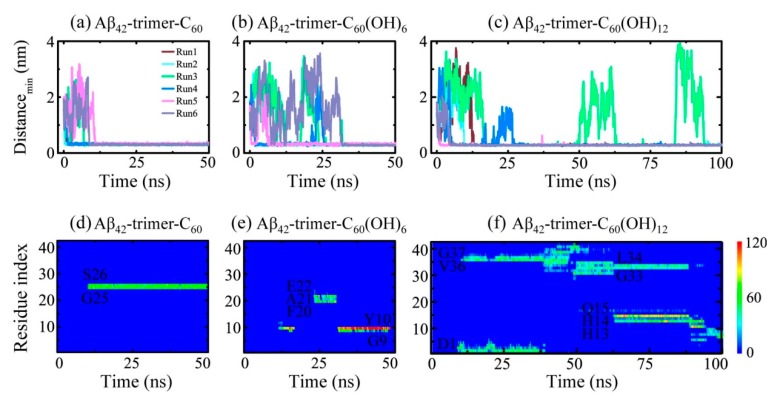
To investigate the binding process of the fullerene/fullerenol molecule to Aβ42-trimer, we first monitored the time evolution of their minimum distance dmin (Figure 2a–c). As for the Aβ42-trimer-C60 system, the C60 molecule was initially placed 2 nm away from the Aβ42-trimer. Once the MD simulations were initiated, dmin started to decrease or increase, depending on the initial velocity distributions. The minimum distances in Run 1, 2 and 4 were observed to decline to ~0.30 nm within the first 3 ns, while those in Run 3, 5 and 6 took ~10 ns to reach ~0.30 nm. Such fast and slow binding processes were also observed in Aβ42-trimer-C60(OH)6 and Aβ42-trimer-C60(OH)12 systems. Similar fast and slow processes were reported in a previous MD study of DMF binding to Aβ fibril [31]. Moreover, we found that the slow binding processes may last tens of nanoseconds for C60(OH)6 and C60(OH)12, much longer than that for C60. It takes over 25 ns for two MD runs of Aβ-C60(OH)6 system (Runs 3, 6) to reach a minimum distance of ~0.30 nm, and the situation was the same in Aβ-C60(OH)12 system (Runs 3, 4). Specially, in Run 3 of Aβ42-trimer-C60(OH)12 system, dmin increased sharply at 49.8 and 83.6 ns, and declined to ~0.30 nm in the next twenty nanoseconds. These indicate that the binding process of the C60(OH)6/C60(OH)12 molecule to Aβ42-trimer is slower than that of C60.
Figure 2.
Dynamics of the fullerene/fullerenol molecule binding to Aβ42-trimer. (a–c) Time evolution of the minimum distance between Aβ42-trimer and fullerene/fullerenol. Six independent molecular dynamics (MD) runs are denoted in different colors. (d–f) Time evolution of the number of contacts between individual residue of Aβ42-trimer and fullerene/fullerenol in a representative MD run for each simulated system.
To further examine the binding status of the fullerene/fullerenol molecule after the initial adsorption to Aβ42-trimer, we monitored the time evolution of the number of contacts between individual residue and the nanoparticle in a representative MD run for each simulated system in Figure 2d–f. The C60 molecule was observed to stay at a relatively fixed location during the remaining simulation time once stable contacts are formed. The C60(OH)6 molecule also had a relatively fixed binding site, while it can shift to other location transiently. As for the C60(OH)12 molecule, its binding location kept changing when simulation time increased, corresponding to a slow move on the protein surface. C60(OH)12 also contacted with more residues at the same time, which indicated a lower specificity of binding sites. These results reflect that with the hydroxylation extent of C60 increased, the binding strength between Aβ42-trimer and the nanoparticle molecule gets weaker.
In order to quantify the binding strength, we calculated in Table 1 the binding free energy and its different components between Aβ42-trimer and the fullerene/fullerenol molecule using the MM/PBSA (molecular mechanics/linear Poisson−Boltzmann surface area) method. The binding energy was calculated over all six MD runs for each simulated system using the last 20 ns data of each MD trajectory. The binding energy components show that the van der Waals interaction (ΔEvdW) has a dominant contribution to the total binding energy (ΔGbind). It is shown that ΔEvdW is -24.02 ± 0.74 kcal/mol in the Aβ-C60 system, -24.02 ± 0.74 kcal/mol in the Aβ-C60(OH)6 system and -18.20 ± 1.02 kcal/mol in the Aβ-C60(OH)12 system. Interestingly, although C60(OH)6 carries six more hydroxyl groups than C60, their ΔEvdW is quite similar, and that of C60(OH)12 became ~6 kcal/mol larger. This reveals that the increment of ΔEvdW is not in proportion to the hydroxylation level of C60 surface. Due to the additional partial charges that hydroxyls bring, the electrostatic interaction (ΔEelec) is strengthened as the hydroxyl number increases. The nonpolar solvation component ΔGnonpolar contributes little to the free energy change. The enhanced hydrophilicity with the addition of hydroxyls results in a positive value of ΔGsolv (solvation effect), indicating that water is favorable for fullerenols and solvation effect goes against the binding of fullerenol to Aβ. Our results are consistent with a previous study on fullerenol C60(OH)16 interacting with Aβ40 [30]. They found that the electrostatics contribution is much increased in fullerenol with respect to that in fullerenes, yet hydroxyl groups contribute a positive amount to the binding free energy. Overall, our free energy calculation demonstrates that the total binding free energy rises with more hydroxyl groups attached to C60. This gives the explanation that higher hydroxylation level leads to slower binding dynamics and weaker binding strength.
Table 1.
Different components of binding free energy (in kcal/mol) between Aβ42-trimer and the fullerene/fullerenol molecule.
| Systems | ΔEvdw | ΔEelec | ΔEMM | ΔGpolar | ΔGnonpolar | ΔGsolv | ΔGbind |
|---|---|---|---|---|---|---|---|
| Aβ42-trimer-C60 | −24.44 ± 0.69 | 0 | −24.44 ± 0.69 | 0 | −3.92 ± 0.16 | −3.92 ± 0.16 | −28.36 ± 0.71 |
| Aβ42-trimer-C60(OH)6 | −24.02 ± 0.74 | −5.16 ± 0.69 | −29.18 ± 0.25 | 15.27 ± 1.68 | −3.61 ± 0.16 | 11.66 ± 1.69 | −17.52 ± 1.71 |
| Aβ42-trimer-C60(OH)12 | −18.20 ± 1.02 | −14.60 ± 1.45 | −32.80 ± 1.77 | 27.06 ± 2.52 | −3.30 ± 0.17 | 23.77 ± 2.53 | −9.03 ± 3.09 |
2.2. Binding Sites of The Fullerene/Fullerenol Molecule to Aβ42-Trimer
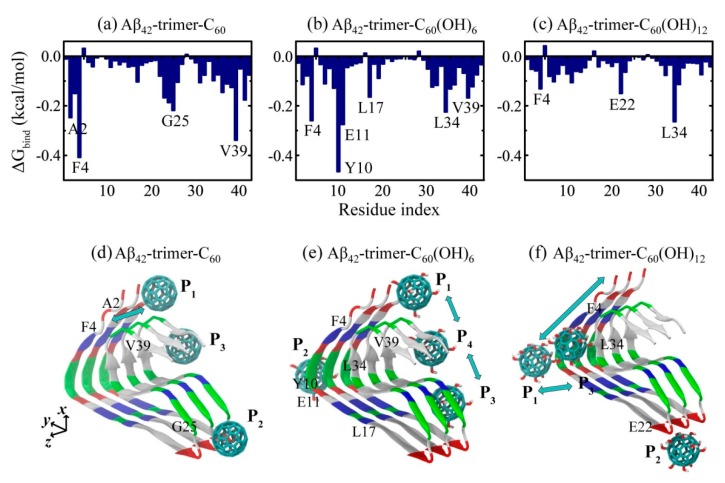
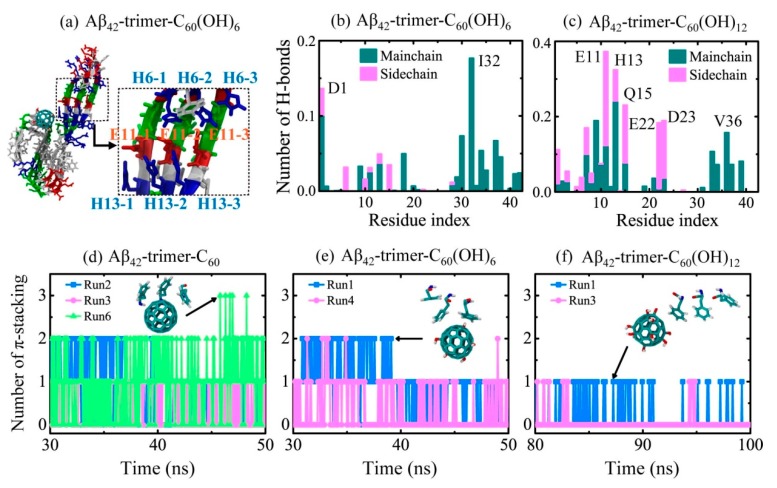
Identifying the binding sites of the C60/C60(OH)6/C60(OH)12 molecule to Aβ42-trimer is the first step to understand the underlying inhibition mechanism. To this aim, we calculated the residue-based binding free energy of the nanoparticles to Aβ42-trimer in Figure 3a–c using last 20 ns data of the simulations. As shown, C60 has the lowest binding energy with aromatic residue F4, and hydrophobic residues V39 and A2, as well as G25 located in the turn region; C60(OH)6 has the lowest binding energy with aromatic Y10 and F4, negatively charged E11, and hydrophobic L34, L17 and V39; C60(OH)12 has the lowest binding energy with hydrophobic L34, negatively charged E22 and aromatic F4. This indicates the critical roles of aromatic stacking and hydrophobic interactions in the interplay between Aβ and all the three nanoparticles. As for fullerenols C60(OH)6 and C60(OH)12, their hydrogen bonding interaction with negatively charged residues of Aβ is also important.
Figure 3.
Analysis of binding sites of the fullerene/fullerenol molecule to Aβ42-trimer. (a–c) Residue-based binding free energy. The binding energy was calculated over all six MD runs for each simulated system using the last 20 ns data of each MD trajectory. (d–f) Schematic diagrams for binding sites of the fullerene/fullerenol molecule to Aβ42-trimer. The positions where the fullerene/fullerenol molecule has high binding affinity are named with P1, P2, etc., from N-termini to C-termini, and z-axis is the fibrillar elongation direction. The color code is consistent with that in Figure 1.
According to the residue-based binding free energy, we found that C60 preferentially interacts with Aβ42-trimer at three different sites: 2AEF4, 23DVG25 and C-terminal residues 31–41. Through the binding energy analysis at each site (Table 2), we found that C-terminal residues 31–41 and 2AEF4 have the lowest binding energy, indicating these two regions are the most favorable binding sites for C60. This finding is in agreement with the binding sites (aromatic residues F4 and C-terminal hydrophobic residues 31–40) identified in DMF interacting with Aβ dimer [34]. The C-terminal hydrophobic region of residues 31–41 was also reported to be the dominant binding site in DMF interacting with Aβ fibrillar hexamer [31]. As for C60(OH)6, it prefers to bind to Aβ42-trimer at four sites: 2AEF4, 9GYE11, 17LVF19 and C-terminal residues 31–41, among which C-terminal residues 31–41 and 9GYE11 have the lowest binding energy. As for C60(OH)12, it has three preferential sites: N-terminal residues 4–14, 22ED23 and 34LM35, among which N-terminal residues 4–14 are the most favorable. The hydrophobic clusters A2-F4-L34-V36, L17-F19-I31 and A30-I32-M35-V40 play critical roles in the structural stability of Aβ42 fibril [5]. The strong binding of C60/C60(OH)6 to these clusters is expected to interfere with the hydrophobic packing of Aβ side chains, and as a result goes against further fibrillization.
Table 2.
Free energy (in kcal/mol) of different binding sites for the fullerene/fullerenol molecule to Aβ42-trimer.
| System | Aβ42-trimer-C60 | Aβ42-trimer-C60(OH)6 | Aβ42-trimer-C60(OH)12 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Binding site | 2–4 | 23–25 | 31–41 | 2–4 | 9–11 | 17–19 | 31–41 | 4–14 | 22–23 | 34–35 |
| ΔGbind | −0.80 | −0.57 | −1.32 | −0.45 | −0.87 | −0.28 | −1.23 | −0.73 | −0.21 | −0.38 |
| Deviation | 0.09 | 0.02 | 0.01 | 0.06 | 0.03 | 0.04 | 0.05 | 0.07 | 0.04 | 0.05 |
To exhibit the relation between binding dynamics and binding sites clearly, we presented the schematic diagrams for binding sites of C60/C60(OH)6/C60(OH)12 to Aβ42-trimer. The positions where the fullerene/fullerenol molecule has high binding affinity are named with P1, P2, etc., from N-termini to C-termini. As shown in Figure 3d, there are three positions P1, P2 and P3 at which C60 prefers to stay when binding to Aβ42-trimer, mainly corresponding to the binding sties 2AEF4, 23DVG25 and C-terminal residues 31–41, respectively. Note that C60 staying at P1 can interact with the region 2AEF4 and the C-terminal residues 31–41 at the same time. Trajectory tracing shows that C60 binds mostly at P1 and P3 with a respective probability of 22.7% and 29.8%, in agreement with the free energy calculation, and the location of C60 is relatively fixed. Moreover, the C60 molecule is able to wander on the surface groove along z-axis of Aβ42-trimer at P1 position. These preferential positions are near two hydrophobic clusters A2-F4-L34-V36 and A30-I32-M35-V40, indicating that the binding of C60 to Aβ42-trimer is dominantly driven by the hydrophobic interaction. The importance of hydrophobic interaction was reported in other studies on the binding processes of fullerene and other small molecules to Aβ [29,37,38]. With respect to P1 and P3, C60 has a relatively lower binding affinity to P2. This binding site is facilitated by the groove of a proper size in the 23DVGS26 region, where the side chains of D23 and S26 are in the outer side of Aβ42-trimer and G25 has no side chain. Similar concave-induced binding sites were observed in the study of fullerenes with Aβ and other proteins [31,39,40].
As for C60(OH)6, it has four preferential binding positions P1, P2, P3 and P4 (Figure 3e), mainly corresponding to the binding sties 2AEF4, 9GYE11, 17LVF19 and C-terminal residues 31–41, respectively. Interestingly, the C60(OH)6 molecule is able to slip on the elongation surface (perpendicular to z-axis), wandering between P1 and P4 or between P3 and P4 with a low probability. The P3 position is adjacent to another hydrophobic cluster L17-F19-I31 of Aβ42-trimer. Besides, C60(OH)6 has high binding affinity to 9GYE11 (P2), facilitated by the hydrogen bonds (H-bonds) formed in between. As for C60(OH)12, it prefers to bind to three positions P1, P2 and P3 (Figure 3f), corresponding to the binding sties N-terminal residues 4–14, 22ED23 and 34LM35, respectively. Different from the binding behaviors of C60 and C60(OH)6, C60(OH)12 is more likely to stay at the hydrophilic parts of protein surface. It is able to move between positions P1 and P3, or slip along the N-terminal β-strand, forming H-bonds with main chain or side chain of amino acids. The C60(OH)12 molecule may also contact with 22ED23 region. As the side chains of E22 and D23 are oriented to water solution, C60(OH)12 is inclined to form H-bonds with them. Considering the important roles of C-terminal hydrophobic residues in Aβ aggregation and toxicity [41,42,43], it is conceivable that the binding of C60 and C60(OH)6 molecules to the C-terminal region can prevent Aβ fibrillization. In addition, the C60(OH)6 molecule has higher affinity to bind to elongation surfaces than C60 and C60(OH)12, which makes C60(OH)6 a more effective inhibitor. As previous computational and experimental studies suggested that binding at fibril ends goes against fibrillar elongation [44,45,46], this binding would block the backbone amide sites for fibril growth and as a result, slows down or inhibits the elongation process. It is noted that the bindings of nanoparticles to protofibril and mature fibril are supposed to be distinct, because the relative area of the exposed ends compared to the entire fibril surface will be greatly decreased in mature fibrils.
2.3. Structural Influence of The Fullerene/Fullerenol Molecule on Aβ42-Trimer
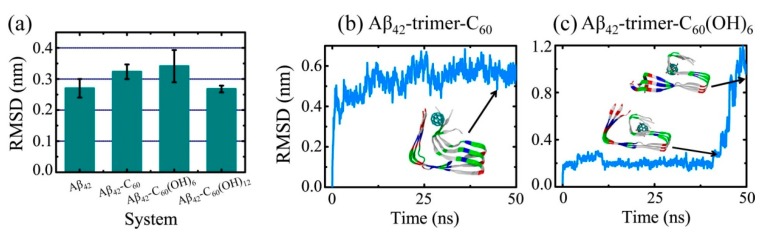
In order to detect the influence of fullerene/fullerenol binding on the Aβ42-trimer structure, we first examined the secondary structural difference relative to the isolated Aβ42-trimer. The β-sheet contents of Aβ42-trimer, Aβ42-trimer-C60, Aβ42-trimer-C60(OH)6 and Aβ42-trimer-C60(OH)12 systems are 80.5%, 83.3%, 81.1% and 80.4%, respectively, showing little difference. Then, we calculated the average Cα-root-mean-square deviation (Cα-RMSD) with respect to the initial coordinates of Aβ42 protofibrillar trimer using the last 20 ns data of each MD trajectory. As shown in Figure 4a, the values of Cα-RMSD in the absence and presence of the C60(OH)12 molecule are 0.27 ± 0.03 nm and 0.27 ± 0.01 nm, showing no statistically significant difference. In the presence of C60/C60(OH)6, Aβ42-trimer has an increased Cα-RMSD of 0.32 ± 0.02 / 0.34 ± 0.05 nm, while the values are still within the error of estimate with respect to that of isolated Aβ. These indicate that the C60/C60(OH)6/C60(OH)12 molecule has a negligible influence on the structural stability of Aβ42-trimer.
Figure 4.
Influence of fullerene/fullerenol molecules on the Aβ42-trimer structure. (a) The average Cα-root-mean-square deviation (Cα-RMSD) relative to the initial coordinates of Aβ42 protofibrillar trimer. The values for Aβ-fullerene/fullerenol systems were calculated over all six MD runs for each simulated system using the last 20 ns data of each MD trajectory, and those for isolated Aβ42-trimer systems were averaged over the last 50 ns data of two independent 200-ns MD runs. (b,c) Time evolution of Cα-RMSD of the MD trajectory that contributes most to the total Cα-RMSD in Aβ42-trimer-C60 and Aβ42-trimer-C60(OH)6 systems, respectively. The color code of the inset snapshots is consistent with that in Figure 1.
Figure 4b,c display the time evolution of Cα-RMSD of the MD trajectory contributing most to the total Cα-RMSD in Aβ42-trimer-C60 and Aβ42-trimer-C60(OH)6 systems, respectively. With C60, the Cα-RMSD value of Aβ42-trimer keeps rising in the first 20 ns and finally fluctuates at around 0.55 nm. During this process, the C60 molecule is observed to contact abundantly with side chains of V39 and I41, and lead to twisted C-termini. In the Aβ42-trimer-C60(OH)6 system, the Cα-RMSD value of Aβ42-trimer keeps at ~0.30 nm until t = 40.8 ns. After that, it rises sharply and increases to >1.0 nm. When Cα-RMSD begins its quick rise, the C60(OH)6 molecule is observed to bind at the C-terminal residues 31–41, and the hydrophobic cluster A2-F4-L34-V36 starts to collapse. Then, the sidechains of A2 and F4 dissociate with those of L34 and V36 one by one, and finally the N-termini and C-termini get separated far away. Note that it is the only MD trajectory among all the simulations we performed in this study that N- and C-termini dissociation is observed. It needs further studying to connect this dissociation with Aβ-C60(OH)6 interaction explicitly.
The detailed interactions between Aβ42-trimer and the fullerene/fullerenol molecule were also investigated. As a previous study suggested that the salt bridges between H6, E11 and H13 stabilize the kink in the N-terminal part of the β-sheets around Y10 [5], we examined the interplay of H6-E11-H13 and found that the interaction pairs stably stay together in all simulated systems except for one MD trajectory of Aβ42-trimer-C60(OH)6 system. This trajectory corresponds to the MD run shown in Figure 4c, and its snapshot of the final state is presented in Figure 5a. Even if the N- and C-termini are dissociated, the interaction pairs of H6, E11 and H13 mostly stay together, and the interactions of side chains are weakened by excluding those of H6-3 (H6 in Chain 3) and E13-1 (E13 in Chain 1).
Figure 5.
Details of interactions between Aβ42-trimer and the fullerene/fullerenol molecule. (a) Disturbance to the interplay of H6-E11-H13 observed in a trajectory of Aβ42-trimer-C60(OH)6 system. (b,c) Number of H-bonds formed between Aβ42-trimer and fullerenols. (d–f) Number of π-stacking structures between Aβ42-trimer and fullerene/fullerenol. The geometrical criterions of H-bonding and π-stacking formations are defined in Model and Methods section.
In Figure 5b,c, we calculated the number of H-bonds formed between individual residue and fullerenols. It shows that C60(OH)6 favors H-bonding with main chains of Aβ42-trimer, and forms H-bonds mostly with residues I32 and D1. The C60(OH)12 molecule forms almost the same amount of H-bonds with main chains and side chains, and it preferentially forms H-bonds with residues E11, H13, Q15, D23, E22 and V36. Previous Thioflavin T (ThT) fluorescence and atomic force microscopy experiments showed that fullerenol C60(OH)16 can prevent Aβ40 fbrillization [30]. The recent study using ThT assay and transmission electron microscope demonstrated that fullerenemalonate can inhibit Aβ42 aggregation [47]. Their computational results showed that the inhibition is attributed to the hydrogen bonding of the fullerenemalonate carboxylate groups with Aβ. Here, the formation of H-bonds between main chains and fullerenols is supposed to block the backbone amide sites for further addition of peptides in β-sheet structure, which goes against the oligomerization or fibrillization of Aβ. The higher affinity of C60(OH)6 bonding with main chains of Aβ peptides makes C60(OH)6 a more efficient inhibitor than C60(OH)12.
The π-stacking interaction is important in the self-assembly of amyloid fibrils, with parallel, T-shaped and herringbone (~50°) orientations suggested for aromatic rings in proteins [48]. The binding energy analysis reveals the important role of F4 in the interaction between Aβ42-trimer and the fullerene/fullerenol molecule. To examine the aromatic stacking interaction between F4 and C60/C60(OH)6/C60(OH)12, we calculated the number of π-stacking structures between Aβ42-trimer and fullerene/fullerenol during the last 20 ns in Figure 5d–f. For the Aβ42-trimer-C60 system, π-stacking structures were observed in three MD trajectories. Run 6 had the largest number of π-stacking structures, and the maximum number was three. This means that the C60 molecule is able to have π-stacking interaction with all the aromatic rings of F4 in Aβ42-trimer at the same time. The inset snapshot displays the corresponding structure, and the aromatic rings of F4 are oriented in parallel or herringbone alignment relative to the C60 surface. As for C60(OH)6, it forms less π-stacking structures with Aβ42-trimer, and the maximum number of π-stacking decreases to two. For the Aβ42-trimer-C60(OH)12 system, π-stacking structures are observed in two trajectories and the total number of π-stacking structures is the least. Only one aromatic ring of F4 can have π-stacking interaction with the C60(OH)12 molecule at one moment, and the ring is mostly oriented in herringbone alignment relative to the carbon surface of C60(OH)12. These results indicate that the more hydroxylated C60 is, the fewer and weaker π-stacking interactions with Aβ42-trimer the nanoparticle has.
2.4. Dynamics, Sites and Interactions of The Fullerene/Fullerenol Molecule Binding to Aβ40-Trimer
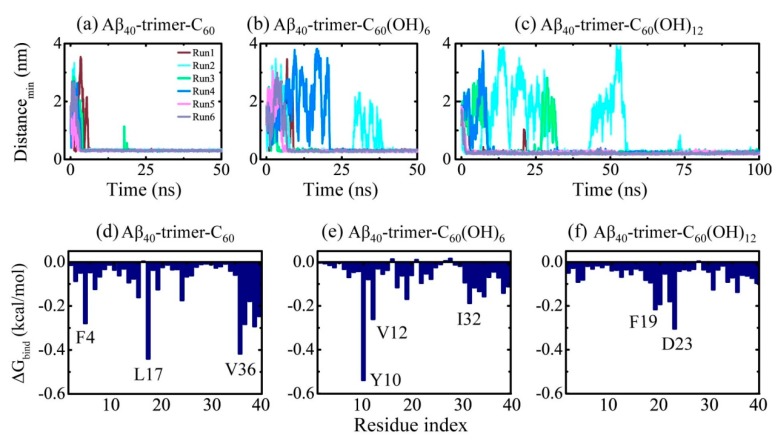
We also carried out multiple MD simulations to examine the binding dynamics, binding sites and interactions of the C60/C60(OH)6/C60(OH)12 molecule with Aβ40-trimer. Although the structure of Aβ40-trimer is different from that of Aβ42-trimer (see Figure 1), the binding behavior of nanoparticles to Aβ40-trimer was found to display a remarkable resemblance with that to Aβ42-trimer. As shown in Figure 6, with the hydroxylation extent increased, the C60 molecule displays slower binding dynamics, corresponding to weakened binding strength. The binding free energy analysis shows that the favorable residues of Aβ40-trimer with which the nanoparticle tend to interact are a little different from those of Aβ42-trimer. Still, these residues are mostly hydrophobic or aromatic, indicating the critical roles of hydrophobic and aromatic interactions in Aβ-nanoparticle interactions. Moreover, the preferential binding regions of the nanoparticles interplaying with Aβ40-trimer resemble with those of the nanoparticles binding to Aβ42-trimer. We also examined the stability of the D23-K28 salt bridge, which is important for the structural stability of Aβ40 [4]. The salt bridge would be interfered by the nanoparticle binding, whereas the connection between the salt bridge disruption and the hydroxylation extent of C60 is not explicit.
Figure 6.
(a–c) Time evolution of the minimum distance between Aβ40-trimer and fullerene/fullerenol. Six independent MD runs are denoted in different colors. (d–f) Residue-based binding free energy. The binding energy was calculated over all six MD runs for each simulated system using the last 20 ns data of each MD trajectory.
3. Materials and Methods
3.1. Aβ40/42 Protofibrillar Trimer and C60/C60(OH)6 /C60(OH)12 Molecules
The Aβ peptide (39–43-amino acid) is derived from the amyloid precursor protein (APP) through proteolytic cleavage by β- and γ-secretase, and the most abundant Aβ are Aβ42 (sequence: DAEFRHDSGY10EVHHQKLVFF20AEDVGSNKGA30IIGLMVGGVV40IA) and Aβ40. The initial coordinate of the Aβ42 protofibrillar trimer was taken from the Aβ42 fibril structure [5] (PDB ID: 5OQV) determined by cryo–electron microscopy (cryo-EM). The coordinate of the Aβ40 protofibrillar trimer was taken from the Aβ40 fibril structure [4] (PDB ID: 2M4J) obtained from solid-state nuclear magnetic resonance (NMR) spectroscopic data. The protonation of the peptide was adjusted to the neutral pH. The N- and C-termini were respectively capped by NH3+ and COO− in accordance with experiments.
The structure of C60/C60(OH)6/C60(OH)12 molecules used in this study is displayed in Figure 1. The force field parameters were taken from a previous MD study on the interaction of Aβ and hydroxylated carbon nanotube [49]. To simplify the modeling, the hydroxyl groups in C60(OH)6/C60(OH)12 molecules are distributed uniformly on the C60 surface.
The Aβ42-trimer-C60 simulation system consists of an Aβ42 protofibrillar trimer and a C60 molecule placed 2.0 nm (minimum distance) away from Aβ, as shown in Figure 1. To remove the bias of the initial position of C60 on the binding site, the C60 molecule was initially placed at three different locations (I, II, III). The other Aβ-fullerene/fullerenol systems were constructed similarly, and were immersed in SPC [50] water. Counterions Na+ and Cl- were added to neutralize the system and provide an additional 0.1 M salt concentration. Systems of isolated Aβ42-trimer and Aβ40-trimer in water were run as control groups.
3.2. Details of MD Simulations
Atomistic MD simulations were performed in isothermal−isobaric (NPT) ensemble using GROMACS-4.5.3 software package [51] with GROMOS96 53a6 force field [52], in accordance with previous computational studies of Aβ peptides [31,33,34,49,53,54]. Periodic boundary conditions were applied in all three directions. The temperature and pressure of the systems were coupled using the Nose−Hoover algorithm [55,56] (310 K, τT = 0.2 ps) and Parinello–Rahman algorithm [57,58] (1 bar, τP = 1.0 ps), respectively. The simulation time step was 2 fs with all bonds constrained using the LINCS algorithm [59]. The electrostatic interactions were treated with the particle mesh Ewald (PME) method [60] with a cutoff of 1.0 nm, and the van der Waals interactions were calculated using a cutoff of 1.4 nm. For Aβ40/42-trimer-C60 and Aβ40/42-trimer-C60(OH)6 systems, six independent copies of each system were carried out, each lasting 50 ns; for Aβ40/42-trimer-C60(OH)12 systems, six independent 100-ns MD runs were carried out; for isolated Aβ40/42-trimer systems, two independent 200-ns MD runs were carried out.
3.3. Analysis Methods
Trajectory analysis was performed using the GROMACS-4.5.3 package toolkits and in-house developed codes. The secondary structure was calculated using the DSSP program [61]. Here, an atomic contact is defined when two non-hydrogen atoms come within 0.54 nm. The H-bond is determined using geometrical criteria: the distance between donor D and acceptor A is less than 0.35 nm and the D-H-A angle is larger than 150°. The π-stacking structure is defined when the centroid of residue aromatic ring is within 0.45 nm from the spherical carbon surface of fullerene/fullerenol [62]. The binding energy between a ligand and a receptor was estimated by means of (MM/PBSA) [63,64]: ΔGbind = ΔEMM + ΔGsolv − TΔS, ΔEMM = ΔEvdW + ΔEelec, ΔGsolv = ΔGpolar + ΔGnonpolar, ΔGnonpolar = γ·SASA + b. Here, EMM is the gas-phase energy, consisting of electrostatic (ΔEelec) and van der Waals (ΔEvdw) terms; ΔGsolv is the sum of polar solvation energy ΔGpolar and nonpolar solvation component ΔGnonpolar; ΔGpolar is estimated by solving the Poisson−Boltzmann equation; ΔGnonpolar is estimated by solvent accessible surface area (SASA). A water probe radius of 0.14 nm was used to calculate SASA, and γ (surface tension of the solvent) and b (fitting parameter) were set to 0.542 kcal/mol/nm2 and 0.92 kcal/mol, respectively. As the binding free energy (ΔGbind) reported here is the relative binding free energy, the contribution of conformational entropy of peptides was ignored in accordance with a number of previous computational studies [33,34,65,66].
4. Conclusions
We investigated the dynamics, sites and interactions of the C60/C60(OH)6/C60(OH)12 nanoparticle binding to Aβ42/40 protofibrillar trimer by performing extensive atomistic MD simulations. To our knowledge, this is the first atomistic explicit-solvent simulation study to investigate the binding behavior of fullerenols to Aβ42/40 protofibril. Our simulations demonstrate that the higher hydroxylation level of C60 leads to slower binding dynamics and weaker binding strength. When binding to Aβ42-trimer, C60 preferentially interacts with C-terminal residues 31–41 and 2AEF4; C60(OH)6 prefers to bind to C-terminal residues 31–41 and 9GYE11; C60(OH)12 favors to bind to N-terminal residues 4–14. In addition, the C60(OH)6 molecule has higher affinity to bind to elongation surfaces than C60 and C60(OH)12. The binding of these nanoparticles has a slight influence on the secondary structure and structural stability of Aβ42-trimer during the simulation time. The hydrophobic interaction plays a critical role in the interplay between Aβ42 and all three nanoparticles; π-stacking interaction gets weakened as C60 carries more hydroxyls. The situations are quite similar when the C60/C60(OH)6/C60(OH)12 nanoparticle binds to Aβ40 protofibrillar trimer. Overall, the proper binding strength and high affinity to form hydrogen bonds with protein backbones make the water-soluble C60(OH)6 molecule an efficient inhibitor. This study provides a detailed picture of fullerene/fullerenols binding to Aβ protofibril and expands the understanding of the underlying inhibitory mechanism, which is helpful to the design of novel agents with anti-amyloid properties.
Author Contributions
Conceptualization, P.C. and Z.Q.; methodology, Y.Z. and Z.Q.; software, Y.Z. and Q.Z.; validation, P.C. and Y.L.; formal analysis, Z.L. and Y.Z.; investigation, Q.Z. and Y.L.; resources, Q.Z.; data curation, Z.L. and Y.Z.; writing—original draft preparation, Z.L.; writing—review and editing, Z.Q.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 11704256.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jucker M., Walker L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowles T.P.J., Vendruscolo M., Dobson C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014;15:384–396. doi: 10.1038/nrm3810. [DOI] [PubMed] [Google Scholar]
- 3.Riek R., Eisenberg D.S. The activities of amyloids from a structural perspective. Nature. 2016;539:227–235. doi: 10.1038/nature20416. [DOI] [PubMed] [Google Scholar]
- 4.Lu J.X., Qiang W., Yau W.M., Schwieters C.D., Meredith S.C., Tycko R. Molecular structure of beta-amyloid fibrils in Alzheimer’s disease brain tissue. Cell. 2013;154:1257–1268. doi: 10.1016/j.cell.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gremer L., Schölzel D., Schenk C., Reinartz E., Labahn J., Ravelli R.B.G., Tusche M., Lopez-Iglesias C., Hoyer W., Heise H., et al. Fibril structure of amyloid-β(1–42) by cryo–electron microscopy. Science. 2017;358:116–119. doi: 10.1126/science.aao2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y., Wang B., Ge X., Ding F. Distinct oligomerization and fibrillization dynamics of amyloid core sequences of amyloid-beta and islet amyloid polypeptide. Phys. Chem. Chem. Phys. 2017;19:28414–28423. doi: 10.1039/C7CP05695H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michaels T.C.T., Šarić A., Habchi J., Chia S., Meisl G., Vendruscolo M., Dobson C.M., Knowles T.P.J. Chemical kinetics for bridging molecular mechanisms and macroscopic measurements of amyloid fibril formation. Annu. Rev. Phys. Chem. 2018;69:273–298. doi: 10.1146/annurev-physchem-050317-021322. [DOI] [PubMed] [Google Scholar]
- 8.Kayed R., Head E., Thompson J.L., McIntire T.M., Milton S.C., Cotman C.W., Glabe C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 9.Haass C., Selkoe D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 10.Straub J.E., Thirumalai D. Toward a molecular theory of early and late events in monomer to amyloid fibril formation. Annu. Rev. Phys. Chem. 2011;62:437–463. doi: 10.1146/annurev-physchem-032210-103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen P., Derreumaux P. Understanding amyloid fibril nucleation and Aβ oligomer/drug interactions from computer simulations. Acc. Chem. Res. 2014;47:603–611. doi: 10.1021/ar4002075. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M., Mao X., Yu Y., Wang C.-X., Yang Y.-L., Wang C. Nanomaterials for reducing amyloid cytotoxicity. Adv. Mater. 2013;25:3780–3801. doi: 10.1002/adma.201301210. [DOI] [PubMed] [Google Scholar]
- 13.Radic S., Davis T.P., Ke P.C., Ding F. Contrasting effects of nanoparticle-protein attraction on amyloid aggregation. RSC Adv. 2015;5:105498. doi: 10.1039/C5RA20182A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B., Pilkington E.H., Sun Y., Davis T.P., Ke P.C., Ding F. Modulating protein amyloid aggregation with nanomaterials. Environ. Sci. Nano. 2017;4:1772–1783. doi: 10.1039/C7EN00436B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young L.M., Saunders J.C., Mahood R.A., Revill C.H., Foster R.J., Tu L.-H., Raleigh D.P., Radford S.E., Ashcroft A.E. Screening and classifying small-molecule inhibitors of amyloid formation using ion mobility spectrometry–mass spectrometry. Nat. Chem. 2014;7:73–81. doi: 10.1038/nchem.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saunders J.C., Young L.M., Mahood R.A., Jackson M.P., Revill C.H., Foster R.J., Smith D.A., Ashcroft A.E., Brockwell D.J., Radford S.E. An in vivo platform for identifying inhibitors of protein aggregation. Nat. Chem. Biol. 2016;12:94–101. doi: 10.1038/nchembio.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z.J., Krause G., Reif B. Structure and orientation of peptide inhibitors bound to β-amyloid fibrils. J. Mol. Biol. 2005;354:760–776. doi: 10.1016/j.jmb.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi T., Mihara H. Peptide and protein mimetics inhibiting amyloid β-peptide aggregation. Acc. Chem. Res. 2008;41:1309–1318. doi: 10.1021/ar8000475. [DOI] [PubMed] [Google Scholar]
- 19.Sevigny J., Chiao P., Bussière T., Weinreb P.H., Williams L., Maier M., Dunstan R., Salloway S., Chen T., Ling Y., et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 20.Liao Q., Owen M.C., Bali S., Barz B., Strodel B. Aβ under stress: The effects of acidosis, Cu2+-binding, and oxidation on amyloid β-peptide dimers. Chem. Commun. 2018;54:7766–7769. doi: 10.1039/C8CC02263A. [DOI] [PubMed] [Google Scholar]
- 21.Xiao L., Aoshima H., Saitoh Y., Miwa N. Highly hydroxylated fullerene localizes at the cytoskeleton and inhibits oxidative stress in adipocytes and a subcutaneous adipose-tissue equivalent. Free Radical Biol. Med. 2011;51:1376–1389. doi: 10.1016/j.freeradbiomed.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Grebowski J., Kazmierska P., Krokosz A. Fullerenols as a new therapeutic approach in nanomedicine. BioMed Res. Int. 2013;2013:9. doi: 10.1155/2013/751913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosi S., Da Ros T., Spalluto G., Prato M. Fullerene derivatives: An attractive tool for biological applications. Eur. J. Med. Chem. 2003;38:913–923. doi: 10.1016/j.ejmech.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Dugan L.L., Turetsky D.M., Du C., Lobner D., Wheeler M., Almli C.R., Shen C.K.F., Luh T.Y., Choi D.W., Lin T.S. Carboxyfullerenes as neuroprotective agents. Proc. Natl. Acad. Sci. USA. 1997;94:9434–9439. doi: 10.1073/pnas.94.17.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J.E., Lee M. Fullerene inhibits β-amyloid peptide aggregation. Biochem. Biophys. Res. Commun. 2003;303:576–579. doi: 10.1016/S0006-291X(03)00393-0. [DOI] [PubMed] [Google Scholar]
- 26.Podolski I.Y., Podlubnaya Z.A., Kosenko E.A., Mugantseva E.A., Makarova E.G., Marsagishvili L.G., Shpagina M.D., Kaminsky Y.G., Andrievsky G.V., Klochkov V.K. Effects of hydrated forms of C60 fullerene on amyloid 1-peptide fibrillization in vitro and performance of the cognitive task. J. Nanosci. Nanotechnol. 2007;7:1479–1485. doi: 10.1166/jnn.2007.330. [DOI] [PubMed] [Google Scholar]
- 27.Ye S., Zhou T., Pan D., Lai Y., Yang P., Chen M., Wang Y., Hou Z., Ren L., Jiang Y. Fullerene C60 derivatives attenuated microglia-mediated prion peptide neurotoxicity. J. Biomed. Nanotechnol. 2016;12:1820–1833. doi: 10.1166/jbn.2016.2281. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh F.-Y., Zhilenkov A.V., Voronov I.I., Khakina E.A., Mischenko D.V., Troshin P.A., Hsu S.-h. Water-soluble fullerene derivatives as brain medicine: Surface chemistry determines if they are neuroprotective and antitumor. ACS Appl. Mat. Interfaces. 2017;9:11482–11492. doi: 10.1021/acsami.7b01077. [DOI] [PubMed] [Google Scholar]
- 29.Huy P.D.Q., Li M.S. Binding of fullerenes to amyloid beta fibrils: Size matters. Phys. Chem. Chem. Phys. 2014;16:20030–20040. doi: 10.1039/C4CP02348J. [DOI] [PubMed] [Google Scholar]
- 30.Bednarikova Z., Huy P.D.Q., Mocanu M.-M., Fedunova D., Li M.S., Gazova Z. Fullerenol C60(OH)16 prevents amyloid fibrillization of Aβ40 – in vitro and in silico approach. Phys. Chem. Chem. Phys. 2016;18:18855–18867. doi: 10.1039/C6CP00901H. [DOI] [PubMed] [Google Scholar]
- 31.Zhou X.Y., Xi W.H., Luo Y., Cao S.Q., Wei G.H. Interactions of a water-soluble fullerene derivative with amyloid-beta protofibrils: Dynamics, binding mechanism, and the resulting salt-bridge disruption. J. Phys. Chem. B. 2014;118:6733–6741. doi: 10.1021/jp503458w. [DOI] [PubMed] [Google Scholar]
- 32.Radic S., Nedumpully-Govindan P., Chen R., Salonen E., Brown J.M., Ke P.C., Ding F. Effect of fullerenol surface chemistry on nanoparticle binding-induced protein misfolding. Nanoscale. 2014;6:8340–8349. doi: 10.1039/C4NR01544D. [DOI] [PubMed] [Google Scholar]
- 33.Xie L., Luo Y., Lin D., Xi W., Yang X., Wei G. The molecular mechanism of fullerene-inhibited aggregation of Alzheimer’s β-amyloid peptide fragment. Nanoscale. 2014;6:9752–9762. doi: 10.1039/C4NR01005A. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y., Qian Z., Wei G. The inhibitory mechanism of a fullerene derivative against amyloid-β peptide aggregation: An atomistic simulation study. Phys. Chem. Chem. Phys. 2016;18:12582–12591. doi: 10.1039/C6CP01014H. [DOI] [PubMed] [Google Scholar]
- 35.Zhao J., Wang Q., Liang G., Zheng J. Molecular dynamics simulations of low-ordered Alzheimer β-amyloid oligomers from dimer to hexamer on self-assembled monolayers. Langmuir. 2011;27:14876–14887. doi: 10.1021/la2027913. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y., Xi W., Wei G. Atomic-level study of the effects of O4 molecules on the structural properties of protofibrillar Abeta trimer: Beta-sheet stabilization, salt bridge protection, and binding mechanism. J. Phys. Chem. B. 2015;119:2786–2794. doi: 10.1021/jp508122t. [DOI] [PubMed] [Google Scholar]
- 37.Ngo S.T., Li M.S. Curcumin binds to Aβ1–40 peptides and fibrils stronger than ibuprofen and naproxen. J. Phys. Chem. B. 2012;116:10165–10175. doi: 10.1021/jp302506a. [DOI] [PubMed] [Google Scholar]
- 38.Thai N.Q., Nguyen H.L., Linh H.Q., Li M.S. Protocol for fast screening of multi-target drug candidates: Application to Alzheimer’s disease. J. Mol. Graphics Modell. 2017;77:121–129. doi: 10.1016/j.jmgm.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Andujar S.A., Lugli F., Hofinger S., Enriz R.D., Zerbetto F. Amyloid-beta fibril disruption by C60-molecular guidance for rational drug design. Phys. Chem. Chem. Phys. 2012;14:8599–8607. doi: 10.1039/c2cp40680b. [DOI] [PubMed] [Google Scholar]
- 40.Benyamini H., Shulman-Peleg A., Wolfson H.J., Belgorodsky B., Fadeev L., Gozin M. Interaction of C60-fullerene and carboxyfullerene with proteins: Docking and binding site alignment. Bioconjugate Chem. 2006;17:378–386. doi: 10.1021/bc050299g. [DOI] [PubMed] [Google Scholar]
- 41.Do T.D., LaPointe N.E., Nelson R., Krotee P., Hayden E.Y., Ulrich B., Quan S., Feinstein S.C., Teplow D.B., Eisenberg D., et al. Amyloid β-protein C-terminal fragments: Formation of cylindrins and β-barrels. J. Am. Chem. Soc. 2016;138:549–557. doi: 10.1021/jacs.5b09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Truex N.L., Wang Y., Nowick J.S. Assembly of peptides derived from β-sheet regions of β-amyloid. J. Am. Chem. Soc. 2016;138:13882–13890. doi: 10.1021/jacs.6b06000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian Z., Zhang Q., Liu Y., Chen P. Assemblies of amyloid-β30–36 hexamer and its G33V/L34T mutants by replica-exchange molecular dynamics simulation. PLoS ONE. 2017;12:e0188794. doi: 10.1371/journal.pone.0188794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kar R.K., Brender J.R., Ghosh A., Bhunia A. Nonproductive binding modes as a prominent feature of Aβ40 fiber elongation: Insights from molecular dynamics simulation. J. Chem. Inf. Model. 2018;58:1576–1586. doi: 10.1021/acs.jcim.8b00169. [DOI] [PubMed] [Google Scholar]
- 45.Brender J.R., Ghosh A., Kotler S.A., Krishnamoorthy J., Bera S., Morris V., Sil T.B., Garai K., Reif B., Bhunia A., et al. Probing transient non-native states in amyloid beta fiber elongation by NMR. Chem. Commun. 2019;55:4483–4486. doi: 10.1039/C9CC01067J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferkinghoff-Borg J., Fonslet J., Andersen C.B., Krishna S., Pigolotti S., Yagi H., Goto Y., Otzen D., Jensen M.H. Stop-and-go kinetics in amyloid fibrillation. Phys. Rev. E. 2010;82:010901. doi: 10.1103/PhysRevE.82.010901. [DOI] [PubMed] [Google Scholar]
- 47.Melchor M.-H., Susana F.-G., Francisco G.-S., Hiram I.B., Norma R.-F., Jorge A.L.-R., Perla Y.L.-C., Gustavo B.-I. Fullerenemalonates inhibit amyloid beta aggregation, in vitro and in silico evaluation. RSC Adv. 2018;8:39667–39677. doi: 10.1039/C8RA07643J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gazit E. A possible role for π-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002;16:77–83. doi: 10.1096/fj.01-0442hyp. [DOI] [PubMed] [Google Scholar]
- 49.Xie L.G., Lin D.D., Luo Y., Li H.Y., Yang X.J., Wei G.H. Effects of Hydroxylated Carbon Nanotubes on the Aggregation of A beta(16-22) Peptides: A Combined Simulation and Experimental Study. Biophys. J. 2014;107:1930–1938. doi: 10.1016/j.bpj.2014.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berendsen H., Postma J., Van Gunsteren W., Hermans J. Interaction models for water in relation to protein hydration. Intermol. Forces. 1981;11:331–342. [Google Scholar]
- 51.Pronk S., Pall S., Schulz R., Larsson P., Bjelkmar P., Apostolov R., Shirts M.R., Smith J.C., Kasson P.M., van der Spoel D., et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics. 2013;29:845–854. doi: 10.1093/bioinformatics/btt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oostenbrink C., Villa A., Mark A.E., van Gunsteren W.F. A biomolecular force field based on the free enthalpy of hydration and solvation: The GROMOS force-field parameter sets 53A5 and 53A6. J. Comput. Chem. 2004;25:1656–1676. doi: 10.1002/jcc.20090. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen P.H., Li M.S., Stock G., Straub J.E., Thirumalai D. Monomer adds to preformed structured oligomers of Aβ-peptides by a two-stage dock-lock mechanism. Proc. Natl. Acad. Sci. USA. 2007;104:111–116. doi: 10.1073/pnas.0607440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krone M.G., Hua L., Soto P., Zhou R., Berne B.J., Shea J.E. Role of water in mediating the assembly of Alzheimer amyloid-β Aβ16-22 protofilaments. J. Am. Chem. Soc. 2008;130:11066–11072. doi: 10.1021/ja8017303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nosé S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984;52:255–268. doi: 10.1080/00268978400101201. [DOI] [Google Scholar]
- 56.Hoover W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A. 1985;31:1695–1697. doi: 10.1103/PhysRevA.31.1695. [DOI] [PubMed] [Google Scholar]
- 57.Parrinello M., Rahman A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981;52:7182–7190. doi: 10.1063/1.328693. [DOI] [Google Scholar]
- 58.Nosé S., Klein M.L. Constant pressure molecular dynamics for molecular systems. Mol. Phys. 1983;50:1055–1076. doi: 10.1080/00268978300102851. [DOI] [Google Scholar]
- 59.Hess B., Bekker H., Berendsen H.J.C., Fraaije J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997;18:1463–1472. doi: 10.1002/(SICI)1096-987X(199709)18:12<1463::AID-JCC4>3.0.CO;2-H. [DOI] [Google Scholar]
- 60.Darden T., York D., Pedersen L. Particle mesh Ewald - an N.Log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993;98:10089–10092. doi: 10.1063/1.464397. [DOI] [Google Scholar]
- 61.Kabsch W., Sander C. Dictionary of protein secondary structure - pattern-recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 62.Xu Z., Lei X., Tu Y., Tan Z.-J., Song B., Fang H. Dynamic cooperation of hydrogen binding and π stacking in ssDNA adsorption on graphene oxide. Chem.-Eur. J. 2017;23:13100–13104. doi: 10.1002/chem.201701733. [DOI] [PubMed] [Google Scholar]
- 63.Luo R., David L., Gilson M.K. Accelerated Poisson–Boltzmann calculations for static and dynamic systems. J. Comput. Chem. 2002;23:1244–1253. doi: 10.1002/jcc.10120. [DOI] [PubMed] [Google Scholar]
- 64.Kumari R., Kumar R., Lynn A. g_mmpbsa—A GROMACS tool for high-throughput MM-PBSA calculations. J. Chem. Inf. Model. 2014;54:1951–1962. doi: 10.1021/ci500020m. [DOI] [PubMed] [Google Scholar]
- 65.Berhanu W.M., Hansmann U.H.E. The stability of cylindrin β-barrel amyloid oligomer models—A molecular dynamics study. Proteins. 2013;81:1542–1555. doi: 10.1002/prot.24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zou Y., Qian Z., Chen Y., Qian H., Wei G., Zhang Q. Norepinephrine inhibits Alzheimer’s amyloid-β peptide aggregation and destabilizes amyloid-β protofibrils: A molecular dynamics simulation study. ACS Chem. Neurosci. 2019;10:1585–1594. doi: 10.1021/acschemneuro.8b00537. [DOI] [PubMed] [Google Scholar]