Abstract
Platelet collagen interactions at sites of vascular injuries predominantly involve glycoprotein VI (GPVI) and the integrin α2β1. Both proteins are primarily expressed on platelets and megakaryocytes whereas GPVI expression is also shown on endothelial and integrin α2β1 expression on epithelial cells. We recently showed that depletion of GPVI improves stroke outcome without increasing the risk of cerebral hemorrhage. Genetic variants associated with higher platelet surface integrin α2 (ITGA2) receptor levels have frequently been found to correlate with an increased risk of ischemic stroke in patients. However until now, no preclinical stroke study has addressed whether platelet integrin α2β1 contributes to the pathophysiology of ischemia/reperfusion (I/R) injury. Focal cerebral ischemia was induced in C57BL/6 and Itga2−/− mice by a 60 min transient middle cerebral artery occlusion (tMCAO). Additionally, wild-type animals were pretreated with anti-GPVI antibody (JAQ1) or Fab fragments of a function blocking antibody against integrin α2β1 (LEN/B). In anti-GPVI treated animals, intravenous (IV) recombinant tissue plasminogen activator (rt-PA) treatment was applied immediately prior to reperfusion. Stroke outcome, including infarct size and neurological scoring was determined on day 1 after tMCAO. We demonstrate that targeting the integrin α2β1 (pharmacologic; genetic) did neither reduce stroke size nor improve functional outcome on day 1 after tMCAO. In contrast, depletion of platelet GPVI prior to stroke was safe and effective, even when combined with rt-PA treatment. Our results underscore that GPVI, but not ITGA2, is a promising and safe target in the setting of ischemic stroke.
Keywords: ischemic stroke, integrin α2, glycoprotein VI, recombinant tissue-type plasminogen activator, intracranial bleeding, transient middle cerebral artery occlusion
1. Introduction
Ischemic stroke (IS) is a major cause of death and invalidity in the aging population with limited treatment options [1,2]. The primary therapeutic goal is the restoration of blood flow by either pharmacological thrombolysis and/or mechanical thrombectomy. However, recanalization by either technique, despite their undisputed potential to induce a favorable clinical outcome, remains futile in a significant number of treated patients. In addition, cerebral hemorrhage may ensue technically successful recanalization. The pathophysiological causes of futile recanalization and of postischemic cerebral hemorrhage after recanalization remain poorly understood. In this context ischemia/reperfusion (I/R), the potentially harmful aspect of blood flow returning to organs after transient ischemia, is arguably one of the most important concepts, which, in particular, applies to the ischemic brain [3]. Platelet activation plays a key role in the pathomechanism underlying IS and I/R injury. Administration of the conventional anti-platelet agent acetylsalicylic acid (ASA) in conjunction with intravenous (IV) recombinant tissue plasminogen activator (rt-PA) thrombolysis led to unacceptable bleeding complications [4]. To improve the outcome in acute ischemic stroke, novel anti-thrombotic/anti-platelet treatment options are urgently needed.
After the initial recruitment of platelets through interactions between the glycoprotein (GP) Ib-V-IX complex and collagen-bound von Willebrand factor (VWF) [5], platelets are immobilized via direct platelet-collagen binding. Thereby, glycoprotein VI (GPVI) functions as the central activating collagen receptor [6,7] which further triggers cellular activation, thus enabling integrin-mediated platelet adhesion and, eventually, aggregation. Of note, recently, other ligands of GPVI, namely fibronectin, vitronectin, laminin, adiponectin, EMMPRIN and fibrin [8] have been identified.
Following platelet activation, integrins undergo conformational changes to mediate firm adhesion via GPIIb/IIIa (αIIbβ3) and vWF or α2β1 (GPIa/IIa) and collagen [6,9]. Utilizing integrin α2-deficient (Itga2−/−) mice as well as an anti-α2β1 antibody (LEN/B Fab-fragment) treatment to analyze the relevance of this integrin for platelet function during hemostatic and thrombotic conditions revealed that α2β1 plays a significant, but only supportive role in platelet adhesion to collagen compared to the major activating collagen receptor GPVI [6,10,11,12]. We and others have previously shown that targeting platelet-collagen interactions during cerebral ischemia, namely antibody mediated depletion of platelet GPVI or blockade of platelet binding to the vessel walls by Revacept, a GPVI-Fc fusion protein competitively inhibiting GPVI, reduced microvascular thrombosis and infarct volumes after a transient middle cerebral artery occlusion (tMCAO) in mice [13,14,15].
With these considerations in mind, the aim of our study was to investigate the pathogenetic relevance of α2β1-mediated collagen binding during the acute phase of IS development using two experimental approaches (LEN/B antibody treatment; Itga2−/− mice). The occurrence of hemorrhagic transformation and intracranial hemorrhages in acute IS is potentially at least in part a consequence of rt-PA induced alterations at the blood brain barrier [16,17,18]. Therefore, in addition we asked whether targeting the collagen receptor GPVI is still safe with regard to bleeding complications when it is performed in conjunction with rt-PA treatment after tMCAO.
2. Results
2.1. Targeting α2β1-Integrin Does Not Improve Outcome after tMCAO
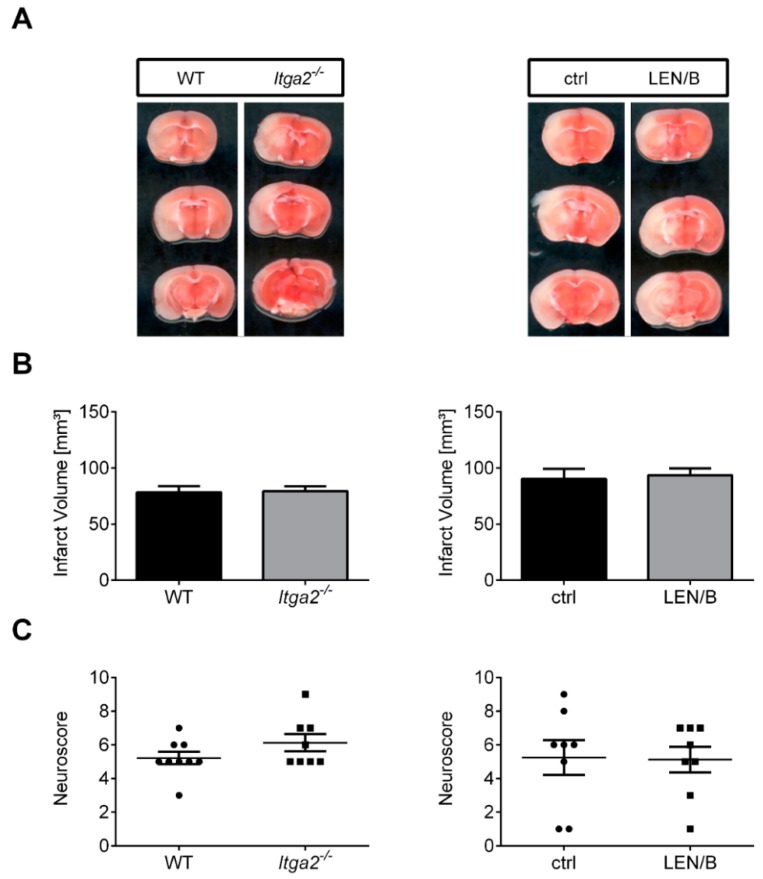
First, we assessed if pharmacologic blockade of α2β1 (LEN/B treatment) before tMCAO influences stroke development in wild-type (WT) mice. Stroke volumes (ctrl: 90.3 ± 9.0 mm3; LEN/B: 93.6 ± 6.1 mm3; p > 0.05; Figure 1A,B, right panel) and functional outcomes as assessed by the Neuroscore (median with (25% and 75%) percentile: ctrl: 6.0 (2.0, 7.5); LEN/B: 5.5 (3.5, 7.0); p > 0.05) on day 1 did not significantly differ (Figure 1C, right panel). In addition, to exclude that the LEN/B antibody treatment influences outcome measures independent of its α2β1-blocking effect, we analyzed Itga2−/− mice. Again, there was no significant difference in infarct volumes (WT: 78.4 ± 5.5 mm3; Itga2−/−: 79.4 ± 4.4 mm3; p > 0.05) (Figure 1A,B, left panel) as well as functional outcomes (median with (25% and 75%) percentile: WT: 5.0 (5.0, 6.0); Itga2−/−: 5.5 (5.0, 7.0); p > 0.05) (Figure 1C, left panel) when comparing Itga2−/− with WT control mice at day 1 after stroke.
Figure 1.
Therapeutic blockade or genetic deficiency of the collagen-binding integrin α2β1 does not alter stroke outcome in a transient middle cerebral artery occlusion (tMCAO) model. (A) Representative 2,3,5-triphenyltetrazolium chloride stains of three corresponding brain sections of a wild-type (WT) mouse in comparison to an Itga2−/− mouse at day 1 after tMCAO (left) and a vehicle-treated C57BL/6 mouse (ctrl) compared to a C57BL/6 mouse treated with a specific α2β1-antigen binding fragment (LEN/B) 1 h before 60 min tMCAO at day 1 after stroke (right). (B) Infarct volumes are similar between the two experimental (pharmacologic; transgenic mice) groups (WT, n = 9; Itga2−/−/ctrl/LEN/B, n = 8/group; unpaired, two-tailed Student’s t-test). (C) Genetic (left) or pharmacologic (right), α2β1 inhibition does not improve functional outcome on day 1 after tMCAO as assessed by the Neuroscore (Mann–Whitney test).
2.2. Blocking the Collagen Receptor GPVI Together with IV rt-PA Treatment after tMCAO Is Safe and Effective
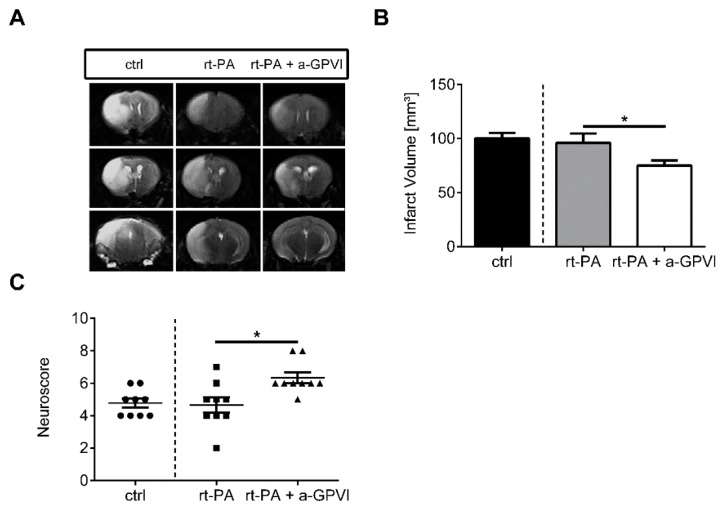
In line with our previous studies [14,15], GPVI depletion by anti-GPVI antibody (JAQ1) treatment significantly reduced infarct volumes at day 1 after tMCAO (rt-PA: 96.1 ± 8.6 mm3; rt-PA + a-GPVI: 75.1 ± 4.7 mm3; p < 0.05; Figure 2A,B) when combined with pharmacological IV rt-PA treatment. Analysis of functional outcome revealed that reduced stroke size in the anti-GPVI treated mice also translated into a better Neuroscore (median with (25% and 75%) percentile: rt-PA: 5.0 (4.0, 5.5); rt-PA + a-GPVI: 5.0 (6.0, 7.0); p < 0.05; Figure 2C). We here conducted magnetic resonance (MR) imaging partly because in addition to the quantification of infarct volumes, this enabled us to quantify the occurrence of cerebral hemorrhages in parallel. Importantly, quantification of intracerebral hemorrhage (ICH) iron-sensitive susceptibility weighted imaging (SWI) sequence MR images at day 1 after tMCAO according to a 0–2 scoring system [16], revealed no increase in the occurrence of cerebral hemorrhages when blocking platelet GPVI during rt-PA treatment (Table 1).
Figure 2.
Blocking of glycoprotein VI (GPVI) mediated collagen binding together with rt-PA treatment improves stroke outcome in a transient middle cerebral artery occlusion (tMCAO) model. (A) Representative serial coronal T2-weighted turbo spin echo MR images of vehicle-treated wild-type mice, mice treated with rt-PA or both rt-PA and JAQ1 (α-GPVI) 24 h after induction of tMCAO. Ischemic infarctions appear hyperintense (bright). (B) Magnetic resonance imaging (MRI)-based infarct volumetry (ctrl/rt-PA/rt-PA + a-GPVI, n = 9/group; unpaired, two-tailed Student’s t-test). (C) Analysis of the functional outcome on day 1 after tMCAO as assessed by the Neuroscore (Mann–Whitney test). * p < 0.05.
Table 1.
Susceptibility weighted imaging (SWI) Score of rt-PA Treated Mice at Day1 after tMCAO.
| SWI Score | 0 | 1 | 2 |
|---|---|---|---|
| rt-PA | 6/9 | 2/9 | 1/9 |
| rt-PA + a-GPVI | 7/9 | 2/9 | 0/9 |
3. Discussion
In the present study, pharmacologic blockade of the platelet integrin α2β1 and Itga2−/− mice was tested independently. Experiments revealed that platelet activation, as well as aggregation in the ischemic brain, does not depend on α2β1-collagen interactions and stroke outcome was comparable. Therefore, this adhesion receptor plays a minor role in acute IS compared to the major activating collagen receptor GPVI [14,15,19]. This is in good agreement with previous in vitro and in vivo studies that found the integrin α2β1 to be of minor relevance in models of arterial thrombosis [10] when compared to GPVI, albeit it also significantly contributes to the adhesion of platelets to collagen [6,12]. A possible explanation for the superiority of targeting GPVI might be due to interactions with ligands other than collagen [8]. Among these, a role of GPVI as a functional platelet receptor for polymerized fibrin has recently been identified. By this, GPVI is critically involved in the amplification of thrombin generation and platelet recruitment to clots [20]. Thrombin formation has been shown to be involved in the induction of inflammation, thrombus formation and infarct development in experimental ischemic stroke [21]. Importantly, when combining GPVI-deficiency together with rt-PA treatment after tMCAO, an experimental setting that closely resembles the current clinical treatment situation where patients with a severe ischemic stroke (caused by large-vessel-occlusion) are treated by mechanical recanalization with the concomitant pharmacological support of IV administered rt-PA, the lack of GPVI was still safe and effective. With respect to the devastating results (bleeding complications) when blocking thromboxane release by ASA [4] or blocking platelet aggregation by GPIIb/IIIa inhibitors [14,15,22,23], our data provide further translational evidence that targeting platelet GPVI is an effective and safe treatment in the setting of acute IS. Despite the strengths of our investigation, additional studies are needed to finally evaluate the safety profile of depleting GPVI together with rt-PA treatment. In the present study rt-PA was infused at 60 min after tMCAO which may be too early to see the bleeding, but the risk of bleeding may dramatically increase at later time points after stroke. Additionally, an embolic stroke model is required in order to assess thrombolysis by rt-PA.
Taken together our study provides evidence that targeting platelet GPVI but not α2β1 integrin protects mice from ischemic stroke in the acute phase after tMCAO.
4. Materials and Methods
4.1. Animals and Ethics
We used 10–12-week old male C57Bl/6 and Itga2−/− mice [11] (backcrossed onto C57Bl/6 > 12 times) throughout this study. All animal experiments were approved by local state authorities (Regierung von Unterfranken) and performed in accordance with Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines (http://www.nc3rs.org/ARRIVE). All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
4.2. Animal Treatment
To inhibit platelet α2β1, mice received 5 mg/kg of anti-α2β1 antibody (LEN/B-Fab fragment) 1 h before tMCAO [12]. To deplete GPVI, mice were given 100 μg JAQ1 intraperitoneally [24], five days prior to tMCAO. After 60 min of tMCAO, 10 mg/kg of human rt-PA (Actilyse; Boehringer-Ingelheim) was injected as an intravenous bolus directly before reperfusion [18].
4.3. Sample Size Calculation
Based on the results of our previous studies using anti-GPVI treated mice, we expected a reduction of infarct volumes of ~32% [14]. Assuming a standard deviation of 20% to the respective mean values, a group size of ≥7 mice was necessary to show this effect with a power of 0.8 and a probability of a type I error of <0.5 (GraphPad StatMate2.0 software).
4.4. tMCAO
Focal cerebral ischemia was induced by 60 min tMCAO, exactly as described previously [14]. To calculate edema-corrected infarct volumes, 2,3,5-triphenyltetrazolium chloride stained brain slices were used. Neurologic function was analyzed calculating a Neuroscore (score 0–10) based on the direct sum of the Grip test (score 0–5) and the inverted Bederson score (score 0–5) [18,25,26].
4.5. Exclusion Criteria
Mice were excluded from endpoint analyses due to: (1) death before the predefined experimental endpoint; (2) Bederson score = 0 (24 h after tMCAO); (3) Operation time >10 min.
4.6. Magnetic Resonance Imaging
To analyze infarct size and to scan for possible intracerebral bleeding, stroke assessment in rt-PA/anti-GPVI treated mice was performed by serial magnetic resonance imaging (MRI) at 3.0 T (MAGNETOM Trio, SIEMENS, Erlangen, Germany) on day 1 after tMCAO. The image protocol comprised a coronal multi-slice TSE (TR/TE (Repetition Time/Echo Time) = 2100/113 ms, 18 slices, BW (Bandwidth) = 120 Hz/px, In-plane-resolution: 0.17 × 0.17 × 1.0 mm3) as well as a 3D CISS (TR/TE = 11.57/4.87 ms, BW = 130 Hz/px, In-plane-resolution: 0.25 × 0.25 × 0.5 mm3, FA (Flip Angle) = 70°) for T2-weighted imaging. Additionally, a 3D GRE SWI (TR/TE = 38 ms, BW = 140 Hz/px, In-plane-resolution: 0.16 × 0.16 × 0.16 mm3, FA = 20°) for susceptibility weighted imaging was acquired. MRIs were visually assessed by researchers blinded to the prior treatment with respect to infarct morphology and, in particular, the occurrence of intracranial hemorrhage [14].
4.7. Evaluation of Bleeding Severity (SWI Score)
The susceptibility weighted imaging (SWI) score was applied to quantify bleeding severity after tMCAO, exactly as described previously [18]. The scoring is as follows: score 0, no bleeding was detectable; score 1, one SWI lesion suggestive for intracerebral hemorrhage; score 2, two or more different SWI lesions were visible.
4.8. Statistical Analysis
For statistical analysis, the GraphPad Prism 5.0 software package (GraphPad Software, La Jolla, CA) was used. Results are given as mean ± standard error of the mean except for the Neuroscore, which is expressed as ordinal values. Data were tested for Gaussian distribution with the D’Agostino and Pearson omnibus normality test and then analyzed by unpaired, two-tailed Student’s t-test. Scores addressing the functional outcome were compared using the Mann–Whitney U-test. p < 0.05 was considered statistically significant.
Acknowledgments
We thank Gabriele Köllner and Burkard Martin for excellent technical assistance.
Author Contributions
G.S. and M.K.S. conceptualized the study. M.K.S., P.K. and M.B. performed experiments. M.K.S., P.K. and M.B. interpreted the data. A.M.K., H.S., B.N., M.P. and D.S. provided support at all stages. All authors wrote and/or critically revised the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (Transregio 240: B02 to G.S. and M.P., Gerok position to A.M.K.; B06 to M.K.S. and D.S.). This publication was funded by the German Research Foundation (DFG) and the University of Wuerzburg in the funding programme Open Access Publishing.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Berkhemer O.A., Fransen P.S., Beumer D., van den Berg L.A., Lingsma H.F., Yoo A.J., Schonewille W.J., Vos J.A., Nederkoorn P.J., Wermer M.J., et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Study Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallenbeck J.M., Dutka A.J. Background review and current concepts of reperfusion injury. Arch. Neurol. 1990;47:1245–1254. doi: 10.1001/archneur.1990.00530110107027. [DOI] [PubMed] [Google Scholar]
- 4.Zinkstok S.M., Beenen L.F., Majoie C.B., Marquering H.A., de Haan R.J., Roos Y.B. Early deterioration after thrombolysis plus aspirin in acute stroke: A post hoc analysis of the antiplatelet therapy in combination with recombinant t-pa thrombolysis in ischemic stroke trial. Stroke. 2014;45:3080–3082. doi: 10.1161/STROKEAHA.114.006268. [DOI] [PubMed] [Google Scholar]
- 5.Savage B., Almus-Jacobs F., Ruggeri Z.M. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94:657–666. doi: 10.1016/S0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 6.Nieswandt B., Brakebusch C., Bergmeier W., Schulte V., Bouvard D., Mokhtari-Nejad R., Lindhout T., Heemskerk J.W., Zirngibl H., Fassler R. Glycoprotein vi but not α2β1 integrin is essential for platelet interaction with collagen. EMBO J. 2001;20:2120–2130. doi: 10.1093/emboj/20.9.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stegner D., Haining E.J., Nieswandt B. Targeting glycoprotein vi and the immunoreceptor tyrosine-based activation motif signaling pathway. Arterioscler. Thromb. Vasc. Biol. 2014;34:1615–1620. doi: 10.1161/ATVBAHA.114.303408. [DOI] [PubMed] [Google Scholar]
- 8.Gawaz M. Novel ligands for platelet glycoprotein vi. Thrombo. Haemost. 2018;118:435–436. doi: 10.1055/s-0038-1635080. [DOI] [PubMed] [Google Scholar]
- 9.Jung S.M., Moroi M. Platelets interact with soluble and insoluble collagens through characteristically different reactions. J. Biol. Chem. 1998;273:14827–14837. doi: 10.1074/jbc.273.24.14827. [DOI] [PubMed] [Google Scholar]
- 10.Gruner S., Prostredna M., Schulte V., Krieg T., Eckes B., Brakebusch C., Nieswandt B. Multiple integrin-ligand interactions synergize in shear-resistant platelet adhesion at sites of arterial injury in vivo. Blood. 2003;102:4021–4027. doi: 10.1182/blood-2003-05-1391. [DOI] [PubMed] [Google Scholar]
- 11.Holtkotter O., Nieswandt B., Smyth N., Muller W., Hafner M., Schulte V., Krieg T., Eckes B. Integrin α 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J. Biol. Chem. 2002;277:10789–10794. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- 12.Shida Y., Rydz N., Stegner D., Brown C., Mewburn J., Sponagle K., Danisment O., Crawford B., Vidal B., Hegadorn C.A., et al. Analysis of the role of von willebrand factor, platelet glycoprotein vi-, and α2β1-mediated collagen binding in thrombus formation. Blood. 2014;124:1799–1807. doi: 10.1182/blood-2013-09-521484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goebel S., Li Z., Vogelmann J., Holthoff H.P., Degen H., Hermann D.M., Gawaz M., Ungerer M., Munch G. The gpvi-fc fusion protein revacept improves cerebral infarct volume and functional outcome in stroke. PLoS ONE. 2013;8:e66960. doi: 10.1371/journal.pone.0066960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinschnitz C., Pozgajova M., Pham M., Bendszus M., Nieswandt B., Stoll G. Targeting platelets in acute experimental stroke: Impact of glycoprotein ib, vi, and iib/iiia blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115:2323–2330. doi: 10.1161/CIRCULATIONAHA.107.691279. [DOI] [PubMed] [Google Scholar]
- 15.Kraft P., Schuhmann M.K., Fluri F., Lorenz K., Zernecke A., Stoll G., Nieswandt B., Kleinschnitz C. Efficacy and safety of platelet glycoprotein receptor blockade in aged and comorbid mice with acute experimental stroke. Stroke. 2015;46:3502–3506. doi: 10.1161/STROKEAHA.115.011114. [DOI] [PubMed] [Google Scholar]
- 16.Ning M., Furie K.L., Koroshetz W.J., Lee H., Barron M., Lederer M., Wang X., Zhu M., Sorensen A.G., Lo E.H., et al. Association between tpa therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006;66:1550–1555. doi: 10.1212/01.wnl.0000216133.98416.b4. [DOI] [PubMed] [Google Scholar]
- 17.Inzitari D., Giusti B., Nencini P., Gori A.M., Nesi M., Palumbo V., Piccardi B., Armillis A., Pracucci G., Bono G., et al. MMP9 variation after thrombolysis is associated with hemorrhagic transformation of lesion and death. Stroke. 2013;44:2901–2903. doi: 10.1161/STROKEAHA.113.002274. [DOI] [PubMed] [Google Scholar]
- 18.Schuhmann M.K., Kraft P., Bieber M., Haarmann A., Homola G.A., Pham M., Nieswandt B., Stoll G. Influence of thrombolysis on the safety and efficacy of blocking platelet adhesion or secretory activity in acute ischemic stroke in mice. Transl. Stroke Res. 2018;9:493–498. doi: 10.1007/s12975-017-0606-7. [DOI] [PubMed] [Google Scholar]
- 19.Cherpokova D., Bender M., Morowski M., Kraft P., Schuhmann M.K., Akbar S.M., Sultan C.S., Hughes C.E., Kleinschnitz C., Stoll G., et al. SLAP/SLAP2 prevent excessive platelet (hem) itam signaling in thrombosis and ischemic stroke in mice. Blood. 2015;125:185–194. doi: 10.1182/blood-2014-06-580597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mammadova-Bach E., Ollivier V., Loyau S., Schaff M., Dumont B., Favier R., Freyburger G., Latger-Cannard V., Nieswandt B., Gachet C., et al. Platelet glycoprotein vi binds to polymerized fibrin and promotes thrombin generation. Blood. 2015;126:683–691. doi: 10.1182/blood-2015-02-629717. [DOI] [PubMed] [Google Scholar]
- 21.Dittmeier M., Wassmuth K., Schuhmann M.K., Kraft P., Kleinschnitz C., Fluri F. Dabigatran etexilate reduces thrombin-induced inflammation and thrombus formation in experimental ischemic stroke. Curr. Neurovasc. Res. 2016;13:199–206. doi: 10.2174/1567202613666160517122605. [DOI] [PubMed] [Google Scholar]
- 22.Adams H.P., Effron M.B., Torner J., Davalos A., Frayne J., Teal P., Leclerc J., Oemar B., Padgett L., Barnathan E.S., et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: Results of an international phase iii trial—Abciximab in emergency treatment of stroke trial (abestt-ii) Stroke. 2008;39:87–99. doi: 10.1161/STROKEAHA.106.476648. [DOI] [PubMed] [Google Scholar]
- 23.Kellert L., Hametner C., Rohde S., Bendszus M., Hacke W., Ringleb P., Stampfl S. Endovascular stroke therapy tirofiban is associated with risk of fatal intracerebral hemorrhage and poor outcome. Stroke. 2013;44:1453–1455. doi: 10.1161/STROKEAHA.111.000502. [DOI] [PubMed] [Google Scholar]
- 24.Nieswandt B., Schulte V., Bergmeier W., Mokhtari-Nejad R., Rackebrandt K., Cazenave J.P., Ohlmann P., Gachet C., Zirngibl H. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein vi in mice. J. Exp. Med. 2001;193:459–469. doi: 10.1084/jem.193.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran P.M., Higgins L.S., Cordell B., Moser P.C. Age-related learning deficits in transgenic mice expressing the 751-amino acid isoform of human β-amyloid precursor protein. Proc. Natl. Acad. Sci. USA. 1995;92:5341–5345. doi: 10.1073/pnas.92.12.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bederson J.B., Pitts L.H., Tsuji M., Nishimura M.C., Davis R.L., Bartkowski H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.STR.17.3.472. [DOI] [PubMed] [Google Scholar]