Abstract
A useful method for the synthesis of 2-acylamino-1,3,4-oxadiazoles was developed. By using potassium iodate as an oxidant in water at 60 °C, a wide range of 2-acylamino-1,3,4-oxadiazoles were afforded in moderate to excellent yields within two hours. This method could provide a facile shortcut to generate a series of 2-acylamino-1,3,4-oxadiazoles in medicinal chemistry. Interestingly, some highly potent antibiotic compounds were found through this synthetic method, and some of them displayed a significant improvement in activity compared with the corresponding 1,4-diacylthiosemicarbazides. Compound 2n was the most active against Staphylococcus aureus with MIC (minimum inhibitory concentration) of 1.56 mg/mL, and compounds 2m and 2q were the most active against Bacillus subtilis with MIC of 0.78 mg/mL. The preliminary cytotoxic activities of the most potent compounds 2m, 2n, and 2q against the androgen-independent (PC-3) prostate cancer cell line were more than 30 μM (IC50 > 30 μM).
Keywords: heterocycles; synthetic method; 2-acylamino-1,3,4-oxadiazoles; antibacterial
1. Introduction
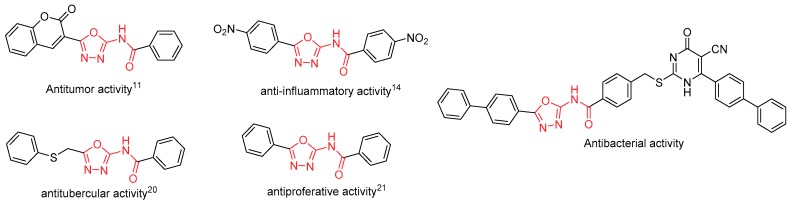
1,3,4-Oxadiazole, a pivotal five-membered nitrogen-containing heterocycle, is an interesting pharmacophore in medicinal chemistry. In particular, 2-acylamino-1,3,4-oxadiazoles have a broad range of biological activities such as anticoagulant [1,2,3], antibacterial [4,5,6,7,8], antifungal [9,10], anticancer [11,12], anti-inflammatory [13,14,15], insecticidal [16,17], antihypertensive [18,19], etc. [20,21] (Figure 1). Thus, the development of facile and efficient approaches to access these 2-acylamino-1,3,4-oxadiazoles is highly valuable to early drug discovery.
Figure 1.
Biologically active compounds containing the 2-acylamino-1,3,4-oxadiazole core motif.
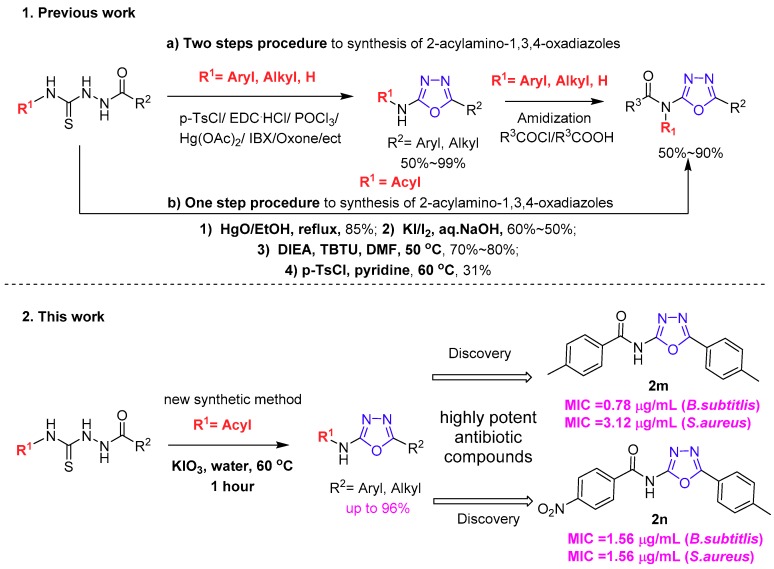
To date, different methods have been developed for the synthesis of these heterocyles. The most straightforward synthesis relies on the cyclization of corresponding thiosemicarbazide derivatives with several desulfurating agents [22], such as 1,3-dibromo-5,5-dimethylhydantoin [23]; p-tosyl chloride [24,25]; alkylating agents [26,27], such as methyl iodide and ethyl bromoacetate; carbodiimides [28,29]; mercury (II) acetate [30,31,32]; oxone [33]; and POCl3 [34] (Scheme 1).
Scheme 1.
Intramolecular cyclodesulfurization of thiosemicarbazides. 1. The previous methods for the synthesis of 2-acylamino-1,3,4-oxadiazoles: (a) Two-step procedure to synthesize 2-acylamino-1,3,4-oxadiazoles by cyclization and amidization [22,23,24,25,26,27,28,29,30,31,32,33,34,35]; (b) One-step procedure to synthesize 2-acylamino-1,3,4-oxadiazoles by desulfurated cyclization [11,36,37,38,39,40,41]. 2. Our new method for the preparation of 2-acylamino-1,3,4-oxadiazoles and the discovery of new antibacterial compounds.
Recently, Sureshbabu and Akamanchi [35] independently utilized hypervalent iodine (IBX) as an oxidant to prepare 2-amino-1,3,4-oxadizale under mild conditions. Chang [36] reported a one-pot procedure to synthesize 2-amino-substituted 1,3,4-oxadiazoles via the condensation of semicarbazide and the corresponding aldehydes followed by I2-mediated oxidative cyclization. As is well known, all of the mentioned methods are extremely dependent on the N-substituted group property, and when the N-substituted groups are aryl, hydrogen, and alkyl groups, the reaction can occur smoothly. When the N-substituted groups are replaced by the acyl groups, these reactions often lead to undesirable by-products. In fact, the preparation of 2-acylamino-substituted 1,3,4-oxadiazoles through the direct oxidative cyclization of thiosemicarbazide is still limited. For example, the 2-acylamino-substituted 1,3,4-oxadiazoles were generated through the cyclodesulfurization of thiosemicarbazide in the presence of I2 /aqueous NaOH as a desulfurization reagent with fair yields [37,38]. Other desulfurization reagents such as stoichiometric mercuric salts [11,39], or lead oxide [40] can be used to induce the cyclization to form the 2-acylamino-substituted 1,3,4-oxadiazoles. More recently, Balalaie and co-workers [41] proved that O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate (TBTU) as a uronium coupling reagent can selectively activate the sulfur moiety to construct disubstituted 1,3,4-oxadiazoles with good yields (Scheme 1). However, these methods suffer from several limitations, such as the handing of harsh and toxic reagents, a poor functional group tolerance, multistep synthetic steps, a long reaction time, and a tedious purified process.
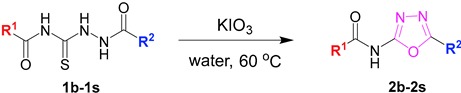
Herein, we reported a useful method for the synthesis of 2-acylamino-1,3,4-oxadiazoles by the direct oxidation of acylthiosemicarbazides using potassium iodate under mild conditions. A wide range of 2-acylamino-substituted 1,3,4-oxadiazoles were afforded in moderate to excellent yields with a short reaction time. We believe that this useful approach provides a facile shortcut for the construction of diverse 2-acylamino-substituted 1,3,4-oxadiaxole libraries in a high throughput manner.
2. Results and Discussion
2.1. Optimization of the Reaction Conditions
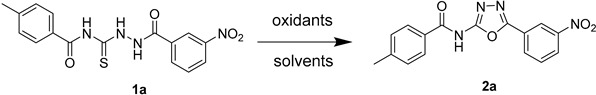
We commenced with the reaction of 4-methyl-N-[2-(3-nitrobenzoyl)hydrazine-1-carbonothioyl]benzamide (1a) in the presence of 1.5 equivalent potassium persulfate or ammonium persulfate as the oxidant in water at 100 °C, but the reaction did not give the corresponding product (Table 1, entry 1–2). Switching to other oxidants such as 2-iodoxybenzoic acid (IBX) and oxone, the cyclodesulfurization product was observed with a low conversion (entry 3–4). To our delight, the reaction obtained the cyclodesulfurization product as 2-acylamino-1,3,4-oxadiazole in 53% yield by using KIO3 as the oxidant (Table 1, entry 5). An encouraging result of an 83% isolated yield was afforded (entry 6) when the reaction temperature was deceased to 80 °C. The screening of different temperatures showed that the reaction temperature had a great influence on this oxidation reaction. The reaction could be performed at a relatively low temperature (60 °C); the isolated yield of the reaction was up to 90% (Table 1, entry 8). Subsequently, various aprotic solvents, such as dichloromethane (DCM) and acetone, were investigated in the presence of 1.5 equivalent KIO3, and the reaction was fully inhibited when the reaction was carried out in DCM and acetone. Thus, we concluded that the optimal condition was the use of KIO3 as the oxidant and water as the solvent at 60 °C (entry 8).
Table 1.
Optimization of cyclization of the thiosemicarbazide.
| Entry | Oxidant | Solvent | Temp. (°C) | Yield (%) |
|---|---|---|---|---|
| 1 | K2S2O8 | Water | 100 | N.O. a,c |
| 2 | (NH4)2S2O8 | Water | 100 | N.O. a,c |
| 3 | IBX | Water | 100 | <30 a |
| 4 | Oxone | Water | 100 | <40 a |
| 5 | KIO3 | Water | 100 | 53 b |
| 6 | KIO3 | Water | 80 | 83 b |
| 7 | KIO3 | Water | 40 | 46 b |
| 8 | KIO3 | Water | 60 | 90 b |
| 9 | KIO3 | DCM | 60 | 5 a |
| 10 | KIO3 | Acetone | 60 | N.O. a,c |
All the reaction were carried out on a 0.4 mmol scale with 1.5 equiv. oxidants within 2 h; a the reaction conversions were monitored by LC-MS; b Isolated yield; c N.O.: No observation.
2.2. Exploring the Substrates’ Scope
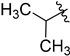
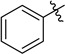
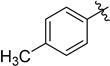
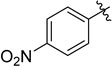
With the optimized reaction condition in hand, we next investigated the scope of 1,4-diacylthiosemicarbazides and the results are depicted in Table 2. Different substituents were well-tolerated and most of the products were synthesized in high yields. The effects of the R2 groups’ properties are important in the cyclization reaction. When the R2 groups were the phenyl substituents, the oxidative reaction resulted in higher yields than the alkyl groups (2b–e, 2i–k, 2n, 2o). Additionally, higher yields of the 2-acylamino-1,3,4-oxadiazoles were observed when the phenyl group bearing an electron-withdrawing substituent (-NO2) was presented on the R2 group (2i, 2j, 2k). Furthermore, the reaction exhibited a good tolerance with the R1 groups in the 1,4-diacylthiosemicarbazides substrates (1b–e, 1i, 1k). When the R1 groups were replaced by a phenyl group, the reaction could generate the desired products with moderate yields (2c, 2f, 2l). A large-scale reaction was conducted to assess the scalability of this desulfurization and cyclization reaction. Under the standard conditions, the thiosemicarbazide 1a (3.0 mmol, 1.07 g) was smoothly converted into the desired products with a 90% yield (See Supplementary Materials).
Table 2.
Reaction scope of cyclization of the thiosemicarbazide.
| Entry | R1 | R2 | Starting Material | Product | Yield (%) |
|---|---|---|---|---|---|
| 1 |
|
|
1b | 2b | 89 |
| 2 |

|

|
1c | 2c | 65 |
| 3 |
|

|
1d | 2d | 90 |
| 4 |
|

|
1e | 2e | 95 |
| 5 |

|
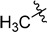
|
1f | 2f | 50 |
| 6 |

|
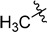
|
1g | 2g | 57 |
| 7 |

|
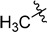
|
1h | 2h | 62 |
| 8 |

|
|
1i | 2i | 90 |
| 9 |

|

|
1j | 2j | 96 |
| 10 |

|

|
1k | 2k | 94 |
| 11 |

|

|
1l | 2l | 68 |
| 12 |

|

|
1m | 2m | 70 |
| 13 |

|

|
1n | 2n | 91 |
| 14 |

|
|
1o | 2o | 93 |
| 15 |

|

|
1p | 2p | 91 |
| 16 |

|

|
1q | 2q | 92 |
| 17 |

|
|
1r | 2r | 90 |
| 18 |

|

|
1s | 2s | 93 |
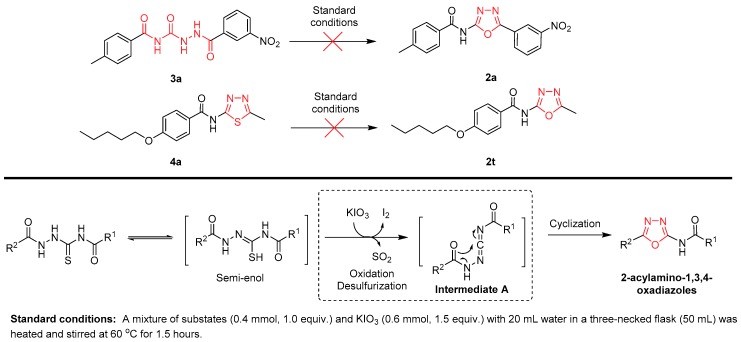
To gain an insight into the reaction mechanism, we set out the control reaction with the semicarbazide (3a) and 2-acylamino-1,3,4-thiadiazoles (4a) under the standard conditions to validate the reaction process. However, neither of them could be converted into the desired 2-acylamino-1,3,4-oxadiazoles (2a, 2t) (Scheme 2). This may suggest that the oxidation and desulfurization step is the key step in this process. Recent reports have proposed that the reaction may be initiated by the nucleophilic attack of S on the electrophilic iodine of IBX, and subsequently, the cyclization and desulfurization step occurred at the same time [42,43]. Very recently, Frederick and co-workers [44] observed that 1-(2-hydroxybenzoyl)-thiosemicarbaziders existed as the keto–enol equilibrium in the 1H-NMR spectrum, and similar results have already been mentioned by Mostafa [45]. However, the details of the corresponding oxidation and desulfurization mechanism remain to be ascertained in this new reaction. On the basis of our studies and recent reports [35,42,43,44,45], a possible mechanism for this reaction is illustrated in Scheme 2. Due to the keto–enol equilibrium of thiosemicarbaziders, hydrosulphonyl could be oxidized by KIO3 to form sulfur dioxide which could be released from the corresponding semi-enol form. An activated carbodiimide intermediate A, generated from the thiosemicarbazides, could subsequently occur to give the desired 2-acylamino-1,3,4-oxiadiazoles. In this reaction, elemental iodine was isolated, and we found that the pH of the reaction solution was less than 6 (pH = 5–6) after the reaction. The keto–enol equilibrium of thiosemicarbaziders could be promoted by the increased reaction temperature, but the higher temperature may induce the decomposition of 2-acylamino-1,3,4-oxadiazoles. Furthermore, the substituted R2 group had a significant effect on this reaction, and when the phenyl groups were replaced by the alkyl groups, the yields were dramatically decreased (2d, 2e, 2g, and 2h). It may suggest that the carbodiimide intermediate A could be stabilized by the phenyl groups. The reaction has a high tolerance for the property of the substituted R1 groups (2o–q).
Scheme 2.
Postulated mechanism of desulfurization for the synthesis of 2-acylamino-1,3,4-oxadiazoles.
2.3. Evaluation of the Antibacterial Activity
The discovery of highly proficient chemical reactions that give functionalized bioactive heterocycles with interesting pharmacophoric properties is a major challenge of medicinal chemistry [46,47]. The development of new synthetic methods for the construction of new scaffolds is an efficient shortcut to accelerate the rate of lead molecule identification [48]. The development of bacterial resistance to currently available antibacterial agents is a growing global health problem [49,50]. The ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) are the cause of severe and difficult-to-treat nosocomial infections due to their antibiotic resistance [50]. One of the most important solutions is the discovery of new antibacterial compounds with a novel mechanism in medicinal chemistry. In our laboratory, we are focusing on the development of new antibacterial agents to protect against the ESKAPE pathogens [51]. As a continuation of our previous work, we further evaluated their antibiotic activity against B. subtilis, S. aureus (Gram-positive bacteria) and E. coli (Gram-negative bacteria) and the results are summarized in Table 3. By comparing the activities of the thiosemicarbazide (1a–1s) and the 2-acylamino-1,3,4-oxadiazoles (2a–2s) against B. subtilis, the potency of the major products (2a–2s) have apparently improved. The activity of the compounds 2e, 2k, 2m, 2n, 2q, and 2s showed six times the potency of their corresponding 1,4-diacylthiosemicarbazides. Furthermore, the compound 2q even showed 64 times the potency of its 1,4-diacylthiosemicarbazides. There were another five compounds, namely 2a, 2j, 2l, 2p, and 2r, which also exhibited better activity. As for antibiotic activity against S. aureus, although some products displayed lower activities, compounds 2e and 2q were also 32 times better than compounds 1e and 1q. We found that the most potent compounds of all of the compounds 1a–1s and 2a–2s were 2m, 2n, and 2q. However, all of the compounds 1a–1s and 2a–2s did not show good antibiotic activity against E. coli (Gram-negative bacteria). At the moment, there is no clear trend regarding activity against E. coli, and the most active compounds (2m, 2n, and 2q) against Gram-positive pathogens do not show the same trend of activity against Gram-negative bacteria. In the literature, some of the 1,3,4-oxadiazoles have been reported to possess cytotoxic activities (PC-3 cell line) [21]. We tested the cytotoxicity of the most potent compounds 2m, 2n, and 2q against the human tumor cell line (androgen-independent prostate cancer cell line, PC-3) to evaluate their potential as antibacterial drugs. The results showed that the in vitro IC50 (median growth inhibitory concentration) of 2m, 2n, and 2q was more than 30 μM (IC50 > 30 μM). Their lower cytotoxic activities may suggest that the compounds 2m, 2n, and 2q are interesting drug-like antibacterial molecules.
Table 3.
The antibiotic activity of compounds 1a–1s and 2a–2s.
| Comp. ID | MIC (μm/mL) | Comp. ID | MIC (μm/mL) | ||||
|---|---|---|---|---|---|---|---|
| B.subtilis | S. aureus | E. coli | B.subtilis | S. aureus | E. coli | ||
| 1a | 12.5 | 12.5 | >100 | 2a | 3.12 | 6.25 | >100 |
| 1b | 12.5 | 25 | 50 | 2b | 50 | 25 | >100 |
| 1c | 6.25 | 6.25 | 25 | 2c | 6.25 | 25 | 100 |
| 1d | 6.25 | 3.12 | >100 | 2d | 3.12 | 12.5 | >100 |
| 1e | 25 | 100 | >100 | 2e | 1.56 | 3.12 | >100 |
| 1f | 25 | 50 | 12.5 | 2f | 100 | >100 | 100 |
| 1g | 12.5 | 25 | 12.5 | 2g | 100 | >100 | 100 |
| 1h | >100 | >100 | >100 | 2h | 50 | 100 | 100 |
| 1i | 25 | 50 | >100 | 2i | 25 | 100 | >100 |
| 1j | 25 | 25 | >100 | 2j | 6.25 | 25 | >100 |
| 1k | 50 | 100 | >100 | 2k | 1.56 | 6.25 | >100 |
| 1l | 3.12 | 6.25 | >100 | 2l | 1.56 | 6.25 | >100 |
| 1m | 6.25 | 3.12 | >100 | 2m | 0.78 | 3.12 | >100 |
| 1n | 12.5 | 12.5 | >100 | 2n | 1.56 | 1.56 | >100 |
| 1o | 25 | 50 | >100 | 2o | >100 | >100 | >100 |
| 1p | 12.5 | 3.12 | >100 | 2p | 6.25 | 12.5 | >100 |
| 1q | 50 | 100 | >100 | 2q | 0.78 | 3.12 | >100 |
| 1r | 6.25 | 3.12 | >100 | 2r | 1.56 | 6.25 | >100 |
| 1s | 25 | 12.5 | >100 | 2s | 3.12 | 3.12 | >100 |
| LEV | 0.05 | 0.20 | 0.02 | 3a | >100 | >100 | >100 |
Levofloxacin (LEV) was used as a positive control.
3. Materials and Methods
3.1. Reagent and Methods
Reagents: unless otherwise indicated, all solvents and organic reagents were obtained from commercially available sources and were used without further purification. Acetone was dried by a molecular sieve. Potassium iodate (99%, Innochem) was used in this oxidative reaction.
Instrument: The reaction process was monitored by thin-layer chromatography (TLC, Yantai Jiangyou Co.Ltd, Tsingdao, Shandong, China) with silica gel plates (thickness = 0.20 mm, GF254) under UV light and LC-MS (Waters Acquity UPLC/SQD, Waters Acquity UPLC/SQD, Milford, MA, USA). Mass spectra were obtained using a Waters Acquity UPLC-SQD mass spectrometer. High-resolution mass spectra (HRMS, Thermo Scientific Exactive. Plus, 81 Wyman Street, Waltham, MA, USA) were recorded on an Agilent Technologies LC/MSD TOF spectrometer. The 1H-NMR spectra were recorded on a Varian Mercury 400 or 500 MHz instrument, and 13C-NMR spectra were recorded at 400 or 500 MHz on a Varian Mercury using dimethyl sulfoxide-d6 (DMSO-d6), methanol-d4 (CD3OD), chloroform-d (CDCl3) as the solvents and tetramethylsilane (TMS) as an internal standard.
3.2. Synthesis of 1,4-Diacylthiosemicarbazide Substrates (1a–1s)
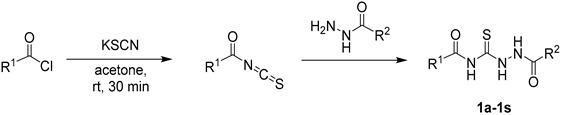
To a solution of acyl chloride (1.0 equiv.) in 20 mL dry acetone, potassium thiocyanate (1.1 equiv.) was added at room temperature. The solution was stirred at room temperature for 0.5 h. Then, relevant acylhydrazine (1.05 equiv.) was added. The reaction was stirred at 60 °C for 2 h until the reaction was complete based on TLC (thin-layer chromatography) (DCM:MeOH = 15:1). Then, it was cooled to room temperature, and concentrated under reduced pressure. The resulting residue was washed by water and recrystallized in MeOH to give the corresponding intermediate as a white solid.
3.3. Synthesis of 2-acylamino-1,3,4-oxadiazole substrates (2a–2s)
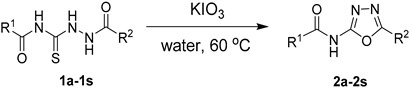
A mixture of 1,4-diacylthiosemicarbazides substrates (0.4 mmol, 1.0 equiv.) and KIO3 (0.6 mmol, 1.5 equiv.) with 20 mL water in a three-necked flask (50 mL) was heated and stirred at 60 °C for 1.5 h. After the reaction was completed, the reaction mixture was cooled to room temperature, and the precipitate was removed by filtration. The aqueous phase was extracted with EtOAc. The combined organic layers were dried by anhydrous Na2SO4, and concentrated under reduced pressure. The residue and the former precipitate were recrystallized in EtOAc to give the desired products.
3.4. Antibacterial Activity and Cell Viability Assay
Microorganisms used for the antibiotic assay were S. aureus ATCC 29213, B. subtilis ATCC 63501, and E. coli ATCC 25922. The MIC (minimum inhibitory concentration) of the compounds was determined according to the macrodilution broth method (National Committee for Clinical Laboratory Standards 2000) in Mueller–Hinton Broth (MHB, Difco, Detroit, MI, USA).
Stock solutions of all compounds were prepared in dimethylsulfoxide. The stock solution was then two-fold diluted in MHB to give an initial concentration of 100 μg/mL, and further dilution was performed until a final concentration of 0.05 μg/mL was obtained.
The microorganisms were incubated overnight in MHB at 37 °C and matched to a 0.5 MCFarland standard and added to make a final volume of 200 μL in each microliter well. A volume of bacterial suspension equal to the volume of diluted antimicrobial solution was added to each well of antimicrobial agent [52]. The 96-well plates were incubated for 18 h under aerobic conditions. The MIC was recorded as the lowest concentration of the test compound which inhibited the growth of bacteria in the broth. Standard drug, media, and bacterial growth control wells were included in each plate. Levofloxacin was used as a positive control. The assay was done in duplicate to confirm results.
The PC-3 cells were kindly provided by the cell bank of the Chinese Academy of Sciences and were maintained in F12K (Wisent) supplemented with 10% fetal bovine serum (Biosera) and 1 unit/mL penicillin−streptomycin (Wisent) at 37 °C under humidified conditions.
Cytotoxicity assays: The in vitro cytotoxicity of the compounds (2m, 2n, and 2q) against PC-3 cells (the androgen-in) was measured by the MTT assays. This assay was based on the cleavage of the yellow tetrazolium salt [3-(4,5-dimethyl-thiazol-2yl]-2,5-diphenyl-tetrazolium bromide, MTT, Sigma) forming purple formazan crystals by viable cells [53]. The cells were plated in 96-well culture plates at a density of 3 × 103 cells per well and incubated for 24 h at 37 °C in a 5% CO2 incubator. The compounds (2m, 2n, and 2q) were dissolved in DMSO and diluted with culture medium. A total of 20 μL of the compound solution was added 24 h post-plating at the indicated concentrations. Cell viability was determined 72-h post-compound treatment. The total number of viable cells was determined using the MTT (biosharp) with the Envision (PekinElmer).
4. Conclusions
To conclude, we have developed an efficient, convenient, and environmentally friendly method to synthesize 2-acylamino-1,3,4-oxadiazoles by the direct oxidation of 1,4-diacylthiosemicarbazides. This oxidative protocol has also shown good functional group tolerance. By mixing the substrates with KIO3, a series of 2-acylamino-1,3,4-oxadiazoles were obtained within two hours. Compared with the classical strategies, this reaction provides a direct approach to construct 2-acylamino-1,3,4-oxadiazoles. Various substituted 2-acylamino-1,3,4-oxadiazoles were synthesized, and evaluated for antibacterial activity. The screening of those compounds revealed that several compounds were active against B. subtilis and S. aureus. The compound 2n is the most active compound with MIC of 1.56 mg/mL against S. aureus, and the compounds 2m and 2q are the most active compounds with MIC of 0.78 mg/mL against B. subtilis. Interestingly, some of the 2-acylamino-1,3,4-oxadiazoles display a significant improvement in activity compared with the corresponding 1,4-diacylthiosemicarbazides. The preliminary cytotoxic effect of the most potent compounds 2m, 2n, and 2q was determined by an MTT assay on an androgen-independent (PC-3) prostate cancer cell line. The results showed that the median growth inhibitory concentrations of 2m, 2n, and 2q are more than 30 μM. Their lower cytotoxic activities may suggest that the compounds 2m, 2n, and 2q are interesting drug-like antibacterial molecules. Further study of this project is currently underway in our laboratory.
Acknowledgments
The authors are grateful to Jie Xia for his assistance in this study.
Supplementary Materials
The supplementary materials are available online.
Author Contributions
Conceptualization, S.W., T.L., and W.Z.; Methodology, G.W., T.L., and J.L.; Data Curation, G.W., J.L., and T.L.; Writing-Original Draft Preparation, T.L. and G.W.; Writing-Review and Editing, S.W. and T.L. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (Grant No: 81703364), Chinese Academy of Medical Sciences-CAMS Innovation Fund for Medical Sciences (Grant No: 2017-I2M-1-011 and 2017-I2M-1-012).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 2m, 2n and 2q are available from the authors.
References
- 1.Sun X., Hong Z., Liu M., Guo S., Yang D., Wang Y., Lan T., Gao L., Qi H., Gong P. Design, synthesis, and biological activity of novel tetrahydropyrazolopyridone derivatives as FXa inhibitors with potent anticoagulant activity. Bioorg. Med. Chem. 2017;25:2800–2810. doi: 10.1016/j.bmc.2017.03.055. [DOI] [PubMed] [Google Scholar]
- 2.Chapleo C.B., Myers M., Myers P.L., Saville J.F., Smith A.C., Stillings M.R., Tulloch I.F., Walter D.S., Welbourn A.P. The oxidation of 1-thioaroylsemicarbazides. J. Med. Chem. 1986;29:2273–2280. doi: 10.1021/jm00161a024. [DOI] [PubMed] [Google Scholar]
- 3.Luszczki J.J., Karpińska M., Matysiak J., Niewiadomy A. Characterization and preliminary anticonvulsant assessment of some 1,3,4-thiadiazole derivatives. Pharmacol. Rep. 2015;67:588–592. doi: 10.1016/j.pharep.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Holla B.S., Gonsalves R., Shenoy S. Synthesis and antibacterial studies of a new series of 1, 2-bis (1, 3, 4-oxadiazol-2-yl) ethanes and 1,2-bis (4-amino-1,2,4-triazol-3-yl) ethanes. Eur. J. Med. Chem. 2000;35:267–271. doi: 10.1016/S0223-5234(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 5.Cesur N., Birteksöz S., Ötük G. Synthesis and biological evaluation of some new thiosemicarbazide, 4-thiazolidinone, 1,3,4-oxadiazole and 1,2,4-triazole-3-thione derivatives bearing imidazo [1, 2-a] pyridine moiety. Acta Pharm. Sci. 2002;44:23–41. [Google Scholar]
- 6.Laddi U., Desai S., Bennur R., Bennur S. Some new 1,3,4-oxadiazoles as antimicrobial agents. Indian J. Heterocycl. Chem. 2002;11:319–322. [Google Scholar]
- 7.Rahman M.A. Ph.D. Thesis. Tennessee State University; Nashville, TN, USA: 2014. ZnX2 (X = Cl, Br) catalyzed efficient regioselective synthesis of 1,3,4-oxadiazole and 1,3,4-thiadiazole rings and their antibacterial studies. [Google Scholar]
- 8.Mishra P., Rajak H., Mehta A. Synthesis of Schiff bases of 2-amino-5-aryl-1,3,4-oxadiazoles and their evaluation for antimicrobial activities. J. Gen. Appl. Microbiol. 2005;51:133–141. doi: 10.2323/jgam.51.133. [DOI] [PubMed] [Google Scholar]
- 9.Stephens C.E., Tanious F., Kim S., Wilson W.D., Schell W.A., Perfect J.R., Franzblau S.G., Boykin D.W. Diguanidino and “reversed” diamidino 2,5-diarylfurans as antimicrobial agents. J. Med. Chem. 2001;44:1741–1748. doi: 10.1021/jm000413a. [DOI] [PubMed] [Google Scholar]
- 10.Zou X.J., Lai L.H., Jin G.Y., Zhang Z.X. Synthesis, fungicidal activity, and 3D-QSAR of pyridazinone-substituted 1,3,4-oxadiazoles and 1,3,4-thiadiazoles. J. Agric. Food Chem. 2002;50:3757–3760. doi: 10.1021/jf0201677. [DOI] [PubMed] [Google Scholar]
- 11.Bondock S., Adel S., Etman H.A., Badria F.A. Synthesis and antitumor evaluation of some new 1,3,4-oxadiazole-based heterocycles. Eur. J. Med. Chem. 2012;48:192–199. doi: 10.1016/j.ejmech.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Dawood K.M., Gomha S.M. Synthesis and Anti-cancer Activity of 1,3,4-Thiadiazole and 1,3-Thiazole Derivatives Having 1,3,4-Oxadiazole Moiety. J. Heterocycl. Chem. 2015;52:1400–1405. doi: 10.1002/jhet.2250. [DOI] [Google Scholar]
- 13.Durgashivaprasad E., Mathew G., Sebastian S., Reddy S.M., Mudgal J., Nampurath G.K. Novel 2,5-disubstituted-1,3,4-oxadiazoles as anti-inflammatory drugs. Indian J. Pharmacol. 2014;46:521–526. doi: 10.4103/0253-7613.140584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh A.K., Lohani M., Parthsarthy R. Synthesis, characterization and anti-inflammatory activity of some 1, 3, 4-oxadiazole derivatives. Iran. J. Pharm. Res. 2013;12:319–323. [PMC free article] [PubMed] [Google Scholar]
- 15.Mullican M.D., Wilson M.W., Conner D.T., Kostlan C.R., Schrier D.J., Dyer R.D. Design of 5-(3,5-di-tert-butyl-4-hydroxyphenyl)-1,3,4-thiadiazoles-1,3,4-oxadiazoles, and-1,2,4-triazoles as orally active, nonulcerogenic antiinflammatory agents. J. Med. Chem. 1993;36:1090–1099. doi: 10.1021/jm00060a017. [DOI] [PubMed] [Google Scholar]
- 16.Zheng X., Li Z., Wang Y., Chen W., Huang Q., Liu C., Song G. Syntheses and insecticidal activities of novel 2,5-disubstituted 1,3,4-oxadiazoles. J. Fluorine Chem. 2003;123:163–169. doi: 10.1016/S0022-1139(03)00168-4. [DOI] [Google Scholar]
- 17.Idoux J.P., Gibbs-Rein K.S., Gupton J.T., Cunningham G.N. Synthesis and insecticidal activity of some 2, 5-(fluoroalkoxyphenyl)-1,3,4-oxadiazoles and their N,N′-dibenzoylhydrazine precursors. J. Chem. Eng. Data. 1988;33:385–388. doi: 10.1021/je00053a044. [DOI] [Google Scholar]
- 18.Tyagi M., Kumar A. Synthesis of 2-[2’-carbonyl-5’-(heteroarylinomethylene)-1’,3’,4’-thiadiazol-2’-yl/oxadiazole-2’-yl)]-4,5-dihydroimidazolines as hypotensive agents. Oriental J. Chem. 2002;18:125–130. [Google Scholar]
- 19.Almasirad A., Tabatabai S.A., Faizi M., Kebriaeezadeh A., Mehrabi N., Dalvandi A., Shafiee A. Synthesis and anticonvulsant activity of new 2-substituted-5-[2-(2-fluorophenoxy) phenyl]-1,3,4-oxadiazoles and 1,2, 4-triazoles. Bioorg. Med.Chem. Lett. 2004;14:6057–6059. doi: 10.1016/j.bmcl.2004.09.072. [DOI] [PubMed] [Google Scholar]
- 20.Ekins S., Freundlich J.S., Hobrath J.V., White E.L., Reynolds R.C. Combining computational methods for hit to lead optimization in Mycobacterium tuberculosis drug discovery. Pharm. Res. 2014;31:414–435. doi: 10.1007/s11095-013-1172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mochona B., Qi X., Euynni S., Sikazwi D., Mateeva N., Soliman K.F. Design and evaluation of novel oxadiazole derivatives as potential prostate cancer agents. Bioorg. Med.Chem. Lett. 2016;26:2847–2851. doi: 10.1016/j.bmcl.2016.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soares de Oliveira C., Lira B.F., Barbosa-Filho J.M., Lorenzo J.G.F., Filgueiras de Athayde-Filho P. Synthetic Approaches and Pharmacological Activity of 1,3,4-Oxadiazoles: A Review of the Literature from 2000–2012. Molecules. 2012;17:10192–10231. doi: 10.3390/molecules170910192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera N.R., Balsells J., Hansen K.B. Synthesis of 2-amino-5-substituted-1,3,4-oxadiazoles using 1,3-dibromo-5,5-dimethylhydantoin as oxidant. Tetrahedron Lett. 2006;47:4889–4891. doi: 10.1016/j.tetlet.2006.05.033. [DOI] [Google Scholar]
- 24.Dolman S.J., Gosselin F., O’Shea P.D., Davies I.W. Superior reactivity of thiosemicarbazides in the synthesis of 2-amino-1,3,4-oxadiazoles. J. Org. Chem. 2006;71:9548–9551. doi: 10.1021/jo0618730. [DOI] [PubMed] [Google Scholar]
- 25.Matsuno K., Masuda Y., Uehara Y., Sato H., Muroya A., Takahashi O., Yokotagawa T., Furuya T., Okawara T., Otsuka M., et al. Identification of a New Series of STAT3 Inhibitors by Virtual Screening. ACS Med. Chem. Lett. 2010;1:371–375. doi: 10.1021/ml1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fülöp F., Semega E., Dombi C., Bernath G. Synthesis of new heterocyclic compounds as potential pharmaceutical agents. J. Heterocycl. Chem. 1990;27:951. doi: 10.1002/jhet.5570270424. [DOI] [Google Scholar]
- 27.Küçükgüzel G., Kocatepe A., De Clercq E., Şahin F., Güllüce M. Synthesis and biological activity of 4-thiazolidinones, thiosemicarbazides derived from diflunisal hydrazide. Eur. J. Med. Chem. 2006;41:353–359. doi: 10.1016/j.ejmech.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Coppo F.T., Evans K.A., Graybill T.L., Burton G. Efficient one-pot preparation of 5-substituted-2-amino-1, 3, 4-oxadiazoles using resin-bound reagents. Tetrahedron Lett. 2004;45:3257. doi: 10.1016/j.tetlet.2004.02.119. [DOI] [Google Scholar]
- 29.Yang S.-J., Choe J.-H., Abdildinova A., Gong Y.-D. A highly efficient diversification of 2-amino/amido-1,3,4-oxadiazole and 1,3,4-thiadiazole derivatives via reagent-based cyclization of thiosemicarbazide intermediate on solid-phase. ACS Comb. Sci. 2015;17:732–741. doi: 10.1021/acscombsci.5b00140. [DOI] [PubMed] [Google Scholar]
- 30.Omar A., Mohsen M., Aboulwafa O.M. Synthesis and anticonvulsant properties of a novel series of 2-substituted amino-5-aryl-1,3,4-oxadiazole derivatives. J. Heterocycl. Chem. 1984;21:1415–1418. doi: 10.1002/jhet.5570210538. [DOI] [Google Scholar]
- 31.Kurzer F., Doyle K.M. The oxidation of 1-thioaroylsemicarbazides. J. Chem. Soc. Perk. Trans. 1. 1986:1873–1880. doi: 10.1039/p19860001873. [DOI] [Google Scholar]
- 32.Zou X., Zhang Z., Jin G. Synthesis and biological activity of 1,3,4-oxadiazole-substituted pyridazinones. J. Chem. Res. 2002;2002:228–230. doi: 10.3184/030823402103171780. [DOI] [Google Scholar]
- 33.Shinde V.N., Ugarkar B.G., Ghorpade S.R. A convenient synthesis of 5-substituted 2-amino-1,3,4-oxadiazoles from corresponding acylthiosemicarbazides using iodine and Oxone. J. Chem. Res. 2013;37:53–54. doi: 10.3184/174751912X13551638283701. [DOI] [Google Scholar]
- 34.Vachal P., Toth L.M. General facile synthesis of 2,5-diarylheteropentalenes. Tetrahedron Lett. 2004;45:7157–7161. doi: 10.1016/j.tetlet.2004.07.132. [DOI] [Google Scholar]
- 35.Chaudhari P.S., Pathare S.P., Akamanchi K.G. o-Iodoxybenzoic Acid Mediated Oxidative Desulfurization Initiated Domino Reactions for Synthesis of Azoles. J. Org. Chem. 2012;77:3716–3723. doi: 10.1021/jo2025509. [DOI] [PubMed] [Google Scholar]
- 36.Niu P., Kang J., Tian X., Song L., Liu H., Wu J., Yu W., Chang J. Synthesis of 2-amino-1,3,4-oxadiazoles and 2-amino-1,3,4-thiadiazoles via sequential condensation and I2-mediated oxidative C-O/C-S bond formation. J. Org. Chem. 2014;80:1018–1024. doi: 10.1021/jo502518c. [DOI] [PubMed] [Google Scholar]
- 37.Holla B.S., Prasanna C.S., Poojary B., Rao K.S., Shridhara K., Bhat U.G. Synthesis and characterization of 1,3,4-thiadiazole and 1,3,4-oxadiazole derivatives containing 2-chloropyridin-5-yl-methyl moiety. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 2004;43B:2170–2174. doi: 10.1002/chin.200505127. [DOI] [Google Scholar]
- 38.Xia Q., He Q., Xu D., Sun D., Peng Z. Synthesis, characterization and antibacterial activity of five-membered heterocycles. Huaxue Xuebao. 2010;68:2414–2420. [Google Scholar]
- 39.Rostom S.A.F., Shalaby M.A., El-Demellawy M.A. Polysubstituted pyrazoles, part 5. Synthesis of new 1-(4-chlorophenyl)-4-hydroxy-1H-pyrazole-3-carboxylic acid hydrazide analogs and some derived ring systems. A novel class of potential antitumor and anti-HCV agents. Eur. J. Med. Chem. 2003;38:959–974. doi: 10.1016/j.ejmech.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Yale H.L., Losee K. 2-Amino-5-substituted 1,3,4-oxadiazoles and 5-imino-2-substituted Δ2-1,3,4-oxadiazolines. A group of novel muscle relaxants. J. Med. Chem. 1966;9:478–483. doi: 10.1021/jm00322a007. [DOI] [PubMed] [Google Scholar]
- 41.Maghari S., Ramezanpour S., Darvish F., Balalaie S., Rominger F., Bijanzadeh H.R. A new and efficient synthesis of 1,3,4-oxadiazole derivatives using TBTU. Tetrahedron. 2013;69:2075–2080. doi: 10.1016/j.tet.2012.11.071. [DOI] [Google Scholar]
- 42.Singh C.B., Ghosh H., Murru S., Patel B.K. Hypervalent iodine(III)-mediated regioselective N-acylation of 1,3-disubstituted thioureas. J. Org. Chem. 2008;78:2924–2927. doi: 10.1021/jo702628g. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh H., Yella R., Nath J., Patel B.K. Desulfurization mediated by hypervalent iodine(III): A novel strategy for the construction of heterocycles. Eur. J. Org. Chem. 2008;36:6189–6196. doi: 10.1002/ejoc.200800901. [DOI] [Google Scholar]
- 44.Ameryckx A., Thabault L., Pochet L., Leimanis S., Poupaert J.H., Wouters J., Joris B., Van Bambeke F., Frederick R. 1-(2-Hydroxybenzoyl)-thiosemicarbazides are promising antimicrobial agents targeting D-alanine-d-alanine ligase in bacterio. Eur. J. Med. Chem. 2018;159:324–338. doi: 10.1016/j.ejmech.2018.09.067. [DOI] [PubMed] [Google Scholar]
- 45.Azhari S.J., Mlahi M.R., Al-Asmy A.A., Mostafa M.M. Synthesis of novel binary and ternary complexes derived from 1-(2-hydroxy benzoyl)-4-phenylthiosemicarbazide (L1) and 2,2′-dipyridyl (L2) with CoII, CuII and ZnII salts. Spectrochim. Acta Part. A. 2015;136:185–191. doi: 10.1016/j.saa.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Prajapat P. Utility of drug discovery in medicinal and organic chemistry. Mod. Chem. Appl. 2017;5:1000e123. doi: 10.4172/2329-6798.1000e123. [DOI] [Google Scholar]
- 47.Nielsen T.E., Schreiber S.L. Towards the optimal screening collection. A synthesis strategy. Angew. Chem. Int. Ed. 2008;47:48–56. doi: 10.1002/anie.200703073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schreiber S.L. Organic synthesis toward small-molecule probes and drugs. Proc. Natl. Acad. Sci. USA. 2011;108:6699–6702. doi: 10.1073/pnas.1103205108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barbachyn M.R., Ford C.W. Oxazolidinone structure-activity relationships leading to linezolid. Angew. Chem. Int. Ed. 2003;42:2010–2023. doi: 10.1002/anie.200200528. [DOI] [PubMed] [Google Scholar]
- 50.Schillaci D., Spano V., Parrino B., Carbone A., Montalbano A., Barraja P., Diana P., Cirrincione G., Cascioferro S. Pharmaceutical Approaches to Target Antibiotic Resistance Mechanisms. J. Med. Chem. 2017;60:8268–8297. doi: 10.1021/acs.jmedchem.7b00215. [DOI] [PubMed] [Google Scholar]
- 51.Wu S., Xia J., Wen G., Feng B., Jia Y.-Q., Zhang W.-X., Yang Q.-Y., Zhang C., Qi Y. Preparation method of 1-benzoyl-4-acyl semicarbazide derivatives as antibacterial drug. Patent CN.108456157 A. 2018 Aug 28;
- 52.Andrews J. M. Determination of minimum inhibitory concentrations. J. Antimicrobial. Chemotherapy. 2001;48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 53.Gao X., Lu Y., Fang L., Fang X., Xing Y., Gou S., Xi T. Synthesis and anticancer activity of some novel 2-phenazinamine derivatives. Eur. J. Med. Chem. 2013;69:1–9. doi: 10.1016/j.ejmech.2013.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.