Abstract
An immunosuppressed man developed rapidly progressive neurologic symptoms resulting in quadriplegia. On magnetic resonance imaging multiple areas of abnormal enhancement were observed in the brain, and spinal cord. Serologic evidence of West Nile Virus (WNV) was discovered in the cerebrospinal fluid. This report highlights the catastrophic complications of WNV in an immunocompromised host.
Keywords: West Nile Virus, Immunocompromised, Quadriplegia, Encephalomyelitis, Renal transplant
Introduction
Since its arrival in North America in 1999, West Nile virus (WNV), has spread to the entire mainland United States of America as well as all Canadian provinces [1]. First recognized in Uganda in 1937, WNV is an enveloped virus with a single-stranded positive-sense RNA genome that belongs to the genus Flavivirus, family Flaviviridae of viruses [2]. Almost 75% of WNV infections are either asymptomatic or cause a mild febrile illness and less than 1% of all WNV infections lead to a neuroinvasive disease including encephalitis, meningitis, and acute flaccid paralysis [3]. Although the latter estimate was repeatedly mentioned in the literature in the past 15 years, recent data suggest that neuroinvasive cases tend to occur with much more frequency than was initially thought [3,4]. Risk factors for WNV neuroinvasive disease include but are not limited to cardiovascular disease, diabetes mellitus, hypertension, chronic kidney disease, and malignancy [1]. Furthermore, a previous history of stroke, cardiovascular disease, chronic kidney disease, hepatitis C, and diabetes mellitus are associated with WNV-related mortality [3]. Immunosuppression is an established independent risk factor for increased mortality [3]. We herein describe a case of an immunocompromised host with a remote history of kidney transplantation who acquired WNV infection through mosquito bites, developed WNV neuroinvasive disease with quadriplegia that has persisted for more than a year.
Case history
A 51-year old male from rural Manitoba, Canada, with type 1 diabetes mellitus on mycophenolate mofetil, tacrolimus, and prednisone for a renal transplant that was done eight years prior to admission, developed a fever of 39.4 °C, a mild headache, and generalized malaise on September 1, 2016. Over the following week, he developed low back pain radiating to the buttocks. Within one week, his leg weakness had progressed such that he could no longer ambulate and had also developed urinary retention. Vertigo, non-bloody diarrhea and vomiting followed. These symptoms prompted him to seek medical attention at his regional health center. The patient was subsequently transferred to an acute care tertiary center in Winnipeg, Manitoba.
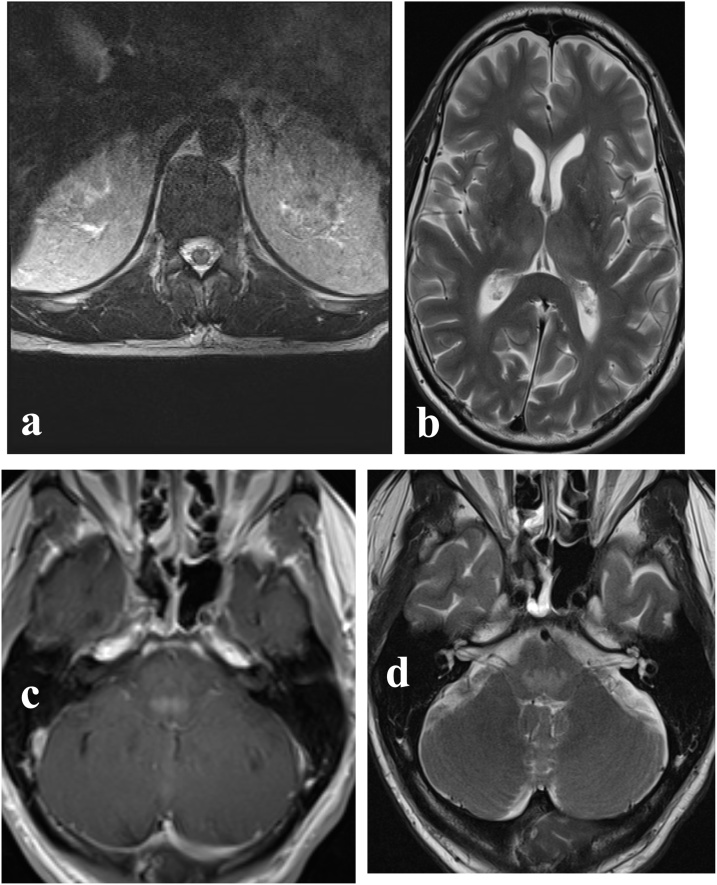
Three days after admission the patient developed respiratory failure requiring intubation and became ventilator dependent; this was followed by a progressive quadriplegia. This prompted Magnetic Resonance Imaging (MRI) scan of the brain and spine that revealed multiple areas of heterogeneously enhancing T2 hyperintensity in the thalami, dorsal pons, medulla, and spinal cord (Fig. 1).
Fig. 1.
Selected axial images from initial MRI (a) Central T2 hyperintensity conforming to gray matter at the level of the conus. (b, c) Patchy areas of increased T2 signal intensity in the bilateral thalami (b) and pontomedullary junction (c) with corresponding enhancement following IV gadolinium contrast administration on T1 weighted series (d).
Multiple blood, urine, and CSF specimens submitted for culture were sterile, not yielding bacteria, mycobacteria, nor fungi. A CSF specimen previously collected at admission was negative for CMV and Herpes simplex viruses-1,2 by PCR and CSF viral culture. Eleven days following onset of symptoms WNV IgM tested positive in serum; however, WNV Plaque Reduction Neutralization Test (PRNT90) performed at National Microbiology Laboratory (NML) in Winnipeg, Canada, result returned negative using the acute serum. Convalescent serum, however, collected 48 days post onset remained positive for WNV IgM and this time tested strongly positive for WNV specific neutralizing antibodies with a WNV PRNT90 titre of >80 indicating recent infection with WNV. Retrospectively, a residual CSF specimen collected at admission later tested positive for WNV RNA by reverse transcriptase (RT)-PCR at NML; this CSF specimen, however, tested negative for WNV IgM. Based on the above results combined, the patient received the diagnosis of confirmed neuroinvasive WNV disease. On admission day 18, a trial of intravenous immunoglobulin was initiated without meaningful improvement.
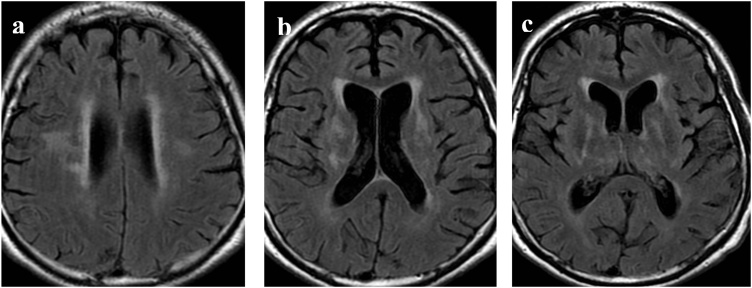
Over the following 5 months, there was some recovery of motor function; particularly the facial and respiratory muscles. By mid-February 2017, the patient was weaned from the ventilator. A serum specimen collected in February 2017, continued to demonstrate WNV IgM but with significantly lower antibody index value from initial diagnosis (6.384–2.637; positivity cutoff: 1.1). Due to the continued neurological impairment and history of renal transplant, a repeat plasma CMV PCR was requested but continued to be negative. Further CSF specimens submitted for cryptococcal antigen testing were non-reactive. Nine months from admission the patient experienced new headache, sluggish pupils, dysarthria, and a decreased level of consciousness concerning for persistent encephalitis. Repeat MRI exams in May and June demonstrated new areas of increased T2 signal intensity centered in the capsuloganglionic regions and hemispheric white matter bilaterally as well as mild generalized atrophy. Lesions involving the brainstem had significantly improved (Fig. 2). A CSF specimen submitted in May 2017 was negative for HSV, Enterovirus, and Polyomaviruses (JC and BK) by PCR. The same CSF specimen was submitted to NML and this time did not demonstrate evidence of WNV RNA, however, WNV IgM tested strongly positive (antibody index value: 25; cutoff: 1.1 using WNV IgM Capture DxSelect™, Focus Diagnostics). This CSF specimen also showed a significant titer of 5 by WNV PRNT90 at NML, the latter two results are strongly suggestive of intrathecal WNV-specific antibody production. More than one year after presentation, the patient remained tetraparetic in a rehabilitation hospital.
Fig. 2.
Selected axial FLAIR images on follow up MRI: Interval development of T2 hyperintensity in the periventricular and subcortical white matter of the bilateral frontal lobes (a) bilateral basal ganglia (b) and stable signal change in the bilateral thalami (c). A mild amount of generalized volume loss is seen at all levels.
Discussion
Immunosuppression, diabetes mellitus, and chronic kidney disease, are all strong risk factors for both neuroinvasive WNV disease and WNV-associated mortality. This patient had all three risk factors and developed a severe and protracted course of neuroinvasive WNV disease from which he has not recovered. Muscle weakness, fatigue, and myalgia are among several symptoms that can persist for extended periods after an acute presentation of WNV disease [5]. Quadriplegia resulting from severe spinal cord involvement is a rare manifestation of WNV disease [6]. In our patient, this developed soon after symptom onset and may be due to the direct viral cytopathic effect; though a potential role for immunopathological mechanisms cannot be entirely excluded. WNV related quadriplegia is associated with a poor prognosis and high risk of mortality [7]. The generalized brain parenchymal atrophy which developed over several months in our patient has, to our knowledge, not been reported in the literature.
There are three proposed mechanisms for central nervous system (CNS) invasion of WNV. The first, direct transcytosis through endothelia cells in the blood-brain barrier (BBB). The second, increased permeability of the BBB due to systemic production of a myriad of cytokines and chemokines in response to the WNV infection; and lastly, the so-called “Trojan Horse” mechanism through which virally-infected monocytes gain access to the immune privileged CNS thereby importing the virus to the CNS [1]. In our patient, similar to other patients with neuroinvasive WNV disease, despite moderate CSF pleocytosis, 32% of cells were monocyte/macrophages further supporting the last mechanism [5,[8], [9], [10]].
Our patient had undergone a kidney transplantation eight years prior to WNV infection. A single report that kidney transplant recipients could shed WNV RNA in urine up to several years post transplantation was never substantiated [1,2,7]. We do not believe our patient developed WNV disease because of occult WNV infection in the donated kidney but instead through mosquito bites up to three weeks prior to his onset of illness. Disease caused by WNV has an incubation period of 2–14 days but in immunocompromised patients, it may take up to three weeks. This suggests that our patient was bitten by infected misquotes sometime after early August 2016. In Manitoba, July and August are typically the peak months of activity for the Culex tarsalis mosquitos and the peak for infection with WNV, further strengthening our finding in addition to acute seroconversion detected by WNV RPNT90.
At least 90% of patients with WNV neuroinvasive disease have detectable WNV IgM in their CSF by 8 days following onset of symptoms [1]. Our patient’s CSF specimen did not demonstrate serologic evidence of WNV IgM until 9 days following onset of symptoms although the antibody index was just below positivity cut-off; however, at the same time the acute CSF specimen demonstrated WNV RNA by RT-PCR. Whether the latter combination of results suggest early direct invasion of the virus to CNS or the role of immunopathological phenomena later in the process causing catastrophic sequelae is not known. It is also possible that the demonstration of WNV RNA in the CSF was due to immune cells, especially monocyte/macrophages that traversed the BBB early in the disease process. Additionally, the down-spiraling WNV IgM antibody index in serum after several months contrasted with what we observed with that of the CSF (detection of persistent IgM) suggesting the presence of the virus within the CNS parenchyma (and not communicating with CSF) continuously triggering immune response ultimately leading to intrathecal production of WNV-specific neutralizing antibodies. The latter findings, however, do not negate the potential role of cell-mediated immunity in destruction of the CNS architecture, particularly through CD8+ T cells as previously shown [2]. Further research is warranted to elucidate the potential persistence of the replicating virus within CNS in immunocompromised individuals. As demonstrated before, WNV is able to directly infect and damage anterior horns of the spinal cord leading to demyelination and non-poliovirus poliomyelitis [6,7]. This happened early during the course of infection in our patient further suggesting the direct cytopathic role of WNV. Inflammation of the conus medularis also explains the initial lower back pain and urinary retention.
This case report highlights the magnitude of WNV disease, and its catastrophic complications in an immunocompromised patient. The case report also reminds us of the importance of mosquito precautions (avoidance) particularly in those who are immunocompromised.
Funding role
There was no funding available for this report.
Conflicts of interest
None declared.
Acknowledgements
The authors would like to thank Kristina Dimitrova at Viral Zoonoses Laboratory at the National Microbiology Laboratory in Winnipeg, Canada, for her kind help.
References
- 1.Petersen L.R., Brault A.C., Nasci R.S. West Nile Virus: review of the literature. JAMA. 2013;310:308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colpitts T.M., Conway M.J., Montgomery R.R., Fikrig E. West Nile Virus: biology, transmission, and human infection. Clin Microbiol Rev. 2012;25:635–648. doi: 10.1128/CMR.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel H., Sander B., Nelder M.P. Long-term sequelae of West Nile Virus-related illness: a systematic review. Lancet Infect Dis. 2015;15:951–959. doi: 10.1016/S1473-3099(15)00134-6. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention . 2017. West Nile Virus disease cases by state– United States. (as of October 24, 2017). https://www.cdc.gov/westnile/statsmaps/preliminarymapsdata2017/disease-cases-state.html#modalIdString_CDCTable_0. Updated October 25, 2017 (Accessed October 28 October, 2017) [Google Scholar]
- 5.Sejvar J.J., Haddad M.B., Tierney B.C. Neurologic manifestations and outcome of West Nile Virus infection. JAMA. 2003;290:511–515. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- 6.Doron S.I., Dashe J.F., Adelman L.S., Brown W.F., Werner B.G., Hadley S. Histopathologically proven poliomyelitis with quadriplegia and loss of brainstem function due to West Nile Virus infection. Clin Infect Dis. 2003;37:74–77. doi: 10.1086/377177. [DOI] [PubMed] [Google Scholar]
- 7.Leis A.A., Stokic D.S. Neuromuscular manifestations of West Nile Virus infection. Front Neurol. 2012;3:1–10. doi: 10.3389/fneur.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winston D.J., Vikram H.R., Rabe I.B. Donor-derived West Nile Virus infection in solid organ transplant recipients: report of four additional cases and review of clinical, diagnostic, and therapeutic features. Transplantation. 2014;97:881–889. doi: 10.1097/TP.0000000000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwamoto M., Jernigan D.B., Guasch A., Trepka M.J., Blackmore C.G., Hellinger W.C. Transmission of West Nile Virus from an organ donor to four transplant recipients. New England J Med. 2003;348:2196–2203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 10.Gilden D.H. Brain imaging abnormalities in CNS virus infections. Neurology. 2008;70:84. doi: 10.1212/01.wnl.0000286937.09760.e4. [DOI] [PubMed] [Google Scholar]