Abstract
Pellagra is caused by cellular deficiency of niacin or its precursor amino acid, tryptophan. Isoniazid preventive therapy (IPT) is the administration of isoniazid (INH) to latent tuberculosis (TB) infection affected people preventing advancement to active TB disease. Although potentially life-saving for human immunodeficiency virus (HIV)-infected people with no active TB, IPT is arguably a possible player in pellagra in addition to well-known malnourishment determinants particularly in developing nations where diagnosis is often overlooked or delayed.
A case study examines clinical presentation and possible causes of pellagra, in HIV + patient on isoniazid prophylaxis. The 30 year old female on routine antiretroviral therapy presented with diarrhea, abdominal discomfort, painful swallowing, and epigastric pain, facial rash spread on the forehead, nose, cheeks and the chin, upper and lower limbs. Withdrawal of isoniazid, administration of nicotinamide and niacin supplements showed clinical improvement in four weeks. Decreased serum tryptophan in persons living with HIV (PLHIV) under IPT and lack of minimum dietary proteins threshold would be pointers to isoniazid induced pellagra risk. Appropriate dietary intake and counseling ought to be emphasized among PLHIV. Tryptophan and nicotinamide serum levels should be part of baseline investigations in PLHIV starting IPT and where feasible clinically, niacin/nicotinamide supplementation be adopted.
Keywords: Pellagra, HIV, Isoniazid, Nicotinamide, Niacin, Supplements
Introduction
Human Immunodeficiency Virus (HIV) infection is considered the strongest risk factor for tuberculosis (TB) disease among people living with HIV (PLHIV) [1,2]. Isoniazid preventive therapy (IPT) is a core intervention for the prevention of active TB infection among PLHIV [2,3], and is usually administered for a period of 6–12 months [2]. Even so, the effectiveness of IPT in PLHIV in high burden TB settings may be limited to the period during which IPT is given, may have suboptimal efficacy in immunocompromised individuals [4] and does not protect against reinfection after therapy cessation [5]. Moreover, high rates of transmission and re-infection among PLHIV in settings with high prevalence of TB increase the risk of development of active TB [6]. In such settings, lifelong IPT may be beneficial for PLHIV. While recognizing the life-saving impact for human immunodeficiency virus (HIV)-infected people who do not have active TB, IPT is arguably a possible player in pellagra in addition to well-known malnourishment determinants particularly in developing nations where diagnosis is often overlooked or delayed.
Pellagra (pelle agra, Italian for leather skin) has long been established to be caused by a cellular deficiency of niacin or its precursor amino acid, tryptophan. Niacin is the generic name for two compounds: nicotinamide and nicotinic acid similarly recognized as vitamin B3, is essential for carbohydrate, fat, protein and alcohol metabolism, detoxification of drugs and reactive oxygen species, cell signaling and DNA repair [7]. Food sources rich in niacin include tuna fish, chicken, beef, peanuts, mushroom, avocado, green peas, among others with recommended intakes of about 14 mg per day [8] while it is advanced that people living with HIV will have higher niacin requirements [9]. Symptoms related to niacin deficiency are often observed in malnourished individuals and as a complication of isoniazid therapy; however as earlier substantiated, the diagnosis is often overlooked or delayed, occasionally with life-threatening consequences [7,[10], [11], [12], [13]]. Furthermore, isoniazid-induced pellagra may occur despite pyridoxine supplementation [[13], [14], [15]].
Clinically, the systemic disease resulting from niacin deficiency is manifested by the 4 Ds: photosensitive dermatitis, diarrhea, dementia, and death if it goes unaddressed [7,13]. The full tetrad of symptoms is usually not well developed in infants and children [16]. HIV infection induces pellagra like state; plasma tryptophan levels are decreased in patients with HIV infection and high dose nicotinamide treatment may successfully reverse this HIV-induced metabolic abnormality [17,18]. It has been hypothesized that, HIV infection induces niacin depletion and therapeutic niacin would be a secondary preventive factor for AIDS in patients with HIV infection [7,13,18]. Isoniazid is known to precipitate pellagra, particularly in people with inadequate diets [19,20]. The structure of isoniazid is very similar to that of nicotinamide and it has been suggested that it competes with the latter at its site of action [10]. Reported findings of pellagra precipitated by isoniazid pointed out that a hydrazine complex is formed with pyridoxine [21,22]. Pyridoxine is essential to the interconversion in the body of tryptophan to nicotinamide [22,23]. Pellagra can be effectively cured with intravenous or oral niacin or nicotinamide. A high protein diet supplemented with B-group vitamins is needed for complete recovery [23]. In lieu of the observations and context challenging clinical realities of most developing nations where diagnosis is often overlooked or delayed, the case study is of particular interest in examining clinical presentation and possible causes of pellagra in a HIV + patient on isoniazid prophylaxis in Kenya and sheds contextual driven insights of relevant clinical value for advancing routine case profiling and subsequent management and outcome.
Case presentation
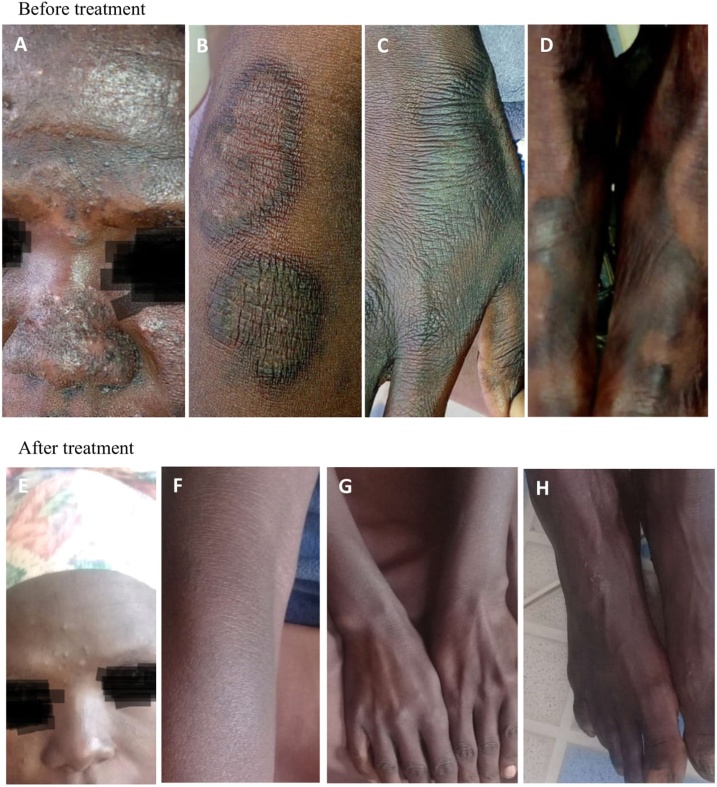
A 30 year old female on a routine antiretroviral clinic visit presented with diarrhea, abdominal discomfort, painful swallowing, epigastric pain and a facial rash spread on the forehead, nose, cheeks and chin. The rash on the face was pruritic (Fig. 1A) especially when the patient was exposed to sunlight. There were also rashes on both fore arms, both hands and both feet (Fig. 1B–D) respectively. The patient reported to be weak and not able to perform her usual chores. At the time of presentation of these symptoms, she was on antiretroviral therapy (lamivudine, tenofovir, and efavirenz) for several years from 2015 and trimethoprim/sulfamethoxazole prophylactic therapy. Isoniazid prophylactic therapy (IPT) (300 mg) and pyridoxine (50 mg) from 4th January 2018. The patient did not have signs/symptoms of tuberculosis and had no history of tuberculosis treatment in the past. There was no history of alcoholism or cigarette smoking. She lived with her five children. A dietary history data collected revealed that the 30 year old female relied on maize as the main staple food that was often consumed with kale (green leafy vegetables locally referred to as sukuma wiki). She rarely consumed beans and meat. The woman was a farmer and did not engage in poultry keeping.
Fig. 1.
The patient’s skin lesions corresponding to before (A–D) and after (E–H) treatment respectively. A: Edematous, hyperpigmented vessiculobullous lesions on the forehead and the nose. B: Hyperkeratotic, hyperpigmented, sharply demarcated plagues on the extensor surface of the right forearm. C: Scaly hyperpigmented rash at the back of the right hand D: Scaly hyperpigmented rashes on both feet.
Clinical examination revealed that the patient was distressed and had hyperpigmented rashes on the forehead, nose, zygomatic and just below zygomatic regions, upper lip, lower lip, and chin. Some parts of the rashes had acne-like appearance; when squeezed gently a white substance (sebum) came out. The facial rash had a butterfly-like appearance. There were rashes on both feet and hands with no definite margins, all hyperpigmented. There were two separate rashes with definite margins on the lateral side of the right forearm just below the elbow joint. The rash closer to the elbow joint had its margins only with hyperpigmentation. The rash below was entirely hyperpigmented. Tests done include complete blood count (Table 1) which had all parameters normal including a negative VDRL. Viral load result done earlier indicated the patient was virally suppressed. An impression of pellagra (isoniazid induced) was made with differentials as sebaceous hyperplasia, acne, auto immune disorder and Systemic Lupus Erythromatosus.
Table 1.
Results of initial laboratory tests.
| Laboratory test | Results | Reference interval (RI) |
|---|---|---|
| White blood cells (×109/L) | 9.7 | 3.5–10.0 |
| Lymphocytes (×109/L) | 1.1 | 0.9–5.0 |
| Granulocytes (×109/L) | 17.1 | 1.2–8.0 |
| Hemoglobin (g/dl) | 11.9 | 11.5–16.5 |
| Mean cell hemoglobin (pg) | 27.3 | 25.0–35.0 |
| Mean corpuscular volume (fl) | 68.1 | 75.0–100.0 |
| Hematocrit (%) | 21.7 | 35.0–55.0 |
| Platelets (×109/L) | 390 | 130–400 |
| HIV-1/2 Antibody test | Positive | |
| Hepatitis B surface antigen | Not detected | |
| Viral Load | Below detectable levels | |
| Treponema pallidum test (RPR) | Not detected |
The management plan included withdrawal of isoniazid and trimethoprim/sulfamethoxazole, nutritional review (dietary counseling and niacin supplements), dermatological review, nicotinamide capsules and prednisolone tablets. A review by a dermatologist confirmed pellagra. Prednisolone was stopped after one week. Nicotinamide capsules of 90 mg three times a day for 8 weeks were recommended. Pyridoxine dose increased to 50 mg twice a day for 8 weeks. The nutritionist recommended increased dietary proteins intake, preferably animal-source protein foods. Niacin supplements- Replace with 5 mg/100 g of niacin was given two level scoops per serving twice a day for 3 weeks and Ensure with 5 mg /100 g of niacin given six level scoops per serving twice a day for 5 weeks. The patient was reviewed weekly for the first 3 weeks then twice weekly. After the second week on treatment the sebum secreting rash on the face disappeared and diarrhea stopped. There was no fatigue. On the fourth week of treatment there was marked improvement on the facial rash. The facial rash was completely gone by the sixth week of treatment. All rashes had cleared by the seventh week. Treatment was extended for a week then stopped.
Discussion
This case demonstrates a near classical case of pellagra, in a patient on antiretroviral and isoniazid prophylactic therapy whose diet largely lacked majorly animal source foods and other foods that are rich in niacin. At the time of diagnosis of pellagra the patient had been on isoniazid for two months while she had been adhering to ART for about 2 years and 1 month. There were neither neurological symptoms nor signs. Withdrawal of isoniazid and high dose of nicotinamide and niacin supplements, led to a notable clinical improvement in four weeks. It has been established, for routine dietary requirements, only 20 mg of niacin is required on a daily basis while the use of nicotinamide for the treatment of HIV-positive patients confirmed that dosages of 3 g/day could be well tolerated [10,18]. In scenarios where the dietary fulfillment is significantly exceeded, then niacin in either form is considered to be a pharmacological agent or drug. The pharmacokinetics of nicotinamide in humans; a study of twice-daily administration of oral nicotinamide in a total daily dose of 25 mg/kg revealed a plasma half-life of 3.5 h, and the mean maximum plasma concentration was 42.1 μg/mL (0.3 mM) [10].
Despite it being apparent that isoniazid had adverse effects on the normal metabolism of Vitamin B complex; B6 and niacin shortly after its initial clinical use [10], it remains less well known by clinicians that isoniazid can significantly affect niacin metabolism in cases like IPT among PLHIV and that it has been observed to induce clinical pellagra (i.e., niacin depletion) [10,12,13]. Decreased tryptophan serum levels in PLHIV accelerate the development of isoniazid induced pellagra [13,17] whereas lack of dietary proteins contributes to development of pellagra in PLHIV [24]. However, the deficiency is still present in cultures that rely on maize as their primary source of sustenance.
Following further understanding of the pathophysiology of pellagra in the 20th century, increased awareness and vitamin fortification endeavors internationally [24], deficiency is rarely seen presently. Nevertheless, sporadic cases have been reported in the last few decades due to varied reasons ranging from alcoholism to tuberculosis therapy and malabsorption disorders among others [12,25]. The populations that are at the greatest risk are those that rely on unfortified corn, or maize, as their primary food source with Sub-Saharan Africa countries being adversely affected [25]. This is in tandem with the fact that IPT is arguably a possible player in pellagra in addition to well-known malnourishment determinants particularly in developing nations where diagnosis is often overlooked or delayed and HIV is prevalent necessitating IPT [10,12,21,26]. Reliance on maize as the main staple food was the case in the reported circumstance under IPT and dietary history accompanied with appropriate counseling was emphasized. In sub-Saharan Africa (SSA) context, more of related chemotherapy challenges are prone to emerge as HIV and related co-infections management and treatment regiments are progressively optimized globally whereas food security challenges continue to persist. Adherence to chemotherapy alongside nutrition counselling efforts need to be underpinned in advancing antiretroviral therapy adherence across gender. Option B + HIV control and management success lessons and gains would be adopted as is being observed in the framework of Prevention of Mother To Child Transmission (PMTCT) [26] in SSA. In which, the infected and affected for instance marital partners or core family keens where applicable are co-opted in clinical visit for not only moral support but to reinforce the clinical treatment and nutritional management knowledge shared for effective uptake at home and in the communities.
In conclusion, isoniazid used in prophylaxis which is not weight dependent may be relatively higher and has a potential of inducing pellagra in maize dependent HIV positive populations. Isoniazid prophylactic dose should be given according to body weight (5 mg/kg body weight). Decreased serum tryptophan in persons living with HIV (PLHIV) under IPT and lack of minimum dietary proteins threshold would be pointers to possible risk of isoniazid induced pellagra. Dietary history and appropriate counseling ought to be emphasized among PLHIV during ART initiation and treatment follow up. Tryptophan and nicotinamide serum levels should be part of baseline investigations in PLHIV starting isoniazid prophylaxis and where feasible clinically, niacin/nicotinamide supplementation be adopted [10,12,17,18,23].
Informed consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Availability of data and material
All data generated or analyzed are included in this published article.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
Not applicable.
Authors’ contributions
JKK and JKC were responsible for diagnosis and clinical management of the patient, drafting of the manuscript, acquisition and analysis of data. PM and CKW participated in formal analysis, writing original draft, review and editing of the manuscript for intellectual content. All authors read and approved the final manuscript.
ICMJE statement
All authors meet the ICMJE authorship criteria.
Acknowledgements
The authors thank Dr. Etemesi Beatrice, Dermatologist Nakuru level 5 Hospital, Nakuru, Kenya; Dr. Eric Langat, Medical Superintendent Ndanai Hospital, Bomet, Kenya; Mr. Stephen Kombich, Henry Jackson Foundation Medical Research International (HJFMRI), Kericho, Kenya; Dr. Jemutai Tarus, Henry Jackson Foundation Medical Research International (HJMRI), Kericho, Kenya and Mr. Francis Rono, Sub County AIDS/Sexually Transmitted Infections(STI) Coordinator, Sotik Sub County, Bomet, Kenya for the technical and specialist assistance during the different facets of this study.
Contributor Information
John Koech Kipsang, Email: johnkoech787@gmail.com.
Joseph K. Choge, Email: jchoge@kabianga.ac.ke.
Pamela A. Marinda, Email: ayiera@yahoo.co.uk.
Christopher Khayeka-Wandabwa, Email: khayekachris@yahoo.com.
References
- 1.World Health Organization . 2011. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. [Google Scholar]
- 2.Den Boon S., Matteelli A., Ford N., Getahun H. Continuous isoniazid for the treatment of latent tuberculosis infection in people living with HIV: a systematic review and meta-analysis. AIDS (London, England) 2016;30(5):797. doi: 10.1097/QAD.0000000000000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Tuberculosis preventive therapy in HIV-infected individuals: A Joint Statement of the WHO Tuberculosis Programme and the Global Programme on AIDS, and the International Union Against Tuberculosis. Wkly Epidemiol Rec. 1993;68(49):361–364. [PubMed] [Google Scholar]
- 4.Houben R.M., Sumner T., Grant A.D., White R.G. Ability of preventive therapy to cure latent Mycobacterium tuberculosis infection in HIV-infected individuals in high-burden settings. Proc Natl Acad Sci U S A. 2014;111(14):5325–5330. doi: 10.1073/pnas.1317660111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Getahun H., Matteelli A., Abubakar I., Aziz M.A., Baddeley A., Barreira D. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46(6):1563–1576. doi: 10.1183/13993003.01245-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonnenberg P., Murray J., Glynn J.R., Shearer S., Kambashi B., Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet. 2001;358(9294):1687–1693. doi: 10.1016/S0140-6736(01)06712-5. [DOI] [PubMed] [Google Scholar]
- 7.Hegyi J., Schwartz R.A., Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43(1):1–5. doi: 10.1111/j.1365-4632.2004.01959.x. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . 2005. Vitamin and mineral requirements in human nutrition. [Google Scholar]
- 9.Lebouché B., Jenabian M.-A., Singer J., Graziani G.M., Engler K., Trottier B. The role of extended-release niacin on immune activation and neurocognition in HIV-infected patients treated with antiretroviral therapy–CTN PT006: study protocol for a randomized controlled trial. Trials. 2014;15(1):390. doi: 10.1186/1745-6215-15-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray M.F. Nicotinamide: an oral antimicrobial agent with activity against both Mycobacterium tuberculosis and human immunodeficiency virus. Clin Infect Dis. 2003;36(4):453–460. doi: 10.1086/367544. [DOI] [PubMed] [Google Scholar]
- 11.Forget E.J., Menzies D. Adverse reactions to first-line antituberculosis drugs. Expert Opin Drug Saf. 2006;5(2):231–249. doi: 10.1517/14740338.5.2.231. [DOI] [PubMed] [Google Scholar]
- 12.Thornton A.M., Drummond C.J. An unexpected case of pellagra. Med J Aust. 2014;200(9):546–548. doi: 10.5694/mja13.11187. [DOI] [PubMed] [Google Scholar]
- 13.Bilgili S.G., Karadag A.S., Calka O., Altun F. Isoniazid-induced pellagra. Cutan Ocul Toxicol. 2011;30(4):317–319. doi: 10.3109/15569527.2011.574303. [DOI] [PubMed] [Google Scholar]
- 14.Desai C. Meyler’s side effects of drugs: the international encyclopedia of adverse drug reactions and interactions. Indian J Pharmacol. 2016;48(2):224. [Google Scholar]
- 15.Darvay A., Basarab T., McGregor J., Russell-Jones R. Isoniazid induced pellagra despite pyridoxine supplementation. Clin Exp Dermatol. 1999;24(3):167–169. doi: 10.1046/j.1365-2230.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- 16.Felípez L., Sentongo T.A. Drug-induced nutrient deficiencies. Pediatr Clin. 2009;56(5):1211–1224. doi: 10.1016/j.pcl.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Murray M. Niacin as a potential AIDS preventive factor. Med Hypotheses. 1999;53(5):375–379. doi: 10.1054/mehy.1999.0908. [DOI] [PubMed] [Google Scholar]
- 18.Murray M.F., Langan M., MacGregor R.R. Increased plasma tryptophan in HIV-infected patients treated with pharmacologic doses of nicotinamide. Nutrition. 2001;17(7-8):654–656. doi: 10.1016/s0899-9007(01)00568-8. [DOI] [PubMed] [Google Scholar]
- 19.Williams A.C., Ramsden D.B. Pellagra: a clue as to why energy failure causes diseases? Med Hypotheses. 2007;69(3):618–628. doi: 10.1016/j.mehy.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Karthikeyan K., Thappa D.M. Pellagra and skin. Int J Dermatol. 2002;41(8):476–481. doi: 10.1046/j.1365-4362.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths W., Greaves M., Meara R. SAGE Publications; 1976. Isoniazid-induced pellagra. [Google Scholar]
- 22.Brenner A., Wapnir R.A. A pyridoxine-dependent behavioral disorder unmasked by isoniazid. Am J Dis Child. 1978;132(8):773–776. doi: 10.1001/archpedi.1978.02120330045011. [DOI] [PubMed] [Google Scholar]
- 23.Thurnham D.I. An overview of interactions between micronutrients and of micronutrients with drugs, genes and immune mechanisms. Nutr Res Rev. 2004;17(2):211–240. doi: 10.1079/NRR200486. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization . World Health Organization; Geneva: 2000. Pellagra and its prevention and control in major emergencies. [Google Scholar]
- 25.Matapandeu G., Dunn S.H., Pagels P. An outbreak of pellagra in the Kasese Catchment area, Dowa, Malawi. Am J Trop Med Hyg. 2017;96(5):1244–1247. doi: 10.4269/ajtmh.16-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marinda P., Chibwe N., Tambo E., Lulanga S., Khayeka-Wandabwa C. Challenges and opportunities of optimal breastfeeding in the context of HIV option B+ guidelines. BMC Public Health. 2017;17(1):541. doi: 10.1186/s12889-017-4457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed are included in this published article.