Abstract
A LC-MS/MS method has been optimized and validated for the determination of aflatoxins (AFB1, AFB2, AFG1 and AFG2) in maize. Extraction was performed using a modified QuEChERS method with little sample preparation without the need for purification procedure. Determination was performed by high pressure liquid chromatography (HPLC) coupled to tandem mass spectrometry (MS/MS). The acquisition was performed using MassHunter software in Multiple Reaction Monitoring (MRM) mode in positive polarity. Different mobile phases were tested to control the degree of the ionization and good performances were obtained for methanol/water with 5 mM ammonium acetate. MRM experiments were optimized for each aflatoxin in order to generate sensitive transitions. Linearity was demonstrated for the aflatoxins in the range 0.225–1.25 μg/L. Limits of detection (LOD) (0.11 and 0.36 μg/Kg) and limits of quantification (LOQ) (0.36–1.19 μg/Kg) of the aflatoxins are below the maximum permitted levels set by the European Union (EU). Aflatoxins have acceptable recoveries using QuEChERS method in the acceptable range of 50–120% for levels below 1 μg/Kg. Satisfactory recoveries were also obtained in the acceptable range of 70–110% for levels between 1 and 10 μg/Kg except for AFB2. Relative standard deviation (RSD) of recoveries for the intra-day precision and inter-day precision were below 11 %. Selectivity of the method was tested and no spectral interferences were observed in the appropriate retention times. The main advantage of the proposed method is its ease of use and requires a smaller solvent consumption that reduces the time and cost of the analysis.
Keywords: Food science, Analytical chemistry, Food technology, Food safety, Food analysis
1. Introduction
Topics related to mycotoxins are of great importance as they pose a real threat to food safety and can cause significant economic losses. They can be found in different commodities of plant origin such as cereals. These grains are extremely vulnerable to fungi and to the production of mycotoxins especially in the field under stress conditions or in storage when conditions like warm temperature and high moisture are met (Bennett and Klich, 2003). It was shown that 25% of cereals approximately consumed in the world are contaminated by mycotoxins especially aflatoxins (Devegowda et al., 1998).
Among cereals, maize is the main agricultural product and the most popular cereal grains. It is widely consumed because is considered as an important nutrient source. For instance, maize contains proteins and antioxidants than many other cereal grains (Panzeri et al., 2011). However, maize is liable to infection with aflatoxigenic fungi and consequently contamination with aflatoxins (Zinedine et al., 2007; Desmarchelier et al., 2010).
During 2017, Morocco imported a total of 2.08 million tons of maize mainly from United States and European Union (ONICL, 2017). The climate in Morocco, characterized by high moisture and temperature, promotes probably the growth of fungi and the secretion of these toxins.
Among aflatoxins currently known, AFB1, AFB2, AFG1 and AFG2 still are of major concern. They are difuranocoumarin derivatives, polar, low-molecular-weight, toxic secondary metabolites produced by some species of filamentous fungi. Aspergillus flavus and Aspergillus parasiticus grow during storage and are the major producers of these mycotoxins (Bennett and Klich, 2003). In addition, toxicological data revealed that many aflatoxins show acute and chronic toxicity including carcinogenic, mutagenic, immunotoxic and hepatotoxic effects in human and animals (Asao et al., 1963; Stora et al., 1983). The AFB1 is the most toxic among them and is classified as a group I human carcinogen by the International Agency for Research on Cancer (International Agency for Research on cancer (IARC), 1993). Both availability of toxicological data and their occurrence in wide range of food commodities intended for human consumption are considered as main factors leading to the establishment of a very stringent tolerance (Van Egmond et al., 2007). In the first place, regulations have been established to minimize human health risks from these compounds. The maximum limits set by EU regulations for AFB1 and sum of aflatoxins (AFB1 + AFB2 + AFG1 + AFG2) in maize range from 2 μg/Kg to 4 μg/Kg, respectively (Commission Regulation (EC) No 1881, 2006). Therefore, monitoring aflatoxins levels in agricultural product is necessary to ensure the quality of the food supply.
Aflatoxins are amenable to determination by different chromatographic techniques such as thin layer chromatography (TLC) (Trucksess et al., 1984) and liquid chromatography with fluorescence detection (LC/FLD) using pre- or post-column derivatization (Huertas-Pérez et al., 2018).
In fact, a few quantitative studies have been published to determine related group aflatoxins in food using liquid chromatography coupled to tandem mass spectrometry (LC MS/MS) (Takino et al., 2004; Cervino et al., 2008; Nonakaa et al., 2009; Sirhan et al., 2013; Zhao et al., 2016).
These techniques require sample preparation (extraction and purification) procedures to ensure accurate quantification and they involve liquid/liquid extraction, solid phase extraction or immunoaffinity chromatography. Recently, QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) method, which was first used for the analysis of pesticides in fruits and vegetables (Anastassiades et al., 2003), has increasingly applied due to its ease of use and suitability for extraction of mycotoxins from complex matrices (Desmarchelier et al., 2010; Yogendrarajaha et al., 2013; Miró-Abella et al., 2017). This procedure is based on an extraction with acetonitrile followed by liquid–liquid partition after the addition of salts (MgSO4 and NaCl).
Actually, LC/FLD in combination with immunoaffinity column (IAC) is a reference method and still remains the method of choice for determination of aflatoxins. However, this method requires not only purification steps that are time consuming but also pre- or post-column derivatization (Fedorowski and LaCourse, 2010).
Liquid chromatography coupled to tandem mass spectrometry (LC MS/MS) is currently the most used technique in the detection of mycotoxins. It can be applied without clean-up procedure and derivatization owing to its high selectivity and sensitivity (Soleimany et al., 2012).
The aim of this study was to optimize and establish a rapid high-performance liquid chromatography tandem mass spectrometry (HPLC–MS/MS) method for the simultaneous determination of aflatoxins (AFB1, AFB2, AFG1 and AFG2) in maize commercialized in Morocco, using a QuEChERS extraction method without any further clean-up steps.
2. Experimental
2.1. Reagents and chemicals
Methanol (MeOH) was purchased from Honey Well (Honey Well International Inc., USA) and acetonitrile (MeCN) from Sigma–Aldrich (St. Louis, USA). All organic solvents were HPLC MS grade. Anhydrous magnesium sulfate (MgSO4), sodium chloride (NaCl) and ammonium acetate (CH3COONH4) were all of analytical grade. Ultrapure water (resistivity 18.2 MΩ.cm) was obtained from ELGA purification system (Veolia water, Solutions and technologies, Germany). Cellulose Syringe filters (15 mm, 0.2 μm) were obtained from ALBET.
The individual standard solution with concentrations of 2.01 μg/mL for AFB1 and AFG1, 0.525 μg/mL for AFB2 and 0.501 μg/mL for AFG2 were purchased from Novakits (Nantes, France).
2.2. Preparation of working solution
Appropriate volumes of individual standard solutions (125 μL for AFB1 and AFG1, 450 μL for AFB2 and AFG2) were diluted to 5 mL with acetonitrile/water (50:50, v/v), resulting in an intermediate mixed solution of 0.05 μg/mL for AFB1 and AFG1, 0.047 μg/mL and 0.045 μg/mL for AFB2 and AFG2, respectively.
2.3. Preparation of solvent calibration curves
In order to prepare the calibration curves in solvent, aliquots of 5, 10, 15, 20 and 25 μL of intermediate mixed solution were taken and diluted to 1 ml with acetonitrile/water (50:50, v/v) yielding a five concentration levels: 0.25, 0.5, 0.75, 1, 1.25 ng/mL for AFB1 and AFG1; 0.235, 0.47, 0.705, 0.94, 1.175 ng/mL for AFB2 and 0.225, 0.45, 0.675, 0.9, 1.125 ng/mL for AFG2. Both individual standard solution and intermediate mixed solution were stored in amber flask and kept in freezer at −18 °C until analysis.
2.4. Mobile phase and gradient elution
Three different mobile phase were investigated. Eluent A was water. Acetonitrile, methanol and methanol containing 5 mM ammonium acetate were tested separately as eluent B. The same gradient elution was applied to these three types of the mobile phase. The proportion of eluent B was linearly increased from 10% to 100% within 4 min, and kept constant for 2 min. The column was re-equilibrated with 10% of eluent B for 3 min.
2.5. Instrumentation
Detection was performed using a HPLC system Agilent 1290 Infinity II (flexible pump, vial sampler, MCT) equipped with an Agilent JetStream electrospray ionization (ESI) source and a 6470 series Triple Quadrupole LC/MS (Agilent technologies, Germany).
Chromatographic separation of aflatoxins was carried out with Zorbax Eclipse C-18 column with 3 mm internal diameter, 50 mm length and 1.8 μm particle size (Agilent technologies, USA). The flow rate of the mobile phase was fixed to 0.6 mL/min and the injection volume for the HPLC system was 5.0 μL. The column oven was maintained at 40 °C.
For MS/MS detection, the ESI interface was used in positive polarity with the following settings: The capillary voltage 3.5 kV, the capillary gas flow 8 L/min, source temperature 300 °C, nozzle voltage 500 V, nebulizer gas pressure 45 psi, desolvation gas flow 12 L/min, desolvation gas temperature 400 °C. Collision-induced dissociation (CID) was performed using nitrogen (10–30 psi) with a high purity (99.99%). The nitrogen used as nebulizer gas and also as desolvation gas was produced by a nitrogen generator.
MRM transitions for each aflatoxin were optimized using single MS full scan mode followed by product ion scan mode after injection of intermediate mixed solution, so as to select one precursor and two product ions per compound for both quantification and confirmation purposes. The response was calculated as the peak area for all compounds in the MRM chromatogram, and was used to determine the concentration of a compound in the sample.
2.6. Samples and sample preparation
Ground maize samples commercialized in Morocco were collected from a local market (Agadir city, Morocco). These samples were extracted and analyzed using the method described below.
Aflatoxins were extracted from maize according to QuEChERS method with some modification (Sirhan et al., 2014). Briefly, in a 50 mL polypropylene tube, 1 g of ground and homogenized maize sample was extracted with 3 mL of methanol-acetonitrile solution (60:40, v/v) and vortexed for 1 min using a VF2 Junkelkunkel (IKA-Labortechnik). Then, anhydrous MgSO4 (1.32 g) and NaCl (0.25 g) were added and the tube was shaken for 1 min to obtain phase separation. The mixture was centrifuged for 5 min at 4000 rpm on an EBA 21 (Hettich Zentrifugen, Germany). Finally, 0.5 mL of the upper organic phase was filtered through a 0.2 μm cellulose syringe filter and directly injected into LC-MS/MS.
2.7. HPLC-MS/MS method optimization
In order to improve the chromatographic separation and to increase the MS response, different mobile phases were tested: Both acetonitrile and methanol mixed with water, and methanol mixed with water containing ammonium acetate buffer. Concerning the optimization of MS/MS conditions, the first step was the determination of precursor ions and the determination of the most intensive and characteristic product ions. For each compound, three transitions were exploited in MRM mode which were also optimized by varying collision energy. The operation began with the injection of 5 μL of intermediate mixed solution containing AFB1 (0.05 μg/mL), AFB2 (0.047 μg/mL), AFG1 (0.05 μg/mL) and AFG2 (0.045 μg/mL).
2.8. Validation study
The optimized method was validated for the four selected aflatoxins in maize according to NF standard T90-210 (NF T-90 210, 2009) and taking into account the respective acceptability criteria in the European Commission regulation (Commission Regulation (EC) No 401, 2006).
The different characteristics of the proposed method such as linearity and linear range, limits of detection (LOD) and quantification (LOQ), recoveries, selectivity and precision were evaluated.
2.8.1. Linearity
In this study, five concentration levels of aflatoxin standard solutions ranging from 0.225 to 1.25 ng/mL were analyzed to evaluate the linearity of the calibration curves. Linearity confirmation was achieved with lack-of-fit Fisher test. Normality was investigated with a Shapiro Wilk test. Outliers and Homogeneity of variances were studied with Grubbs test (Maximum Normed Residual) and Cochran test, respectively.
2.8.2. LOD and LOQ
LODs and LOQs were estimated for a signal to noise ratio (S/N) of 3 and 10 using Eqs. (1) and (2) respectively, by spiking samples at the lowest level validated of each target analyte.
| (1) |
| (2) |
2.8.3. Recovery
Recovery experiments were studied by spiking ground maize at levels: 0.5, 2.5, 5 and 10 μg/Kg for AFB1 and AFG1; 0.47, 2.35, 4.70 and 9.40 μg/Kg for AFB2; 0.45, 2.25, 4.5 and 9 μg/Kg for AFG2. References values adopted for recovery evaluation were set by the EC (2006) (Commission Regulation (EC) No 401, 2006). Recoveries were calculated using Eq. (3).
| (3) |
2.8.4. Precision
For the estimation of the method precision, the mean recoveries were compared with theoretical spiked concentration. The recovery study was also used to evaluate repeatability (precision), and four replicates were carried out for each level (intra-day precision). Three separates runs were performed for a period of three days to evaluate intermediate reproducibility (inter-day precision).
2.8.5. Selectivity
Selectivity of the method was studied by analyzing both blank and spiked samples based on the monitoring of characteristic transition of each analyte in the appropriate retention time.
3. Results and discussion
3.1. HPLC-MS/MS method optimization
3.1.1. Effects of mobile phase composition
Aflatoxins were analyzed in electropray positive ionization (ESI+) operating in single-MS full scan mode.
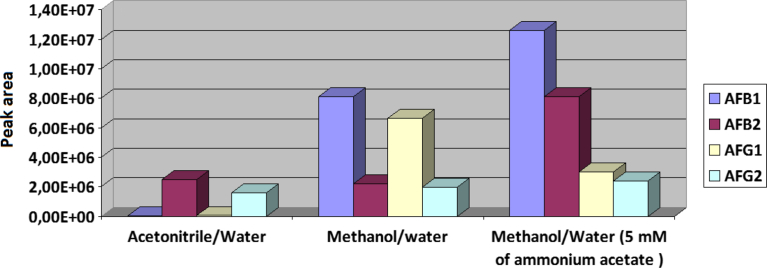
In order to attain the optimal conditions and to control the degree of the ionization of aflatoxins in ESI+ mode, mobile phases consisting of acetonitrile/water, methanol/water and methanol/water containing 5 mM CH3COONH4 were tested. It has been widely reported that aflatoxins are better ionized in positive mode (Sirhan et al., 2013; Miró-Abella et al., 2017). Methanol and acetonitrile are usually used as the organic mobile phase eluent in liquid chromatography coupled to mass spectrometry analysis since they affect quality of chromatographic separation and improve analyte ionization (Proctor and Todd, 1983). Also, the addition of buffers to the mobile phase often results in more efficient ionization and gives better peak shapes. However, higher concentrations should be avoided to prevent ion suppression in ESI (Zimmer, 2003). Fig. 1 indicates that there is a significant influence of the mobile phase composition on the ionization efficiency. The mobile phase consisting of methanol/water is favourable to the ionization and leads to an increase in observed signals (peak area), while the use of acetonitrile leads to much lower signals. In addition, the use of 5 mM ammonium acetate buffer enhances sensitivity and this is in agreement with previous studies (Sirhan et al., 2013; Campone et al., 2015). AFG2 was the single compound for which the intensities remained relatively stable whatever the mobile phase used. Thus, methanol/water containing 5 mM CH3COONH4 was selected as the best mobile phase eluent.
Fig. 1.
Evolution of the Aflatoxin peak area versus the composition of the mobile phase (ESI+, Full scan mode).
3.1.2. Optimization of MRM transitions
Determination of the optimal MRM transitions for each aflatoxin was conducted using MS full scan mode followed by product ion scan mode using aflatoxin intermediate mixed solution.
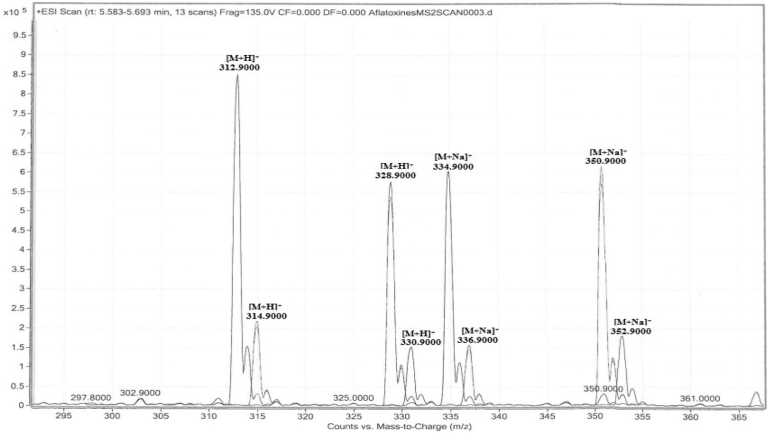
Scanning between 100 and 500 m/z shows the molecular ion of aflatoxin B1 at m/z 312.9, aflatoxin B2 m/z at 314.9, aflatoxin G1 at m/z 328.9 and aflatoxin G2 at m/z 330.9. All precursors ions exhibited responses in ESI+ mode and lead to a protonated molecular ions [M + H]+. Molecular ions with sodium adducts [M + Na]+ were also formed. The full mass spectra obtained show that aflatoxins are easily ionized in ESI+ mode. AFB1 and AFB2 were better ionized as hydrogen adducts [M+ H]+ representing the more abundant form. The abundance of the two forms of precursor ions for AFG1 and AFG2 were relatively similar (Fig. 2). Hence, the protonated molecular ions were finally chosen for all analytes.
Fig. 2.
Full-scan mass spectra of [M + H]+ and [M + Na]+ precursor ions for Aflatoxin B1 (m/z 312.9 and 334.9), Aflatoxin B2 (m/z 314.9 and 336.9), Aflatoxin G1 (m/z 328.9 and 350.9) and Aflatoxin G2 (m/z 330.9 and 352.9).
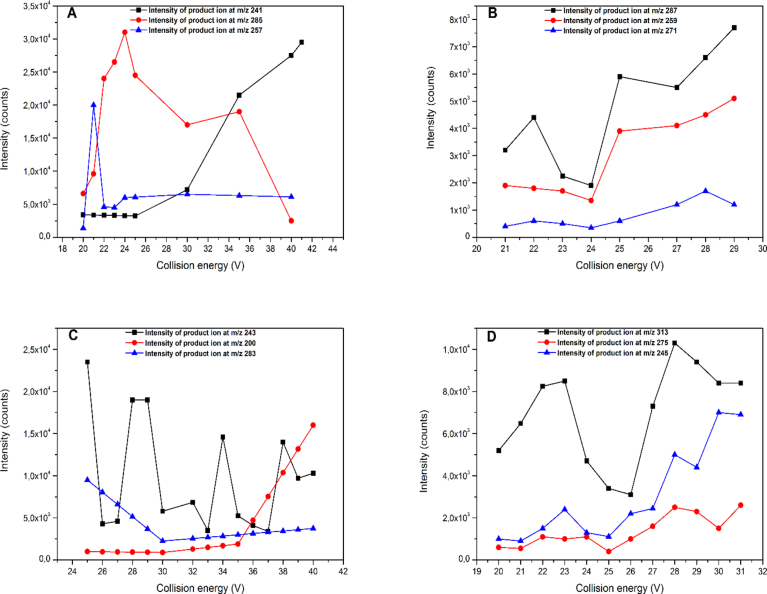
Once the precursor ions were identified, the optimum collision energy for MRM transitions for each aflatoxin was performed. Only three product ions for each precursor ion were selected after using product ion scan mode and optimized by varying collision energy between 20 and 41 V. The most of these fragment ions have previously been reported in the literature (Sulyok et al., 2006; Sirhan et al., 2013; Li et al., 2017). The most prominent product ions from each aflatoxin are shown in Fig. 3. Finally, two MRM transitions for each compound were selected according to the criteria for MS detection established by the European Commission (Commission Decision (EC) No 657, 2002). The most intense MRM transition was monitored for quantification and the second most intense transition for confirmation. Optimized MS/MS parameters (precursor ions, product ions and collision energies) as well as retention times are shown in Table 1.
Fig. 3.
Intensities of the most abundant product ions (nominal mass) corresponding to: AFB1 (A), AFB2 (B), AFG1 (C) and AFG2 (D).
Table 1.
LC-ESI-MS/MS parameters for the analysis of aftatoxin B1, B2, G1 and G2 in multiple reactions monitoring (MRM) mode.
| Aflatoxin | Molecular formula | Molecular weight (g/mol) | Retention time (min) | Precursor ion [M + H]+ (m/z) | Product ion (m/z) | Dwell time (ms) | Fragmentor voltage (V) | Collision energy (V) |
|---|---|---|---|---|---|---|---|---|
| B1 | C17H12O6 | 312 | 3.382 | 313.1 | 284.7* | 50 | 135 | 24 |
| 240.8 | 41 | |||||||
| B2 | C17H14O6 | 314 | 3.277 | 315.1 | 286.7* | 50 | 135 | 29 |
| 258.7 | 29 | |||||||
| G1 | C17H12O7 | 328 | 3.145 | 329.1 | 242.7* | 50 | 135 | 25 |
| 199.9 | 40 | |||||||
| G2 | C17H14O7 | 330 | 3.026 | 331.1 | 312.7* | 50 | 135 | 28 |
| 244.7 | 30 |
* Quantification product ions.
Highlighted in bold are Retention times 3.026 min for AFG2, 3.145 min for AFG1, 3.277 min for AFB2 and 3.382 min for AFB1.
Aflatoxins were eluted within 9 min, including cleaning and re-equilibration steps, without interfering peaks. Retention times were 3.026 min for AFG2, 3.145 min for AFG1, 3.277 min for AFB2 and 3.382 min for AFB1.
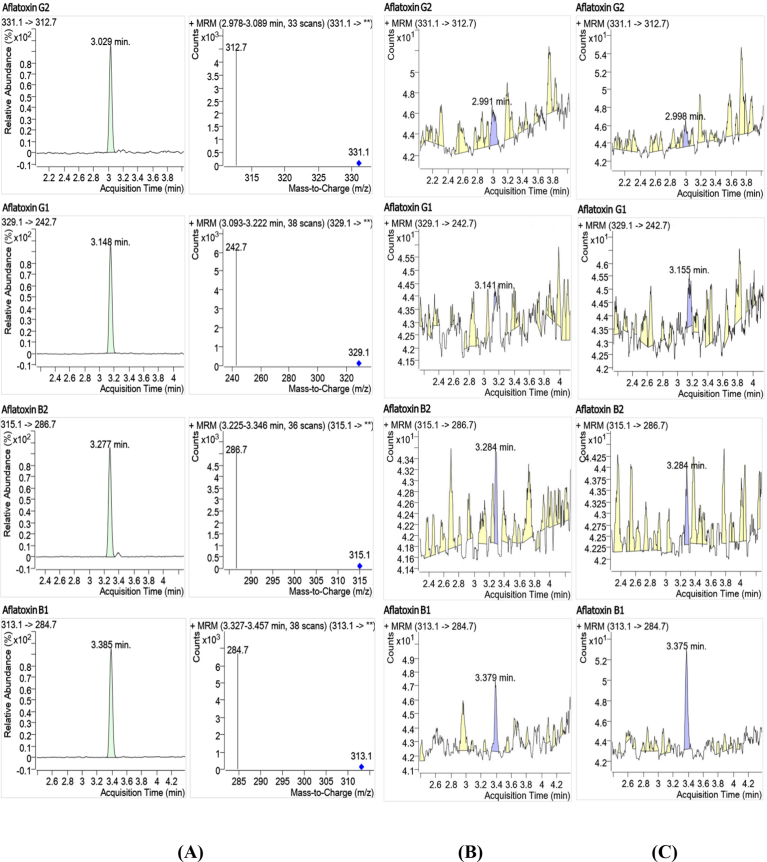
In Fig. 4 A, the obtained MRM chromatograms and mass spectra of quantitative transitions for blank maize sample contaminated with AFB1 (2.5 μg/Kg), AFB2 (2.35 μg/Kg), AFG1 (2.5 μg/Kg) and AFG2 (2.25 μg/Kg) are illustrated.
Fig. 4.
(A) Typical MRM chromatograms and mass spectra of quantitative transitions for aflatoxins. Blank maize sample contaminated with AFB1 (2.5 μg/Kg), AFB2 (2.35 μg/Kg), AFG1 (2.5 μg/Kg) and AFG2 (2.25 μg/Kg). (B) and (C) Real samples.
3.2. HPLC-MS/MS method validation
The responses of each analyte (peak area) obtained in ESI+ MRM mode were plotted versus five concentration levels ranging from 0.225 to 1.25 ng/ml. The intercept (a), slope (b) and correlation coefficient (R2) were estimated with least-squares method.
3.2.1. Linearity
Several published studies describing method validation reported the correlation coefficient (R2) as indicator for linearity (Spanjer et al., 2008; Lattanzio et al., 2007). Correlation coefficient (R2) is often used to assume the relationship between variables of the model and to assess the quality of the fit. It is noteworthy that this approach is not appropriate for evaluation of linearity (Thompson et al., 2002).
In the present study, the calibration curves established were linear for all analytes over the studied range with satisfactory correlation coefficients (R2) between 0.9917 and 0.9946. All statistical tests were performed at significance level α = 0.01. Normal distribution of responses was confirmed with the Shapiro Wilk test . Verification of outliers was conducted with Maximum Normed Residual Test (Grubbs test) for different responses and no outliers were detected . Homogeneity of variances was confirmed in all cases after applying Cochran test .
In order to evaluate linearity, the results obtained were submitted to a lack-of-fit Fisher test (F test) based on the analysis of variance (ANOVA). The F-values calculated for regression and lack-of-fit were compared with F-distribution values corresponding to and , respectively, with a significance level α = 0.01 and 1, p-2, N-p degrees of freedom . The linear regression model explains variation and the studied range was acceptable confirming the linearity for the four analytes. The regression parameters obtained are summarized in Table 2.
Table 2.
Calibration curve equations (mean ± standard deviation, n = 5) and statistical tests used for linearity assessment.
| Aflatoxins | Equation | R2 | Normality |
Maximum normed residual |
Homogeneity of variances |
Regression |
Lack-of-fit |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WTest (5 levels) | WCritical | GTest (5 levels) | GCritical | CTest | CCritical | F | FCritical | F | FCritical | |||
| AFB1 | y = (893.67 ± 67.05)x – (17.94 ± 33.88) | 0.9934 | 0.815–0.963 | 0.686 | 1.35–1.70 | 1.764 | 0.406 | 0.633 | 1155.9 | 7.823 | 2.82 | 3.67 |
| AFB2 | y = (1086.24 ± 76.90)x – (24.71 ± 28.18) | 0.9946 | 0.722–0.982 | 0.686 | 1.14–1.64 | 1.764 | 0.336 | 0.633 | 1228.5 | 7.823 | 1.95 | 3.67 |
| AFG1 | y = (710.97 ± 41.57)x + (0.94 ± 9.80) | 0.9923 | 0.809–0.918 | 0.686 | 1.53–1.71 | 1.764 | 0.325 | 0.633 | 992.9 | 7.823 | 1.08 | 3.67 |
| AFG2 | y = (731.35 ± 29.03)x – (14.08 ± 25.20) | 0.9917 | 0.740–0.946 | 0.686 | 1.20–1.74 | 1.764 | 0.386 | 0.633 | 1245.7 | 7.823 | 1.84 | 3.67 |
R2 correlation coefficient, (Wtest, Wcritical) Shapiro Wilk statistic, (Ctest, Ccritical) Cochran statistic, (Gtest, Gcritical) Grubbs statistic, (F, Fcritical) F statistic.
Shapino Wilk statistic (normality) : Values in bold represent the coefficients of Shapino Wilk test (W test for 5 levels) and critical value (W critical) at 1%.
Grubbs statistic (Maximum normed residual): Values in bold represent the coefficients of Grubbs test (G test for 5 levels) and critical value (G critical) at 1%.
Cochran statistic (Homogeneity of variances): Values in bold represent the coefficients of Cochran test and critical value (C critical) at 1%.
F statistic (Analysis of variance) : Values in bold represent F values and F critical for Regression, F values and F critical for lack-of-fit at %1.
3.2.2. LOD and LOQ
The LOD and LOQ for each aflatoxin were determined using signal-to-noise ratio (S/N) of 3 and 10 at the lowest validated level. Signal-to-noise ratio (S/N) for each compound was provided by Agilent MassHunter Software. The LODs and LOQs estimated in maize are listed in Table 3. A comparison of the obtained LODs and LOQs with the maximum limits set by EU for aflatoxins in maize (2 μg/Kg for AFB1 and 4 μg/Kg for total aflatoxins) reveals the suitability of the proposed method for the application of the regulation. This approach is the commonly adopted to estimate LOD and LOQ (Spanjer et al., 2008; Capriotti et al., 2010; Malachová et al., 2014; Zhao et al., 2016). Some authors mentioned linearity based method which consists of 3 and 10 times the standard deviation slope divided by the intercept of the calibration curve (Thompson et al., 2002; Paschoal et al., 2016). The LODs obtained in this study are slightly higher or comparable with those reported by other authors using MRM mode for the analysis of aflatoxins in maize without purification procedures (Spanjer et al., 2008; Beltran et al., 2009; Malachová et al., 2014).
Table 3.
Mean recoveries of aflatoxins spiked at levels in the range 0.45–10 μg/Kg in maize sample, Relative Standard Deviation value (RSD), limit of detection (LOD) and limit of quantification (LOQ). Aflatoxins detected in analyzed maize samples.
| Aflatoxins | Concentration (μg/Kg) | Mean recovery (%) | RSD (%) Intra-day precision (n = 4) |
RSD (%) Inter-day precision |
LOD (μg/Kg) | LOQ (μg/Kg) | Sample 1 | Sample 2 |
|---|---|---|---|---|---|---|---|---|
| AFB1 | 0.5 | 60.15 | 3.2 | 6.9 | 0.16 | 0.54 | <LOQ | <LOQ |
| 2.5 | 91.79 | 1.5 | 6.3 | |||||
| 5 | 99.80 | 1.6 | 2.4 | |||||
| 10 | 89.90 | 1.1 | 3.3 | |||||
| AFB2 | 0.47 | 62.62 | 7.6 | 7.6 | 0.11 | 0.36 | <LOQ | <LOQ |
| 2.35 | 62.13 | 2.4 | 4.8 | |||||
| 4.70 | 67.60 | 1.2 | 2.3 | |||||
| 9.40 | 62.10 | 0.9 | 1 | |||||
| AFG1 | 0.5 | 62.92 | 3.6 | 10.1 | 0.36 | 1.19 | <LOQ | <LOQ |
| 2.5 | 102.14 | 1.8 | 2 | |||||
| 5 | 109.40 | 1.7 | 1.9 | |||||
| 10 | 101.70 | 1.4 | 1.8 | |||||
| AFG2 | 0.45 | 76.60 | 10.8 | 10.8 | 0.16 | 0.52 | <LOQ | <LOQ |
| 2.25 | 80.86 | 2.9 | 4.8 | |||||
| 4.50 | 89.9 | 2 | 2.5 | |||||
| 9.00 | 82.2 | 1.6 | 1.6 |
3.2.3. Selectivity
The selectivity of the method was studied both by the specificity of the retention times and by the detection based on the monitoring of the MRM transition for each target analyte. No co-eluting peaks were observed in the appropriate retention time and this allows a selective determination of aflatoxin (AFB1, AFB2, AFG1 and AFG2).
3.2.4. Recovery and precision
Satisfactory recoveries were achieved in case of the addition of different concentration of aflatoxins (B1, B2, G1 and G2) in blank maize sample. For recoveries at concentrations below 1 μg/Kg, the four compounds were in the acceptable range of 50–120%. Concerning recoveries at levels between 1 and 10 μg/Kg, only recoveries obtained for AFB2 were slightly below the acceptable range of 70–110% (Commission Regulation (EC) No 401, 2006).
As shown in Table 3, the mean recoveries values were between 60.15 % and 109.40 % for AFB1 and AFG1 at concentrations of 0.5, 2.5, 5 and 10 μg/Kg. Recoveries ranged from 62.10 % to 67.60 % for AFB2 over the concentrations of 0.47, 2.35, 4.70 and 9.40 μg/Kg, and from 76.60 % to 89.9 % for AFG2 at concentrations of 0.45, 2.25, 4.5 and 9 μg/Kg. Relative standard deviations (RSD) values for the intra-day precision and inter-day precision were acceptable as they were below 20% (Commission regulation (EC), 2010).
These results for recoveries fulfil the requirements as regulated by the European Commission regulation EC 401/2006 (Commission Regulation (EC) No 401, 2006) at concentration levels in the range 0.45–10 μg/Kg except for AFB2, for which the recoveries were in the range of 62.10–67.60 %.
On the one hand, purification step can be avoided as shown in Table 3, especially for concentrations between 2 and 10 ppb. Therefore, this method will allow the application of the regulation (2 μg/Kg for AFB1 and 4 μg/Kg for total aflatoxins), saving time and expenses. On the other hand, extraction yields obtained for levels below 1 μg/Kg need some improvements and indicate that a specific purification step is recommended.
Recoveries for AFB1 and AFG1 in maize were comparable with those achieved by Soleimany et al. (2012), with recoveries of 106.1% and 85.8% for AFB1 and AFG1 at concentration level of 2.5 μg/Kg with a RSD under repeatability conditions of 7.1% and 8.4%, respectively. The authors also reported recoveries of 84.1 % for AFG2 and 89.9 % for AFB2 at concentration level of 0.75 μg/Kg. In the study developed by Sirhan et al. (2013) to determine aflatoxins in food, the recovery obtained in maize samples ranged from 66.9 % to 108.2 % with a RSD under repeatability conditions from 8.2 to 12 % at concentration levels from 2 to 9 μg/Kg for AFB1 and AFG1, from 2.7 to 4.5 μg/Kg for AFB2 and AFG2.
Analytical figures of merit obtained in this study such as limit of detection, limit of quantification and recoveries were summarized and compared with previously reported approaches (Table 4).
Table 4.
Analytical figures of merit of the determination of aflatoxins in maize compared with previously reported approaches: LOD, LOQ, Recovery (%) and RSD (% in the parentheses).
| Aflatoxins | LOD (μg/Kg) | LOQ (μg/Kg) | References | Approach used | Maximum limits | Spiked level (μg/Kg) | Recovery (%) | Spiked level (μg/Kg) | Recovery (%) | Spiked level (μg/Kg) | Recovery (%) | References | Performance criteria for aflatoxins (Recovery) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFB1 AFB2 AFG1 AFG2 |
0.16 0.11 0.36 0.16 |
0.54 0.36 1.19 0.52 |
Our result | Signal to noise ratio of 3 and 10 (S/N) | 2 μg/Kg for AFB1 and 4 μg/Kg for total aflatoxins in maize (Commission Regulation (EC) No 1881, 2006) | 0.50 0.47 0.50 0.45 |
60.15 (3.2) 60.62 (7.6) 62.92 (3.6) 76.60 (10.8) |
2.50 2.35 2.50 2.25 |
91.79 (1.5) 62.13 (2.4) 102.14 (1.8) 80.86 (2.9) |
5.00 4.70 5.00 4.50 |
99.8 (1.6) 67.6 (1.2) 109.4 (1.7) 89.9 (2.0) |
Our result | 60–120% for concentrations <1.0 μg/Kg 70–110% for concentrations 1–10 μg/Kg RSD <20% (Commission Regulation (EC) No 401, 2006; EC 2010) |
| AFB1 AFB2 AFG1 AFG2 |
0.50 1.00 1.00 0.40 |
- - - - |
Spanjer et al. (2008) | - 0.75 - 0.75 |
- 89.9 (9.2) - 84.1 (8.3) |
2.50 - 2.50 - |
106.10 (7.1) - 85.80 (8.4) - |
Soleimany et al. (2012) | |||||
| AFB1 AFB2 AFG1 AFG2 |
0.60 0.60 1.20 3.60 |
1.90 2.00 4.10 12.00 |
Malachová et al. (2014) | 0.50 - - - |
96.5 (4.7) - - - |
2.50 - - - |
103.8 (1.3) - - - |
5.00 - - - |
105.8 (5.6) - - - |
Zhao et al. (2016) | |||
| AFB1 AFB2 AFG1 AFG2 |
0.20 0.70 0.10 0.40 |
0.70 2.50 0.30 1.50 |
Beltràn et al. (2009) | - 0.6 - 0.6 |
- 103.7 (11.8) - 63.6 (2.5) |
2.00 - 2.00 - |
66.9 (8.2) - 74.70 (10.2) - |
Sirhan et al. (2013) | |||||
| AFB1 AFB2 AFG1 AFG2 |
0.117 0.141 0.176 0.211 |
0.391 0.469 0.586 0.703 |
Sirhan et al. (2013) | 2.00 2.00 2.00 2.00 |
108.00 (23) 120.00 (21) 101.00 (11) 96.00 (19) |
Beltrán et al. (2009) | |||||||
3.3. Application to real samples
The optimized method was applied to determine aflatoxins (AFB1, AFB2, AFG1 and AFG2) in two maize samples obtained from a local market (Agadir City, Morocco). Fig. 4B and C show the MRM chromatograms of two maize samples and aflatoxins were detected at levels below LOQ (Table 3). Therefore, these levels comply with the European Union regulation (Commission Regulation (EC) No 1881, 2006).
4. Conclusion
A HPLC-MS/MS method using ESI+ in MRM mode was optimized and validated for determination of aflatoxins. Ground and homogenized maize samples were extracted using a modified QuEChERS method without the need for pre-concentration or laborious purification procedure. The proposed method was easy to handle and requires smaller amounts of solvents. Firstly, chromatographic and mass spectrometric conditions in the positive ion mode were optimized in order to increase sensitivity. Secondly, the linearity was demonstrated for the four aflatoxins in the studied concentration ranges. The LOQs obtained were lower than the maximum levels set by EU. Satisfactory recoveries were achieved at concentrations below 1 μg/Kg for all compounds. Concerning the recoveries at concentrations between 1 and 10 μg/Kg, the extraction recoveries were in the acceptable ranges of 70–110 % except for AFB2. The use of triple quadrupole LC/MS system with the acquisition of MRM transitions provided good sensitivity and selectivity, as well as reliable confirmation and determination. Validation data in terms of linearity, limits of quantification (LOQ), recoveries, selectivity and precision showed that this method is acceptable to be used for routine analysis of aflatoxins in maize.
Declarations
Author contribution statement
Abdallah Ouakhssase: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Adil Chahid: Conceived and designed the experiments; Wrote the paper.
Hanane Choubbane: Contributed reagents, materials, analysis tools or data.
Abdelmajid Aitmazirt: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Elhabib Ait Addi: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors gratefully acknowledge Laboratory of Analysis and research (National Health Security Office Food Products, ONSSA) for technical support.
References
- Anastassiades M., Lehotay S.J., Stajnbaher D., Schenck F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003;86:412–431. [PubMed] [Google Scholar]
- Asao T., Büchi G., Abdel-Kadre M., Chang S., Wick E., Wogan G. Aflatoxins B and G. J. Am. Chem. Soc. 1963;85:1706–1707. doi: 10.1021/ja01082a031. [DOI] [PubMed] [Google Scholar]
- Beltran E., Ibanez M., Sancho J.V., Hernandez F. Determination of mycotoxins in different food commodities by ultra-high-pressure liquid chromatography coupled to triple quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. 2009;23:1801–1809. doi: 10.1002/rcm.4077. [DOI] [PubMed] [Google Scholar]
- Bennett J.W., Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campone L., Piccinelli A.L., Celano R., Russo M., Valdés A., Ibáñez C., Rastrelli L. A fully automated method for simultaneous determination of aflatoxins and ochratoxin A in dried fruits by pressurized liquid extraction and online solid-phase extraction cleanup coupled to ultra-high-pressure liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2015;407(10):2899–2911. doi: 10.1007/s00216-015-8518-4. [DOI] [PubMed] [Google Scholar]
- Capriotti A.L., Foglia P., Gubbiotti R., Roccia C., Samperi R., Laganà A. Development and validation of a liquid chromatography/atmospheric pressure photoionization-tandem mass spectrometric method for the analysis of mycotoxins subjected to commission regulation (EC) No. 1881/2006 in cereals. J. Chromatogr. A. 2010;1217:6044–6051. doi: 10.1016/j.chroma.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Cervino C., Asam S., Knopp D., Rychlik M., Niessner R. Use of isotope-labeled Aflatoxins for LC-MS/MS stable isotope dilution analysis of foods. J. Agric. Food Chem. 2008;56:1873–1879. doi: 10.1021/jf073231z. [DOI] [PubMed] [Google Scholar]
- Commission Decision (EC) No 657 Commission Decision 657/2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Union. 2002;221:8–36. [Google Scholar]
- Commission regulation (EC) Commission regulation (EU) No 178/2010 of 2 March 2010 amending regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union. 2010;L52:32. [Google Scholar]
- Commission Regulation (EC) No 1881 Commission Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union. 2006;L364:5–24. [Google Scholar]
- Commission Regulation (EC) No 401 Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union. 2006;70:12–34. [Google Scholar]
- Desmarchelier A., Oberon J., Tella P., Gremaud E., Seefelder W., Mottier P. Development and comparison of two multiresidue methods for the analysis of 17 mycotoxins in cereals by liquid chromatography electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2010;58:7510–7519. doi: 10.1021/jf100891m. [DOI] [PubMed] [Google Scholar]
- Devegowda G., Raju M., Swang H. Mycotoxins: novel solutions for their counteraction. Feedstuffs. 1998;70:12–15. [Google Scholar]
- Fedorowski J., LaCourse W.R. A review of post-column photochemical reaction systems coupled to electrochemical detection in HPLC. Anal. Chim. Acta. 2010;657(1):1–8. doi: 10.1016/j.aca.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Huertas-Pérez J., Arroyo-Manzanares N., Hitzler D., Castro-Guerrero F., Gámiz-Gracia L., Garcia Camparia A.M. Simple determination of aflatoxins in rice by UHPLC coupled to chemical post-column derivatization and fluorescence detection. Food Chem. 2018;245:189–195. doi: 10.1016/j.foodchem.2017.10.041. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on cancer (IARC) vol. 56. 1993. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins; p. 19. (Monographs on the Evaluation of Carcinogenic Risks to Humans). Lyon, France. [Google Scholar]
- Lattanzio V., Solfrizzo M., Powers S., Visconti A. Simultaneous determination of aflatoxins, ochratoxin A and fusarium toxins in maize by liquid chromatography/tandem mass spectrometry after multitoxin immunoaffinity cleanup. Rapid Commun. Mass Spectrom. 2007;21:3253–3261. doi: 10.1002/rcm.3210. [DOI] [PubMed] [Google Scholar]
- Li X., Liu B., Wang F., Ma X., Li Z., Guo D., Zhang S. Determination of 16 mycotoxins in maize by ultrahigh-performance liquid chromatography–tandem mass spectrometry. Anal. Lett. 2017 [Google Scholar]
- Malachová A., Sulyok M., Beltrán E., Berthiller F., Krska R. Optimization and validation of a quantitative liquid chromatography–tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chromatogr. A. 2014:145–156. doi: 10.1016/j.chroma.2014.08.037. [DOI] [PubMed] [Google Scholar]
- Miró-Abella E., Herrero P., Canela N., Arola L., Borrull F., Ras R., Fontanals N. Determination of mycotoxins in plant-based beverages using quechers and liquid chromatography-tandem mass spectrometry. Food Chem. 2017 doi: 10.1016/j.foodchem.2017.02.078. [DOI] [PubMed] [Google Scholar]
- NF T-90 210 . 2009. Protocole d'évaluation initiale des performances d'une méthode dans un laboratoire. [Google Scholar]
- Nonakaa Y., Saito K., Haniokaa N., Narimatsua S., Kataoka H. Determination of aflatoxins in food samples by automated on-line in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. J. Chromatogr. A. 2009;1216:4416–4422. doi: 10.1016/j.chroma.2009.03.035. [DOI] [PubMed] [Google Scholar]
- ONICL Importations des quatre céréales principales au Maroc. Bull. Inf. Marché Céréales Légumineuses. 2017 [Google Scholar]
- Panzeri D.V., Cesari I., Toschi, Pilu R. Seed calorific value in different maize genotypes. Energy Sources Part A. 2011;33:1700–1705. [Google Scholar]
- Paschoal F.N., Silva D.A., Souza R.S., Oliveira M.S., Pereira D.A., Souza S.C. A rapid single-extraction method for the simultaneous determination of aflatoxins B1, B2, G1, G2, Fumonisin B1, and Zearalenone in corn meal by ultra performance liquid chromatography tandem mass spectrometry. Food Anal. Methods. 2016;10(6):1631–1644. [Google Scholar]
- Proctor C.J., Todd J.F. Atmospheric pressure ionization mass spectrometry. Org. Mass Spectrom. 1983;18(12):509–516. [Google Scholar]
- Sirhan A.Y., Tan G.H., Al-Shunnaq A., Abdulra'uf L., Won R.C. QuEChERS-HPLC method for aflatoxin detection of domestic and imported food in Jordan. J. Liq. Chromatogr. Relat. Technol. 2014;37:321–342. [Google Scholar]
- Sirhan A., Tan G., Wong R. Determination of aflatoxins in food using liquid chromatography coupled with electrospray ionization quadrupole time of flight mass spectrometry (LC-ESI-QTOF-MS/MS) Food Control. 2013;31:35–44. [Google Scholar]
- Soleimany F., Jinap S., Abas F. Determination of mycotoxins in cereals by liquid chromatography tandem mass spectrometry. Food Chem. 2012;130:1055–1060. [Google Scholar]
- Spanjer M.C., Rensen P., Scholten J. LC-MS/MS multi-method for mycotoxins after single extraction, with validation data for peanut, pistachio, wheat, maize, raisins and figs. Food Addit. Contam. 2008;25:1–18. doi: 10.1080/02652030701552964. [DOI] [PubMed] [Google Scholar]
- Stora C., Dvorackova I., Ayraud N. Aflatoxin and Reye's syndrome. J. Med. 1983;14(1):47–54. [PubMed] [Google Scholar]
- Sulyok M., Berthiller F., Krska R., Schuhmacher R. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun. Mass Spectrom. 2006;20:2649–2659. doi: 10.1002/rcm.2640. [DOI] [PubMed] [Google Scholar]
- Takino M., Tanaka T., Yamaguchi K., Nakahara T. Atmospheric pressure photo-ionization liquid chromatography/mass spectrometric determination of aflatoxins in food. Food Addit. Contam. 2004;21(1):76–84. doi: 10.1080/02652030310001632538. [DOI] [PubMed] [Google Scholar]
- Thompson M., Ellison S., Wood R. Harmonized guidelines for single-laboratory validation of methods of analysis. Pure Appl. Chem. 2002;74:835–855. [Google Scholar]
- Trucksess M.W., Brumley W.C., Nesheim S. Rapid quantitation and confirmation of aflatoxins in corn and peanut butter, using a disposable silica gel column, thin layer chromatography, and gas chromatography/mass spectrometry. J. Assoc. Off. Anal. Chem. 1984;67:973–975. [PubMed] [Google Scholar]
- Van Egmond H.P., Schothorst R., Jonker M.A. Regulations relating to mycotoxins in food: perspectives in a global and European context. Anal. Bioanal. Chem. 2007;389:147–157. doi: 10.1007/s00216-007-1317-9. [DOI] [PubMed] [Google Scholar]
- Yogendrarajaha P., Van Poucke C., De Meulenaer B., De Saeger S. Development and validation of a QuEChERS based liquid chromatography tandem mass spectrometry method for the determination of multiple mycotoxins in spices. J. Chromatogr. A. 2013;1297:1–11. doi: 10.1016/j.chroma.2013.04.075. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Huang J., Ma L., Wang F. Development and validation of a simple and fast method for simultaneous determination of aflatoxin B1 and sterigmatocystin in grains. Food Chem. 2016 doi: 10.1016/j.foodchem.2016.10.036. [DOI] [PubMed] [Google Scholar]
- Zimmer D. Introduction to quantitative liquid chromatography-tandem mass spectrometry (LC-MS-MS) Chromatogr. Suppl. 2003;57:s325–s332. [Google Scholar]
- Zinedine A., Juan C., Soriano J. Limited survey for the occurrence of aflatoxins in cereals and poultry feeds from Rabat, Morocco. Int. J. Food Microbiol. 2007;115:124–127. doi: 10.1016/j.ijfoodmicro.2006.10.013. [DOI] [PubMed] [Google Scholar]