Abstract
Aroma profiles, key aroma compound quantification, and cluster analysis of 15 brands of extra-virgin olive oils (EVOOs) from three countries (Spain, Italy, and Greece) were investigated in the current study. Aroma compounds were isolated from the oil by using solvent-assisted flavor evaporation (SAFE) and solid-phase micro-extraction (SPME) and analyzed by gas chromatography-olfactometry mass spectrometry (GC-MS/O). A total of 89 compounds were screened by SPME/SAFE-GC-MS/O with chromatographic columns in 15 brands of samples. Eighty and 54 compounds were respectively identified by SPME- and SAFE-GC-MS/O. Of those, 44 compounds were detected by both methods. Undecanol, (Z)-4-decenal, (E)-2-dodecenal, and 2-nonanone extracted by SAFE were not found in EVOOs before. Eight classes of aroma compounds were identified, including 17 alcohols, 22 aldehydes, 9 ketones, 4 acids, 14 esters, 5 aromatics, 12 alkene, and 6 others. Eleven compounds were identified as the key aroma compounds in alternative brands of EVOOs by SAFE-aroma extract dilution analysis (AEDA). Hexanal, (E)-2-hexenal, (E)-3-hexenol, acetic acid, and (E)-2-heptenal were the common key aroma compounds by AEDA and odor activity values (OAVs). From the cluster analysis of the heatmap, the aroma compounds of all the Spain EVOOs were similar, and there were some differences from the samples of Italy and Greece. It suggested that both the amount and concentration of aroma compounds determine the similarity of aroma in EVOOs.
Keywords: extra-virgin olive oils (EVOOs), headspace solid-phase micro-extraction (SPME), solvent-assisted flavor evaporation (SAFE), cluster analysis, quantification
1. Introduction
Extra-virgin olive oil (EVOO) is a kind of high-grade edible vegetable oil obtained by cold mechanical extraction from fresh olives (Olea europaea) without use of solvents or refining methods [1]. It can be consumed in original form without refining, possessing good stability as well as nutritional and healthy features with respect to other vegetable oils [2]. Thanks to its excellent nutritional and organoleptic properties, EVOO has become one of the most popular oils in the world. The demand for it has increased rapidly in the past decade. In 2014, 3.05 million tons of olive oil was produced worldwide, and the production of olives increased to 19.27 million tons in 2016 [3].
Aroma is an important criterion for EVOO. Its characteristic flavor is one of the main f distinguishing it from the other edible vegetable oils [4]. Meanwhile, the identification of the compounds contributing to the aroma is considered to be a key for quality and authentication control. The aroma compounds of EVOO derive from the enzymatic reactions and autoxidation of unsaturated fatty acids; they occur by the lipoxygenase (LOX) pathway comprising mainly the actuation of LOX and hydroperoxide-lyase enzymes [5] after crushing and during malaxation, a basic step of EVOO extraction process, aimed to improve oil yield and quality [6,7]. From the qualitative and quantitative results, C6 aldehydes and alcohols and the corresponding esters are considered to be the most crucial aroma compounds of EVOO [8,9]. Reasonable amounts of various classes of C5 compounds are also contained in the aroma of EVOO [10].
Aroma of EVOO consists of a complex mixture of volatile compounds, which includes mainly aldehydes, alcohol, ketones, and esters [2,11,12]. (E)-2-Hexenal is the most important compound in olive oil, followed by hexanal and (E)-2-heptenal using the extraction methods of headspace SPME, purge and trap (P&T), simultaneous distillation and extraction (SDE), and headspace (HS) [2,11,13,14]. According to the flavor dilution (FD) factor, the most potent aromatic active compound is hexanal (FD = 512) in Fakhari olive oil, (FD = 256) in Touffehi oils, and (FD = 128) in Jemri olive oil [12]. (E)-2-Hexenal, (E)-2-hex-1-enol, (Z)-3-hexen-1-ol, and 1-hexanol were also identified in the virgin olive oils from Greece and Tunisia of Koroneiki variety using SPME-GC/MS [8]. Although the percentage of C6 alcohols [hexan-1-ol, (E)-2-hexen-1-ol, and (E)-3-hexen-1-ol] were higher than that of C6 aldehydes [hexanal and (E)-2-hexenal], C6 aldehydes contributed to the virgin olive oil from four cultivars greatly grown in southern of Tunisia with the lower thresholds [15]. C6 and C5 volatile compounds were considered to be the potent odorants in most studies previously, but there were some differences of varieties and content of the compounds because of the different extraction methods [2,11,13,14,16].
The selective adsorption coating of SPME results in the incomplete extraction of aroma compounds. The high temperature of SDE affects the accuracy during the extraction process. Solvent-assisted flavor evaporation (SAFE) has become a preferred method because of the low extraction temperature and high vacuum characteristics (about 10−5 Pa), which can extract more low-boiling compounds. SAFE allows the fast and careful isolation of volatiles from either solvent extracts of foods, oil samples, or even fruit pulps. Application of SAFE to model solutions of selected aroma compounds resulted in higher yield from both solvent extracts and fatty matrices (50% fat) compared to previously used techniques. Besides its efficiency in aroma isolation, the use of the equipment saves time and reduces costs due to the stability of the compact distillation unit [17]. The volatile profiles of pomelo flower, leaf, peel, and juice were comparatively analyzed using HS-SPME and SAFE [18]. To our knowledge, SAFE has not been applied to the aroma extraction of EVOO until now. In addition, the difference among EVOO brands from different countries was not evaluated previously. From this viewpoint, in this study, (1) SAFE coupled with aroma extract dilution analysis (AEDA) was applied to identify the key aroma compounds of 15 brands of EVOO from Spain, Italy, and Greece; (2) the most contributing compounds were quantified by standard curve method using selective ion monitoring (SIM) mode by SPME; (3) the EVOOs were classified based on their volatile compounds.
2. Results and Discussion
2.1. Aroma Compounds of 15 Brands of EVOOs Extracted by SPME and SAFE
Eighty-nine compounds were screened by SPME/SAFE-GC-MS/O with chromatographic columns of DB-Wax and DB-5 (Table 1). Eight classes of flavor compounds were identified in the 15 brands of EVOOs, including 17 alcohols, 22 aldehydes, 9 ketones, 4 acids, 14 esters, 5 aromatics, 12 alkene, 6 others. The important aroma compounds in EVOOs are the C6 compounds, which were generated from lipoxygenase pathway through a series of enzymatic acting on fatty acids, such as linoleic and linolenic acids initiated by the tissue disruption [5]. The hydroperoxide-lyase enzyme produces aldehydes, subsequently reduced to alcohols by the alcohol dehydrogenase enzyme [19].
Table 1.
All the flavor compounds identified in 15 EVOOs by SPME and SAFE.
| Compounds | CAS | LRI | Odor Property | ID 1 | Extraction Method | Brands of EVOOs 2 | |
|---|---|---|---|---|---|---|---|
| DB-Wax | DB-5 | ||||||
| Alcohols | |||||||
| (Z)-2-Pentenol | 1576-95-0 | 1157 | green, plastic | LRI, MS | SPME | 11 | |
| 1-Pentene-3-ol | 616-25-1 | 1157 | 686 | fruity, nut | LRI, MS, O | SPME/SAFE | 5, 10, 12-15/6, 8-10, 14, 15 |
| Pentanol | 71-41-0 | 1206 | balsamic | LRI, MS | SPME | 3, 6, 7 | |
| Eucalyptol | 470-82-6 | 1206 | mint, sweet | LRI, MS | SPME | 15 | |
| Hexanol | 111-27-3 | 1345 | 868 | resin, flower, green | LRI, MS | SPME/SAFE | 1-4, 6-9, 11-14/1-15 |
| (E)-3-Hexenol | 928-97-2 | 1376 | moss, fresh | LRI, MS, O | SAFE | 1-15 | |
| (E)-2-Hexenol | 928-95-0 | 1397 | 866 | green, leaf, walnut | LRI, MS | SPME/SAFE | 1, 9, 12/1, 5-15 |
| 2-Ethyl hexanol | 104-76-7 | 1481 | 1030 | rose, green | LRI, MS | SPME/SAFE | 4-6, 13, 14/4-15 |
| Linalool | 78-70-6 | 1538 | 1099 | flower, lavender | LRI, MS | SPME/SAFE | 5, 13/1-6, 9, 10, 13, 15 |
| Octanol | 111-87-5 | 1549 | chemical, metal, burnt | LRI, MS | SPME/SAFE | 2, 3, 8/1-3, 9, 10, 12-15 | |
| Nonanol | 143-08-8 | 1556 | 1172 | fat, green | LRI, MS | SPME | 2, 4, 7-10, 12-15 |
| Undecanol | 112-42-5 | 1653 | mandarin | LRI, MS | SAFE | 3, 6, 9, 10, 12-15 | |
| Benzyl alcohol | 100-51-6 | 1863 | 1038 | sweet, flower | LRI, MS | SPME/SAFE | 4, 7, 8, 12, 13, 15/1-5, 7-15 |
| Phenylethyl alcohol | 60-12-8 | 1899 | 1115 | honey, spice, rose, lilac | LRI, MS | SPME/SAFE | 1-15/1-15 |
| (Z)-2-Hexenol | 928-94-9 | 866 | leaf, green, wine, fruit | LRI, MS | SPME | 8, 15 | |
| (Z)-3-Hexenol | 928-96-1 | 1382 | 855 | grass | LRI, MS, O | SPME/SAFE | 1-15/1-15 |
| 1-Octen-3-ol | 3391-86-4 | 979 | mushroom | LRI, MS | SPME | 2, 5, 6, 12, 15 | |
| Aldehydes | |||||||
| 2-Methylbutyraldehyde | 96-17-3 | 905 | cocoa, almond | LRI, MS | SPME | 13 | |
| Hexanal | 66-25-1 | 1081 | 801 | grass, tallow, fat | LRI, MS, O, STD | SPME/SAFE | 1-15/1-15 |
| (E)-2-Pentenal | 1576-87-0 | 1126 | strawberry, fruit, tom | LRI, MS, O | SPME | 3-5, 12, 13, 15 | |
| (E)-2-Hexenal | 6728-26-3 | 1214 | 852 | apple, green | LRI, MS, O, STD | SPME/SAFE | 1-15/1-15 |
| (Z)-2-Hexenal | 505-57-7 | 848 | fat, rancid | LRI, MS, O | SPME | 7-12, 14, 15 | |
| Octanal | 124-13-0 | 1285 | 1003 | fat, soap, lemon, green | LRI, MS | SAFE | 1-15/1-3, 5-15 |
| (E)-2-Heptenal | 18829-55-5 | 1319 | 956 | soap, fat, almond | LRI, MS, O, STD | SPME/SAFE | 1-3, 5-13/1-3, 5-15 |
| 2,6-Dimethyl-5-heptenal | 106-72-9 | 1361 | fruit, green, melon | LRI, MS | SPME | 3 | |
| Nonanal | 124-19-6 | 1388 | 1105 | fat, citrus, green | LRI, MS, O | SPME/SAFE | 1-15/1-15 |
| (E)-2-Octenal | 2548-87-0 | 1425 | 1059 | green, nut, fat | LRI, MS, O | SPME/SAFE | 1-15/1-3, 5-8, 10-12 |
| (E,E)-2,4-Heptadienal | 4313-03-5 | 1456 | 998 | nut, fat | LRI, MS, O | SPME/SAFE | 1-15/1-15 |
| 2-Furanaldehyde | 98-01-1 | 1457 | bread, almond, sweet | LRI, MS | SPME | 3 | |
| Benzaldehyde | 100-52-7 | 1521 | almond, burnt sugar | LRI, MS | SPME | 1-12/15 | |
| Phenylacetaldehyde | 122-78-1 | 1044 | hawthorn, honey, sweet | LRI, MS | SPME | 1, 4, 6, 10, 14 | |
| (E)-2-Nonenal | 18829-56-6 | 1530 | 1161 | cucumber, fat, green | LRI, MS, O, STD | SPME/SAFE | 1-3, 5-15/1-3, 5-15 |
| (Z)-2-Decenal | 2497-25-8 | 1263 | tallow | LRI, MS | SPME | 10 | |
| (Z)-4-Decenal | 21662-09-9 | 1605 | green, must | LRI, MS | SAFE | 6, 11, 14 | |
| (E)-2-Decenal | 3913-81-3 | 1640 | 1264 | tallow | LRI, MS | SPME/SAFE | 1-9, 11-15/1-15 |
| 2-Undecenal | 2463-77-6 | 1748 | 1369 | soap, fat, green | LRI, MS | SPME/SAFE | 3/1, 6, 8, 14 |
| (E)-2-Dodecenal | 20407-84-5 | 1749 | green, fat, sweet | LRI, MS | SAFE | 3, 7 | |
| (E,E)-2,4-Decadienal | 25152-84-5 | 1803 | 1321 | fried, wax, fat | LRI, MS | SPME/SAFE | 1-3, 5-7, 9-13/1-3, 5-11, 13, 14 |
| Heptanal | 111-71-7 | 1181 | 902 | fat, citrus, rancid | LRI, MS | SPME/SAFE | 1-3, 5, 11, 13, 15/1, 3, 6, 7, 13, 15 |
| Ketones | |||||||
| 2-Pentanone | 107-87-9 | 977 | ether, fruit | LRI, MS | SPME | 1-6 | |
| 3-Pentanone | 96-22-0 | 980 | ether | LRI, MS | SPME | 4-8, 10, 13-15 | |
| 1-Penten-3-one | 1629-58-9 | 1017 | <800 | fish, pungent | LRI, MS, O | SPME | 4, 13-15 |
| 2-Octanone | 111-13-7 | 1281 | soap, gasoline | LRI, MS, O, STD | SPME/SAFE | 1, 3, 12, 13/3 | |
| 1-Octen-3-one | 4312-99-6 | 1302 | mushroom, metal | O, STD | SAFE | 1-15 | |
| 6-Methyl-5-hepten-2-one | 110-93-0 | 1331 | 987 | pepper, mushroom, rubber | LRI, MS, O, STD | SPME/SAFE | 1-15/1-15 |
| 2-Nonanone | 821-55-6 | 1404 | hot milk, soap | O, STD | SAFE | 3, 6-8, 13-15 | |
| 3,5-Octadien-2-one | 38284-27-4 | 1511 | 1072 | fruit, fat, mushroom | LRI, MS | SPME/SAFE | 1, 5-9, 11-15/1-10, 12-15 |
| Acetophenone | 98-86-2 | 1649 | must, flower, almond | LRI, MS | SPME | 2, 5 | |
| Acids | |||||||
| Acetic acid | 64-19-7 | 1435 | sour | LRI, MS, O, STD | SPME/SAFE | 1, 4, 5, 8, 11, 13, 15/1-13, 15 | |
| Propionic acid | 79-09-4 | 1524 | pungent, rancid, soy | LRI, MS, O | SPME/SAFE | 1-3, 5, 6, 9, 11-15/2, 3, 6-10, 12, 14, 15 | |
| Hexanoic acid | 142-62-1 | 1832 | 990 | sweat | LRI, MS, O | SPME/SAFE | 1, 7, 11, 13, 15/2, 3, 7, 9, 11, 13, 15 |
| Nonanoic acid | 112-05-0 | 1273 | green, fat | LRI, MS | SPME/SAFE | 1, 2, 5, 7, 9, 10, 12, 13, 15/1, 6, 8-10, 13-15 | |
| Esters | |||||||
| Ethyl acetate | 141-78-6 | <900 | pineapple | LRI, MS | SPME | 2-4, 8, 10, 13 | |
| Methyl hexanoate | 106-70-7 | 922 | fruit, fresh, sweet | LRI, MS, O | SPME | 3, 6 | |
| Ethyl Hexanoate | 123-66-0 | 1231 | apple peel, fruit | LRI, MS | SPME/SAFE | 2, 3, 6/2, 3, 5 | |
| Hexyl acetate | 142-92-7 | 1268 | 1013 | fruit, herb | LRI, MS | SPME/SAFE | 1-15/1-15 |
| (Z)-3-Hexenyl acetate | 3681-71-8 | 1311 | 1007 | green, banana | LRI, MS, STD | SPME/SAFE | 1-15/1-15 |
| Linalyl acetate | 115-95-7 | 1544 | sweet, fruit | LRI, MS | SPME | 5 | |
| Methyl benzoate | 93-58-3 | 1613 | 1098 | prune, lettuce, herb, sweet | LRI, MS, O | SPME/SAFE | 1-15/1-7, 9, 10, 12-14 |
| γ-Butyrolactone | 96-48-0 | 1626 | caramel, sweet | LRI, MS | SPME | 2, 3 | |
| Methyl octanoate | 111-11-5 | 1123 | orange | LRI, MS | SPME | 2, 3, 6 | |
| Ethyl benzoate | 93-89-0 | 1661 | 1172 | chamomile, flower, celery, fruit | LRI, MS | SPME/SAFE | 1-7, 9, 11/2-7, 9 |
| γ-Hexanolide | 695-06-7 | 1701 | coumarin, sweet | LRI, MS | SPME | 7 | |
| Methyl salicylate | 119-36-8 | 1769 | 1199 | peppermint | LRI, MS | SPME/SAFE | 4-10, 12-15/2-5, 7, 9-11, 13-15 |
| Nonyl ethanoate | 143-13-5 | 1308 | sweet, fruit | LRI, MS | SPME | 7 | |
| Butyl acetate | 123-86-4 | 815 | pear | LRI, MS | SAFE | 1, 4, 5 | |
| Aromatics | |||||||
| Methylbenzene | 108-88-3 | 1041 | <800 | paint | LRI, MS, O | SPME/SAFE | 1, 2, 4-8, 10, 12-15/1, 2, 5, 6 |
| 1,2-Dimethylbenzene | 95-47-6 | 1138 | 870 | geranium | LRI, MS, O | SPME/SAFE | 3, 5-8, 12, 13, 15/1-3, 5-15 |
| 1,3-Dimethylbenzene | 108-38-3 | 1182 | 861 | plastic | LRI, MS, O | SPME/SAFE | 1-3, 5-8, 10-15/1, 2, 5, 7, 9, 10 |
| 1,2,4-Trimethylbenzene | 95-63-6 | 1277 | 969 | plastic | LRI, MS | SAFE | 1-3, 5, 6, 8-15/1, 5, 6, 8, 10, 12-15 |
| 1,2,4,5-Tetramethylbenzene | 95-93-2 | 1436 | rancid, sweet | LRI, MS | SPME | 1, 7 | |
| Terpenes | |||||||
| Cinene | 138-86-3 | 1029 | lemon, orange | LRI, MS | SPME | 1, 2, 7-9, 15 | |
| (+)-Dipentene | 5989-27-5 | 1198 | 1032 | citrus, mint | LRI, MS | SPME/SAFE | 3, 8, 10, 11, 13, 15/1-3, 6-11, 13, 15 |
| 3-Carene | 13466-78-9 | 1233 | 1049 | lemon, resin | LRI, MS, O | SPME | 1-4, 6-12, 14, 15 |
| (E)-Ocimene | 3779-61-1 | 1235 | sweet, herb | LRI, MS | SPME | 7 | |
| (Z)-3,7-Dimethyl-1,3,6-octatriene | 3338-55-4 | 1249 | 1054 | citrus, herb, flower | LRI, MS | SPME/SAFE | 1-13, 15/1-4, 9, 11, 15 |
| Perillen | 539-52-6 | 1116 | wood | LRI, MS | SPME | 2, 3, 6, 7, 9, 13, 15 | |
| Ethenylbenzene | 100-42-5 | 1252 | 892 | balsamic, gasoline | LRI, MS | SPME/SAFE | 1-3, 5, 6, 11/1-6, 11-15 |
| (-)-α-Cubebene | 17699-14-8 | 1495 | herb, wax | LRI, MS | SPME | 8, 15 | |
| (-)-α-Copaene | 3856-25-5 | 1495 | 1387 | wood, spice | LRI, MS | SPME/SAFE | 1-15/1-15 |
| 4-Methoxystyrene | 637-69-4 | 1675 | sweet | LRI, MS | SPME | 5 | |
| (+)-Valencene | 4630-07-3 | 1717 | green, oil | LRI, MS | SPME | 5 | |
| α-Farnesene | 502-61-4 | 1743 | 1512 | wood, sweet | LRI, MS, O | SPME/SAFE | 1-15/1-15 |
| Others | |||||||
| 2-Pentylfuran | 3777-69-3 | 1231 | green bean, butter | LRI, MS | SPME | 1, 3, 5, 11 | |
| Benzyl methyl ether | 538-86-3 | 1386 | 988 | metal | LRI, MS, O | SPME/SAFE | 2-6, 10/2, 6, 10 |
| Dimethyl sulfoxide | 67-68-5 | 1568 | garlic | LRI, MS | SPME/SAFE | 1-7, 11/2-5, 7, 11 | |
| Naphthalene | 91-20-3 | 1734 | 1189 | tar | LRI, MS | SPME/SAFE | 5,7, 11-15/2, 5-14 |
| 4-Ethylphenol | 123-07-9 | 2159 | phenol, spice | LRI, MS | SPME/SAFE | 3-6, 11/1-3, 5, 7, 8, 10, 11, 13 | |
| 4-Allylanisole | 140-67-0 | 1275 | licorice, anise | LRI, MS | SPME | 13 | |
1 Each compound was identified by comparing it with an authentic standard based on the following criteria: (i) matching linear retention index on the same column; (ii) mass spectrum on NIST 14.0 database; (iii) description of its odor description; (iv) injection of reference standards. 2 1-PL, 2-BDS, 3-OL, 4-BLN, 5-YGY, 6-OLWL, 7-OS, 8-ALCF, 9-MNN, 10-AN, 11-XBK, 12-DMDN, 13-MSWN, 14-AGL, 15-DNLE.
2.1.1. Alcohols
In this study, 17 alcohols were identified from the 15 brands of EVOOs (Table 1). Among them, hexanol, (E)-3-hexenol, (Z)-3-hexenol, and phenylethyl alcohol existed in all the 15 EVOOs. Compared to previous studies, these four compounds were also detected in virgin olive oils produced in Iran, Turkey, Spain, Greece, Tunisia, and Italy [2,8,11,12,15,16,20,21,22,23]. (E)-3-Hexenol and (Z)-3-hexenol are derived from the reduction of (E)-3-hexenal and (Z)-3-hexenal by the action of an alcohol dehydrogenase (ADH). They present the most obvious green, fresh, and grass note. However, these aroma compounds were not thought to be responsible for the significant effect on olive oil odor, owning to their high odor threshold values in oil [19,24]. 2-Ethyl hexanol, linalool, octanol, 1-nonanol, undecanol, and benzyl alcohol were found in most of the 15 brands of EVOOs. 2-Ethyl hexanol, octanol, 1-nonanol, and benzyl alcohol were also reported in the previous studies about EVOOs from Turkey, Spain, Greece, Tunisia [2,8,16,23]. Nonanol presents fat or green note, which also derives from lipoxygenase pathway of unsaturated fatty acids [19]. Linalool with the flower and lavender note was detected in the samples of Turkey [2], while undecanol was not found in previous studies on EVOO flavor.
2.1.2. Aldehydes
In this study, 22 aldehydes were identified from the 15 brands of EVOOs (Table 1). Among them, hexanal, (E)-2-hexenal, octanal, nonanal, (E,E)-2,4-heptadienal, and (E)-2-decenal were detected in all the 15 brands of EVOOs. Also, these compounds were reported in the previous studies on EVOOs from Iran, Turkey, Greece, Tunisia, and Italy [2,8,9,11,15,23]. The enzymatic breakdown of the 13-hydroperoxide of linolenic acid in leaf homogenates causes to produce the (E)-2-hexenal, while the action of the aldehyde-lyase generates hexanal [25]. Hexanal, (E)-2-hexenal, octanal, and nonanal present grass or green note, while (E,E)-2,4-heptadienal and (E)-2-decenal show the fat or tallow note. (E)-2-Heptenal, (E)-2-octenal, benzaldehyde, (E)-2-nonenal, and (E,E)-2,4-decadienal were existed in most of the 15 brands of EVOOs. These aroma compounds also found in the EVOOs from Turkey and Spain [2,16,23]. (E)-2-Heptenal, (E)-2-octenal, (E)-2-nonenal, and (E,E)-2,4-decadienal all possess the fat note in their flavor quality, and benzaldehyde showed almond or burnt sugar note. While the other compounds, for example (E)-2-pentenal, (Z)-2-hexenal, 2-furanaldehyde, and phenylacetaldehyde, only existed in some brands of EVOOs, most of the aldehydes have the low odor threshold values in common, which contribute to the whole flavor of EVOOs greatly. However, hexanal, (E)-2-hexenal, octanal, nonanal, (E,E)-2,4-heptadienal, and (E)-2-decenal were also reported as being potentially associated with off-flavor sensations and organoleptic defects in their elevated concentrations, such as winey, fusty, frozen, musty, or hay-wood as in note [26,27]. In the next research, the panel test will be used to verify if the 15 brands of EVOOs present olfactory defects due to the compounds above, because the perceived intensity of the defect can compromise the quality of the oils and determine a downgrading based on the law.
2.1.3. Ketones
In this study, 9 ketones were identified from the 15 brands of EVOOs (Table 1). Among them, 1-octen-3-one and 6-methyl-5-hepten-2-one, both with the mushroom note, were found in all the 15 brands of EVOOs. In the previous studies, 6-methyl-5-hepten-2-one was also reported in the virgin olive oil samples from Iran, Turkey, Spain, Tunisia [2,11,12,15,16,20,23,27]. While 1-octen-3-one was only detected in 1 kind of Turkish olive oils until now [22], 3-Pentanone and 3,5-octadien-2-one existed in the 15 brands of EVOOs comprehensively. 3-Pentanone showing ether note was also found in the samples of Spain, Greece, Tunisia [7,15,19,22,28], whereas 3,5-octadien-2-one with fruit, fat, and mushroom note only appeared in the 3 kinds of Turkish olive oils in the previous investigations [23]. 1-Penten-3-one, 2-octanone, 2-nonanone, and acetophenone existed in few samples of the 15 brands of EVOOs.
2.1.4. Acids
In this study, 4 acids were identified from the 15 brands of EVOOs, including acetic acid, propionic acid, hexanoic acid, and nonanoic acid (Table 1). These 4 acid compounds were not detected in all the 15 brands of EVOOs. They were all found in the study on Turkish olive oils as well [23]. Many kinds of virgin olive oils did not contain acid compounds [8,11,15,16,20,22,28]. Acetic acid was reported in the olive oil samples of Turkey, Tunisian [2,12,29]. Propionic acid and hexanoic acid existed in Iranian olive oil [11]. Nonanoic acid and acetic acid were related to fusty and winey-vinegary defects, respectively [26]. Negative aromas such as rancid, fusty, winey-vinegary, and frozen are sensory attributes of defective virgin olive oil recognized by the International Olive Council. In the next research, the panel test will also be applied to confirm if the 15 brands of EVOOs show olfactory defects due to nonanoic acid and acetic acid, as the oil quality will be compromised by the perceived intensity of the defect and lead to a downgrading.
2.1.5. Esters
In this study, 14 ketones were identified from the 15 brands of EVOOs (Table 1). Among these compounds, hexyl acetate, (Z)-3-hexenyl acetate, methyl benzoate, and methyl salicylate were found in all the 15 brands of EVOOs. They were also detected in the virgin olive oil from Italy, Greece, and Tunisia [8,9]. Besides, hexyl acetate and (Z)-3-hexenyl acetate were reported in the sample of Portugal, Turkey, Tunisia, and Spain, which possess the fruit and green note [2,12,13,15,20,22,23]. Methyl benzoate in the samples of Iran and Spain, and methyl salicylate in the ones of Tunisia and Turkey presented the herb and peppermint note [11,12,16,23]. Ethyl benzoate existed in several kinds of EVOOs with chamomile or flower note, which was reported in the samples from Iran [11].
2.1.6. Aromatics
In this study, 5 aromatics were identified from the 15 brands of EVOOs (Table 1). Thereinto, methylbenzene, 1,2-dimethylbenzene, 1,3-dimethylbenzene, and 1,2,4-trimethylbenzene were detected in most of the samples. In previous study, 1,2-dimethylbenzene and 1,3-dimethylbenzene with geranium and plastic note existed in the samples from Greece and Tunisia [8], and 1,2,4-trimethylbenzene with plastic note was found in the virgin olive oil from Spain and Italy as well [9,16,20,28]. Methylbenzene and 1,2,4,5-tetramethylbenzene with the paint and rancid note were not reported in the former investigations.
2.1.7. Terpenes
In this study, 12 terpenes were identified from the 15 brands of EVOOs (Table 1). (-)-α-Copaene and α-farnesene existed in all the 15 brands of EVOOs with the wood, spice, and sweet note. (-)-α-Copaene was reported in the virgin olive oil from Turkey, Spain, Greece, Tunisia, and Italy [2,8,9,16,23], and α-farnesene was found in the samples of Iran, Spain, Tunisia, and Italy as well [9,11,12,16,30]. (+)-Dipentene, 3-carene, (Z)-3,7-dimethyl-1,3,6-octatriene, and ethynylbenzene were detected in most of the 15 brands of EVOOs. 3-Carene with the lemon and resin note was also reported in the virgin olive oil of Spain [16,20]. While (E)-ocimene was only detected in the sample, OS were found in several kinds of olive oil from Spain, Tunisia, and Turkey [12,20,23].
2.1.8. Others
In this study, 6 other compounds were identified from the 15 brands of EVOOs (Table 1). All these compounds (2-pentylfuran, benzyl methyl ether, dimethyl sulfoxide, naphthalene, 4-ethylphenol and 4-allylanisole) only existed in some brands of samples. They had no contribution to the aroma of all the samples by FDs. Based on the studies before, there was no report about the compounds above in EVOOs. These six compounds were not the important aroma compounds in EVOOs.
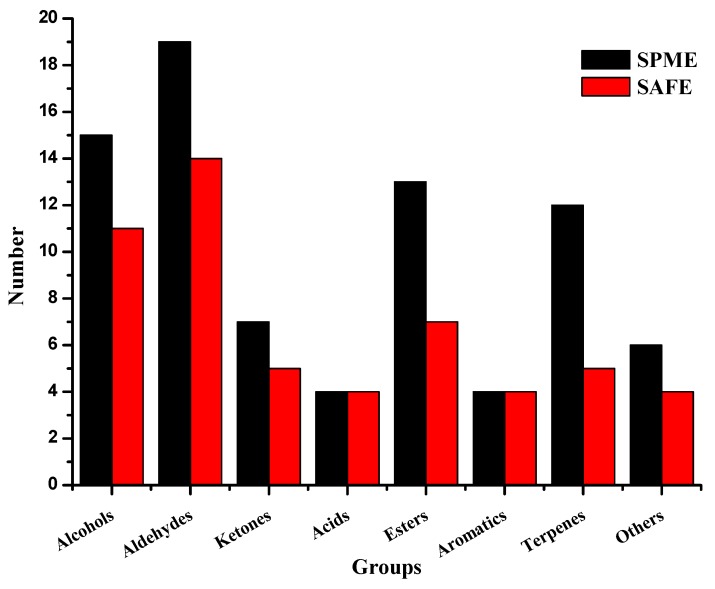
2.2. Comparison of Extraction Effect of SPME and SAFE
Eighty compounds in EVOOs were identified by SPME-GC-MS/O and 54 by SAFE-GC-MS/O method (Table 1). Forty-four compounds were detected by both methods. Two extraction methods (SPME and SAFE) had different extraction efficiency for different kinds of volatile compounds (Figure 1).
Figure 1.
Number of different groups of flavor compounds extracted by SPME and SAFE.
It can be seen from Figure 1 that more alcohols, aldehydes, ketones, esters, terpenes, and others were detected by SPME compared to SAFE. The same numbers of acids and aromatics were extracted by these two methods. The reasons can be the following: formyl groups of aldehydes and carbonyl groups of ketones were unstable, which could get oxidized or reduced in organic solvent. Alcohols in EVOOs are derived from the reduction of aldehydes by dehydrogenase, for example, from (E)-2-pentenal to 1-pentene-3-ol, from (E)-2-hexenal to (E)-2-hexenol, from octanal to octanol, which resulted in the more alcohols by SPME [31]. This result was in accordance with that of watermelon juice [32]. It indicated that SPME had better extraction effect on aldehydes, ketones, and alcohols just because of the different systems of EVOOs and watermelon juice.
Similarly, SPME could extract more esters than that by SAFE, while the result was just opposite in natto [33]. In natto, all the esters were ethyl esters, and there were more complicated constitutes of esters in EVOOs including ethyl, methyl, hexyl, and linalyl ester, which might result in the different extraction effect. SPME also had better extraction effect to terpenes. The previous studies showed that SPME with the fiber of DVB/CAR/PDMS (divinylbenzene/carbon/polydimethylsiloxane) had better extraction effect to terpenes compared to the other fibers (100 μm PDMS, 85 μm PA (polyacrylate), and 7 μm PDMS) or method (hydrodistillation), which confirmed the result in this study to some extent [34,35]. In conclusion, SPME had better extraction efficiency to the aroma compounds in EVOOs.
However, nine compounds were only extracted by SAFE, including (E)-3-hexenol, undecanol, octanal, (Z)-4-decenal, (E)-2-dodecenal, 1-octen-3-one, 2-nonanone, butyl acetate, and 1,2,4-trimethylbenzene. In particular, undecanol (mandarin), (Z)-4-decenal (green, must), (E)-2-dodecenal (green, fat, sweet), and 2-nonanone (hot milk, soap) were found in EVOOs for the first time to the best of our knowledge. All these compounds had a significant aroma property, and 2-nonanone was the key aroma compounds identified by AEDA. (E)-3-Hexenol and 1-octen-3-one reported in the previous studies were also the potent aroma compounds in this study.
2.3. Identification and Quantification of Key Aroma Compounds of 15 Brands of EVOOs
Eleven compounds were identified as the key aroma compounds in alternative brands of EVOOs by SAFE-AEDA (Table 2). FD value reflects the flavor contribution degree of each compound. Higher value indicates greater contribution to EVOO flavor. Among them, four compounds [hexanal (FD: 16 to 64), (E)-2-hexenal (FD: 2 to 64), 1-octen-3-one (FD: 4 to 32), and (E)-3-hexenol (FD: 0 to 8)] were the joint key aroma compound in the 15 brands of EVOOs, and the former two compounds contributed to the flavor greatly with the highest FD values, which was in accordance with the low odor thresholds of aldehydes. In particular, (E)-2-hexenal in all the Spain EVOOs had high FD (64 in PL, BDS, OL, and YGY/16 in BLN), suggesting its great contribution to Spain samples. The flavor character of hexanal and (E)-2-hexenal were grass, fat, apple, or green note represented the flavor of EVOOs to a great extent. In addition, the mushroom/metal note of 1-octen-3-one and moss/fresh note of (E)-3-hexenol also impacted the whole flavor of EVOOs significantly. The other three kinds of compounds, 2-octanone (FD: 0 to 16, does not exist in BLN, soap or gasoline note), (E)-2-heptenal (FD: 2 to 16, does not exist in PL, fat or almond note), and (E)-2-Nonenal (FD: 0 to 16, does not exist in BDS, cucumber and green note) affected the EVOO flavor notably. These three brands of EVOOs were both from Spain. Nonanal (FD: 0 to 8) and 2-nonanone (FD: 0 to 4) existed in most of Italy and Greece EVOOs, but they both did not consist in the Spain samples. It indicated that the EVOOs from Spain were lack of the flavor of fat, citrus, green, milk, or soap. Acetic acid (FD: 0 to 4, 16 in ALCF) and methyl benzoate (FD: 2 to 4, 8 in YGY) only existed in several brands of EVOOs from these three countries, and they had little effect to the flavor of EVOOs.
Table 2.
Key aroma compounds identified in 15 EVOOs by FD.
| Compounds | FD | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PL | BDS | OL | BLN | YGY | OLWL | OS | ALCF | MNN | AN | XBK | DMDN | MSWN | AGL | DNLE | |
| Hexanal | 32 | 32 | 16 | 64 | 16 | 16 | 64 | 32 | 32 | 64 | 32 | 32 | 64 | 32 | 32 |
| (E)-2-Hexenal | 64 | 64 | 64 | 16 | 64 | 16 | 8 | 8 | 16 | 16 | 64 | 8 | 4 | 4 | 2 |
| 2-Octanone | 4 | 2 | 4 | - | 4 | 2 | 4 | 16 | 2 | 4 | 0 | 4 | 8 | 4 | 4 |
| 1-Octen-3-one | 8 | 8 | 4 | 4 | 16 | 8 | 32 | 16 | 16 | 8 | 4 | 32 | 16 | 16 | 16 |
| (E)-2-Heptenal | - | 2 | 2 | 4 | 4 | 4 | 8 | 4 | 8 | 8 | 4 | 8 | 4 | 16 | 8 |
| (E)-3-Hexenol | 8 | 4 | 4 | 4 | 4 | 4 | 8 | 0 | 2 | 2 | 0 | 0 | 4 | 4 | 4 |
| Nonanal | - | - | - | - | - | 2 | 2 | 2 | 0 | 4 | 8 | - | 4 | 2 | 0 |
| 2-Nonanone | - | - | 0 | - | - | 4 | 4 | 0 | - | - | - | - | 2 | 0 | 2 |
| Acetic acid | 4 | 2 | 4 | - | 4 | - | 4 | 16 | 2 | 2 | 2 | 4 | 0 | - | - |
| (E)-2-Nonenal | 16 | - | 2 | 0 | 2 | 4 | 2 | 2 | 2 | 4 | 4 | 2 | 4 | 2 | 0 |
| Methyl benzoate | - | 2 | - | - | 8 | 4 | - | - | - | 2 | 4 | 2 | - | - | - |
Among these key aroma compounds identified by SAFE-AEDA, 1-octen-3-one could not be quantified because it had no peak in total ion chromatogram. 2-Octanone could be detected only in four samples by SAFE, so it could not be quantified by SPME with external standard; nonanal, 2-nonanone, (E)-2-nonenal and methyl benzoate could only be detected in several samples with the weak contribution (lower FD). Therefore, they were not quantified precisely. Except for the compounds above, all the other five compounds were quantified by external standard. Besides, (Z)-3-hexenyl acetate and 6-methyl-5-hepten-2-one were also identified as the key aroma compounds by SPME with internal standard, although they could not be sniffed by SAFE. Hence, all these seven compounds were quantified precisely (Table 3).
Table 3.
Standard curve, characteristic ions, and concentration of key aroma compounds in 15 EVOOs by external standard.
| Compounds | Ion Selection (m/z) | Standard Curve | R 2 | Concentration (mg/L) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PL | BDS | OL | BLN | YGY | OLWL | OS | ALCF | MNN | AN | XBK | DMDN | MSWN | AGL | DNLE | ||||
| Hexanal | 56.2, 72.2, 82.2 | y = 0.1546x | 0.9950 | 1.06 ± 0.17 | 0.92 ± 0.12 | 1.88 ± 0.39 | 0.64 ± 0.14 | 2.16 ± 0.22 | 1.23 ± 0.09 | 4.27 ± 0.03 | 1.69 ± 0.09 | 1.72 ± 0.03 | 1.84 ± 0.03 | 4.31 ± 0.10 | 1.37 ± 0.07 | 1.90 ± 0.04 | 1.76 ± 0.18 | 2.00 ± 0.11 |
| (E)-2-Hexenal | 69.2, 83.2, 98.2 | y = 0.5272x | 0.9953 | 0.30 ± 0.01 | 1.01 ± 0.14 | 0.33 ± 0.07 | 0.69 ± 0.11 | 1.58 ± 0.03 | 1.52 ± 0.18 | 4.61 ± 0.77 | 3.05 ± 0.12 | 2.67 ± 0.14 | 3.81 ± 0.12 | 3.92 ± 0.00 | 3.25 ± 0.46 | 1.66 ± 0.01 | 3.87 ± 0.17 | 6.99 ± 0.35 |
| (Z)-3-Hexenyl acetate | 67.2, 82.2 | y = 0.8552x | 0.9940 | 0.86 ± 0.09 | 2.22 ± 0.25 | 0.72 ± 0.16 | 2.32 ± 0.29 | 1.01 ± 0.02 | 1.87 ± 0.20 | 3.00 ± 0.65 | 1.19 ± 0.01 | 1.92 ± 0.19 | 2.22 ± 0.23 | 1.80 ± 0.01 | 2.10 ± 0.42 | 1.93 ± 0.09 | 3.70 ± 0.19 | 3.96 ± 0.08 |
| (E)-3-Hexenol | 55.2, 67.2, 82.2 | y = 0.3685x | 0.9974 | 0.72 ± 0.01 | 1.63 ± 0.24 | 0.63 ± 0.10 | 1.60 ± 0.16 | 1.32 ± 0.05 | 1.41 ± 0.09 | 1.69 ± 0.33 | 1.09 ± 0.00 | 1.19 ± 0.05 | 1.20 ± 0.15 | 0.83 ± 0.05 | 1.03 ± 0.10 | 0.74 ± 0.03 | 0.64 ± 0.91 | 0.83 ± 0.01 |
| Acetic acid | 60.2 | y = 0.1964x | 0.9970 | 2.32 ± 0.18 | 3.94 ± 0.58 | 2.21 ± 0.33 | 4.59 ± 0.38 | 2.14 ± 0.09 | 0.64 ± 0.05 | 3.05 ± 0.50 | 2.38 ± 0.10 | 3.07 ± 0.08 | 1.54 ± 0.18 | 0.82 ± 0.03 | 0.81 ± 0.04 | 0.86 ± 0.02 | 0.55 ± 0.03 | 1.21 ± 0.04 |
| (E)-2-Heptenal | 55.2, 83.2, 112.2 | y = 8.4945x | 0.9948 | 0.30 ± 0.01 | 0.13 ± 0.01 | 0.48 ± 0.03 | 0.06 ± 0.01 | 0.24 ± 0.02 | 0.36 ± 0.01 | 0.44 ± 0.04 | 0.26 ± 0.02 | 0.39 ± 0.01 | 0.31 ± 0.01 | 0.43 ± 0.03 | 0.43 ± 0.03 | 0.31 ± 0.01 | 0.14 ± 0.02 | 0.26 ± 0.01 |
| 6-Methyl-5-hepten-2-one | 69.2, 108.2, 126.2 | y = 30.406x | 0.9982 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.07 ± 0.01 | 0.02 ± 0.00 | 0.09 ± 0.01 | 0.06 ± 0.00 | 0.11 ± 0.01 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.08 ± 0.00 | 0.03 ± 0.00 | 0.08 ± 0.01 | 0.06 ± 0.00 | 0.03 ± 0.00 | 0.07 ± 0.00 |
As shown in Table 4, (Z)-3-hexenyl acetate showed the highest odor activity value (OAV), indicating the strong contribution to the flavor of EVOOs. (E)-2-heptenal, hexanal, acetic acid, and (E)-2-hexenal were also identified as the key aroma compounds based on OAVs by SPME. It was in accordance with the results by SAFE-AEDA. While (E)-3-hexenol and 6-methyl-5-hepten-2-one had little aroma contribution due to the lower OAVs, there were some differences between the results based on AEDA and OAV. Hence, the key aroma compounds could be identified precisely by both methods.
Table 4.
Odor activity values (OAVs) of key aroma compounds identified in 15 EVOOs.
| Compounds | Threshold [19] | OAV | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PL | BDS | OL | BLN | YGY | OLWL | OS | ALCF | MNN | AN | XBK | DMDN | MSWN | AGL | DNLE | ||
| Hexanal | 80 | 12 | 11 | 22 | 7 | 25 | 14 | 49 | 19 | 20 | 21 | 50 | 16 | 22 | 20 | 23 |
| (E)-2-Hexenal | 420 | 1 | 2 | 1 | 2 | 3 | 3 | 10 | 7 | 6 | 8 | 9 | 7 | 4 | 8 | 15 |
| (Z)-3-Hexenyl acetate | 6.9 | 115 | 296 | 96 | 309 | 135 | 249 | 400 | 159 | 256 | 296 | 240 | 280 | 257 | 493 | 528 |
| (E)-3-Hexenol | 1100 | <1 | 1 | <1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Acetic acid | 500 | 4 | 7 | 4 | 8 | 4 | 1 | 6 | 4 | 6 | 3 | 2 | 1 | 2 | 1 | 2 |
| (E)-2-Heptenal | 5 | 55 | 24 | 88 | 11 | 44 | 66 | 81 | 48 | 72 | 57 | 79 | 79 | 57 | 26 | 48 |
| 6-Methyl-5-hepten-2-one | 1000 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
2.4. Comparison of Aroma Compounds of 15 Brands of EVOOs
As shown in Table 5, the detection frequency of compounds was compared based on the kinds of EVOOs and producing areas. The detection frequency of alcohols in EVOOs of Italy (subtotal: 54) and Greece (subtotal: 58) were higher than that of Spain (subtotal: 39) generally. In particular, there were only five and seven kinds of alcohols in PL and BDS. There was a big difference on that of esters among the samples from these three countries, EVOOs of Spain showed the highest detection frequency (39). Similarly, for the other compounds, there was also the highest detection frequency from samples of Spain. While the ones of aldehydes, ketones, acids, aromatics, and terpenes were very close among the samples of these three areas, for the brand of sample, the range of detection frequency was from 41 to 59. The highest one (OL: 59) and the lowest one (BLN: 41) were both from Spain. The impact factor of aroma compound of EVOOs included the cultivar of olive, growing environment, producing technology, storage condition, and so on. From this aspect, these factors might impact EVOO aroma of Spain greatly.
Table 5.
The detection frequency of compounds based on the kinds of EVOOs and compound groups.
| Number (EVOOs of Spain) | Subtotal | Number (EVOOs of Italy) | Subtotal | Number (EVOOs of Greece) | Subtotal | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PL | BDS | OL | BLN | YGY | OLWL | OS | ALCF | MNN | AN | XBK | DMDN | MSWN | AGL | DNLE | ||||
| Alcohols | 5 | 7 | 9 | 8 | 10 | 39 | 10 | 9 | 11 | 12 | 12 | 54 | 8 | 12 | 12 | 11 | 15 | 58 |
| Aldehydes | 13 | 12 | 17 | 10 | 13 | 65 | 13 | 13 | 12 | 11 | 14 | 63 | 14 | 13 | 13 | 12 | 13 | 65 |
| Ketones | 5 | 5 | 6 | 6 | 6 | 28 | 6 | 5 | 5 | 3 | 4 | 23 | 3 | 4 | 7 | 6 | 6 | 26 |
| Acids | 4 | 4 | 3 | 1 | 3 | 15 | 3 | 4 | 3 | 4 | 3 | 17 | 3 | 3 | 4 | 2 | 4 | 16 |
| Esters | 5 | 9 | 10 | 7 | 8 | 39 | 8 | 7 | 5 | 5 | 5 | 30 | 5 | 4 | 5 | 4 | 3 | 21 |
| Aromatics | 4 | 4 | 3 | 1 | 4 | 16 | 4 | 3 | 4 | 3 | 4 | 18 | 3 | 4 | 4 | 4 | 4 | 19 |
| Terpenes | 7 | 8 | 7 | 5 | 4 | 31 | 7 | 8 | 7 | 7 | 5 | 34 | 6 | 5 | 6 | 4 | 9 | 30 |
| Others | 3 | 4 | 4 | 3 | 5 | 19 | 3 | 3 | 2 | 1 | 3 | 12 | 4 | 1 | 3 | 1 | 0 | 9 |
| Subtotal | 46 | 53 | 59 | 41 | 53 | 54 | 52 | 49 | 46 | 50 | 46 | 46 | 54 | 44 | 54 | |||
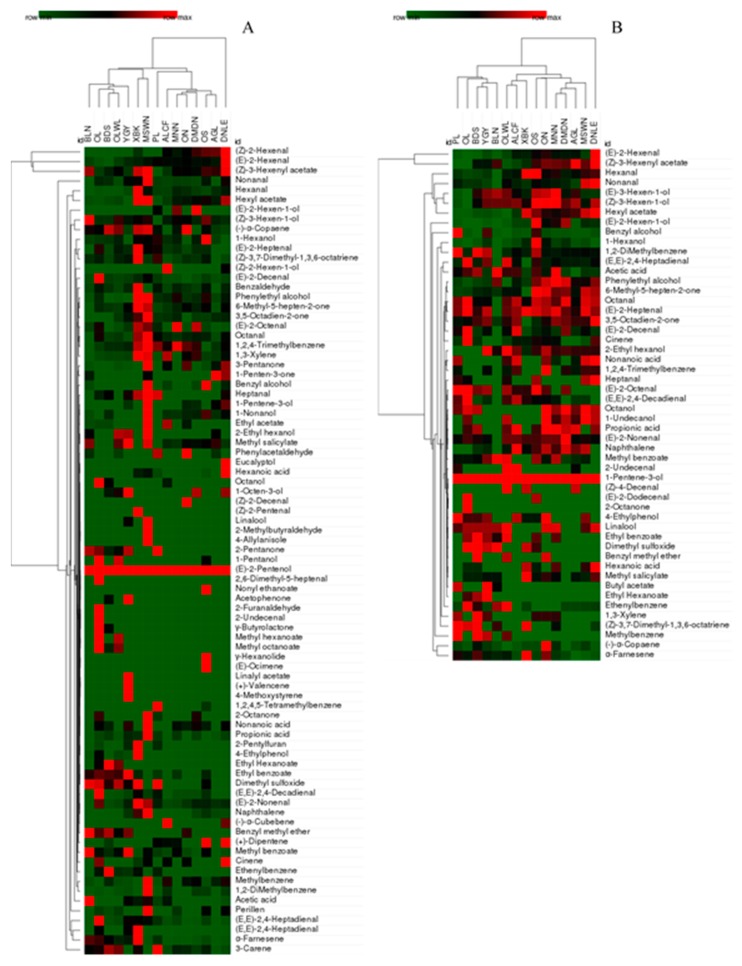
Grouping different types of oil is meaningful by flavor because it is an important criterion for EVOO. In the previous studies, cluster analyses were applied to distinguish the different types of edible oil based on their flavor. Sesame oils, soybean oils, and peanut oils could be completely classified using cluster analysis of volatiles [36]. Also, hierarchical cluster analysis showed similarities between EVOO, VOO and LOO (lampante olive oil) samples based on HS-GC-IMS fingerprints [37]. It can be seen from Figure 2, EVOOs were clustered in the heatmap based on their aroma compounds extracted by SPME and SAFE. From Figure 2A, EVOOs of BLN, OL, BDS, OLWL, and YGY had the similar aroma components, all these samples were from Spain except for OLWL. In addition, the flavor was similar between the samples from Italy (ALCF, MNN, AN, and OS) and Greece (DMDN and AGL). From Figure 2B, the aroma compounds of five brands of EVOOs from Spain (PL, OL, BDS, YGY, and BLN) were alike, and they were analogous between the ones from Italy (OS, AN, and MNN) and Greece (DMDN, AGL, and MSWN). Based on these results by SPME and SAFE, the flavor of Spain EVOOs was similar, and that of some of samples from Italy and Greece was alike. It indicated that there was big difference between samples of Spain and the other countries. These results seemed to contradict with that of detection frequency, but it just suggested that the similarity depended not only on the number of compounds but also on the content of ones.
Figure 2.
Heatmap indicating the aroma compounds from 15 EVOOs by SPME (A) and SAFE (B).
3. Materials and Methods
3.1. Samples
Fifteen varieties of EVOOs from the three largest export countries of olive oil in the world were purchased from the import supermarket. All the samples were authenticated by “Inspection and Quarantine Certificate of Entry Goods” from Entry-exit Inspection and Quarantine Bureau of the People’s Republic of China. EVOO samples were stored at 15 °C in dark. Specific brands were used in this study as follows, Spain: PL (MUELOLIVA), BDS (BETIS), OL (EURO GOLD), BLN (BELLINA), and YGY (IGAURIN); Italy: OLWL (OLIVOILÀ), OS (OUSA), ALCF (EMROW KITCHEN), MNN (MONINI), and AN (OLITALIA); Greece: XBK (HIPPOCRATES), DMDN (DIAMANDINO), MSWN (MESA VOUNOS), AGL (AEGLE), and DNLE (DONLIAR). All the EVOOs were imported with original packaging from the three countries mentioned above. The quality of all these samples complied with the “Trade Standard Applying to Olive Oils and Olive-Pomace Oils” (COI/T. 15/NC No. 3/Rev. 7 2013) by International Olive Council.
3.2. Chemicals
Dichloromethane, anhydrous sodium sulfate, n-hexane were purchased from Yifengtiancheng Scientific Instruments Co. Ltd. (Beijing, China); n-Alkanes (C7~C30) and 4-methyl-2-pentanol were purchased from Sigma-Aldrich (St Louis, MO, USA); Nitrogen (99.9992% purity) was purchased from Beijing Haipu Beifen Gas Industry Co. Ltd. (Beijing, China).
Standards of volatile compounds, hexanal, trans-2-hexenal, (Z)-3-hexenyl acetate, trans-2-heptenal, 6-methyl-5-hepten-2-one, (Z)-3-hexen-1-ol, acetic acid, 2-octanone, and 1-octen-3-one were purchased from Sigma-Aldrich Co. Ltd. (Santa Clara, CA, USA).
3.3. Aroma Extraction of EVOO by SPME
The method was in accordance with Wang et al. with minor modification [38]. After being selected, a manual SPME (Supelco, Inc., Bellefonte, PA, USA) with a 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) SPME fiber was used for volatile extraction after the fiber had been conditioned at 250 °C for 30 min. Ten milliliter of EVOO sample was quickly transferred into a 40 mL vial, 1 μL of 4-methyl-2-pentanol was added as an internal standard at the concentration of 401 μg/μL. After the equilibrium of 60 °C for 20 min, a stainless steel needle, housing the SPME fiber, was placed through the hole to expose the fiber at the position of 1 cm over the liquid surface for 40 min. The vials were sealed tightly with screw caps fitted with a Teflon/silicon septum. Vials were continuously swirled during SPME exposure with an agitation speed of 100 rpm.
3.4. Aroma Extraction of EVOO by SAFE
The method was in accordance with Usami et al. with minor modification [39]. Fifty milliliters of EVOO was transferred into 250 mL Teflon bottle, then 150 mL of dichloromethane and 1 μL of 4-methyl-2-pentanol was added as an internal standard at the concentration of 401 μg/μL. The bottle was placed in a shaker for 8h at 4 °C and 180r/min. The volatile compounds were separated from the solvent extracts using SAFE [17]. The filtrate was vacuum distilled using SAFE apparatus as previously described [40].
3.5. Gas Chromatography-Olfactometry-Mass Spectrometry (GC-MS/O) Analysis
The method was in accordance with Nuzzi et al. with minor modification [41]. The qualitative and quantitative analyses of the volatile compounds were conducted using Agilent 7890A gas chromatograph coupled with an Agilent model 7000B series mass spectrometer (GC-MS) and desorbed for 7 min in a split/splitless GC injection port, which was equipped with an inlet linear specific for SPME use (Agilent Technologies, Wilmington, DE, USA). The GC-MS was equipped with a sniffer 9000 Olfactometer (Brechbühler, Switzerland). The volatiles were separated on DB-5 and DB-Wax (30 m × 0.25 mm i.d. × 0.25 μm, J&W Scientific), a type of fused silica capillary columns.
The oven temperature was initially at 40 °C, held for 3 min, ramped at 5 °C/min to 200 °C, then ramped at 10 °C /min to 230 °C and held for 3 min, then baked at 250 °C for 3 min. The injection port and ionizing source were kept at 250 and 230 °C, respectively; the carrier gas was helium at 1.2 mL/min. The injector mode was splitless. Electron-impact mass spectra were generated at 70 eV, with an m/z scan range from 35 to 350 amu. Compounds were identified according to NIST 14.0 mass spectra libraries installed in the GC-MS equipment.
A sniffing port (Sniffer 9000) coupled to a GC-MS instrument was used for odor-active compound characterization. At the exit of the capillary column, the effluents were split 1:1 (by volume) into a sniffing port and a MS detector by employing the Agilent capillary flow technology; the transfer line to the GC/O sniffing port was held at 280 °C. GC/O was performed by three experienced panelists.
3.6. Aroma Extract Dilution Analysis (AEDA)
The highest sample concentration after SAFE was assigned with a FD factor of 1. The volatile components were stepwise diluted at the ratio of 1: 2 with dichloromethane, and aliquots of the dilutions (1 μL) were subjected to analysis. The process was stopped when aromas ceased to be detected by the evaluators. The result was expressed as the FD factor, which was the ratio of initial and final concentration of the odorant in the sample.
3.7. Identification of Volatile Aroma Compounds
The method was in accordance with Xu et al. with minor modification [42]. The chemical identification was performed using a mass spectrum database, the linear retention index (LRI), and aromatic characteristics by sniffing. Some important aroma compounds were identified by comparison with standard compounds. LRI was calculated using normal alkane series and compared with the references. Mass spectra identification was performed based on the NIST 2.0 mass spectra libraries. The formula for the calculation of LRI was (1).
| (1) |
where “n” represents the number of carbon atoms of n-paraffins; “tn” represents the retention time of n-paraffins Cn, “tn+1” represents the retention time of n-paraffins Cn+1, “ta” represents the retention of an unknown compound in the sample time (to be satisfied “ta” is between “tn” and “tn+1”).
3.8. Quantification of Volatile Aroma Compounds
SPME was used to extract the volatile aroma components in olive oil for quantification analysis. At the same time, two quantitative methods were used, including an internal standard method for total aroma compounds and an external standard method in SIM mode for the key aroma compounds. In the internal standard method, 4-methyl-2-pentanol (401 μg/μL in hexane) was added as an internal standard to the sample to calculate the target compound concentration.
| (2) |
where “CIS” represents the concentration of internal standard; “AIS” represents the peak area of the internal standard; “CX” represents the concentration of the target compound; “AX” represents the peak area of the target compound.
Hexanal, (E)-2-hexenal, (Z)-3-hexenyl acetate, (E)-2-heptenal, 6-methyl-5-hepten-2-one, (Z)-3-hexenol, and acetic acid were quantified using SIM mass spectrometry by standard curve method. The solutions of the mixture of 4-methyl-2-pentanol and reference compounds at different concentrations were prepared and analyzed by GC-MS. The standard curves were prepared by plotting the ratio of the peak areas of the reference compound relative to 4-methyl-2-pentanol against their concentration ratio.
3.9. Calculation of OAV
OAV was calculated using the following equation:
| (3) |
where Ci is the concentration of the compound in the watermelon juice and OTi is its odor threshold. Compounds with OAV equal to or greater than 1 actually contribute to aroma as an odor-active compound because they are above their odor threshold, whereas those with OAV smaller than 1 may not.
3.10. Statistical Analysis
Analysis of variance (ANOVA) were carried out by using the software SAS (SAS Institute Inc., Cary, NC, USA). The ANOVA test was performed for all experimental runs, to determine the significance at 95% confidence interval. All experiments were performed in triplicate. The cluster analysis was applied to the concentration of volatile compounds data, and it was conducted by using the software of Morpheus online (https://software.broadinstitute.org/morpheus/). In this process, hierarchical clustering was selected, “Euclidean distance” and “Average” were applied to calculate the distance of samples and groups.
4. Conclusions
Eighty-nine compounds were screened by SPME/SAFE-GC-MS/O with chromatographic columns of DB-Wax and DB-5 in 15 brands of EVOOs. Eighty compounds were identified by SPME-GC-MS/O and 54 by SAFE-GC-MS/O method. Forty-four compounds were detected by both methods. Undecanol, (Z)-4-decenal, (E)-2-dodecenal, and 2-nonanone were found in EVOOs for the first time only by SAFE. 2-Nonanone was the key aroma compounds identified by AEDA. SPME had better extraction efficiency to aroma compounds in EVOOs, while SAFE could extract the most effective components (seven key aroma compounds). Hence, it is feasible and advantageous that SAFE is applied to the aroma extraction of EVOOs.
Eight classes of flavor compounds were identified in the 15 brands of EVOOs, including 17 alcohols, 22 aldehydes, 9 ketones, 4 acids, 14 esters, 5 aromatics, 12 alkene, and 6 others. Eleven compounds were identified as the key aroma compounds in alternative brands of EVOOs by SAFE-AEDA. Key aroma compounds authenticated by AEDA had some differences from those by OAVs. The aroma compounds of Spain EVOOs were similar, and those of some of samples from Italy and Greece were alike.
Author Contributions
Q.Z. collected samples, drafted and revised the manuscript; S.L. carried out the laboratory work and performed statistical analysis; Y.L. conceived, designed and coordinated the study; H.S. helped to edited and revised the manuscript. All authors gave final approval for publication.
Funding
Financial support was provided by The National Key Research and Development Program of China (No. 2018YFD040050204), National Natural Science Foundation of China (No. 31871838), and Project of High-level Teachers in Beijing Municipal Universities (No. IDHT20180506).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Padilla M.N., Martinez-Rivas J.M., Perez A.G., Sanz C. Thermal inactivation kinetics of recombinant proteins of the lipoxygenase pathway related to the synthesis of virgin olive oil volatile compounds. J. Agric. Food Chem. 2012;60:6477–6482. doi: 10.1021/jf3016738. [DOI] [PubMed] [Google Scholar]
- 2.Kesen S., Kelebek H., Selli S. Characterization of the key aroma compounds in Turkish olive oils from different geographic origins by application of aroma extract dilution analysis (AEDA) J. Agric. Food Chem. 2014;62:391–401. doi: 10.1021/jf4045167. [DOI] [PubMed] [Google Scholar]
- 3.Food and Agriculture Organization, statistical database. [(accessed on 6 May 2019)]; Available online: http://www.fao.org/faostat/en/#data.
- 4.Kesen S., Kelebek H., Selli S. Characterization of the volatile, phenolic and antioxidant properties of monovarietal olive oil obtained from cv. Halhali. J. Am. Oil Chem. Soc. 2013;90:1685–1696. doi: 10.1007/s11746-013-2327-8. [DOI] [Google Scholar]
- 5.Sanchez-Ortiz A., Perez A.G., Sanz C. Synthesis of aroma compounds of virgin olive oil: Significance of the cleavage of polyunsaturated fatty acid hydroperoxides during the oil extraction process. Food Res. Int. 2013;54:1972–1978. doi: 10.1016/j.foodres.2013.03.045. [DOI] [Google Scholar]
- 6.Amirante P., Clodoveo M.L., Tamborrino A., Leone A. A New designer malaxer to improve thermal exchange enhancing virgin olive oil quality. Acta Hortic. 2012;949:455–462. doi: 10.17660/ActaHortic.2012.949.67. [DOI] [Google Scholar]
- 7.Amirante P., Clodoveo M.L., Tamborrino A., Leone A., Paice A.G. Influence of the Crushing System: Phenol Content in Virgin Olive Oil Produced from Whole and De-stoned Pastes. In: Preedy V.R., Watson R.R., editors. Olives and Olive Oil in Health and Disease Prevention. Elsevier; Amsterdam, The Netherlands: 2010. pp. 69–76. [Google Scholar]
- 8.Kandylis P., Vekiari A.S., Kanellaki M., Kamoun N.G., Msallem M., Kourkoutas Y. Comparative study of extra virgin olive oil flavor profile of Koroneiki variety (Olea europaea var. Microcarpa alba) cultivated in Greece and Tunisia during one period of harvesting. Lwt-Food Sci. Tech. 2011;44:1333–1341. doi: 10.1016/j.lwt.2010.12.021. [DOI] [Google Scholar]
- 9.Cecchi T., Alfei B. Volatile profiles of Italian monovarietal extra virgin olive oils via HS-SPME-GC-MS: Newly identified compounds, flavors molecular. Food Chem. 2013;141:2025–2035. doi: 10.1016/j.foodchem.2013.05.090. [DOI] [PubMed] [Google Scholar]
- 10.Salch Y.P., Grove M.J., Takamura H., Gardner H.W.J.P.P. Characterization of a C-5,13-cleaving enzyme of 13(S)-hydroperoxide of linolenic acid by soybean seed. Plant Physiol. 1995;108:1211–1218. doi: 10.1104/pp.108.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amanpour A., Kelebek H., Kesen S., Selli S. Characterization of aroma-active compounds in Iranian cv. mari olive oil by aroma extract dilution analysis and GC-MS-Olfactometry. J. Am. Oil Chem. Soc. 2016;93:1595–1603. doi: 10.1007/s11746-016-2906-6. [DOI] [Google Scholar]
- 12.Ben Brahim S., Amanpour A., Chtourou F., Kelebek H., Selli S., Bouaziz M. Gas chromatography-mass spectrometry-olfactometry to control the aroma fingerprint of extra virgin olive oil from three Tunisian cultivars at three harvest times. J. Agric. Food Chem. 2018;66:2851–2861. doi: 10.1021/acs.jafc.7b06090. [DOI] [PubMed] [Google Scholar]
- 13.Peres F., Jelen H.H., Majcher M.M., Arraias M., Martins L.L., Ferreira-Dias S. Characterization of aroma compounds in Portuguese extra virgin olive oils from Galega Vulgar and Cobrancosa cultivars using GC-O and GC x GC-ToFMS. Food Res. Int. 2013;54:1979–1986. doi: 10.1016/j.foodres.2013.06.015. [DOI] [Google Scholar]
- 14.Tahri K., Duarte A.A., Carvalho G., Ribeiro P.A., da Silva M.G., Mendes D., El Bari N., Raposo M., Bouchikhi B. Distinguishment, identification and aroma compound quantification of Portuguese olive oils based on physicochemical attributes, HS-GC/MS analysis and voltammetric electronic tongue. J. Sci. Food Agric. 2018;98:681–690. doi: 10.1002/jsfa.8515. [DOI] [PubMed] [Google Scholar]
- 15.Gargouri O.D., Ben Rouina Y., Ben Mansour A., Flamini G., Ben Rouina B., Bouaziz M. Comparative study of oil quality and aroma profiles from Tunisian olive cultivars growing in saharian oasis using chemometric analysis. J. Oleo Sci. 2016;65:1033–1044. doi: 10.5650/jos.ess15286. [DOI] [PubMed] [Google Scholar]
- 16.Vichi S., Guadayol J.M., Caixach J., Lopez-Tamames E., Buxaderas S. Comparative study of different extraction techniques for the analysis of virgin olive oil aroma. Food Chem. 2007;105:1171–1178. doi: 10.1016/j.foodchem.2007.02.018. [DOI] [Google Scholar]
- 17.Engel W., Bahr W., Schieberle P. Solvent assisted flavor evaporation-a new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Food Res. Technol. 1999;209:237–241. doi: 10.1007/s002170050486. [DOI] [Google Scholar]
- 18.Goh R.M.V., Lau H., Liu S.Q., Lassabliere B., Guervilly R., Sun J.C., Bian Y.L., Yu B. Comparative analysis of pomelo volatiles using headspace-solid phase micro-extraction and solvent assisted flavour evaporation. Lwt-Food Sci. Tech. 2019;99:328–345. [Google Scholar]
- 19.Kalua C.M., Allen M.S., Bedgood D.R., Bishop A.G., Prenzler P.D., Robards K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007;100:273–286. doi: 10.1016/j.foodchem.2005.09.059. [DOI] [Google Scholar]
- 20.Reboredo-Rodríguez P., González-Barreiro C., Cancho-Grande B., Simal-Gándara J. Dynamic headspace/GC–MS to control the aroma fingerprint of extra-virgin olive oil from the same and different olive varieties. Food Control. 2012;25:684–695. doi: 10.1016/j.foodcont.2011.12.005. [DOI] [Google Scholar]
- 21.Tura D., Failla O., Bassi D., Attilio C., Serraiocco A. Regional and cultivar comparison of Italian single cultivar olive oils according to flavor profiling. Eur. J. Lipid Sci. Tech. 2013;115:196–210. doi: 10.1002/ejlt.201200104. [DOI] [Google Scholar]
- 22.Reboredo-Rodriguez P., Gonzalez-Barreiro C., Cancho-Grande B., Simal-Gandara J. Concentrations of aroma compounds and odor activity values of odorant series in different olive cultivars and their oils. J. Agric. Food Chem. 2013;61:5252–5259. doi: 10.1021/jf400804m. [DOI] [PubMed] [Google Scholar]
- 23.Kesen S., Kelebek H., Sen K., Ulas M., Selli S. GC-MS-olfactometric characterization of the key aroma compounds in Turkish olive oils by application of the aroma extract dilution analysis. Food Res. Int. 2013;54:1987–1994. doi: 10.1016/j.foodres.2013.09.005. [DOI] [Google Scholar]
- 24.Morales M.T., Rios J.J., Aparicio R. Changes in the volatile composition of virgin olive oil during oxidation: Flavors and off-flavors. J. Agric. Food Chem. 1997;45:2666–2673. doi: 10.1021/jf960585+. [DOI] [Google Scholar]
- 25.Kiritsakis A.K. Flavor components of olive oil-a review. J. Am. Oil Chem. Soc. 1998;75:673–681. doi: 10.1007/s11746-998-0205-6. [DOI] [Google Scholar]
- 26.Zhu H., Wang S.C., Shoemaker C.F. Volatile constituents in sensory defective virgin olive oils. Flavour Frag. J. 2016;31:22–30. doi: 10.1002/ffj.3264. [DOI] [Google Scholar]
- 27.Romero I., García-González D.L., Aparicio-Ruiz R., Morales M.T. Validation of SPME–GCMS method for the analysis of virgin olive oil volatiles responsible for sensory defects. Talanta. 2015;134:394–401. doi: 10.1016/j.talanta.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 28.Reboredo-Rodriguez P., Gonzalez-Barreiro C., Cancho-Grande B., Simal-Gandara J. Effects of sedimentation plus racking process in the extra virgin olive oil aroma fingerprint obtained by DHS-TD/GC-MS. Food Bioprocess Tech. 2013;6:1290–1301. doi: 10.1007/s11947-011-0751-z. [DOI] [Google Scholar]
- 29.Tura D., Failla O., Bassi D., Pedo S., Serraiocco A. Cultivar influence on virgin olive (Olea europea L.) oil flavor based on aromatic compounds and sensorial profile. Sci. Hortic. 2008;118:139–148. doi: 10.1016/j.scienta.2008.05.030. [DOI] [Google Scholar]
- 30.Reboredo-Rodriguez P., Gonzalez-Barreiro C., Cancho-Grande B., Valli E., Bendini A., Toschi T.G., Simal-Gandara J. Characterization of virgin olive oils produced with autochthonous Galician varieties. Food Chem. 2016;212:162–171. doi: 10.1016/j.foodchem.2016.05.135. [DOI] [PubMed] [Google Scholar]
- 31.Bezysov A., Dubova H., Rogova N. New methods of plant selection for food aroma recovery aided by oxidation processes. Acta Univ. CibiniensisSer. E: Food Tech. 2015;19:15–26. doi: 10.1515/aucft-2015-0011. [DOI] [Google Scholar]
- 32.Liu Y., He C.C., Song H.L. Comparison of SPME versus SAFE processes for the analysis of flavor compounds in watermelon juice. Food Anal. Method. 2018;11:1677–1689. doi: 10.1007/s12161-018-1153-x. [DOI] [Google Scholar]
- 33.Liu Y., Su H., Song H.L. Comparison of four extraction methods, SPME, DHS, SAFE, versus SDE, for the analysis of flavor compounds in natto. Food Anal. Method. 2018;11:343–354. doi: 10.1007/s12161-017-1005-0. [DOI] [Google Scholar]
- 34.Lei F., Zheng S.L., Xin Y.S., Shu H.Z. Study of four coating materials of SPME fiber on extraction of aroma compounds in strawberry. Sci. Agric. Sin. 2010;43:4472–4481. [Google Scholar]
- 35.Bajer T., Ligor M., Ligor T., Buszewski B. Design of the extraction process for terpenes and other volatiles from allspice by solid-phase microextraction and hydrodistillation. J. Sep. Sci. 2016;39:769–775. doi: 10.1002/jssc.201500605. [DOI] [PubMed] [Google Scholar]
- 36.Zhao F.F., Liu J.K., Wang X.P., Li P.W., Zhang W., Zhang Q. Detection of adulteration of sesame and peanut oils via volatiles by GC×GC-TOF/MS coupled with principal components analysis and cluster analysis. Eur. J. Lipid Sci. Technol. 2013;115:337–347. doi: 10.1002/ejlt.201200133. [DOI] [Google Scholar]
- 37.Gerhardt N., Schwolow S., Rohn S., Pérez-Cacho P.R., Galán-Soldevilla H., Arce L., Weller P. Quality assessment of olive oils based on temperature-ramped HS-GC-IMS and sensory evaluation: Comparison of different processing approaches by LDA, kNN, and SVM. Food Chem. 2019;278:720–728. doi: 10.1016/j.foodchem.2018.11.095. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y., Song H.L., Zhang Y., Tang J.N., Yu D.H. Determination of aroma compounds in pork broth produced by different processing methods. Flavour Frag. J. 2016;31:319–328. doi: 10.1002/ffj.3320. [DOI] [Google Scholar]
- 39.Usami A., Nakahashi H., Marumoto S., Miyazawa M. Aroma evaluation of Setonojigiku (Chrysanthemum japonense var. debile) by hydrodistillation and solvent-assisted flavour evaporation. Phytochem. Anal. 2014;25:561–566. doi: 10.1002/pca.2528. [DOI] [PubMed] [Google Scholar]
- 40.Schirack A.V., Drake M.A., Sanders T.H., Sandeep K.P. Characterization of aroma-active compounds in microwave blanched peanuts. J. Food Sci. 2006;71:513–520. doi: 10.1111/j.1750-3841.2006.00173.x. [DOI] [Google Scholar]
- 41.Nuzzi M., Lo Scalzo R., Testoni A., Rizzolo A. Evaluation of fruit aroma quality: Comparison between gas chromatography-olfactometry (GC-O) and odour activity value (OAV) aroma patterns of strawberries. Food Anal. Method. 2008;1:270–282. doi: 10.1007/s12161-008-9039-y. [DOI] [Google Scholar]
- 42.Xu Y.Q., Wang C., Li C.W., Liu S.H., Zhang C.X., Li L.W., Jiang D.H. Characterization of aroma-active compounds of Pu-erh tea by headspace solid-phase microextraction (HS-SPME) and simultaneous distillation-extraction (SDE) coupled with GC-Olfactometry and GC-MS. Food Anal. Method. 2016;9:1188–1198. doi: 10.1007/s12161-015-0303-7. [DOI] [Google Scholar]