This editorial refers to ‘Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double-blind, sham-controlled CHART-1 clinical trial’†, by J. Bartunek et al., on page 648.
Two-thirds of all heart failure is due to ischaemic heart disease.1 These patients receive lifelong medication, experience significant morbidity and mortality, and often progress to heart transplantation or insertion of a left ventricular assist device,1,2 In terms of population impact, a successful regenerative medicine strategy will have enormous impact upon patients with heart failure, who number in the millions worldwide. The staggering impact upon patients and healthcare systems provides extraordinary impetus for the development of a regenerative approach to treat these patients.2,3 Indeed, the notion of affecting an improvement in left ventricular structure as a substrate for enhancing patients’ quality of life and functional capacity (ultimately leading to reducing morbidity and mortality) is extraordinarily attractive.2
Cell-based therapy: the future for heart failure patients
This quest has spawned several clinical investigative efforts to devise an effective and efficient way to deliver cell-based therapy to this large patient population with major unmet needs.4,5 This resulted in the testing of a vast array of cells (Table 1).2 Currently, there are few decisive successes in the field. However, all of the current efforts are small, with no published studies exceeding 200 patients, and very importantly lack standardization. For reference, an early important study in the development of biventricular pacing enrolled 453 patients.6
Table 1.
Cell types under investigation in cell-based therapy trials for heart failure
| Cell types | Application |
|---|---|
| Unfractionated BM-MNCs | Ischaemic heart failure and chronic heart failure |
| CD34+ stem cells | Chronic ischaemic failure |
| Mesenchymal precursor cells | Ischaemic heart failure |
| Adipose tissue derived MSCs | Ischaemic and non-ischaemic heart failure |
| Bone marrow-derived MSCs | Ischaemic and non-ischaemic heart failure |
| Cardiosphere-derived cells | Ischaemic heart failure |
| Cardiac c-Kit cells | Ischaemic heart failure |
| Cardiopoietic stem cells | Chronic heart failure |
| Induced pluripotent cell-derived cardiomyocytes | Chronic heart failure |
BM-MNCs, bone marrow-derived mononuclear cells; MSCs, mesenchymal stem cells.
The use of cardiopoietic stem cells in heart failure
In 2010, Behfar and colleagues demonstrated that mesenchymal stem cells (MSCs) could be guided to become cardiac progenitor cells in mice.7 Furthermore, this study demonstrated that there are individuals that harbour stem cells with unique reparative capabilities, which are characterized by high expression of Nkx2.5, Tbx5, Mesp-1, and Mef2C and had unique cardio reparative properties [cardiopoietic stem cells (CpSCs)].7 This study led to the design of a cardiac cocktail [transforming growth factor β1 (TGFβ1), bone morphogenetic protein 4 (BMP-4), Activin-A, retinoic acid, insulin-like growth factor-1 (IGF-1), fibroblast growth factor-2 (FGF-2), α-thrombin, and interleukin-6 (IL-6)] capable of guiding MSCs towards CpSCs.7 This pre- clinical study formed the basis for a clinical trial: the C-CURE (Cardiopoietic Stem Cell Therapy in Heart Failure) trial which implemented CpSCs in humans with ischaemic cardiomyopathy,8 demonstrating safety, as well as provisional efficacy including increased ejection fraction (EF), reduced left ventricular end-systolic volume (LVESV), and improved 6-min walk distance (6MWD) by cell therapy compared with standard of care.8
CHART-1 clinical trial
The C-CURE trial formed the basis for the CHART-1 trial, which is presented in the current issue of the journal.9 CHART-1 (The Congestive Heart Failure Cardiopoietic Regenerative Therapy) is a double-blind, sham-controlled study in patients with ischaemic heart failure that randomized 315 subjects into treatment or sham procedure.10 To date this is the largest trial of transendocardial stem cell injection (TESI) therapy in patients with ischaemic cardiomyopathy. Despite the promising results of C-CURE, the CHART-1 trial produced a negative result—no improvement was seen in the pre-specified composite endpoint relative to sham controls.
The primary endpoint utilized a Finkelstein–Schoenfeld hierarchical composite8 that encompasses all-cause mortality, worsening heart failure, Minnesota Living with Heart Failure Score (MLHFS), 6-MWD, LVESV, and EF.9 Of note, a subset of patients with ischaemic heart failure had favourable results provided that their left ventricular end- diastolic volume (LVEDV) was between 200 and 370 mL. In this subset of patients, an improvement in 6-MWD and reverse heart remodelling measured by LVESV were demonstrated. Although the overall results of this trial were neutral, the findings potentially form the basis for a larger study with a group of patients with greater LV remodelling.
Impact of negative clinical trials
Although negative studies can be disappointing, we learn and use them as a guide to help move a field forward. The first thing to be said is that negative clinical trials are far more common than positive ones, and have helped guide the field of heart failure therapeutics substantially over the past few decades. In fact, negative heart failure trials have assisted investigators in developing successful therapies and have helped greatly in modifying pathophysiological paradigms that form the basis for new therapeutic principles (e.g. targeting neurohormonal activation in heart failure).11
Factors for consideration
In the context of the CHART-1 results, it is valuable to take stock of the present state of the field, and consider some important factors regarding the state-of-the-art.
Cell preparations
There are multiple choices currently for cell preparation (Table 1), but almost no rigorous comparison studies. In order to identify the best therapeutic cells for each clinical application, there is a need for more direct cell comparison studies to be conducted in each specific heart condition. Several groups have conducted clinical trials with potentially promising results (Figure 1; Table 1). Despite promising early stage results, positive larger trials are lacking, with a notable exception in the Ixmyelocel-T trial.12 An example of a cell comparison trial is the TAC-HFT trial, which suggested greater potency of MSCs compared with autologous whole bone marrow; arguably such a finding enhances the successful design of subsequent trials. To date, the CpSCs have not been directly compared with other MSCs in pre-clinical or clinical studies, nor have autologous vs. allogenic comparisons been conducted.
Figure 1.
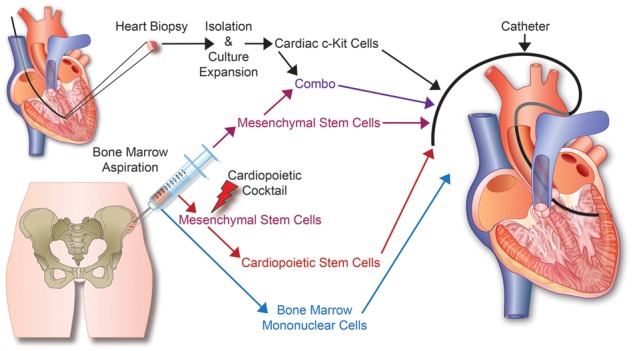
Stem cell therapy for heart failure: From tissue acquisition to stem cell expansion and delivery. Currently, autologous or allogeneic stems cells are derived from heart biopsies or bone marrow aspirations for various types of cell based therapies used in heart failure.
The quest to optimize cell potency is the underpinning of current trials that use cardiac stem cells or a combination of cardiac stem cells and MSCs in an attempt to enhance improvements in cardiac function, cell engraftment, and endogenous cardiac repair programmes,13–16 which in turn could augment clinical outcomes.
Autologous vs. allogeneic
The issue of using allogeneic vs. autologous cell therapy requires further exploration. On first principles, an allogeneic cell source can be mass-produced in a quality-controlled fashion, whereas autologous preparations are more cumbersome and costly. Importantly, when using autologous therapy, each patient is receiving a unique cell-based drug. CHART-1 employed an autologous strategy; in this trial 12% of patients failed to expand their own bone marrow MSCs and could not be treated if randomized to the active group. Importantly, the underlying disease process could impair endogenous stem cell compartments as it has been shown that chronic inflammation such as occurs in heart failure patients can impair stem cells and potentially decrease their effectiveness.17
Pre-clinical testing
Limited testing of the cardiopoietic cell has been performed with only one animal trial in mice. Although rodents have served as a great model for generating valuable data in cardiovascular biology, there are significant differences between mice and humans that limit extrapolation of results in to clinical trials.18 It is important to emphasize that pre-clinical trials, particularly in large animals, are crucial to the success of clinical trials, allowing investigators to work out issues with dosing and delivery before getting to the clinical stage.19 The CHART-1 study design could have been optimized potentially from additional large animal studies.
Dosing and delivery
A final and critical issue in the burgeoning field of cell-based therapy is dosing and delivery.19 Several doses have been compared, from 20 to 200 million cells. Paradoxically, some studies indicate that lower doses or concentrations could yield superior phenotypic outcomes relative to higher doses.4 In the case of the CHART-1 study, the target dose was 600 million cells.10 An analysis of dose vs. outcome will be of great value.
The other point of importance is the route of delivery, i.e. intracoronary infusion, intramyocardial injection, or transendocardial injection. In the case of chronic heart failure, intracoronary infusion may not be the best route as underperfused regions would not be treated. In order to get the cells to the ischaemic regions, intramyocardial or transendocardial injections are of greatest effect. The CHART-1 investigators opted for intramyocardial injection in order to ensure delivery to the ischaemic regions.10 Importantly, however, the CHART-1 study employed a unique injection catheter, not previously used in other clinical or pre-clinical studies.9
Conclusion
Although there is much to support enthusiasm for ongoing development efforts of cell-based therapeutics for heart failure, translation into positive late stage clinical trials with clinical efficacy has been a challenge. Here we have attempted to highlight some key areas of attention required for the field to advance, and have addressed issues of cell preparation, pre-clinical models, dose, and delivery for consideration. Important ongoing studies such as the CONCERT-HF (ClinicalTrials.gov Identifier: NCT02501811), BAMI [The Effect of Intracoronary Reinfusion of Bone Marrow-derived Mononuclear Cells (BM-MNC) on All Cause Mortality in Acute Myocardial Infarction (ClinicalTrials.gov Identifier: NCT01569178)], and DREAM-HF (ClinicalTrials.gov Identifier: NCT02032004) are underway which will further advance the field. While the CHART-1 trial produced a negative primary endpoint, this trial provides an important opportunity for the field to reappraise several critical issues that can enhance the success of future trials. Moreover, given the size of the trial, important hypothesis-generating subgroup analyses can be expected from the CHART-1 investigators that can further guide this field forward.
Conflict of interest: A.M.L. and J.L.H. disclose a relationship with Longeveron LLC that includes consulting. J.L.H. also discloses a relationship with Vestion Inc. that includes equity, board membership, and consulting, and is currently funded by the National Institutes of Health grant R01HL084275, R01HL107110, UM1HL113460, and R01HL110737; and grants from the Starr Foundation and the Soffer Family Foundation.
References
- 1. Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2016 update: a report from the American Heart Association. Circulation 2016;133:e38–360. [DOI] [PubMed] [Google Scholar]
- 2. Braunwald E. The war against heart failure: the Lancet lecture. Lancet 2015;385:812–824. [DOI] [PubMed] [Google Scholar]
- 3. Golpanian S, Wolf A, Hatzistergos KE, Hare JM.. Rebuilding the damaged heart: mesenchymal stem cells, cell-based therapy, and engineered heart tissue. Physiol Rev 2016;96:1127–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A.. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischaemic cardiomyopathy: the POSEIDON randomized trial. JAMA 2012;308: 2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM.. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischaemic cardiomyopathy: the TAC-HFT randomized trial. JAMA 2014;311:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J, MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845–1853. [DOI] [PubMed] [Google Scholar]
- 7. Behfar A, Yamada S, Crespo-Diaz R, Nesbitt JJ, Rowe LA, Perez-Terzic C, Gaussin V, Homsy C, Bartunek J, Terzic A.. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol 2010;56:721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A.. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol 2013;61:2329–2338. [DOI] [PubMed] [Google Scholar]
- 9. Bartunek J., Terzic A,, Davison B,, Filippatos G,, Radovanovic S,, Beleslin B,, Merkely B,, Musialek P,, Wojakowski W,, Andreka P,, Horvath I,, Katz A,, Dolatabadi D,, El Nakadi B,, Arandjelovic A,, Edes I,, Seferovic P,, Obradovic S,, Vanderheyden M,, Jagic N,, Petrov I,, Atar S,, Halabi M,, Gelev V,, Shochat M,, Kasprzak J,, Sanz Ruiz R,, Heyndrickx GR,, Nyolczas N,, Legrand V,, Guedes A,, Heyse A,, Moccetti T,, Fernandez-Aviles F,, Jimenez-Quevedo P,, Bayes-Genis A,, Hernandez-Garcia JM,, Ribichini F,, Gruchala M,, Waldman S,, Teerlink J,, Gersh B,, Povsic T,, Henry T,, Metra M,, Hajjar R,, Behfar A,, Alexandre B,, Seron A,, Gattis Stough W,, Sherman W,, Cotter G,, Wijns W.. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J 2017;38:648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bartunek J, Davison B, Sherman W, Povsic T, Henry TD, Gersh B, Metra M, Filippatos G, Hajjar R, Behfar A, Homsy C, Cotter G, Wijns W, Tendera M, Terzic A.. Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) trial design. Eur J Heart Fail 2016;18:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katz AM. The cardiomyopathy of overload: an unnatural growth response in the hypertrophied heart. Ann Intern Med 1994;121:363–371. [DOI] [PubMed] [Google Scholar]
- 12. Patel AN, Henry TD, Quyyumi AA, Schaer GL, Anderson RD, Toma C, East C, Remmers AE, Goodrich J, Desai AS, Recker D, DeMaria A, ixCELL-DCM Investigators. Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial. Lancet 2016;387:2412–2421. [DOI] [PubMed] [Google Scholar]
- 13. Sanganalmath SK, Bolli R.. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res 2013;113:810–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karantalis V, Suncion-Loescher VY, Bagno L, Golpanian S, Wolf A, Sanina C, Premer C, Kanelidis AJ, McCall F, Wang B, Balkan W, Rodriguez J, Rosado M, Morales A, Hatzistergos K, Natsumeda M, Margitich I, Schulman IH, Gomes SA, Mushtaq M, DiFede DL, Fishman JE, Pattany P, Zambrano JP, Heldman AW, Hare JM.. Synergistic effects of combined cell therapy for chronic ischaemic cardiomyopathy. J Am Coll Cardiol 2015;66:1990–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM.. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation 2013;127:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hatzistergos KE, Saur D, Seidler B, Balkan W, Breton M, Valasaki K, Takeuchi LM, Landin AM, Khan A, Hare JM.. Stimulatory effects of mesenchymal stem cells on cKit+ cardiac stem cells are mediated by SDF1/CXCR4 and SCF/cKit signaling pathways. Circ Res 2016;119:921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan L, Hu C, Chen J, Cen P, Wang J, Li L.. Interaction between mesenchymal stem cells and B-cells. Int J Mol Sci 2016;17:650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCall FC, Telukuntla KS, Karantalis V, Suncion VY, Heldman AW, Mushtaq M, Williams AR, Hare JM.. Myocardial infarction and intramyocardial injection models in swine. Nat Protoc 2012;7:1479–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Golpanian S, Schulman IH, Ebert RF, Heldman AW, DiFede DL, Yang PC, Wu JC, Bolli R, Perin EC, Moye L, Simari RD, Wolf A, Hare JM, Cardiovascular Cell Therapy Research Network. Concise review: review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem Cells Transl Med 2016;5:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]


