Abstract
Two California state prisons (A and B) have very high rates of coccidioidomycosis (Valley Fever). The prison health care service sought to improve their prevention strategy by risk stratification with a newly available spherulin-based Coccidioides delayed-type hypersensitivity test. Of the 36,789 voluntarily screened inmates, 4.7% experienced adverse reactions. A positive test (8.6% of those tested) was independently associated with (1) incarceration at prisons A and B, (2) admission to prison from a Coccidioides-endemic county, (3) length of stay at prisons A and B, and (4) increasing age. These findings suggest that the test is safe and performing well at risk stratification; the prison system now restricts inmates with negative tests from prisons A and B. This novel use of the test might benefit other coccidioidomycosis prevention programs.
Keywords: Valley Fever, coccidioidomycosis, Coccidioides, spherulin, hypersensitivity, prisoners
Introduction
Coccidioidomycosis (Valley Fever) is a disease caused by inhalation of Coccidioiodes spp., a fungus endemic to certain semiarid regions in the Americas. Although most patients requiring medical care have pulmonary disease, about 1% to 5% have disseminated disease that may require lifelong treatment or be fatal (Galgiani et al., 2016). Risk factors for severe disease include immunodeficiency, diabetes, advanced age, pregnancy, and African American or Filipino race/ethnicity.
Ten California Department of Corrections and Rehabilitation (CDCR) men’s prisons are located in Coccidioides endemic areas, including prisons A and B where coccidioidomycosis rates were 75 times higher than in non-endemic prisons and nearly 20 times higher than in Kern county (the county with the highest rate in California) in 2011 (California Department of Public Health, 2016; Wheeler, Lucas, & Mohle-Boetani, 2015). For more than a decade, prevention of this disease has been a high priority for California Correctional Health Care Services (CCHCS), the agency responsible for CDCR inmate health care.
Coccidioides exposure occurs by inhaling dirt that contains microscopic fungal spores. In endemic areas, complete avoidance of inhalation of contaminated particulates is impossible since the spores are unevenly distributed (Fisher, Bultman, Johnson, Pappagianis, & Zaborsky, 2007; Lacy & Swatek, 1974; Maddy, 1958), travel long distances (Flynn et al., 1979; Pappagianis & Einstein, 1978; Schneider et al., 1997), and may pass through ventilation filters and face masks (de Perio & Burr, 2014; Nicas & Hubbard, 2002). In 2007, CCHCS restricted inmates with immunocompromising medical conditions (e.g., HIV infection) from being incarcerated in endemic prisons (Wheeler et al., 2015). Despite this restriction, high morbidity persisted with a majority of coccidioidomycosis cases occurring in prisons A and B. In 2013, CCHCS restricted other groups at high risk of disseminated disease (African Americans and Filipinos) and severe pulmonary disease (diabetes mellitus [DM]) from prisons A and B (Plata v. Brown, 2013).
An alternative approach to prevention is to restrict non-immune persons from areas with a high likelihood of exposure. Although prior infection confers lifelong immunity, less than 40% of infected persons are symptomatic. Since most have undiagnosed, self-limited illnesses, those with diagnosed coccidioidomycosis are a small fraction of those with immunity. Because humoral immunity is not sustained, standard serologic testing detects only those with recent infections. In contrast, tests detecting a cell-mediated immune response (e.g., tests for delayed type hypersensitivity [DTH]) can detect past infections (and patients with presumed lifelong immunity).
In 2013, the Centers for Disease Control and Prevention (CDC) developed a statistical model comparing two strategies for reducing the risk of coccidioidomycosis to CDCR inmates (Purfield, Derado, Mohle-Boetani, Wheeler, & Park, 2014). The model suggested that coccidioidomycosis incidence could be reduced by employing a test-based strategy to identify inmates with previous exposure, using a Coccidioides DTH skin test. According to CDC’s model, populating prisons A and B with inmates with positive DTH skin tests would result in an estimated 61% decline in coccidioidomycosis incidence, substantially more than the 10% expected decrease from restriction based on race/ethnicity and clinical criteria.
Coccidioides DTH tests have not been available since 1999, but a new test, Spherusol®, was developed and approved by the Food and Drug Administration (FDA) in 2014 (Neilsen BioSciences, Inc., 2013; Wack, Ampel, Sunenshine, & Galgiani, 2015). Although Spherusol® was approved for persons with a history of pulmonary coccidioidomycosis, past skin tests had been widely used for establishing Coccidioides exposure history (e.g., among military personnel training in the San Joaquin Valley Army airfields in the 1940s; Smith, Beard, Whiting, & Rosenberger, 1946). These seminal studies established that Coccidioides infections could be asymptomatic and that individuals with positive skin tests were protected from clinical infections (Smith, Beard, Rosenberger, & Whiting, 1946).
In 2015, based on the history of the skin test’s performance as an indicator of immunity as well as evidence that the new test was safe, sensitive, and specific (Johnson et al., 2012; Neilsen BioSciences, Inc., 2013), CCHCS adopted a policy to screen inmates with the Coccidioides DTH skin test and exclude all inmates with negative skin test results and inmates with DM or of African American or Filipino race/ethnicity who refused testing from prisons A and B. To our knowledge, this CCHCS program is the first use of the skin test in risk stratification to reduce the risk of coccidioidomycosis in a specific population versus use in clinical settings (e.g., predicting treatment outcome). In this report, we describe the rates of test acceptance, test positivity, and adverse reactions, as well as factors associated with skin test positivity.
Method
Inmates in all of CDCR’s 33 men’s prisons were offered the skin test if they had no medical or custody restriction on being incarcerated at prisons A and B and were 18 to 64 years old (to comply with FDA Spherusol® approval parameters).
We defined a skin test that was read within 48 + 4 hours after administration as completed and a mean of > 5 millimeters (mm) of induration as a positive test result. We defined an adverse reaction as any localized or systemic signs or symptoms reported by the inmate or observed by the nurse at the time of test reading, and an immediate adverse reaction as signs or symptoms that occurred within 30 minutes of test administration.
We defined incarceration at prisons A and B as posing a high risk of coccidioidomycosis, at the eight other endemic prisons as medium risk, and at the non-endemic prisons as low risk. As a proxy for past community exposures, we used custody data indicating whether an inmate was ever admitted to CDCR from an endemic county. We defined endemic California counties as those with the highest coccidioidomycosis rates during 2009 to 2012 (California Department of Public Health, 2014). We defined Kern and Kings counties as posing a high risk for coccidioidomycosis; Fresno, San Luis Obispo, Tulare, and Madera counties as medium risk; and all other California counties as non-endemic.
During December 2014, nurse trainers instructed nurses and technicians on skin test placement, skin test reading procedures, and real-time documentation using a CCHCS custom-built computer application. Nurses at CDCR’s 33 men’s institutions then used standardized teaching materials, including brochures and a video, to educate inmates about the voluntary skin test and the new restriction policy.
On January 12, 2015, nurses and technicians offered eligible inmates the skin test and administered tests according to the Spherusol® package insert instructions, using a tuberculin syringe with a 0.5½-in. 26- to 27-gauge needle to inject 0.1 ml of Spherusol® intradermally into the volar surface of the arm (Nielsen Biosciences, 2013). Using millimeter rulers to measure the orthogonal diameters of any palpable induration, nurses and technicians read tests on January 14 and calculated the mean. At the time of reading, nurses and technicians questioned inmates about 10 specific signs and symptoms since test placement and offered a chance to report additional symptoms.
Statistical Analysis
We compiled demographic data, data on test acceptance, mean mm test results, and adverse reactions. We evaluated trends in acceptance by age group and positivity by race/ethnicity with the Cochran-Armitage test. To determine past CDCR institutional exposures, we examined custody data including each inmate’s history of prison(s) of incarceration and lengths of stay. For exposure during incarceration, we created separate variables for prison A, prison B, and any other endemic prison. For prison A, we categorized inmates into the following mutually exclusive categories based on their risk of exposure to Coccidioides: (1) incarceration at prison A during 2010 to 2011 (the years with the highest coccidioidomycosis rates) and other years, (2) incarceration at prison A in 2010 to 2011 only, (3) incarceration at prison A in other years only, and (4) never incarcerated at prison A. For prison B, we categorized inmates into the same mutually exclusive categories. We categorized incarceration at any other endemic prison as a binary variable (ever vs. never at another endemic prison). For exposure prior to incarceration, we categorized inmates in mutually exclusive categories based on their risk of exposure to Coccidioides: (1) ever admitted from a high-risk county, (2) ever admitted from a medium-risk county, and (3) never admitted from an endemic county. We collapsed inmate self-reported race/ethnicity on incarceration into six mutually exclusive categories: White (non-Hispanic), African American, Filipino, Hispanic, Asian/Pacific Islander, and Other (including multiracial). We compared test results by inmate age group (≤ 30, 31–45, 46–64 years), race/ethnicity, and institutional and community exposures.
We used logistic regression to test for association (unadjusted and adjusted) between coccidioidomycosis skin test positivity and categorical or continuous variables of interest as potential risk factors. All statistical tests were conducted at a significance level of .05. We applied a stepwise forward mode selection approach to identify our final model. We tested for effect modification for each pair of variables in the final model. We used SAS software (Version 9.4, ©2013 SAS Institute Inc., Cary, NC) for all statistical analyses.
Ethical Considerations
This voluntary screening program was adopted as policy. All data used for analysis were collected in the course of clinical practice and record keeping. Analyses of de-identified data were conducted for evaluation purposes and thus were considered nonresearch and exempt from institutional review board review.
Results
Of 112,829 inmates in 33 men’s institutions, 98,348 (87.1%) were eligible for skin test screening, and of these, 96,987 (98.6%) were offered the test. The test was accepted by 37,089 (38.2%) of those offered. Test acceptance differed between those who would not be placed in prisons A and B on refusal of testing (African American and Filipino inmates) and those who would (White [nonHispanic] and Hispanic inmates; 24.7% vs. 44.6%; OR = 0.41; 95 % CI [0.39, 0.42]). Skin test acceptance significantly increased with age, from 34.3% among inmates < 30 years of age to 38.0% among those aged 31 to 45 and 43.1% among those > 45 years of age (p < .001).
Of the 37,089 inmates who accepted testing, 36,789 (98.6%) completed the test. Nurses and technicians reported six (0.016%) inmates with immediate adverse reactions. Of the six, five were reported to have a wheal, itching, and/or pain at the injection site with no systemic reactions. The sixth patient, who was in his 40s with no underlying medical conditions, was reported to experience dizziness, nausea, dry mouth, and chest pain. The emergency medical responder who evaluated the patient in the prison’s treatment and triage area found him to be alert and oriented with normal vital signs. The inmate continued to be stable over the next days. He, and all of the others with immediate adverse reactions, reported on time for skin test reading on January 14, 2015. His test result, as well as those of all but one of the others with an immediate reaction, was negative.
At the time of test reading, nurses and technicians reported that 1,713 (4.7%) inmates experienced an adverse reaction (Table 1). The most common adverse reactions were itching (3.2%) or rash at the injection site (1.1%). The rate of adverse reactions varied by institution (median = 4.5%; range = 1.3–9.7%). Inmates with positive test results were significantly more likely than those with negative results to report an adverse reaction at the time of test reading (36.9% vs. 1.6%; OR = 35.7; 95% CI [31.9, 39.9]).
Table 1.
Coccidioides Skin Test Adverse Reactions Reported by 36,789 California State Prison Inmates, January 2015.
| Effect | N | % |
|---|---|---|
| Individuals with any adverse reaction | 1,713 | 4.7 |
| Itching at site | 1,180 | 3.2 |
| Itching elsewhere | 97 | 0.3 |
| Rash at site | 423 | 1.1 |
| Rash elsewhere | 42 | 0.1 |
| Pain at site | 299 | 0.8 |
| Necrosis at site | 8 | 0.0 |
| Fever | 56 | 0.2 |
| Chills | 73 | 0.2 |
| Nausea | 97 | 0.3 |
| Arthralgias | 67 | 0.2 |
| Other | 159 | 0.4 |
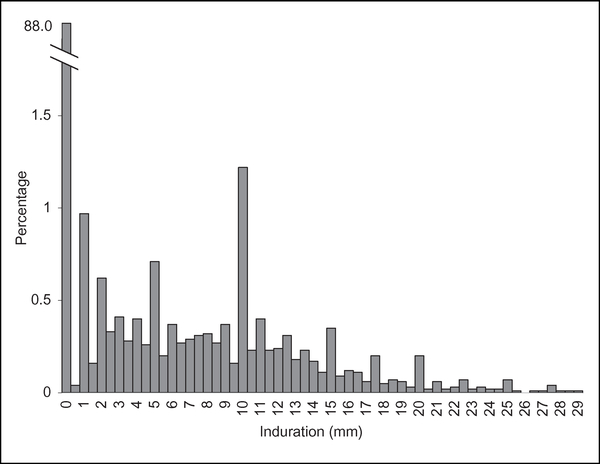
Of the 36,789 inmates who completed the test, 3,169 (8.6%) had a positive result. The average mean induration of the positive tests was 12 mm, and 99% of the mean indurations ranged from 5 to 29 mm (Figure 1). Positive skin tests were significantly less common among African American than White (non-Hispanic) inmates (8.0% vs. 9.1%; OR = 0.88; 95% CI [0.78,0.98]). The positive skin test rate increased significantly with age, from 4.9% among inmates who were < 30 years of age to 9.0% among those aged 31 to 45 and 11.1% among those > 45 years of age (p < .001).
Figure 1.
Coccidioides skin test indurationa size distribution among 36,789 California state prison inmatesb, January 2015. “Millimeters of induration were calculated as the mean of orthogonal diameters of induration at the skin test site and expressed in increments of 0.5 mm. bEighty inmates (0.21% of those tested) are not represented on the graph as they had mean indurations greater than 29 mm (range = 30–82 mm).
Across the 33 prisons, the median proportion of inmates with positive skin tests was 7.8% (range = 3.4–17.7%). The highest proportions were in inmates incarcerated at prisons A (17.7%) and B (15.3%) at the time of testing, and the lowest rates were in prisons in nonendemic areas (7.6%). A significantly higher proportion of inmates at prisons A and B, at the time of testing, had positive skin tests (16.2%) compared with those at non-endemic prisons (OR = 2.3; 95% CI [2.1, 2.6]). Similarly, a significantly higher proportion of inmates at prisons other than prisons A and B in endemic areas (8.8%) had positive skin tests compared with those at non-endemic prisons (OR = 1.2; 95% CI [1.1, 1.3]).
Comparing inmates by incarceration histories revealed that the odds of skin test positivity for inmates who had been incarcerated at prisons A or B as well as other endemic prisons were more than 6 times greater than for inmates who had never been incarcerated at an endemic prison (OR = 6.2; 95% CI [5.1, 7.5]; Table 2).
Table 2.
Positive Coccidioides Skin Tests Among California State Prison Inmates With Mutually Exclusive Incarceration Histories, January 2015.
| Exposure Prison | N | % | Unadjusted OR [95% CI] |
|---|---|---|---|
| Ever in A or B and in other endemic | 1,573 | 17.1 | 6.2 [5.1–7.5] |
| Ever in A or B/not in other endemic | 110 | 14.1 | 4.9 [3.8–6.4] |
| Ever in other endemic/not in A or B | 1,363 | 5.9 | 1.9 [1.6–2.3] |
| Non-endemic only | 123 | 3.2 | Reference |
Note. CI = confidence interval; OR = odds ratio.
The difference between test reactivity of African American inmates when compared to White (non-Hispanic) inmates became insignificant once race/ethnicity was adjusted for incarceration and pre-incarceration community exposures and age (Table 3). Incarceration at prison A was significantly associated with skin test positivity compared with no incarceration history at prison A, especially if the incarceration at prison A occurred during the epidemic years 2010 to 2011 and other years (OR = 3.3; 95% CI [2.8, 4.0]). Incarceration at prison B during 2010 to 2011 and other years also posed a greater risk of skin test positivity than no incarceration history at prison B (OR = 2.1; 95% CI [1.7, 2.7]). Admission to prison from any endemic county was significantly associated with skin test positivity when compared to admission from a non-endemic county only, and this was especially seen for inmates with admissions from highly endemic counties (OR = 2.6; 95% CI [2.3, 2.8]). Lengths of stay at prison A (OR = 1.4 per 2-year increment; 95% CI [1.3, 1.5]) and prison B (OR = 1.3; 95% CI [1.2, 1.4]) were associated with skin test positivity, as was increasing age (OR = 1.03 per 2-year increment; 95% CI [1.02, 1.04]).
Table 3.
Risk Factors for a Positive Coccidioides Skin Test Among California State Prison Inmates, January 2015.
| Characteristics | Positive N (%) | Unadjusted OR [95% CI] | Adjusted OR [95% CI] |
|---|---|---|---|
| Race/ethnicity | |||
| African American | 561 (8.0) | 0.9 [0.8–1.0] | 1.0 [0.9–1.1] |
| Hispanic | 1,567 (8.8) | 1.0 [0.9–1.1] | 1.1 [0.9–1.2] |
| Asian Pacific Islandera | 25 (6.2) | 0.7 [0.4–1.0] | 1.0 [0.7–1.5] |
| Other | 195 (8.0) | 0.9 [0.7–1.0] | 0.9 [0.8–1.1] |
| White (non-Hispanic) | 821 (9.1) | Reference | Reference |
| Incarceration at prison A | |||
| Prison A 2010–2011 and other years | 437 (35.9) | 8.0 [7.0–9.0] | 3.3 [2.8–4.0] |
| Prison A 2010–2011 only | 25 (13.5) | 2.2 [1.5–3.4] | 1.7 [1.1–2.6] |
| Prison A other years only | 644 (16.7) | 2.8 [2.6–3.1] | 1.8 [1.6–2.0] |
| Never at prison A | 2,063 (6.6) | Reference | Reference |
| Incarceration at prison B | |||
| Prison B 2010–2011 and other years | 226 (26.1) | 4.5 [3.8–5.2] | 2.1 (1.7–2.7] |
| Prison B 2010–2011 only | 14 (11.0) | 1.6 [0.9–2.7] | 1.4 (0.8–2.5] |
| Prison B other years only | 683 (13.7) | 2.0 [1.8–2.2] | 1.3 (1.2-1.5] |
| Never at prison B | 2,246 (7.3) | Reference | Reference |
| Incarceration at other endemic prison | |||
| Ever at other endemic prison | 2,936 (9.1) | 1.9 [1.6–2.2] | 1.3 [1.2–1.5] |
| Never at other endemic prison | 233 (5.1) | Reference | Reference |
| County admitted from | |||
| Ever high risk | 656 (19.2) | 3.1 [2.8–3.4] | 2.6 [2.3–2.8] |
| Ever medium risk (never high) | 184 (15.0) | 2.3 [1.9–2.7] | 2.1 [1.8–2.5] |
| Never from endemic county | 2,329 (7.2) | Reference | Reference |
| Prison A stay (units = 2 years) | 1.4 [1.3–1.5] | ||
| Prison B stay (units = 2 years) | 1.3 [1.2–1.4] | ||
| Age (units = 2 years) | 1.03 [1.02–1.04] | ||
Note. CI = confidence interval; OR = odds ratio.
Includes Filipino.
Discussion
This is the largest Coccidioides skin test screening ever performed with a goal of preventing morbidity and mortality and the first to use a spherulin-based test on a large scale. Almost 40,000 California inmates were screened with very few adverse events and no serious complications, and approximately 9% of screened inmates had positive skin tests. We found that skin test positivity was independently associated with history of incarceration at the prisons with the highest coccidioidomycosis rates, admission to prison from California’s Coccidioides-endemic counties, length of stay at prisons A and B, and increasing age. Most importantly for public health and safety, skin test screening has been integrated into routine opt-out tests for all inmates entering CDCR: Inmates with negative tests are restricted from incarceration at the two prisons with the highest coccidioidomycosis rates. Further evaluation is needed to determine the impact of using skin test results to medically restrict inmates from prisons A and B on morbidity and mortality (e.g., coccidioidomycosis rates and cost-benefit analysis).
Our results are consistent with earlier findings that suggested that the skin test is a reliable indicator of past exposure to Coccidioides. Specifically, the analysis demonstrates that (1) the years 2010 and 2011 were years of high Coccidioides exposures (CDC, 2013), (2) pre-incarceration residence in California counties in the Southern San Joaquin Valley poses a higher risk of infection than in other counties (Edwards & Palmer, 1957), and (3) race/ethnicity is not associated with skin test reactivity (Thorner, 1941; Willett & Weiss, 1945). The finding that older age was independently associated with infection was likely related to the amount of time an individual has spent in an endemic area, consistent with hypotheses previously proposed (Emmett, 1952; Fiese, 1958).
We found the newly available spherulin-based Coccidioides DTH skin test to be safe and to produce skin reactions similar to those associated with the previously available coccidioidin skin tests. We noted adverse reactions considerably lower than rates reported by the manufacturer, who reported that 85% of respondents complained of itching at the injection site at any time over 7 days. Of the six reported immediate reactions we identified, four involved localized wheals, itching, or pain at the test site, similar to immediate wheal and flair reactions reported in the past for coccidioidin-based skin tests (Smith et al., 1948) and for tuberculosis skin tests (Tarlo, Day, Mann, & Day, 1977). The average positive skin test reaction sizes (mean = 12 mm; range = 5–29+ mm) were consistent with previous reports for coccidioidin (mean = 10.6–11.1 mm; range = 5–24+ mm; Edwards & Palmer, 1957).
This is the first large-scale use of any Coccidioides skin test as a tool to identify non-immune individuals and reduce their exposure to infection based on a negative skin test result. Our analyses indicate that for the CDCR inmate population, largely composed of people from non-endemic metropolitan areas, the threat of exposure increases with time in the prison system, especially time spent in the two highest risk prisons. Therefore, it is incumbent upon CCHCS to take steps to mitigate high risks of exposure to Coccidioides. The strategy of screening a population with a skin test to identify those who have had a past infection, even if they were asymptomatic or had a selflimiting undiagnosed illness, permits risk stratification. Skin tests can be used to help communicate risk of infection; they could allow selection of skin test–positive persons for work details in highly endemic areas, reducing the risk that others would acquire an infection.
More than 60% of eligible inmates declined to participate in the Coccidioides screening program. This may reflect reluctance to accept an unfamiliar medical test or other considerations. The complex messages about the consequences of taking the test based on one’s race/ethnicity (i.e., Filipinos and African Americans were excluded from prisons A and B if they refused testing; all others were not) may have affected inmates’ decisions. However, the significantly lower uptake by inmates of African American and Filipino descent—inmates whose test refusals ensured that they would not be sent to these two prisons—as compared to inmates of White (non-Hispanic) and Hispanic descent, could indicate that many inmates understood the message and preferred to remain untested to ensure that they would be medically restricted from prisons A and B.
Our analysis was limited primarily by the difficulty in assessing possible Coccidioides exposures and length of time each inmate resided in endemic areas before incarceration. Pre-incarceration residence was based on the county of an inmate’s prosecution, a variable that may not be equivalent to the inmate’s county of residence. Therefore, community exposures may have been inadequately weighted and may have accounted for more or less risk than the model predicted. The imperfect measurements of community exposures do not invalidate the results, however. In fact, these flaws would likely bias results toward the null; hence, findings would likely have been strengthened had there been a way to precisely measure length of exposure in endemic areas.
We hope that the experience with this new skin test will provide helpful data for organizations that would like to develop a prevention strategy for coccidioidomycosis. Individuals or groups that could benefit from risk stratification including those in occupations that may require high-risk activities (e.g., digging in dirt) in Coccidioides endemic areas, such as archaeologists, agricultural workers, construction workers, and firefighters. Risk stratification could also be useful to individuals who plan to travel to endemic areas and who may be at high risk of severe disease. Coccidioidomycosis prevention strategies based on risk of infection (using skin test results) and risk of exposure (using coccidioidomycosis case surveillance) may be especially useful for other systems with residential facilities in endemic areas such as nursing homes, mental health hospitals, county jails, and state and federal prisons.
Acknowledgments
At California Correctional Health Care Services, we thank the Nursing Services Branch for developing and conducting Spherusol® administration training, the nurses and technicians for performing the testing, the Public Health Branch, Program Development Section for developing inmate educational materials, the public health nurses in the prisons for facilitating inmate outreach and educational sessions, the Quality Management Branch for determining mass screening eligibility, and the Information Services Technology Division for deploying a web-based application for real-time capture of skin test administration, adverse reactions, and reading results. Finally, we thank the CDCR administration and correctional officers for ensuring smooth and timely operations that allowed thousands of inmates to participate in the mass screening.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The authors disclosed no conflicts of interest with respect to the research, authorship, or publication of this article. For information about JCHC’s disclosure policy, please see the Self-Study Program.
References
- California Department of Public Health. (2014). Epidemiologic summary of coccidioidomycosis in California, 2009–2012. Retrieved from https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/CocciEpiSummary09-12.pdf
- California Department of Public Health. (2016). Yearly summaries of selected general communicable diseases in California, 2011–2015. Retrieved from https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/YearlySummRptsofSelectedGenCommDisinCA2011-2015.pdf
- Centers for Disease Control and Prevention. (2013). Increase in reported coccidioidomycosis—United States, 1998–2011. Morbidity and Mortality Weekly Report, 62, 217–221. [PMC free article] [PubMed] [Google Scholar]
- de Perio MA, & Burr GA (2014). Health hazard evaluation report: Evaluation of Coccidioides exposures and coccidioidomycosis infections among prison employees (NIOSH Report No. 2013–0113–3198). Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Retrieved from https://www.cdc.gov/niosh/hhe/reports/pdfs/2013-0113-3198.pdf [Google Scholar]
- Edwards PQ, & Palmer CE (1957). Prevalence of sensitivity to coccidioidin, with special reference to specific and nonspecific reactions to coccidioidin and to histoplasmin. Diseases of the Chest, 31, 35–60. [DOI] [PubMed] [Google Scholar]
- Emmett J (1952). Coccidioidin sensitivity among school children in Phoenix (skin test and x-ray survey). American Journal of Public Health and the Nation’s Health, 42, 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiese MJ (1958). Coccidioidomycosis. Springfield, IL: Charles C. Thomas. [Google Scholar]
- Fisher FS, Bultman MW, Johnson SM, Pappagianis D, & Zaborsky E (2007). Coccidioides niches and habitat parameters in the southwestern United States: A matter of scale. Annals of the New York Academy of Sciences, 1111, 47–72. doi: 10.1196/annals.1406.031 [DOI] [PubMed] [Google Scholar]
- Flynn NM, Hoeprich PD, Kawachi MM, Lee KK, Lawrence RM, Goldstein E,... Wong GA (1979). An unusual outbreak of windborne coccidioidomycosis. New England Journal of Medicine, 301, 358–361. [DOI] [PubMed] [Google Scholar]
- Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Geertsma F, Hoover SE,... Theodore N (2016). 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clinical Infectious Diseases, 63, e112–e146. doi: 10.1093/cid/ciw360 [DOI] [PubMed] [Google Scholar]
- Johnson R, Kernerman SM, Sawtelle BG, Rastogi SC, Nielsen HS, & Ampel NM (2012). A reformulated spherule-derived coccidioidin (Spherusol) to detect delayed-type hypersensitivity in coccidioidomycosis. Mycopathologia, 174, 353–358. doi: 10.1007/s11046-012-9555-6 [DOI] [PubMed] [Google Scholar]
- Lacy GH, & Swatek FE (1974). Soil ecology of Coccidioides immitis at Amerindian middens in California. Applied Microbiology, 27, 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddy KT (1958). The geographic distribution of Coccidioides immitis and possible ecologic implications. Arizona Medicine, 15, 178–188. [PubMed] [Google Scholar]
- Neilsen BioSciences, Inc. (2013). Highlights of prescribing information. Coccidioides immitis spherule-derived skin test antigen-Spherusol®. Retrieved from http://nielsenbiosciences.dmclientportal.com/files/Spherusol-full-prescribing.pdf [Google Scholar]
- Nicas M, & Hubbard A (2002). A risk analysis for airborne pathogens with low infectious doses: Application to respirator selection against Coccidioides immitis spores. Risk Analysis, 22, 1153–1163. [DOI] [PubMed] [Google Scholar]
- Pappagianis D, & Einstein H (1978). Tempest from Tehachapi takes toll or coccidioides conveyed aloft and afar. The Western Journal of Medicine, 129, 527–530. [PMC free article] [PubMed] [Google Scholar]
- Plata v. Brown. No. C01–1351 TEH. (Dist. Court, ND Cal June 24, 2013).
- Purfield A, Derado G, Mohle-Boetani J, Wheeler C, & Park B (2014). Preventing coccidioidomycosis (Valley Fever) at highly endemic prisons in California: Estimating the effect of a screening skin test to identify immune inmates. Open Forum Infectious Diseases, 1, S464. doi: 10.1093/ofid/ofu052.1275 [DOI] [Google Scholar]
- Schneider E, Hajjeh RA, Spiegel RA, Jibson RW, Harp EL, Marshall GA,... Werner SB (1997). A coccidioidomycosis outbreak following the Northridge, Calif, earthquake. Journal of the American Medical Association, 277, 904–908. [PubMed] [Google Scholar]
- Smith CE, Beard RR, Rosenberger HG, & Whiting EG (1946). Effect of season and dust control on coccidioidomycosis. Journal of the American Medical Association, 132, 833–838. [DOI] [PubMed] [Google Scholar]
- Smith CE, Beard RR, Whiting EG, & Rosenberger HG (1946). Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. American Journal of Public Health and the Nation’s Health, 36, 1394–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE, Whiting EG, Baker EE, Rosenberger HG, Beard RR, & Saito MT (1948). The use of coccidioidin. American Review of Tuberculosis, 57, 330–360. [DOI] [PubMed] [Google Scholar]
- Tarlo SM, Day JH, Mann P, & Day MP (1977). Immediate hypersensitivity to tuberculin. In vivo and in vitro studies. Chest, 71, 33–37. [DOI] [PubMed] [Google Scholar]
- Thorner JE (1941). Coccidioidomycosis: Relative values of coccidioidin and tuberculin testing among children of the San Joaquin valley. California and Western Medicine, 54, 12–15. [PMC free article] [PubMed] [Google Scholar]
- Wack EE, Ampel NM, Sunenshine RH, & Galgiani JN (2015). The return of delayed-type hypersensitivity skin testing for coccidioidomycosis. Clinical Infectious Diseases, 61, 787–791. doi: 10.1093/cid/civ388 [DOI] [PubMed] [Google Scholar]
- Wheeler C, Lucas KD, & Mohle-Boetani JC (2015). Rates and risk factors for coccidioidomycosis among prison inmates, California, USA, 2011. Emerging Infectious Diseases, 21, 70–75. doi: 10.3201/eid2101.140836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett FM, & Weiss A (1945). Coccidioidomycosis in southern California: Report of a new endemic area with a review of 100 cases. Annals of Internal Medicine, 23, 349–375. [Google Scholar]