Dear Editors,
The incidence of thrombosis is dramatically enhanced in sepsis patients and under conditions of hypoxia (reduced oxygenation), but the mechanisms that regulate sepsis-induced thrombosis are incompletely understood. Meanwhile, current treatments for sepsis-associated thrombosis may lead to increased bleeding or re-thrombosis. A better understanding of the mechanisms that control sepsis-induced thrombosis could lead to the development of novel treatments that aim to reduce thrombosis in sepsis patients.
Deep vein thrombosis has an annual incidence of approximately 1 in 500 in the general population [1], while the incidence of venous thromboembolism increases in sepsis patients to ~40% [2]. Mortality is also increased by more than 10% in sepsis patients with venous thromboembolism compared with thrombosis-free sepsis patients [2]. Notably, sepsis patients suffer from increased propensity for thrombosis in the pulmonary vasculature as well as the deep veins [3–6]. Sepsis not only leads to an increased risk of thrombosis in humans [1, 2], but also enhances thrombus formation in rodents [7–9]. Along with platelet-neutrophil aggregation and the formation of cross- linked fibrin, thrombosis involves endothelial activation, which is triggered by sepsis challenge [10]. Mechanisms that regulate sepsis-associated thrombus formation have been elucidated previously, including inflammatory cytokine- and toll-like receptor-induced thrombosis [7, 9, 11–13], but the effect of cell-specific and hypoxia-responsive signalling pathways on sepsis-induced thrombosis remains unclear.
Hypoxia signalling and sepsis-induced thrombosis
Thrombi are more likely to form under hypoxia compared with normoxia in experimental animal studies and humans [14–16]. Under hypoxic or inflammatory conditions, hypoxia-inducible factors 1α and 2α (HIF1α and HIF2α) accumulate and translocate to the cell nucleus, where they bind with HIFβ to form HIF1 and HIF2 respectively [17]. The active HIF1 or HIF2 complex then binds to the hypoxia-responsive element of its target genes, causing transcriptional upregulation [17]. Although HIF1α expression is reduced in the leukocytes of sepsis patients compared with healthy volunteers, this may be a consequence of chronic stimulation [18], and HIF1α expression is acutely induced by sepsis challenge in human monocytes [18] and murine macrophages [19]. Subsequently, HIF1 signalling is highly involved in the vascular response to sepsis challenge [20–22]. Cell- and species- specific expression patterns of the HIFα isoforms following acute versus chronic sepsis challenge could be investigated in future studies. Given that the vascular response to inflammatory and hypoxic stimuli is regulated by HIF1 and HIF2, future studies should also aim to investigate whether sepsis leads to increases in the production of factors that control thrombosis via increased activation of cell-specific HIFs. For example, it would be interesting to determine whether sepsis challenge leads to increases in endothelial and myeloid cell-specific HIF1α and HIF2α, which in turn increase the levels of HIF1 and HIF2 targets that control sepsis-induced thrombus formation (Fig 1). Endothelial and myeloid HIF1 and HIF2 targets include factors that are highly expressed during sepsis and regulate coagulation/thrombosis, such as pro-thrombotic tissue factor (TF) [23–25] and plasminogen activator inhibitor (PAI) 1 [26–28], and anti-thrombotic TF pathway inhibitor (TFPI) [29, 30] and matrix metalloproteinases (MMPs) 2 and 9 [17, 31, 32]. Despite evidence that systemic and local hypoxia stimulates sepsis-free thrombosis [16, 23, 33, 34], and that HIF1 activation could promote sepsis-free thrombosis [35] for instance via upregulation of TF and PAI1 [23, 36, 37], direct evidence for a role of cell-specific HIFs in sepsis-induced thrombosis is lacking.
Figure 1: Investigations of HIF signalling pathways in sepsis-induced thrombosis.

Proposed model of sepsis-induced thrombus formation. Abbreviations: EC, endothelial cell; Mac, macrophage; MMP, matrix metalloproteinase; PAI, plasminogen activator inhibitor; PMN, polymorphonuclear cell; TF, tissue factor; TFPI, tissue factor pathway inhibitor.
To elucidate the roles of endothelial or myeloid HIF1α or HIF2α in sepsis-induced thrombus formation, venous [38, 39] or pulmonary [40] thrombosis could be assessed in sepsis-challenged cell-specific HIF1α or HIF2α knockout mice and compared with wild type littermates. If a HIF- mediated pathway was identified as a potential therapeutic candidate for reducing sepsis- associated thrombosis, then the effect of targeting this pathway could be investigated in prevention or treatment studies of wild type mice. Such studies could ultimately identify a cell-specific HIF- mediated pathway that regulates sepsis-induced thrombus formation and would therefore represent a putative therapeutic target. If so and given that HIF agonists and antagonists are already in clinical trials, such drugs could eventually be tested for their efficacy against sepsis-associated thrombosis in humans.
Inflammation-targeting strategies in sepsis-induced thrombosis
Future studies could also aim to assess whether sepsis-associated thrombus formation can be reduced using recently-developed inflammation-targeting strategies [41]. These strategies include drug-loaded nanoparticles that are double-coated with antibodies and proteins to enable inflammation targeting and phagocytosis evasion [41]. Given that inflammatory endothelial and myeloid cells overexpress intercellular adhesion molecule (ICAM) 1 [42, 43], and that sepsis- induced thrombosis is dependent upon ICAM1 [7], therapies could be delivered not only in their free form, but also encapsulated in modified nanoparticles coated with anti-ICAM1 antibody and CD47 peptides [41]. Anti-ICAM1 antibody improves nanoparticle delivery to inflammatory ICAM1- expressing endothelial cells and macrophages, and CD47 reduces phagocytic clearance of the drug-loaded nanoparticle from the circulation [41]. Given that molecule-, cell-, and tissue-targeting strategies are currently being developed to enhance drug effectiveness against inflammatory diseases (e.g. bacterial infection [44], breast cancer [45], and autoimmune disease [46]), it would be intriguing to assess whether such nanotechnological advances could be used effectively in thrombosed tissue (Fig 2) [47].
Figure 2: Potential treatment of sepsis-induced thrombosis with modified nanoparticles.
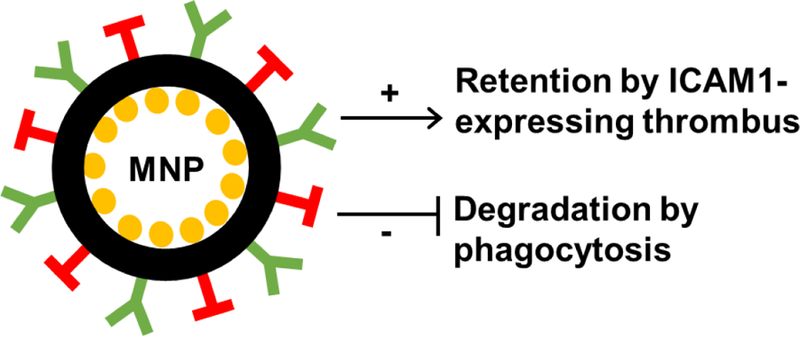
Experimental studies could assess whether sepsis-induced thrombosis could be treated with drug (yellow)-loaded nanoparticles (black) modified by the additions of (i) anti-ICAM1 antibody (green) to target ICAM1-expressing inflammatory/thrombosed tissue and (ii) CD47 (red) to reduce phagocytotic clearance from the circulation. Abbreviations: ICAM, intercellular adhesion molecule; MNP, modified nanoparticle.
The potential anti-thrombotic impact of signalling pathways that are identified and targeted in experimental studies of sepsis-induced thrombosis should ultimately be assessed in human cells or tissues. Nevertheless, studies employing experimental models of thrombus formation could facilitate translational studies of other diseases linked with increased thrombosis, including chronic thromboembolic pulmonary hypertension [48] and lung cancer [40]. Importantly, parallel preclinical and clinical investigations of sepsis-induced thrombus formation could lead to the development of new therapies against thrombosis in patients with sepsis.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflicts of Interest
None.
References
- [1].Fowkes FJ, Price JF, Fowkes FG, Incidence of diagnosed deep vein thrombosis in the general population: systematic review, Eur J Vasc Endovasc Surg 25(1) (2003) 1–5. [DOI] [PubMed] [Google Scholar]
- [2].Kaplan D, Casper TC, Elliott CG, Men S, Pendleton RC, Kraiss LW, Weyrich AS, Grissom CK, Zimmerman GA, Rondina MT, VTE Incidence and Risk Factors in Patients With Severe Sepsis and Septic Shock, Chest 148(5) (2015) 1224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dixon B, The role of microvascular thrombosis in sepsis, Anaesth Intensive Care 32(5) (2004) 619–29. [DOI] [PubMed] [Google Scholar]
- [4].Ware LB, Matthay MA, The acute respiratory distress syndrome, N Engl J Med 342(18) (2000) 1334–49. [DOI] [PubMed] [Google Scholar]
- [5].de Stoppelaar SF, van ‘t Veer C, van der Poll T, The role of platelets in sepsis, Thromb Haemost 112(4) (2014) 666–77. [DOI] [PubMed] [Google Scholar]
- [6].Aihara M, Nakazawa T, Dobashi K, Joshita T, Kojima M, Onai M, Mori M, A selective pulmonary thrombosis associated with sepsis-induced disseminated intravascular coagulation, Intern Med 36(2) (1997) 97–101. [DOI] [PubMed] [Google Scholar]
- [7].Obi AT, Andraska E, Kanthi Y, Kessinger CW, Elfline M, Luke C, Siahaan TJ, Jaffer FA, Wakefield TW, Henke PK, Endotoxaemia-augmented murine venous thrombosis is dependent on TLR-4 and ICAM-1, and potentiated by neutropenia, Thromb Haemost (2016). [DOI] [PMC free article] [PubMed]
- [8].Obi AT, Andraska E, Kanthi Y, Luke CE, Elfline M, Madathilparambil S, Siahaan TJ, Jaffer FA, Wakefield TW, Raghavendran K, Henke PK, Gram-Negative Pneumonia Alters Large-Vein Cell-Adhesion Molecule Profile and Potentiates Experimental Stasis Venous Thrombosis, J Vasc Res 53(3–4) (2016) 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Patel KN, Soubra SH, Lam FW, Rodriguez MA, Rumbaut RE, Polymicrobial sepsis and endotoxemia promote microvascular thrombosis via distinct mechanisms, Journal of thrombosis and haemostasis : JTH 8(6) (2010) 1403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Saha P, Humphries J, Modarai B, Mattock K, Waltham M, Evans CE, Ahmad A, Patel AS, Premaratne S, Lyons OT, Smith A, Leukocytes and the natural history of deep vein thrombosis: current concepts and future directions, Arterioscler Thromb Vasc Biol 31(3) (2011) 506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Konecny FA, Review of cellular and molecular pathways linking thrombosis and innate immune system during sepsis, Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences 15(6) (2010) 348–58. [PMC free article] [PubMed] [Google Scholar]
- [12].Levi M, Schultz M, van der Poll T, Sepsis and thrombosis, Semin Thromb Hemost 39(5) (2013) 559–66. [DOI] [PubMed] [Google Scholar]
- [13].Semeraro N, Ammollo CT, Semeraro F, Colucci M, Sepsis, thrombosis and organ dysfunction, Thromb Res 129(3) (2012) 290–5. [DOI] [PubMed] [Google Scholar]
- [14].Hamer JD, Malone PC, Silver IA, The PO2 in venous valve pockets: its possible bearing on thrombogenesis, Br J Surg 68(3) (1981) 166–70. [DOI] [PubMed] [Google Scholar]
- [15].Yan SF, Mackman N, Kisiel W, Stern DM, Pinsky DJ, Hypoxia/Hypoxemia-Induced activation of the procoagulant pathways and the pathogenesis of ischemia-associated thrombosis, Arterioscler Thromb Vasc Biol 19(9) (1999) 2029–35. [DOI] [PubMed] [Google Scholar]
- [16].Brill A, Suidan GL, Wagner DD, Hypoxia, such as encountered at high altitude, promotes deep vein thrombosis in mice, Journal of thrombosis and haemostasis : JTH (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Semenza GL, Vascular responses to hypoxia and ischemia, Arterioscler Thromb Vasc Biol 30(4) (2010) 648–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schafer ST, Frede S, Winning S, Bick A, Roshangar P, Fandrey J, Peters J, Adamzik M, Hypoxia-inducible factor and target gene expression are decreased in patients with sepsis: prospective observational clinical and cellular studies, Anesthesiology 118(6) (2013) 1426–36. [DOI] [PubMed] [Google Scholar]
- [19].Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M, NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha, Nature 453(7196) (2008) 807–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nizet V, Johnson RS, Interdependence of hypoxic and innate immune responses, Nature reviews. Immunology 9(9) (2009) 609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Palazon A, Goldrath AW, Nizet V, Johnson RS, HIF transcription factors, inflammation, and immunity, Immunity 41(4) (2014) 518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shalova IN, Lim JY, Chittezhath M, Zinkernagel AS, Beasley F, Hernandez-Jimenez E, Toledano V, Cubillos-Zapata C, Rapisarda A, Chen J, Duan K, Yang H, Poidinger M, Melillo G, Nizet V, Arnalich F, Lopez-Collazo E, Biswas SK, Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1alpha, Immunity 42(3) (2015) 484–98. [DOI] [PubMed] [Google Scholar]
- [23].Matsuura Y, Yamashita A, Iwakiri T, Sugita C, Okuyama N, Kitamura K, Asada Y, Vascular wall hypoxia promotes arterial thrombus formation via augmentation of vascular thrombogenicity, Thromb Haemost 114(1) (2015) 158–72. [DOI] [PubMed] [Google Scholar]
- [24].Pawlinski R, Mackman N, Cellular sources of tissue factor in endotoxemia and sepsis, Thromb Res 125 Suppl 1 (2010) S70–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Grover SP, Mackman N, Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis, Arterioscler Thromb Vasc Biol 38(4) (2018) 709–725. [DOI] [PubMed] [Google Scholar]
- [26].Liao H, Hyman MC, Lawrence DA, Pinsky DJ, Molecular regulation of the PAI-1 gene by hypoxia: contributions of Egr-1, HIF-1alpha, and C/EBPalpha, FASEB J 21(3) (2007) 935–49. [DOI] [PubMed] [Google Scholar]
- [27].Pinsky DJ, Liao H, Lawson CA, Yan SF, Chen J, Carmeliet P, Loskutoff DJ, Stern DM, Coordinated induction of plasminogen activator inhibitor-1 (PAI-1) and inhibition of plasminogen activator gene expression by hypoxia promotes pulmonary vascular fibrin deposition, J Clin Invest 102(5) (1998) 919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Westrick RJ, Eitzman DT, Plasminogen activator inhibitor-1 in vascular thrombosis, Current drug targets 8(9) (2007) 966–1002. [DOI] [PubMed] [Google Scholar]
- [29].Mousa SA, Fareed J, Iqbal O, Kaiser B, Tissue factor pathway inhibitor in thrombosis and beyond, Methods Mol Med 93 (2004) 133–55. [DOI] [PubMed] [Google Scholar]
- [30].Winckers K, ten Cate H, Hackeng TM, The role of tissue factor pathway inhibitor in atherosclerosis and arterial thrombosis, Blood reviews 27(3) (2013) 119–32. [DOI] [PubMed] [Google Scholar]
- [31].Cui XY, Skretting G, Tinholt M, Stavik B, Dahm AEA, Sahlberg KK, Kanse S, Iversen N, Sandset PM, A novel hypoxia response element regulates oxygen-related repression of tissue factor pathway inhibitor in the breast cancer cell line MCF-7, Thromb Res 157 (2017) 111–116. [DOI] [PubMed] [Google Scholar]
- [32].Henke PK, Plasmin and matrix metalloproteinase system in deep venous thrombosis resolution, Vascular 15(6) (2007) 366–71. [DOI] [PubMed] [Google Scholar]
- [33].Ogawa S, Gerlach H, Esposito C, Pasagian-Macaulay A, Brett J, Stern D, Hypoxia modulates the barrier and coagulant function of cultured bovine endothelium. Increased monolayer permeability and induction of procoagulant properties, The Journal of clinical investigation 85(4) (1990) 1090–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gupta N, Ashraf MZ, Exposure to high altitude: a risk factor for venous thromboembolism?, Seminars in thrombosis and hemostasis 38(2) (2012) 156–63. [DOI] [PubMed] [Google Scholar]
- [35].Gupta N, Sahu A, Prabhakar A, Chatterjee T, Tyagi T, Kumari B, Khan N, Nair V, Bajaj N, Sharma M, Ashraf MZ, Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia, Proc Natl Acad Sci U S A 114(18) (2017) 4763–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Evans CE, Bendahl PO, Belting M, Branco C, Johnson RS, Diverse roles of cell-specific hypoxia-inducible factor 1 in cancer-associated hypercoagulation, Blood 127(10) (2016) 1355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kaneko M, Minematsu T, Yoshida M, Nishijima Y, Noguchi H, Ohta Y, Nakagami G, Mori T, Sanada H, Compression-induced HIF-1 enhances thrombosis and PAI-1 expression in mouse skin, Wound Repair Regen 23(5) (2015) 657–63. [DOI] [PubMed] [Google Scholar]
- [38].Evans CE, Humphries J, Mattock K, Waltham M, Wadoodi A, Saha P, Modarai B, Maxwell PH, Smith A, Hypoxia and upregulation of hypoxia-inducible factor 1{alpha} stimulate venous thrombus recanalization, Arterioscler Thromb Vasc Biol 30(12) (2010) 2443–51. [DOI] [PubMed] [Google Scholar]
- [39].Evans CE, Grover SP, Humphries J, Saha P, Patel AP, Patel AS, Lyons OT, Waltham M, Modarai B, Smith A, Antiangiogenic therapy inhibits venous thrombus resolution, Arteriosclerosis, thrombosis, and vascular biology 34(3) (2014) 565–70. [DOI] [PubMed] [Google Scholar]
- [40].Evans CE, Palazon A, Sim J, Tyrakis PA, Prodger A, Lu X, Chan S, Bendahl PO, Belting M, Von Euler L, Rundqvist H, Johnson RS, Branco C, Modelling pulmonary microthrombosis coupled to metastasis: distinct effects of thrombogenesis on tumorigenesis, Biology open 6(5) (2017) 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kim J, Sinha S, Solomon M, Perez-Herrero E, Hsu J, Tsinas Z, Muro S, Co-coating of receptor-targeted drug nanocarriers with anti-phagocytic moieties enhances specific tissue uptake versus non-specific phagocytic clearance, Biomaterials 147 (2017) 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hubbard AK, Giardina C, Regulation of ICAM-1 expression in mouse macrophages, Inflammation 24(2) (2000) 115–25. [DOI] [PubMed] [Google Scholar]
- [43].Lawson C, Wolf S, ICAM-1 signaling in endothelial cells, Pharmacological reports : PR 61(1) (2009) 22–32. [DOI] [PubMed] [Google Scholar]
- [44].Chen Y, Chen M, Zhang Y, Lee JH, Escajadillo T, Gong H, Fang RH, Gao W, Nizet V, Zhang L, Broad-Spectrum Neutralization of Pore-Forming Toxins with Human Erythrocyte Membrane- Coated Nanosponges, Advanced healthcare materials 7(13) (2018) e1701366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jin H, Pi J, Zhao Y, Jiang J, Li T, Zeng X, Yang P, Evans CE, Cai J, EGFR-targeting PLGA-PEG nanoparticles as a curcumin delivery system for breast cancer therapy, Nanoscale 9(42) (2017) 16365–16374. [DOI] [PubMed] [Google Scholar]
- [46].Jiang Y, Fang RH, Zhang L, Biomimetic Nanosponges for Treating Antibody-Mediated Autoimmune Diseases, Bioconjugate chemistry 29(4) (2018) 870–877. [DOI] [PubMed] [Google Scholar]
- [47].Greineder CF, Johnston IH, Villa CH, Gollomp K, Esmon CT, Cines DB, Poncz M, Muzykantov VR, ICAM-1-targeted thrombomodulin mitigates tissue factor-driven inflammatory thrombosis in a human endothelialized microfluidic model, Blood advances 1(18) (2017) 1452–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Matthews DT, Hemnes AR, Current concepts in the pathogenesis of chronic thromboembolic pulmonary hypertension, Pulm Circ 6(2) (2016) 145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]


