Key Points
Question
Does preventive, low-dose aspirin increase the frequency of intracranial hemorrhage in the general population?
Findings
In this systematic review and meta-analysis that included 13 randomized clinical trials, low-dose aspirin was associated with an increased risk of any intracranial bleeding.
Meaning
Use of low-dose aspirin was associated with an overall increased risk of intracranial hemorrhage among people without symptomatic cardiovascular disease.
Abstract
Importance
Use of low-dose aspirin for the primary prevention of cardiovascular events remains controversial because increased risk of bleeding may offset the overall benefit. Among major bleeding events, intracranial hemorrhage is associated with high mortality rates and functional dependency.
Objective
To assess the risk of intracranial hemorrhage associated with low-dose aspirin among individuals without symptomatic cardiovascular disease.
Data Sources
PubMed, Embase, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov were searched from January 1966 to October 30, 2018.
Study Selection
Randomized clinical trials that compared low-dose aspirin (daily dose ≤100 mg) vs control and recorded the end points of intracranial hemorrhage separately for active treatment and control groups were included.
Data Extraction and Synthesis
A random-effect estimate was computed based on the Mantel-Haenszel method. Relative risk with 95% CI was used as a measure of aspirin vs control on risk of intracranial hemorrhage.
Main Outcomes and Measures
The main outcomes were any intracranial hemorrhage, intracerebral hemorrhage, subdural or extradural hemorrhage, and subarachnoid hemorrhage, for aspirin vs control.
Results
The search identified 13 randomized clinical trials of low-dose aspirin use for primary prevention, enrolling 134 446 patients. Pooling the results from the random-effects model showed that low-dose aspirin, compared with control, was associated with an increased risk of any intracranial bleeding (8 trials; relative risk, 1.37; 95% CI, 1.13-1.66; 2 additional intracranial hemorrhages in 1000 people), with potentially the greatest relative risk increase for subdural or extradural hemorrhage (4 trials; relative risk, 1.53; 95% CI, 1.08-2.18) and less for intracerebral hemorrhage and subarachnoid hemorrhage. Patient baseline features associated with heightened risk of intracerebral hemorrhage with low-dose aspirin, compared with control, were Asian race/ethnicity and low body mass index.
Conclusions and Relevance
Among people without symptomatic cardiovascular disease, use of low-dose aspirin was associated with an overall increased risk of intracranial hemorrhage, and heightened risk of intracerebral hemorrhage for those of Asian race/ethnicity or people with a low body mass index.
This systematic review and meta-analysis assesses the risk of intracranial hemorrhage associated with low-dose aspirin among individuals without symptomatic cardiovascular disease.
Introduction
The benefits of low-dose aspirin for secondary prevention of myocardial infarction and ischemic stroke are well established, with prevention of recurrent ischemic events outweighing the risk of hemorrhage.1,2 However, the value of aspirin for primary prevention of symptomatic cardiovascular disease is controversial because the risk of cardiovascular events among event-free individuals is typically lower than that of patients with symptomatic atherosclerotic disease, and the increased risk of bleeding may offset the overall benefit of aspirin use.3,4,5 Of the various major bleeding events related to the use of aspirin, intracranial hemorrhage is a special concern because it is strongly associated with a high risk of mortality6 and poorer health over a lifetime.7,8
Published meta-analyses have provided conflicting findings regarding whether the use of aspirin for primary prevention of symptomatic cardiovascular disease increases the risk of intracranial hemorrhage.4,5,9,10,11,12 Furthermore, the link between aspirin use for primary prevention and specific subtypes of intracranial hemorrhage, such as intracerebral hemorrhage or subdural hemorrhage, was not investigated in published meta-analyses, to our knowledge. Finally, 3 recently published large randomized clinical trials of different populations have provided important new data, with individual trials showing mixed results on the association of aspirin use and intracranial hemorrhage.13,14,15
Because of the devastating nature of intracranial hemorrhage outcomes, inconsistent published data on the risk of intracranial hemorrhage for individuals taking aspirin for primary prevention, the paucity of information on specific subtypes of intracranial hemorrhage, and recently published clinical trials, we conducted a systematic review and meta-analysis of randomized clinical trials to clarify the qualitative and quantitative links of aspirin for primary prevention with the risk of intracranial hemorrhage and its subtypes.
Methods
This study was performed in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.16
Data Sources and Searches
We searched PubMed (1966 to October 30, 2018), Embase (1966 to October 30, 2018), the Cochrane Central Register of Controlled Trials (1966 to October 30, 2018), and the clinical trial registry maintained at ClinicalTrials.gov with the terms aspirin or acetylsalicylic acid or ASA and primary prevention or cardiovascular disease or diabetes or healthy or elderly or hypertension and intracranial hemorrhage or intracerebral hemorrhage or intraparenchymal hemorrhage or subdural hemorrhage or epidural hemorrhage or subarachnoid hemorrhage or brain hemorrhage or brain bleeding or hemorrhagic stroke or ICH or major bleeding or adverse effect or side effect or safety. We restricted our search to studies conducted in humans and clinical trials. There were no language restrictions. We also reviewed the introduction and discussion sections of retrieved trials, relevant review articles, and published meta-analysis to identify additional trials. Two of us (W.-Y.H. and M.L.) independently conducted the literature search, screening of abstracts, and selection of included trials.
Study Selection
Criteria for inclusion of a study were as follows: (1) the study design was a randomized clinical trial; (2) the study included a comparison of aspirin with placebo or aspirin with no aspirin; (3) at least 1 of the following end points was reported: all intracranial hemorrhage, all hemorrhagic stroke, intracerebral hemorrhage, subdural or extradural hemorrhage, or subarachnoid hemorrhage; (4) total participants and the number of participants who reached an end point were reported separately in each group; (5) aspirin dosage was equal to or less than 100 mg once daily; and (5) treatment duration of at least 6 months. Criteria for exclusion of a study were (1) a participant had preexisting symptomatic cardiovascular diseases (eg, coronary heart disease, stroke, or peripheral artery disease); (2) use of antithrombotic agents other than aspirin in the active arm of the study; (3) use of other antithrombotic agents in the control arm of the study; or (4) aspirin dosage greater than 100 mg once daily.
Data Abstraction
We abstracted data about baseline characteristics, which included age, sex, duration of follow-up, study populations, and patient number in each group. We also abstracted data on cranial hemorrhagic safety outcomes from each trial, which included the number of patients with intracranial hemorrhage, intracerebral hemorrhage, subdural or extradural hemorrhage, or subarachnoid hemorrhage in each group (aspirin and no-aspirin treatment). Two of us (W.-Y.H. and Y.-L.W.) independently abstracted data from eligible studies. Any discrepant judgments were resolved by joint discussion.
Quality Assessment
The risk of bias (eg, sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other issues) in each trial was independently assessed by 2 of us (W.-Y.H. and M.L.), using the Cochrane risk of bias tool.17 Any discrepant judgments were resolved by joint discussion.
Statistical Analysis
Data were analyzed according to the intention-to-treat principle. The primary end point was the association of aspirin, compared with placebo or a no-aspirin group, and risks for any intracranial bleeding. The secondary end points were risks of intracerebral hemorrhage, subdural or extradural hemorrhage, and subarachnoid hemorrhage. The definitions of any intracranial bleeding, intracerebral hemorrhage, subdural or extradural hemorrhage, and subarachnoid hemorrhage were consistent across trials. Relative risk (RR) with 95% CI was used as a measure of aspirin vs no aspirin on the risk of any intracranial bleeding, intracerebral hemorrhage, subdural or extradural hemorrhage, or subarachnoid hemorrhage. We computed a random-effect estimate based on the Mantel-Haenszel method when 2 or more studies provided sufficient data for a given outcome.
Subgroup analyses for the primary and secondary end points were conducted for different patient characteristics, based on the extent of their representation in different trials: higher proportion of patients with type 1 or 2 diabetes (>15% of trial patients with diabetes), race/ethnicity (all enrolled patients Asian vs minority of enrolled patients Asian), age (mean age of trial population, <65 years vs ≥65 years), body mass index (BMI; mean BMI of trial population <25 vs ≥25 [calculated as weight in kilograms divided by height in meters squared]) and blinding of the trial (blinding vs nonblinding). The subgroup analysis of Asian and non-Asian race/ethnicity was driven by the fact that Asian populations have a higher risk of intracerebral hemorrhage compared with non-Asian populations.18,19,20 Also, a recent individual-level patient analysis suggested that, for people taking low-dose aspirin, major bleeding events were increased with low-dose aspirin in individuals weighing less than 90 kg but not those weighing 90 kg or more.21 Because most trials reported BMI, but not body weight, we conducted subgroup analysis based on BMI less than 25 vs BMI of 25 or more.
Heterogeneity was assessed by P value of χ2 statistics and I2, which describes the percentage of variability in the estimates that is due to heterogeneity rather than chance.22,23 We considered study-level estimates to be heterogeneous if the I2 statistic was greater than 50%. We reported absolute risks in terms of the difference in the number of events per 1000 patients and the respective 95% CI. All P values were from 2-sided tests and results were deemed statistically significant at P < .05. Publication bias was assessed by visual examination of funnel plots. The Cochrane Collaboration’s Review Manager Software Package (RevMan, version 5) was used for this meta-analysis.
Results
We identified 18 full articles for detailed assessment,13,14,15,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38 of which 4 were excluded for using an aspirin dose greater than 100 mg once daily (higher than current standard treatment)24,27,36,38 and 1 was excluded because some enrolled patients had symptomatic rather than asymptomatic peripheral arterial disease.28 Our final principal analysis included 13 randomized clinical trials comparing aspirin and control, with 134 446 individuals enrolled (eFigure 1 in the Supplement).13,14,15,25,26,29,30,31,32,33,34,35,37
The characteristics of these 13 randomized clinical trials are shown in the Table. Three trials compared aspirin, 81 to 100 mg, once daily with placebo or no aspirin in patients with diabetes.14,26,35 Four trials compared aspirin, 75 to 100 mg, once daily with placebo or no aspirin in patients with at least 1 cardiovascular risk factor.13,29,32,33 One trial compared aspirin, 100 mg, once daily with placebo in patients with polycythemia vera.34 One trial compared aspirin, 81 mg, once daily with placebo in patients with asymptomatic, persistently antiphospholipid antibody–positive test results.30 One trial compared aspirin, 100 mg, once daily with placebo in healthy elderly individuals.15 One trial compared aspirin, 75 mg, once daily with placebo in men.25 One trial compared aspirin, 100 mg, every other day with placebo in initially healthy women.37 One trial compared aspirin, 100 mg, once daily with placebo in patients with a low ankle brachial index (≤0.95).31
Table. Characteristics of Included Trials.
| Source, Year | Population | Dosage of Aspirin in Active Arm | Sample Size (Men, %) | Mean | Baseline SBP/DBP, mm Hg | Population With HTN, % | Population With Diabetes, % | Follow-up, y | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | Weight, kg | BMIa | ||||||||
| Hansson et al (HOT),32 1998 | HTN; 26 countries | 75 mg Once daily | 18 790 (53) | 61.5 | NA | 28.4 | 170/105 | 100 | 8 | 3.8 |
| Thrombosis Prevention Trial,25 1998 | Men; United Kingdom | 75 mg Once daily | 2540 (100) | 57.5 | NA | 27.4 | 139/NA | NA | NA | 6.7 |
| de Gaetano (PPP),29 2001 | At least 1 of the major recognized cardiovascular risk factors; Italy | 100 mg Once daily | 4495 (43) | 64.4 | NA | 27.6 | 145/85 | 68.5 | 16.5 | 3.6 |
| Landolfi et al (ECLAP),34 2004 | Polycythemia vera; 12 countries | 100 mg Once daily | 518 (60) | 60.9 | NA | 25.6 | NA/NA | 34.7 | 6.8 | 3 |
| Ridker et al (WHS),37 2005 | Initially healthy women; United States | 100 mg Every other day | 39 876 (0) | 54.6 | NA | 26 | NA/NA | 25.9 | 2.6 | 10.1 |
| Erkan et al (APLASA),30 2007 | Asymptomatic, persistently aPL-positive individuals; United States | 81 mg Once daily | 98 (10) | 42.9 | NA | NA | NA/NA | 22.4 | 6.1 | 2.3 |
| Belch et al (POPADAD),26 2008 | Type 1 and 2 diabetes with ankle brachial pressure index ≤0.99; Scotland | 100 mg Once daily | 636 (43) | 60.1 | NA | 29 | 145/79 | NA | 100 | 6.7 |
| Ogawa et al (JPAD),35 2008 | Type 2 diabetes; Japan | 81 or 100 mg Once daily | 2539 (55) | 64.5 | NA | 24 | 135/77 | 58 | 100 | 4.4 |
| Fowkes et al (AAA),31 2010 | Low ankle brachial pressure index (≤0.95); Scotland | 100 mg Once daily | 3350 (29) | 61.8 | NA | NA | 148/84 | NA | 3 | 8.2 |
| Ikeda et al (JPPP),33 2014 | HTN, diabetes, or dyslipidemia; Japan | 100 mg Once daily | 14 464 (42) | 70.5 | 58.7 | 24.2 | 137/78 | 84.9 | 33.9 | 5 |
| Bowman et al (ASCEND),14 2018 | Diabetes (any type); United Kingdom | 100 mg Once daily | 15 480 (63) | 63.3 | NA | 30.7 | 136/77 | 61.6 | 100 | 7.4 |
| McNeil et al (ASPREE),15 2018 | Healthy elderly individuals; Australia and United States | 100 mg Once daily | 19 114 (44) | 74 | NA | NA | NA/NA | 74.5 | 11 | 4.7 |
| Gaziano et al (ARRIVE),13 2018 | Men ≥55 y with 2-4 risk factors; women ≥60 y with ≥3 risk factors; 7 countries | 100 mg Once daily | 12 546 (71) | 63.9 | 82 | 28.4 | NA/NA | 62.7 | 0 | 5 |
Abbreviations: AAA, the Aspirin for Asymptomatic Atherosclerosis trial; APLASA, the Antiphospholipid Antibody Acetylsalicylic Acid study; aPL, antiphospholipid antibody; ARRIVE, the Aspirin to Reduce Risk of Initial Vascular Events study; ASCEND, A Study of Cardiovascular Events in Diabetes; ASPREE, the Aspirin in Reducing Events in the Elderly trial; BMI, body mass index; DBP, diastolic blood pressure; ECLAP, the European Collaboration on Low-Dose Aspirin in Polycythemia Vera; HOT, the Hypertension Optimal Treatment Study; HTN, hypertension; JPAD, the Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes Trial; JPPP, the Japanese Primary Prevention Project; NA, not available; POPADAD, the Prevention of Progression of Arterial Disease and Diabetes trial; PPP, the Primary Prevention Project; SBP, systolic blood pressure; WHS, the Women’s Health Study.
Calculated as weight in kilograms divided by height in meters squared.
Mean patient age ranged from 42.9 to 74.0 years. Mean body weight was reported in 2 trials: 58.7 kg in one trial and 82.0 kg in the other trial. Mean BMI was reported in 10 trials and ranged from 24.0 to 30.7. Follow-up duration ranged from 2.3 to 8.2 years. The Cochrane risk of bias assessment for the included trials is summarized in eFigure 2 in the Supplement. Three trials had performance bias due to nonblinding of intervention,29,33,35 and 3 trials had attrition bias due to incomplete outcomes data.25,30,34
Any Intracranial Hemorrhage
Pooling the results from the random-effects model showed that low-dose aspirin compared with control was associated with increased risk of any intracranial hemorrhage (8 trials; 0.63% vs 0.46%; RR, 1.37; 95% CI, 1.13-1.66; I2 = 0%) (Figure 1A).14,15,29,30,31,32,33,34 Low-dose aspirin, compared with control, would be associated with an additional 2 (from 1 to 3) intracranial hemorrhages in 1000 people. Because patients in the ASPREE (Aspirin in Reducing Events in the Elderly) trial15 were elderly and had more intracranial hemorrhages, and therefore shifted the overall risk ratio, we conducted a sensitivity analysis by excluding the ASPREE trial and found that low-dose aspirin compared with control was associated with a nonsignificant increased risk of any intracranial hemorrhage (RR, 1.28; 95% CI, 0.99-1.65) (eFigure 3 in the Supplement). There was no evidence of publication bias on funnel plot analysis (eFigure 4A in the Supplement).
Figure 1. Relative Risk (RR) for Intracranial Hemorrhage (Aspirin vs Placebo or No Aspirin).
The sizes of the data markers indicate the weight of each trial. AAA indicates the Aspirin for Asymptomatic Atherosclerosis trial; APLASA, the Antiphospholipid Antibody Acetylsalicylic Acid study; ARRIVE, the Aspirin to Reduce Risk of Initial Vascular Events study; ASCEND, A Study of Cardiovascular Events in Diabetes; ASPREE, the Aspirin in Reducing Events in the Elderly trial; ECLAP, the European Collaboration on Low-Dose Aspirin in Polycythemia Vera; HOT, the Hypertension Optimal Treatment Study; JPAD, the Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes Trial; JPPP, the Japanese Primary Prevention Project; POPADAD, the Prevention of Progression of Arterial Disease and Diabetes trial; PPP, the Primary Prevention Project; and WHS, the Women’s Health Study.
Intracerebral Hemorrhage
Pooling the results from the random-effects model showed that low-dose aspirin compared with control showed a strong but nonsignificant association with a higher risk of intracerebral hemorrhage (10 trials; 0.30% vs 0.24%; RR, 1.23; 95% CI, 0.98-1.54; I2 = 0%) (Figure 1B).13,14,15,25,26,30,31,33,35,37 There was no evidence of publication bias on funnel plot analysis (eFigure 4B in the Supplement).
Subdural or Extradural Hemorrhage
Pooling the results from the random-effects model showed that low-dose aspirin compared with control was associated with an increased risk of subdural or extradural hemorrhage (4 trials; 0.31% vs 0.20%; RR, 1.53; 95% CI, 1.08-2.18; I2 = 0%) (Figure 1C).14,15,30,33 Low-dose aspirin, compared with control, would be associated with an additional 1 (from 0 to 2) subdural or extradural hemorrhage in 1000 people. There was no evidence of publication bias on funnel plot analysis (eFigure 4C in the Supplement).
Subarachnoid Hemorrhage
Pooling the results from the random-effects model showed that low-dose aspirin compared with control had a similar risk of subarachnoid hemorrhage (5 trials; 0.14% vs 0.12%; RR, 1.13; 95% CI, 0.70-1.83; P = .83 for heterogeneity; I2 = 0%) (Figure 1D).14,15,25,30,33 There was no evidence of publication bias on funnel plot analysis (eFigure 4D in the Supplement).
Subgroup Analysis
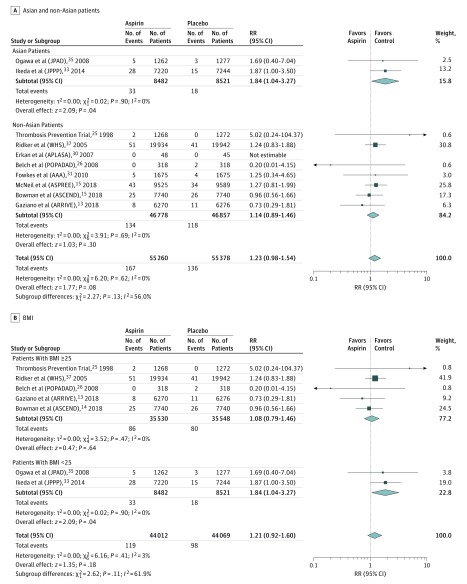
For intracerebral hemorrhage, indications of potential heterogeneity were noted for 2 of the subgroup analyses: race/ethnicity and BMI. Low-dose aspirin, compared with control, was associated with a magnified risk of intracerebral hemorrhage in trials enrolling only Asian populations vs trials with preponderantly non-Asian populations (I2 = 56%) (Figure 2A). The RR of intracerebral hemorrhage in Asian-populations trials was 1.84 (95% CI, 1.04-3.27; 2 trials),33,35 whereas in preponderantly non-Asian population trials the RR was 1.14 (95% CI, 0.89-1.46; 8 trials).13,14,15,25,26,30,31,37 Low-dose aspirin, compared with control, was also associated with a magnified risk of intracerebral hemorrhage in populations with a mean BMI less than 25 vs populations with a mean BMI of 25 or greater (I2 = 62%) (Figure 2B). The RR of intracerebral hemorrhage in trials with a mean population BMI less than 25 was 1.84 (95% CI, 1.04-3.27; 2 trials),33,35 whereas in trials with a mean population BMI of 25 or higher, the RR was 1.08 (95% CI, 0.79-1.46; 5 trials).13,14,25,26,37 Otherwise, no obvious heterogeneity was found in other subgroup analyses (eFigures 5-9 in the Supplement).
Figure 2. Subgroup Analysis of Intracerebral Hemorrhage (Aspirin vs Placebo or No Aspirin).
The sizes of the data markers indicate the weight of each trial. AAA indicates the Aspirin for Asymptomatic Atherosclerosis trial; APLASA, the Antiphospholipid Antibody Acetylsalicylic Acid study; ARRIVE, the Aspirin to Reduce Risk of Initial Vascular Events study; ASCEND, A Study of Cardiovascular Events in Diabetes; ASPREE, the Aspirin in Reducing Events in the Elderly trial; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); JPAD, the Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes Trial; JPPP, the Japanese Primary Prevention Project; POPADAD, the Prevention of Progression of Arterial Disease and Diabetes trial; RR, relative risk; and WHS, the Women’s Health Study.
Discussion
The current meta-analysis, comprising 13 randomized clinical trials with more than 130 000 individuals, revealed that low-dose aspirin use in individuals without symptomatic cardiovascular disease increased the risk of intracranial hemorrhage, when compared with control. In terms of specific types of intracranial hemorrhage, intracerebral hemorrhage, subdural or extradural hemorrhage, and subarachnoid hemorrhage all showed increased risk point estimates, with a suggestion of the greatest risk increase for subdural and extradural hemorrhage and the lowest risk increase for subarachnoid hemorrhage. Population subgroup analyses indicated that low-dose aspirin is associated with a magnified level of increased risk of intracerebral hemorrhage associated with Asian race/ethnicity and populations with a mean BMI less than 25.
The absolute magnitude of these adverse effects is modest, but clinically relevant. Among every 1000 people treated with low-dose aspirin instead of control, 2 more had intracranial hemorrhage events. Intracranial hemorrhage events are generally associated with higher mortality and greater disability than ischemic events associated with atherosclerotic cardiovascular disease, such as ischemic stroke.7,8 In NEMESIS (the North East Melbourne Stroke Incidence Study), the mean loss of quality-adjusted life-years was 6.2 for each intracerebral hemorrhage.7 Given that the many individuals in the general population have a very low risk of atherosclerotic cardiovascular events, if low-dose aspirin is given universally, adverse outcomes from intracranial hemorrhage may outweigh the beneficial effects of low-dose aspirin.
The increased propensity to intracerebral hemorrhage with low-dose aspirin in Asian compared with non-Asian populations in these randomized trial cohorts is consonant with findings in observational epidemiologic studies. Multiple studies have found that, in general, the ratio of hemorrhagic stroke to ischemic stroke is higher in Asian than in non-Asian populations.39 Large case-control studies in Spain and Australia found that use of antiplatelet agents was not associated with a substantial increased risk of intracerebral hemorrhage in general populations.40,41 In contrast, epidemiologic studies have found a higher risk for anticoagulation-associated intracranial hemorrhage among Asian patients with atrial fibrillation.42 The elevated risk of intracerebral hemorrhage in Asian individuals has been suggested to arise from multiple potential reasons, including higher frequencies of cerebral amyloid angiopathy, uncontrolled blood pressure, and smoking; a diet with higher fish intake (including ω-3 fatty acids with mild antithrombotic effects); and environmental factors.18,19,43,44
The association of lower BMI with a higher propensity to intracerebral hemorrhage with low-dose aspirin intake in randomized clinical trials is also consistent with findings of observational studies that have identified low weight and low BMI as predisposing factors to a higher ratio of hemorrhagic to ischemic stroke.45,46,47 It also accords to a degree with an individual participant pooled data analysis of primary prevention trials that found that major bleeding events, including both intracranial and extracranial events, were increased with low-dose aspirin in individuals weighing less than 90 kg but not in those weighing 90 kg or more.21 Potential mediators of this association include the association of lower BMI with lower triglyceride and total cholesterol levels,48 both known risk factors for intracerebral hemorrhage.18 Asian race/ethnicity and lower BMI are known to covary. As the current investigation was a study-level, not individual patient-level, meta-analysis, analyses could not be pursued to determine the degree to which each factor independently contributed to increased risk of intracerebral hemorrhage.
The current study provides an updated perspective on the association of preventive low-dose aspirin and subdural and extradural hemorrhage. A prior meta-analysis of randomized trials of aspirin used for both primary and secondary prevention of cardiovascular diseases found that aspirin did not significantly increase the risk of subdural hemorrhage.9 However, that meta-analysis included only 1 relevant primary prevention trial. Subsequently, 3 additional primary prevention trials have been reported with data on subdural and extradural hemorrhage events, and pooling the results of all 4 trials showed that low-dose aspirin use significantly increased the risk of subdural and extradural hemorrhage.14,15,30,33 A main driver of this finding was the ASPREE trial,15 in which participants had a more advanced mean age of 74 years. Epidemiologic studies have suggested that older age, as well as male sex, history of head injury, and alcoholism are common risk factors for subdural hemorrhage.49,50 Elderly individuals may be prone to falling or head injury, and may have more atrophic brain retraction placing stress on bridging veins, which may raise the risk of subdural and extradural hemorrhages; these risks may be potentiated by taking antithrombotic agents.
Limitations
Our study has several limitations. First, meta-analysis may be biased when the literature search fails to identify all relevant trials or the selection criteria for including trials were applied in a subjective manner. To reduce these risks, we performed thorough searches across multiple literature databases and clinical trial databases and used explicit criteria for study selection, data abstraction, and data analysis. Second, the trials enrolling solely Asian populations were both performed in Japan, and may not be fully representative of other Asian populations. Third, as this was a study-level rather than participant-level meta-analysis, we were able to analyze only univariate, not multivariate, associations of baseline features with outcomes.
Conclusions
The use of low-dose aspirin for primary prevention of cardiovascular events in individuals without symptomatic cardiovascular disease was associated with an increased risk of overall intracranial hemorrhage. The risk of intracerebral hemorrhage was particularly elevated in Asian populations and in populations with lower mean BMI. Because the benefits of low-dose aspirin for primary prevention of cardiovascular events are not well established, and the outcomes of intracranial hemorrhage are often catastrophic, these findings suggest caution regarding using low-dose aspirin in individuals without symptomatic cardiovascular disease.
eFigure 1. Flow of Study Selection
eFigure 2. Risk of Bias of Included Trials
eFigure 3. Sensitivity Analysis by Excluding ASPREE Trial for Relative Risk with 95% Confidence Interval for Intracranial Hemorrhage (Aspirin vs Placebo or No Aspirin)
eFigure 4. Funnel Plots of Each Outcome
eFigure 5. Relative Risk With 95% Confidence Interval of Subgroup Analysis (Asian and non-Asian)
eFigure 6. Relative Risk With 95% Confidence Interval of Subgroup Analysis (Diabetes and Mostly Nondiabetes)
eFigure 7. Relative Risk With 95% Confidence Interval of Subgroup Analysis (Age≧65 Years and Age <65 Years)
eFigure 8. Relative Risk With 95% Confidence Interval of Subgroup Analysis (Body Mass Index≧25 kg/m2 and <25 kg/m2)
eFigure 9. Relative Risk With 95% Confidence Interval of Subgroup Analysis (Blinding and Nonblinding)
References
- 1.Baigent C, Blackwell L, Collins R, et al. ; Antithrombotic Trialists’ (ATT) Collaboration . Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849-1860. doi: 10.1016/S0140-6736(09)60503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorelick PB, Weisman SM. Risk of hemorrhagic stroke with aspirin use: an update. Stroke. 2005;36(8):1801-1807. doi: 10.1161/01.STR.0000174189.81153.85 [DOI] [PubMed] [Google Scholar]
- 3.Capodanno D, Angiolillo DJ. Aspirin for primary cardiovascular risk prevention and beyond in diabetes mellitus. Circulation. 2016;134(20):1579-1594. doi: 10.1161/CIRCULATIONAHA.116.023164 [DOI] [PubMed] [Google Scholar]
- 4.Guirguis-Blake JM, Evans CV, Senger CA, Rowland MG, O’Connor EA, Whitlock EP. Aspirin for the Primary Prevention of Cardiovascular Events: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2015. [PubMed] [Google Scholar]
- 5.Lei H, Gao Q, Liu SR, Xu J. The benefit and safety of aspirin for primary prevention of ischemic stroke: a meta-analysis of randomized trials. Front Pharmacol. 2016;7:440. doi: 10.3389/fphar.2016.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HC, Choi DP, Ahn SV, Nam CM, Suh I. Six-year survival and causes of death among stroke patients in Korea. Neuroepidemiology. 2009;32(2):94-100. doi: 10.1159/000177034 [DOI] [PubMed] [Google Scholar]
- 7.Cadilhac DA, Dewey HM, Vos T, Carter R, Thrift AG. The health loss from ischemic stroke and intracerebral hemorrhage: evidence from the North East Melbourne Stroke Incidence Study (NEMESIS). Health Qual Life Outcomes. 2010;8:49. doi: 10.1186/1477-7525-8-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HY, Hwang JS, Jeng JS, Wang JD. Quality-adjusted life expectancy (QALE) and loss of QALE for patients with ischemic stroke and intracerebral hemorrhage: a 13-year follow-up. Stroke. 2010;41(4):739-744. doi: 10.1161/STROKEAHA.109.573543 [DOI] [PubMed] [Google Scholar]
- 9.Connolly BJ, Pearce LA, Kurth T, Kase CS, Hart RG. Aspirin therapy and risk of subdural hematoma: meta-analysis of randomized clinical trials. J Stroke Cerebrovasc Dis. 2013;22(4):444-448. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 10.Hart RG, Halperin JL, McBride R, Benavente O, Man-Son-Hing M, Kronmal RA. Aspirin for the primary prevention of stroke and other major vascular events: meta-analysis and hypotheses. Arch Neurol. 2000;57(3):326-332. doi: 10.1001/archneur.57.3.326 [DOI] [PubMed] [Google Scholar]
- 11.Raju N, Sobieraj-Teague M, Hirsh J, O’Donnell M, Eikelboom J. Effect of aspirin on mortality in the primary prevention of cardiovascular disease. Am J Med. 2011;124(7):621-629. doi: 10.1016/j.amjmed.2011.01.018 [DOI] [PubMed] [Google Scholar]
- 12.Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277-287. doi: 10.1001/jama.2018.20578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaziano JM, Brotons C, Coppolecchia R, et al. ; ARRIVE Executive Committee . Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi: 10.1016/S0140-6736(18)31924-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowman L, Mafham M, Wallendszus K, et al. ; ASCEND Study Collaborative Group . Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi: 10.1056/NEJMoa1804988 [DOI] [PubMed] [Google Scholar]
- 15.McNeil JJ, Wolfe R, Woods RL, et al. ; ASPREE Investigator Group . Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi: 10.1056/NEJMoa1805819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Chichester, England: The Cochrane Collaboration; 2011. [Google Scholar]
- 18.An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. 2017;19(1):3-10. doi: 10.5853/jos.2016.00864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. ; Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010); GBD Stroke Experts Group . Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1(5):e259-e281. doi: 10.1016/S2214-109X(13)70089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueshima H, Sekikawa A, Miura K, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118(25):2702-2709. doi: 10.1161/CIRCULATIONAHA.108.790048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392(10145):387-399. doi: 10.1016/S0140-6736(18)31133-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ETDRS Investigators Aspirin effects on mortality and morbidity in patients with diabetes mellitus: Early Treatment Diabetic Retinopathy Study report 14. JAMA. 1992;268(10):1292-1300. doi: 10.1001/jama.1992.03490100090033 [DOI] [PubMed] [Google Scholar]
- 25.The Medical Research Council’s General Practice Research Framework Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet. 1998;351(9098):233-241. doi: 10.1016/S0140-6736(97)11475-1 [DOI] [PubMed] [Google Scholar]
- 26.Belch J, MacCuish A, Campbell I, et al. ; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh . The Prevention of Progression of Arterial Disease and Diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. doi: 10.1136/bmj.a1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Côté R, Battista RN, Abrahamowicz M, Langlois Y, Bourque F, Mackey A; The Asymptomatic Cervical Bruit Study Group . Lack of effect of aspirin in asymptomatic patients with carotid bruits and substantial carotid narrowing. Ann Intern Med. 1995;123(9):649-655. doi: 10.7326/0003-4819-123-9-199511010-00002 [DOI] [PubMed] [Google Scholar]
- 28.Catalano M, Born G, Peto R; Critical Leg Ischaemia Prevention Study (CLIPS) Group . Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med. 2007;261(3):276-284. doi: 10.1111/j.1365-2796.2006.01763.x [DOI] [PubMed] [Google Scholar]
- 29.de Gaetano G; Collaborative Group of the Primary Prevention Project . Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Lancet. 2001;357(9250):89-95. doi: 10.1016/S0140-6736(00)03539-X [DOI] [PubMed] [Google Scholar]
- 30.Erkan D, Harrison MJ, Levy R, et al. Aspirin for primary thrombosis prevention in the antiphospholipid syndrome: a randomized, double-blind, placebo-controlled trial in asymptomatic antiphospholipid antibody-positive individuals. Arthritis Rheum. 2007;56(7):2382-2391. doi: 10.1002/art.22663 [DOI] [PubMed] [Google Scholar]
- 31.Fowkes FG, Price JF, Stewart MC, et al. ; Aspirin for Asymptomatic Atherosclerosis Trialists . Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303(9):841-848. doi: 10.1001/jama.2010.221 [DOI] [PubMed] [Google Scholar]
- 32.Hansson L, Zanchetti A, Carruthers SG, et al. ; HOT Study Group . Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351(9118):1755-1762. doi: 10.1016/S0140-6736(98)04311-6 [DOI] [PubMed] [Google Scholar]
- 33.Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312(23):2510-2520. doi: 10.1001/jama.2014.15690 [DOI] [PubMed] [Google Scholar]
- 34.Landolfi R, Marchioli R, Kutti J, et al. ; European Collaboration on Low-Dose Aspirin in Polycythemia Vera Investigators . Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350(2):114-124. doi: 10.1056/NEJMoa035572 [DOI] [PubMed] [Google Scholar]
- 35.Ogawa H, Nakayama M, Morimoto T, et al. ; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators . Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300(18):2134-2141. doi: 10.1001/jama.2008.623 [DOI] [PubMed] [Google Scholar]
- 36.Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. BMJ (Clin Res Ed). 1988;296(6618):313-316. doi: 10.1136/bmj.296.6618.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293-1304. doi: 10.1056/NEJMoa050613 [DOI] [PubMed] [Google Scholar]
- 38.Steering Committee of the Physicians’ Health Study Research Group Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321(3):129-135. doi: 10.1056/NEJM198907203210301 [DOI] [PubMed] [Google Scholar]
- 39.Klatsky AL, Friedman GD, Sidney S, Kipp H, Kubo A, Armstrong MA. Risk of hemorrhagic stroke in Asian American ethnic groups. Neuroepidemiology. 2005;25(1):26-31. doi: 10.1159/000085310 [DOI] [PubMed] [Google Scholar]
- 40.García-Rodríguez LA, Gaist D, Morton J, Cookson C, González-Pérez A. Antithrombotic drugs and risk of hemorrhagic stroke in the general population. Neurology. 2013;81(6):566-574. doi: 10.1212/WNL.0b013e31829e6ffa [DOI] [PubMed] [Google Scholar]
- 41.Thrift AG, McNeil JJ, Forbes A, Donnan GA; Melbourne Risk Factor Study (MERFS) Group . Risk factors for cerebral hemorrhage in the era of well-controlled hypertension. Stroke. 1996;27(11):2020-2025. doi: 10.1161/01.STR.27.11.2020 [DOI] [PubMed] [Google Scholar]
- 42.Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50(4):309-315. doi: 10.1016/j.jacc.2007.01.098 [DOI] [PubMed] [Google Scholar]
- 43.Chen YW, Lee MJ, Smith EE. Cerebral amyloid angiopathy in East and West. Int J Stroke. 2010;5(5):403-411. doi: 10.1111/j.1747-4949.2010.00466.x [DOI] [PubMed] [Google Scholar]
- 44.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167-176. doi: 10.1016/S1474-4422(09)70340-0 [DOI] [PubMed] [Google Scholar]
- 45.Kroll ME, Green J, Beral V, et al. ; Million Women Study Collaborators . Adiposity and ischemic and hemorrhagic stroke: prospective study in women and meta-analysis. Neurology. 2016;87(14):1473-1481. doi: 10.1212/WNL.0000000000003171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, Katzmarzyk PT, Horswell R, et al. Body mass index and stroke risk among patients with type 2 diabetes mellitus. Stroke. 2015;46(1):164-169. doi: 10.1161/STROKEAHA.114.006718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price AJ, Wright FL, Green J, et al. Differences in risk factors for 3 types of stroke: UK prospective study and meta-analyses. Neurology. 2018;90(4):e298-e306. doi: 10.1212/WNL.0000000000004856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denke MA, Sempos CT, Grundy SM. Excess body weight: an underrecognized contributor to high blood cholesterol levels in white American men. Arch Intern Med. 1993;153(9):1093-1103. doi: 10.1001/archinte.1993.00410090045006 [DOI] [PubMed] [Google Scholar]
- 49.Baechli H, Nordmann A, Bucher HC, Gratzl O. Demographics and prevalent risk factors of chronic subdural haematoma: results of a large single-center cohort study. Neurosurg Rev. 2004;27(4):263-266. doi: 10.1007/s10143-004-0337-6 [DOI] [PubMed] [Google Scholar]
- 50.de Araújo Silva DO, Matis GK, Costa LF, et al. Chronic subdural hematomas and the elderly: surgical results from a series of 125 cases: old ‘horses’ are not to be shot! Surg Neurol Int. 2012;3:150. doi: 10.4103/2152-7806.104744 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow of Study Selection
eFigure 2. Risk of Bias of Included Trials
eFigure 3. Sensitivity Analysis by Excluding ASPREE Trial for Relative Risk with 95% Confidence Interval for Intracranial Hemorrhage (Aspirin vs Placebo or No Aspirin)
eFigure 4. Funnel Plots of Each Outcome
eFigure 5. Relative Risk With 95% Confidence Interval of Subgroup Analysis (Asian and non-Asian)
eFigure 6. Relative Risk With 95% Confidence Interval of Subgroup Analysis (Diabetes and Mostly Nondiabetes)
eFigure 7. Relative Risk With 95% Confidence Interval of Subgroup Analysis (Age≧65 Years and Age <65 Years)
eFigure 8. Relative Risk With 95% Confidence Interval of Subgroup Analysis (Body Mass Index≧25 kg/m2 and <25 kg/m2)
eFigure 9. Relative Risk With 95% Confidence Interval of Subgroup Analysis (Blinding and Nonblinding)