This cohort study analyzes long-term outcomes in a patients with multiple sclerosis according to initial treatment strategy.
Key Points
Question
How do 5-year disability outcomes compare between people with multiple sclerosis who have an early intensive approach to disease-modifying therapy vs those who have an escalation approach?
Findings
In this cohort study including 592 people, those who received high-efficacy treatment initially had a smaller increase in Expanded Disability Status Scale score at 5 years vs those who first received moderate-efficacy disease-modifying therapy.
Meaning
These findings suggest that real-world escalation approaches may be inadequate to prevent unfavorable long-term outcomes and support the need for a prospective clinical trial to compare disease-modifying therapy algorithms.
Abstract
Importance
Uncertainty remains about how aggressively to treat early multiple sclerosis. High-efficacy disease-modifying therapies (DMTs) are often reserved for individuals expressing poor prognostic features at baseline.
Objective
To analyze long-term outcomes in a population-based cohort according to initial treatment strategy.
Design, Setting and Participants
In this cohort study, data were derived from January 1998 to December 2016, and analysis was performed in January 2017. From a total of 720 patients prescribed a DMT, 592 (82%) were included in analysis. Reasons for exclusion were first treated elsewhere or privately (n = 39), clinical trial participant (n = 25), and insufficient clinical data (n = 45).
Exposures
Patients were classified according to first-line treatment strategy: high-efficacy (early intensive treatment [EIT]) or moderate-efficacy DMT (escalation [ESC]).
Main Outcomes and Measures
Primary outcome was 5-year change in Expanded Disability Status Scale score. Secondary outcome was time to sustained accumulation of disability (SAD). Models were adjusted for sex, age at treatment, year of starting DMT, and escalation to high-efficacy treatment in the ESC group.
Results
Mean (SD) age of 592 patients at symptom onset was 27.0 (9.4) years. Mean (SD) 5-year change in Expanded Disability Status Scale score was lower in the EIT group than the ESC group (0.3 [1.5] vs 1.2 [1.5]); this remained significant after adjustment for relevant covariates (β = −0.85; 95% CI, −1.38 to −0.32; P = .002). Median (95% CI) time to SAD was 6.0 (3.17-9.16) years for EIT and 3.14 (2.77-4.00) years for ESC (P = .05). For those within the ESC group who escalated to high-efficacy DMT as second-line treatment, median (95% CI) time to SAD was 3.3 years (1.8-5.6; compared with EIT group log-rank test P = .08). After adjustment for relevant covariates, there was no difference in hazard of SAD between the groups. However, 60% of those who escalated to high-efficacy DMTs were observed to develop SAD while still receiving initial moderate-efficacy treatment before escalation.
Conclusions and Relevance
In a real-life setting, long-term outcomes were more favorable following early intensive therapy vs first-line moderate-efficacy DMT. Contemporary surveillance strategies and escalation protocols may be insufficiently responsive. This finding is particularly relevant as patients in real-world practice are typically selected for an EIT approach to therapy on the basis of clinical and radiological features predictive of a poor outcome. These data support the need for a prospective randomized clinical trial.
Introduction
The introduction of disease-modifying therapies (DMTs) for multiple sclerosis (MS) has been associated with little measurable improvement in long-term disability outcomes.1,2,3,4,5 The reasons for the limited quantifiable impact of these drugs on long-term disability are likely to relate in part to the rapid evolution of the therapeutic landscape in MS and a lack of detailed, prospective population-based disability data with follow-up durations sufficient to capture long-term outcomes. However, it may also be that current treatment strategies are not optimized to deliver the best possible long-term outcomes.
Permanent disability in MS is thought to occur through a number of mechanisms including the sequelae of relapses, the insidious effects of subclinical inflammation, and neurodegeneration. Current DMTs are primarily directed at the inflammatory phase of disease. The modest impact of MS DMTs on long-term disability has led to speculation that the neurodegenerative phase of MS may represent a separate, parallel pathology. However, DMTs have been most effective when aggressive treatments have been applied very early in the clinical course of MS.6,7 It is possible that an early window of therapeutic opportunity exists in which the biology of disease can be modified for longer-term benefit8; however, after a certain period, a threshold is crossed beyond which cumulative immune-mediated injury leads to sustained and progressive neurological disability.
Disease-modifying therapies in MS can be broadly divided according to the efficacy with which they prevent MS relapses. The licensed DMTs with the highest efficacy are associated with more complex safety profiles, monitoring requirements, and the need for hospital or day unit admission. These factors tend to lead clinicians or treatment guidelines to recommend that they be reserved for use in individuals with the most aggressive or resistant forms of MS.9 In those with moderately active MS, clinicians often adopt an escalation approach whereby a DMT is selected that is considered to be most safe, subsequently escalating to more efficacious therapies, with more complex safety profiles, in the event of continued disease activity. However, in light of current knowledge, it is possible that the inevitable delay imposed by escalation strategies may result in a lost therapeutic opportunity.
Several consensus working groups in MS9,10,11 have highlighted the need for further research to establish optimum treatment and monitoring strategies in MS. In this study, we report long-term, real-life clinical outcomes in a large, well-characterized cohort of patients with MS, according to whether they were treated initially with high-efficacy or moderate-efficacy DMT (with subsequent escalation if indicated).
Methods
Patients and Data Collection
This study was undertaken on a population-based cohort of patients with MS in southeast Wales, United Kingdom, which has a total population of 1.4 million from the cities of Cardiff and Newport and the surrounding communities. Data collection for this population has been conducted by means of a cross-sectional epidemiological study in 1985,12 with periodic updates thereafter.13,14 Since 1999, longitudinal data have been gathered prospectively on this population and is estimated to have captured more than 97% of the MS cases in this region15 with a total of more than 3000 patients. At each encounter, data are gathered including relapse history and DMT prescription. Patients undergo a comprehensive clinical assessment at least annually. Disability is measured using the Expanded Disability Status Scale (EDSS).16
Data storage is within a secure National Health Service–hosted web-based departmental database, registered under the Data Protection Act. Written consent was obtained from all patients. The study is approved by the South East Wales Research Ethics Committee (ref No.05/WSE03/111).
Inclusion and Exclusion Criteria
All patients who had ever been prescribed a licensed DMT for MS while living within the region and who had comprehensive long-term follow-up data were included. Patients in whom a DMT had been commenced outside of area, with insufficient data, or who were part of an externally sponsored clinical trial were excluded.
Disease-modifying therapies were classified as follows: monoclonal antibodies (alemtuzumab and natalizumab) were categorized as high efficacy and all other DMTs, as moderate efficacy (interferons, glatiramer acetate, dimethyl fumarate, fingolimod, and teriflunomide).9 Patients’ initial treatment strategy was classified according to whether their first-line treatment was high efficacy (early intensive treatment [EIT] group) or moderate efficacy (escalation [ESC] group). Individuals who received EIT were selected on the basis of poor prognostic factors including higher relapse rates and radiological evidence of recent MS activity. Those individuals who embarked on an ESC strategy with a first-line moderate-efficacy DMT had regular clinical and radiological monitoring and could escalate to a high-efficacy agent if required.
Prospectively recorded data on dates of starting and stopping DMTs (if not current) were used for analysis. Treatment discontinuation was defined as a period of treatment of 90 days or more.17 Where the same DMT was restarted within 90 days of discontinuation, prescriptions were amalgamated. Alemtuzumab was an exception since a standard regimen consists of 2 short courses 1 year apart and no further treatment if patients remain clinically and radiologically stable. Indication and reasons for discontinuation of DMTs were analyzed, noting that more than 1 discontinuation reason may be classified per event. Systematic review of clinical records of all individuals was performed to validate the data set. Data capture began in January 1998 and ended in December 2016. Analysis began in January 2017.
Statistical Analysis
After patient classification, demographic details were compared between EIT and ESC groups using t test or Mann-Whitney U test (if data were not normally distributed) and χ2 test for categorical data. Annualized relapse rates before and after treatment were compared using Mann-Whitney U test. Significance was set at P = .05.
Primary outcome was change in EDSS score at 5 years. Baseline EDSS score was defined as that closest to starting DMT, and final EDSS score was measured 5 years later (both ±1 year). The change in EDSS score from baseline to 5-year follow-up was compared between EIT and ESC groups using a linear regression model. The secondary outcome was sustained accumulation of disability (SAD), defined as an increase in EDSS score of 1.5 if baseline was 0, an increase of 1.0 if baseline was 1.0 to 5.5, or an increase of 0.5 if baseline was 5.5 or higher, sustained for at least 6 months.18 Cox proportional hazards regression modeling was used to compare the hazard of SAD in the EIT and ESC groups. All models were performed both without any adjustment for confounders and also adjusted a priori for known confounders of outcome: sex and age at first starting DMT and calendar year of starting DMT (to address whether secular trends in treatment may have affected the results given the long standby period). For the adjusted models, backward stepwise regression was used to generate a final model containing only treatment strategy and covariates that significantly contributed to the model. Escalation to a high-efficacy DMT for those in the ESC group was included in the linear regression model for change in EDSS score as a binary variable and in the Cox proportional hazards regression model for hazard of SAD as a time-dependent variable.
Two sensitivity analyses were performed. First, we recognize that dichotomizing treatments into 2 levels of efficacy is somewhat arbitrary but is not without precedent.9 In recognition of the fact that a switch from injectables to fingolimod might be regarded as escalation, we classified fingolimod as a high-efficacy treatment and analyzed the data accordingly. Second, an analysis including only those patients treated since 2005 was performed; this is the date from which high-efficacy treatment was freely available in Cardiff, so that a choice between high- and moderate-efficacy DMT at disease onset or diagnosis was always possible. Prior to that date, patients received high-efficacy treatment mainly by referral to another specialist center.
Results
At the time of data extraction, there were 2568 registered patients with MS with complete clinical data from disease onset. Of 720 patients prescribed a DMT before January 2017, 109 (15.1%) were excluded from the analysis (45 had insufficient clinical data, 25 were treated in a clinical trial, 38 were first treated out of area, and 1 was treated via private prescription), leaving 592 (23.1% of the total cohort and 82% of those prescribed a DMT) eligible for inclusion in the current analysis. Of 592 patients, 104 (17.6%) had been prescribed a high-efficacy DMT as first-line (EIT), while 488 patients (82.4%) had commenced DMT with a moderate-efficacy agent (ESC). Individuals who received EIT were most likely to receive alemtuzumab than natalizumab (70 [67%] vs 34 [33%]), while individuals who received high-efficacy therapy second line (as part of an escalation algorithm) were most likely to receive natalizumab than alemtuzumab (43 [74%] vs 15 [26%]).
Of 488 patients who initially embarked on moderate-efficacy treatment, 58 patients (11.9%) subsequently went on to receive a high-efficacy DMT (Figure 1). The reason for escalation was clinical disease activity (relapses) in 52 cases, subclinical radiological evidence of disease activity in 5 cases, and 1 case had no evidence of clinical or radiological evidence of disease activity during the 12 months before escalation.
Figure 1. Flowchart of Participants.
DMT indicates disease-modifying therapy; ESC, escalation approach; EIT, early intensive.
Median time spent on any single disease-modifying drug was 2.0 years (95% CI, 1.8-2.4). In those patients from the ESC group who later escalated to a high-efficacy DMT, the median time to escalation was 2.4 years (95% CI, 2.1-3.5). Sixty-one individuals received fingolimod in the overall cohort, and in the sensitivity analysis were reclassified to EIT (n = 2) and escalation from moderate- to high-efficacy DMT (n = 59).
Comparison of Treatment Strategies
Demographics and Relapse Rates
The baseline characteristics of all patients in the 2 groups are shown in Table 1. There was no difference in the sex ratio (EIT 1:3.2; ESC 1:2.5; P = .37), mean (SD) age at symptom onset (EIT: 29.8 [9.2] years; ESC: 30.3 [9.4] years; P = .73), median (IQR) baseline EDSS score (EIT: 3.5 [2.0-5.0]; ESC: 3.5 [2.0-5.0]; P = .55), or median (SD) follow-up time (EIT: 5.8 [3.6] years; ESC: 6.9 [5.3] years; P = .30) between the EIT and ESC groups. As expected, the pretreatment annualized relapse rate (ARR) was higher in the EIT group (1.7; interquartile range [IQR], 0.9-2.8) than the ESC group (0.7; IQR, 0.4-1.3; P < .001). In retrospect, those who commenced a moderate-efficacy DMT but subsequently escalated to a high-efficacy agent had a pretreatment ARR of 1.2 (IQR, 0.7-2.1) compared with 0.7 (IQR, 0.4-1.2) in those who continued with moderate-efficacy DMT (P < .001).
Table 1. Clinical and Demographic Features of the Escalation and Early Intensive Treatment Cohorts.
| Variable | Treatment Group | P Value | |
|---|---|---|---|
| Early Intensive (n = 104) | Escalation (n = 488) | ||
| Women, No. (%) | 79 (76) | 346 (71) | .37 |
| Age at symptom onset, mean (SD), y | 29.8 (9.2) | 30.2 (9.4) | .73 |
| Age at first DMT, mean (SD), y | 34.0 (9.0) | 38.5 (9.7) | <.001 |
| EDSS score at DMT onset, median (IQR) | 3.5 (2.0-5.0) | 3.5 (2.0-5.0) | .55 |
| Follow-up duration, mean (SD), y | 5.8 (3.6) | 6.9 (5.3) | .30 |
| Baseline (pretreatment) ARR, median (IQR) | 1.7 (0.9-2.8) | 0.7 (0.4-1.3) | <.001 |
| Posttreatment ARR, median (IQR) | 0 (0-0.3) | 0.16 (0-0.5) | .02 |
| Median calendar year of first DMT | 2010 | 2011 | .84 |
Abbreviations: ARR, annualized relapse rate; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; IQR, interquartile range.
The ARR fell in all groups following the introduction of a DMT (Table 1). Following DMT, the ARR was 0.16 (IQR, 0-0.5) in the ESC group and 0 (IQR, 0-0.3) in the EIT group (P = .02). Within the ESC group, the ARR following initial DMT was 0.9 (IQR, 0.7-1.5) in those patients who later escalated to high-efficacy treatment, and ARR was 0 (IQR, 0-0.2) in that group following escalation to high-efficacy DMT. The ARR was 0.11 (IQR, 0-0.4) in the group who continued with moderate-efficacy DMTs (P = .01).
Change in EDSS Score Over 5 Years
Overall, 179 patients (41 in the EIT group and 138 in the ESC group) had EDSS scores available at baseline and at 5-year follow-up. The mean (SD) baseline EDSS score was 4.2 (1.7) for those in the EIT group and 3.5 (1.7) in the ESC group. Similarly, in those patients with both baseline and 5-year EDSS scores available, median (IQR) baseline EDSS was 4.5 (3.0-5.5) in the EIT group and 3.5 (2.0-4.5) in the ESC group. Mean change in EDSS score at 5 years was +0.3 in the EIT group, and +1.2 in the ESC group (β = −0.92; 95% CI, −1.45 to −0.41; P < .001). After adjustment for relevant covariates, the EIT group had a significantly lower change in EDSS score at 5 years compared with the ESC group (β = −0.85; 95% CI, −1.38 to −0.32; P = .002). The only additional covariate to be retained in the final model was age at first DMT treatment (β = 0.03; 95% CI, 0.002-0.05; P = .03). The unadjusted and final adjusted linear regression models are shown in Table 2. There was no change in this finding for either of the sensitivity analyses (data not shown).
Table 2. Association of First-Line DMT Strategy and Change in EDSS Score at 5 Years: Adjusted Linear Regression Modela.
| Covariate | β Estimate (95% CI) | P Value |
|---|---|---|
| Unadjusted model | ||
| EIT treatment strategy | −0.92 (−1.45 to −0.41) | <.001 |
| Final adjusted model | ||
| EIT treatment strategy | −0.85 (−1.38 to −0.32) | .002 |
| Age at starting DMT | 0.03 (−0.002 to 0.05) | .03 |
Abbreviations: DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; EIT, early intensive treatment.
First-line DMT strategy included EIT (n = 41) vs escalation approach (n = 138).
Sustained Accumulation of Disability
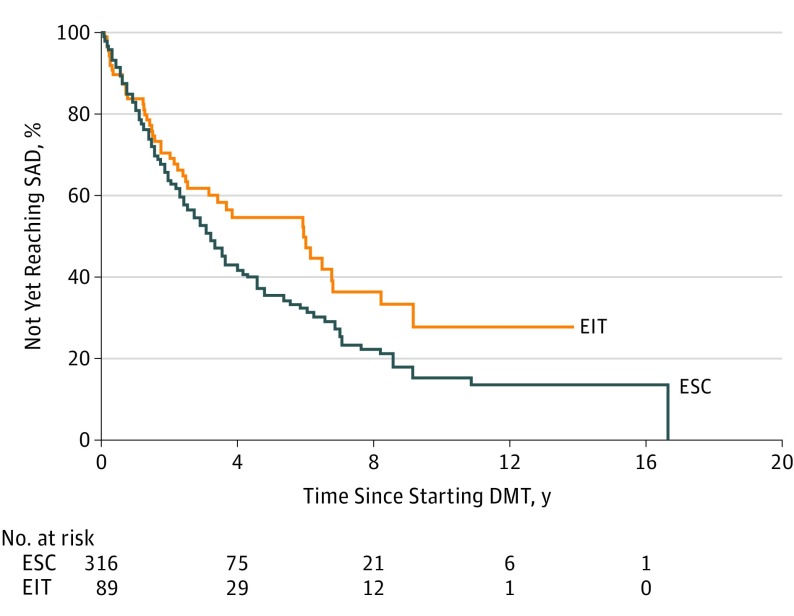
In the total cohort, median time to SAD was 6.0 years (95% CI, 3.4-8.2) for the EIT group, and 3.1 (95% CI, 2.8-4.0) for the ESC group (log-rank test P = .05). For those within the ESC group who escalated to high-efficacy DMT as second-line treatment, median time to SAD was 3.3 years (95% CI, 1.8-5.6; compared with EIT group log-rank test P = .08), and 60% of this group reached SAD while on initial moderate-efficacy treatment before escalation of treatment. For those who continued with moderate-efficacy DMT, median time to SAD was 3.1 years (95% CI, 2.6-4.0; compared with EIT group log-rank test P = .07). Treatment strategy was not associated with hazard of SAD either analyzed alone (hazard ratio, 0.72; 95% CI, 0.52-1.01; P = .05) or after adjustment for relevant covariates (hazard ratio, 0.74; 95% CI, 0.52-1.06; P = .10) (Figure 2). Neither unadjusted nor adjusted model violated the proportional hazards assumption. The results of the Cox proportional hazards regression models are shown in Table 3. Again, results were unchanged in both sensitivity analyses (data not shown).
Figure 2. Time to Sustained Accumulation of Disability by Initial Treatment Strategy .
Adjusted hazard ratio, 0.74; 95% CI, 0.52-1.06; P = .10. DMT indicates disease-modifying therapy; EIT, early intensive treatment; ESC, escalation approach; SAD, sustained accumulation of disability.
Table 3. Association of Treatment Strategy and Hazard of Sustained Accumulation of Disability: Adjusted Cox Proportional Hazards Regression Modela.
| Covariate | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Unadjusted model | ||
| EIT treatment strategy | 0.72 (0.52-1.01) | .05 |
| Final adjusted model | ||
| EIT treatment strategy | –0.74 (0.52-1.06) | .10 |
| Age at starting DMT | 1.02 (1.01- 1.04) | .005 |
Abbreviations: DMT, disease-modifying therapy; EIT, early intensive treatment.
Treatment strategy included EIT (n = 104) vs escalation approach (n = 488).
Safety
Adverse event data on patients receiving alemtuzumab within this cohort has been published previously (n = 100)19: 87% developed infusion-related adverse events, and 47% developed autoimmunity (35 thyroid, 3 immune thrombocytopenic purpura, and 13 other), but there were no serious infections and no treatment-related deaths. In patients receiving natalizumab, there were no serious adverse events, no cases of progressive multifocal leukoencephalopathy, and no treatment-related deaths. In patients receiving moderate-efficacy DMTs, there were no treatment-related deaths but 7 serious adverse events (1.4%): 3 cases of necrotic skin reactions, 1 case of anaphylaxis while receiving injectable DMTs, and 3 severe infections while receiving fingolimod.
Discussion
The concept of escalation vs early intensive treatment strategies has arisen largely as a result of concerns over the complex safety profiles of the high-efficacy DMTs. Contemporary treatment algorithms often suggest reserving first-line high-efficacy treatments for individuals considered at highest risk of accumulating disability, usually those who meet an arbitrarily high level of clinical or radiological MS activity. For an escalation approach to be successful in the remaining cases, it is necessary that adequate procedure is in place to detect and respond to “failure” of first-line moderate-efficacy DMTs without the individual accumulating permanent disability in the interim and that any delay does not diminish the efficacy of subsequent DMTs. These assumptions have not been tested in a randomized clinical trial. The benefit of reserving high-efficacy interventions for those patients perceived to have the most active disease therefore remains unclear.
In this study, we compared long-term outcomes in patients who started receiving EIT with a high-efficacy DMT vs those who commenced receiving an ESC strategy. We found that although patients were selected to receive EIT on the basis of poor prognostic factors including more active disease, it was this patient group that had better long-term outcomes. In patients who started to receive an ESC treatment strategy, there was a mean increase in EDSS score of 1.2 over 5 years despite clinical surveillance and targeted escalation, compared with only 0.3 in the EIT group. Time to SAD appeared delayed in those receiving EIT (6.0 years) compared with those who had an escalation approach (3.1 years), but the hazard of reaching SAD did not remain statistically significant after adjustment for potential confounders. Interestingly, age at first DMT was the only variable identified to be associated with hazard of SAD, whereas in the analysis of 5-year change in EDSS score, the effect size of initial treatment strategy was much greater than that of age at first DMT. This discrepancy may be explained by the observation that in early MS, SAD appears to be driven by relapse activity,20 whereas 5-year EDSS score may better capture the longer-term neurodegenerative component of disability. Our results therefore support the premise that DMT efficacy on relapse activity is largely age dependent21 but raise the possibility that the effect of DMT may be greater than the effect of age on the evolution of longer-term disability.
There could be a number of reasons for the difference in EDSS score outcome identified between EIT and ESC approaches in this study. While it is possible that compliance (time spent on treatment) may have been lower in those on moderate-efficacy DMTs, the data points toward several other contributors. First, those individuals who embarked on an escalation approach but subsequently required escalation to a high-efficacy agent had a higher baseline ARR (1.2) than those who continued with moderate-efficacy therapy (0.7). These data suggest that existing thresholds for using an EIT approach (eg, rapidly evolving severe MS) may be too high. Second, the majority of disability accumulation in the escalation group occurred while these individuals were receiving moderate-efficacy therapies, implying that contemporary methods of clinical and magnetic resonance imaging surveillance were insufficiently responsive to trigger escalation. Meanwhile, the reduction in relapse rate experienced after high-efficacy therapy appeared to be similarly great whether it was given as first-line or as escalation therapy, suggesting that the benefit of high-efficacy therapy on relapse rate does not diminish after a mean delay of 2.4 years.
Limitations
This study is subject to limitations that are common to population cohort data, such as a lack of uniformly acquired imaging or adverse event data compared with clinical trials. Various monitoring algorithms have been proposed to prompt escalation of DMT,22,23,24,25 but none is universally accepted and some are not feasible in all real-world clinical settings.10 We feel that this cohort is likely to represent the manner in which the majority of contemporary cohorts in developed countries were managed during the period. The data have practical relevance and may also provide a measure of the translatability of clinical trial results into general clinical practice, where resources tend to be more limited.
Conclusions
Despite evidence from phase 3 trials that monoclonal antibodies are likely to have superior efficacy than more established DMTs,26,27 the widespread uptake of first-line, high-efficacy DMT has not emerged. Our study undermines the prevalent belief that an escalation approach represents a lower-risk strategy to MS treatment and suggests that in the real world, an escalation approach to DMT may be inadequate to prevent unfavorable long-term outcomes. These data should prompt a more detailed study of whether refined selection and escalation criteria could negate the long-term risk of disability accumulation observed in this escalation cohort.
References
- 1.Shirani A, Zhao Y, Karim ME, et al. . Association between use of interferon beta and progression of disability in patients with relapsing-remitting multiple sclerosis. JAMA. 2012;308(3):247-256. doi: 10.1001/jama.2012.7625 [DOI] [PubMed] [Google Scholar]
- 2.Palace J, Duddy M, Bregenzer T, et al. . Effectiveness and cost-effectiveness of interferon beta and glatiramer acetate in the UK Multiple Sclerosis Risk Sharing Scheme at 6 years: a clinical cohort study with natural history comparator. Lancet Neurol. 2015;14(5):497-505. doi: 10.1016/S1474-4422(15)00018-6 [DOI] [PubMed] [Google Scholar]
- 3.Tilling K, Lawton M, Robertson N, et al. . Modelling disease progression in relapsing-remitting onset multiple sclerosis using multilevel models applied to longitudinal data from two natural history cohorts and one treated cohort. Health Technol Assess. 2016;20(81):1-48. doi: 10.3310/hta20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cree BAC, Gourraud PA, Oksenberg JR, et al. ; University of California, San Francisco MS-EPIC Team . Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol. 2016;80(4):499-510. doi: 10.1002/ana.24747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg BM, Balcer L, Calabresi PA, et al. . Interferon beta use and disability prevention in relapsing-remitting multiple sclerosis. JAMA Neurol. 2013;70(2):248-251. doi: 10.1001/jamaneurol.2013.1017 [DOI] [PubMed] [Google Scholar]
- 6.Coles AJ, Cox A, Le Page E, et al. . The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol. 2006;253(1):98-108. doi: 10.1007/s00415-005-0934-5 [DOI] [PubMed] [Google Scholar]
- 7.Edan G, Comi G, Le Page E, Leray E, Rocca MA, Filippi M; French–Italian Mitoxantrone Interferon-beta-1b Trial Group . Mitoxantrone prior to interferon beta-1b in aggressive relapsing multiple sclerosis: a 3-year randomised trial. J Neurol Neurosurg Psychiatry. 2011;82(12):1344-1350. doi: 10.1136/jnnp.2010.229724 [DOI] [PubMed] [Google Scholar]
- 8.Tremlett H, Yousefi M, Devonshire V, Rieckmann P, Zhao Y; UBC Neurologists . Impact of multiple sclerosis relapses on progression diminishes with time. Neurology. 2009;73(20):1616-1623. doi: 10.1212/WNL.0b013e3181c1e44f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scolding N, Barnes D, Cader S, et al. . Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol. 2015;15(4):273-279. doi: 10.1136/practneurol-2015-001139 [DOI] [PubMed] [Google Scholar]
- 10.Wattjes MP, Rovira À, Miller D, et al. ; MAGNIMS study group . Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis: establishing disease prognosis and monitoring patients. Nat Rev Neurol. 2015;11(10):597-606. doi: 10.1038/nrneurol.2015.157 [DOI] [PubMed] [Google Scholar]
- 11.Lublin FD, Reingold SC, Cohen JA, et al. . Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286. doi: 10.1212/WNL.0000000000000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swingler RJ, Compston DA. The prevalence of multiple sclerosis in south east Wales. J Neurol Neurosurg Psychiatry. 1988;51(12):1520-1524. doi: 10.1136/jnnp.51.12.1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennessy A, Swingler RJ, Compston DA. The incidence and mortality of multiple sclerosis in south east Wales. J Neurol Neurosurg Psychiatry. 1989;52(9):1085-1089. doi: 10.1136/jnnp.52.9.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hennessey A, Robertson NP, Swingler R, Compston DA. Urinary, faecal and sexual dysfunction in patients with multiple sclerosis. J Neurol. 1999;246(11):1027-1032. doi: 10.1007/s004150050508 [DOI] [PubMed] [Google Scholar]
- 15.Hirst C, Ingram G, Pickersgill T, Swingler R, Compston DA, Robertson NP. Increasing prevalence and incidence of multiple sclerosis in South East Wales. J Neurol Neurosurg Psychiatry. 2009;80(4):386-391. doi: 10.1136/jnnp.2008.144667 [DOI] [PubMed] [Google Scholar]
- 16.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. doi: 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 17.Jokubaitis VG, Spelman T, Lechner-Scott J, et al. ; Australian Msbase Study Group . The Australian Multiple Sclerosis (MS) immunotherapy study: a prospective, multicentre study of drug utilisation using the MSBase platform. PLoS One. 2013;8(3):e59694. doi: 10.1371/journal.pone.0059694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coles AJ, Compston DAS, Selmaj KW, et al. ; CAMMS223 Trial Investigators . Alemtuzumab vs interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359(17):1786-1801. doi: 10.1056/NEJMoa0802670 [DOI] [PubMed] [Google Scholar]
- 19.Willis MD, Harding KE, Pickersgill TP, et al. . Alemtuzumab for multiple sclerosis: Long term follow-up in a multi-centre cohort. Mult Scler. 2016;22(9):1215-1223. doi: 10.1177/1352458515614092 [DOI] [PubMed] [Google Scholar]
- 20.Hirst C, Ingram G, Pearson O, Pickersgill T, Scolding N, Robertson N. Contribution of relapses to disability in multiple sclerosis. J Neurol. 2008;255(2):280-287. doi: 10.1007/s00415-008-0743-8 [DOI] [PubMed] [Google Scholar]
- 21.Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577. doi: 10.3389/fneur.2017.00577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sormani MP, Rio J, Tintorè M, et al. . Scoring treatment response in patients with relapsing multiple sclerosis. Mult Scler. 2013;19(5):605-612. doi: 10.1177/1352458512460605 [DOI] [PubMed] [Google Scholar]
- 23.Freedman MS, Selchen D, Arnold DL, et al. ; Canadian Multiple Sclerosis Working Group . Treatment optimization in MS: Canadian MS Working Group updated recommendations. Can J Neurol Sci. 2013;40(3):307-323. [DOI] [PubMed] [Google Scholar]
- 24.Healy BC, Glanz BI, Stankiewicz J, Buckle G, Weiner H, Chitnis T. A method for evaluating treatment switching criteria in multiple sclerosis. Mult Scler. 2010;16(12):1483-1489. doi: 10.1177/1352458510379245 [DOI] [PubMed] [Google Scholar]
- 25.Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord. 2015;4(4):329-333. doi: 10.1016/j.msard.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 26.Polman CH, O’Connor PW, Havrdova E, et al. ; AFFIRM Investigators . A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899-910. doi: 10.1056/NEJMoa044397 [DOI] [PubMed] [Google Scholar]
- 27.Cohen JA, Coles AJ, Arnold DL, et al. ; CARE-MS I investigators . Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819-1828. doi: 10.1016/S0140-6736(12)61769-3 [DOI] [PubMed] [Google Scholar]