Key Points
Question
What are the time trends and associated factors for the incidence of aneurysmal subarachnoid hemorrhage (SAH)?
Findings
In this systematic review and meta-analysis including 75 studies and 8176 patients, the crude global incidence of SAH declined from 10.2 per 100 000 person-years in 1980 to 6.1 in 2010, but large variation according to region, age, and sex exists. The global incidence of SAH decreased by 7.1% with every millimeter of mercury decrease in systolic blood pressure, 11.5% for every millimeter of mercury decrease in diastolic blood pressure, and 2.4% for every percentage decrease in smoking prevalence.
Meaning
Understanding determinants for regional differences and further reducing blood pressure and smoking prevalence may yield a diminished SAH burden.
This systematic review and meta-analysis assesses worldwide aneurysmal subarachnoid hemorrhage incidence according to region, age, sex, time period, blood pressure, and smoking prevalence.
Abstract
Importance
Subarachnoid hemorrhage (SAH) from ruptured intracranial aneurysms is a subset of stroke with high fatality and morbidity. Better understanding of a change in incidence over time and of factors associated with this change could facilitate primary prevention.
Objective
To assess worldwide SAH incidence according to region, age, sex, time period, blood pressure, and smoking prevalence.
Data Sources
We searched PubMed, Web of Science, and Embase for studies on SAH incidence published between January 1960 and March 2017. Worldwide blood pressure and smoking prevalence data were extracted from the Noncommunicable Disease Risk Factor and Global Burden of Disease data sets.
Study Selection
Population-based studies with prospective designs representative of the entire study population according to predefined criteria.
Data Extraction and Synthesis
Two reviewers independently extracted data according to PRISMA guidelines. Incidence of SAH was calculated per 100 000 person-years, and risk ratios (RRs) including 95% CIs were calculated with multivariable random-effects binomial regression. The association of SAH incidence with blood pressure and smoking prevalence was assessed with linear regression.
Main Outcomes and Measures
Incidence of SAH.
Results
A total of 75 studies from 32 countries were included. These studies comprised 8176 patients with SAH were studied over 67 746 051 person-years. Overall crude SAH incidence across all midyears was 7.9 (95% CI, 6.9-9.0) per 100 000 person-years; the RR for women was 1.3 (95% CI, 0.98-1.7). Compared with men aged 45 to 54 years, the RR in Japanese women older than 75 years was 2.5 (95% CI, 1.8-3.4) and in European women older than 75 years was 1.5 (95% CI, 0.9-2.5). Global SAH incidence declined from 10.2 (95% CI, 8.4-12.5) per 100 000 person-years in 1980 to 6.1 (95% CI, 4.9-7.5) in 2010 or by 1.7% (95% CI, 0.6-2.8) annually between 1955 and 2014. Incidence of SAH declined between 1980 and 2010 by 40.6% in Europe, 46.2% in Asia, and 14.0% in North America and increased by 59.1% in Japan. The global SAH incidence declined with every millimeter of mercury decrease in systolic blood pressure by 7.1% (95% CI, 5.8-8.4) and with every percentage decrease in smoking prevalence by 2.4% (95% CI, 1.6-3.3).
Conclusions and Relevance
Worldwide SAH incidence and its decline show large regional differences and parallel the decrease in blood pressure and smoking prevalence. Understanding determinants for regional differences and further reducing blood pressure and smoking prevalence may yield a diminished SAH burden.
Introduction
Subarachnoid hemorrhage (SAH) from a ruptured intracranial aneurysm accounts for 5% of all strokes and carries an exceptionally high disease-specific burden; half of patients with SAH are younger than 55 years, one-third die within the initial days to weeks after the hemorrhage, and most survivors have long-term disability or cognitive impairment.1 On a community level, the loss of productive life-years after SAH is similar in magnitude to that of ischemic stroke. The crude incidence of SAH was previously estimated to be 9 per 100 000 person-years but varied considerably according to geographic location, age, and sex.2 More recently, register-based or regional studies have reported conflicting data on reduction of SAH incidence over time.3,4 If SAH incidence has indeed declined and potential determinants for such a decline could be detected on a population-based level, then this would have important implications for primary prevention strategies and thus reduction of the burden of disease in patients with SAH. We aimed to investigate worldwide and age-specific and sex-specific incidences of SAH according to region, age, sex, time period, blood pressure, and smoking prevalence in the population.5,6
Methods
Search Strategy and Selection Criteria
To identify population-based studies on the incidence of SAH published between January 1960 and March 2017, we performed a systematic review of PubMed, Web of Science, and Embase using the keywords “subarachnoid hemorrhage” or “subarachnoid haemorrhage” and “incidence,” “epidemiology,” or “population,” building on our previous studies.2,7 In addition, we cross-referenced the list of studies with the personal database of references of one of the authors (G.J.E.R.) to include missing studies. Our study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Inclusion criteria were (1) a prospective study design, (2) the study population being representative of the studied population in general, (3) SAH reported as an individual entity, (4) the data including crude figures or enabling crude calculation of SAH incidence, (5) case findings permitting inclusion of all hospitals in the region and either involvement of general practitioners or review of death certificates by the study investigator(s), (6) diagnostic verification, including a neuroimaging rate greater than 80% or at least lumbar puncture or autopsy, (7) an upper age limit for the study not lower than 75 years, and (8) a lower age limit not greater than 25 years. Registry-based, hospital-based, and cohort studies, studies on nonaneurysmal SAH, and studies reporting data only for specific ethnic groups were excluded. In studies reporting on similar study populations or study periods, we only included the most recent study or the study with the greater amount of person-years or more sophisticated case finding methods.
Data Extraction
For studies published between October 2005 and March 2017, 2 reviewers (H.-S.C. and K.H.) assessed all newly retrieved studies independent from each other and registered the following items in a data extraction form: (1) size of study population; (2) study region; (3) midyear and study period; (4) number of patients with SAH; (5) case finding methods for SAH; (6) diagnostic criteria for SAH; and (7) age-specific and sex-specific incidence. For previously identified studies, we used data we had extracted in the same fashion.2,7 Excellent diagnostics was defined as greater than 90% of patients with SAH being diagnosed with brain imaging (computed tomography [CT] or magnetic resonance imaging). In the event of disagreement on extracted data, the article was assessed by 2 other authors (N.E. and M.D.I.V.) independently from each other and discussed between the 4 authors until agreement was achieved. We used incidence rates relating to the entire population, without adjustment for age or sex. To determine the association of SAH incidence with age and sex, we collected age-specific and sex-specific incidence from the subset of studies that provided sufficient data. Study investigators were contacted for missing data on crude incidence of SAH when required. Since only 14% of studies included in our meta-analysis reported data on smoking or hypertension prevalence in patients with SAH and not for the entire reported population, we extracted age-specific, sex-specific, midyear-specific, and country-specific systolic and diastolic blood pressure values from the Noncommunicable Disease Risk Factor (NCD) data set5 and smoking prevalence data from the Global Burden of Disease Study6 to analyze their association with SAH incidence within the age-specific and sex-specific SAH incidence data set.
Data Analyses
For each of the included studies, the crude SAH incidence with corresponding 95% CIs was calculated with Poisson methods. For all subsequent analyses, we used random-effects binomial regression, with the number of SAHs and the number of person-years for each study as variables (RMA.GLMN module in R version 3.4.3 [The R Foundation]). Overall and regional incidences were estimated based on the models’ intercept; in addition, I2 was calculated as a measure of variation, ie, heterogeneity, between the included studies.8 We considered an I2 of 25% to 49% as low, 50% to 74% as moderate, and 75% or greater as high heterogeneity. To assess regional differences, we compared studies by continent or country. Next, we determined the association of age, sex, and time period with the incidence of SAH. In addition, we studied regional patterns of the association of age, sex, and time period with SAH incidence. To estimate current SAH incidence vs previous SAH incidence, we used the regression models and midyear cut-off of all studies (1996) within 15 years, which, after rounding, corresponded to 2010 (current SAH incidence) and 1980 (historic SAH incidence). In the age-specific and sex-specific data subset, we adjusted the time trend analyses for age and sex. In addition, we determined the association of blood pressure and smoking prevalence with SAH incidence. To describe patterns of blood pressure and smoking prevalence over time, we used linear regression analysis. Multivariable regression was used to estimate the (independent) contribution of continent, age, and sex as well as time trends to SAH incidence. Since our previous meta-analysis found a higher SAH incidence in Finland and Japan compared with the reference population,2 we specifically analyzed the SAH incidence according to time for these countries. Included studies were reviewed for adherence to core and/or supplemental criteria for population-based stroke studies in relation to the midyear cutoff of all studies (1996).9 In sensitivity analyses, we restricted the time trend analysis to studies with at least 90% cranial scanning as well as studies with a midyear of study of 1985 at the earliest and with data on percentage of CT scanning.
Results
Data from 75 population-based studies and 84 study periods were included (eFigure 1 in the Supplement). For all 75 studies (range of midyears, 1955-2014), 55 (73%) fulfilled core criteria for population-based studies. For the 56 studies published after 1996, 48 studies (86%) fulfilled these criteria.
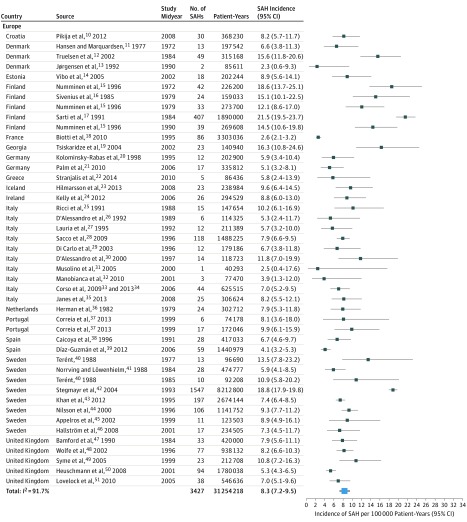
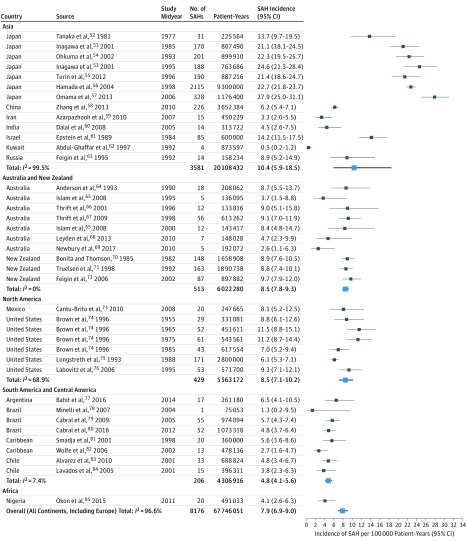
These studies described 8176 patients with SAH over 67 746 051 person-years from 32 countries and 6 continents. The median percentage of cranial imaging for detection of SAH was 91% (interquartile range, 78-97). Incidences of SAH by region, population size, study midyear, and case finding method as well as the diagnostic criteria for each study are listed in eTable 1 in the Supplement. Summary characteristics of the overall and age-specific and sex-specific data sets are provided in eTable 2 in the Supplement. Overall crude SAH incidence across all midyears was 7.9 (95% CI, 6.9-9.0) per 100 000 person-years (I2 = 96.6%) (Figure 110,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51 and Figure 252,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85).
Figure 1. Crude Subarachnoid Hemorrhage (SAH) Incidence by Country and Midyear in Europe.
Crude SAH incidence per 100 000 person-years with 95% CIs are presented according to country and midyear in Europe.
Figure 2. Crude Subarachnoid Hemorrhage (SAH) Incidence by Continent, Country, and Midyear.
Crude SAH incidence per 100 000 person-years with 95% CIs are presented according to continent, country, and midyear. I2 values are calculated per continent and overall including Europe (Figure 1).
SAH Incidence by Region and Time Trends
The crude worldwide SAH incidence (84 study periods) was 6.1 (95% CI, 4.9-7.5) per 100 000 person-years for 2010 and 10.2 (95% CI, 8.4-12.5) for 1980 and declined annually by 1.7% (95% CI, 0.6-2.8) between 1955 and 2014. Figure 1 and Figure 2 display the crude SAH incidences by continent and region. The SAH incidence for 2010 in Europe (45 study periods) was estimated to be 6.3 (95% CI, 4.9-8.1) per 100 000 person-years and declined annually by 1.7% (95% CI, 0.4-3.1) since 1972.10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51 For Finland (5 study periods), the SAH incidence was 16.6 (95% CI, 13.4-20.5) per 100 000 person-years, with no clear change over time (1972-1990); after 1990, no new studies fulfilling our inclusion criteria appeared. For Asia (13 study periods), the SAH incidence for 2010 was estimated to be 7.7 (95% CI, 2.8-21.7) per 100 000 person-years, and the annual decline was 2.0% (95% CI, −3.9 to 7.9) since 1977.52,53,54,55,56,57,58,59,60,61,62,63 Since 7 of 13 study periods from Asia were from Japan alone and there was a large heterogeneity between Japan and the remainder of Asia, data for Japan are presented separately. The SAH incidence in Japan was estimated to be 28.0 (95% CI, 25.3-31.0) per 100 000 person-years and increased annually by 1.6% (95% CI, 0.8-2.3) since 1977.52,53,54,55,56,57 In Asia excluding Japan, the SAH incidence for 2010 was estimated to be 3.7 (95% CI, 0.1-13.3) per 100 000 person-years, and the annual decline since 1984 was 1.3% (95% CI, −7.2 to 9.8).58,59,60,61,62,63 For North America (7 study periods), the SAH incidence for 2010 was estimated to be 6.9 (95% CI, 4.8-10.0) per 100 000 person-years, and the annual decline was 0.7% (95% CI, −0.4 to 1.8) since 1955.73,74,75,76 Crude incidences for SAH, risk ratios (RRs) for SAH incidence, and time trends for all geographical locations, including Middle and South America (8 study periods),77,78,79,80,81,82,83,84 Australia and New Zealand (10 study periods),64,65,66,67,68,69,70,71,72 and Africa (Nigeria),85 are summarized in Table 1. Continental and regional SAH incidences and time trends from studies reporting incidence data in the same population over several time periods are highlighted in eFigure 2 in the Supplement. In this subset of studies, there was a decrease in SAH incidence in most studies from Europe11,13,15,40,47,48,50,51 and the study from North America74 and an increase in SAH incidence in the 2 studies from Japan.53,86
Table 1. Subarachnoid Hemorrhage (SAH) Incidence, Risk Ratios (RRs), Midyear Range, and Time Trends by Region.
| Geographic Location | No. of Study Periods | Overall SAH Incidence (95% CI) | I2, % | RR of Overall Incidence (95% CI) | Time Trends | |||
|---|---|---|---|---|---|---|---|---|
| Range of Midyears | SAH Incidence (95% CI) | Annual Change, % (95% CI) | ||||||
| 1980 | 2010 | |||||||
| Global | 84 | 7.9 (6.9 to 9.0) | 96.6 | NA | 1955-2014 | 10.2 (8.4 to 12.5) | 6.1 (4.9 to 7.5) | −1.7 (−2.8 to −0.6) |
| Europe overall | 45 | 8.3 (7.2 to 9.5) | 91.7 | 1 [Reference] | 1972-2010 | 10.6 (8.5 to 13.3) | 6.3 (4.9 to 8.1) | −1.7 (−3.1 to −0.4) |
| Europe without Finland | 40 | 7.5 (6.6 to 8.6) | 87.6 | 1 [Reference]a | 1972-2010 | 8.4 (6.4 to 11.1) | 6.9 (5.4 to 8.8) | −0.7 (−2.2 to 0.8) |
| Finland | 5 | 16.6 (13.4 to 20.5)b | 65.8 | 2.1 (1.5 to 3.1)a | 1972-1990 | 16.6 (13.4 to 20.6) | ND | −0.1 (−3.5 to 3.5) |
| Asia overall | 13 | 10.4 (5.9 to 18.6) | 99.5 | 1.4 (1.0 to 1.9) | 1977-2010 | 14.3 (4.9 to 41.5) | 7.7 (2.8 to 21.7) | −2.0 (−7.9 to 3.9) |
| Asia without Japan | 6 | 4.3 (0.5 to 36.4) | 95.5 | 0.6 (0.4 to 0.9) | 1984-2010 | 5.5 (0.9 to 32.2) | 3.7 (0.1 to 13.3) | −1.3 (−9.8 to 7.2) |
| Japan | 7 | 22.5 (20.3 to 24.9) | 78.5 | 2.6 (1.8 to 3.9) | 1977-2006 | 17.6 (15.4 to 20.1) | 28.0 (25.3 to 31.0) | 1.6 (0.8 to 2.3) |
| Australia/New Zealand | 10 | 8.5 (7.8 to 9.3) | 0 | 0.9 (0.7 to 0.9) | 1982-2010 | 9.4 (0.8 to 11.0) | 7.4 (6.0 to 9.2) | −0.8 (−1.8 to 0.3) |
| North America | 7 | 8.5 (7.1 to 10.2) | 68.9 | 0.7 (0.6 to 0.8) | 1955-2008 | 8.0 (7.2 to 10.1) | 6.9 (4.8 to 10.0) | −0.7 (−1.8 to 0.4) |
| South/Middle America | 8 | 4.8 (4.1 to 5.6) | 7.4 | 0.5 (0.4 to 0.8) | 1998-2014 | 3.3 (1.6 to 7.1) | 5.1 (4.2 to 6.1) | 1.4 (−1.5 to 4.3) |
| Africa | 1 | 4.1 (2.6 to 6.3) | NA | 0.5 (0.2 to 1.5) | 2011 | NA | NA | NA |
Abbreviations: NA, not applicable; ND, no data.
Europe without Finland; the RR for Finland is in comparison with this reference. For the remainder of the comparison, Europe as a whole including Finland is used as the reference value.
The overall Finnish SAH incidence is derived from incidence studies with a midyear range from 1972 to 1990.
The sensitivity analysis for the overall decline in the 40 studies that had at least 90% CT scanning14,18,20,21,22,23,24,27,28,29,30,32,34,35,37,39,44,49,51,53,54,56,57,58,59,62,66,68,69,71,72,75,76,77,78,79,80,81,82,84 demonstrated an annual decrease in SAH incidence of 2.1% (95% CI, −1.0 to 5.0). The 63 studies with a midyear of study after 1985 and data on percentage of CT scanning10,13,14,15,18,19,20,21,22,23,24,25,27,28,29,30,31,32,33,34,35,37,38,39,42,43,44,45,48,49,50,51,53,54,55,56,57,58,59,60,62,63,64,65,66,67,68,69,71,72,73,75,76,77,78,79,80,81,82,83,84 showed an annual decrease in SAH incidence of 1.4% (95% CI, −1.0 to 3.7) and an annual decrease in SAH incidence after adjustment for percentage of CT scanning of 1.3% (95% CI, −1.2 to 3.8).
SAH Incidence Stratified by Age, Sex, Region, and Time Trends
Twenty-nine studies10,19,20,27,28,29,32,34,36,43,51,52,53,57,58,64,66,67,72,73,77,78,79,83,84,85,86,87,88 with data on 34 study periods reported age-specific and sex-specific SAH incidence for 2133 patients with SAH over 17 029 016 person-years in 18 countries and 6 continents (eTable 2 in the Supplement). In this subset of studies, the overall incidence was 10.3 (95% CI, 9.0-11.9). Irrespective of geographical location, the incidence of SAH increased with increasing age but increased distinctly more in women older than 55 years (eFigure 3 and eTables 3 and 4 in the Supplement). The increase of SAH incidence associated with increasing age was higher in Japanese women older than 75 years (RR, 2.5; 95% CI, 1.8-3.4) than in European women older than 75 years (RR, 1.5; 95% CI, 0.9-2.5) compared with men aged 45 to 54 years from the same region.
The overall sex-specific incidence of SAH was 11.5 (95% CI, 9.5-13.9) per 100 000 person-years in women vs 9.3 (95% CI, 7.7-11.3) in men; the RR for women was 1.3 (95% CI, 0.98-1.7), which remained essentially the same after adjustment for midyear of study. In Europe, the SAH incidence was 12.5 (95% CI, 10.1-15.4) per 100 000 person-years in women and 10.7 (95% CI, 8.2-13.9) in men; the RR for women was 1.1 (95% CI, 0.8-1.5). In Japan, the incidence in women was 22.9 (95% CI, 15.7-33.5) per 100 000 person-years and in men was 19.5 (95% CI, 14.2-26.8); the RR for women was 1.3 (95% CI, 0.8-2.1). In Asia overall, the SAH incidence in women was 17.8 (95% CI, 12.4-25.7) per 100 000 person-years and in men was 14.8 (95% CI, 10.8-20.3); the RR for women was 1.3 (95% CI, 0.8-2.1) (eTable 4 in the Supplement). The annual decline in overall SAH incidence was 2.2% (95% CI, 0.7-3.7) in the sex-adjusted analysis and 2.3% (95% CI, 1.2-3.3) in the age-adjusted analysis (eTable 5 in the Supplement). This decline was more apparent in men (3.4%; 95% CI, 1.9-4.8) than women (1.3%; 95% CI, −0.3 to 2.8). After adjustment for age, SAH incidence in Europe tended to decline (annual decline, 0.7%; 95% CI, −1.3 to 2.7); this tendency was more visible in men. In Japan, there was an increase in crude SAH incidence (annual increase, 4.3%; 95% CI, 1.3-7.3) and sex-adjusted SAH incidence (annual increase, 4.2%; 95% CI, 1.3-7.2), which was no longer statistically significant after adjustment for age (annual increase, 0.9%; 95% CI, −0.6 to 2.4).
Association of Blood Pressure Levels and Smoking Prevalence With SAH Incidence by Region and Time Trends
Data on systolic and diastolic blood pressures were available for 18 countries and 34 study periods for which age-specific, sex-specific, midyear-specific, and country-specific SAH incidence data were available. Mean systolic and diastolic blood pressures, smoking prevalence, annual changes, and 1980 and 2010 estimates are given in Table 2. The annual changes in systolic and diastolic blood pressure by age category and sex between 1980 and 2010 are given in eTable 6 in the Supplement.
Table 2. Association of Mean Blood Pressure (BP) and Smoking Prevalence Time Trends With Subarachnoid Hemorrhage (SAH) Incidencea.
| Measure | Mean (Range) | Annual Change, % (95% CI) | 1980 Estimate (Range) | 2010 Estimate (Range) | Annual Decrease in SAH Incidence per Unit Decrease, % (95% CI) |
|---|---|---|---|---|---|
| Systolic BP, mm Hg | 134.9 (106.4 to 161.8) | −0.2 (−0.3 to −0.1) | 120.4 (118.6 to 122.2) | 114.6 (112.9 to 116.3) | 7.1 (5.8 to 8.4) |
| Diastolic BP, mm Hg | 79.1 (65.1 to 88.1) | 0.01 (−0.02 to 0.04) | 70.6 (69.5 to 71.6) | 70.8 (69.8 to 71.7) | 11.5 (8.8 to 14.3) |
| Smoking prevalence, % | 19.3 (0.3 to 72.9) | −0.5 (−0.6 to −0.4) | 26.5 (22.7 to 30.3) | 11.9 (8.4. to 15.4) | 2.4 (1.6 to 3.3) |
All analyses are adjusted for age and sex.
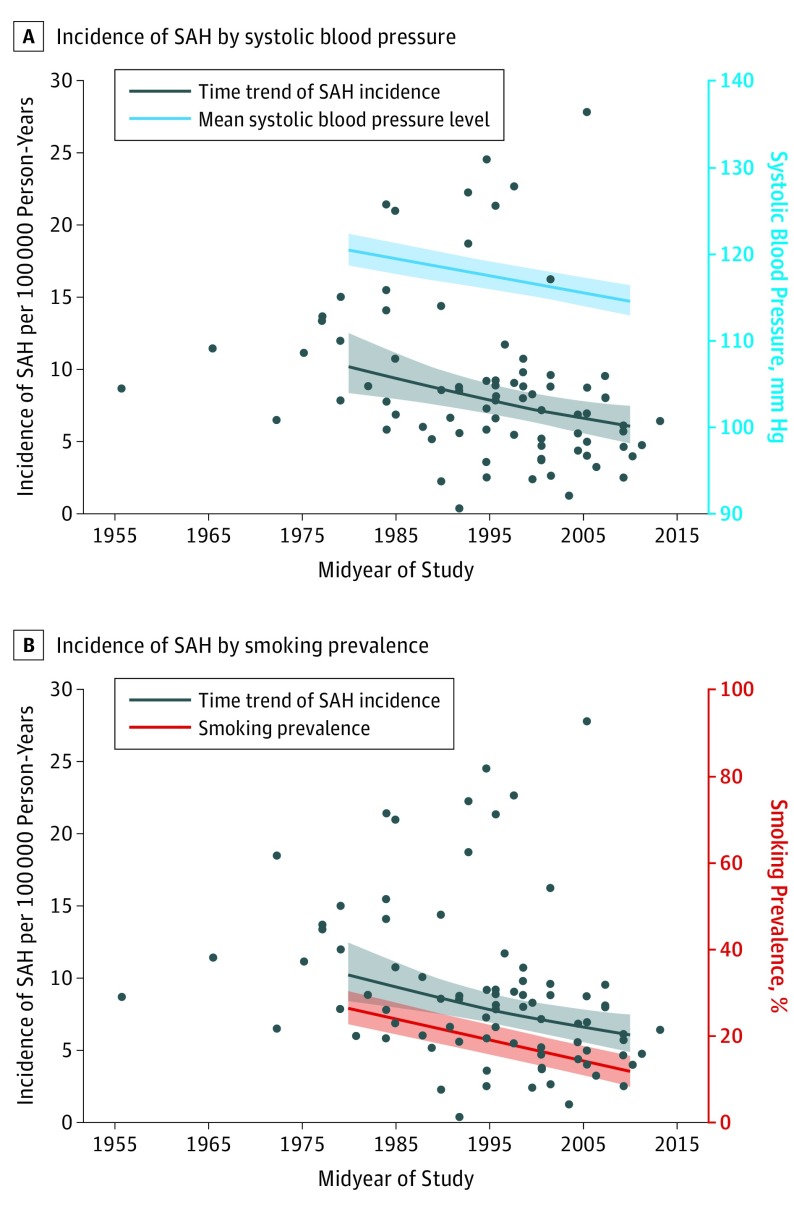
With every millimeter of mercury decrease in systolic blood pressure, the overall age-adjusted and sex-adjusted incidence of SAH declined by 7.1% (95% CI, 5.8-8.4) (Table 2) (Figure 3A). With every millimeter of mercury decrease in diastolic blood pressure, the overall age-adjusted and sex-adjusted incidence of SAH declined by 11.5% (95% CI, 8.8-14.3). With every percentage decrease in smoking prevalence, the overall age-adjusted and sex-adjusted incidence of SAH decreased by 2.4% (95% CI, 1.6-3.3) (Figure 3B). The limited number of data points for individual countries did not permit robust analyses on the association of blood pressure and smoking prevalence with SAH incidence by country, age, sex, and time period.
Figure 3. Association of Time Trends of Blood Pressure and Smoking Prevalence With Subarachnoid Hemorrhage (SAH) Incidence.
A, Time trends in SAH incidence in all studies by midyear are presented irrespective of age and sex (black dots). The black line indicates the regression/time trend of SAH incidence with markers for mean estimated incidence for 1980 and 2010. The blue line indicates mean systolic blood pressure levels in studies included in the age-specific and sex-specific analyses, with markers for mean estimated systolic blood pressure levels for 1980 and 2010. B, Time trends in SAH incidence in all studies by midyear are presented irrespective of age and sex (black dots). The black line indicates the regression/time trend of SAH incidence with markers for mean estimated incidence for 1980 and 2010. The red line indicates smoking prevalence in studies included in the age-specific and sex-specific analyses, with markers for smoking prevalence in 1980 and 2010.
Discussion
The crude global incidence of SAH has declined by 40% between 1980 and 2010, but there is large variation of SAH incidence according to age, sex, region, time period, blood pressure, and smoking prevalence. Between 1980 and 2010, SAH incidence declined by 40.6% in Europe, 46.2% in Asia, and 14.0% in North America. The global decrease in SAH incidence paralleled a global decrease in mean blood pressure and smoking prevalence. In Japan, the SAH incidence increased by 59.1% over the last 3 decades. The higher SAH incidence in women older than 55 years was striking in Japan and no longer statistically significant in Europe after adjustment.
A 2014 meta-analysis on worldwide stroke incidence including 56 population-based studies89—to our knowledge, the most recent—did not detect a decrease in crude SAH incidence between 1980 and 2008. However, the overall proportion of studies reporting specific data on aneurysmal SAH incidence in that study may have been too limited to permit robust analysis of time trends. For Finland, a country with a previously reported high SAH incidence, no studies fulfilled our inclusion criteria after 1990, and therefore we could not assess a change over time. A recent register-based Finnish study,3 which also did not fulfill our inclusion criteria, reported an age-standardized decrease in SAH incidence by 24% between 1998 and 2012 along with a simultaneous decrease in smoking prevalence by 30%. The crude SAH incidence for 2010 in Finland was estimated in that study to be 9.1 per 100 000 person-years.3 This incidence is lower than our current estimate for the pre-1990 studies (ie, 16.6 per 100 000 person-years), which suggests that the incidence has also declined in Finland. Nevertheless, the estimated incidence from this registry study is still higher than the current SAH incidence in other European countries in our data.
There are several potential explanations for our findings of a decline in global SAH incidence over the last decades. First, the global SAH incidence may have declined because of a parallel decline in blood pressure and smoking prevalence in the underlying populations. Because of the nature of our study, we cannot draw causal conclusions, but it is highly likely that the evident decrease in the global prevalence of 2 major risk factors for SAH resulted in the decline of its incidence.90 For smoking alone, such an association has been recently reported in a registry-based incidence study from Finland.3 However, it remains uncertain why the SAH incidence in Japan has increased substantially, despite the concomitant global decline. Hypothetically, this could be a consequence of the distinctly higher crude prevalence of smoking in Japan (26.1%) compared with the global population (19.3%) in our data set. Unfortunately, the limited age-specific, sex-specific, midyear-specific, and country-specific SAH incidence data did not permit further regression analyses in this respect.
Second, preventive repair of unruptured intracranial aneurysms (UIAs) could have resulted in a subsequent decrease of SAH incidence in the underlying populations. However, we consider it unlikely as the sole explanation because of the enormous number of interventions that should have been performed to reach such a reduction; to achieve a 10% reduction in SAH incidence, at least 15 million UIAs should have been treated preventively (assuming a UIA prevalence of 3% in the adult population and 165 million UIAs worldwide). In the United States alone, only about 12 000 Medicare patients underwent preventive aneurysm treatment in 1 decade.91 Third, a decrease in the prevalence of UIAs over the past decades could have resulted in a decline of SAH incidence, but such a trend was not found in the most recent pooled analysis.92
Fourth, one could argue that our data on SAH incidence time trends are explained by the trend of increased proportions of CT scanning over time, which was the main explanation for the decline in SAH incidence almost 2 decades ago.8 However, our sensitivity analyses underline that the decline in SAH incidence in our study is genuine and is only partially explained by the increased use of CT scanning over time, especially because of the generally high proportion of cranial imaging (mean percentage of CT scanning, 89.6%) in studies published after 1985. Theoretically, a change in incidence over time within a population may also be caused by a change in proportions of race/ethnicity within that population over time. However, because none of the populations where incidence was studied in several time periods provided data on change in race/ethnicity over time within the population, we were not able to further analyze this aspect.
Strengths and Limitations
A strength of our study is that it comprises, to our knowledge, the most rigorous and geographically and chronologically dispersed data set on SAH incidence specifically and solely derived from population-based studies to date. We had strict inclusion criteria to ensure detection of patients dying before reaching the hospital. Since we also found little variation in case ascertainment and diagnostic criteria in our meta-analysis and since the proportion of patients who die suddenly is around 12%, it is unlikely that the large regional variation in incidence found in this systematic review is explained only by differences in case finding and diagnostic criteria between the studies.93 Furthermore, our meta-analysis is the first to our knowledge to find an association of time trends of blood pressure and smoking prevalence with SAH incidence.
Our study had limitations. There were limited population-based data on SAH incidence for most of Africa and large Eastern populations, including China, Russia, and India. We chose the population-based study design over a registry-based design in favor of high-quality data and under the premise that study populations are representative of the population of that country. However, variations in SAH incidence on a regional level may exist, which may not be captured by the study population that represented a specific country. Further, a limitation of such an ecological study design is that one cannot study causal relationships on an individual patient level, and such a design harbors the risk of confounding relations. We minimized the risk of confounding by means of adjusted regression analyses. Nevertheless, we underline that our findings are no more than associations and that causal relationships between decline in blood pressure or smoking prevalence and SAH incidence can only be accurately studied when quantitative data for these risk factors become available on a population-based or individual patient level. Finally, not all patients with SAH included in the parent studies underwent angiography. On the one hand, this may have led to an overestimation of the incidence because instances of nonaneurysmal SAH may have been included. On the other hand, restricting to angiographically confirmed aneurysmal SAH inevitably induces an underestimation of the actual incidence because not all patients reach hospitals alive, and in the pre-CT angiography era, catheter angiography was only done if the patient was eligible for aneurysm treatment.
Conclusions
The association we found between blood pressure and smoking prevalence reduction with decrease in SAH incidence further supports control of these risk factors to reduce SAH burden. Future studies should address the regional differences in SAH incidence and its decline, regional differences in age-specific and sex-specific incidences, and their association with actual quantitative data on smoking. Explanations for these differences may help to further decrease SAH incidence. The reasons for the increasing incidence of SAH in Japan remain unclear.
eTable 1. Summary of all 75 included studies and 84 study periods, according to ascending midyear of study.
eTable 2. Characteristics of the overall and age-specific and sex-specific datasets.
eTable 3. Crude SAH incidence with 95% CIs and risk ratios for SAH in the age-specific and sex-specific dataset.
eTable 4. Overall and age-specific risk ratios in women vs men in the age-specific and sex-specific dataset (34 study periods).
eTable 5. Time trends in SAH incidence in the in the age-specific and sex-specific dataset (34 study periods).
eTable 6. Estimated blood pressures in 1980 and 2010 for women and men aged 45-54 years and 55-74 years including annual change from 1977 to 2014.
eFigure 1. Selection of studies.
eFigure 2. Regional time trends for studies reporting on consecutive SAH incidence for the same study population.
eFigure 3. Crude incidence of SAH in age-specific and sex-specific dataset overall, in Europe, and in Japan only.
References
- 1.Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8(7):635-642. doi: 10.1016/S1474-4422(09)70126-7 [DOI] [PubMed] [Google Scholar]
- 2.de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78(12):1365-1372. doi: 10.1136/jnnp.2007.117655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korja M, Lehto H, Juvela S, Kaprio J. Incidence of subarachnoid hemorrhage is decreasing together with decreasing smoking rates. Neurology. 2016;87(11):1118-1123. doi: 10.1212/WNL.0000000000003091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackey J, Khoury JC, Alwell K, et al. . Stable incidence but declining case-fatality rates of subarachnoid hemorrhage in a population. Neurology. 2016;87(21):2192-2197. doi: 10.1212/WNL.0000000000003353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet. 2017;389(10064):37-55. doi: 10.1016/S0140-6736(16)31919-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GBD 2015 Tobacco Collaborators Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017;389(10082):1885-1906. doi: 10.1016/S0140-6736(17)30819-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linn FH, Rinkel GJ, Algra A, van Gijn J. Incidence of subarachnoid hemorrhage: role of region, year, and rate of computed tomography: a meta-analysis. Stroke. 1996;27(4):625-629. doi: 10.1161/01.STR.27.4.625 [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudlow CL, Warlow CP. Comparing stroke incidence worldwide: what makes studies comparable? Stroke. 1996;27(3):550-558. doi: 10.1161/01.STR.27.3.550 [DOI] [PubMed] [Google Scholar]
- 10.Pikija S, Cvetko D, Malojčić B, et al. . A population-based prospective 24-month study of stroke: incidence and 30-day case-fatality rates of first-ever strokes in Croatia. Neuroepidemiology. 2012;38(3):164-171. doi: 10.1159/000336114 [DOI] [PubMed] [Google Scholar]
- 11.Hansen BS, Marquardsen J. Incidence of stroke in Frederiksberg, Denmark. Stroke. 1977;8(6):663-665. doi: 10.1161/01.STR.8.6.663 [DOI] [PubMed] [Google Scholar]
- 12.Truelsen T, Grønbaek M, Schnohr P, Boysen G. Stroke case fatality in Denmark from 1977 to 1992: the Copenhagen City Heart Study. Neuroepidemiology. 2002;21(1):22-27. doi: 10.1159/000048610 [DOI] [PubMed] [Google Scholar]
- 13.Jørgensen HS, Plesner AM, Hübbe P, Larsen K. Marked increase of stroke incidence in men between 1972 and 1990 in Frederiksberg, Denmark. Stroke. 1992;23(12):1701-1704. doi: 10.1161/01.STR.23.12.1701 [DOI] [PubMed] [Google Scholar]
- 14.Vibo R, Kõrv J, Roose M. The Third Stroke Registry in Tartu, Estonia: decline of stroke incidence and 28-day case-fatality rate since 1991. Stroke. 2005;36(12):2544-2548. doi: 10.1161/01.STR.0000189633.33623.69 [DOI] [PubMed] [Google Scholar]
- 15.Numminen H, Kotila M, Waltimo O, Aho K, Kaste M. Declining incidence and mortality rates of stroke in Finland from 1972 to 1991: results of three population-based stroke registers. Stroke. 1996;27(9):1487-1491. doi: 10.1161/01.STR.27.9.1487 [DOI] [PubMed] [Google Scholar]
- 16.Sivenius J, Heinonen OP, Pyörälä K, Salonen J, Riekkinen P. The incidence of stroke in the Kuopio area of East Finland. Stroke. 1985;16(2):188-192. doi: 10.1161/01.STR.16.2.188 [DOI] [PubMed] [Google Scholar]
- 17.Sarti C, Tuomilehto J, Salomaa V, et al. . Epidemiology of subarachnoid hemorrhage in Finland from 1983 to 1985. Stroke. 1991;22(7):848-853. doi: 10.1161/01.STR.22.7.848 [DOI] [PubMed] [Google Scholar]
- 18.Biotti D, Jacquin A, Boutarbouch M, et al. . Trends in case-fatality rates in hospitalized nontraumatic subarachnoid hemorrhage: results of a population-based study in Dijon, France, from 1985 to 2006 [published correction appears in Neurosurgery. 2010;67(3):F878]. Neurosurgery. 2010;66(6):1039-1043. doi: 10.1227/01.NEU.0000369512.58898.99 [DOI] [PubMed] [Google Scholar]
- 19.Tsiskaridze A, Djibuti M, van Melle G, et al. . Stroke incidence and 30-day case-fatality in a suburb of Tbilisi: results of the first prospective population-based study in Georgia. Stroke. 2004;35(11):2523-2528. doi: 10.1161/01.STR.0000144683.96048.98 [DOI] [PubMed] [Google Scholar]
- 20.Kolominsky-Rabas PL, Sarti C, Heuschmann PU, et al. . A prospective community-based study of stroke in Germany—the Erlangen Stroke Project (ESPro): incidence and case fatality at 1, 3, and 12 months. Stroke. 1998;29(12):2501-2506. doi: 10.1161/01.STR.29.12.2501 [DOI] [PubMed] [Google Scholar]
- 21.Palm F, Urbanek C, Rose S, et al. . Stroke incidence and survival in Ludwigshafen am Rhein, Germany: the Ludwigshafen Stroke Study (LuSSt). Stroke. 2010;41(9):1865-1870. doi: 10.1161/STROKEAHA.110.592642 [DOI] [PubMed] [Google Scholar]
- 22.Stranjalis G, Kalamatianos T, Gatzonis S, Loufardaki M, Tzavara C, Sakas DE. The incidence of the first-ever stroke in a Mediterranean island population: the isle of Lesvos Stroke Study. Neuroepidemiology. 2014;43(3-4):206-212. doi: 10.1159/000365849 [DOI] [PubMed] [Google Scholar]
- 23.Hilmarsson A, Kjartansson O, Olafsson E. Incidence of first stroke: a population study in Iceland. Stroke. 2013;44(6):1714-1716. doi: 10.1161/STROKEAHA.111.000222 [DOI] [PubMed] [Google Scholar]
- 24.Kelly PJ, Crispino G, Sheehan O, et al. . Incidence, event rates, and early outcome of stroke in Dublin, Ireland: the North Dublin Population Stroke Study. Stroke. 2012;43(8):2042-2047. doi: 10.1161/STROKEAHA.111.645721 [DOI] [PubMed] [Google Scholar]
- 25.Ricci S, Celani MG, La Rosa F, et al. . A community-based study of incidence, risk factors and outcome of transient ischaemic attacks in Umbria, Italy: the SEPIVAC study. J Neurol. 1991;238(2):87-90. doi: 10.1007/BF00315687 [DOI] [PubMed] [Google Scholar]
- 26.D’Alessandro G, Di Giovanni M, Roveyaz L, et al. . Incidence and prognosis of stroke in the Valle d’Aosta, Italy: first-year results of a community-based study. Stroke. 1992;23(12):1712-1715. doi: 10.1161/01.STR.23.12.1712 [DOI] [PubMed] [Google Scholar]
- 27.Lauria G, Gentile M, Fassetta G, et al. . Incidence and prognosis of stroke in the Belluno province, Italy: first-year results of a community-based study. Stroke. 1995;26(10):1787-1793. doi: 10.1161/01.STR.26.10.1787 [DOI] [PubMed] [Google Scholar]
- 28.Sacco S, Totaro R, Toni D, Marini C, Cerone D, Carolei A. Incidence, case-fatalities and 10-year survival of subarachnoid hemorrhage in a population-based registry. Eur Neurol. 2009;62(3):155-160. doi: 10.1159/000226617 [DOI] [PubMed] [Google Scholar]
- 29.Di Carlo A, Inzitari D, Galati F, et al. . A prospective community-based study of stroke in Southern Italy: the Vibo Valentia Incidence of Stroke Study (VISS): methodology, incidence and case fatality at 28 days, 3 and 12 months. Cerebrovasc Dis. 2003;16(4):410-417. doi: 10.1159/000072565 [DOI] [PubMed] [Google Scholar]
- 30.D’Alessandro G, Bottacchi E, Di Giovanni M, et al. . Temporal trends of stroke in Valle d’Aosta, Italy: incidence and 30-day fatality rates. Neurol Sci. 2000;21(1):13-18. doi: 10.1007/s100720070113 [DOI] [PubMed] [Google Scholar]
- 31.Musolino R, La Spina P, Serra S, et al. . First-ever stroke incidence and 30-day case fatality in the Sicilian Aeolian archipelago, Italy. Stroke. 2005;36(12):2738-2741. doi: 10.1161/01.STR.0000190907.88846.df [DOI] [PubMed] [Google Scholar]
- 32.Manobianca G, Zoccolella S, Petruzzellis A, Miccoli A, Logroscino G. The incidence of major stroke subtypes in southern Italy: a population-based study. Eur J Neurol. 2010;17(9):1148-1155. doi: 10.1111/j.1468-1331.2010.02983.x [DOI] [PubMed] [Google Scholar]
- 33.Corso G, Bottacchi E, Giardini G, et al. . Community-based study of stroke incidence in the Valley of Aosta, Italy: CARe—Cerebrovascular Aosta Registry: years 2004-2005. Neuroepidemiology. 2009;32(3):186-195. doi: 10.1159/000195688 [DOI] [PubMed] [Google Scholar]
- 34.Corso G, Bottacchi E, Giardini G, et al. . Epidemiology of stroke in northern Italy: the Cerebrovascular Aosta Registry, 2004-2008. Neurol Sci. 2013;34(7):1071-1081. doi: 10.1007/s10072-012-1185-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janes F, Gigli GL, D’Anna L, et al. . Stroke incidence and 30-day and six-month case fatality rates in Udine, Italy: a population-based prospective study. Int J Stroke. 2013;8(suppl A100):100-105. doi: 10.1111/ijs.12000 [DOI] [PubMed] [Google Scholar]
- 36.Herman B, Leyten AC, van Luijk JH, Frenken CW, Op de Coul AA, Schulte BP. Epidemiology of stroke in Tilburg, the Netherlands. the population-based stroke incidence register: 2. incidence, initial clinical picture and medical care, and three-week case fatality. Stroke. 1982;13(5):629-634. doi: 10.1161/01.STR.13.5.629 [DOI] [PubMed] [Google Scholar]
- 37.Correia M, Magalhães R, Silva MR, Matos I, Silva MC. Stroke types in rural and urban northern Portugal: incidence and 7-year survival in a community-based study. Cerebrovasc Dis Extra. 2013;3(1):137-149. doi: 10.1159/000354851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caicoya M, Rodríguez T, Lasheras C, Cuello R, Corrales C, Blázquez B. Stroke incidence in Asturias, 1990-1991 [in Spanish]. Rev Neurol. 1996;24(131):806-811. [PubMed] [Google Scholar]
- 39.Díaz-Guzmán J, Egido JA, Gabriel-Sánchez R, Barberá-Comes G, Fuentes-Gimeno B, Fernández-Pérez C; IBERICTUS Study Investigators of the Stroke Project of the Spanish Cerebrovascular Diseases Study Group . Stroke and transient ischemic attack incidence rate in Spain: the IBERICTUS study. Cerebrovasc Dis. 2012;34(4):272-281. doi: 10.1159/000342652 [DOI] [PubMed] [Google Scholar]
- 40.Terént A. Increasing incidence of stroke among Swedish women. Stroke. 1988;19(5):598-603. doi: 10.1161/01.STR.19.5.598 [DOI] [PubMed] [Google Scholar]
- 41.Norrving B, Löwenhielm P. Epidemiology of stroke in Lund-Orup, Sweden, 1983-85: incidence of first stroke and age-related changes in subtypes. Acta Neurol Scand. 1988;78(5):408-413. doi: 10.1111/j.1600-0404.1988.tb03677.x [DOI] [PubMed] [Google Scholar]
- 42.Stegmayr B, Eriksson M, Asplund K. Declining mortality from subarachnoid hemorrhage: changes in incidence and case fatality from 1985 through 2000. Stroke. 2004;35(9):2059-2063. doi: 10.1161/01.STR.0000138451.07853.b6 [DOI] [PubMed] [Google Scholar]
- 43.Khan FA, Engstrom G, Jerntorp I, Pessah-Rasmussen H, Janzon L. Seasonal patterns of incidence and case fatality of stroke in Malmo, Sweden: the STROMA study. Neuroepidemiology. 2005;24(1-2):26-31. doi: 10.1159/000081046 [DOI] [PubMed] [Google Scholar]
- 44.Nilsson OG, Lindgren A, Ståhl N, Brandt L, Säveland H. Incidence of intracerebral and subarachnoid haemorrhage in southern Sweden. J Neurol Neurosurg Psychiatry. 2000;69(5):601-607. doi: 10.1136/jnnp.69.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Appelros P, Nydevik I, Seiger A, Terént A. High incidence rates of stroke in Orebro, Sweden: further support for regional incidence differences within Scandinavia. Cerebrovasc Dis. 2002;14(3-4):161-168. doi: 10.1159/000065680 [DOI] [PubMed] [Google Scholar]
- 46.Hallström B, Jönsson AC, Nerbrand C, Norrving B, Lindgren A. Stroke incidence and survival in the beginning of the 21st century in southern Sweden: comparisons with the late 20th century and projections into the future. Stroke. 2008;39(1):10-15. doi: 10.1161/STROKEAHA.107.491779 [DOI] [PubMed] [Google Scholar]
- 47.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project—1981-86. 2. incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1990;53(1):16-22. doi: 10.1136/jnnp.53.1.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolfe CD, Rudd AG, Howard R, et al. . Incidence and case fatality rates of stroke subtypes in a multiethnic population: the South London Stroke Register. J Neurol Neurosurg Psychiatry. 2002;72(2):211-216. doi: 10.1136/jnnp.72.2.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Syme PD, Byrne AW, Chen R, Devenny R, Forbes JF. Community-based stroke incidence in a Scottish population: the Scottish Borders Stroke Study. Stroke. 2005;36(9):1837-1843. doi: 10.1161/01.STR.0000177873.82478.1c [DOI] [PubMed] [Google Scholar]
- 50.Heuschmann PU, Grieve AP, Toschke AM, Rudd AG, Wolfe CD. Ethnic group disparities in 10-year trends in stroke incidence and vascular risk factors: the South London Stroke Register (SLSR). Stroke. 2008;39(8):2204-2210. doi: 10.1161/STROKEAHA.107.507285 [DOI] [PubMed] [Google Scholar]
- 51.Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: population-based study and systematic review. Neurology. 2010;74(19):1494-1501. doi: 10.1212/WNL.0b013e3181dd42b3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka H, Ueda Y, Date C, et al. . Incidence of stroke in Shibata, Japan: 1976-1978. Stroke. 1981;12(4):460-466. doi: 10.1161/01.STR.12.4.460 [DOI] [PubMed] [Google Scholar]
- 53.Inagawa T. Trends in incidence and case fatality rates of aneurysmal subarachnoid hemorrhage in Izumo City, Japan, between 1980-1989 and 1990-1998. Stroke. 2001;32(7):1499-1507. doi: 10.1161/01.STR.32.7.1499 [DOI] [PubMed] [Google Scholar]
- 54.Ohkuma H, Fujita S, Suzuki S. Incidence of aneurysmal subarachnoid hemorrhage in Shimokita, Japan, from 1989 to 1998. Stroke. 2002;33(1):195-199. doi: 10.1161/hs0102.101891 [DOI] [PubMed] [Google Scholar]
- 55.Turin TC, Kita Y, Rumana N, et al. . Ambient air pollutants and acute case-fatality of cerebro-cardiovascular events: Takashima Stroke and AMI Registry, Japan (1988-2004). Cerebrovasc Dis. 2012;34(2):130-139. doi: 10.1159/000339680 [DOI] [PubMed] [Google Scholar]
- 56.Hamada J, Morioka M, Yano S, Kai Y, Ushio Y. Incidence and early prognosis of aneurysmal subarachnoid hemorrhage in Kumamoto Prefecture, Japan. Neurosurgery. 2004;54(1):31-37. doi: 10.1227/01.NEU.0000097196.55204.0B [DOI] [PubMed] [Google Scholar]
- 57.Omama S, Yoshida Y, Ogasawara K, et al. . Incidence rate of cerebrovascular diseases in northern Japan determined from the Iwate Stroke Registry with an inventory survey system. J Stroke Cerebrovasc Dis. 2013;22(8):e317-e322. doi: 10.1016/j.jstrokecerebrovasdis.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 58.Zhang J, Liu G, Arima H, et al. ; CHERISH Investigators . Incidence and risks of subarachnoid hemorrhage in China. Stroke. 2013;44(10):2891-2893. doi: 10.1161/STROKEAHA.113.002599 [DOI] [PubMed] [Google Scholar]
- 59.Azarpazhooh MR, Etemadi MM, Donnan GA, et al. . Excessive incidence of stroke in Iran: evidence from the Mashhad Stroke Incidence Study (MSIS), a population-based study of stroke in the Middle East. Stroke. 2010;41(1):e3-e10. doi: 10.1161/STROKEAHA.109.559708 [DOI] [PubMed] [Google Scholar]
- 60.Dalal PM, Malik S, Bhattacharjee M, et al. . Population-based stroke survey in Mumbai, India: incidence and 28-day case fatality. Neuroepidemiology. 2008;31(4):254-261. doi: 10.1159/000165364 [DOI] [PubMed] [Google Scholar]
- 61.Epstein L, Rishpon S, Bental E, et al. . Incidence, mortality, and case-fatality rate of stroke in northern Israel. Stroke. 1989;20(6):725-729. doi: 10.1161/01.STR.20.6.725 [DOI] [PubMed] [Google Scholar]
- 62.Abdul-Ghaffar NU, el-Sonbaty MR, el-Din Abdul-Baky MS, Marafie AA, al-Said AM. Stroke in Kuwait: a three-year prospective study. Neuroepidemiology. 1997;16(1):40-47. doi: 10.1159/000109669 [DOI] [PubMed] [Google Scholar]
- 63.Feigin VL, Wiebers DO, Nikitin YP, O’Fallon WM, Whisnant JP. Stroke epidemiology in Novosibirsk, Russia: a population-based study. Mayo Clin Proc. 1995;70(9):847-852. doi: 10.1016/S0025-6196(11)63942-6 [DOI] [PubMed] [Google Scholar]
- 64.Anderson CS, Jamrozik KD, Burvill PW, Chakera TM, Johnson GA, Stewart-Wynne EG. Determining the incidence of different subtypes of stroke: results from the Perth Community Stroke Study, 1989-1990. Med J Aust. 1993;158(2):85-89. [DOI] [PubMed] [Google Scholar]
- 65.Islam MS, Anderson CS, Hankey GJ, et al. . Trends in incidence and outcome of stroke in Perth, Western Australia during 1989 to 2001: the Perth Community Stroke Study. Stroke. 2008;39(3):776-782. doi: 10.1161/STROKEAHA.107.493643 [DOI] [PubMed] [Google Scholar]
- 66.Thrift AG, Dewey HM, Macdonell RA, McNeil JJ, Donnan GA. Incidence of the major stroke subtypes: initial findings from the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke. 2001;32(8):1732-1738. doi: 10.1161/01.STR.32.8.1732 [DOI] [PubMed] [Google Scholar]
- 67.Thrift AG, Dewey HM, Sturm JW, et al. . Incidence of stroke subtypes in the North East Melbourne Stroke Incidence Study (NEMESIS): differences between men and women. Neuroepidemiology. 2009;32(1):11-18. doi: 10.1159/000170086 [DOI] [PubMed] [Google Scholar]
- 68.Leyden JM, Kleinig TJ, Newbury J, et al. . Adelaide stroke incidence study: declining stroke rates but many preventable cardioembolic strokes. Stroke. 2013;44(5):1226-1231. doi: 10.1161/STROKEAHA.113.675140 [DOI] [PubMed] [Google Scholar]
- 69.Newbury J, Kleinig T, Leyden J, et al. . Stroke Epidemiology in an Australian Rural Cohort (SEARCH). Int J Stroke. 2017;12(2):161-168. doi: 10.1177/1747493016670174 [DOI] [PubMed] [Google Scholar]
- 70.Bonita R, Thomson S. Subarachnoid hemorrhage: epidemiology, diagnosis, management, and outcome. Stroke. 1985;16(4):591-594. doi: 10.1161/01.STR.16.4.591 [DOI] [PubMed] [Google Scholar]
- 71.Truelsen T, Bonita R, Duncan J, Anderson NE, Mee E. Changes in subarachnoid hemorrhage mortality, incidence, and case fatality in New Zealand between 1981-1983 and 1991-1993. Stroke. 1998;29(11):2298-2303. doi: 10.1161/01.STR.29.11.2298 [DOI] [PubMed] [Google Scholar]
- 72.Feigin V, Carter K, Hackett M, et al. ; Auckland Regional Community Stroke Study Group . Ethnic disparities in incidence of stroke subtypes: Auckland Regional Community Stroke Study, 2002-2003. Lancet Neurol. 2006;5(2):130-139. doi: 10.1016/S1474-4422(05)70325-2 [DOI] [PubMed] [Google Scholar]
- 73.Cantu-Brito C, Majersik JJ, Sánchez BN, et al. . Hospitalized stroke surveillance in the community of Durango, Mexico: the Brain Attack Surveillance in Durango study. Stroke. 2010;41(5):878-884. doi: 10.1161/STROKEAHA.109.577726 [DOI] [PubMed] [Google Scholar]
- 74.Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27(3):373-380. [PubMed] [Google Scholar]
- 75.Longstreth WT Jr, Nelson LM, Koepsell TD, van Belle G. Clinical course of spontaneous subarachnoid hemorrhage: a population-based study in King County, Washington. Neurology. 1993;43(4):712-718. doi: 10.1212/WNL.43.4.712 [DOI] [PubMed] [Google Scholar]
- 76.Labovitz DL, Halim AX, Brent B, Boden-Albala B, Hauser WA, Sacco RL. Subarachnoid hemorrhage incidence among whites, blacks and Caribbean Hispanics: the Northern Manhattan Study. Neuroepidemiology. 2006;26(3):147-150. doi: 10.1159/000091655 [DOI] [PubMed] [Google Scholar]
- 77.Bahit MC, Coppola ML, Riccio PM, et al. . First-ever stroke and transient ischemic attack incidence and 30-day case-fatality rates in a population-based study in Argentina. Stroke. 2016;47(6):1640-1642. doi: 10.1161/STROKEAHA.116.013637 [DOI] [PubMed] [Google Scholar]
- 78.Minelli C, Fen LF, Minelli DP. Stroke incidence, prognosis, 30-day, and 1-year case fatality rates in Matão, Brazil: a population-based prospective study. Stroke. 2007;38(11):2906-2911. doi: 10.1161/STROKEAHA.107.484139 [DOI] [PubMed] [Google Scholar]
- 79.Cabral NL, Gonçalves AR, Longo AL, et al. . Incidence of stroke subtypes, prognosis and prevalence of risk factors in Joinville, Brazil: a 2 year community based study. J Neurol Neurosurg Psychiatry. 2009;80(7):755-761. doi: 10.1136/jnnp.2009.172098 [DOI] [PubMed] [Google Scholar]
- 80.Cabral NL, Cougo-Pinto PT, Magalhaes PS, et al. . Trends of stroke incidence from 1995 to 2013 in Joinville, Brazil. Neuroepidemiology. 2016;46(4):273-281. doi: 10.1159/000445060 [DOI] [PubMed] [Google Scholar]
- 81.Smadja D, Cabre P, May F, et al. ; ERMANCIA Study Group . ERMANCIA: Epidemiology of Stroke in Martinique, French West Indies: part I: methodology, incidence, and 30-day case fatality rate. Stroke. 2001;32(12):2741-2747. doi: 10.1161/hs1201.099385 [DOI] [PubMed] [Google Scholar]
- 82.Wolfe CD, Corbin DO, Smeeton NC, et al. . Estimation of the risk of stroke in black populations in Barbados and South London. Stroke. 2006;37(8):1986-1990. doi: 10.1161/01.STR.0000230578.10937.a6 [DOI] [PubMed] [Google Scholar]
- 83.Alvarez G, Cox P, Pairoa M, García M, Delgado I, Lavados PM. Incidence of subarachnoid haemorrhage in the Aconcagua Valley, Chile: a community-based, prospective surveillance project. J Neurol Neurosurg Psychiatry. 2010;81(7):778-782. doi: 10.1136/jnnp.2009.192971 [DOI] [PubMed] [Google Scholar]
- 84.Lavados PM, Sacks C, Prina L, et al. . Incidence, 30-day case-fatality rate, and prognosis of stroke in Iquique, Chile: a 2-year community-based prospective study (PISCIS project). Lancet. 2005;365(9478):2206-2215. doi: 10.1016/S0140-6736(05)66779-7 [DOI] [PubMed] [Google Scholar]
- 85.Okon M, Adebobola NI, Julius S, et al. . Stroke incidence and case fatality rate in an urban population. J Stroke Cerebrovasc Dis. 2015;24(4):771-777. doi: 10.1016/j.jstrokecerebrovasdis.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 86.Kita Y, Turin TC, Ichikawa M, et al. . Trend of stroke incidence in a Japanese population: Takashima Stroke Registry, 1990-2001. Int J Stroke. 2009;4(4):241-249. doi: 10.1111/j.1747-4949.2009.00293.x [DOI] [PubMed] [Google Scholar]
- 87.ACROSS Group Epidemiology of aneurysmal subarachnoid hemorrhage in Australia and New Zealand: incidence and case fatality from the Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS). Stroke. 2000;31(8):1843-1850. doi: 10.1161/01.STR.31.8.1843 [DOI] [PubMed] [Google Scholar]
- 88.Vemmos KN, Bots ML, Tsibouris PK, et al. . Stroke incidence and case fatality in southern Greece: the Arcadia Stroke Registry. Stroke. 1999;30(2):363-370. doi: 10.1161/01.STR.30.2.363 [DOI] [PubMed] [Google Scholar]
- 89.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. ; Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group . Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383(9913):245-254. doi: 10.1016/S0140-6736(13)61953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol. 2016;12(12):699-713. doi: 10.1038/nrneurol.2016.150 [DOI] [PubMed] [Google Scholar]
- 91.Jalbert JJ, Isaacs AJ, Kamel H, Sedrakyan A. Clipping and coiling of unruptured intracranial aneurysms among Medicare beneficiaries, 2000 to 2010. Stroke. 2015;46(9):2452-2457. doi: 10.1161/STROKEAHA.115.009777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10(7):626-636. doi: 10.1016/S1474-4422(11)70109-0 [DOI] [PubMed] [Google Scholar]
- 93.Huang J, van Gelder JM. The probability of sudden death from rupture of intracranial aneurysms: a meta-analysis. Neurosurgery. 2002;51(5):1101-1105. doi: 10.1097/00006123-200211000-00001 [DOI] [PubMed] [Google Scholar]
- 94.Bamford J, Dennis M, Sandercock P, Burn J, Warlow C. The frequency, causes and timing of death within 30 days of a first stroke: the Oxfordshire Community Stroke Project. J Neurol Neurosurg Psychiatry. 1990;53(10):824-829. doi: 10.1136/jnnp.53.10.824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feigin VL, Wiebers DO, Whisnant JP, O’Fallon WM. Stroke incidence and 30-day case-fatality rates in Novosibirsk, Russia, 1982 through 1992. Stroke. 1995;26(6):924-929. doi: 10.1161/01.STR.26.6.924 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Summary of all 75 included studies and 84 study periods, according to ascending midyear of study.
eTable 2. Characteristics of the overall and age-specific and sex-specific datasets.
eTable 3. Crude SAH incidence with 95% CIs and risk ratios for SAH in the age-specific and sex-specific dataset.
eTable 4. Overall and age-specific risk ratios in women vs men in the age-specific and sex-specific dataset (34 study periods).
eTable 5. Time trends in SAH incidence in the in the age-specific and sex-specific dataset (34 study periods).
eTable 6. Estimated blood pressures in 1980 and 2010 for women and men aged 45-54 years and 55-74 years including annual change from 1977 to 2014.
eFigure 1. Selection of studies.
eFigure 2. Regional time trends for studies reporting on consecutive SAH incidence for the same study population.
eFigure 3. Crude incidence of SAH in age-specific and sex-specific dataset overall, in Europe, and in Japan only.