Abstract
Background
Helminthiases are very prevalent worldwide, yet their treatment and control rely on a handful of drugs. Emodepside, a marketed broad-spectrum veterinary anthelminthic with a unique mechanism of action, undergoing development for onchocerciasis is an interesting anthelmintic drug candidate. We tested the in vitro and in vivo activity of emodepside on nematode species that serve as models for human soil-transmitted helminth infection as well as on schistosomes.
Methods
In vitro viability assays were performed over a time course of 72 hours for Trichuris muris, Necator americanus, Ancylostoma ceylanicum, Heligmosomoides polygyrus, Strongyloides ratti, Schistosoma mansoni and Schistosoma haematobium. The drug effect was determined by the survival rate for the larvae and by phenotypical scores for the adult worms. Additionally, mice infected with T. muris and hamsters harboring hookworm infection (N. americanus or A. ceylanicum) were administered orally with emodepside at doses ranging from 1.25 to 75 mg/kg. Expelled worms in the feces were counted until 3 days post-drug intake and worms residing in the intestines were collected and counted after dissection.
Results
After 24 hours, emodepside was very active in vitro against both larval and adult stages of the nematodes T. muris, A. ceylanicum, N. americanus, H. polygyrus and S. ratti (IC50 < 4 µM). The good in vitro activity was confirmed in vivo. Hamsters infected with the hookworms were cured when administered orally with 2.5 mg/kg of the drug. Emodepside was also highly active in vivo against T. muris (ED50 = 1.2 mg/kg). Emodepside was moderately active on schistosomula in vitro (IC50 < 8 µM) 24 h post-drug incubation and its activity on adult S. mansoni and S. haematobium was low (IC50: 30–50 µM).
Conclusions
Emodepside is highly active against a broad range of nematode species both in vitro and in vivo. The development of emodepside for treating soil-transmitted helminth infections should be pursued.
Electronic supplementary material
The online version of this article (10.1186/s13071-019-3476-x) contains supplementary material, which is available to authorized users.
Keywords: Emodepside, Drug repurposing, Soil-transmitted helminthiases (STH), Hookworms, Trichuris spp., Nematodes, Trematodes, Schistosoma spp.
Background
Helminths affect a fifth of the world population and their associated morbidities include general fatigue, food malabsorption or iron deficiency anemia [1–4]. They are an important public health issue in low and middle income countries, where they enhance the vicious cycle of poverty notably by reducing school attendance and productivity [5, 6]. The most prevalent helminthiases are schistosomiasis (primarily caused by Schistosoma haematobium, S. japonicum and S. mansoni) that affects more than 250 million people and soil-transmitted helminthiases (STH) that account for more than 1.5 billion infected cases worldwide [2, 4, 6, 7]. Infections with the hookworms Ancylostoma duodenale and Necator americanus, the whipworm Trichuris trichiura, the roundworm Ascaris lumbricoides and the threadworm Strongyloides stercoralis are grouped as soil-transmitted helminths, based on their mode of transmission [8, 9].
Preventive chemotherapy is the strategy of choice, recommended by the World Health Organization (WHO) to control these helminth infections. Schistosomiasis control relies on praziquantel while albendazole, mebendazole, levamisole and pyrantel pamoate are used against STH [7, 10, 11]. A recent meta-analysis showed that all four drugs used against STH have a limited and even decreasing efficacy against the parasites [12]. Also, the recent epidemiological survey from Crellen et al. [13] reported that the efficacy of praziquantel against S. mansoni was reduced, likely because of frequent mass drug administration campaigns (MDA). Together with the rising risk of drug resistance due to an intense use of the same drugs and the lack of lead molecules in the development pipeline, the discovery of new anthelmintic treatments is urgent [12, 14, 15].
As the expected return on investment for helminthiases is negligible, drug repurposing represents a sustainable approach and an effective strategy to expand the pool of active molecules, in particular when using veterinary anthelmintics as starting point [16]. Emodepside, is a broad-spectrum veterinary anthelmintic licensed under the name of Profender® and Procox® and is used in combination with praziquantel and toltrazuril, respectively [17]. Its activity has been demonstrated against a wide range of nematodes in the veterinary field [18–25]. Repurposing emodepside for human use started more than ten years ago with pre-clinical studies against filarial nematodes which may be considered surrogates of human filarial infections [26]. These promising results triggered in 2016 a phase I study with emodepside in healthy volunteers, which was then completed by single and multiple ascending dose studies [17, 27].
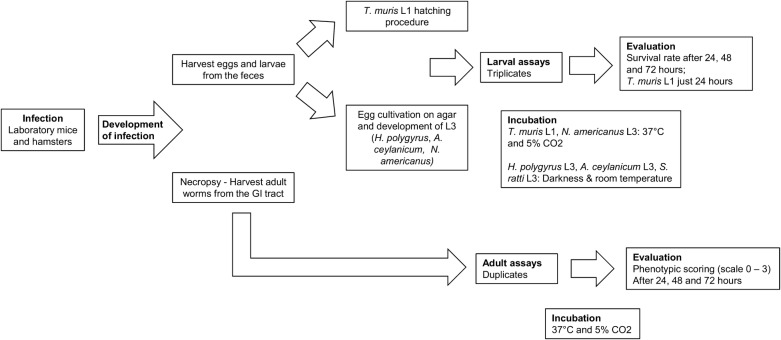
Aiming at possibly expanding its range of application in human medicine and in order to broaden previous work on laboratory models, we thoroughly tested emodepside against seven species of helminths in vitro and in vivo [28–30]. The drug was tested first in vitro on both larval and adult stages of T. muris, A. ceylanicum, N. americanus, S. ratti and H. polygyrus, as well as on S. mansoni and S. haematobium. The activity of emodepside was next tested in vivo in animal models infected with T. muris, A. ceylanicum and N. americanus. The experimental flow for the study on nematodes is presented in Fig. 1.
Fig. 1.
Workflow for the nematode assays
Methods
Drugs
Emodepside was purified by preparative high-performance liquid chromatography (HPLC) from the commercially available topical solution for cats Profender® (Bayer, Leverkusen, Germany). Profender® was diluted 1:1 (v/v) with acetonitrile (ACN), filtered (0.22 µm) and injected (10 mL) into a preparative HPLC (Varian ProStar system). The purification was performed on a ReproSil® 100 C18, 7 µm, 250 × 40 mm column (Dr. Maisch GmbH, Ammerbuch-Entringen, Germany) with double distilled water, 0.1% Trifluoroacetic acid (TFA) as solvent A and HPLC grade ACN (Buchs, Sigma-Aldrich) as solvent B at 20 ml/min flowrate. The following linear gradient was used: 0 min 20% B; 0.5 min 20% B; 34 min 100% B; 40 min 100% B. Fractions containing emodepside were then combined and lyophilized.
Emodepside’s 1H- and 13C-NMR spectra were recorded on Bruker 400 and 500 spectrometers and referenced to residual solvent peaks. LC-MS spectra of the purified compound were measured on an Acquity™ (Waters system) coupled to an Esquire HCT from Bruker (Bremen, Germany) using an Acquity UPLC BEH C18 column (2.1 × 50 mm, 1.7 μm) at 0.6 ml/min flow with a linear gradient of A (double distilled water 0.1% v/v formic acid) and B (ACN 0.1% v/v formic acid); t = 0 min, 5% B; t = 0.25 min, 5% B; t = 1.5 min, 100% B; t = 2.5 min, 100% B. NMR and MS spectra corresponded to that previously reported for emodepside (Segment solid-phase total synthesis of the anthelmintic cyclooctadepsipeptides PF1022A and emodepside) [31].
Levamisole was purchased in powder from Sigma-Aldrich (Buchs, Switzerland). Stock solutions of emodepside and levamisole (10 mM) were dissolved in pure dimethyl sulfoxide (DMSO, Sigma-Aldrich, Buchs, Switzerland) and stored until use at − 20 °C.
Culture media
RPMI 1640 (Gibco, Waltham MA, USA) medium supplemented with 5% amphotericin B (250 µg/ml, Sigma-Aldrich, Buchs, Switzerland) and 1% penicillin 10,000 U/ml, and streptomycin 10 mg/ml solution (Sigma-Aldrich, Buchs, Switzerland) was used for the assays with T. muris adults and stage 1 larvae (L1), H. polygyrus adults and supplemented additionally with 10% inactivated fetal calf serum (iFCS; Bioconcept AG, Allschwil, Switzerland) for T. muris L1 hatching medium. RPMI 1640 supplemented with 5% amphotericin B (250 µg/ml), 1% penicillin (10,000 U/ml) and streptomycin (10 mg/ml) solution and 1% of the antibiotics mixture developed by Mäser et al. [32] was used for the H. polygyrus third stage larval (L3) assays. Adult S. mansoni, S. haematobium and S. ratti were incubated in RPMI 1640 medium supplemented with 5% iFCS and 1% penicillin (10,000 U/ml) and streptomycin (10 mg/ml) solution. Phosphate-buffered saline (PBS, Sigma-Aldrich, Buchs, Switzerland) supplemented with 1% penicillin (10,000 U/ml) and streptomycin (10 mg/ml) solution was used to incubate S. ratti L3 and to wash the adult worms. For the S. mansoni newly transformed schistosomula (NTS) assays, M199 medium (Gibco, Waltham MA, USA) supplemented with 5% iFCS and a mixture of antibiotics was used for the incubation [33]. Ancylostoma ceylanicum and N. americanus L3 stages were incubated in Hanksʼ balanced salt solution (HBSS; Gibco, Waltham MA, USA) supplemented with 10% amphotericin B and 1% penicillin (10,000 U/ml) and streptomycin (10 mg/ml) solution. The adult hookworms were kept in HBSS supplemented with 10% iFCS, 5% amphotericin B (250 µg/ml) and 1% penicillin (10,000 U/ml) and streptomycin (10 mg/ml) solution.
Laboratory animals
Before the infection, all animals were left one week for acclimation in our facility. Three-week-old male Syrian golden hamsters (Charles River, Sulzfeld, Germany) were orally infected with 150 L3 of A. ceylanicum or subcutaneously with 150 N. americanus L3. Four-week-old female NMRI mice (Charles Rivers, Sulzfeld, Germany) were used for S. mansoni infection and injected subcutaneously with 100 cercariae. The same mice strain was used for growing H. polygyrus which were administered orally with 88 L3. Six-week-old female C57BL/6NRj mice (Janvier labs, Le Genest-Saint-Isle, France) were orally infected with 200 embryonated T. muris eggs. Three-week-old male Wistar rats (Janvier labs, Le Genest-Saint-Isle, France), were infected subcutaneously with 1300 S. ratti L3. Three-week-old male LVG Syrian Golden hamsters (Charles River, USA) were infected with S. haematobium cercariae at the Biomedical Research Institute (Atlanta, USA) before being sent to the Swiss Tropical and Public Health Institute.
All animals were kept in polycarbonate cages under environmentally-controlled conditions (temperature: 25 °C, humidity: 70%, 12:12 h light/dark photocycle) and had free access to tap water and rodent food. To guarantee a sustainable infection, dexamethasone (Sigma-Aldrich, Buchs, Switzerland) was supplied in the drinking water until 2 days before treatment for the NMRI mice infected with H. polygyrus (0.25 mg/l dexamethasone), the C57BL/6NRj mice infected with T. muris (1 mg/l dexamethasone) and the hamsters infected with the hookworms (0.5 mg/l dexamethasone). Animals were killed using the CO2 method to collect the adult worms for the in vitro studies as described below.
Drug assays
To determine the half maximal inhibitory concentration (IC50) of the drug, each compound and concentration was tested in triplicates in the larval assays and in duplicates in adult worm assays. Parasites incubated in wells containing culture medium and DMSO corresponding to the highest drug concentration, served as negative controls and were included in every in vitro assay.
Heligmosomoides polygyrus, S. ratti and A. ceylanicum L3 larvae were then kept in the dark and at room temperature for 72 h. Strongyloides ratti L3 plates were sealed with Parafilm (Faust AG, Schaffhausen, Switzerland) before incubation. T. muris L1, N. americanus L3, S. mansoni NTS and the adult assays of all seven species were kept in the incubator for 72 h at 37 °C and 5% CO2.
In vitro tests on N. americanus and A. ceylanicum L3
Necator americanus and A. ceylanicum L3 were obtained from infected hamsters by the cultivation of eggs, gained by repeated filtration and centrifugation of the infected feces. The eggs were washed with tap water and cultivated on agar plates protected from light for 8 to 10 days at room temperature. The L3 were then kept in tap water supplemented with 5% amphotericin B (250 µg/ml), 1% penicillin (10,000 U/ml) and streptomycin (10 mg/ml) solution and used within 3 weeks. In each well of a 96-well plate (Sarstedt, Nümbrecht, Germany), 30 L3 were exposed to 4 serial dilutions (1:4) ranging from 0.016 µM up to 1 µM emodepside concentrations in a final volume of 200 µl.
In vitro tests on adult N. americanus and A. ceylanicum
Five weeks to six weeks post-infection (p.i.), the worms were collected directly from the hamster’s intestines. In a 24-well plate (Sarstedt, Nümbrecht, Germany), 3 to 4 adult worms per well were incubated in 2.5 ml drug solution with 4 different concentrations ranging from 0.005 µM to 0.5 µM for A. ceylanicum and with concentrations of 0.01 µM, 0.1 µM and 1 µM for N. americanus.
In vitro tests on T. muris L1
Six weeks after the infection of the mice, T. muris eggs were gained by filtration of their feces and storage in tap water for three months in the dark. To obtain the first stage larvae, egg hatching was triggered by incubation with Escherichia coli (BL21 strain) in hatching medium for 3 to 4 h at 37 °C in a wet chamber. For the assay, the L1 suspended in a total volume of 100 µl medium were placed in each well of a 96-well plate containing 14 emodepside concentrations ranging from 0.098 µM to 100 µM. Wells that contained levamisole (25 µM or 100 µM) served as positive control. The assays were kept in the incubator for 24 hours.
In vitro tests on adult T. muris
Trichuris muris adult worms were collected manually from the intestines of infected mice, 41 days p.i. The drug assays were performed in 24-well plates. In each well, 2 to 3 adult worms were incubated with the drug (1:4 serial dilutions ranging from 0.039 µM to 10 µM) in a final volume of 2.5 ml.
In vitro tests on H. polygyrus L3
The eggs were collected 2 weeks p.i. from mice feces and placed on agar at room temperature for 8 to 10 days in the dark. Forty L3 were exposed to emodepside at 3 different concentrations (0.625 µM, 2.5 µM and 10 µM) in a final volume of 100 µl.
In vitro assay on adult H. polygyrus
Heligmosomoides polygyrus adults were collected 2 weeks p.i. when dissecting mice intestines. In each well of 24-well plates 3 to 4 adult worms were exposed to emodepside (1:2 serial dilutions ranging from 0.125 µM to 1 µM) in 2.5 ml culture medium.
In vitro studies on S. mansoni NTS
Schistosoma mansoni (Liberian strain) cercariae were harvested from infected Biomphalaria glabrata snails and were then transformed into NTS [33, 34]. The drug assays and phenotypic screening were performed as described previously [33].
In vitro studies on adult S. mansoni and S. haematobium
Seven weeks p.i. the worms were extracted from the rodent mesenteric veins. Three single flukes or 2 pairs were placed in a final volume of 2.4 ml in each well of a 24-well plate exposed to 1:3 serial dilutions ranging from 3.7 µM up to 33.33 µM emodepside.
In vitro studies on S. ratti L3
The L3 were obtained following the procedure described by Garcia (1998) [35]. Thirty L3 were exposed to emodepside (1:4 serial dilutions ranging from 0.039 µM to 10 µM) in a final volume of 100 µl.
In vitro studies on adult S. ratti
The infected rats were dissected 3 weeks p.i. The intestines of the rats were excised, opened and immerged in PBS supplemented with penicillin/streptomycin. They were incubated for 4 h at 37 °C and 5% CO2 in order to detach the nematodes from the intestinal wall. A maximum of 15 worms were then transferred into the wells of 24-well plates and placed in 2 ml culture medium containing emodepside at 6 different concentrations ranging from 50 µM to 0.25 µM.
Evaluation of the assays
The drug effect was evaluated 24, 48 and 72 h post-exposure. For evaluating the L3 assays, the larvae were stimulated if necessary by the addition of 50 µl (T. muris L1) to 100 µl (others spp.) hot water (≈ 80 °C) and the percentage of survival was determined by the ratio of moving larvae to the total number of larvae present in the well. The N. americanus L3 assay was an exception as the wells were stimulated by vigorous up and down pipetting. The adult worms of each parasite species were scored microscopically based on their phenotype, using a viability scale ranging from 3 to 0 (3: good motility and no morphological changes; 2: low motility and light changes in morphology; 1: very low motility and morphologically impaired; and 0: death). In case the adult worms did not move enough for a clear scoring, they were stimulated with hot water at the last evaluation time-point.
Trichuris muris in vivo studies
C57BL/6NRj mice were orally infected with 200 embryonated T. muris eggs. At 42 days p.i., the feces were collected and soaked for 1 hour in 0.9% sodium chloride, before the filtered suspension was examined under the microscope to determine the success of the infection. According to their infection intensities, the mice were equally assigned to the different groups. The compound was dissolved in 70:30 Tween 80-ethanol in ultrapure water (10% v/v) and was administered by gavage first at a dose of 75 mg/kg based on results from a previous study [28] followed by lower dosages from 1.25 to 10 mg/kg. Untreated mice served as controls. Until 3 days after drug administration the feces of the mice were examined for expelled worms. Six to seven days post-treatment, the animals were killed, their intestines were dissected, and the adult worms were collected and counted.
Ancylostoma ceylanicum and N. americanus in vivo studies
Hamsters were orally infected with 150 A. ceylanicum L3 or subcutaneously with 150 N. americanus L3. A fecal sample was collected from each hamster, just before treatment. The fecal samples were processed using an in-house sedimentation method to determine the infection intensity of each animal [36]. The different dosage and control groups were composed of hamsters evenly distributed depending on their infection status. A. ceylanicum infected hamsters were then treated on day 28 p.i. with a single oral dose of 2.5 mg/kg emodepside and N. americanus infected hamsters with a single oral dose of 1.25–10 mg/kg emodepside. Expelled worms were counted from each hamster from the collected feces until 72 h after treatment. One week post-treatment, the hamsters were euthanized and the worms remaining in their intestines were collected and counted.
Data analysis
For the in vitro drug sensitivity assays, all viability scores and larval survival counts were averaged across replicates and normalized to the control wells. The effect of emodepside was determined by normalizing the mean parasite survival rate to the control wells. Based on these values, the IC50 values were calculated using CompuSyn software (ComboSyn Inc., version 1.0), as well as the r-values (the linear correlation coefficient) that reflects the goodness of the fit. For each assay, a minimal r value and viability of the controls was required. The detailed selection criteria are presented in Table 1.
Table 1.
Mean IC50 values in vitro of emodepside tested on larval and adult stages of different helminths
| Species | Replicates | No. of parasites per wella | 24 hours | 48 hours | 72 hours |
|---|---|---|---|---|---|
| Mean IC50 ± SD (µM) | Mean IC50 ± SD (µM) | Mean IC50 ± SD (µM) | |||
| T. muris (L1) | 9 | 20–40 | 3.73 ± 6.54 | – | – |
| T. muris (adults) | 2 | 2–3 | 0.28 ± 0.15 | 0.043 ± 0.0089 | 0.022 ± 0.013 |
| H. polygyrus (L3) | 2 | 30 | 0.78 ± 0.086 | 0.9 ± 0.034 | 0.48 ± 0.05 |
| H. polygyrus (adults) | 3 | 3–4 | 0.57 ± 0.42 | 0.21 ± 0.13 | 0.25 ± 0.16 |
| A. ceylanicum (L3) | 2 | 30 | 0.14 ± 0.041 | 0.086 ± 0.08 | 0.25 ± 0.051 |
| A. ceylanicum (adults) | 3 | 2–3 | 0.0044 ± 0.0021 | 0.0015 ± 0.00078 | 0.0024 ± 0.002 |
| N. americanus (L3) | 2 | 30 | 0.77 ± 0.52 | 0.15 ± 0.069 | 0.083 ± 0.033 |
| N. americanus (adults) | 2 | 2–3 | 0.0031 ± 0.0011 | 0.0029 ± 0.0018 | 0.0021 ± 0.0012 |
| S. ratti (L3) | 4 | 30 | 0.73 ± 0.5 | 0.27 ± 0.21 | 0.25 ± 0.14 |
| S. ratti (adults) | 3 | 5–15 | 0.75 ± 0.57 | 0.21 ± 0.29 | 0.36 ± 0.32 |
| S. mansoni (NTS) | 2 | 100 | 7.79 ± 1.57 | 6.92 ± 0.21 | 2.48 ± 0.78 |
| S. mansoni (adults) | 2 | 2–3 | 50.4 ± 3.32 | 37.27 ± 10.47 | 34.1 ± 9.18 |
| S. haematobium (adults) | 2 | 2–3 | 40.51 ± 24.96 | 40.25 ± 6.49 | 36.73 ± 6.49 |
aEach assay included 2 to 3 wells per concentration/condition
Notes: The inclusion criteria used in our analysis were different for each stage and parasite. Minimal survival rates (larvae) or viability scores (adults and NTS) and IC50 r-values considered acceptable were as follows: T. muris L1 (survival rate: 60%; R = 0.7), adults (score: 2.5; R = 0.8); H. polygyrus L3 (70%; 0.9), adults (1.9; 0.8); A. ceylanicum L3 (55%; 0.7), adults (2; 0.7); N. americanus L3 (60%; 0.75), adults (2; 0.8); S. ratti L3 (60%; 0.75), adults (2; 0.7); S. mansoni NTS (2; 0.75), adults (1.5; 0.85); S. haematobium adults (2; 0.7)
Abbreviation: SD, standard deviation
To assess the effect of the drug in vivo, the mean numbers of living worms recovered in treated animals were compared to the controls. The worm burden reduction (WBR) was calculated as described previously [16]. The worm expulsion rate (WER) was determined by the number of dead worms excreted in the feces during 72 h after treatment, over the total number of worms (alive and dead) recovered during the necropsy. The Kruskal-Wallis test (Statsdirect version 3.1.20) were used to determine statistical significance of WBR at a level of 0.05. The median effective dose (ED50) values were calculated using CompuSyn software (ComboSyn Inc., version 1.0).
Results
In vitro studies
Table 1 and Additional files 1 and 2 summarize the mean IC50 values for each helminth species over 3 days post-drug exposure, except for T. muris L1 that were assessed after a 24 h incubation period. Emodepside showed IC50 values below 1 µM, within 24 h for all nematode species with the exception of T. muris L1 that had an IC50 of 3.7 µM. The highest drug activity was observed for adult hookworms. After one day of incubation, emodepside was highly active against adult A. ceylanicum and N. americanus with IC50 values below 0.005 µM, which were reduced by half over the incubation period (IC50 < 0.0025 µM). Decreasing IC50 values were also recorded over the 72 h incubation period for the adult worms of every species tested. Against T. muris adults emodepside showed an IC50 value below 0.3 µM after 24 h of drug exposure. This value decreased below 0.05 µM after another day of incubation. IC50 values in the range of 0.2 µM to 0.8 µM were observed for adult H. polygyrus and S. ratti.
IC50 values for all nematode L3 ranged from 0.9 µM to 0.08 µM. The IC50 values of S. ratti and N. americanus L3 decreased, while they decreased and increased over the 3 days incubation period for H. polygyrus and A. ceylanicum larvae, respectively. For the schistosomes S. mansoni and S. haematobium, IC50 values above 30 µM were calculated for adult worms while decreasing IC50 from 7.8 µM after 24 h to 2.5 µM after 72 h were observed for S. mansoni NTS.
For all the nematode species, the IC50 values were higher for the larval stages than for the adult worms. Strongyloides ratti was the only species where the difference between the two life-stages was less than 2-fold. The IC50 on adult worms was of about twice as high than for the larval stage for H. polygyrus, 13 times for T. muris and between 30 to 250 times for the hookworms N. americanus and A. ceylanicum. The exact opposite was observed for S. mansoni. At the 24 h and 48 h evaluation time-points, the IC50 values on NTS were 5 to 7 times lower than the ones measured on adult S. mansoni. When assessed after 3 days, a 13-fold difference was observed between S. mansoni NTS and the adult worms.
Emodepside was lethal (100% effect) in vitro on A. ceylanicum adults, S. ratti L3 and both life stages of N. americanus. Drug effects above 90% were observed at a concentration of 25 µM emodepside for T. muris L1 and at a concentration of 2.5 µM for adult worms. This was also the case for A. ceylanicum when incubated at 1 µM (L3) or 0.1 µM (adults) and N. americanus at 0.25 µM (L3) or 0.1 µM (adults). Effects of more than 75% were reached at 2.5 µM for H. polygyrus L3 and at 0.5 µM for adult worms. Emodepside had an effect above 75% at a concentration of 2.5 µM on S. ratti (L3) and showed a similar effect at a 10 times lower concentration when tested on adult worms.
In vivo studies
The worm expulsion rates and worm burden reductions obtained with single-dose, oral emodepside against T. muris are summarized in Table 2. A high dose of 75 mg/kg emodepside resulted in complete elimination of all worms. At doses of 10 mg/kg and 2.5 mg/kg worm burden reductions of 85.9% and 69.6% and worm expulsion rates of 62.0% and 60.9%, respectively were observed. The lowest dose tested (1.25 mg/kg) showed low activity, with a worm burden reduction of 73.9% and worm expulsion rate of 5.3%. The worm burden reductions obtained with emodepside (all doses versus controls) were statistically significant (t = 7.18, P = 0.0073). Based on worm burden reductions we calculated an ED50 value of 1.2 mg/kg.
Table 2.
In vivo dose response relationships of emodepside on A. ceylanicum, N. americanus and T. muris
| Dose (mg/kg) | Mean no. of worms ± SD | Worm expulsion rate (%) | Worm burden reduction (%) | P-value | ED50 (mg/kg) | |
|---|---|---|---|---|---|---|
| T. muris | ||||||
| Emodepside | 75c | 0 | 100 | 100 | 0.007a | 1.2 |
| 10c | 18.8 ± 20.8 | 62.0 | 85.9 | |||
| 2.5c | 36.5 ± 30.8 | 60.9 | 69.6 | |||
| 1.25d | 133 ± 27.9 | 5.3 | 73.9 | |||
| Control 1c | 120.8 ± 12.0 | 0 | – | |||
| Control 2d | 121.7 ± 4.7 | 0 | – | |||
| A. ceylanicum | ||||||
| Emodepside | 2.5 | 0 | 100 | 100 | 0.014a | |
| Control | 21.3 ± 2.6 | 0.6 | – | |||
| N. americanus | ||||||
| Emodepside | 10c | 0 | 100 | 100 | 0.060a | 0.5 |
| 5c | 0 | 100 | 100 | |||
| 2.5d | 0.25 ± 0.5 | 87.5 | 93.8 | |||
| 1.25d | 2.25 ± 2.3 | 40.0 | 43.8 | |||
| Control 1c | 5.5 ± 6.1 | 5.6 | – | |||
| Control 2d | 4.0 ± 1.4 | 0 | – | |||
aKruskal-Wallis test was applied to determine statistical significance on worm burden reduction of all doses versus controls
cControl 1 was used for this dose
dControl 2 was used for this dose
Single doses of 10 mg/kg and 5 mg/kg cured all N. americanus-infected hamsters. A worm burden reduction of 93.8% and a worm expulsion rate of 87.5% were observed at a dose of 2.5 mg/kg. Moderate activity (worm burden reduction of 43.8% and worm expulsion rate of 40.0%) was observed in N. americanus-infected hamsters with the lowest dose tested of 1.25 mg/kg (all doses, t = 3.52, P = 0.06). An ED50 value of 0.5 mg/kg was determined for emodepside in N. americanus-infected hamsters. To confirm that emodepside also acts on Ancylostoma spp. the minimum effective dose on N. americanus of 2.5 mg/kg was tested in the A. ceylanicum hamster model, which resulted in cure of all animals (t = 6.05; P = 0.014).
Discussion
Given a promising activity against a wide range of resistant worm infections and its unique mode of action, it is worthwhile to evaluate the activity of emodepside against other helminth infections including STH and schistosomiasis. For the first time we thoroughly tested emodepside against a wide range of laboratory models for these diseases.
Both the larval and the adult nematode and schistosome stages were screened phenotypically in presence of emodepside over a time course of 72 hours followed by in vivo studies. As emodepside belongs to the group of cyclooctadepsipeptides that are known to be very active against different animal gastrointestinal nematodes and filarial parasites, good antinematicidal activity was expected [20, 30, 37–40]. The drug showed a high efficacy in vitro against all the nematode species and was highly effective against the two hookworms (A. ceylanicum and N. americanus) and the whipworm (T. muris). On the contrary, the effect of emodepside on schistosomes, remained only moderate.
We further investigated the efficacy of emodepside in vivo on rodents infected with T. muris, A. ceylanicum and N. americanus. Overall, the promising in vitro activity of emodepside was confirmed in vivo, where the drug demonstrated high worm burden reduction rates, even when administered orally as a low, single dose regimen.
These results were consistent with previous findings. The ED50 value obtained in vivo for T. muris (ED50 of 1.2 mg/kg) was very similar to the one reported by Kulke et al. [28]. Our study also confirms the good activity of emodepside in vitro against larval and especially adult stages of the nematodes S. ratti and H. polygyrus that was so far only described in vivo [29].
Emodepside performed much better in vitro and in vivo than albendazole, levamisole and pyrantel pamoate, the standard drugs used against STH infections tested in a previous study [36], where none of the standard drugs showed in vitro activity against adult A. ceylanicum (Table 3). Moreover, only a moderate in vitro efficacy against T. muris and N. americanus was reached by levamisole and pyrantel pamoate. In contrast, emodepside was very active in vitro against the larvae and adult worms of all three species. In vivo, albendazole was the only drug that performed as well as emodepside on A. ceylanicum infected hamsters (Table 4). While none of the three standard drugs cured mice harboring a T. muris infection, emodepside was fully active at a concentration of 75 mg/kg.
Table 3.
Mean IC50 values (µg/ml) after 72 hours drug exposure on L3 and adult stages of A. ceylanicum, N. americanus and T. muris of emodepside compared to the ones of albendazole, levamisole and pyrantel pamoate
| Species | Mean IC50 (µg/ml) after 72 hours of drug incubation | |||
|---|---|---|---|---|
| Emodepside | Albendazolea | Levamisole-HCla | Pyrantel pamoatea | |
| T. muris (L1) after 24 h | 4.18 | |||
| T. muris (L3) | – | ≥ 200 | 33.1 | 95.5 |
| T. muris (adults) | 0.022 | ≥ 200 | 16.5 | 34.1 |
| A. ceylanicum (L3) | 0.28 | 32.40 | 1.60 | 90.9 |
| A. ceylanicum (adults) | 0.0027 | ≥ 100 | ≥ 100 | ≥ 100 |
| N. americanus (L3) | 0.090 | ≥ 100 | 0.50 | 2.0 |
| N. americanus (adults) | 0.0024 | ≥ 100 | 13.40 | 7.6 |
aAll values for this drug are taken from the study of Tritten et al. [36]
Table 4.
In vivo dose response relationships of emodepside, albendazole, levamisole and pyrantel pamoate on A. ceylanicum, N. americanus and T. muris
| Drug | Dose (mg/kg) | Worm expulsion rate (%) | Worm burden reduction (%) |
|---|---|---|---|
| T. muris | |||
| Emodepside | 75 | 100 | 100 |
| 10 | 62.0 | 85.9 | |
| 2.5 | 60.9 | 69.6 | |
| 1.25 | 5.3 | 73.9 | |
| Albendazolea | 600 | 49.4 | 20.2 |
| Levamisole-HCla | 200 | 90.5 | 95.9 |
| Pyrantel pamoatea | 300 | 9.4 | 0 |
| A. ceylanicum | |||
| Emodepside | 2.5 | 100 | 100 |
| Albendazolea | 1.25 | 70.5 | 87.8 |
| 2.5 | 100 | 100 | |
| 5 | 100 | 100 | |
| Levamisole-HCla | 10 | 44.3 | 60.2 |
| Pyrantel pamoatea | 10 | 63.4 | 87.2 |
| N. americanus | |||
| Emodepside | 10 | 100 | 100 |
| 5 | 100 | 100 | |
| 2.5 | 87.5 | 100 | |
| 1.25 | 40.0 | 62.5 | |
| Albendazolea | 10 | 100 | 100 |
| 5 | 69.6 | 70.8 | |
aAll values for this drug are taken from the study of Tritten et al. [36]
Although emodepside was also very active in vitro on H. polygyrus L3 (with IC50 values below 1 µM), previous studies reported lower in vivo sensitivity of H. polygyrus larvae compared to the larval stages of other nematode species. This decreased activity against H. polygyrus larvae in vivo was explained by their presence burrowed deep into the gastro-intestinal tissues which was likely to protect them from the drug [29, 41]. Aiming at a formulation of emodepside active on all parasite stages, in vivo studies should preferably be performed at both early and late stages of infection. Hence future in vivo studies should evaluate the activity against the early developmental stages of the nematodes.
In our study, the in vitro activity of emodepside against the nematode species was higher in adult worms than in the larvae. Such difference in anthelmintic susceptibility between the early and the late developmental stages of the parasite was reported previously [42–44]. A differential expression of emodepside molecular target(s) between the parasites life-stages or differences in the permeability of the cuticle (or both) may account for it [42, 45]. Moreover, a similar trend was observed in vivo in other studies on Nippostrongylus brasiliensis, S. ratti and H. polygyrus [29]. However, for S. mansoni, we documented the opposite finding, with revealing lower IC50 values than the adults.
We observed in our in vitro studies that whereas the morphology of the parasites seemed not affected by the drug, often no motility or pharyngeal pumping movement could be detected. This observation corroborates the suggested mechanism of action of emodepside [30, 37, 46–49]. Although its exact mechanism of action is not fully understood yet, the drug is known to bind to two different targets of the neuromuscular junction, the evolutionary conserved calcium-activated potassium channel slowpoke 1 (SLO-1) and the latrophilin receptors LAT-1/LAT-2 [30, 37, 47, 50, 51]. In nematodes, the over activation of the SLO-1 receptors by emodepside is likely to induce a potassium efflux triggering a hyperpolarization of the neurons that results in a decreased synaptic transmission and muscle contraction, leading notably to a paralysis of the worm pharynx [37, 42, 47, 50, 52]. The specificity of emodepside towards the nematode channel subunits might account for its lack of efficacy against the trematodes [49].
The in vitro assay read-out methods varied among the different parasites and life-stages. While visual scoring of adult worms is generally straightforward for a trained operator, evaluating larval assays was more challenging, especially for T. muris and S. ratti larval assays. This led to a high variability between the different assays and explained a higher number of replicates reported compared to the other parasites. This finding urges the optimization and development of more accurate assessment methods.
Conclusions
Our study confirms that emodepside represents a promising broad spectrum human anthelmintic drug candidate with intriguing activity against a wide range of nematodes. Since emodepside is already well characterized in veterinary medicine and undergoing clinical development for onchocerciasis, and the activity observed in this study against different nematodes was similar to previous findings on filarial worms [26] this will allow a significant shortcut developing this drug for human STH. A drug development plan should therefore be established to fill the missing gaps required so that emodepside will soon be available for the treatment of both filarial and STH infections.
Additional files
Additional file 1: Figure S1. Mean IC50 overtime for the nematode L3.
Additional file 2: Figure S2. Mean IC50 overtime for the adult nematodes.
Acknowledgments
The authors are also grateful to Yvette Endriss for her help in maintaining the parasite life-cycles.
Abbreviations
- IC50
half maximal inhibitory concentration
- ED50
median effective dose
- STH
soil-transmitted helminthiases
- WHO
World Health Organization
- MDA
mass drug administration
- HPLC
high-performance liquid chromatography
- ACN
acetonitrile
- TFA
trifluoroacetic acid
- LC-MS
liquid chromatography-mass spectrometry
- NMR
nuclear magnetic resonance
- MS
mass spectrometry
- DMSO
dimethyl sulfoxide
- RPMI
Roswell Park Memorial Institute
- L1
stage 1 larvae
- L3
stage 3 larvae
- iFCS
inactivated fetal calf serum
- PBS
phosphate-buffered saline
- NTS
newly transformed schistosomula
- HBSS
Hanksʼ balanced salt solution
- CO2
carbon dioxide
- p.i.
post-infection
- WBR
worm burden reduction
- WER
worm expulsion rate
- SLO-1
calcium-activated potassium channel slowpoke 1
- LAT-1/LAT-2
latrophilin receptors
- 3 R
Replacement, Reduction, Refinement
Authorsʼ contributions
TK, VP and JK designed the study. TK and VP performed the experiments in vitro. CH infected, treated and dissected the animals involved in this study. AN synthetized emodepside powder. TK, VP and JK analyzed the data and wrote the first draft of the manuscript that was revised and completed by IS and AN. All authors read and approved the final manuscript.
Funding
JK is grateful to the European Research Council (ERC-2013-CoG 614739-A_HERO) for financial support.
Availability of data and materials
The datasets supporting the conclusion of this article are included within the article and its additional files.
Ethics approval and consent to participate
All in vivo studies were carried out at the Swiss Tropical Institute (Basel, Switzerland), in accordance with both cantonal (license no. 2070) and Swiss national regulations on animal experimentation and complied with the 3Rs principles.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tanja Karpstein, Email: tanja.karpstein@gmx.de.
Valérian Pasche, Email: valerian.pasche@swisstph.ch.
Cécile Häberli, Email: cecile.haeberli@swisstph.ch.
Ivan Scandale, Email: iscandale@dndi.org.
Anna Neodo, Email: anna.neodo@gmail.com.
Jennifer Keiser, Email: jennifer.keiser@swisstph.ch, Email: jennifer.keiser@unibas.ch.
References
- 1.Geary TG, Woo K, McCarthy JS, Mackenzie CD, Horton J, Prichard RK, et al. Unresolved issues in anthelmintic pharmacology for helminthiases of humans. Int J Parasitol. 2010;40:1–13. doi: 10.1016/j.ijpara.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasite Vectors. 2014;7:37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weatherhead JE, Hotez PJ, Mejia R. The global state of helminth control and elimination in children. Pediatr Clin North Am. 2017;64:867–877. doi: 10.1016/j.pcl.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conteh L, Engels T, Molyneux DH. Socioeconomic aspects of neglected tropical diseases. Lancet. 2010;375:239–247. doi: 10.1016/S0140-6736(09)61422-7. [DOI] [PubMed] [Google Scholar]
- 6.WHO . Research priorities for helminth infections: technical report of the TDR Disease Reference Group on Helminth Infections. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 7.WHO . Investing to overcome the global impact of neglected tropical diseases: third WHO report on neglected tropical diseases. Geneva: World Health Organization; 2015. [Google Scholar]
- 8.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 9.Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG. Soil-transmitted helminth infections. Lancet. 2018;391:252–265. doi: 10.1016/S0140-6736(17)31930-X. [DOI] [PubMed] [Google Scholar]
- 10.WHO. Preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups. http://www.who.int/nutrition/publications/guidelines/deworming/en/. Accessed 10 Oct 2018. [PubMed]
- 11.WHO. Accelerating work to overcome the global impact of neglected tropical diseases: a roadmap for implementation: executive summary. Geneva: World Health Organization; 2012. http://apps.who.int/iris/bitstream/handle/10665/70809/WHO_HTM_NTD_2012.1_eng.pdf?sequence=1&isAllowed=y. Accessed 15 Nov 2018.
- 12.Moser W, Schindler C, Keiser J. Efficacy of recommended drugs against soil transmitted helminths: systematic review and network meta-analysis. BMJ. 2017;358:j4307. doi: 10.1136/bmj.j4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crellen T, Walker M, Lamberton PHL, Kabatereine NB, Tukahebwa EM, Cotton JA, et al. Reduced efficacy of praziquantel against Schistosoma mansoni is associated with multiple rounds of mass drug administration. Clin Infect Dis. 2016;63:1151–1159. doi: 10.1093/cid/ciw506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keiser J, Utzinger J. The drugs we have and the drugs we need against major helminth infections. Adv Parasitol. 2010;73:197–230. doi: 10.1016/S0065-308X(10)73008-6. [DOI] [PubMed] [Google Scholar]
- 15.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 16.Panic G, Vargas M, Scandale I, Keiser J. Activity profile of an FDA-approved compound library against Schistosoma mansoni. PLoS Negl Trop Dis. 2015;9:e0003962. doi: 10.1371/journal.pntd.0003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emodepside - DNDi. https://www.dndi.org/diseases-projects/portfolio/emodepside/. Accessed 9 Oct 2018.
- 18.Altreuther G, Schimmel A, Schroeder I, Bach T, Charles S, Kok DJ, et al. Efficacy of emodepside plus praziquantel tablets (Profender® tablets for dogs) against mature and immature infections with Toxocara canis and Toxascaris leonina in dogs. Parasitol Res. 2009;105:1–8. doi: 10.1007/s00436-009-1489-7. [DOI] [PubMed] [Google Scholar]
- 19.Altreuther G, Borgsteede FHM, Buch J, Charles SD, Cruthers L, Epe C, et al. Efficacy of a topically administered combination of emodepside and praziquantel against mature and immature Ancylostoma tubaeforme in domestic cats. Parasitol Res. 2005;97:S51–S57. doi: 10.1007/s00436-005-1444-1. [DOI] [PubMed] [Google Scholar]
- 20.Epe C, Kaminsky R. New advancement in anthelmintic drugs in veterinary medicine. Trends Parasitol. 2013;29:129–134. doi: 10.1016/j.pt.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Mehlhorn H, Schmahl G, Frese M, Mevissen I, Harder A, Krieger K. Effects of a combinations of emodepside and praziquantel on parasites of reptiles and rodents. Parasitol Res. 2005;97:S65–S69. doi: 10.1007/s00436-005-1446-z. [DOI] [PubMed] [Google Scholar]
- 22.Petry G, Kruedewagen E, Bach T, Gasda N, Krieger KJ. Efficacy of Procox® oral suspension for dogs (0.1% emodepside and 2% toltrazuril) against experimental nematode (Toxocara cati and Ancylostoma tubaeforme) infections in cats. Parasitol Res. 2011;109:37–43. doi: 10.1007/s00436-011-2401-9. [DOI] [PubMed] [Google Scholar]
- 23.Schimmel A, Altreuther G, Schroeder I, Charles S, Cruthers L, Kok DJ, et al. Efficacy of emodepside plus praziquantel tablets (Profender® tablets for dogs) against mature and immature adult Trichuris vulpis infections in dogs. Parasitol Res. 2009;105:17–22. doi: 10.1007/s00436-009-1491-0. [DOI] [PubMed] [Google Scholar]
- 24.Schimmel A, Schroeder I, Altreuther G, Settje T, Charles S, Wolken S, et al. Efficacy of emodepside plus toltrazuril (Procox® oral suspension for dogs) against Toxocara canis, Uncinaria stenocephala and Ancylostoma caninum in dogs. Parasitol Res. 2011;109:1–8. doi: 10.1007/s00436-011-2397-1. [DOI] [PubMed] [Google Scholar]
- 25.Schimmel A, Altreuther G, Schroeder I, Charles S, Cruthers L, Ketzis J, et al. Efficacy of emodepside plus praziquantel tablets (Profender® tablets for dogs) against mature and immature adult Ancylostoma caninum and Uncinaria stenocephala infections in dogs. Parasitol Res. 2009;105:9–16. doi: 10.1007/s00436-009-1490-1. [DOI] [PubMed] [Google Scholar]
- 26.Townson S, Freeman A, Harris A, Harder A. Activity of the cyclooctadepsipeptide emodepside against Onchocerca gutturosa, Onchocerca lienalis and Brugia pahangi. Am J Trop Med Hyg. 2005;73:93. [Google Scholar]
- 27.Kuesel AC. Research for new drugs for elimination of onchocerciasis in Africa. Int J Parasitol Drugs Drug Resist. 2016;6:272–286. doi: 10.1016/j.ijpddr.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulke D, Krücken J, Harder A, von Samson-Himmelstjerna G. Efficacy of cyclooctadepsipeptides and aminophenylamidines against larval, immature and mature adult stages of a parasitologically characterized trichurosis model in mice. PLoS Negl Trop Dis. 2014;8:e2698. doi: 10.1371/journal.pntd.0002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harder A, Samson-Himmelstjerna G. Activity of the cyclic depsipeptide emodepside (BAY 44–4400) against larval and adult stages of nematodes in rodents and the influence on worm survival. Parasitol Res. 2001;87:924–928. doi: 10.1007/s004360100479. [DOI] [PubMed] [Google Scholar]
- 30.Krücken J, Harder A, Jeschke P, Holden-Dye L, O’Connor V, Welz C, et al. Anthelmintic cyclooctadepsipeptides: complex in structure and mode of action. Trends Parasitol. 2012;28:385–394. doi: 10.1016/j.pt.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Scherkenbeck J, Lüttenberg S, Ludwig M, Brücher K, Kotthaus A. Segment solid-phase total synthesis of the anthelmintic cyclooctadepsipeptides PF1022A and emodepside. Eur J Org Chem. 2012;2012:1546–1553. doi: 10.1002/ejoc.201101421. [DOI] [Google Scholar]
- 32.Mäser P, Grether-Bühler Y, Kaminsky R, Brun R. An anti-contamination cocktail for the in vitro isolation and cultivation of parasitic protozoa. Parasitol Res. 2002;88:172–174. doi: 10.1007/s00436-001-0511-5. [DOI] [PubMed] [Google Scholar]
- 33.Lombardo FC, Pasche V, Panic G, Endriss Y, Keiser J. Life cycle maintenance and drug-sensitivity assays for early drug discovery in Schistosoma mansoni. Nat Protoc. 2019;14:461–481. doi: 10.1038/s41596-018-0101-y. [DOI] [PubMed] [Google Scholar]
- 34.Milligan JN, Jolly ER. Cercarial transformation and in vitro cultivation of Schistosoma mansoni schistosomules. J Vis Exp JoVE. 2011;54:3191. doi: 10.3791/3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia LS. Diagnostic medical parasitology. Parasitol Today. 1998;14:125–126. doi: 10.1016/s0169-4758(97)01175-7. [DOI] [PubMed] [Google Scholar]
- 36.Tritten L, Silbereisen A, Keiser J. In vitro and in vivo efficacy of monepantel (AAD 1566) against laboratory models of human intestinal nematode infections. PLoS Negl Trop Dis. 2011;5:e1457. doi: 10.1371/journal.pntd.0001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harder A, Holden-Dye L, Walker R, Wunderlich F. Mechanisms of action of emodepside. Parasitol Res. 2005;97:S1–S10. doi: 10.1007/s00436-005-1438-z. [DOI] [PubMed] [Google Scholar]
- 38.Von Samson-Himmelstjerna G, Harder A, Sangster NC, Coles GC. Efficacy of two cyclooctadepsipeptides, PF1022A and emodepside, against anthelmintic-resistant nematodes in sheep and cattle. Parasitology. 2005;130:343–347. doi: 10.1017/S0031182004006523. [DOI] [PubMed] [Google Scholar]
- 39.Harder A, Schmitt-Wrede H-P, Krücken J, Marinovski P, Wunderlich F, Willson J, et al. Cyclooctadepsipeptides - an anthelmintically active class of compounds exhibiting a novel mode of action. Int J Antimicrob Agents. 2003;22:318–331. doi: 10.1016/S0924-8579(03)00219-X. [DOI] [PubMed] [Google Scholar]
- 40.Zahner H, Taubert A, Harder A, von Samson-Himmelstjerna G. Effects of Bay 44-4400, a new cyclodepsipeptide, on developing stages of filariae (Acanthocheilonema viteae, Brugia malayi, Litomosoides sigmodontis) in the rodent Mastomys coucha. Acta Trop. 2001;80:19–28. doi: 10.1016/S0001-706X(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 41.Stepek G, Lowe AE, Buttle DJ, Duce IR, Behnke JM. The anthelmintic efficacy of plant-derived cysteine proteinases against the rodent gastrointestinal nematode, Heligmosomoides polygyrusin vivo. Parasitology. 2007;134:1409–1419. doi: 10.1017/S0031182007002867. [DOI] [PubMed] [Google Scholar]
- 42.Bull K, Cook A, Hopper NA, Harder A, Holden-Dye L, Walker RJ. Effects of the novel anthelmintic emodepside on the locomotion, egg-laying behaviour and development of Caenorhabditis elegans. Int J Parasitol. 2007;37:627–636. doi: 10.1016/j.ijpara.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Schürmann S, Harder A, Schnieder T, von Samson-Himmelstjerna G. Effects of emodepside on egg hatching, larval development and larval motility in parasitic nematodes. Parasitol Res. 2007;101:45–56. doi: 10.1007/s00436-007-0610-z. [DOI] [Google Scholar]
- 44.Bernt U, Junkersdorf B, Londershausen M, Harder A, Schierenberg E. Effects of anthelminthics with different modes of action on the behavior and development of Caenorhabditis elegans. Fundam Appl Nematol. 1998;3:251–263. [Google Scholar]
- 45.Krüger N, Harder A, von Samson-Himmelstjerna G. The putative cyclooctadepsipeptide receptor depsiphilin of the canine hookworm Ancylostoma caninum. Parasitol Res. 2009;105:91–100. doi: 10.1007/s00436-009-1500-3. [DOI] [PubMed] [Google Scholar]
- 46.Guest M, Bull K, Walker RJ, Amliwala K, O’Connor V, Harder A, et al. The calcium-activated potassium channel, SLO-1, is required for the action of the novel cyclo-octadepsipeptide anthelmintic, emodepside, in Caenorhabditis elegans. Int J Parasitol. 2007;37:1577–1588. doi: 10.1016/j.ijpara.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Holden-Dye L, O’Connor V, Hopper NA, Walker RJ, Harder A, Bull K, et al. SLO, SLO, quick, quick, slow: calcium-activated potassium channels as regulators of Caenorhabditis elegans behaviour and targets for anthelmintics. Invert Neurosci. 2007;7:199–208. doi: 10.1007/s10158-007-0057-z. [DOI] [PubMed] [Google Scholar]
- 48.Kulke D, von Samson-Himmelstjerna G, Miltsch SM, Wolstenholme AJ, Jex AR, Gasser RB, et al. Characterization of the Ca2+-gated and voltage-dependent K+-channel Slo-1 of nematodes and its interaction with emodepside. PLoS Negl Trop Dis. 2014;8:e3401. doi: 10.1371/journal.pntd.0003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crisford A, Ebbinghaus-Kintscher U, Schoenhense E, Harder A, Raming K, O’Kelly I, et al. The cyclooctadepsipeptide anthelmintic emodepside differentially modulates nematode, insect and human calcium-activated potassium (SLO) channel alpha subunits. PLoS Negl Trop Dis. 2015;9:e0004062. doi: 10.1371/journal.pntd.0004062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holden-Dye L, Crisford A, Welz C, von Samson-Himmelstjerna G, Walker RJ, O’Connor V. Worms take to the slo lane: a perspective on the mode of action of emodepside. Invert Neurosci. 2012;12:29–36. doi: 10.1007/s10158-012-0133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin RJ, Buxton SK, Neveu C, Charvet CL, Robertson AP. Emodepside and SL0-1 potassium channels: a review. Exp Parasitol. 2012;132:40–46. doi: 10.1016/j.exppara.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willson J, Amliwala K, Harder A, Holden-Dye L, Walker RJ. The effect of the anthelmintic emodepside at the neuromuscular junction of the parasitic nematode Ascaris suum. Parasitology. 2003;126:79–86. doi: 10.1017/S0031182002002639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Mean IC50 overtime for the nematode L3.
Additional file 2: Figure S2. Mean IC50 overtime for the adult nematodes.
Data Availability Statement
The datasets supporting the conclusion of this article are included within the article and its additional files.