Abstract
We present a novel mouse-model for the study of skeletal structure and evolution, based on selective breeding for high levels of voluntary wheel running. Whereas traditional models (originally inbred strains, more recently knockouts and transgenics) rely on the study of mutant or laboratory-manipulated phenotypes, we have studied changes in skeletal morphometrics resulting from many generations of artificial selection for high activity in the form of wheel running, in which mice engage voluntarily. Mice from the four replicate High Runner (HR) lines run nearly three times as many revolutions during days 5 and 6 of a 6-day exposure to wheels (1.12 m circumference). We have found significant changes in skeletal dimensions of the hind limbs, including decreased directional asymmetry, larger femoral heads, and wider distal femora. The latter two have been hypothesized as evolutionary adaptations for long-distance locomotion in hominids. Exercise-training studies involving experimental groups with and without access to wheels have shown increased diameters of both femora and tibiafibulae, and suggest genetic effects on trainability (genotype-by-environment interactions). Reanalysis of previously published data on bone masses of hind limbs revealed novel patterns of change in bone mass associated with access to wheels for 2 months. Without access to wheels, HR mice have significantly heavier tibiafibulae and foot bones, whereas with chronic access to wheels, a significant increase in foot bone mass that was linearly related to increases in daily wheel running was observed. Mice exhibiting a recently discovered small-muscle phenotype (“mini-muscle,” [MM] caused by a Mendelian recessive gene), in which the mass of the triceps surae muscle complex is ∼50% lower than in normal individuals, have significantly longer and thinner bones in the hind limb. We present new data for the ontogenetic development of muscle mass in Control, HR, and MM phenotypes in mice of 1–7 weeks postnatal age. Statistical comparisons reveal highly significant differences both in triceps surae mass and mass-corrected triceps surae mass between normal and MM mice at all but the postnatal age of 1 week. Based on previously observed differences in distributions of myosin isoforms in adult MM mice, we hypothesize that a reduction of myosin heavy-chain type-IIb isoforms with accounts for our observed ontogenetic changes in muscle mass.
Introduction
Mouse-models for skeletal biology
For decades, rodents have been used as model organisms to study skeletal biology, to address hypotheses about human skeletal physiology and disease, and to understand the genetic underpinnings of skeletal traits. The benefits of using mice (Silver 1995; Eisen 2005) as models include their rapid generation times, large litters, ease of care, recently decoded genome, and similarities to human skeletons in structure and function (but see Bagi et al. 1997 for discussion of limitations).
Inbred strains of mice are the predominant model system in which the effects of treatments (e.g., experimental loading, exercise, limb unloading) on both skeletal form (i.e., morphology) and skeletal function (i.e., fracture mechanics) have been studied (e.g., Hallgrímsson 2008; Vinyard and Payseur this issue; Forwood 2008). Echoing studies of human twins, which have shown that 50–80% of the mineral density of adult bones is genetically determined (Eisman 1999; Ferrari et al. 1999), basic differences in mineralization among 11 inbred strains of mice were reported by Beamer et al. (1996). These authors found significant inter-strain differences in mineralization and, importantly, identified strains that exhibited extremely high (C3H/HeJ) and extremely low (C57BL/6J) mineralization of bone. Studies of these two strains have been instrumental in understanding the genetic underpinnings of the response of bone to loading (Akhter et al. 1998, 2000; Robling and Turner 2002; Koller et al. 2003; Robling et al. 2003).
More recently, knockout and transgenic mouse-models have come to the forefront of mouse skeletal biology. Specific gene knockouts have been produced that cause either increased or decreased bone mass, mineralization, and strength (López et al. 2008). Knockouts leading to increased mineralization include ob (leptin; Ducy et al. 2000), GDF-8 (myostatin; Whittemore et al. 2003; Hamrick et al. 2000, 2006), Dkk1 (MacDonald et al. 2007), and cav-1 (Rubin et al. 2007). Conversely, knockouts leading to decreased mineralization of bone similar to that seen in human osteoporosis and osteopenia include estrogen receptor α (ERα; Curtis Hewitt et al. 2000; Parikka et al. 2005), the P2X7 receptor (Ke et al. 2003), and COX-2 (Robertson et al. 2006). Similarly, using double-knockout (Myf5 and MyoD) mice that completely lacked striated skeletal muscle, Rot-Nikcevic et al. (2007) showed that normal skeletal morphology is under significant influence of skeletal muscle forces, which had been shown in previous studies of muscle denervation and disuse (Selye and Bajusz 1958; Dietz 1989; Dysart et al. 1989; Biewener and Bertram 1994).
In knockout models, the expression or function of a gene is blocked, whereas in transgenic models, genetic material from one organism is inserted into the genome of another. Transgenic mouse-models (e.g., López et al. 2008) have led to increased understanding of the role of the Wnt signaling pathway in bone formation and regulation (reviewed by Karsenty and Wagner 2002). Activating or deactivating a co-receptor in the Wnt pathway, low-density lipoprotein receptor-related protein 5 (Lrp5) can result in either high bone mass (Boyden et al. 2002; Babij et al. 2003; Akhter et al. 2004) or low bone mass (Ferrari et al. 2004; MacDonald et al. 2007; reviewed by Glass and Karsenty 2007).
Whereas genetic studies of skeletal biology have predominantly relied upon inbred, knockout, and transgenic mouse-models, herein we argue for the value of the original form of genetic manipulation—selective breeding (Garland and Rose manuscript in review). Selection experiments can be designed in many different ways, but all have a primary goal of changing the frequency of alleles that are segregating at any or all loci that affect a trait of interest (Swallow and Garland 2005). One type of selection experiment that is commonly used with rodents is artificial selection, in which, each generation, individuals are measured for some trait of interest and then the highest- or lowest-scoring are used as breeders to produce the next generation of a high- or low-selected line, respectively. In addition, Control (C) lines can be maintained in which individuals are measured (although perhaps not in all generations), but breeders are chosen without regard to the trait being selected in the high- or low-selected lines.
We summarize recent work on skeletal morphology and biomechanics of mice that are part of a long-term selection experiment for high levels of voluntary wheel running (Swallow et al. 1998; Garland 2003; Rhodes et al. 2005). An interesting and unexpected result from this selection experiment has been the observation of a Mendelian recessive allele that causes a 50% reduction in the muscle mass of the hind limb in homozygotes, has been favored by the selection regime, and has many other pleiotropic effects, including some effects on the hind limbs that mimic classic “cursorial” adaptations (Garland et al. 2002; Kelly et al. 2006). We present new data on the ontogeny of muscle mass in affected individuals, and interpret the results in the context of the development of muscle fiber types.
Selection experiments as a tool
Genetic modification via selective breeding has been used informally for thousands or tens of thousands of years to produce plants and animals with desired phenotypes, such as high milk yield, large reproductive output, ease of domestication, low toxicity, or high nutritional content (Vilà et al. 1997; Brotherstone and Goddard 2005; Burke et al. 2007; Casas et al. 2007; Pickersgill 2007; Bell 2008). Evolution in the laboratory (or garden) by way of selection experiments is an increasingly accessible methodology used to study traits of interest, both morphologic and physiologic (Garland and Carter 1994; Bennett and Lenski 1999; Gibbs 1999; Feder et al. 2000; Bennett 2003; Garland 2003; Swallow and Garland 2005; Garland and Rose manuscript in review). Well-documented selection experiments (Hill and Caballero 1992) on morphological traits in vertebrates began >60 years ago with the work of MacArthur, Rutledge, Eisen, Atchley, and their collaborators (MacArthur 1944a, 1944b; Rutledge et al. 1973, 1974; Eisen and Bandy 1977; Atchley et al. 1982, 1984; Eisen 1986, 1987a, 1987b). Most early studies involved selection for body mass or growth rate, and several of these examined the correlated response of skeletal morphology. For example, significant changes in tail length (MacArthur 1944a, 1944b; Rutledge et al 1973, 1974) and foot length (MacArthur 1944a, 1944b) were found during selection on body size in mice.
Several research programs have recently undertaken selection experiments for physiologic traits and for behaviors in rodents (Rhodes and Kawecki manuscript in review; Swallow et al. manuscript in review). For example, Koch and Britton have selected for both increased and decreased endurance during treadmill running in rats (Koch et al. 1998; Koch and Britton 2001). However, the inferences that can be drawn from these studies are limited because (1) an unselected C line was not maintained and (2) the selected lines were not replicated (Henderson 1989, 1997; but see example in Konarzewski et al. 2005). By the sixth generation of selection, Koch and Britton (2001) found ∼170% difference in distance run to exhaustion between the high- and low-selected lines. These lines have been the basis for studies of physiologic traits related to endurance exercise, such as maximum oxygen uptake (Henderson et al. 2002; Gonzalez et al. 2006), mitochondrial function (Walsh et al. 2006), and lipid metabolism (Hawley 2007; Spargo et al. 2007). It would also be of interest to examine skeletal architecture in these lines.
Selective breeding for high levels of voluntary wheel running
In 1993, Garland and colleagues began a selection experiment for high levels of voluntary exercise (wheel running) in mice (reviewed in Swallow et al. 1998, 2005; Garland 2003; Rhodes et al. 2005; Rhodes and Kawecki manuscript in review; details of the experimental protocol are provided subsequently). The overall goal of this experiment is to better understand the heritable aspects of behavior and physiology in a mammalian model, including neurobiologic and physiologic processes. It also seeks to understand how a purely voluntary behavior evolves with respect to both the brain (i.e., motivation and reward) and the body (i.e., exercise abilities). Voluntary exercise influences energy expenditure and energy balance, body composition, and overall physical fitness, as well as psychologic well-being. Wheel running was chosen because mice will engage in it voluntarily to levels that are potentially physiologically taxing, the amount of daily wheel running is heritable (Dunnington et al. 1981; Swallow et al. 1998; references therein), and it has analogies to human exercise (Eikelboom 1999), including being rewarding (Belke and Garland 2007; Brené et al. 2007).
Wheel running in the four replicate High Runner (HR) lines diverged rapidly from the four nonselected C lines for the first 16 generations, at which point HR lines ran, on average, 170–200% more revolutions than C lines (Fig. 1; see also Garland 2003). The fold difference for HR/C is virtually identical for males and females (e.g., see Fig. 2 in Garland 2003). Behavioral changes in the HR lines, in addition to increased wheel running, include differences in thermoregulatory nesting (Carter et al. 2000) and predatory aggression (Gammie et al. 2003), as well as the details of running speeds and bout lengths (Girard et al. 2001). Physiological changes associated with selection include increased insulin-stimulated glucose uptake in some muscles (Dumke et al. 2001) and increased maximum aerobic capacity as measured during forced treadmill exercise (Rezende et al. 2006a, 2006b). Hormonal changes have also been observed, including increased circulating baseline corticosterone (Malisch et al. 2007, 2008), increased circulating adiponectin (Vaanholt et al. 2007), and decreased circulating leptin (Girard et al. 2007). For a complete list of studies involving the HR mice, see http://biology.ucr.edu/people/faculty/Garland.html.
Fig. 1.
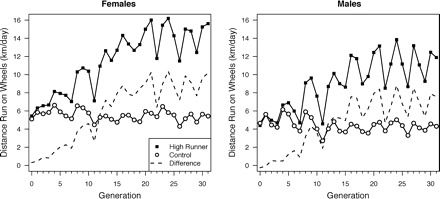
Voluntary wheel running (mean revolutions per day) on days 5 and 6 of a 6-day exposure to wheels (1.12 m circumference). Solid line with black squares is average value of the four replicate HR lines, solid line with open circles is average of four nonselected C lines, and dashed line is the differences between those averages. For both the HR and C lines, about one-third of the total revolutions (and hence distance) per day are attributable to “coasting” (Koteja et al. 1999). After generation 31, mice were moved from Wisconsin to California, and wheel running was not recorded for four generations. Data for subsequent generations are not shown.
Fig. 2.
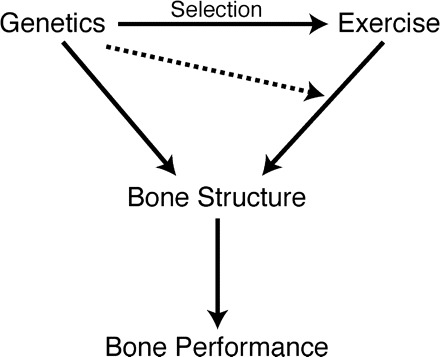
Bone performance during loading is primarily governed by bone structure (from microstructure to macrostructure). Genetics and history of loading (exercise) are the two main determinants of bone structure (Eisman 1999; Ferrari et al. 1999). Genetic background can mediate the effect of exercise in two ways. First, the propensity to exercise has a genetic basis, and this desire for activity is the target of selection (gray line). Second, the physiological response to exercise can be modulated by genetic background (dashed line). For example, Kodama et al. (2000) showed differential sensitivity to exercise in C57BL/6J mice compared with C3H/HeJ mice, with a greater response to loading in the former.
Wheel running as a model to study skeletal response to loading
Studies of the skeletal response to loading that employ rodent models have typically involved either externally applied loads (e.g., using external loading apparatuses; Robling and Turner 2002; Robling et al. 2003; Kesavan et al. 2005; Lau et al. 2006; Sawakami et al. 2006) or impact loads (e.g., training via jumping; Honda et al. 2001, 2003; Umemura et al. 2002; Welch et al. 2004). Although externally applied loads are attractive for their precise magnitudes and large potential response, such loads often are 5 to 10 times higher than those that animals likely experience in the wild. Thus, the response to loading in these experiments may be viewed as pathologic rather than part of normal skeletal homeostasis (Middleton et al. 2008). We propose that loads produced during wheel running may more closely mimic loads encountered in a natural setting and that the response of bone to such loads is more representative of responses that occur in wild populations and is more homologous to normal human locomotion. Differences resulting from either selective breeding for high voluntary wheel running (see subsequently) or from chronic access to wheels thus may be more reflective of natural processes that occur as bones adapt to loads (Fig. 2).
Because wheel running can require considerable energy, any skeletal adaptations that minimize the cost of oscillating the limbs tens or hundreds of thousands of times a day, as required to run the distances observed in the HR lines, might be favored by selection. However, mice always have ad libitum access to food during their tests on wheels, which might eliminate, or at least ameliorate, any selection related to overall energy budgets per se (Koteja et al. 2003; Rezende 2005; and references therein). In any case, significant alterations in skeletal morphology, resulting from either selective breeding, voluntary locomotory activity, or their combined effects, may mimic the skeletal changes observed in natural populations that are hypothesized to be adaptations minimizing the cost of locomotion. For example, among species of mammals, “cursoriality” is generally associated with relative lengthening of distal limb segments and an increased metatarsal/femur (MT/F) ratio. Furthermore, phylogenetic analyses have indicated a positive correlation between home range area (corrected for body mass) and both length of the hind limb (one-tailed P = 0.010, r2 = 0.279) and the MT/F ratio (one-tailed P = 0.037, r2 = 0.176) in a sample of 19 species of Carnivora, although only the length of the hind limb is statistically significant in a multiple regression that includes both length of the hind limb and the MT/F ratio (Kelly et al. 2006).
Counter to our expectations based on previously documented cursorial adaptations, mice from HR lines have thicker and heavier bones in the hind limb, as compared with C lines, when housed without access to wheels. These differences could be attributable to elevated home-cage activity of HR lines when housed without access to wheels (Rhodes et al. 2005; Malisch et al. 2008), as discussed by Kelly et al. (2006), or could possibly be related to overall energy balance. The skeleton may play a role in the homeostasis of energy via the interaction between bone remodeling processes and the endocrine system (Ducy et al. 2000; Lee et al. 2007). We are currently investigating whether HR and C lines differ in their rates of formation and remodeling of bone when housed with or without access to wheels.
Skeletal evolution in response to selection and the effects of chronic access to wheels
In a study of both sexes from generation 11, Garland and Freeman (2005) found that the classic indicators of “cursoriality” had not evolved in concert with high levels of activity. Instead, HR mice had significantly larger femoral condyles and significantly reduced directional asymmetry in lengths of the bones of the hind limbs, as compared with C lines. They hypothesized that greater limb symmetry and larger femoral heads may be general adaptations associated with sustained, high-speed locomotion.
Three subsequent studies, involving later generations, have examined mice housed both with and without chronic access to wheels. Kelly et al. (2006) report data for male mice given access to wheels for 8 weeks beginning at 25–28 days of age. They measured the length, diameter, and mass of the femora, tibiafibulae, and metatarsals. Total hind-limb length was computed as the sum of lengths of the femur, tibiafibula, and metatarsal; the MT/F ratio was also calculated. Some highlights of their results are as follows.
An analysis of covariance (ANCOVA) revealed that body mass was a statistically significant predictor of all bone measures except the MT/F ratio; therefore, all results reported were from ANCOVAs with body mass as the covariate. This finding and approach is important given that not all studies investigating the effects of loading on bone include body mass in the statistical analyses (e.g., Gordon et al. 1989; Kannus et al. 1995; Järvinen et al. 2003; Binkley and Specker 2004), even when body mass differs significantly between control and treatment groups (e.g., Shaw et al. 1987; Niehoff et al. 2004; Wu et al. 2004). See Middleton et al. (2008) for further discussion of the importance of controlling for body mass in studies investigating skeletal architecture.
Once the effects of body mass were controlled statistically, no bone length was significantly affected by either 21 generations of selective breeding or 8 weeks of access to wheels. MT/F ratio, the classic indicator of cursoriality in mammals, was also unaffected by either factor (and did not correlate with body mass). The lack of classic cursorial adaptations in the HR lines is somewhat surprising (see also Garland and Freeman 2005), given that on behavioral performance grounds they can reasonably be considered cursors. However, the classic indicators of cursoriality are mainly based on large-bodied animals, and it may be germane to redefine “cursors” according to body-size classes. Therefore, it is a long-term goal of the authors to correlate behavioral indicators of cursoriality (e.g., distance of daily movement, home-range size) with skeletal and kinematic (e.g., Reilly et al. 2006) characteristics among small-bodied animals in a phylogenetic context (Garland et al. 2005; Kelly et al. 2006).
Conversely, both (1) selection for high level of voluntary running, and (2) wheel access positively affected a variety of femoral, tibiafibular, and metatarsal widths (see Table 2 in Kelly et al. 2006). Particularly notable was a positive effect of selection (HR versus C, P = 0.0119; and of access to wheels, P = 0.0349) on the anterior–posterior depth of the femoral condyle (i.e., the femoral head). This positive effect of selection history has also been observed in other studies of the skeletons of these lines of mice (Garland and Freeman 2005; Middleton et al. 2008). This increase in surface area is presumably an evolutionary adaptation that reduces stress on the joint during endurance running, defined as running many kilometers aerobically (Bramble and Lieberman 2004). Such an increase has also been observed in Homo (compared with Pan and Australopithecus), along with an increase in other lower-body joint surfaces (e.g., femoral head and knee, the sacroiliac joint, and the lumbar centra; Bramble and Lieberman 2004).
Table 2.
Simple means and standard errors from a two-way ANOVA on linetype (C versus HR) and activity (wheel versus no wheel), with MM as an additional factor
| Trait | N | Simple mean | SE | Difference (%) |
|---|---|---|---|---|
| Femur | ||||
| C—no wheel | 20 | 0.1434 | 0.0084 | 1.79 |
| wheel | 20 | 0.1460 | 0.0104 | |
| HR—no wheel | 16 | 0.1534 | 0.0108 | −1.47 |
| wheel | 14 | 0.1512 | 0.0137 | |
| MM—no wheel | 4 | 0.1346 | 0.0086 | 9.12 |
| wheel | 5 | 0.1469 | 0.0170 | |
| Tibiafibula | 0.0000 | |||
| C—no wheel | 19 | 0.1159 | 0.0078 | 9.97 |
| wheel | 19 | 0.1274 | 0.0084 | |
| HR—no wheel | 16 | 0.1295 | 0.0088 | 6.18 |
| wheel | 15 | 0.1375 | 0.0116 | |
| MM—no wheel | 4 | 0.1208 | 0.0077 | 11.14 |
| wheel | 5 | 0.1342 | 0.0125 | |
| Foot (all bones) | 0.0000 | |||
| C—no wheel | 20 | 0.1151 | 0.0068 | 13.23 |
| wheel | 20 | 0.1326 | 0.0091 | |
| HR - no wheel | 16 | 0.1314 | 0.0085 | 9.54 |
| wheel | 15 | 0.1452 | 0.0122 | |
| MM—no wheel | 4 | 0.1406 | 0.0047 | 5.17 |
| wheel | 5 | 0.1483 | 0.0141 |
As expected from previous studies of voluntary running on wheels (Newhall et al. 1991; Notomi et al. 2000a), locomotion on treadmills (Iwamoto et al. 1998, 1999), climbing (Siegel and Jones 1975; Notomi et al. 2001; Mori et al. 2003), lever pressing (Westerlind et al. 1998), jumping (Kodama et al. 2000; Notomi et al. 2000a, 2000b), and swimming (Hart et al. 2001), access to wheels generally increased diameters of both the femora and tibia-fibulae, as well as the masses of femora, tibiafibulae, and metatarsals in both HR and C lines (Kelly et al. 2006). Although we might have expected mice from the HR lines to experience greater effects of training (“more pain, more gain”) as compared with C mice, given that they run significantly more revolutions per day (Swallow et al. 2005; Kelly et al. 2006), we found that for no skeletal trait was the interaction between selection history and access to wheels (i.e., indicating a genotype-by-environment interactions) statistically significant at P < 0.05. Similarly, Middleton et al (2008) found an interaction significant at P < 0.05 for only 1 of 19 femoral traits. Several functional explanations for the lack of statistical interactions are outlined by Kelly et al. (2006). For example, throughout the 8 weeks of access to wheels, both HR and C mice presumably experienced a redundant loading environment, which may have made bone cells less responsive to the routine mechanical stimuli, regardless of how much HR lines ran versus C lines (Robling et al. 2001, 2002a, 2002b; Srinivasan et al. 2002). The lack of significant interactions may also have a statistical explanation: ANOVAs typically have relatively low power to detect interactions (Neyman 1935; Rodger 1974; Traxler 1976; Wahlsten 1990). For example, according Wahlsten (1991), for a 2 × 2 design, power to detect an interaction is only 16%, whereas power to detect a main effect is 87%.
Therefore, instead of only performing a two-way ANOVA and checking for interactions that are significant at P < 0.05 (as in Garland and Kelly 2006), we have begun to consider the use of alternative statistical tests for detecting biologically important interactions between selective breeding and exercise training. For example, comparison of the linetype effect (HR versus C lines) in separate analyses of the “sedentary” and “wheel-access” groups may more reliably point out the existence of differential plasticity between HR and C lines.
As an example of this approach, we have reanalyzed the data for masses of the femur, tibiafibula, and foot (all bones) that were presented by Kelly et al. (2006). In the original study, the P-value for the interaction between linetype and access to wheels was 0.68, 0.37, and 0.07, respectively, for the three bone masses. As shown in Table 1, when housed without access to wheels, HR lines had significantly heavier tibiafibulae (P = 0.0256) and feet (P = 0.0012) as compared with C lines. When housed with access to wheels for 8 weeks, these differences became less significant (P = 0.0702 and 0.0248, respectively). Table 2 shows the simple means from a two-way ANOVA on linetype (C versus HR) and activity (wheel versus no wheel), with mini-muscle (MM) as an additional factor and demonstrates that the C lines exhibit a larger positive training effect resulting from access to wheels as compared with the HR lines. This result is surprising, given that the former run fewer revolutions per day.
Table 1.
Results of nested ANCOVA [body mass as covariate, line (replicate line 1–8) as random effect nested within linetype (C versus HR): SAS Procedure mixed] comparing mice from C and HR lines when housed either without or with access to wheels for 8 weeks (data from Kelly et al. 2006)
| Trait | N | ln maximum likelihood | −2 × ln maximum likelihood | Likelihood ratio testa, P | P for HR versus. Cb | P for MMb | P for Revs. in final 6 days | −2 × ln restricted maximum likelihood; first iteration | −2 × ln restricted maximum likelihood; last iteration | Line likelihood ratio testc | P for linec |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No wheel | |||||||||||
| Femur | 40 | 0.1055+ | 0.0050− | −232.75 | −238.00 | 5.24 | 0.0220 | ||||
| Tibiafibula | 39 | 0.0256+ | 0.0885− | −236.70 | −244.56 | 7.86 | 0.0050 | ||||
| Foot (all bones) | 40 | 0.0012+ | 0.0438+ | −251.00 | −251.36 | 0.37 | 0.5446 | ||||
| Wheel | |||||||||||
| Femur | 39 | 118.26 | −236.52 | 0.726, 0.3942 | 0.8183+ | 0.8567− | 0.4499+ | −180.66 | −183.35 | 2.69 | 0.1011 |
| 39 | 117.90 | −235.79 | 0.5213+ | 0.9712− | −205.89 | −208.98 | 3.10 | 0.0785 | |||
| Tibiafibula | 39 | 125.85 | −251.70 | 1.835, 0.1755 | 0.2492+ | 0.6328− | 0.2456+ | −195.63 | −195.94 | 0.30 | 0.5814 |
| 39 | 124.93 | −249.86 | 0.0702+ | 0.8377− | −220.37 | −221.18 | 0.81 | 0.3681 | |||
| Foot (all bones) | 40 | 127.43 | −254.87 | 4.270, 0.0388 | 0.2170+ | 0.7043+ | 0.0549+ | −199.07 | −199.08 | 0.00 | 0.9436 |
| 40 | 125.30 | −250.60 | 0.0248+ | 0.5255+ | −221.60 | −221.83 | 0.22 | 0.6375 |
aTwice the difference in ln maximum likelihood is distributed as a χ2 with 1 df, i.e., 3.841 for P = 0.05. Values larger than this indicate that the model including amount of running during final week as a covariate (full model) fits the data significantly better than a model that does not include this covariate (reduced model).
b P-values ≤ 0.05 (unadjusted, two-tailed) are noted in bold. Signs following P values indicate direction of effect based on the partial regression from the mixed model: + indicates HR lines > C, and MM > non-MM.
cIn each one-way ANCOVA, to determine if significant variation among replicate lines was present, the −2 ln restricted maximum likelihoods of the initial and last iteration evaluations within each ANCOVA were examined. The difference in −2 ln REMLs can be compared with a χ2 distribution with 1 df, for which the critical value for P = 0.05 is 3.841.
An alternative to the “more pain, more gain” hypothesis is the “principle of initial value,” which states that the percentage gain via training will be inversely proportional to the initial value (Winters-Stone and Snow 2003; Koch et al. 2005; Garland and Kelly 2006; and references therein). The results for masses of bones of the hind limbs are generally in accord with this hypothesis, as can be seen from the changes in mean values indicated in Table 2 (also see Fig. 3); mice from the C lines exhibit a greater positive effect of training than do the HR lines for every bone. For the mice housed with access to wheels, models can also be analyzed, which include the quantitative amount of running during the final 6 days as a covariate. For the foot, such a model is significantly better (likelihood ratio test) than the simpler model. This result indicates that, for the feet of mice with access to wheels, the significantly heavier bones of HR as compared with C mice (Table 2) can be explained, in statistical terms, as a function of their greater amount of running (Fig. 3C; Table 1). Note that the difference is less than when they are housed without wheels, in accordance with the principle of initial value. We did not formally test for interactions between linetype (or MM) and amount of running within the wheel-access group because of convergence problems (or small sample sizes).
Fig. 3.
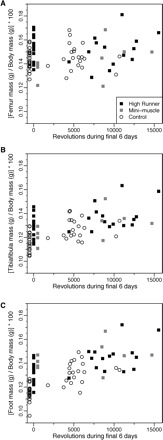
Bone mass in relation to amount of running in a wheel (revolutions/day) during the last 6 days of an 8-week access to wheels (1.12 m circumference) as described by Kelly et al. (2006). Individuals plotted near the left axis, above the value of zero revolutions, were housed in ordinary cages without wheels (points are offset from zero for clarity). Data based on Kelly et al. (2006).
Most recently, Middleton et al. (2008) compared bone morphometrics and fracture mechanics of the femur in female HR and C mice that were allowed or denied access to wheels for 20 months. Total revolutions increased rapidly, peaking between 70 and 80 km/week at 5–10 weeks of age (see Fig. 3 in Morgan et al. 2003; Middleton et al. 2008). The amount of running in the wheel declined steadily throughout the 20-month period of access, but a difference between HR and C lines was maintained throughout the experimental period.
The femoral neck was loaded to fracture in cantilever bending using an Instron-4220 materials-testing apparatus. Load at fracture was then correlated with body mass, suborganismal traits (height of the femoral neck), and microCT-derived measurements (second-moment of area and cross-sectional area of the femoral neck). We found strong correlations between fracture load and all traits studied, suggesting that the strength of the femoral neck is primarily determined by the geometry of the bone (see Table 4 in Middleton et al. 2008). We did not find widespread effects of either chronic access to wheels or linetype, and we hypothesized that the beneficial effects of loading usually associated with long-term exercise may have been lost in the aged mice because of decreasing running activity and presumed postmenopausal status of the experimental group.
Skeletal effects of the MM phenotype
At generation 22 of selection for high levels of voluntary wheel running, a gene of major effect on muscle mass, named MM, was identified, and some of its pleiotropic phenotypic effects were noted (Garland et al. 2002). The MM phenotype was initially observed in one of the four replicate C lines (laboratory designation line 5) and in two of the four replicate HR lines (laboratory designation lines 3 and 6; Garland et al. 2002; Houle-Leroy et al. 2003). Analyses showed that the MM allele must have been favored by the selection regime. Thus, its increase in frequency is a fundamental part of the response to selective breeding for high wheel running, but restricted to two of the four lines; the other two HR lines apparently lost it by random genetic drift early in the experiment (Garland et al. 2002).
Since publication of the original description, the MM allele has apparently been lost by drift in the only C line that ever showed the phenotype, has gone to fixation in one of the HR lines (laboratory designation line 3) by generation 36 (Syme et al. 2005), and remains polymorphic in the other HR line (laboratory designation line 6) as of generation 51. A Mendelian recessive (Garland et al. 2002; Hannon et al. 2008), the predominant phenotypic effect of the MM allele is a 50% reduction in mass of the triceps surae muscle complex (comprising the gastrocnemius, soleus, and plantaris; Fig. 4; also see Garland et al. 2002). In addition to reduced mass of the triceps surae muscle complex, MM muscles are physiologically different from unaffected or “wild type” muscles in many ways. For example, Houle-Leroy et al. (2003) showed that muscles with the MM phenotype have higher mass-specific aerobic capacities and resembled, physiologically, muscles trained for endurance exercise. MMs have also been shown to have altered contractile properties, including slower twitch times and approximately half of the mass-corrected force output as unaffected muscles (Syme et al. 2005). Recently, Guderley et al. (2006, 2008) characterized the mitochondrial density (greater in MMs) and alterations in myosin isoforms and fiber types (reduced populations of type-IIb fibers, increased proportion of oxidative fibers), which are hypothesized to result in increased resistance to fatigue (double the mass-specific aerobic output) and decreased force output in these muscles. Additional pleiotropic effects in mice with the MM phenotype include significantly larger ventricles, livers, and spleens, even after correcting for body mass (Garland et al. 2002; Hannon et al. 2008). The gene that causes the MM phenotype has recently been mapped to a 2.6 Mb interval on MMU11 (Hartmann et al. 2008). Here, we present new data on the ontogeny of muscle mass in both normal and MM individuals.
Fig. 4.
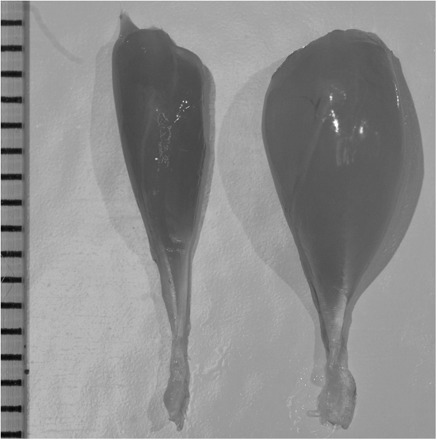
Comparison of the size of the triceps surae muscle in the MM phenotype (left; Mouse ID 53283; body mass = 20.4 g, age = 66 days) and in normal (right; Mouse ID 53277; body mass = 26.1 g, age = 66 days) mice. The muscles of the MM phenotype are ∼50% the mass of normal muscles (0.055 versus 0.129 g). Scale bar = 1 mm. Supplementary online figure is in color.
Several skeletal traits were affected by the presence of the MM phenotype. MM individuals generally had longer and thinner, but not lighter hind-limb bones (femur and tibia-fibula). Interestingly, these single-gene effects are in accordance with previously identified “cursorial” adaptations, sensu Hildebrand (1982): “Animals that travel far, fast, or easily on the ground are said to be cursorial.” According to Carrano et al. (1999), to facilitate increases in cursoriality or running performance animals should have thinner individual limb segments, long distal limb segments, and more proximal insertions of the muscles. The MM mice exhibit two out of the three former “adaptations” and the third will be the focus of future investigations. Although MM mice do not routinely run longer distances than do normal muscle HR mice (but see Hannon et al. 2008), they do typically run significantly faster on wheels (see Table 1 in Kelly et al. 2006). Therefore, the longer and thinner hindlimb bones of the MM individuals may be beneficial for running at significantly higher speeds. Thus, it is plausible that the MM phenotype has been favored by selection in part because of its effects on the size and shape of bones. Given the ontogenetic trajectory of the MM (see subsequently), the muscle itself may be causing the alteration in the size and shape of the bones.
Materials and methods
Establishment of C and selected HR lines
The complete experimental design is described by Swallow et al. (1998) and Garland (2003), and only a brief outline is provided here. From a base population of genetically heterogeneous, outbred mice (Mus domesticus; Hsd:ICR strain; Harlan Sprague Dawley), eight closed lines were established. In four replicate-selected HR lines, the parents for a subsequent generation are those that exhibit the highest levels of voluntary running on a wheel on days 5 and 6 of a 6-day access to wheels (1.12 m circumference Wahman type activity wheels). In four replicate-C lines, parents are randomly chosen, but otherwise treated identically. Mice from the ICR strain were chosen as the founding population because they exhibit high levels of genetic variation approximating those in natural populations and had been selected for large litter sizes and high weaning success (Swallow et al. 1998; and references therein). Within-family selection is performed to reduce maternal effects and reduce the rate of inbreeding.
Muscle ontogeny in mice with the MM phenotype
To determine the ontogenetic growth of muscles exhibiting the MM phenotype, 34 mating pairs from three lines from generation 45 of selection were bred. These lines include a C line (laboratory designation line 2), a HR line in which the MM phenotype has never been observed (HR; line 8), and a HR line in which the MM phenotype is fixed (MM; line 3). For 7 weeks, beginning at 1 week postnatal, one male and one female mouse from each family were dissected after sacrificed via CO2 inhalation. As in previous studies (Garland et al. 2002; Kelly et al. 2006), left and right triceps surae muscle complexes were dissected free of the surrounding tissue, excised, and weighed to the nearest 0.0001 g. In total, 332 mice were dissected (n = 62, 61, 57, 47 40, 33, 32 for weeks 1–7, respectively). All experimental procedures were in accordance with the guidelines of, and approved by, the University of California, Riverside IACUC.
We used ANOVA to compare differences among the three lines in three characteristics: mean triceps surae muscle mass, body mass, and muscle mass expressed as a percentage of total body mass, with Tukey's HSD used for pairwise post hoc comparisons between lines. We also performed an additional ANCOVA comparing triceps surae mass between lines with body mass as a covariate. Sexes were analyzed separately. All analyses were performed using R (version 2.6.2; R Core Development Team 2008). We did not correct for multiple comparisons (Curran-Everett and Benos 2004) because each time point (e.g., weeks 1–7) constituted a separate sample of mice.
Results and discussion
Ontogeny of the masses of the body and of muscle
Body mass increased rapidly during the first 7 postnatal weeks (Fig. 5A). Body mass was not significantly different among the three lines (C, HR, and MM) for either sex during weeks 1–7, except for female HR mice being smaller than C mice at week 3 (Table 3). In previous studies on older populations of male and female mice, both HR (Swallow et al. 2005; Kelly et al. 2006) and sometimes MM (Swallow et al. 2005) mice were found to have significantly lower body mass than did C mice (see also Hannon et al. 2008).
Fig. 5.
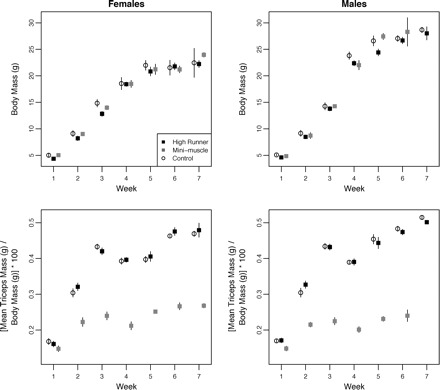
Ontogenetic change in body mass (top row) and in mass of the triceps surae muscle normalized to body mass (bottom row) during postnatal weeks 1–7 in C, normal HR, and MM-phenotype mice for females (left) and males (right). Values are simple means ± 1 SD (some of which are so small as to be obscured by the mean point). Points are slightly offset in age (weeks) for clarity.
Table 3.
Results of ANOVA of body mass, triceps surae mass, and (triceps surae mass/Body mass) × 100
| Overall |
Pairwise comparisons |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week | df | F | P | HR versus C | MM versus C | MM versus HR | ||||
| Body mass | ||||||||||
| 1 | Female | 2, 28 | 2.6 | 0.0944 | 0.1297 | − | 0.9976 | + | 0.1486 | + |
| Male | 2, 28 | 1.0 | 0.3864 | 0.3532 | − | 0.7722 | − | 0.7667 | + | |
| 2 | Female | 2, 27 | 1.1 | 0.3464 | 0.3727 | − | 0.9928 | − | 0.4711 | + |
| Male | 2, 28 | 0.5 | 0.6393 | 0.6215 | − | 0.8220 | − | 0.9414 | + | |
| 3 | Female | 2, 26 | 0.3 | 0.0550 | 0.0436 | − | 0.5387 | − | 0.3525 | + |
| Male | 2, 25 | 0.2 | 0.7902 | 0.8092 | − | 1.0000 | − | 0.8363 | + | |
| 4 | Female | 2, 20 | 0.0 | 0.9935 | 0.9928 | − | 0.9982 | − | 0.9988 | + |
| Male | 2, 21 | 2.0 | 0.1617 | 0.2852 | − | 0.2030 | − | 0.9432 | + | |
| 5 | Female | 2, 15 | 0.3 | 0.7308 | 0.7086 | − | 0.8604 | − | 0.9445 | − |
| Male | 2, 19 | 2.8 | 0.0859 | 0.1577 | − | 0.8256 | + | 0.1236 | + | |
| 6 | Female | 2, 10 | 0.2 | 0.8402 | 0.9811 | + | 0.9666 | − | 0.8270 | − |
| Male | 2, 17 | 0.5 | 0.5925 | 0.9401 | − | 0.6887 | + | 0.5667 | + | |
| 7 | Female | 2, 11 | 1.6 | 0.2376 | 0.9853 | − | 0.5503 | + | 0.2322 | + |
| Male | 1, 16 | 0.3 | 0.5803 | 0.5803 | − | |||||
| Triceps surae mass | ||||||||||
| 1 | Female | 2, 28 | 1.7 | 0.1943 | 0.1728 | − | 0.5591 | − | 0.7510 | + |
| Male | 2, 28 | 1.9 | 0.1756 | 0.5927 | − | 0.1509 | − | 0.6341 | − | |
| 2 | Female | 2, 27 | 5.0 | 0.0139 | 0.8408 | − | 0.0144 | − | 0.0560 | − |
| Male | 2, 28 | 9.8 | 0.0006 | 0.9988 | + | 0.0016 | − | 0.0018 | − | |
| 3 | Female | 2, 26 | 60.3 | <0.0001 | 0.0040 | − | <0.0001 | − | <0.0001 | − |
| Male | 2, 25 | 62.5 | <0.0001 | 0.7422 | − | <0.0001 | − | <0.0001 | − | |
| 4 | Female | 2, 20 | 30.3 | <0.0001 | 0.9930 | + | <0.0001 | − | <0.0001 | − |
| Male | 2, 21 | 78.8 | <0.0001 | 0.3546 | − | <0.0001 | − | <0.0001 | − | |
| 5 | Female | 2, 15 | 21.5 | <0.0001 | 0.9156 | − | 0.0002 | − | 0.0001 | − |
| Male | 2, 19 | 53.2 | <0.0001 | 0.0356 | − | <0.0001 | − | <0.0001 | − | |
| 6 | Female | 2, 10 | 50.7 | <0.0001 | 0.8448 | + | 0.0002 | − | <0.0001 | − |
| Male | 2, 17 | 62.8 | <0.0001 | 0.5810 | − | <0.0001 | − | <0.0001 | − | |
| 7 | Female | 2, 11 | 43.5 | <0.0001 | 0.9869 | + | 0.0002 | − | <0.0001 | − |
| Male | 1, 16 | 1.6 | 0.2308 | 0.2308 | − | |||||
| (Triceps surae mass/body mass) × 100 | ||||||||||
| 1 | Female | 2, 28 | 1.5 | 0.2312 | 0.8098 | − | 0.2053 | − | 0.5223 | − |
| Male | 2, 28 | 4.3 | 0.0228 | 0.9841 | + | 0.0467 | − | 0.0367 | − | |
| 2 | Female | 2, 27 | 19.3 | <0.0001 | 0.5556 | + | 0.0001 | − | <0.0001 | − |
| Male | 2, 28 | 32.4 | <0.0001 | 0.2807 | + | <0.0001 | − | <0.0001 | − | |
| 3 | Female | 2, 26 | 130.7 | <0.0001 | 0.5974 | − | <0.0001 | − | <0.0001 | − |
| Male | 2, 25 | 168.9 | <0.0001 | 0.9866 | − | <0.0001 | − | <0.0001 | − | |
| 4 | Female | 2, 20 | 133.2 | <0.0001 | 0.9447 | + | <0.0001 | − | <0.0001 | − |
| Male | 2, 21 | 210.6 | <0.0001 | 0.9955 | + | <0.0001 | − | <0.0001 | − | |
| 5 | Female | 2, 15 | 74.5 | <0.0001 | 0.8629 | + | <0.0001 | − | <0.0001 | − |
| Male | 2, 19 | 48.4 | <0.0001 | 0.8444 | − | <0.0001 | − | <0.0001 | − | |
| 6 | Female | 2, 10 | 125.5 | <0.0001 | 0.8022 | + | <0.0001 | − | <0.0001 | − |
| Male | 2, 17 | 142.2 | <0.0001 | 0.6887 | − | <0.0001 | − | <0.0001 | − | |
| 7 | Female | 2, 11 | 64.1 | <0.0001 | 0.9323 | + | <0.0001 | − | <0.0001 | − |
| Male | 1, 16 | 1.6 | 0.2200 | 0.2200 | − | |||||
P-values for the overall ANOVA as well as for pair-wise comparisons between groups are shown, with + and − indicating that the first group in the comparison is larger (+) or smaller (−) than the second. P-values ≤0.05 are noted in bold.
All statistical tests of the mass of the triceps surae, including the simplest one-way ANOVA model (Table 3), with muscle mass normalized to body mass (Table 3), and with body mass included as a covariate (results not shown), yielded similar results overall for both sexes. At week 1, mass of the triceps surae in MM mice was not significantly different from that in the other groups (C and HR), with one exception. Triceps surae mass normalized to body mass in males at week 1 was significantly smaller than in either C (P = 0.0467) or HR mice (P = 0.0367). In weeks 2 through 7, mass of the triceps surae in MM mice was significantly lower than in either C or HR mice (Fig. 5B; Table 3), and triceps surae mass corrected for body mass was never significantly different between C and HR mice.
The absence of differences in muscle mass in MM versus C and HR mice at 1 week postnatal age appears to be consistent with early postnatal muscle development and its associated changes in myosin isoforms. At birth, skeletal muscle is dominated by developmental myosin isoforms (embryonic and perinatal or neonatal), which are gradually replaced by the adult myosin isoforms (Allen and Leinwand 2001). In mice, this transition spans postnatal days 5–20, by which point over 90% of the muscle consists of adult fast-myosin isoforms (IIa, IIb, IId; Allen and Leinwand 2001). In a study of muscle development in mice, using high-resolution gel electrophoresis, Agbulut et al. (2003) found that neonatal myosin was completely absent 14 days after birth.
We hypothesize that C and normal HR mice as well as those with the MM phenotype, are all born with embryonic and perinatal myosin isoforms, as has been reported for other strains of mice (genetic basis of myosin differentiation reviewed in Weiss and Leinwand 1996; Lu et al. 1999). In MM-phenotype muscle, Guderley et al. (2006, 2008) observed large populations of small muscle cells that may represent undifferentiated myosin type-IIb muscle fibers. Our findings of coincident decreases in body-mass-corrected muscle mass (MM relative to HR and C) from 2 weeks postnatal age onward (Fig. 5) may potentially be explained by the nondifferentiation of type-IIb fibers and their ∼50% reduction in adult skeletal muscle in MM-phenotype mice (Guderley et al. 2006, 2008). Future studies will examine the specific myosin isoforms present in this ontogenetic series of MM and normal phenotypes.
Conclusions
Long-term selective breeding for high levels of voluntary running in a wheel has been used to examine the physiologic and behavior changes that underlie the evolution of increased activity levels, and now can be used to study changes in skeletal morphology. Here, we reviewed our initial studies that demonstrated significant evolutionary change in skeletal traits in response to selection for a greater amount of running in wheels, as well as significant phenotypic plasticity in response to as little as 8 weeks of access to wheels (e.g., see Fig. 8 in Kelly et al. 2006). Thus, selection experiments represent a viable and arguably more natural model for studying skeletal form, function, and evolution than do more traditional inbred strain models or newer knockout and transgenic models. For example, knockout and transgenic mouse-models typically address only a single locus. Evolution both in the wild and in the context of selection experiments potentially involves changes at many loci. Because selection experiments typically operate on high-level traits (e.g., such behaviors as the propensity to exercise), which are almost certainly polygenic, phenotypic change resulting from selective breeding may be more representative of change occurring in natural populations.
We reanalyzed some of the previously published data on the masses of bone in the hind limbs (Kelly et al. 2006) and found that, when active and sedentary experimental groups are analyzed separately, some previously masked patterns emerge. For example, in sedentary mice, femoral mass is significantly lower in MM mice, but mass of the foot bones is significantly higher in the same group (Table 1). We discuss these results from the perspective of “more pain, more gain” versus the “principle of initial value.”
We also present new data on ontogenetic changes in the mass of the triceps surae muscle in both normal and MM mice, and hypothesize that observed differences beginning at 2 weeks postnatal age reflect differences in myosin isoforms and fiber types. We hypothesize that ontogenetic changes in muscle mass in MM mice are explained by patterns of replacement of myosin isoforms observed in rodents (Allen and Leinwand 2001; Agbulut et al. 2003) and adult distributions of myosin isoforms in MM mice (Guderley et al. 2006, 2008). Once identified, the recessive MM allele will offer varied opportunities for studies of gene targeting (Hannon et al. 2008; Hartmann et al. 2008).
Supplementary Material
Acknowledgments
We thank Kristian Carlson and Craig Byron for their persistence in organizing the symposium and for the invitation to participate. We are grateful to Katie M. Blank, Patrick A. Carter, Polly P. Czech, Patricia W. Freeman, Douglas C. Moore, Corinne E. Shubin, Sharon M. Swartz, and Jeffrey T. Wight, who assisted with some of the studies described here. This study was supported by National Science Foundation (IOB-0543429 to T.G.); National Institutes of Health (1F32AR053008-01 to K.M.M.).
References
- Agbulut O, Noirez P, Beaumont F, Butler-Browne GS. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol Cell. 2003;95:399–406. doi: 10.1016/s0248-4900(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Akhter MP, Cullen DM, Pedersen EA, Kimmel DB, Recker RR. Bone response to mechanical loading in two breeds of mice. Calcif Tissue Int. 1998;63:442–9. doi: 10.1007/s002239900554. [DOI] [PubMed] [Google Scholar]
- Akhter MP, Iwaniec UT, Covey MA, Cullen DM, Kimmel DB, Recker RR. Genetic variation in bone density, histomorphometry, and strength in mice. Calcif Tissue Int. 2000;67:337–44. doi: 10.1007/s002230001144. [DOI] [PubMed] [Google Scholar]
- Akhter MP, et al. Bone biomechanical properties in LRP5 mutant mice. Bone. 2004;35:162–9. doi: 10.1016/j.bone.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Allen DL, Leinwand LA. Postnatal myosin heavy chain isoform expression in normal mice and mice null for IIb or IId myosin heavy chains. Dev Biol. 2001;229:383–95. doi: 10.1006/dbio.2000.9974. [DOI] [PubMed] [Google Scholar]
- Atchley WR, Riska B, Kohn LAP, Plummer AA, Rutledge JJ. A quantitative genetic analysis of brain and body size associations, their origin and ontogeny: data from mice. Evolution. 1984;38:1165–79. doi: 10.1111/j.1558-5646.1984.tb05640.x. [DOI] [PubMed] [Google Scholar]
- Atchley WR, Rutledge JJ, Cowley DE. Direct and correlated response to selection in osteometric traits in the rat. Bioscience. 1982;32:684. doi: 10.1111/j.1558-5646.1982.tb05435.x. [DOI] [PubMed] [Google Scholar]
- Babij P, et al. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18:960–74. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- Bagi CM, Wilkie D, Georgelos K, Williams D, Bertolini D. Morphological and structural characteristics of the proximal femur in human and rat. Bone. 1997;21:261–7. doi: 10.1016/s8756-3282(97)00121-x. [DOI] [PubMed] [Google Scholar]
- Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- Belke TW, Garland T., Jr A brief opportunity to run does not function as a reinforcer for mice selected for high daily wheel-running rates. J Exp Anal Behav. 2007;88:199–213. doi: 10.1901/jeab.2007.62-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. Selection: the mechanism of evolution. 2nd. Oxford, UK: University Press; 2008. [Google Scholar]
- Bennett AF. Experimental evolution and the Krogh Principle: generating biological novelty for functional and genetic analyses. Physiol Biochem Zool. 2003;76:1–11. doi: 10.1086/374275. [DOI] [PubMed] [Google Scholar]
- Bennett AF, Lenski RE. Experimental evolution and its role in evolutionary physiology. Am Zool. 1999;39:346–62. [Google Scholar]
- Biewener AA, Bertram JEA. Structural response of growing bone to exercise and disuse. J Appl Physiol. 1994;76:946–55. doi: 10.1152/jappl.1994.76.2.946. [DOI] [PubMed] [Google Scholar]
- Binkley T, Specker B. Increased periosteal circumference remains present 12 months after an exercise intervention in preschool children. Bone. 2004;35:1383–8. doi: 10.1016/j.bone.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. New Eng J Med. 2002;346:1513–21. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345–52. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- Brené S, Bjørnebekk A, Åberg E, Mathé A, Olson L, Werme M. Running is rewarding and antidepressive. Physiol Behav. 2007;92:136–40. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherstone S, Goddard M. Artificial selection and maintenance of genetic variance in the global dairy cow population. Philos Trans R Soc Lond B. 2005;360:1479–88. doi: 10.1098/rstb.2005.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Burger JC, Chapman MA. Crop evolution: from genetics to genomics. Curr Opin Genet Dev. 2007;17:525–32. doi: 10.1016/j.gde.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Carrano MT. What, if anything, is a cursor? Categories versus continua for determining locomotor habit in mammals and dinosaurs. J Zool. 1999;247:29–42. [Google Scholar]
- Carter PA, Swallow JG, Davis SJ, Garland T., Jr Nesting behavior of house mice (Mus domesticus) selected for increased wheel-running activity. Behav Genet. 2000;30:85–94. doi: 10.1023/a:1001967019229. [DOI] [PubMed] [Google Scholar]
- Casas A, Otero-Arnaiz A, Pérez-Negrón E, Valiente-Banuet A. In situ management and domestication of plants in Mesoamerica. Ann Bot. 2007;100:1101–15. doi: 10.1093/aob/mcm126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran-Everett D, Benos DJ. Guidelines for reporting statistics in journals published by the American Physiological Society. J Appl Physiol. 2004;97:457–9. doi: 10.1152/physiolgenomics.00155.2004. [DOI] [PubMed] [Google Scholar]
- Curtis Hewitt S, Couse JF, Korach KS. Estrogen receptor transcription and transactivation—estrogen receptor knockout mice: what their phenotypes reveal about mechanisms of estrogen action. Breast Cancer Res. 2000;2:345–52. doi: 10.1186/bcr79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz FR. Effect of denervation on limb growth. J Orthop Res. 1989;7:292–303. doi: 10.1002/jor.1100070218. [DOI] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Bell FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control on bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- Dumke CL, Rhodes JS, Garland T, Jr, Maslowski E, Swallow JG, Wetter AC, Cartee GD. Genetic selection of mice for high voluntary wheel running: effect on skeletal muscle glucose uptake. J Appl Physiol. 2001;91:1289–97. doi: 10.1152/jappl.2001.91.3.1289. [DOI] [PubMed] [Google Scholar]
- Dunnington EA, White JM, Vinson WE. Selection for serum cholesterol, voluntary physical activity, 56-day weight and feed intake in randombred mice. I. Direct responses. Can J Genet Cytol. 1981;23:533–43. doi: 10.1139/g81-059. [DOI] [PubMed] [Google Scholar]
- Dysart PS, Harkness EM, Herbison GP. Growth of the humerus after denervation. An experimental study in the rat. J Anat. 1989;167:147–59. [PMC free article] [PubMed] [Google Scholar]
- Eikelboom R. Human parallel to voluntary wheel running: exercise. Anim Behav. 1999;57:F11–2. doi: 10.1006/anbe.1998.1045. [DOI] [PubMed] [Google Scholar]
- Eisen EJ. Maturing patterns of organ weights in mice selected for rapid postweaning gain. Theor Appl Genet. 1986;73:148–57. doi: 10.1007/BF00273732. [DOI] [PubMed] [Google Scholar]
- Eisen EJ. Selection for components related to body composition in mice: direct responses. Theor Appl Genet. 1987;74:793–801. doi: 10.1007/BF00247559. [DOI] [PubMed] [Google Scholar]
- Eisen EJ. Selection for components related to body composition in mice: correlated responses. Theor Appl Genet. 1987;75:177–88. doi: 10.1007/BF00247559. [DOI] [PubMed] [Google Scholar]
- Eisen EJ, editor. The mouse in animal genetics and breeding research. London: Imperial College Press; 2005. [Google Scholar]
- Eisen EJ, Bandy T. Correlated responses in growth and body composition of replicated single-trait and index selected lines of mice. Theor Appl Genet. 1977;49:133–44. doi: 10.1007/BF00281711. [DOI] [PubMed] [Google Scholar]
- Eisman JA. Genetics of osteoporosis. Endocr Rev. 1999;20:788–804. doi: 10.1210/edrv.20.6.0384. [DOI] [PubMed] [Google Scholar]
- Feder ME, Bennett AF, Huey RB. Evolutionary physiology. Annu Rev Ecol Syst. 2000;31:315–41. [Google Scholar]
- Ferrari S, Deutsch S, Choudhury U, Chevalley T, Bonjour J, Dermitzakis ET, Rizzoli R, Antonarakis SE. Polymorphisms in the low-density lipoprotein receptor–related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites. Am J Hum Genet. 2004;74:866–75. doi: 10.1086/420771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Rizzoli R, Bonjour J. Genetic aspects of osteoporosis. Curr Opin Rheumatol. 1999;11:294–300. doi: 10.1097/00002281-199907000-00013. [DOI] [PubMed] [Google Scholar]
- Forwood MR. Physical activity and bone development during childhood: insights from animal models. J Appl Physiol. 2008 doi: 10.1152/japplphysiol.00040.2008. Available at: http://jap.physiology.org/cgi/content/abstract/00040.2008v1. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Hasen NS, Rhodes JS, Girard IA, Garland T., Jr Predatory aggression, but not maternal or intermale aggression, is associated with high voluntary wheel-running behavior. Horm Behav. 2003;44:209–21. doi: 10.1016/s0018-506x(03)00140-5. [DOI] [PubMed] [Google Scholar]
- Garland T., Jr . Selection experiments: an under-utilized tool in biomechanics and organismal biology. In: Bels VL, Gasc J-P, Casinos A, editors. Vertebrate biomechanics and evolution. Oxford: BIOS Scientific Publishers Limited; 2003. pp. 23–56. [Google Scholar]
- Garland T, Jr, Carter PA. Evolutionary physiology. Annu Rev Physiol. 1994;56:579–621. doi: 10.1146/annurev.ph.56.030194.003051. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Freeman PW. Selective breeding for high endurance running increases hindlimb symmetry. Evolution. 2005;59:1851–4. [PubMed] [Google Scholar]
- Garland T, Jr, Morgan MT, Swallow JG, Rhodes JS, Girard IA, Belter JG, Carter PA. Evolution of a small-muscle polymorphism in line of house mice selected for high activity levels. Evolution. 2002;56:1267–75. doi: 10.1111/j.0014-3820.2002.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Rose M. Berkeley: University of California Press; Manuscript in review. Experimental evolution: concepts, methods, and applications. [Google Scholar]
- Gibbs AG. Laboratory selection for the comparative physiologist. J Exp Biol. 1999;202:2709–18. doi: 10.1242/jeb.202.20.2709. [DOI] [PubMed] [Google Scholar]
- Girard IA, McAleer MW, Rhodes JS, Garland T., Jr Selection for high voluntary wheel-running increases speed and intermittency in house mice (Mus domesticus) J Exp Biol. 2001;204:4311–20. doi: 10.1242/jeb.204.24.4311. [DOI] [PubMed] [Google Scholar]
- Girard IA, Rezende EL, Garland T., Jr Leptin levels and body composition of mice selectively bred for high voluntary locomotor activity. Physiol Biochem Zool. 2007;80:568–79. doi: 10.1086/521086. [DOI] [PubMed] [Google Scholar]
- Glass DA, Karsenty G. In vivo analysis of Wnt signaling in bone. Endocrinology. 2007;148:2630–4. doi: 10.1210/en.2006-1372. [DOI] [PubMed] [Google Scholar]
- Gonzalez NC, Kirkton SD, Howlett RA, Britton SL, Koch LG, Wagner HE, Wagner PD. Continued divergence in VO2max of rats selected for running endurance in mediated by greater convective blood O2 delivery. J Appl Physiol. 2006;101:1288–96. doi: 10.1152/japplphysiol.01527.2005. [DOI] [PubMed] [Google Scholar]
- Gordon KR. Adaptive nature of skeletal design. Bioscience. 1989;39:784–90. [Google Scholar]
- Guderley H, Houle-Leroy P, Diffee GM, Camp DM, Garland T., Jr Morphometry, ultrastructure, myosin isoforms, and metabolic capacities of the “Mini muscles” favoured by selection for high activity in house mice. Comp Biochem Physiol B. 2006;144:271–82. doi: 10.1016/j.cbpb.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Guderley H, Joanisse DR, Mokas S, Bilodeau GM, Garland T., Jr Altered fibre types in gastrocnemius muscle of high wheel-running selected mice with mini-muscle phenotypes. Comp Biochem Physiol B. 2008;149:490–500. doi: 10.1016/j.cbpb.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Hallgrimsson B, Lieberman D. Mouse models and the evolutionary biology of the skull. 2008 doi: 10.1093/icb/icn076. Proceedings of the Society for Integrative and Comparative Biology, January 2–6 in San Antonio, TX ( http://www.sicb.org/meetings/2008/schedule) [DOI] [PubMed]
- Hamrick MW, McPherron AC, Lovejoy CO, Hudson J. Femoral morphology and cross-sectional geometry of adult myostatin-deficient mice. Bone. 2000;27:343–9. doi: 10.1016/s8756-3282(00)00339-2. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Samaddar T, Pennington C, McCormick J. Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. J Bone Miner Res. 2006;21:477–83. doi: 10.1359/JBMR.051203. [DOI] [PubMed] [Google Scholar]
- Hannon RM, Kelly SA, Middleton KM, Kolb EM, Pomp D, Garland T., Jr Phenotypic effects of the “Mini-muscle” allele in a large HR x C57BL/6J mouse backcross. J Hered. 2008 doi: 10.1093/jhered/esn011. Available at: http://jhered.oxfordjournals.org/cgi/content/full/esn011. [DOI] [PubMed]
- Hart KJ, Shaw JM, Vajda EG, Hegsted M, Miller SC. Swim-trained rats have greater bone mass, density, strength, and dynamics. J Appl Physiol. 2001;91:1663–8. doi: 10.1152/jappl.2001.91.4.1663. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Garland T, Jr, Hannon RM, Kelly SA, Muñoz G, Pomp D. Fine mapping of “Mini-muscle,” a recessive mutation causing reduced hind-limb muscle mass in mice. J Hered. 2008 doi: 10.1093/jhered/esn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley JA. Retraction. Am J Physiol (Endocr Metab) 2007;293:E1845. doi: 10.1152/ajpendo.zh1-5190-corr.2007. [DOI] [PubMed] [Google Scholar]
- Henderson ND. Interpreting studies that compare high- and low-selected lines on new characters. Behav Genet. 1989;19:473–502. doi: 10.1007/BF01066250. [DOI] [PubMed] [Google Scholar]
- Henderson ND. Spurious associations in unreplicated selected lines. Behav Genet. 1997;27:145–54. doi: 10.1023/a:1025689425738. [DOI] [PubMed] [Google Scholar]
- Henderson KK, Wagner HE, Favret F, Britton SL, Koch LG, Wagner PD, Gonzalez NC. Determinants of maximal O2 uptake in rats selective bred for endurance running capacity. J Appl Physiol. 2002;93:1265–74. doi: 10.1152/japplphysiol.00809.2001. [DOI] [PubMed] [Google Scholar]
- Hildebrand M. Analysis of vertebrate structure. 2nd. New York: John Wiley and Sons; 1982. [Google Scholar]
- Hill WG, Caballero A. Artificial selection experiments. Annu Rev Ecol Syst. 1992;23:287–310. [Google Scholar]
- Honda A, Sogo N, Nagasawa S, Shimizu T, Umemura Y. High-impact exercise strengthens bone in osteopenic ovariectomized rats with the same outcome as sham rats. J Appl Physiol. 2003;95:1032–7. doi: 10.1152/japplphysiol.00781.2002. [DOI] [PubMed] [Google Scholar]
- Honda A, Umemura Y, Nagasawa S. Effect of high-impact and low-repetition training on bones in ovariectomized rats. J Bone Miner Res. 2001;16:1688–93. doi: 10.1359/jbmr.2001.16.9.1688. [DOI] [PubMed] [Google Scholar]
- Houle-Leroy P, Guderley H, Swallow JG, Garland T., Jr Artificial selection for high activity favors mighty mini-muscle in house mice. Am J Physiol (Regul Integr Comp Physiol) 2003;284:R433–43. doi: 10.1152/ajpregu.00179.2002. [DOI] [PubMed] [Google Scholar]
- Iwamoto J, Takeda T, Ichimura S. Effects of exercise on bone mineral density in mature osteopenic rats. J Bone Miner Res. 1998;13:1308–17. doi: 10.1359/jbmr.1998.13.8.1308. [DOI] [PubMed] [Google Scholar]
- Iwamoto J, Yeh JK, Aloia JF. Differential effect of treadmill exercise on three cancellous bone sites in the young growing rat. Bone. 1999;24:163–9. doi: 10.1016/s8756-3282(98)00189-6. [DOI] [PubMed] [Google Scholar]
- Järvinen TLN, Pajamäkai I, Sievänen H, Vuohelainen T, Tuukkanen J, Järvinen M, Kannus P. Femoral neck response to exercise and subsequent deconditioning in young and adult rats. J Bone Miner Res. 2003;18:1292–9. doi: 10.1359/jbmr.2003.18.7.1292. [DOI] [PubMed] [Google Scholar]
- Kannus P, Haapsalo H, Sankelo M, Sievänen H, Pasanen M, Heinonen A, Oja P, Vuori I. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med. 1995;123:27–31. doi: 10.7326/0003-4819-123-1-199507010-00003. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Ke HZ, et al. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol. 2003;17:1356–67. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- Kelly SA, Czech PP, Wight JT, Blank KM, Garland T., Jr Experimental evolution and phenotypic plasticity of hindlimb bones in high-activity house mice. J Morphol. 2006;267:360–74. doi: 10.1002/jmor.10407. [DOI] [PubMed] [Google Scholar]
- Kesavan C, Mohan S, Oberholtzer S, Wergedal JE, Baylink DJ. Mechanical loading-induced gene expression and BMD changes are different in two inbred mouse strains. J Appl Physiol. 2005;99:1951–7. doi: 10.1152/japplphysiol.00401.2005. [DOI] [PubMed] [Google Scholar]
- Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- Koch LG, Green CL, Lee AD, Hornyak JE, Cicila GT, Britton SL. Test of the principle of initial value in rat genetic models of exercise capacity. Am J Physiol (Regul Integr Comp Physiol) 2005;288:R466–72. doi: 10.1152/ajpregu.00621.2004. [DOI] [PubMed] [Google Scholar]
- Koch LG, Meredith TA, Fraker TD, Metting PJ, Britton SL. Heritability of treadmill running endurance in rats. Am J Physiol (Regul Integr Comp Physiol) 1998;275:R1455–60. doi: 10.1152/ajpregu.1998.275.5.R1455. [DOI] [PubMed] [Google Scholar]
- Kodama Y, Umemura Y, Nagasawa S, Beamer WG, Donahue LR, Rosen CJ, Baylink DJ, Farley JR. Exercise and mechanical loading increase periosteal bone formation and whole bone strength in C57BL/6J but not in C3H/Hej mice. Calcif Tissue Int. 2000;66:298–306. doi: 10.1007/s002230010060. [DOI] [PubMed] [Google Scholar]
- Koller DL, Schreifer J, Sun Q, Shultz KL, Donahue LR, Rosen CJ, Foroud T, Beamer WG, Turner CH. Genetic effects for femoral biomechanics, structure, and density in C57BL/6J and C3H/Hej inbred mouse strains. J Bone Miner Res. 2003;18:1758–65. doi: 10.1359/jbmr.2003.18.10.1758. [DOI] [PubMed] [Google Scholar]
- Konarzewski M, Książek A, Łapo I. Artificial selection on metabolic rates and related traits in rodents. Integr Comp Biol. 2005;45:416–25. doi: 10.1093/icb/45.3.416. [DOI] [PubMed] [Google Scholar]
- Koteja P, Garland T, Jr, Sax JK, Swallow JG, Carter PA. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Anim Behav. 1999;58:1307–18. doi: 10.1006/anbe.1999.1270. [DOI] [PubMed] [Google Scholar]
- Koteja P, Swallow JG, Carter PA, Garland T., Jr Different effects of intensity and duration of locomotor activity on circadian period. J Biol Rhythms. 2003;18:491–501. doi: 10.1177/0748730403256998. [DOI] [PubMed] [Google Scholar]
- Lau KHW, Kapur S, Kesavan C, Baylink DJ. Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/Hej osteoblasts in part contributes to the differential anabolic response to fluid shear. J Biol Chem. 2006;281:9576–88. doi: 10.1074/jbc.M509205200. [DOI] [PubMed] [Google Scholar]
- Lee NK, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López E, Stock S, Chenn A, Ravosa M. A novel transgenic mouse model of fetal encephalization and craniofacial development. Integr Comp Biol. 2008 doi: 10.1093/icb/icn047. doi:10.1093/icb/icn047. [DOI] [PubMed] [Google Scholar]
- Lu BD, Allen DL, Leinwand LA, Lyons GE. Spatial and temporal changes in myosin heavy chain gene expression in skeletal muscle development. Dev Biol. 1999;216:312–26. doi: 10.1006/dbio.1999.9488. [DOI] [PubMed] [Google Scholar]
- MacArthur JW. Genetics of body size and related characters. I. Selecting small and large races of the laboratory mouse. Am Nat. 1944;78:142–57. [Google Scholar]
- MacArthur JW. Genetics of body size and related characters. II. Satellite characters associated with body size in mice. Am Nat. 1944;78:224–37. [Google Scholar]
- MacDonald BT, Joiner DM, Oyserman SM, Sharma P, Goldstein SA, He X, Hauschka PV. Bone mass is inversely proportional to Dkk1 levels in mice. Bone. 2007;41:331–9. doi: 10.1016/j.bone.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisch JL, Breuner CW, Gomes FR, Chappell MA, Garland T., Jr Circadian pattern of total and free corticosterone concentrations, corticosteroid-binding globulin, and physical activity in mice selectively bred for high voluntary wheel-running behavior. Gen Comp Endocrinol. 2008;156:210–7. doi: 10.1016/j.ygcen.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Malisch JL, Saltzman W, Gomes FR, Rezende EL, Jeske DR, Garland T., Jr Baseline and stress-induced plasma corticosterone concentrations of mice selectively bred for high voluntary wheel running. Physiol Biochem Zool. 2007;80:146–56. doi: 10.1086/508828. [DOI] [PubMed] [Google Scholar]
- Middleton KM, Shubin CE, Moore DC, Carter PA, Garland T, Jr, Swartz SM. The relative importance of genetics and phenotypic plasticity in dictating bone morphology and mechanics in aged mice: evidence from an artificial selection experiment. Zoology. 2008;111:135–47. doi: 10.1016/j.zool.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TJ, Garland T, Jr, Carter PA. Ontogenies in mice selected for high voluntary wheel-running activity. I. Mean ontogenies. Evolution. 2003;57:646–57. doi: 10.1111/j.0014-3820.2003.tb01556.x. [DOI] [PubMed] [Google Scholar]
- Mori T, Okimoto N, Sakai A, Okazaki Y, Nakura N, Notomi T, Nakamura T. Climbing exercise increases bone mass and trabecular bone turnover through transient regulation of marrow osteogenic and osteoclastogenic potentials in mice. J Bone Miner Res. 2003;18:2002–9. doi: 10.1359/jbmr.2003.18.11.2002. [DOI] [PubMed] [Google Scholar]
- Newhall KM, Rodnick KJ, van der Meulen MCH, Carter DR, Marcus R. Effects of voluntary exercise on bone mineral content in rats. J Bone Miner Res. 1991;6:289–96. doi: 10.1002/jbmr.5650060311. [DOI] [PubMed] [Google Scholar]
- Neyman J. Comments on Mr. Yates’ paper. J R Stat Soc Suppl. 1935;2:235–41. [Google Scholar]
- Niehoff A, Kersting UG, Zaucke F, Morlock MM, Brüggemann G. Adaptation of mechanical, morphological, and biochemical properties of the rat growth plate to dose-dependent voluntary exercise. Bone. 2004;35:899–908. doi: 10.1016/j.bone.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Notomi T, Okazaki Y, Okimoto N, Saitoh S, Nakamura T, Suzuki M. A comparison of resistance and aerobic training for mass, strength and turnover of bone in growing rats. Eur J Appl Physiol. 2000;83:469–474. doi: 10.1007/s004210000316. [DOI] [PubMed] [Google Scholar]
- Notomi T, Lee SJ, Okimoto N, Okazaki Y, Takamoto T, Nakamura T, Suzuki M. Effects of resistance exercise training on mass, strength, and turnover of bone in growing rats. Eur J Appl Physiol. 2000;82:268–74. doi: 10.1007/s004210000195. [DOI] [PubMed] [Google Scholar]
- Notomi T, Okimoto N, Okazaki Y, Tanaka Y, Nakamura T, Suzuki M. Effects of tower climbing exercise on bone mass, strength, and turnover in growing rats. J Bone Miner Res. 2001;16:166–74. doi: 10.1359/jbmr.2001.16.1.166. [DOI] [PubMed] [Google Scholar]
- Parikka V, Peng Z, Hentunen T, Risteli J, Elo T, Väänänen HK, Härkönen P. Estrogen responsiveness of bone formation in vitro and altered bone phenotype in aged estrogen receptor-α-deficient male and female mice. Eur J Endocrinol. 2005;152:301–14. doi: 10.1530/eje.1.01832. [DOI] [PubMed] [Google Scholar]
- Pickersgill B. Domestication of plants in the Americas: insights from Mendelian and molecular genetics. Ann Bot. 2007;100:925–40. doi: 10.1093/aob/mcm193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2008 ISBN 3-900051-07-0. Available from: http://www.R-project.org.
- Reilly SM, McElroy EJ, Odum RA, Hornyak VA. Tuataras and salamanders show that walking and running mechanics are ancient features of tetrapod locomotion. Proc R Soc B. 2006;273:1563–8. doi: 10.1098/rspb.2006.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende EL, Chappell MA, Gomes FR, Malisch JL, Garland T., Jr Maximal metabolic rates during voluntary exercise, forced exercise, and cold exposure in house mice selectively bred for high wheel-running. J Exp Biol. 2005;208:2447–58. doi: 10.1242/jeb.01631. [DOI] [PubMed] [Google Scholar]
- Rezende EL, Garland T, Chappell MA, Malisch JL, Gomes FR. Maximum aerobic performance in lines of Mus selected for high wheel-running activity: effects of selection, oxygen availability and the mini-muscle phenotype. J Exp Biol. 2006;209:115–27. doi: 10.1242/jeb.01883. [DOI] [PubMed] [Google Scholar]
- Rezende EL, Kelly SA, Gomes FR, Chappell MA, Garland T., Jr Effects of size, sex, and voluntary running speeds on costs of locomotion in line of laboratory mice selectively bred for high wheel-running activity. Physiol Biochem Zool. 2006;79:83–99. doi: 10.1086/498187. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Gammie SC, Garland T., Jr Neurobiology of mice selected for high voluntary wheel-running activity. Integr Comp Biol. 2005;45:438–55. doi: 10.1093/icb/45.3.438. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Kawecki TJ. Manuscript in review. Behavior and neurobiology. In: In: Garland T Jr, Rose MR, editors. Berkeley: University of California Press; Experimental evolution: concepts, methods, and applications. [Google Scholar]
- Robertson G, Xie C, Chen D, Awad H, Schwarz EM, O’Keefe RJ, Guldberg RE, Zhang X. Alteration of femoral bone morphology and density in COX-2–/– mice. Bone. 2006;39:767–72. doi: 10.1016/j.bone.2006.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robling AG, Burr DB, Turner CH. Recovery periods restore mechanosensitivity to dynamically loaded bone. J Exp Biol. 2001;204:3389–99. doi: 10.1242/jeb.204.19.3389. [DOI] [PubMed] [Google Scholar]
- Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17:1545–54. doi: 10.1359/jbmr.2002.17.8.1545. [DOI] [PubMed] [Google Scholar]
- Robling AG, Hinant FM, Burr DB, Turner CH. Shorter, more frequent mechanical loading sessions enhance bone mass. Med Sci Sports Exerc. 2002;34:196–202. doi: 10.1097/00005768-200202000-00003. [DOI] [PubMed] [Google Scholar]
- Robling AG, Li J, Shultz KL, Beamer WG, Turner CH. Evidence for a skeletal mechanosensitivity gene on mouse chromosome 4. FASEB J. 2003;17:324–6. doi: 10.1096/fj.02-0393fje. [DOI] [PubMed] [Google Scholar]
- Rodger RS. Multiple contrasts, factors, error rate and power. Br J Math Stat Psychol. 1974;27:179–98. [Google Scholar]
- Rot-Nikcevic I, Downing KJ, Hall BK, Kablar B. Development of the mouse mandibles and clavicles in the absence of skeletal myogenesis. Histol Histopathol. 2007;22:51–60. doi: 10.14670/HH-22.51. [DOI] [PubMed] [Google Scholar]
- Rubin J, et al. Caveolin-1 knockout mice have increased bone size and stiffness. J Bone Miner Res. 2007;22:1408–18. doi: 10.1359/jbmr.070601. [DOI] [PubMed] [Google Scholar]
- Rutledge JJ, Eisen EJ, Legates JE. An experimental evaluation of genetic correlation. Genet. 1973;75:709–26. doi: 10.1093/genetics/75.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge JJ, Eisen EJ, Legates JE. Correlated response in skeletal traits and replicate variation in selected lines of mice. Theor Appl Genet. 1974;45:26–31. doi: 10.1007/BF00281170. [DOI] [PubMed] [Google Scholar]
- Sawakami K, et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281:23698–711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- Selye H, Bajusz E. Effect of denervation on experimentally induced changes in the growth of bone and muscle. Am J Physiol. 1958;192:297–300. doi: 10.1152/ajplegacy.1958.192.2.297. [DOI] [PubMed] [Google Scholar]
- Shaw SR, Zernicke RF, Vailas AC, DeLuna D, Thomason DB, Baldwin KM. Mechanical, morphological and biochemical adaptations of bone and muscle to hindlimb suspension and exercise. J Biomech. 1987;20:225–34. doi: 10.1016/0021-9290(87)90289-2. [DOI] [PubMed] [Google Scholar]
- Siegel MI, Jones CL. The skeletal correlates of behavioral modification in the laboratory mouse (Mus musculus) Am J Phys Anthropol. 1975;42:141–4. doi: 10.1002/ajpa.1330420117. [DOI] [PubMed] [Google Scholar]
- Silver LM. Mouse genetics: concepts and applications. New York: Oxford University Press; 1995. [Google Scholar]
- Spargo FJ, McGee SL, Dzamko N, Watt MJ, Kemp BE, Britton SL, Koch LG, Hargreaves M, Hawley JA. Dysregulation of muscle lipid metabolism in rats selectively bred for low aerobic running capacity. Am J Physiol (Endocr Metab) 2007;292:E1631–6. doi: 10.1152/ajpendo.00702.2006. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res. 2002;17:1613–20. doi: 10.1359/jbmr.2002.17.9.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow JG, Carter PA, Garland T., Jr Artificial selection for increased wheel-running behavior in house mice. Behav Genet. 1998;28:227–37. doi: 10.1023/a:1021479331779. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Garland T., Jr Selection experiments as a tool in evolutionary and comparative physiology: insights into complex traits - an introduction to the symposium. Integr Comp Biol. 2005;45:387–90. doi: 10.1093/icb/45.3.387. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Hayes JP, Koteja P, Garland T., Jr . Manuscript in review. Performance and physiology. In: In: Garland T Jr, Rose MR, editors. Berkeley: University of California Press; Experimental evolution: concepts, methods, and applications. [Google Scholar]
- Swallow JG, Rhodes JS, Garland T., Jr Phenotypic and evolutionary plasticity of organ masses in response to voluntary exercise in house mice. Integr Comp Biol. 2005;45:426–37. doi: 10.1093/icb/45.3.426. [DOI] [PubMed] [Google Scholar]
- Syme DA, Evashuk K, Grintuch B, Rezende EL, Garland T., Jr Contractile abilities of normal and “Mini” Triceps surae muscle from mice (Mus domesticus) selectively bred for high voluntary wheel running. J Appl Physiol. 2005;99:1308–16. doi: 10.1152/japplphysiol.00369.2005. [DOI] [PubMed] [Google Scholar]
- Traxler RH. A snag in the history of factorial experiments. In: Owen DB, editor. On the history of statistics and probability. New York: Marcel Dekker; 1976. pp. 283–95. [Google Scholar]
- Umemura Y, Baylink DJ, Wergedal JE, Mohan S, Srivastava AK. A time course of bone response to jump exercise in C57BL/6J mice. J Bone Miner Metab. 2002;20:209–15. doi: 10.1007/s007740200030. [DOI] [PubMed] [Google Scholar]
- Vaanholt LM, Meerlo P, Garland T, Jr, Visser GH, van Dijk G. Plasma adiponectin is increased in mice selectively bred for high wheel-running activity, but not by wheel running per sé. Horm Metab Res. 2007;39:377–83. doi: 10.1055/s-2007-976542. [DOI] [PubMed] [Google Scholar]
- Vilà C, Savolainen P, Maldonado JE, Amorim IR, Rice JE, Honeycutt RL, Crandall KA, Lundeberg J, Wayne RK. Multiple and ancient origins of the domestic dog. Science. 1997;276:1687–9. doi: 10.1126/science.276.5319.1687. [DOI] [PubMed] [Google Scholar]
- Wahlsten D. Insensitivity of the analysis of variance to heredity-environment interaction. Behav Brain Sci. 1990;13:109–61. [Google Scholar]
- Wahlsten D. Sample size to detect a planned contrast and a one degree-of-freedom interaction effect. Psychol Bull. 1991;110:587–95. [Google Scholar]
- Walsh B, Hooks RB, Hornyak JE, Koch LG, Britton SL, Hogan MC. Enhanced mitochondrial sensitivity to creatine in rats bred for high aerobic capacity. J Appl Physiol. 2006;100:1765–9. doi: 10.1152/japplphysiol.01533.2005. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Leinwand LA. The mammalian myosin heavy chain gene family. Annu Rev Cell Dev Biol. 1996;12:417–39. doi: 10.1146/annurev.cellbio.12.1.417. [DOI] [PubMed] [Google Scholar]
- Welch JM, Weaver CM, Turner CH. Adaptations to free-fall impact are different in the shafts and bone ends of rat forelimbs. J Appl Physiol. 2004;97:1859–65. doi: 10.1152/japplphysiol.00438.2004. [DOI] [PubMed] [Google Scholar]
- Westerlind KC, Fluckey JD, Gordon SE, Kraemer WJ, Farrell PA, Turner RT. Effect of resistance exercise training on cortical and cancellous bone in mature male rats. J Appl Physiol. 1998;84:459–64. doi: 10.1152/jappl.1998.84.2.459. [DOI] [PubMed] [Google Scholar]
- Whittemore LA, et al. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun. 2003;300:965–71. doi: 10.1016/s0006-291x(02)02953-4. [DOI] [PubMed] [Google Scholar]
- Winters-Stone K, Snow C. Musculoskeletal response to exercise is greatest in women with low initial values. Med Sci Sports Exerc. 2003;35:1691–6. doi: 10.1249/01.MSS.0000089338.66054.A5. [DOI] [PubMed] [Google Scholar]
- Wu J, Wang X, Chiba H, Higuchi M, Nakatani T, Ezaki O, Cui H, Yamada K, Ishimi Y. Combined intervention of soy isoflavone and moderate exercise prevents body fat elevation and bone loss in ovariectomized mice. Metabolism. 2004;53:942–8. doi: 10.1016/j.metabol.2004.01.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


