Abstract
Enhanced recovery after surgery (ERAS) protocols are a combination of multimodal evidence-based strategies, applied to the conventional perioperative techniques, to reduce postoperative complications and to achieve early recovery. These strategies or protocols, require a dedicated and organized team effort for their implementation to enable early discharge and thus reduce the length of hospital stay. Anesthesiologists play an important role in facilitating these protocols as some of the key elements such as preoperative patient preparation and assessment, perioperative fluid management, and perioperative pain relief are handled by them. This article discusses in detail the various components of ERAS and the anesthesiologist's role in implementing them.
Keywords: Anesthesia, enhanced recovery after surgery, evidence
Introduction
Prolonged hospital stay and increased postoperative morbidity following major surgery has always been a cause of concern to the attending perioperative physician [Figure 1]. In recent times, postoperative outcome is considered to be positive only when it is associated with a shortened length of hospital stay and absence of postoperative functional incapacitation. Despite all the recent advances in perioperative patient management, ensuring a positive postoperative outcome after major surgery is still a difficult goal to achieve.[1]
Figure 1.
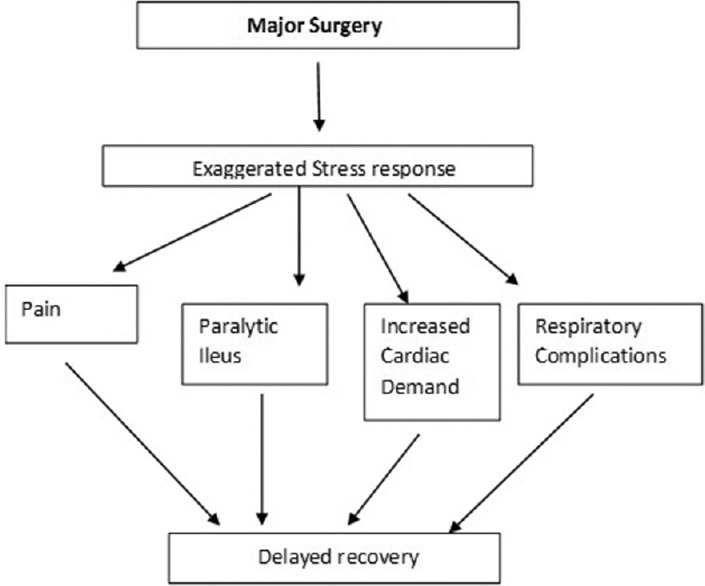
Effects of major surgery on recovery
Enhanced recovery after surgery (ERAS) is a protocolized scientific pathway applied to boost the outcome and enhance the recovery phase after surgery. This encompasses multimodal evidence based strategies at every step of perioperative care including the rehabilitation phase. The background milieu/requisites include patient education and a dedicated team approach for implementing these protocols.[2]
The concept of ERAS protocol was first pioneered by Prof. Kehlet and Wilmore somewhere in the last decade of the twentieth century.[3] His area of work was in colorectal surgeries where delay in recovery and lengthened hospital stay could be attributed to multifactorial reasons, the most prominent one being delay in return of bowel function.[4] In the years that followed a lot of research regarding the same was carried out in different areas such as colorectal,[5] vascular,[6] thoracic,[7] urosurgery,[8] spine,[9] neurosurgery,[10] orthopedic,[11] liver,[12] pancreatic,[13] and cardiac surgery.[14] Between 2001 and 2004, an ERAS study group was formed by Prof. Ken Fearon (UK) and Prof. Olle Ljungqvist (Sweden) which was later registered in the name of ERAS society (registered in 2010, based in Stockholm, Sweden) with a mission to develop perioperative care and to improve recovery through research, education, audit, and implementation of evidence-based practice. The ERAS® society soon discovered that there was a great discrepancy between the actual practices and what was already known to be best-practice, based on the literature. This prompted the society to examine the process of change from tradition to best-practice. The first ERAS symposium was held thereafter in 2003 and the first consensus guidelines for ERAS were published in 2005 based on evidence obtained from colorectal surgeries.[15] In the recent past, evidence based guidelines have been established by Association of Surgeons of Great Britain and Ireland (2009)[16] and The Royal College of Anesthetists (2011)[17] for effective implementation and understanding of the concept. In the year 2013, the Society Guidelines on radical cystectomy were published in Clinical Nutrition,[18] whereas in 2016, ERAS guidelines in gynecological and bariatric surgery were presented in two different symposia which are yet to be published.
ERAS protocols have rapidly gained a lot of interest since then and have been shown to modify the physiological and psychological response to major surgery. They have also lead to a reduction in complications and hospital stay, improvements in cardiopulmonary function, earlier return of bowel function and earlier resumption of normal activities.[19] Although the requirement and conditions for different surgeries may differ, the elements applied in the three phases of perioperative period remain almost the same.[20]
Successful implementation of ERAS protocol requires proper coordination between the surgeon, the anesthesiologist, the nursing personnel, the patient and the people looking after the patient in the perioperative period.[2] Here, an anesthesiologist plays the most vital role of guiding and monitoring some important elements such as preoperative patient selection and optimization, choice of anesthetic regimen, fluid and pain management and thus facilitates and bridges the gap between pre- and post-operative care.
Our search for ERAS protocols was mainly from PubMed, published guidelines, Google Search and EndNote 1X. The level of evidence for all these elements varies from low to high and most of the recommendations are strong.[16,17,21,22,23] [Table 1].
Table 1.
Evidence-based ERAS elements with recommendations as per various guidelines
| ERAS elements | Level of evidence | Recommendations |
|---|---|---|
| Patient education | Low | Strong |
| Preoperative optimization | Low | |
| Cessation of smoking - 1 month | Moderate | Strong |
| Alcohol abstinence - 1 month | Low | Strong |
| Preoperative fasting | ||
| Liquids | High | Strong |
| Solids | Low | |
| Carbohydrate loading | Low | Strong |
| Premedication Avoid long acting sedative agents |
Moderate | Weak |
| Prophylaxis for thromboembolism | High | Strong |
| Mechanical bowel preparation (abdominal surgery) | Moderate | Strong |
| Intra- and post-operative elements | ||
| Antibiotic prophylaxis and skin preparation | High | Strong |
| Anesthetic protocol | Low to high | Strong |
| Multimodal analgesia | ||
| Regional blocks like epidurals, TAP block, etc. | Low to high Low to moderate |
Strong Weak |
| PONV prophylaxis | Low | Strong |
| Minimally invasive approach | Low to high | Strong |
| Prevention of intraoperative hypothermia | High | Strong |
| Perioperative fluid management | ||
| Zero fluid balance | High | Strong |
| Goal-directed therapy | Moderate | Strong |
| Use of balanced crystalloids | Moderate | Strong |
| Use of drainage catheters like nasogastric drains, biliary drainage, and surgical drains-minimal usage or avoided | Moderate to high | Strong |
| Perioperative nutritional care | ||
| Screening of patients; high risk patients-active nutritional support | Low to high | Strong recommendation (for feeding) |
| Curtailed fasting duration | ||
| Early feeding in the postoperative period | ||
| Perioperative glycemic control | Weak to strong | |
| Early mobilization | Strong | |
| Early bowel movement | ||
| Use of chewing gum | Low | Weak to strong |
| Postoperative laxatives and prokinetics | Low | Weak |
| Audit | Low | Strong |
ERAS = Enhanced recovery after surgery, PONV = Postoperative nausea and vomiting
Preoperative Components in Enhanced Recovery after Surgery
Application of the preoperative components is mainly to achieve an informed, prepared, physiologically optimized and fed-state patient.[24]
Preoperative evaluation aims at identifying risk factors, and co-morbidities. This subsequently enables choice of surgical technique, choice of anesthetic technique, and determination of appropriate postoperative location for nursing.[25] Chronic diseases such as diabetes mellitus, asthma, hypertension, anemia and ischemic heart disease, can be identified and a suitable plan can be chalked out after optimization.
Patient education is an essential part of an ERAS program. The aim is to educate the patient about the program, to set realistic expectations for postoperative recovery and to psychologically prepare the patient as well as family members for the care program. Written and oral information at an appropriate literacy level in the language which they best understand should be provided in detail. Patients and their families should receive information on risk factors associated with the procedure, approximate length of stay, preoperative fasting and carbohydrate loading, pain control, early ambulation, postoperative feeding/ileus, and timing of catheter removal.[26] Anesthesiologist is the most caring perioperative physician who guides and monitors the patient to achieve the realistic goals in a step-wise and dedicated manner.
Recent studies have shown preoperative nutritional deficiency to be a strong predictor of 90 days mortality and poor overall survival.[27] Correction of these nutritional deficiencies either through prolonged enteral or sometimes through a combination of parenteral and enteral nutrition, depending on the severity of the problem is an important part of preparation for early recovery after surgery.
One of the primary aims of ERAS protocol is to blunt the body's stress response to surgery which is characterized by its catabolic effect.[28] Catecholamines tend to induce a relative lack of insulin, peripheral insulin resistance and defects in insulin receptor/intracellular signaling pathway, thus leading to hyperglycemia.[29] Hyperglycemia, in turn, is a major variable influencing length of stay, poor wound healing and increased risk of postoperative complications.[30] Prolonged preoperative fasting actually increases the metabolic stress, hyperglycemia, and insulin resistance.[31] Methods which can reduce this insulin resistance include adequate pain relief, early postoperative alimentation and the use of carbohydrate loading.[32]
Carbohydrate loading not only reduces insulin resistance but also improves muscle function by reducing nitrogen and protein loss. It is also seen to reduce preoperative thirst, hunger, and anxiety.[33,34] Allowing solid food up to 6 h preoperatively and a carbohydrate rich drink (12.5%), preferably containing complex carbohydrates, 800 ml at bedtime and 400 ml 2 h prior to surgery is the recommended protocol.[35] Cochrane systematic review by Smith et al. has shown decreased length of stay without any significant effect on the postoperative complications with preoperative carbohydrate treatment.[36] Any commercially available carbohydrate alternative can be used, such as clear fruit juices, clear tea, carbonated beverages, and black coffee; however, care should be taken that the formulation used is clear and residue free.[37]
Carbohydrate loading can be safely used in noninsulin dependent diabetics. In insulin dependent diabetics, a preoperative carbohydrate load has not been shown to result in hyperglycemia or delayed gastric emptying. However, monitoring of blood glucose levels should be carried out at regular intervals.[38] The patients are counseled and explained about its benefits, at the time of preanesthetic checkup and accordingly strict orders are advised regarding carbohydrate loading and its timings.
According to the Best Practice in General Surgery guidelines, selective mechanical bowel preparation also helps in ERAS.[39] All patients having an open or laparoscopic colorectal procedure do not require mechanical bowel preparation, are not put on dietary restrictions and receive fleet enema only if the anastomosis planned is left-sided. As an exception to the above rule, patients having an open or laparoscopic low anterior resection with or without diverting stoma, should receive mechanical bowel preparation, with no diet restrictions prior to bowel preparation and then put on clear oral fluids and enema.[40] As mechanical bowel preparation is associated with dehydration, electrolyte disturbances, and is unpleasant for the patient,[41] its routine use is not recommended in colon and other abdominal surgeries.
Preoperative preparation is not complete without cessation of smoking. One month of abstinence from smoking is required to reduce the incidence of postoperative pulmonary complications.[42] In addition, prehabilitation to improve physical fitness before surgery and cardiopulmonary exercise testing to objectively evaluate exercise capacity can be used for preoperative optimization. At least 1 month of abstinence from alcohol has shown to benefit the patient outcome.[43]
The occurrence of deep vein thrombosis in the perioperative period carries a significant morbidity and mortality in surgical patients. Evidence speaks for adequate prophylaxis as an important requisite in the perioperative period to combat this serious complication.[44] The recommended prophylaxis includes mechanical devices such as intermittent compression devices and use of low molecular weight heparin.
In the fast – track surgical setting, premedication is not mandatory. If still prescribed, the ideal agent should be aimed at reducing the surgical stress response and without any sedative properties. Use of β blockers and α2 agonists is gaining a lot of interest in this aspect. According to the ACC/AHA 2009 guidelines for perioperative use of beta blockade in noncardiac elective surgery, β blockers suppress surgically induced increase in circulating catecholamines and thus, reduces predisposition to cardiac complications thereby reducing the risk of myocardial infarction and death in high risk patients (Class IIB). Beta blockers can be started at least 1 week before surgery and the dose titrated to achieve a heart rate of 60–80 beats/min in the absence of hypotension.[45] Alpha 2 agonists improve pain relief and shorten the duration of ileus. Both are known to reduce analgesic requirement, have anticatabolic properties, reduce intraoperative blood loss and reduce postoperative nausea vomiting (PONV).[46,47] Use of paracetamol, nonsteroid anti-inflammatory drug or dexamethasone as a premedicant is effective as a primer in reducing the intraoperative opioid requirement.[48]
Intraoperative Components in Enhanced Recovery After Surgery
Both surgical and anesthetic factors in the intraoperative period are key elements for a successful ERAS program. These factors lay the groundwork for early mobilization and feeding.
Laparoscopic surgery within an ERAS protocol has been shown to give superior recovery when compared to open surgery with ERAS protocol or laparoscopic surgery with standard care.[49] Lin et al. in 2009 conducted a prospective study in which they found out that laparoscopic surgery allows earlier ambulation and resultant better postoperative outcomes in comparison with standard open surgery.[50] Oblique or transverse short in length incisions are preferred as they are less painful and heal faster.[51] Minimally invasive approaches including endoscopy in varied fields such as spine surgery, orthopedic, neurosurgery have shown added benefits of decreased blood loss, decreased pain intensity, early ambulation, and cosmetic gains.[52]
A single dose of antibiotic covering both aerobes and anaerobes is recommended for infection prophylaxis and is administered just before the surgical incision is given.[53] A second dose is administered for surgical procedures lasting more than 4 h or when there is blood loss more than 1500 ml.
The goal of intraoperative fluid management is to maintain central euvolemia and minimize salt and water excess.[54] In order to achieve this, an individual fluid management plan for each patient is recommended in the ERAS protocol. As a part of this protocol, excess fluid administration should be avoided as it results in fluid shifting out of the circulation into the interstitium leading to intestinal edema and prolonged postoperative ileus.[55] Maintenance of fluid requirements during surgery can be performed with 1–3 ml/kg/h infusion of balanced salt solution with the aim of maintaining preoperative body weight. This therapy also called as zero-balance fluid therapy is sufficient for a low risk patient undergoing low risk surgery.[56] During major surgery, goal-directed fluid therapy (GDFT) can be used. GDFT refers to individualized fluid therapy using a minimally invasive cardiac output monitor.[57] It uses algorithms to optimize stroke volume and to avoid episodes of hypovolemia and postoperative oxygen debt. GDFT has been shown to significantly reduce length of hospital stay and postoperative pulmonary complications.[58]
An esophageal Doppler probe if used allows guided fluid management targeted against indicators of cardiac output. The intraoperative use of an esophageal Doppler probe has been shown to accelerate the return of gut function and expedite discharge after surgery.[59]
Anesthetic regimen
Recent literature has included decreased opiate usage and decreased patient controlled analgesia for early discharge to home in orthopedic patients as a part of enhanced recovery protocols.[60] There is little evidence to favor one anesthetic technique over another but the general principles of enhanced recovery, support the use of medications which have minimal postoperative hangover and minimal effects on gastric motility.[61] Thus, short acting premedicants and volatile anesthetics or total intravenous anesthesia with short acting agents is preferred. Remifentanil is the recommended opioid. Regional anesthetic techniques to support intraoperative and postoperative pain relief can be used, for example peripheral nerve blocks, thoracic epidural catheters, etc.[62] The aim of their use is to reduce the dose of the general anesthetic and reduce the stress response to surgery. They are also known to reduce the incidence of postoperative ileus by blocking sympathetic nervous system. For musculoskeletal surgeries, regional anesthetic techniques can be used alone or in combination with general anesthesia. The use of low concentration anesthetic mixtures reduces motor block and promotes early mobilization.[63]
Avoidance of PONV is also very important as it is one of the most incapacitating and undesired side effects of anesthesia.[64] The ERAS group recommends risk stratification of patients for PONV based on these factors (APFEL score): female sex, previous PONV or motion sickness, nonsmokers, and use of opioids.[65] Two risk factors constitute moderate risk and high risk patients have three or more. The ASA guidelines (2014) suggest a multimodal approach with different strategies, such as reduction of baseline risks (e.g., adequate hydration, intraoperative use of propofol and dexmedetomidine etc.), combination antiemetic therapy using a 5HT3 antagonist with droperidol or dexamethasone to effectively reduce incidental or established PONV.[66] The ERAS group have recommended use of dexamethasone at induction or a 5HT3 receptor antagonist, for example ondansetron, at the end of surgery for moderate risk and TIVA, dexamethasone at induction and a 5HT3 receptor antagonist or droperidol or metoclopramide near the end of surgery for high risk cases.[67] The Cochrane review of antiemetic prophylaxis did not show a beneficial effect of one agent over another so choice of drug is dependent on patient factors, cost, and practical considerations.[68] Novel antiemetics such as palonosetron are being tried for more evidence based information.[69]
There is increasing evidence that at least for mid-to lower abdominal procedures, the use of nasogastric tubes is not indicated as they may actually hinder recovery by prolonging paralytic ileus and predisposing to pulmonary aspiration.[70] Similarly, surgical drains may also slow the recovery of bowel function and make pain control difficult.[71] Hence, these should be avoided as far as possible.
Hypothermia is associated with increased wound infection, blood loss and high stress response predisposing to cardiac events due to release of catecholamines and cortisol.[72] It also causes patient discomfort. Forced warmed air devices, warmed intravenous fluids and warmed humidified gases should be used. Temperature monitoring is mandatory.
Postoperative Components
Early feeding decreases the incidence of ileus and negates the need for postoperative intravenous fluid administration.[73] Adequate nutrition is important to enhance wound healing, reduce infection, maintain muscle strength for mobilization and to counter fatigue. The Cochrane collaboration reviewed all the relevant randomized control trials regarding early enteral feeding until August 2006.[74] They concluded that the earlier fed group showed significant reduction in mortality, probably due to reduction in conditions leading to death such as sepsis, cardiac dysfunction, and anastomotic leak and also showed significant reduction in hospital stay.
Early mobilization aims to reduce skeletal muscle loss and improve respiratory function and oxygen delivery to tissues.[75] Patients should be encouraged to achieve daily procedure specific goals which can be guided by specific day-by-day proformas to ensure all areas of care receive attention. Ideally, patients should sit out of bed for 2 h on the day of surgery and 6 h a day until discharge.[76] The involvement of the physiotherapy and rehabilitation departments is vital to help with patient motivation and care.
Maintenance of hydration involves encouraging discontinuation of intravenous fluids and early commencement of oral fluid intake, including carbohydrate drinks. Avoidance of postoperative fluid overload continues to be as important as intraoperative fluid management strategies. However, this can pose a challenge to the clinician due to the lack of adequate fluid monitoring in wards.
Some extent of permissible oliguria until decrease in antidiuretic hormone or permissive relative hypotension within 10–15 systolic points of baseline, whereas the peripheral tone is decreased due to epidural, allows for minimal fluid infusion when it is not needed.[77]
Multimodal analgesia should consist of a combination of regular acetaminophen, nonsteroidal anti-inflammatory drugs unless contraindicated, local wound infiltration techniques and regional blocks, epidural opioids, patient controlled analgesia, and patient controlled epidural analgesia. Postoperative optimum pain relief facilitates early mobilization, early feeding and reduces stress related complications.[78] Excessive intravenous opioids should be avoided because of increased sedation, ileus, and respiratory complications; although, small doses of oral opioids for breakthrough pain are given wherever appropriate.
Urinary catheters are to be removed as early as possible. All patients undergoing lower abdominal – pelvic surgeries should have their catheters removed within 72 h of surgery and in most of the other surgeries the urinary catheter should be removed within 24 h.[79] The concern regarding urinary retention due to epidural analgesia was studied by Zaouter et al. in 2009 only to conclude that epidural analgesia did not interfere with bladder function, on the other hand, there was increased evidence of urinary tract infection in patients who had their bladder catheterized for more than 24 h.[80]
Gut motility enhancers such as chewing gum have been extensively studied.[81,82] Evidence-based data favors the use of chewing gum for reduction in incidence of postoperative ileus. It is an inexpensive, well tolerated and widely available method which has been included in enhanced recovery protocols.[83] The use of chewing gum should be encouraged starting on postoperative day 1, and each patient should chew one stick of gum for at least 5 min ≥3 times a day.
All the perioperative elements of ERAS protocols stated so far are of equal and utmost importance to achieve enhanced recovery. Nevertheless, some specific elements play a vital role in specific surgeries, where they should be necessarily applied [Table 2]
Table 2.
Enhanced recovery after surgery elements related to specific surgeries
| Type of surgery | Important ERAS elements |
|---|---|
| Neurosurgical procedures | Goal-directed fluid therapy[84] |
| Cardiothoracic procedures | Postoperative multimodal analgesia[14] |
| Abdominal surgery (open and minimally invasive) | Selective bowel preparation, early feeding, use of chewing gum, postoperative multimodal analgesia, minimal or avoiding usage of drains[4,5] |
| Pediatric surgery | Carbohydrate drink, goal-directed fluid therapy, prevention of hypothermia[85,86] |
| Orthopedic procedures | Early mobilization, multimodal analgesia[87] |
| Gynecological procedures | Early feeding, early mobilization, screen for malnutrition[88] |
| Obstetrics | Early mobilization, early feeding[89] |
ERAS = Enhanced recovery after surgery
The Benefits of Enhanced Recovery Protocols
Benefits of ERAS are to both the patient and the health services as a whole. Significant reductions in median length of hospital stay causes a significant reduction in hospital costs and more and more patients can be benefited by making hospital beds available. The most recent meta-analysis showed that ERAS protocols shorten the average hospital length of stay by approximately 2.3 days.[90]
ERAS protocols also reduce complications by approximately 40% and consequently reduce expenses related to them.[91] ERAS has widened its application effectively in the geriatric cohort undergoing orthopedic surgeries.[92] In an era of pay for performance programs, ERAS is beneficial to the attending anesthesiologist as well, by reducing postoperative complications without compromising patient safety. The anesthesiologist by participating in these protocols can improve the hospital's health-care quality with the added benefit of patient satisfaction.
Critical Appraisal
ERAS protocol guidelines have been established on the evidence based protocols used and tested in abdominal and colorectal surgeries The same guidelines are being increasingly used in arthroplasty, joint replacement and other orthopedic surgeries, as specific guidelines for specific surgeries have not yet been protocolized. In the current practice, similar guidelines are being used in all types of surgeries without any clinical evidence of their benefit. Specific ERAS guidelines for specific surgeries such as orthopedic, spine, vascular, thoracic, and neurosurgical procedures need to be formulated on the basis of evidence based data.
Although implementation of these guidelines emphasizes on a protocolized approach for benefitting the outcome, individual elements of ERAS protocols are also beneficial if advocated in isolation or wherever needed.[93] Very sparse literature is available regarding the cost effectiveness, availability of experienced health personnel for implementing the protocols and patient satisfaction in relation to an ERAS program. Systematic audit is beneficial in improving the outcome through feedbacks. This helps in identifying the fallacies, improving the compliance and successful implementation of the protocols.
Increased blood loss and massive blood transfusion are like two sides of a sharp knife and there is evidence that they affect the outcome and increase hospital stay.[94] Minimizing blood loss and avoiding transfusion can be considered as a part of ERAS in the future. Tranexamic acid or the use of advancements in surgery like ultrasonic blade, curettage and surgical aspirator, and stapler techniques which minimize the intraoperative blood loss can also be added in the protocols.[95,96,97]
Summary
ERAS protocols are multimodal perioperative care protocols that apply evidence based medicine to reduce length of hospital stay and postoperative complications. To play in the preoperative preparation, intraoperative management and postoperative period, and is one of the chief participants. The anesthesiologist and plays an active role, especially in the preanesthetic area for premedication, curtailed fasting guidelines, carbohydrate loading and optimization of risk factors, second in the choice of anesthesia and anesthetic drugs, multimodal analgesia, fluid management, prevention of hypothermia and avoidance of PONV, and finally also in the postoperative period in early feeding and early mobilization. Further, specific surgery based protocols should be streamlined to ensure its proper application and maximum benefits of ERAS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Novotny V, Hakenberg OW, Wiessner D, Heberling U, Litz RJ, Oehlschlaeger S, et al. Perioperative complications of radical cystectomy in a contemporary series. Eur Urol. 2007;51:397–401. doi: 10.1016/j.eururo.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Kahokehr A, Sammour T, Zargar-Shoshtari K, Thompson L, Hill AG. Implementation of ERAS and how to overcome the barriers. Int J Surg. 2009;7:16–9. doi: 10.1016/j.ijsu.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630–41. doi: 10.1016/s0002-9610(02)00866-8. [DOI] [PubMed] [Google Scholar]
- 4.Eskicioglu C, Forbes SS, Aarts MA, Okrainec A, McLeod RS. Enhanced recovery after surgery (ERAS) programs for patients having colorectal surgery: A meta-analysis of randomized trials. J Gastrointest Surg. 2009;13:2321–9. doi: 10.1007/s11605-009-0927-2. [DOI] [PubMed] [Google Scholar]
- 5.Wind J, Polle SW, Fung Kon Jin PH, Dejong CH, von Meyenfeldt MF, Ubbink DT, et al. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. 2006;93:800–9. doi: 10.1002/bjs.5384. [DOI] [PubMed] [Google Scholar]
- 6.Podore PC, Throop EB. Infrarenal aortic surgery with a 3-day hospital stay: A report on success with a clinical pathway. J Vasc Surg. 1999;29:787–92. doi: 10.1016/s0741-5214(99)70204-1. [DOI] [PubMed] [Google Scholar]
- 7.Tovar EA, Roethe RA, Weissig MD, Lloyd RE, Patel GR. One-day admission for lung lobectomy: An incidental result of a clinical pathway. Ann Thorac Surg. 1998;65:803–6. [PubMed] [Google Scholar]
- 8.Melnyk M, Casey RG, Black P, Koupparis AJ. Enhanced recovery after surgery (ERAS) protocols: Time to change practice? Can Urol Assoc J. 2011;5:342–8. doi: 10.5489/cuaj.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wainwright TW, Immins T, Middleton RG. Enhanced recovery after surgery (ERAS) and its applicability for major spine surgery. Best Pract Res Clin Anaesthesiol. 2016;30:91–102. doi: 10.1016/j.bpa.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Hagan KB, Bhavsar S, Raza SM, Arnold B, Arunkumar R, Dang A, et al. Enhanced recovery after surgery for oncological craniotomies. J Clin Neurosci. 2016;24:10–6. doi: 10.1016/j.jocn.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Tucker A, McCusker D, Gupta N, Bunn J, Murnaghan M. Orthopaedic enhanced recovery programme for elective hip and knee arthroplasty – Could a regional programme be beneficial? Ulster Med J. 2016;85:86–91. [PMC free article] [PubMed] [Google Scholar]
- 12.Stoot JH, van Dam RM, Busch OR, van Hillegersberg R, De Boer M, Olde Damink SW, et al. The effect of a multimodal fast-track programme on outcomes in laparoscopic liver surgery: A multicentre pilot study. HPB (Oxford) 2009;11:140–4. doi: 10.1111/j.1477-2574.2009.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pecorelli N, Nobile S, Partelli S, Cardinali L, Crippa S, Balzano G, et al. Enhanced recovery pathways in pancreatic surgery: State of the art. World J Gastroenterol. 2016;22:6456–68. doi: 10.3748/wjg.v22.i28.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rafiq S, Steinbrüchel DA, Wanscher MJ, Andersen LW, Navne A, Lilleoer NB, et al. Multimodal analgesia versus traditional opiate based analgesia after cardiac surgery, a randomized controlled trial. J Cardiothorac Surg. 2014;9:52. doi: 10.1186/1749-8090-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fearon KC, Ljungqvist O, Von Meyenfeldt M, Revhaug A, Dejong CH, Lassen K, et al. Enhanced recovery after surgery: A consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24:466–77. doi: 10.1016/j.clnu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Khan S, Gatt M, Horgan A, Anderson I, MacFie J. 1st ed. London: Association of Surgeons of Great Britain and Ireland (ASGBI); 2009. Issues in Professional Practice: Guidelines for Implementation of Enhanced Recovery Protocols; pp. 1–19. [Google Scholar]
- 17.White L, Rivett K. 1st ed. London: The Royal College of Anaesthetists (RCoA); 2012. Guidelines for Patients Undergoing Surgery as Part of an Enhanced Recovery Programme (ERP): Helping You to Get Better Sooner after Surgery. [Google Scholar]
- 18.Cerantola Y, Valerio M, Persson B, Jichlinski P, Ljungqvist O, Hubner M, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS®) society recommendations. Clin Nutr. 2013;32:879–87. doi: 10.1016/j.clnu.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144:961–9. doi: 10.1001/archsurg.2009.170. [DOI] [PubMed] [Google Scholar]
- 20.Miller TE, Thacker JK, White WD, Mantyh C, Migaly J, Jin J, et al. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg. 2014;118:1052–61. doi: 10.1213/ANE.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 21.Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:783–800. doi: 10.1016/j.clnu.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Lassen K, Coolsen MM, Slim K, Carli F, de Aguilar-Nascimento JE, Schäfer M, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:817–30. doi: 10.1016/j.clnu.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg. 2014;101:1209–29. doi: 10.1002/bjs.9582. [DOI] [PubMed] [Google Scholar]
- 24.American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011;114:495–511. doi: 10.1097/ALN.0b013e3181fcbfd9. [DOI] [PubMed] [Google Scholar]
- 25.Adamina M, Kehlet H, Tomlinson GA, Senagore AJ, Delaney CP. Enhanced recovery pathways optimize health outcomes and resource utilization: A meta-analysis of randomized controlled trials in colorectal surgery. Surgery. 2011;149:830–40. doi: 10.1016/j.surg.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Mondloch MV, Cole DC, Frank JW. Does how you do depend on how you think you'll do? A systematic review of the evidence for a relation between patients' recovery expectations and health outcomes. CMAJ. 2001;165:174–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Gregg JR, Cookson MS, Phillips S, Salem S, Chang SS, Clark PE, et al. Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer. J Urol. 2011;185:90–6. doi: 10.1016/j.juro.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–17. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 29.Mathur S, Plank LD, Hill AG, Rice MA, Hill GL. Changes in body composition, muscle function and energy expenditure after radical cystectomy. BJU Int. 2008;101:973–7. doi: 10.1111/j.1464-410X.2007.07337.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen L. A literature review of intensive insulin therapy and mortality in critically ill patients. Clin Nurse Spec. 2010;24:80–6. doi: 10.1097/NUR.0b013e3181cf55af. [DOI] [PubMed] [Google Scholar]
- 31.Abebe WA, Rukewe A, Bekele NA, Stoffel M, Dichabeng MN, Shifa JZ. Preoperative fasting times in elective surgical patients at a referral hospital in Botswana. Pan Afr Med J. 2016;23:102. doi: 10.11604/pamj.2016.23.102.8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svanfeldt M, Thorell A, Hausel J, Soop M, Rooyackers O, Nygren J, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342–50. doi: 10.1002/bjs.5919. [DOI] [PubMed] [Google Scholar]
- 33.Soop M, Nygren J, Thorell A, Weidenhielm L, Lundberg M, Hammarqvist F, et al. Preoperative oral carbohydrate treatment attenuates endogenous glucose release 3 days after surgery. Clin Nutr. 2004;23:733–41. doi: 10.1016/j.clnu.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Hausel J, Nygren J, Lagerkranser M, Hellström PM, Hammarqvist F, Almström C, et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93:1344–50. doi: 10.1097/00000539-200111000-00063. [DOI] [PubMed] [Google Scholar]
- 35.Nygren J, Thorell A, Ljungqvist O. Preoperative oral carbohydrate nutrition: An update. Curr Opin Clin Nutr Metab Care. 2001;4:255–9. doi: 10.1097/00075197-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Smith MD, McCall J, Plank L, Herbison GP, Soop M, Nygren J. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst Rev. 2014;8:CD009161. doi: 10.1002/14651858.CD009161.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ljungqvist O. Modulating postoperative insulin resistance by preoperative carbohydrate loading. Best Pract Res Clin Anaesthesiol. 2009;23:401–9. doi: 10.1016/j.bpa.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Gustafsson UO, Nygren J, Thorell A, Soop M, Hellström PM, Ljungqvist O, et al. Pre-operative carbohydrate loading may be used in type 2 diabetes patients. Acta Anaesthesiol Scand. 2008;52:946–51. doi: 10.1111/j.1399-6576.2008.01599.x. [DOI] [PubMed] [Google Scholar]
- 39.Eskicioglu C, Forbes SS, Fenech DS, McLeod RS Best Practice in General Surgery Committee. Preoperative bowel preparation for patients undergoing elective colorectal surgery: A clinical practice guideline endorsed by the Canadian Society of Colon and Rectal Surgeons. Can J Surg. 2010;53:385–95. [PMC free article] [PubMed] [Google Scholar]
- 40.Guenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2011;9:CD001544. doi: 10.1002/14651858.CD001544.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slim K, Vicaut E, Launay-Savary MV, Contant C, Chipponi J. Updated systematic review and meta-analysis of randomized clinical trials on the role of mechanical bowel preparation before colorectal surgery. Ann Surg. 2009;249:203–9. doi: 10.1097/SLA.0b013e318193425a. [DOI] [PubMed] [Google Scholar]
- 42.Lindström D, Sadr Azodi O, Wladis A, Tønnesen H, Linder S, Nåsell H, et al. Effects of a perioperative smoking cessation intervention on postoperative complications: A randomized trial. Ann Surg. 2008;248:739–45. doi: 10.1097/SLA.0b013e3181889d0d. [DOI] [PubMed] [Google Scholar]
- 43.Burnham EL. Identification of risky alcohol consumption in the preoperative assessment: Opportunity to diagnose and intervene. Anesthesiology. 2008;109:169–70. doi: 10.1097/ALN.0b013e31817f587a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bell BR, Bastien PE, Douketis JD Thrombosis Canada. Prevention of venous thromboembolism in the Enhanced Recovery After Surgery (ERAS) setting: An evidence-based review. Can J Anaesth. 2015;62:194–202. doi: 10.1007/s12630-014-0262-2. [DOI] [PubMed] [Google Scholar]
- 45.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2009;120:e169–276. doi: 10.1161/CIRCULATIONAHA.109.192690. [DOI] [PubMed] [Google Scholar]
- 46.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–9. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 47.Wu CT, Jao SW, Borel CO, Yeh CC, Li CY, Lu CH, et al. The effect of epidural clonidine on perioperative cytokine response, postoperative pain, and bowel function in patients undergoing colorectal surgery. Anesth Analg. 2004;99:502–9. doi: 10.1213/01.ANE.0000117146.46373.51. [DOI] [PubMed] [Google Scholar]
- 48.Jakobsson JG. Pain management in ambulatory surgery – A review. Pharmaceuticals (Basel) 2014;7:850–65. doi: 10.3390/ph7080850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: A randomized clinical trial (LAFA-study) Ann Surg. 2011;254:868–75. doi: 10.1097/SLA.0b013e31821fd1ce. [DOI] [PubMed] [Google Scholar]
- 50.Lin JH, Whelan RL, Sakellarios NE, Cekic V, Forde KA, Bank J, et al. Prospective study of ambulation after open and laparoscopic colorectal resection. Surg Innov. 2009;16:16–20. doi: 10.1177/1553350608330478. [DOI] [PubMed] [Google Scholar]
- 51.Seiler CM, Deckert A, Diener MK, Knaebel HP, Weigand MA, Victor N, et al. Midline versus transverse incision in major abdominal surgery: A randomized, double-blind equivalence trial (POVATI: ISRCTN60734227) Ann Surg. 2009;249:913–20. doi: 10.1097/SLA.0b013e3181a77c92. [DOI] [PubMed] [Google Scholar]
- 52.Swanson TV. Posterior single-incision approach to minimally invasive total hip arthroplasty. Int Orthop. 2007;31(Suppl 1):S1–5. doi: 10.1007/s00264-007-0436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alfonsi P, Slim K, Chauvin M, Mariani P, Faucheron JL, Fletcher D, et al. Guidelines for enhanced recovery after elective colorectal surgery. Ann Fr Anesth Reanim. 2014;33:370–84. doi: 10.1016/j.annfar.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Mythen MG, Swart M, Acheson N, Crawford R, Jones K, Kuper M, et al. Perioperative fluid management: Consensus statement from the enhanced recovery partnership. Perioper Med (Lond) 2012;1:2. doi: 10.1186/2047-0525-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacob M, Chappell D, Rehm M. The ‘third space’ – Fact or fiction? Best Pract Res Clin Anaesthesiol. 2009;23:145–57. doi: 10.1016/j.bpa.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M. A rational approach to perioperative fluid management. Anesthesiology. 2008;109:723–40. doi: 10.1097/ALN.0b013e3181863117. [DOI] [PubMed] [Google Scholar]
- 57.Srinivasa S, Taylor MH, Singh PP, Yu TC, Soop M, Hill AG. Randomized clinical trial of goal-directed fluid therapy within an enhanced recovery protocol for elective colectomy. Br J Surg. 2013;100:66–74. doi: 10.1002/bjs.8940. [DOI] [PubMed] [Google Scholar]
- 58.Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: A randomized clinical trial and systematic review. JAMA. 2014;311:2181–90. doi: 10.1001/jama.2014.5305. [DOI] [PubMed] [Google Scholar]
- 59.Noblett SE, Snowden CP, Shenton BK, Horgan AF. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg. 2006;93:1069–76. doi: 10.1002/bjs.5454. [DOI] [PubMed] [Google Scholar]
- 60.Talboys R, Mak M, Modi N, Fanous N, Cutts S. Enhanced recovery programme reduces opiate consumption in hip hemiarthroplasty. Eur J Orthop Surg Traumatol. 2016;26:177–81. doi: 10.1007/s00590-015-1722-2. [DOI] [PubMed] [Google Scholar]
- 61.White PF, Kehlet H, Neal JM, Schricker T, Carr DB, Carli F Fast-Track Surgery Study Group. The role of the anesthesiologist in fast-track surgery: From multimodal analgesia to perioperative medical care. Anesth Analg. 2007;104:1380–96. doi: 10.1213/01.ane.0000263034.96885.e1. [DOI] [PubMed] [Google Scholar]
- 62.Levy BF, Scott MJ, Fawcett W, Fry C, Rockall TA. Randomized clinical trial of epidural, spinal or patient-controlled analgesia for patients undergoing laparoscopic colorectal surgery. Br J Surg. 2011;98:1068–78. doi: 10.1002/bjs.7545. [DOI] [PubMed] [Google Scholar]
- 63.Pöpping DM, Elia N, Marret E, Remy C, Tramèr MR. Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: A meta-analysis. Arch Surg. 2008;143:990–9. doi: 10.1001/archsurg.143.10.990. [DOI] [PubMed] [Google Scholar]
- 64.Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003;362:1921–8. doi: 10.1016/S0140-6736(03)14966-5. [DOI] [PubMed] [Google Scholar]
- 65.Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350:2441–51. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118:85–113. doi: 10.1213/ANE.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 67.Gan TJ, Meyer TA, Apfel CC, Chung F, Davis PJ, Habib AS, et al. Society for ambulatory anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105:1615–28. doi: 10.1213/01.ane.0000295230.55439.f4. [DOI] [PubMed] [Google Scholar]
- 68.Karanicolas PJ, Smith SE, Kanbur B, Davies E, Guyatt GH. The impact of prophylactic dexamethasone on nausea and vomiting after laparoscopic cholecystectomy: A systematic review and meta-analysis. Ann Surg. 2008;248:751–62. doi: 10.1097/SLA.0b013e3181856024. [DOI] [PubMed] [Google Scholar]
- 69.Li Y, Wei X, Zhang S, Zhou L, Zhang J. A meta-analysis of palonosetron for the prevention of postoperative nausea and vomiting in adults. J Perianesth Nurs. 2015;30:398–405. doi: 10.1016/j.jopan.2015.05.116. [DOI] [PubMed] [Google Scholar]
- 70.Nelson R, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev. 2005;1:CD004929. doi: 10.1002/14651858.CD004929.pub2. [DOI] [PubMed] [Google Scholar]
- 71.Petrowsky H, Demartines N, Rousson V, Clavien PA. Evidence-based value of prophylactic drainage in gastrointestinal surgery: A systematic review and meta-analyses. Ann Surg. 2004;240:1074–84. doi: 10.1097/01.sla.0000146149.17411.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong PF, Kumar S, Bohra A, Whetter D, Leaper DJ. Randomized clinical trial of perioperative systemic warming in major elective abdominal surgery. Br J Surg. 2007;94:421–6. doi: 10.1002/bjs.5631. [DOI] [PubMed] [Google Scholar]
- 73.Han-Geurts IJ, Hop WC, Kok NF, Lim A, Brouwer KJ, Jeekel J. Randomized clinical trial of the impact of early enteral feeding on postoperative ileus and recovery. Br J Surg. 2007;94:555–61. doi: 10.1002/bjs.5753. [DOI] [PubMed] [Google Scholar]
- 74.Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database Syst Rev. 2006;4:CD004080. doi: 10.1002/14651858.CD004080.pub2. [DOI] [PubMed] [Google Scholar]
- 75.Papaspyros S, Uppal S, Khan SA, Paul S, O'Regan DJ. Analysis of bedside entertainment services' effect on post cardiac surgery physical activity: A prospective, randomised clinical trial. Eur J Cardiothorac Surg. 2008;34:1022–6. doi: 10.1016/j.ejcts.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 76.Massey RL. A randomized trial of rocking-chair motion on the effect of postoperative ileus duration in patients with cancer recovering from abdominal surgery. Appl Nurs Res. 2010;23:59–64. doi: 10.1016/j.apnr.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 77.MacKay G, Fearon K, McConnachie A, Serpell MG, Molloy RG, O'Dwyer PJ. Randomized clinical trial of the effect of postoperative intravenous fluid restriction on recovery after elective colorectal surgery. Br J Surg. 2006;93:1469–74. doi: 10.1002/bjs.5593. [DOI] [PubMed] [Google Scholar]
- 78.Liu SS, Wu CL. Effect of postoperative analgesia on major postoperative complications: A systematic update of the evidence. Anesth Analg. 2007;104:689–702. doi: 10.1213/01.ane.0000255040.71600.41. [DOI] [PubMed] [Google Scholar]
- 79.Baldini G, Bagry H, Aprikian A, Carli F. Postoperative urinary retention: Anesthetic and perioperative considerations. Anesthesiology. 2009;110:1139–57. doi: 10.1097/ALN.0b013e31819f7aea. [DOI] [PubMed] [Google Scholar]
- 80.Zaouter C, Kaneva P, Carli F. Less urinary tract infection by earlier removal of bladder catheter in surgical patients receiving thoracic epidural analgesia. Reg Anesth Pain Med. 2009;34:542–8. doi: 10.1097/aap.0b013e3181ae9fac. [DOI] [PubMed] [Google Scholar]
- 81.Chan MK, Law WL. Use of chewing gum in reducing postoperative ileus after elective colorectal resection: A systematic review. Dis Colon Rectum. 2007;50:2149–57. doi: 10.1007/s10350-007-9039-9. [DOI] [PubMed] [Google Scholar]
- 82.Noble EJ, Harris R, Hosie KB, Thomas S, Lewis SJ. Gum chewing reduces postoperative ileus? A systematic review and meta-analysis. Int J Surg. 2009;7:100–5. doi: 10.1016/j.ijsu.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 83.Fitzgerald JE, Ahmed I. Systematic review and meta-analysis of chewing-gum therapy in the reduction of postoperative paralytic ileus following gastrointestinal surgery. World J Surg. 2009;33:2557–66. doi: 10.1007/s00268-009-0104-5. [DOI] [PubMed] [Google Scholar]
- 84.Ract C, Le Moigno S, Bruder N, Vigué B. Transcranial Doppler ultrasound goal-directed therapy for the early management of severe traumatic brain injury. Intensive Care Med. 2007;33:645–51. doi: 10.1007/s00134-007-0558-6. [DOI] [PubMed] [Google Scholar]
- 85.Smith I, Kranke P, Murat I, Smith A, O'Sullivan G, Søreide E, et al. Perioperative fasting in adults and children: Guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:556–69. doi: 10.1097/EJA.0b013e3283495ba1. [DOI] [PubMed] [Google Scholar]
- 86.Mittnacht AJ, Hollinger I. Fast-tracking in pediatric cardiac surgery – The current standing. Ann Card Anaesth. 2010;13:92–101. doi: 10.4103/0971-9784.62930. [DOI] [PubMed] [Google Scholar]
- 87.Kehlet H, Søballe K. Fast-track hip and knee replacement – What are the issues? Acta Orthop. 2010;81:271–2. doi: 10.3109/17453674.2010.487237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wodlin NB, Nilsson L. The development of fast-track principles in gynecological surgery. Acta Obstet Gynecol Scand. 2013;92:17–27. doi: 10.1111/j.1600-0412.2012.01525.x. [DOI] [PubMed] [Google Scholar]
- 89.Orji EO, Olabode TO, Kuti O, Ogunniyi SO. A randomised controlled trial of early initiation of oral feeding after cesarean section. J Matern Fetal Neonatal Med. 2009;22:65–71. doi: 10.1080/14767050802430826. [DOI] [PubMed] [Google Scholar]
- 90.Gouvas N, Tan E, Windsor A, Xynos E, Tekkis PP. Fast-track vs standard care in colorectal surgery: A meta-analysis update. Int J Colorectal Dis. 2009;24:1119–31. doi: 10.1007/s00384-009-0703-5. [DOI] [PubMed] [Google Scholar]
- 91.Sammour T, Zargar-Shoshtari K, Bhat A, Kahokehr A, Hill AG. A programme of Enhanced Recovery After Surgery (ERAS) is a cost-effective intervention in elective colonic surgery. N Z Med J. 2010;123:61–70. [PubMed] [Google Scholar]
- 92.Aw D, Sahota O. Orthogeriatrics moving forward. Age Ageing. 2014;43:301–5. doi: 10.1093/ageing/afu011. [DOI] [PubMed] [Google Scholar]
- 93.Simpson JC, Moonesinghe SR, Grocott MP, Kuper M, McMeeking A, Oliver CM, et al. Enhanced recovery from surgery in the UK: An audit of the enhanced recovery partnership programme 2009-2012. Br J Anaesth. 2015;115:560–8. doi: 10.1093/bja/aev105. [DOI] [PubMed] [Google Scholar]
- 94.Sibia US, MacDonald JH, King PJ. Predictors of hospital length of stay in an enhanced recovery after surgery program for primary total hip arthroplasty. J Arthroplasty. 2016;31:2119–23. doi: 10.1016/j.arth.2016.02.060. [DOI] [PubMed] [Google Scholar]
- 95.Drosos GI, Ververidis A, Valkanis C, Tripsianis G, Stavroulakis E, Vogiatzaki T, et al. A randomized comparative study of topical versus intravenous tranexamic acid administration in enhanced recovery after surgery (ERAS) total knee replacement. J Orthop. 2016;13:127–31. doi: 10.1016/j.jor.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guild GN, 3rd, Runner RP, Castilleja GM, Smith MJ, Vu CL. Efficacy of hybrid plasma scalpel in reducing blood loss and transfusions in direct anterior total hip arthroplasty. J Arthroplasty. 2017;32:458–62. doi: 10.1016/j.arth.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 97.Usas A, Usaite D, Gao X, Huard J, Clymer JW, Malaviya P. Use of an ultrasonic blade facilitates muscle repair after incision injury. J Surg Res. 2011;167:e177–84. doi: 10.1016/j.jss.2010.12.042. [DOI] [PubMed] [Google Scholar]


