Abstract
Improvement in patient outcomes has become a significant consideration with our limited resources in the surgical setting. The implementation of enhanced recovery pathway protocols has resulted in significant benefits to both the patients and hospitals, such as shorter length of hospital stays, reduction in the rate of complications, and fewer hospital readmissions. An emerging component and a key element for the success of Enhanced Recovery After Surgery (ERAS) protocols has been the concept of goal-directed fluid therapy (GDT). GDT related to ERAS protocols attempts to minimize complications associated with fluid imbalance during surgery. We performed a literature search for articles that included the terms enhanced recovery and GDT. We evaluated methods for appropriate volume status assessment, such as heart rate, blood pressure, end-tidal CO2, central venous pressure, urine output, stroke volume, cardiac output, and their derivatives. Some invasive, minimally invasive, and non-invasive monitors of hemodynamic evaluation are now being used to assess volume status and predict fluid responsiveness and fluid need during various surgical procedures. Regardless of monitoring technique, it is important for the clinician to effectively plan and implement preoperative and intraoperative fluid goals. Excess crystalloid fluid should be avoided. In some low-risk patients undergoing low-risk surgery, a “zero-balance” approach is encouraged. For the majority of patients undergoing major surgery, GDT is recommended. Optimal perioperative fluid management is an important component of the ERAS pathways and it can reduce postoperative complications.
Keywords: Arterial contour analysis, bioimpedance, ERAS, goal-directed therapy, length of hospital stay, volume status
Introduction
The Enhanced Recovery After Surgery (ERAS)© protocols are increasingly applied in perioperative services worldwide.[1,2] ERAS adopts a multimodal, multidisciplinary approach to provide seamless perioperative care of the surgical patient. ERAS pathways should include a team consisting of surgeons, anesthesiologists, an ERAS coordinator, nursing and other staff from units that care for the surgical patient. The ERAS Society develops, promotes, and implements recommended ERAS programs, which can be modified based on each individual institution's conditions. The ERAS Society also regularly publishes updated and evidence-based guidelines for various surgical procedures. The implementation of ERAS protocols has resulted in significant benefits to both the patients and hospitals with a shorter length of hospital stay by 30–50%, a similar rate of complication decrease, and a significantly reduced re-admissions rate to the hospital.[1,2,3]
Perioperative fluid management is a key element for the success of ERAS protocols. The use of goal-directed fluid therapy (GDT) is steadily gaining popularity for the appropriate perioperative fluid/volume management.[4] A PubMed literature search was performed for articles that included the terms: enhanced recovery and goal-directed fluid therapy. Key terms that were also included in this search: volume status assessment, heart rate (HR), blood pressure (BP), end-tidal CO2, central venous pressure (CVP), urine output, stroke volume (SV), and cardiac output (CO). All relevant information, regardless of publication year, was included although the authors tried to focus on manuscripts that were published in the last 5 years.
This review article will discuss the pertinent aspects of GDT and its role in the successful implementation of ERAS protocols.
Perioperative Volume Status and Monitoring
Factors affecting preoperative intravascular volume
Intravenous fluid therapy is an important and integrated treatment of patients undergoing surgery. Traditionally, surgical patients have been required to fast for 8 h. This can potentially lead to preoperative hypovolemia. The resulting surgical stress can induce multiple endocrine responses, including the release of vasopressin (antidiuretic hormone). The re-absorptive actions of vasopressin on the collecting duct in the kidneys can cause water retention, which can, to some extent offset the hypovolemic effect of fasting.[5] The majority of perioperative patients experience a certain degree of preoperative hypovolemia.[6] This has been shown to be associated with poorer clinical outcome.[5] The usual etiologies of preoperative hypovolemia are summarized in Table 1. Hypovolemia can lead to vasoconstriction and inadequate perfusion with decreased oxygen delivery to organs and peripheral tissues causing organ dysfunction. On the other hand, fluid overload can lead to interstitial edema and local inflammation and likely impair the regeneration of collagen, thus negatively affecting tissue healing and increasing the risk of wound dehiscence, wound infections, and anastomotic leakage,[5] as illustrated in Figure 1. Therefore, it is imperative to manage each patient's fluid therapy in an individualized manner.[4,5,6]
Table 1.
Factors affecting preoperative volume status or preoperative hypovolemia
| Factors | Notes |
|---|---|
| Traditional preoperative fasting protocol | Usually 8 h nothing by mouth |
| Unable to have oral intake | Due to disease status |
| Preoperative hemorrhage | Trauma patient |
| Other preoperative volume loss | Fever, diuresis, diarrhea |
Figure 1.
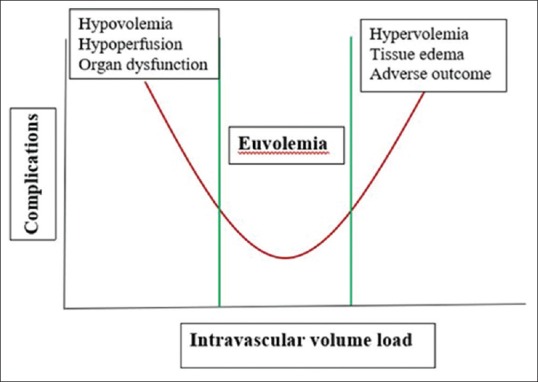
A depiction of how fluid overload can lead to interstitial edema and local inflammation, impairing the regeneration of collagen, and thus negatively affecting tissue healing and increasing the risk of wound dehiscence, wound infections, and anastomotic leakage
Is urine output a valid indicator of perioperative volume status?
Urine output has traditionally been considered as an indicator of intravascular volume status, because urine output generally reflects adequate renal perfusion that is closely associated with systemic intravascular volume. Undoubtedly, preoperative anuria is abnormal and warrants aggressive clinical investigation. Oliguria is defined as urine output <0.5 ml/kg/h, and caused by various etiologies. Though low urine output can occur when there is decreased blood flow to the kidney (as with dehydration or excessive blood loss and hypotension), urinary obstruction of outflow of the urine, such as seen in prostate enlargement can also reduce urine output. Since urine output can be affected by multiple factors, it is not a sensitive indicator of circulating blood volume. On the other hand, urine output has proven to be a sensitive and early marker for acute kidney injury, which can be associated with adverse outcomes in perioperative patients.[7] Some studies suggest high postoperative urine volume may predict early readiness for discharge among patients undergoing colorectal surgery.[8] Johnson et al. recommended that isolated low urine output should not trigger fluid therapy and extensive diagnostic efforts.[8]
Monitoring of intravascular volume status
The purpose of monitoring intravascular volume status is to guide fluid administration in order to maintain adequate tissue perfusion. Reduced tissue perfusion can be associated with hypovolemia (hemorrhage) or hypervolemia (i.e., a patient with severe myocardial dysfunction and compensatory fluid retention). Volume status assessment can be achieved with continuous intraoperative monitoring of factors such as HR, BP, end-tidal CO2, CVP, urine output, SV, CO, and their derivatives, as summarized in Table 2.[9] However, these are not reliable measures of volume status.[10] Thus, some invasive, minimally invasive, and non-invasive monitors of hemodynamic parameters are used to assess volume status and predict fluid responsiveness in various surgical procedures.[11,12,13] Regardless of the monitoring methods employed, accurate intraoperative determination of intravascular volume status remains challenging because of continuously changing cardiovascular responses to anesthetic drugs, variable surgical volume losses that are often difficult to quantify, a preoperative hypovolemia or an unknown preoperative volume status, as well as the manifestations of the normal physiological responses to surgery.[14] Additionally, not all patients who are fluid responders require volume expansion and clinicians often have difficulty estimating the preload condition of their patients. The decision to administer fluid should be supported by an apparent need for hemodynamic improvement in the context of a likely volume deficit and by the lack of associated risk.[1]
Table 2.
Commonly used volume status measurement techniques
| Category | Parameters |
|---|---|
| Vital signs | Blood pressure |
| Heart rate | |
| Orthostatic changes | |
| Physical examinations | Mental status |
| Capillary refill | |
| Extremity temperature | |
| Skin turgor | |
| Skin perfusion | |
| Urine output | |
| Laboratory tests | Fractional excretion of sodium, urea |
| Blood lactate level | |
| Mixed venous oxygen saturation | |
| Intravascular/cardiac catheterization | CVP |
| PAWP | |
| SVV | |
| LVEDP | |
| Doppler/echocardiography | LVEDV |
| SV | |
| CO | |
| CI |
Volume status measurement techniques separated into categories and parameters. CVP=Central venous pressure, PAWP=Pulmonary artery wedge pressure, SVV=Stroke volume variation, SV=Stroke volume, LVEDP=Left ventricular end-diastolic pressure, LVDEV=Left ventricular end-diastolic volume, CO=Cardiac output, CI=Cardiac index
Intraoperative fluid management within enhanced recovery after surgery protocols
Intraoperative fluid management within ERAS protocols should be viewed as a continuum through the preoperative, intraoperative, and postoperative period.[1] The goal of preoperative fluid management is for the patient to be in a hydrated and euvolemic state when arriving in the operating room. This is usually achieved by avoidance of prolonged fasting and mechanical bowel preparation and encouraging patients to ingest a clear carbohydrate drink approximately 2 h prior to surgery. The goals of intraoperative fluid management are to avoid excess salt and water and to maintain central euvolemia. As such, patients undergoing surgery within an ERAS protocol should have an individualized fluid management plan. Excess crystalloid should be avoided for all the patients. In some low-risk patients undergoing low-risk surgery, a “zero-balance” approach is encouraged. For the majority of patients undergoing major surgery, GDT is recommended. Optimal perioperative fluid management is an important component of the ERAS protocol. In one study, a change in fluid management alone on the day of surgery was shown to reduce perioperative complications by 50%.[1]
Goal-Directed Fluid Therapy
What is the goal in the “goal-directed fluid therapy”?
There is no consensus reported in the literature in terms of the goals in the “Goal-directed fluid therapy”. For many years, the parameters originally used in critical care medicine for septic patients are listed in Table 3. Various parameters have been investigated, Table 4 summarizes the reported “Goals” and reported outcomes in the literature.
Table 3.
Parameters in early goal-directed therapy[15]
| Parameters | Range to target | Interventions |
|---|---|---|
| CVP | 8-12 cmH2O | Early use of mechanical ventilation |
| MAP | 65-90 mmHg | Fluid resuscitation |
| SvO2 | >70% | Use of vasoactive agents |
| ScvO2 | >65% | Noradrenaline |
| Urine output | >0.5 ml/kg/h | Dobutamine |
| Hematocrit | >30% | Transfusion |
Parameters, range to target, and interventions in goal-directed fluid therapy. CVP=Central venous pressure, MAP=Men arterial pressure, SvO2=Mixed venous oxygen saturation, ScvO2=Central venous oxygen saturation
Table 4.
| Parameters | Technique | Surgical procedures | GDT results | Year reported |
|---|---|---|---|---|
| CI, SVV, SvO2, SVR | EV1000 (Edwards Life Science, USA) | Off-pump CABG | LOHS ↓ ICU stay ↓ | 2017[16] |
| SV | Transesophageal Doppler (Deltex, UK) | Colorectal surgery | Postoperative ileus: Not better | 2017[17] |
| SVV | Vigileo/Flotrac (Edwards Life Science, USA) | Spine surgery | EBL/transfusion ↓ ICU stay ↓ Bowel function ↑ | 2016[18] |
| SVV | LidCO (UK) | Bariatric surgery | IVF ↓ | 2010[19] |
| SV, SVV | Flotrac (Edwards Life Science) | Major abdominal | Complications ↓ | 2017[20] |
| ScvO2 | PreSep Oximetry (Edwards Life Science, USA) | Sepsis | Mortality ↓ | 2014[21] |
| PVI | Masimo (USA) | Roux-en-Y gastric bypass | IVF ↓ | 2017[22] |
↓= Decrease; ↑= Increase. CI=cardiac index, SVV=stroke volume variation, SvO2=Mixed venous oxygen saturation, SVR=Systemic vascular resistance, CABG=Coronary artery bypass graft, LOHS=Length of hospital stay, EBL=Estimated blood loss, IVF=Intravenous fluid, ScvO2=Central venous oxygen saturation, PVI=Plethysmography variability index, ICU=Intensive Care Unit, GDT=Goal-directed fluid therapy
Fluid responsiveness
GDT extrapolates a patient's fluid responsiveness from measurable hemodynamic changes according to the Frank–Starling curve. Fluid responders will generally demonstrate an increase in their SV by ≥10–15% after a fluid challenge. The exact definition of a “fluid challenge” varies, yet in most sources it typically refers to a certain volume of fluid administered over a short period of time, for example, a bolus of 500 ml or more, administered in 10 min or less. A fluid challenge can simultaneously identify and treat volume depletion while avoiding deleterious consequence of large fluid overload. However, it is important to realize that being a fluid responder is not equal to being hypovolemic. For example, when a fluid challenge is given on clinical grounds, only 50% of those hemodynamically unstable patients will prove to be volume responders.[1] Due to the risks associated with excessive fluid administration and the challenge in identifying hypovolemic patients, it is important to be able to predict whether a patient will be fluid responsive without actually giving fluid. One such method is to see the response to a 30° Trendlenburg position.
Commonly used techniques in goal-directed fluid therapy
Transesophageal echocardiography
Transesophageal echocardiography can measure SV, CO, CVP, thus it can be used intraoperatively to provide parameters for GDT.[12] The less invasive transthoracic echocardiography can be used preoperatively and postoperatively; however, it is often not convenient to use transthoracic echocardiography intraoperatively.
Pulmonary artery catheterization
Pulmonary artery catheterization was used initially perioperative when early GDT was proposed to guide intensive care unit (ICU) management of septic patients. Pulmonary artery catheterization can provide those hemodynamic parameters needed for GDT, which include CVP, mixed venous oxygen saturation (SvO2), left ventricular end-diastolic pressure (LVEDP), left ventricular end-diastolic volume (LVEDV), SV, CO, cardiac index (CI), and systemic vascular resistance (SVR).[12]
Arterial waveform analysis-based techniques
There are different technologies that can be used to measure the parameters for GDT-guided management. Examples include:
ClearSight/EV and 1000/Vigileo/Flotrac by Edwards Life Science are series of products introduced over a two decades' span. Clearsight is completely non-invasive and it can continuously monitor BP, CO, SV, CVV, PPV, SVR by using a digital sensor and wrist cuff[23]
The CNAP is relatively new, but its basic theory “the Peñáz principle” was described by Dr. Saugel as early as 1973 as a method to generate arterial waveform.[23] This system offers continuous non-invasive beat-by-beat recording of the arterial pressure waveform, CNAP system also uses an inflatable finger cuff, so the patient's finger artery's diameter is measured by an integrated photo-plethysmograph. The pressure needed to keep the blood volume constant corresponds to the arterial blood pressure waveform. The new CNAP algorithm also analyzes the arterial blood pressure waveforms and it can respond to any deviations from the set point.[23]
Esophageal Doppler
Transesophageal Doppler is basically measuring the thoracic aortic blood velocity to calculate SV, CO, and other hemodynamic parameters. It is relatively easy to perform and the required training is significantly less than transesophageal echocardiography.
Bioimpedance-based technologies
Thoracic Electrical Bioimpedance (TEB): TEB determines the change of impedance via delivering a low-amplitude high frequency electrical current across the thorax. The TEB electrodes are placed on the upper and lower thorax. TEB provided hemodynamic parameters are based on changes in the thoracic electrical conductivity to changes of thoracic aortic blood flow during the cardiac cycle. TEB is an alternative technique to measure SV, CO, and CI[24]
Electrical Bioreactance-based Technology: Electric bioreactance (EB) analysis is also based on alterations in frequency of electrical resistivity across the thorax. EB is significantly less susceptible to interference from chest wall movement, lung edema, and pleural effusion.[25] EB measures CO centrally. When an alternative current is applied to the thorax, the pulsatile blood flow in the large thoracic arteries induces phase shifts or time delays between the measured thoracic voltage and the applied alternative current. By continuously measuring these phase shifts, EB can determine SV and other derivative parameters such as CO, CI, SVI, and SVRI.[25]
Application of goal-directed fluid therapy and clinical outcomes
GDT has been associated with improved clinical outcomes based on some clinical investigations. Hamilton et al. conducted a meta-analysis that included 29 studies, 23 of which reported the incidence of surgical complications. The total 4805 patients enrolled in the 29 studies suffered an overall mortality of 7.6%. The use of preemptive hemodynamic intervention guided by GDT significantly decreased mortality and surgical complications.[26] They also found that subgroup analysis further showed significant reductions in mortality for investigations using a pulmonary artery catheter, supra-normal resuscitation targets, and studies using CI or oxygen delivery as goals in GDT, and the combined use of fluids and inotropes versus fluids alone. Thus, they concluded that the use of a preemptive strategy of hemodynamic monitoring and coupled GDT can reduce surgical mortality and morbidity.[26] However, there are also reports that indicated no clinical benefits from GDT. Gómez-Izquierdo et al. performed a randomized and assessor-blind controlled clinical trial (GDT vs traditional) in adult patients undergoing laparoscopic colorectal surgery. Postoperative ileus was used as the primary outcome. 128 patients were included and analyzed (both GDT group and control group enrolled 64 patients each). The incidence of primary postoperative ileus was 22% in the GDT group and 22% in the control group. Intraoperatively, patients in the GDT group received less intravenous fluids (mainly less crystalloids) but a greater volume of colloids. And GDT group had more pronounced increase of SV and CO. No difference was identified in length of hospital stay, 30-day postoperative morbidity, and mortality in the two groups. Therefore, the authors believe intraoperative GDT had no advantage over traditional fluid therapy in reducing primary postoperative ileus in patients undergoing laparoscopic colorectal surgery.[17] The additional benefit of GDT should be determined based on surgical and patient risk factors, meaning it may not be applicable to all patients undergoing surgery. GDT should not be used in isolation, instead, perioperative hemodynamic trends and the fluid priorities of the patient should always be considered. The principle behind GDT is to maximize tissue oxygen delivery by achieving a maximum hemodynamic status with the required amount of fluid therapy. In the discussion of GDT, it is essential that an individualized GDT plan includes optimization of flow-related parameters.
Conclusion
Decisions regarding fluid therapy are among the most challenging and important tasks that clinicians face on a daily basis.[27] The theory of GDT encourages clinicians to manage fluid/volume administration based on objective goals of hemodynamic parameters that are evidence based. The principle behind GDT is to maximize tissue oxygen delivery without fluid overload by achieving measurable optimal hemodynamic indices. CO will usually increase in response to a fluid challenge, which is in contrast to the historical method of predicting fluid losses based on fasting duration and insensible losses that may occur during surgery, in addition to titrating fluid administration based on static parameters such as urine output, HR, and BP. It is well established that both hypovolemia and hypervolemia are associated with postoperative morbidity.[28] Maintenance of intravascular euvolemia throughout the perioperative period is ideal. Assessment of volume status can be achieved by various techniques, including traditional measures (HR, BP, laboratory test), invasive methods like pulmonary artery catheterization, minimally invasive approaches namely, arterial waveform-based analysis, thoracic bioimpedance-based technologies, and echocardiography. ERAS Society publishes evidence-based guidelines regularly. Individual institutions can establish their own ERAS protocols based on these guidelines.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: A review. JAMA Surg. 2017;152:292–8. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 2.ERAS Compliance Group. The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: Results from an international registry. Ann Surg. 2015;261:1153–9. doi: 10.1097/SLA.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 3.Savaridas T, Serrano-Pedraza I, Khan SK, Martin K, Malviya A, Reed MR, et al. Reduced medium-term mortality following primary total hip and knee arthroplasty with an enhanced recovery program. A study of 4,500 consecutive procedures. Acta Orthop. 2013;84:40–3. doi: 10.3109/17453674.2013.771298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiele RH, Raghunathan K, Brudney CS, Lobo DN, Martin D, Senagore A, et al. American society for enhanced recovery (ASER) and perioperative quality initiative (POQI) joint consensus statement on perioperative fluid management within an enhanced recovery pathway for colorectal surgery. Perioper Med (Lond) 2016;5:24. doi: 10.1186/s13741-016-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voldby AW, Brandstrup B. Fluid therapy in the perioperative setting – A clinical review. J Intensive Care. 2016;4:27. doi: 10.1186/s40560-016-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bundgaard-Nielsen M, Jørgensen CC, Secher NH, Kehlet H. Functional intravascular volume deficit in patients before surgery. Acta Anaesthesiol Scand. 2010;54:464–9. doi: 10.1111/j.1399-6576.2009.02175.x. [DOI] [PubMed] [Google Scholar]
- 7.Macedo E, Malhotra R, Claure-Del Granado R, Fedullo P, Mehta RL. Defining urine output criterion for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2011;26:509–15. doi: 10.1093/ndt/gfq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson BL, 3rd, Davis BR, Rafferty JF, Paquette IM. Postoperative predictors of early discharge following laparoscopic segmental colectomy. Int J Colorectal Dis. 2015;30:703–6. doi: 10.1007/s00384-015-2153-6. [DOI] [PubMed] [Google Scholar]
- 9.Kaye AD. Basics of Anesthesia. Philadelphia: Elselvier; 2011. Fluid management; pp. 364–71. [Google Scholar]
- 10.Miller TE, Roche AM, Mythen M. Fluid management and goal-directed therapy as an adjunct to enhanced recovery after surgery (ERAS) Can J Anaesth. 2015;62:158–68. doi: 10.1007/s12630-014-0266-y. [DOI] [PubMed] [Google Scholar]
- 11.Kalantari K, Chang JN, Ronco C, Rosner MH. Assessment of intravascular volume status and volume responsiveness in critically ill patients. Kidney Int. 2013;83:1017–28. doi: 10.1038/ki.2012.424. [DOI] [PubMed] [Google Scholar]
- 12.Sangkum L, Liu GL, Yu L, Yan H, Kaye AD, Liu H, et al. Minimally invasive or noninvasive cardiac output measurement: An update. J Anesth. 2016;30:461–80. doi: 10.1007/s00540-016-2154-9. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Kaye AD, Venakatesh AG, Green MS, Asgarian CD, Luedi MM, et al. Enhanced recovery after cardiac surgery: An update on clinical implications. Int Anesthesiol Clin. 2017;55:148–62. doi: 10.1097/AIA.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 14.Brandstrup B, Tønnesen H, Beier-Holgersen R, Hjortsø E, Ørding H, Lindorff-Larsen K, et al. Effects of intravenous fluid restriction on postoperative complications: Comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–8. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 16.Kapoor PM, Magoon R, Rawat RS, Mehta Y, Taneja S, Ravi R, et al. Goal-directed therapy improves the outcome of high-risk cardiac patients undergoing off-pump coronary artery bypass. Ann Card Anaesth. 2017;20:83–9. doi: 10.4103/0971-9784.197842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gómez-Izquierdo JC, Trainito A, Mirzakandov D, Stein BL, Liberman S, Charlebois P, et al. Goal-directed fluid therapy does not reduce primary postoperative ileus after elective laparoscopic colorectal surgery: A randomized controlled trial. Anesthesiology. 2017;127:36–49. doi: 10.1097/ALN.0000000000001663. [DOI] [PubMed] [Google Scholar]
- 18.Bacchin MR, Ceria CM, Giannone S, Ghisi D, Stagni G, Greggi T, et al. Goal-directed fluid therapy based on stroke volume variation in patients undergoing major spine surgery in the prone position: A cohort study. Spine (Phila Pa 1976) 2016;41:E1131–7. doi: 10.1097/BRS.0000000000001601. [DOI] [PubMed] [Google Scholar]
- 19.Jain AK, Dutta A. Stroke volume variation as a guide to fluid administration in morbidly obese patients undergoing laparoscopic bariatric surgery. Obes Surg. 2010;20:709–15. doi: 10.1007/s11695-009-0070-x. [DOI] [PubMed] [Google Scholar]
- 20.Joosten A, Delaporte A, Ickx B, Touihri K, Stany I, Barvais L, et al. Crystalloid versus colloid for intraoperative goal-directed fluid therapy using a closed-loop system: A randomized, double-blinded, controlled trial in major abdominal surgery. Anesthesiology. 2018;128:55–66. doi: 10.1097/ALN.0000000000001936. [DOI] [PubMed] [Google Scholar]
- 21.Sankar J, Sankar MJ, Suresh CP, Dubey NK, Singh A. Early goal-directed therapy in pediatric septic shock: Comparison of outcomes “with” and “without” intermittent superior venacaval oxygen saturation monitoring: A prospective cohort study*. Pediatr Crit Care Med. 2014;15:e157–67. doi: 10.1097/PCC.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 22.Demirel İ, Bolat E, Altun AY, Özdemir M, Beştaş A. Efficacy of goal-directed fluid therapy via pleth variability index during laparoscopic roux-en-Y gastric bypass surgery in morbidly obese patients. Obes Surg. 2018;28:358–63. doi: 10.1007/s11695-017-2840-1. [DOI] [PubMed] [Google Scholar]
- 23.Li MQ, Yang LQ, Zhou L, Liu J, Liu H. Non-invasive cardiac output measurement: Where are we now? J Anesth Perioper Med. 2018;5:221–7. doi:10.24015/JAPM.2018.0076. [Google Scholar]
- 24.Jordan HS, Ioannidis JP, Goudas LC, Chung M, Kupelnick B, Miller K, et al. Thoracic Electrical Bioimpedance. Rockville (MD): Agency for Healthcare Research and Quality (US); 2002. [PubMed] [Google Scholar]
- 25.Kim JY, Kim BR, Lee KH, Kim KW, Kim JH, Lee SI, et al. Comparison of cardiac output derived from floTrac™/Vigileo™ and impedance cardiography during major abdominal surgery. J Int Med Res. 2013;41:1342–9. doi: 10.1177/0300060513487649. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112:1392–402. doi: 10.1213/ANE.0b013e3181eeaae5. [DOI] [PubMed] [Google Scholar]
- 27.Marik PE, Lemson J. Fluid responsiveness: An evolution of our understanding. Br J Anaesth. 2014;112:617–20. doi: 10.1093/bja/aet590. [DOI] [PubMed] [Google Scholar]
- 28.Thacker JK, Mountford WK, Ernst FR, Krukas MR, Mythen MM. Perioperative fluid utilization variability and association with outcomes: Considerations for enhanced recovery efforts in sample US surgical populations. Ann Surg. 2016;263:502–10. doi: 10.1097/SLA.0000000000001402. [DOI] [PubMed] [Google Scholar]


