Abstract
Context:
The role of saliva in detection of pregnancy has not received the due importance it deserves.
Aims:
The present paper aims at determining the role of saliva in detection of pregnancy using home-based pregnancy detection kits to provide an alternative biofluid that is more user friendly, acceptable, noninvasive, rapid, and easy for home use.
Settings and Design:
The study was conducted among 35 women visiting a gynecology clinic for confirmation of pregnancy, who gave a history of a missed menstrual cycle not more than 4 weeks.
Subjects and Methods:
A home-based pregnancy detection kit meant for urinary human chorionic gonadotropin (hCG) detection with 20 mIU specification was used in the study for estimation of salivary hCG. Routine test that was prescribed to the patient such as laboratory-based urine hCG and/or ultrasound examination was used for confirmation of pregnancy status and correlation with the saliva-based result.
Results:
A positive test was obtained in 74% participants, with a dark band in 43% participants and a light band in 31% participants. A negative result was noted in 26% participants. We observed that salivary hCG estimation showed a 77% accuracy and 23% false-negative results.
Conclusions:
An astounding 74% of participants showed a positive result with an existing pregnancy kit which indicates a strong plausibility of using salivary hCG as a biomarker for detection of pregnancy. With the findings obtained from our study, we could positively affirm that salivary hCG can be used as a potential, user friendly, and more acceptable biomarker for early detection of pregnancy.
Keywords: Human chorionic gonadotropin, pregnancy, saliva, salivary diagnostics
INTRODUCTION
Saliva is a clinically important biologic fluid that can be utilized for novel approaches in prognosis, diagnosis, monitoring, and management of patients with oral and systemic diseases. Saliva can be easily collected and stored and is ideal for early detection of disease. It is known to contain specific soluble biologic markers commonly referred to as biomarkers. The presence of multiple biomarkers makes it useful for multiplexed assays that can be developed as point-of-care devices, rapid tests, and for centralized clinical laboratory operations.[1]
The measurement beta-human chorionic gonadotropin (β-hCG) in plasma and urine are currently used in the detection of pregnancy. β-hCG levels in saliva are usually detectable at about 3–4 weeks of pregnancy and continue to increase throughout the pregnancy. However, only a few studies have been carried out to evaluate the role of saliva as a noninvasive, rapid, and more acceptable biofluid for pregnancy detection. With this objective, the present study was carried out to determine the role of saliva in the detection of pregnancy using home-based pregnancy detection kits.
SUBJECTS AND METHODS
After taking informed consent, the study was conducted among 35 women visiting a gynecology clinic for confirmation of pregnancy with a history of a missed menstrual cycle not more than 4 weeks. A home-based pregnancy detection kit meant for urinary hCG detection with 20 mIU specification was used in the study for estimation of salivary hCG. Routine test that was prescribed for the patient such as laboratory-based urine hCG and/or ultrasound examination was used for confirmation of pregnancy and for correlation with the saliva-based result.
RESULTS
All the participants evaluated were in the age group 21–27 years of age, with 77% patients in the age group of 23–26 years. About 43% participants had a history of missed period of less than or equal to 10 days, 49% between 11 and 15 days, and 9% more than 15 and 30 days [Table 1].
Table 1.
Results of the salivary hCG test
| Saliva results | Days after missed periods | |||
|---|---|---|---|---|
| ≤10 days | 11-15 days | >15 days | Total | |
| Dark Band | 7 | 8 | 0 | 15 |
| Light Band | 4 | 4 | 3 | 11 |
| Negative | 4 | 5 | 0 | 9 |
| Total | 15 | 17 | 3 | 35 |
The confirmation (presence of pregnancy) was obtained in 23% participants by an ultrasound examination and in 77% participants by a laboratory-based urine hCG level. About 74% participants with a confirmed pregnancy showed a positive result, of which 43% showed a dark band [Figure 1] and a light band was noted in 31% participants [Figure 2]. A negative result was noted in 26% participants [Chart 1]. Of the 9 subjects who showed a negative result, 8 showed a positive confirmation with a urine test and 1 was confirmed negative on further evaluation.
Figure 1.
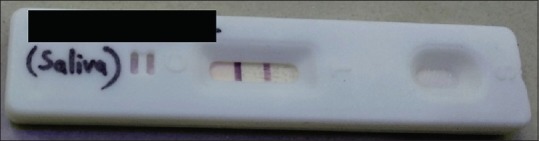
Test kit showing a dark band (positive)
Figure 2.
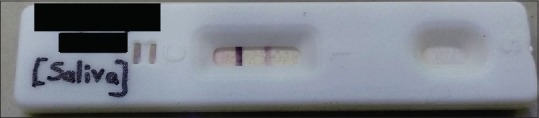
Test kit showing a light band (positive)
Chart 1.
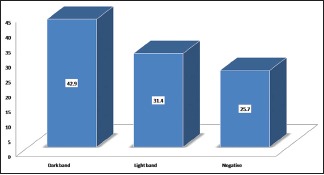
Gestational age when the salivary hCG test was performed
Hence, in the present study, we observed that salivary hCG estimation showed a 77% accuracy and 23% false-negative results.
DISCUSSION
Saliva is an exceptional biological fluid that is useful for noninvasive diagnosis and monitoring of the diseases and physiological phenomenon.[2]
In addition, it offers an edge over the other body fluids that it is a more patiently acceptable method and it may be retrieved many times a day, making repetition possible along with a very high durability. Although it mainly contains water, the other constituents include cellular elements, organic and inorganic substances, and biological markers that are also known to be present blood and urine. All these may be used in the early detection and monitoring of many diseases.[3]
Some other advantages of saliva include the characteristic that hormonal analysis of saliva is not influenced by changes in concentrations of binding globulins as free concentration of the hormones in saliva is measured.[4]
Liu et al. in their study on quantification of steroid hormones in human saliva, stated that steroid hormones follow the same metabolic pathway and therefore have an unequivocal degree of correlation with respect to their concentrations.[5]
Saibaba et al. in 2016 in their study on proteomic analysis of human saliva, an approach to find the marker protein for ovulation, reported that theirs was the first study on salivary proteins as biomarkers of ovulation in the human. They observed that of all the ovulation-specific proteins that have been found, cystatin-S was the highly expressed protein.[6]
Detection of pregnancy is based on the measurement of hCG and its variants. In a normal menstrual cycle, ovulation occurs on an average of 14 days before the next anticipated menses, and the corpus luteum then starts to develop. If the oocyte is fertilized, implantation takes place approximately during the next 7 days, resulting in an increase in the concentrations of hCG and its variants. Pregnancy tests utilizing urine or serum can detect hCG from the time of the missed menses. Hence, pregnancy tests for hCG are widespread in hospital and home settings. A urinary hCG level of 25 U/l is generally considered positive urine pregnancy test result.[7]
Based on the results of the previous studies, we intended at evaluating if saliva could be used as a potential biomarker for detection of pregnancy. In the present study, a home-based pregnancy detection kit meant for urinary hCG detection with 20 mIU specification was used in the study for estimation of salivary hCG.
Saliva is considered as an alternative matrix for monitoring of biochemical parameters. A graphene-based chemiluminescence resonance energy transfer immunoassay for the detection of hCG in the serum and saliva samples had been utilized. The results of their study indicated that hCG level could be detected in the saliva samples in the concentrations ranging from 0.3 mIU/mL to 0.8 mIU/mL. The authors hence stated that it was necessary to develop simple and sensitive analytical method for detection of hCG in saliva samples that would have widespread clinical applications.[8]
In the present study, although we used home-based pregnancy detection kit meant for urinary hCG detection, we still noted that salivary hCG estimation using these kits showed a 77% accuracy and 23% false-negative results.
It was interesting to find that 43% subjects had a history of missed period of ≤10 days, 49% between 11 and 15 days, and 8% more than 15–30 days [Table 1], and hence, the test was able to detect pregnancy status in most women at a gestational age of 2–3 weeks or lesser.
Although this is a pilot study, the results from the study are encouraging. Some of the limitations of the study were a small sample size and since the study setting was a rural one, many pregnant women were not willing for an ultrasound due to educational and financial barriers though the ultrasonography examination would have provided a more confirmatory result. With a larger sample size and more sensitive kits meant for salivary hCG, saliva has a promising potential as an early, user-friendly biomarker of pregnancy.
In the dearth of literature, it was not possible to compare the results of our study with other studies. Second, the initiative was taken by an undergraduate student, and with limited resources, the study design was a simple one. However, the idea holds promise in that a user-friendly home-based salivary hCG-based pregnancy detection kit may be developed with further research.
CONCLUSION
The results from this study, though preliminary, can go a long way in replacing the traditionally used bio fluids with more user friendly and acceptable bio fluid i.e. saliva as a biomarker for detection of pregnancy.
Though this was a pilot study and the kit used was a commercially available kit meant for urinary hCG, strong positive data obtained is encouraging and opens new avenues towards development of new saliva based diagnostic kits using salivary hCG as a diagnostic bio fluid for early detection of pregnancy. With the findings obtained from our study we could positively affirm that Salivary hCG can be used as a potential, user friendly, more acceptable home based biomarker for early detection of pregnancy
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This research was supported by the Department of Oral Pathology, School of Dental Sciences, Krishna Institute of Medical Sciences “Deemed to be University,” Karad.
REFERENCES
- 1.Malamud D. Saliva as a diagnostic fluid. Dent Clin North Am. 2011;55:159–78. doi: 10.1016/j.cden.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong DT. Towards a simple, saliva-based test for the detection of oral cancer ‘oral fluid (saliva), which is the mirror of the body, is a perfect medium to be explored for health and disease surveillance’. Expert Rev Mol Diagn. 2006;6:267–72. doi: 10.1586/14737159.6.3.267. [DOI] [PubMed] [Google Scholar]
- 3.Chojnowska S, Baran T, Wilińska I, Sienicka P, Cabaj-Wiater I, Knaś M. Human saliva as a diagnostic material. Adv Med Sci. 2018;63:185–91. doi: 10.1016/j.advms.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Langelaan ML, Kisters JM, Oosterwerff MM, Boer AK. Salivary cortisol in the diagnosis of adrenal insufficiency: Cost efficient and patient friendly. Endocr Connect. 2018;7:560–6. doi: 10.1530/EC-18-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Qiu X, Wang D, Li Y, Zong Y, Liu Y, et al. Quantification of 10 steroid hormones in human saliva from Chinese adult volunteers. J Int Med Res. 2018;46:1414–27. doi: 10.1177/0300060517752733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saibaba G, Rajesh D, Muthukumar S, Sathiyanarayanan G, Padmanabhan P, Akbarsha MA, et al. Proteomic analysis of human saliva: An approach to find the marker protein for ovulation. Reprod Biol. 2016;16:287–94. doi: 10.1016/j.repbio.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Berger P, Sturgeon C. Pregnancy testing with hCG – Future prospects. Trends Endocrinol Metab. 2014;25:637–48. doi: 10.1016/j.tem.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Lei J, Jing T, Zhou T, Zhou Y, Wu W, Mei S, et al. A simple and sensitive immunoassay for the determination of human chorionic gonadotropin by graphene-based chemiluminescence resonance energy transfer. Biosens Bioelectron. 2014;54:72–7. doi: 10.1016/j.bios.2013.10.033. [DOI] [PubMed] [Google Scholar]


