Abstract
Atrial fibrillation (AF) is a leading preventable cause of ischemic stroke for which early detection and treatment are critical. The risk of stroke in people with AF can be stratified by the use of such validated prediction instruments such as CHADS2 or CHA2 DS2–VASc. The CHA2 DS2–VASc adds to the evaluation of the risk of stroke by reliably identifying patients at very low risk. Additional points are assigned for an additional age category of 65–74 years (1 point), female sex (1 point), and vascular disease other than cerebrovascular disease (1 point). Two points are awarded for age ≥75 years. The risk of stroke increases according to point score: 0.5% per year (0 points), 1.5% per year (1 point), 2.5% per year (2 points), 5% per year (3 points), 6% per year (4 points), and 7% per year (5–6 points). For decades, Vitamin K antagonists were the only class of oral anticoagulants available to clinicians for the prevention of stroke in AF. However, new oral anticoagulants (NOACs), such as apixaban, dabigatran, and rivaroxaban, are currently available and have proved to be safe and effective in preventing stroke in patients with nonvalvular AF. In addition, a nonpharmacologic procedure like left atrial appendage occlusion is a possible option in selected patients. In this article, we have reviewed the stratification of stroke risk in AF, prevention of stroke in nonvalvular AF, warfarin versus NOACs, weighting risk of bleeding versus stroke risk when deciding on the anticoagulation protocol in patients with AF, and the use of nonpharmacologic therapy for stroke prevention.
Keywords: Atrial fibrillation, prevention, stroke risk stratification
Introduction
Atrial fibrillation (AF) is estimated to affect 33 million people worldwide.[1,2] This number is likely to be an underestimate since many people do not know that they have AF until they develop symptoms or present with an ischemic thromboembolic stroke or systemic thromboembolism. A rising prevalence is related to an increase in elderly population and the increased prevalence of risk factors, such as diabetes, hypertension, obesity, and alcohol consumption.[3]
AF is one of the ten potentially modifiable risk factors associated with acute stroke.[3] The risk of stroke in patients with AF has been estimated as between 1% and 20% annually.[4,5] In the United States, this arrhythmia may be responsible for >70,000 ischemic strokes each year representing 10%–12% of all ischemic strokes.[6,7] Accordingly, risk stratification for the risk of stroke in AF and early initiation of therapy that aims to reduce the risk of AF-associated stroke is a crucial component in the management of this arrhythmia.
Stratification of Stroke Risk in Atrial Fibrillation
The risk of stroke in people with AF can be stratified by the use of validated prediction scores such as CHADS2 or CHA2 DS2–VASc.[8,9] All available scores use selected clinical characteristics to predict the risk of stroke and provide a rough estimate of the risk of thrombosis in a population at similar risk as patients are reviewed.
CHADS2 is the most widely used scoring system for stratifying the risk of stroke in AF.[8] In CHADS2, the risk of stroke is assessed by means of a scoring system that awards points for congestive heart failure (1 point), hypertension (1 point), age ≥75 years (1 point), diabetes mellitus (1 point), and prior stroke or transient ischemic attack (2 points) [Table 1]. The risk of stroke increases according to point score per year: 1.9% (0 points), 2.8% (1 point), 4.0% (2 points), 5.9% (3 points), 8.5% (4 points), 12.5% (5 points), and 18.2% (6 points).[8] Based on CHADS2 cumulative score, the risk of stroke is divided into three strata: low-risk patients with a score of 0, moderate-risk patients with scores of 1–2, and high-risk patients with scores of 3–6 [Table 2].[9]
Table 1.
Components of the CHADS2 and CHA2DS2-VASc scores that are used to assess the risk of stroke in atrial fibrillation and guide the decision of anticoagulation therapy
| Condition | CHADS2 | Points | CHA2DS2-VASc | Points |
|---|---|---|---|---|
| Congestive heart failure (or left ventricular systolic dysfunction) | C | 1 | C | 1 |
| Hypertension (BP above 140/90 or treated hypertension on medication) | H | 1 | H | 1 |
| Age >75 | A | 1 | A2 | 2 |
| Diabetes mellitus | D | 1 | D | 1 |
| Stroke or TIA or thromboembolism in history | S2 | 2 | S2 | 2 |
| Vascular disease (peripheral vascular disease, myocardial infarction, and aortic plaque) | V | 1 | ||
| Age >65 | A | 1 | ||
| Sex (Female) | SC | 1 |
AF=Atrial fibrillation, BP=Blood pressure, TIA=Transient ischemic attack
Table 2.
Stroke risk according to CHADS2 and CHA2DS2-VASc stratification
| Stroke risk Category | CHADS2 score | 1-year event rate (%) | CHA2DS2-VASc | 1-year event rate (%) |
|---|---|---|---|---|
| Low risk | 0 | 1.67 | 0 | 0.78 |
| Intermediate risk | 1-2 | 4.75 | 1 | 2.0 |
| High risk | 3-6 | 12.3 | 2-9 | 8.8 |
The CHA2 DS2–VASc is the most recent risk stratification score. It improves the risk stratification in patients with CHADS2= 0 or 1 and allows for identification of truly low-risk patients. Additional points are assigned for an additional age category of 65–74 years (1 point), female sex (1 point), and vascular disease other than cerebrovascular disease (1 point). Two points are awarded for age ≥75 years [Table 1]. The risk of stroke increases according to point score per year: 0.5% (0 points), 1.5% (1 point), 2.5% (2 points), 5% (3 points), 6% (4 points), and 7% (5–6 points).[9]
By being more inclusive of risk factors, CHA2 DS2–VASc help identify truly low risk patients with score of 0 who who do not require antithrombotics while for all other patients ((CHA2DS2–VASc ≥1) oral anticoagulation (OAC) therapy should be considered. It is hoped that these improvements will lead to the appropriate use of OAC for the treatment of more patients, resulting in fewer strokes over time.
For practical reasons, CHA2 DS2–VASc should be used in conjunction with CHADS2. Patients with CHADS2≥2 should clearly be considered high risk for stroke. However, for patients with a CHADS2 of 0–1, it is argued that there is a need to refine the patients' risk using CHA2 DS2–VASc. The overall risk of stroke can be stratified into low, intermediate, or high according to the cumulative points from CHADS2 or CHA2 DS2–VASc [Table 2] which helps in guiding the decision of the strategy of stroke prevention.
Stroke Prevention in Nonvalvular Atrial Fibrillation
The prevention of stroke related to AF is a global public health priority. Strokes due to AF are common and associated with very poor outcome (70%–80% of patients die or become disabled).[10,11] Proper risk stratification and early initiation of appropriate preventive therapy result in a significant reduction of AF-related ischemic strokes and its associated mortality.[12]
Antiplatelet therapy with aspirin or clopidogrel has been indicated in patients with nonvalvular AF categorized as low risk for ischemic stroke according to CHA2 DS2–VASc [Table 3]. Aspirin therapy, according to data from three trials, resulted in an estimated relative risk reduction of 21% compared with placebo (95% confidence interval [CI], 0%–38%).[13] The largest aspirin effect was observed in the Stroke Prevention in Atrial Fibrillation (SPAF-1) trial, which used aspirin 325 mg/d. However, based on the results of studies done in multiple vascular indications, the best balance of efficacy and safety of aspirin appears to be low dose at 75–100 mg/d.[13]
Table 3.
Stroke risk category according to CHA2DS2-VASc and recommended stroke preventive therapy
| Risk category | CHA2DS2-VASc score | Recommended antithrombotic therapy |
|---|---|---|
| One “major” risk factor or >2 “clinically relevant nonmajor” risk factors | >2 | OAC |
| One “clinically relevant nonmajor” risk factor | 1 | Either OAC or aspirin 75-325 mg daily Preferred: OAC rather than aspirin |
| No risk factors | 0 | Either aspirin 75-325 mg daily or no antithrombotic therapyPreferred: No antithrombotic therapy rather than aspirin |
OAC=Oral anticoagulation
Multiple clinical trials have demonstrated the superior therapeutic effect of warfarin compared with antiplatelets in patients with nonvalvular AF.[12] Therefore, in patients with intermediate and high stroke risk according to CHA2 DS2–VASc, anticoagulation with warfarin is the preferred therapy for stroke prevention.[14,15,16,17] An analysis of pooled data from five primary prevention trials demonstrated a relative risk reduction of 68% (95% CI, 50%–79%) and an absolute reduction in annual stroke rate from 4.5% for control patients to 1.4% in patients assigned to adjusted-dose warfarin.[17]
The new oral anticoagulants (NOACs) such as dabigatran, apixaban, or rivaroxaban have shown to be equivalent to warfarin in the prevention of ischemic stroke in nonvalvular AF and might have a better adherence profile.[18,19,20] The direct thrombin inhibitor, dabigatran etexilate, is one of the NOACs that represent a prodrug of dabigatran, which reversibly inhibits the active site of thrombin with an oral bioavailability of 6%.[21]
Dabigatran has a half-life of 14–17 h, which permits once- or twice-daily administration, and 80% of the drugs are excreted unchanged by the kidneys. It has been evaluated for the prevention of stroke in patients with AF in the RE-LY trial.[18] The RE-LY trial investigators randomized 18,113 patients with AF and at least one additional risk factor for stroke to receive dabigatran etexilate (at doses of 110 or 150 mg twice daily) or warfarin, which was adjusted to achieve an international normalized ratio (INR) of 2 to 3. Rates of the primary efficacy outcome, stroke, or systemic embolism were 1.69%/year in the warfarin group, 1.53%/year in the group that received 110 mg of dabigatran (P < 0.001 for noninferiority), and 1.11%/year in the group given 150 mg of dabigatran (P < 0.001 for both noninferiority and superiority).
Rivaroxaban (Xarelto) is a direct coagulation factor Xa inhibitor that reversibly binds to the active site of coagulation factor Xa without antithrombin mediation affecting free and platelet-bound factor Xa. The rivaroxaban once-daily oral direct factor Xa inhibition compared with Vitamin K antagonism for the prevention of stroke and embolism trial in AF (ROCKET AF) study evaluated rivaroxaban for the prevention of stroke or systemic embolization in patients with nonvalvular AF (intermediate to high risk of stroke).[19] Patients were randomly assigned to receive either rivaroxaban 20 mg/day (15 mg/d in patients with CrCl of 30–49 mL/min per 1.73 m2) or warfarin (target INR, 2.0–3.0). Rivaroxaban was noninferior to warfarin (2.1% vs. 2.4% per year; P < 0.001 for noninferiority) in the intention-to-treat analysis for the primary efficacy endpoint of stroke and systemic embolism. There was no difference between patients taking rivaroxaban and those taking warfarin in terms of all bleeding events (14.9% vs. 14.5% per 100 patient-years; P1/4.44) and major bleeding events (3.6% vs. 3.4% per 100 patient-years; P1/4.58).
Apixaban, another NOAC, which works as a direct coagulation factor Xa inhibitor is absorbed rapidly, and maximal plasma concentrations are achieved 3 h after oral administration with a terminal half-life of 8–14 h.[19] Elimination of apixaban involves multiple pathways, including hepatic, renal, and intestinal routes.[19] Efficacy of apixaban in SPAF-1 patients was tested in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial.[20] In this trial, 18,201 patients with nonvalvular AF and at least one other stroke risk factor were randomized to apixaban 5 mg twice daily or adjusted-dose warfarin.[20] After a median follow-up of 1.8 years, the primary outcome of ischemic stroke, hemorrhagic stroke, or systemic embolism occurred in 212 patients assigned to apixaban compared with 265 assigned to warfarin (Hazard Ratio with apixaban, 0.79; 95% CI, 0.66–0.95; P < 0.001 for noninferiority and P = 0.01 for superiority).[20]
Warfarin Versus New Oral Anticoagulants
The narrow therapeutic margin and drug or food interactions of warfarin with requirements of frequent INR testing and dose adjustments represent major limitations of the use of warfarin [Table 4]. To overcome the limitations of warfarin, several NOACs have been developed, including direct thrombin inhibitors and factor Xa inhibitors as discussed above. Evidence from multiple clinical trials has led to the replacement of warfarin with the NOACs in stroke prevention in nonvalvular AF.[18,19,20,21,22]
Table 4.
Major differences of warfarin and new oral anticoagulants and clinical implications of such differences
| Characteristics | Warfarin | NOACs | Clinical implication |
|---|---|---|---|
| Onset and offset of action | Slow onset and offset of action | Rapid onset of action | Warfarin need bridging with a rapidly acting anticoagulant |
| Therapeutic window | Narrow therapeutic index | Wide therapeutic index | Need for routine coagulation monitoring with warfarin but not with NOACs |
| Food and drug interactions | Frequent food and drug interactions | No food and drug interactions | Dietary precautions with warfarin and need for frequent coagulation monitoring but not with NOACs |
| Variability in anticoagulant effect | Inter individual variability in anticoagulant effect | Predictable anticoagulant effect | Variability in dosing requirements for warfarin compared to relatively fixed stable dosing for NOACs |
NOACs=New oral anticoagulants
The currently unique contraindications to NOACs are patients with mechanical heart valves and those with moderate-to-severe mitral stenosis (valvular AF). This is supported by the Phase II trial of dabigatran etexilate against warfarin treatment, which was stopped prematurely because of increased rates of thromboembolism as well as increased bleeding associated with dabigatran.[23]
Unlike warfarin, for which Vitamin K and fresh frozen plasma may be used to reverse anticoagulation during acute bleeding, no similar antidotes are available for the newer oral anticoagulants with the exception of dabigatran. However, the short half-life of these agents offers some protection that limits the need for antidote.
Another contraindication to the use of NOACs for SPAF-1 is advanced renal failure with CrCl <15 mL/min per 1.73 m2.[24]
Milder renal failure will need caution and adjustment of the NOAC dosing, which is variable from one agent to another.[24,25] For example, in patients with CrCl of 15–30 mL/min per 1.73 m2, the recommended dose of dabigatran is 75 mg twice daily, apixaban is 2.5 mg twice daily, and rivaroxaban is15 mg once daily.[25]
Weighting the Risk of Bleeding Versus Stroke Risk When Determining the Anticoagulation Protocol in a Patient With Atrial Fibrillation
Preventing ischemic stroke in patients with AF is a major therapeutic goal. The achievement of this goal should be balanced with the risk of bleeding that can complicate the use of anticoagulation therapy. The underlisted factors have emerged as a result of multiple systematic reviews. The strongest risk factors for developing a bleed while on OAC are:
Age (>75 years)
Concomitant use of antiplatelet drugs (such as aspirin, clopidogrel, prasugrel, or nonsteroidal anti-inflammatory agents)
Uncontrolled hypertension
History of previous bleeding episode or bleeding disorders
Poorly controlled anticoagulation therapy.
HAS-BLED is one of the validated risk scoring systems used to assess the risk of bleeding in patients receiving OAC.[26] The acronym HAS-BLED represents each of the bleeding risk factors and assigns one point for the presence of each of the following: hypertension (uncontrolled systolic blood pressure, 160 mmHg), abnormal renal and/or liver function, previous stroke, bleeding history or predisposition, labile INRs, elderly, and concomitant drugs and/or excessive use of alcohol [Table 5]. HAS-BLED patients are categorized into low, moderate, or high bleeding risk [Table 5]. A HAS-BLED score of >3 (high bleeding risk) indicates that caution is necessary when prescribing OAC and regular review is recommended.[27] The use of the HAS-BLED score can help to identify modifiable bleeding risks that need to be addressed but should not be used on its own to exclude patients from anticoagulation therapy.[28]
Table 5.
HAS-BLED score for bleeding risk on oral anticoagulation in atrial fibrillation*
| Condition | Points |
|---|---|
| Hypertension (systolic ≥160 mmHg) | 1 |
| Abnormal renal function | 1 |
| Abnormal liver function | 1 |
| Previous stroke | 1 |
| Bleeding | 1 |
| Labile INRs | 1 |
| Elderly ≥65 years | 1 |
| Taking other drugs as well | 1 |
*Risk categories: 0 low risk; 1-3 moderate risk; >3 high risk. INRs=International normalized ratios
Nonpharmacologic Option for Stroke Prevention in Atrial Fibrillation
Systemic anticoagulation provides effective reduction in the risk of stroke in AF patients. However, it has several drawbacks, including inconvenience and cost associated with daily administration of medication, the need for frequent blood tests and dietary restrictions in patients treated with warfarin, the increased risk of bleeding complications, and the difficulty in the management of anticoagulants in the setting of elective or nonelective invasive/surgical procedures or trauma. As a result of these limitations, nonpharmacologic option such as left atrial appendage (LAA) occlusion has been suggested as a possible alternative for SPAF-1 with the approval of the first device (Watchman, Boston Scientific) by the United States Food and Drug Administration in 2015 [Figure 1].
Figure 1.
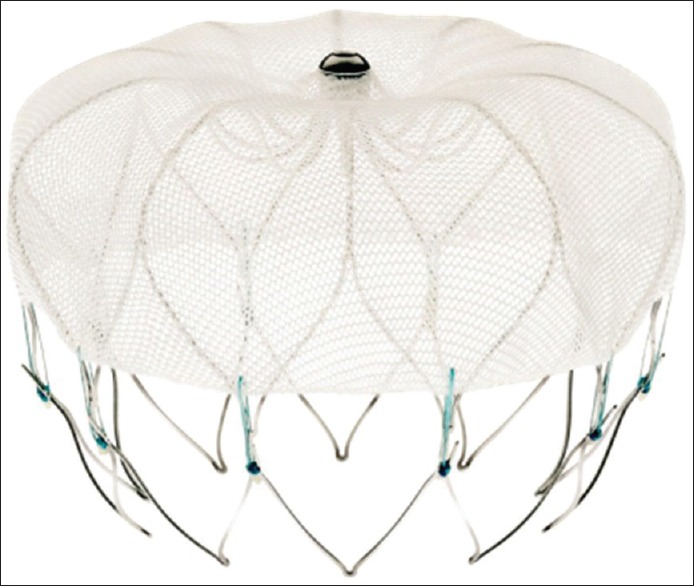
The Watchman device for the left atrial appendage occlusion, the polyester fabric covers the proximal 50% of the devise depth and is designed to prevent thrombi from embolizing out of the left atrial appendage with the anchors seen on the bottom (Image reproduced with permission from Boston Scientific)
The first randomized trial evaluating the Watchman device was the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation).[29] The composite primary endpoint consisted of stroke, systemic embolism, and cardiovascular death that occurred in 3.0 per 100 patient-years in the Watchman group as compared to 4.3 in the warfarin group that meets the noninferiority endpoint. However, in this study, the safety endpoints of life-threatening bleeding and significant bleeding as well as procedure-related complications presented a significantly higher risk (7.7%) in the Watchman group.[29] In another randomized trial, PREVAIL study (Evaluation of the Watchman LAA Closure Device in Patients with Atrial Fibrillation Versus Long Term Warfarin Therapy), randomized patients with CHADS score of ≥2 or 1 with one additional risk factor in a 2:1 fashion to the implant of a device or continuation of warfarin therapy, the primary endpoints consisted of stroke, systemic embolism, or cardiovascular death/unexplained death and was not met at 18 months.[30] However, the second endpoint of stroke or systemic embolism >7 days postrandomization met noninferiority. The procedure-related complications occurred in 4.5% of Watchman patients in PREVAIL as compared to 8.7% in PROTECT AF. In summary, LAA occlusion offers a well-established and cost-effective mechanical option for stroke prevention in patients with nonvalvular AF who cannot tolerate long-term OAC.[31,32]
Conclusion
AF is a very common cardiac arrhythmia with significant cardiovascular morbidity and mortality. The associated risk of embolic events, particularly embolic cerebrovascular accidents, is its most serious complication. Careful risk stratification and estimation of the risk of stroke using CHADS2 or CHA2 DS2–VASc can help to identify the high-risk patients who will benefit from OAC. The NOACs are preferable for stroke prevention in nonvalvular AF while warfarin is still the best option in valvular AF. The risk of bleeding should be assessed in every patient with AF prior to initiating anticoagulation to help guide the appropriate determination of the method of stroke prevention and avoid bleeding complications. LAA appendage occlusion has emerged as an aid in reducing embolic complications in AF who cannot tolerate OAC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 study. Circulation. 2014;129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics-2014 update: A report from the American Heart Association. Circulation. 2014;129:e28–92. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): A case-control study. Lancet. 2016;388:761–75. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 4.Furie KL, Goldstein LB, Albers GW, Khatri P, Neyens R, Turakhia MP, et al. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: A science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:3442–53. doi: 10.1161/STR.0b013e318266722a. [DOI] [PubMed] [Google Scholar]
- 5.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ, et al. Validation of clinical classification schemes for predicting stroke: Results from the national registry of atrial fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 6.Flint AC, Banki NM, Ren X, Rao VA, Go AS. Detection of paroxysmal atrial fibrillation by 30-day event monitoring in cryptogenic ischemic stroke: The stroke and monitoring for PAF in real time (SMART) registry. Stroke. 2012;43:2788–90. doi: 10.1161/STROKEAHA.112.665844. [DOI] [PubMed] [Google Scholar]
- 7.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham study. Stroke. 1991;22:983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 8.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 9.Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: Nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gladstone DJ, Bui E, Fang J, Laupacis A, Lindsay MP, Tu JV, et al. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke. 2009;40:235–40. doi: 10.1161/STROKEAHA.108.516344. [DOI] [PubMed] [Google Scholar]
- 11.Saposnik G, Gladstone D, Raptis R, Zhou L, Hart RG. Investigators of the Registry of the Canadian Stroke Network (RCSN) and the Stroke Outcomes Research Canada (SORCan) Working Group. Atrial fibrillation in ischemic stroke: Predicting response to thrombolysis and clinical outcomes. Stroke. 2013;44:99–104. doi: 10.1161/STROKEAHA.112.676551. [DOI] [PubMed] [Google Scholar]
- 12.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 13.Singer DE, Albers GW, Dalen JE, Fang MC, Go AS, Halperin JL, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition) Chest. 2008;133:546S–92S. doi: 10.1378/chest.08-0678. [DOI] [PubMed] [Google Scholar]
- 14.Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med. 1996;335:540–6. doi: 10.1056/NEJM199608223350802. [DOI] [PubMed] [Google Scholar]
- 15.Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke prevention in atrial fibrillation III randomised clinical trial. Lancet. 1996;348:633–8. [PubMed] [Google Scholar]
- 16.van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ. Effect of study setting on anticoagulation control: A systematic review and metaregression. Chest. 2006;129:1155–66. doi: 10.1378/chest.129.5.1155. [DOI] [PubMed] [Google Scholar]
- 17.Laupacis A, Boysen G, Connolly S, Ezekowitz MD, Hart H, James KE, et al. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–57. [PubMed] [Google Scholar]
- 18.Ezekowitz MD, Connolly S, Parekh A, Reilly PA, Varrone J, Wang S, et al. Rationale and design of RE-LY: Randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157:805–10. 810.e1–2. doi: 10.1016/j.ahj.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 20.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor Xa inhibitors in development. Clin Pharmacokinet. 2009;48:1–22. doi: 10.2165/0003088-200948010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Fox KA, Piccini JP, Wojdyla D, Becker RC, Halperin JL, Nessel CC, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32:2387–94. doi: 10.1093/eurheartj/ehr342. [DOI] [PubMed] [Google Scholar]
- 23.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 24.Harel Z, Sholzberg M, Shah PS, Pavenski K, Harel S, Wald R, et al. Comparisons between novel oral anticoagulants and vitamin K antagonists in patients with CKD. J Am Soc Nephrol. 2014;25:431–42. doi: 10.1681/ASN.2013040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andò G, Capranzano P. Non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with chronic kidney disease: A systematic review and network meta-analysis. Int J Cardiol. 2017;231:162–9. doi: 10.1016/j.ijcard.2016.11.303. [DOI] [PubMed] [Google Scholar]
- 26.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: The HAS-BLED (Hypertension, abnormal renal/Liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/Alcohol concomitantly) score. J Am Coll Cardiol. 2011;57:173–80. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: The Swedish atrial fibrillation cohort study. Eur Heart J. 2012;33:1500–10. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 28.Olesen JB, Lip GY, Lindhardsen J, Lane DA, Ahlehoff O, Hansen ML, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: A net clinical benefit analysis using a 'real world' nationwide cohort study. Thromb Haemost. 2011;106:739–49. doi: 10.1160/TH11-05-0364. [DOI] [PubMed] [Google Scholar]
- 29.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet. 2009;374:534–42. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 30.Holmes DR, Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, et al. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J Am Coll Cardiol. 2014;64:1–2. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 31.Reddy VY, Akehurst RL, Armstrong SO, Amorosi SL, Beard SM, Holmes DR, Jr, et al. Time to cost-effectiveness following stroke reduction strategies in AF: Warfarin versus NOACs versus LAA closure. J Am Coll Cardiol. 2015;66:2728–39. doi: 10.1016/j.jacc.2015.09.084. [DOI] [PubMed] [Google Scholar]
- 32.Boersma LV, Ince H, Kische S, Pokushalov E, Schmitz T, Schmidt B, et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-year follow-up outcome data of the EWOLUTION trial. Heart Rhythm. 2017;14:1302–8. doi: 10.1016/j.hrthm.2017.05.038. [DOI] [PubMed] [Google Scholar]


