Abstract
Background:
There is a need to develop and periodically evaluate new treatment strategies in major depression due to the high burden of nonresponse and inadequate response to antidepressants.
Aim:
We aimed to assess the effect of vitamin D supplementation on depression symptom scores among individuals with clinically diagnosed major depression.
Materials and Methods:
Electronic search of databases was carried out for published randomized controlled trials in English language, peer-reviewed journals from inception till August 2017. Outcome measure used for effect size calculation was depression symptom scores. Effect sizes for the trials were computed using standardized mean difference (Cohen's d), and I2 test was used to assess sample heterogeneity. Pooled mean effect sizes were derived using both fixed and random-effects model. Critical appraisal of studies was done using the Cochrane risk of bias assessment tool.
Results:
A total of four trials involving 948 participants were included in the study. In three trials, the intervention group received oral vitamin D supplementation whereas in one parenteral vitamin D was given. Pooled mean effect size for vitamin D supplementation on depressive symptom ratings in major depression was 0.58 (95% confidence interval, 0.45–0.72). The I2 value for heterogeneity was 0 suggesting low heterogeneity among studies. Egger plot intercept indicated minimal publication bias.
Conclusion:
Vitamin D supplementation favorably impacted depression ratings in major depression with a moderate effect size. These findings must be considered tentative owing to the limited number of trials available and inherent methodological bias noted in few of them.
KEY WORDS: Depressive disorder, meta-analysis, randomized controlled trials, vitamin D
Introduction
Worldwide, it is estimated that more than 300 million people are affected by depression.[1] Unipolar depressive disorders were the third leading cause of disability-adjusted life years (DALY) lost based on the WHO report of 2004,[2] and were projected to rise to the second leading cause of DALY across all age group by 2020.[3] Despite burgeoning research on its neurobiological basis in the last decade, a significant gap exists in our understanding of the origins and progression of depressive disorders. Studies have suggested that depression may have multifactorial origins involving dysfunction of multiple brain areas and alteration in many biochemical functions such as gene expression and immune response.[4,5] However, classical antidepressants mainly function by correction of monoamine imbalance. This may be a possible reason for the low rates of treatment-induced remission often noted in clinical trials on depressed patients.[6,7] Therefore, there is a pressing need to look at other treatment targets that can optimize clinical management of depression.
A growing body of literature links vitamin D to the pathophysiology of depression.[8,9,10,11] This has mainly come from three lines of evidence; first, lower serum vitamin D levels in depressed persons compared to controls;[8,10,12,13] second, presence of vitamin D receptors in various parts of the brain limbic system, cerebellum, and cortex,[14,15,16] which controls emotions and behavior; and third, the important modulatory role that vitamin D plays in regulating immunoinflammatory pathways that have been found to be relevant to the pathophysiology of depression.[5,17,18] This evidence has spawned a series of trials that have tried to answer the question whether vitamin D supplementation among depressed patients might improve their depression scores with mixed results. Thus far, a few meta-analyses have included studies assessing the effect of vitamin D on depressive symptom scores in individuals without a clinical diagnosis of major depression.[19,20,21,22,23] Further, as the authors of these studies point out, many of the included patients had very low depression scores to begin with which may be the reason for the marginal effects noted. Hence, there is a need to separately examine the effects of vitamin D supplementation in populations with major depression.
With this background, we aimed to combine the evidence from available randomized trials to provide a quantitative estimate of the effect of vitamin D supplementation on depression symptom ratings among patients with clinically diagnosed depression compared to a control group. We also aimed to highlight the research gaps in this area to inform future trials.
Materials and Methods
Inclusion and exclusion criteria
Using the patient, intervention, comparison, outcomes, and study design criteria, all studies that assessed the association between vitamin D and depression or vice versa were included provided they met the following a priori criteria:
Population – Patients clinically diagnosed with unipolar depression as per the standard diagnostic criteria
Intervention – Vitamin D supplementation in any dosage formulation
Comparison – With a control group of any nature
Outcomes – Primary outcome expressed in terms of depression symptom scores
We included only randomized controlled trials meeting the above criteria [Table 1]. Studies done on healthy volunteers or nonpsychiatric populations were excluded.[8,24,25,26,27] The excluded studies with relevant details are given in Table 2.
Table 1.
Characteristics of included studies (n=4)
| Study | Region | Type of RCT | Sample size (intervention vs control) | Intervention | Comparator | Duration of study | Primary Outcome measure for effect size calculation | Effect size (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Wang et al.,[35] 2016 | China | Double blind | 746 (373 vs 373) | 50,000 IU/wk oral Vitamin D3 | Placebo | 52 weeks | BDI score | 0.5632 (0.4167-0.7097) |
| Sepehrmanesh et al.,[34] 2016 | Iran | Double blind | 40 (20 vs 20) | 50,000 IU/wk oral Vitamin D3 | Placebo | 8 weeks | BDI score | 0.4876 (−0.1465-1.1217) |
| Mozaffari-Khosravi et al.,[32] 2013 | Iran | Nonblinded | 120 (40 vs 40 vs 40) | 300,000/150,000 IU I.M single dose Vitamin D3 | No treatment | 12 weeks | BDI score | 0.6988 (0.2437-1.1539) |
| Khoraminya et al.,[33] 2013 | Iran | Double blind | 42 (21 vs 21) | 1500 IU oral Vitamin D3 + 20 mg Fluoxetine daily | Fluoxetine alone | 8 weeks | HAM-D score | 1.0268 (0.1294-1.9242) |
RCT: Randomized controlled trial, CI: Confidence intervals, BDI: Beck depression inventory, HAM-D: Hamilton depression rating scale, IU: International units
Table 2.
Characteristics of excluded studies (n=13)
| Study | Region | Type of trial | Sample size | Intervention | Comparator | Comments |
|---|---|---|---|---|---|---|
| Hogberg et al.,[8] 2012 | Sweden | Case series | 48 | Daily dosage of oral Vitamin D3 | None | Depressed adolescents were investigated for vitamin D deficiency. Those found deficient were supplemented by vitamin D |
| Stokes et al.,[27] 2015 | Germany | Cross-sectional & interventional analysis. | 111 | 20000 IU/week oral Vitamin D3 | None | Patients with chronic liver disease were assessed for vitamin D deficiency and depressive symptoms. Depressive symptoms were reassessed after vitamin D supplementation |
| Shipowick et al.,[49] 2009 | USA | Prospective interventional analysis | 9 | Oral vitamin D3 | None | A pilot study to assess effect of vitamin D supplementation on depressive symptoms in women during winter |
| Bertone Johnson et al.,[26] 2012 | USA | Double blinded RCT | 36282 | 400 IU/day oral Vitamin D3 | Placebo | Improvement in depression scores in postmenopausal women with vitamin D supplementation were assessed |
| Jorde et al.,[24] 2008 | Norway | Double blind RCT | 441 | 20000 vs 40000 IU/week oral vitamin D3 | Placebo | Improvement in depressive symptom scores after vitamin D supplementation |
| Yalamanchi et al.,[25] 2012 | USA | Double blind RCT | 488 | Hormone therapy, Oral Vitamin D3 | Placebo | Examine the effect of hormone therapy and calcitriol on depression scores in older postmenopausal women and to determine whether the response was associated with polymorphisms of estrogen receptor > and vitamin D receptor |
| Vaziri et al.,[50] 2016 | Iran | RCT | 169 | 2000 IU/day oral vitamin D3 | None | Effect of vitamin D supplementation on depressive symptoms in pregnant women were examined |
| Mousa et al.,[51] 2017 | Australia | Double blind RCT | 63 | Oral vitamin D3 | Placebo | Whether vitamin D levels were associated with depressive symptoms and whether supplementation reduced depressive symptoms who are obese and vitamin D deficient, but otherwise healthy i.e., Not clinically depressed |
| Kjaergaard et al,[13] 2012 | Norway | Case-control study & RCT | 243 | Oral vitamin D3 | Placebo | Effect of vitamin D supplement on depression scores in people with low vitamin D levels were evaluated |
| Frandsen et al.,[52] 2017 | Denmark | Double blind RCT | 34 | 70 microgram oral vitamin D3 daily | Placebo | Vitamin D supplementation was evaluated for treatment of seasonal affective symptoms in healthcare professionals |
| Zanetidou et al.,[53] 2011 | Italy | Non-randomized trial | 39 | 300000 IU oral vitamin D along with Anti-depressants | Routine anti-depressants | Vitamin D was additionally given to the routine anti-depressants in the study group to compare those on only antidepressants who were not willing to take oral vitamin D |
| Marsh et al.,[54] 2017 | USA | Double blind RCT | 33 | 5000 IU vitamin D/day | Placebo | Patients with DSM IV bipolar depression and vitamin D deficiency were selected and randomized to give vitamin D to evaluate whether vitamin D adjunct reduces bipolar depression |
| de Koning et al.,[55] 2015 | Netherlands | Double blind RCT | 155 | 1200 IU vitamin D/day | Placebo | Study aims to elucidate effects of vitamin D supplementation on depressive symptoms and physical functioning in older adults |
RCT: Randomized controlled trial, DSM IV: Diagnostic and Statistical Manual of Mental Disorders, IU: International units
Search strategy and study selection
Electronic searches of database such as PubMed, ScienceDirect, and Google Scholar were carried out from inception till August 2017. Articles were generated using random combinations of the following search terms: “depression,” “depressive disorder,” “depressed individuals,” “vitamin D,” “vitamin d,” “25-hydroxyvitamin D,” “vitamin D3,” “Cholecalciferol,” or “Calcitriol.” The search was limited to articles published in English language, peer reviewed journals. The title and abstracts of the generated studies were examined by two of the authors independently (Vikas Menon and Favaz V), and a consolidated list of abstracts were drawn up after eliminating duplicates. In case of inadequate information in the abstract, the corresponding full texts of potentially relevant articles were retrieved to screen for inclusion criteria. The reference lists of included studies were also manually examined for potential articles. We did not include gray literature such as conference proceedings primarily due to concerns about study quality and inadequate reporting. The flow chart for the literature search is presented in Figure 1.
Figure 1.
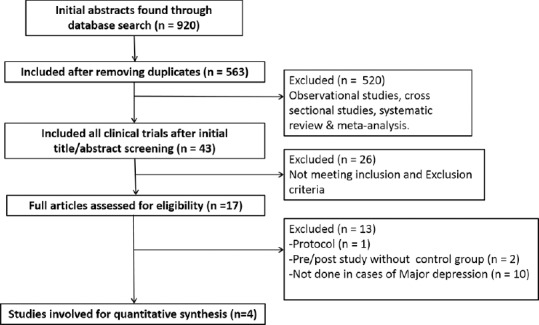
Flowchart for literature search
Data extraction and selection of outcome measure
Data were abstracted from articles meeting the inclusion criteria on the following items: author and year of study, region of study, total sample size as well as sample size in each group, dosing regimen of vitamin D followed, nature and dose of antidepressant received by the comparator arm, assessment time points, total duration of study, and primary and secondary outcome measures. The outcome measure used for calculation of effect size was the depression symptom score which had to be a primary outcome measure of all the included studies. Data extraction was done by two of the authors (Vikas Menon and Favaz Vellekkatt). We relied only on published information and did not contact the authors for additional data.
Study quality assessment
We assessed the quality of individual trials using the Cochrane collaboration tool which summarizes the risk of bias under various heads.[28] These included information about random sequence generation (selection bias), details about allocation concealment (selection bias), blinding of participants and study personnel (performance bias), blinding of outcome rater (detection bias), handling incomplete data (attrition bias), and selectively reporting originally mentioned outcomes (reporting bias). The authors, after examining the full texts of the included articles, categorized every trial on the above parameters which were reported as present, absent, or unclear [Table 3].
Table 3.
Risk of bias assessment for included trials (n=4)
| Study | Random sequence generation (selection bias) | Allocation concealment (Selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data addressed (attrition bias) | Selective reporting (reporting bias) |
|---|---|---|---|---|---|---|
| Wang et al.,[35] 2016 | Yes | Yes | Yes | Yes | No | No |
| Sepehrmanesh et al.,[34] 2016 | Yes | Yes | Yes | Yes | No | No |
| Mozaffari-Khosravi et al.,[32] 2013 | Yes | Not mentioned | No | No | No | No |
| Khoraminya et al.,[33] 2013 | Yes | Not mentioned | Yes | Yes | No | No |
Statistical analysis
For every trial that assessed the efficacy of vitamin D supplementation against a comparator in major depression, effect sizes were computed as standardized mean difference (Cohen's d) with 95% confidence interval.[29]
Pooled mean effect sizes for the intervention was calculated using both the fixed and random-effects model for the entire sample. Both the models threw up similar values for the present meta-analysis. We used the I2 statistic to assess heterogeneity among the selected studies. Its value may range from 0 to 100, with higher values suggesting greater heterogeneity.[30]
Assessment of publication bias was done using the Funnel plot. This is essentially a scatter plot of the intervention effect estimates of each study against some measure of its size or precision indicating its overall weight in the meta-analysis. Here, we studied the scatter between the computed effect size for each study against its standard error. In the absence of a publication bias, effect estimates from smaller studies will scatter more widely towards the bottom of the graph while the larger studies aggregate more closely above. The result is the appearance of an inverted funnel. If there is a publication bias, the funnel plot tends to be proportionately asymmetrical with gaps in the bottom of the graph. Analysis was performed using STATA version 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).[31]
Results
Characteristics of selected studies
A total of four trials met the inclusion criteria and were included in the present meta-analysis. Characteristics of the included studies are shown in Table 1. Three of the included trials were done[32,33,34] in Iran and the remaining one[35] was done in southeast China. Two of the trials[32,35] were done on laboratory proven vitamin D deficiency, whereas in the remaining two trials[33,34] participants were not examined for vitamin D deficiency before recruitment. The sample size of individual trials ranged from 40[34] to 746,[35] and the pooled sample size was 948. Among the four trials, three were double-blind randomized control trial[33,34,35] and one was a nonblinded randomized control trial.[32] In one of the trials,[32] intervention group received parenteral vitamin D supplementation, whereas in all other trials,[33,34,35] oral vitamin D was provided. In two trials,[34,35] the intervention group received weekly oral vitamin D supplementation, whereas in the one other trial,[33] they received daily oral vitamin D supplementation. Duration of follow-up ranged from a minimum of 8 weeks[34] to a maximum of 52 weeks.[35]
Efficacy outcomes
Effect sizes of individual trials ranged from 0.49[34] to 1.03.[33] The effect sizes, confidence intervals, and respective weights for included trials are depicted in Figure 2 (Forest plot). Meta-analysis of the four trials using the random effects model yielded a pooled mean effect size of 0.58 [n = 948, confidence Intervals (CI) 0.45–0.72] for vitamin D supplementation on depressive symptom ratings in major depression. The I2 value for heterogeneity of the analyzed studies was 0 in both fixed and random-effects model (P = 0.720). This indicates low levels of heterogeneity among the sample of included studies.
Figure 2.
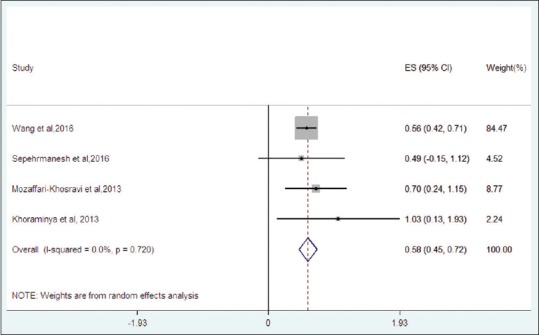
Forest plot for included trials
Quality assessment of studies
All were randomized controlled trials, of which all were double-blind trials except one which was a nonblinded study.[32] Details regarding allocation concealment were not mentioned in two of the studies.[32,33] In all four trials, the outcome data was complete and there was no reporting bias.
Evidence of publication bias
To assess publication bias, a funnel plot was derived [Figure 3]. Though the number of trials were few, it was observed that less precise studies were skewed towards the right side of the graph (favoring the intervention). To test the asymmetry of the funnel plot, Egger's test was carried out.[36] The standard normal deviate was regressed against its precision, and the regression equation for the sample of studies was derived as follows:
Figure 3.
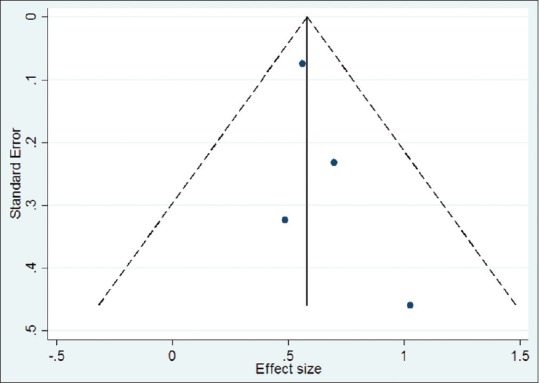
Funnel plot for publication bias
Standard normal deviate (SND) =0.613 + 0.516 × (precision)
The Y-intercept (constant) for the equation was 0.613 (confidence intervals −1.558–2.784, P = 0.349, adjusted R2 = 0.95), which suggested minimal publication bias among the selected sample of studies.
Discussion
The findings of the present meta-analysis suggest that vitamin D supplementation has clinical benefits in patients with syndromal depression. The pooled mean effect size was 0.58 which suggest a moderate effect size. Of the four studies included in the meta-analysis, only in one did the confidence intervals for the effect size span zero.[34] In the remaining three studies,[32,33,35] the confidence intervals were noninclusive of the null value (zero) and can be regarded as clinically effective trials. Notably, parenteral vitamin D supplementation was not found to be superior to oral vitamin D. The study by Khoraminya et al.[33] had a relatively high effect size (standardized mean difference, 1.03), and was the only study where patients were administered adjunctive daily vitamin D along with antidepressant (fluoxetine). However, on sensitivity analysis, we observed that the pooled effect size did not change much when this study was dropped from the random-effects model. The effect size continued to be moderate in magnitude (0.57, 95% confidence interval 0.44–0.71), which indicates that vitamin D supplementation on a nondaily basis may also be as effective as a daily supplementation regimen. Visual inspection of the funnel plot indicated that there was some publication bias particularly among smaller studies though the Egger test suggested minimal publication bias.
To our knowledge, no prior meta-analysis has tried to answer the question whether supplementation of vitamin D is beneficial in clinically depressed individuals. The reason for the benefit seen with vitamin D could be due to the fact that depression has multifactorial origins including oxidative stress.[37,38] Therefore, the antioxidant properties of vitamin D could be relevant[39,40] and may contribute to therapeutic effects observed. Moreover, neuroinflammation is an upcoming theory for depression[5,17,18] and is postulated to be an important factor in modulating treatment response.[5,41] Certain studies have shown low levels of vitamin D to be associated with elevated levels of systemic inflammatory markers.[42,43,44,45,46] This provides a basis for understanding the mechanisms underpinning improvement in depressive symptoms upon addition of vitamin D to the therapeutic regimen. Future studies with prospective designs must investigate whether change in levels of inflammatory markers parallel that of serum vitamin D levels in major depression to confirm and establish the directionality of these associations.
Some limitations of the current meta-analysis should be considered before drawing firm conclusions. First, only four studies met the inclusion criteria, and therefore, the results must be viewed as tentative. Second, in the current meta-analysis, one study[35] had disproportionately higher weightage owing to large sample size. To see how the other studies behaved as a group, we did a sensitivity analysis and noticed that the effect size showed a marginal appreciation (0.68, 95% confidence interval 0.34–1.03) after dropping the Wang et al. study from the final model. This continues to support the benefits of supplementing vitamin D in depression. Having observed this, we must add that the other three studies had small sample sizes, and therefore, group differences had to be of comparatively higher magnitude to achieve statistical significance. This implies that the therapeutic effect of vitamin D was substantial. Third, we have employed the funnel plot with Egger test to detect publication bias. However, the power of this test to detect bias is low with a small number of studies. Fourth, because patients with depression have an appreciable placebo response rate,[47,48] lack of allocation concealment in two of the studies[32,33] and lack of blinding of intervention in one trial[32] could have influenced the results. These were among several sources of bias noted in the current meta-analysis. The included trials differed in the dosages of adjunctive vitamin D used and this may have influenced the overall results. It is also unclear if vitamin D supplementation is beneficial only among deficient subjects or across the board in major depression and this needs to be answered through future studies comparing depressed patients with and without a concurrent diagnosis of vitamin D deficiency.
To conclude, our findings show that vitamin D supplementation favorably impacts depression ratings in major depression. The effect sizes noted were in the moderate range for this outcome. These conclusions are certainly impacted by the limited number of trials analyzed and the inherent methodological bias noted in a few of them. Clearly, more well-designed trials that address these flaws as well as concurrently investigate the possible mechanisms underpinning clinical benefits with vitamin D are required to improve our understanding and therapeutic strategy. Considering the high burden of poor response and nonresponse in depression, this will inform and expand the therapeutic armamentarium to deal with this condition better.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.WHO|Depression [Internet]. WHO. [Last accessed on 2017 Sep 23]. Available from: http://www.who.int/mediacentre/factsheets/fs369/en/
- 2.World Health Organization. The global burden of disease: 2004 update. In: Mathers C, Fat DM, Boerma JT, editors. Geneva, Switzerland: World Health Organization; 2008. p. 146. [Google Scholar]
- 3.Reddy MS. Depression: The Disorder and the Burden. Indian J Psychol Med. 2010;32:1–2. doi: 10.4103/0253-7176.70510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufmann FN, Costa AP, Ghisleni G, Diaz AP, Rodrigues ALS, Peluffo H, et al. NLRP3 inflammasome-driven pathways in depression: Clinical and preclinical findings. Brain Behav Immun. 2017;64:367–83. doi: 10.1016/j.bbi.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Mauskopf JA, Simon GE, Kalsekar A, Nimsch C, Dunayevich E, Cameron A. Nonresponse, partial response, and failure to achieve remission: Humanistic and cost burden in major depressive disorder. Depress Anxiety. 2009;26:83–97. doi: 10.1002/da.20505. [DOI] [PubMed] [Google Scholar]
- 7.Otte C. Incomplete remission in depression: Role of psychiatric and somatic comorbidity. Dialogues Clin Neurosci. 2008;10:453–60. doi: 10.31887/DCNS.2008.10.4/cotte. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Högberg G, Gustafsson SA, Hällström T, Gustafsson T, Klawitter B, Petersson M. Depressed adolescents in a case-series were low in vitamin D and depression was ameliorated by vitamin D supplementation: Depressed adolescents and vitamin D status. Acta Paediatr. 2012;101:779–83. doi: 10.1111/j.1651-2227.2012.02655.x. [DOI] [PubMed] [Google Scholar]
- 9.Imai CM, Halldorsson TI, Eiriksdottir G, Cotch MF, Steingrimsdottir L, Thorsdottir I, et al. Depression and serum 25-hydroxyvitamin D in older adults living at northern latitudes – AGES-Reykjavik Study. J Nutr Sci. 2015;4:e37. doi: 10.1017/jns.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Känel R, Fardad N, Steurer N, Horak N, Hindermann E, Fischer F, et al. Vitamin D Deficiency and Depressive Symptomatology in Psychiatric Patients Hospitalized with a Current Depressive Episode: A Factor Analytic Study. PloS One. 2015;10:e0138550. doi: 10.1371/journal.pone.0138550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jhee JH, Kim H, Park S, Yun H-R, Jung S-Y, Kee YK, et al. Vitamin D deficiency is significantly associated with depression in patients with chronic kidney disease. PloS One. 2017;12:e0171009. doi: 10.1371/journal.pone.0171009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoogendijk WJG, Lips P, Dik MG, Deeg DJH, Beekman ATF, Penninx BWJH. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65:508–12. doi: 10.1001/archpsyc.65.5.508. [DOI] [PubMed] [Google Scholar]
- 13.Kjaergaard M, Waterloo K, Wang CEA, Almas B, Figenschau Y, Hutchinson MS, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: Nested case-control study and randomised clinical trial. Br J Psychiatry. 2012;201:360–8. doi: 10.1192/bjp.bp.111.104349. [DOI] [PubMed] [Google Scholar]
- 14.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 15.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Prüfer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16:135–45. doi: 10.1016/s0891-0618(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 17.Muthuramalingam A, Menon V, Rajkumar R, Negi V. Is depression an inflammatory disease? findings from a cross-sectional study at a tertiary care center. Indian J Psychol Med. 2016;38:114–9. doi: 10.4103/0253-7176.178772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raison CL, Miller AH. Is Depression an Inflammatory Disorder? Curr Psychiatry Rep. 2011;13:467–75. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erhard SM, Knitter S, Westphale R, Roll S, Keil T, et al. Re: “Vitamin D supplementation to reduce depression in adults: Meta-analysis of randomized controlled trials.” Gouda U. Nutrition. 2015;31:421–9. doi: 10.1016/j.nut.2014.06.017. Nutrition 2017;38:94. [DOI] [PubMed] [Google Scholar]
- 20.Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208:56–61. doi: 10.1016/j.jad.2016.08.082. [DOI] [PubMed] [Google Scholar]
- 21.Spedding S. Vitamin D and depression: A systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients. 2014;6:1501–18. doi: 10.3390/nu6041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaffer JA, Edmondson D, Wasson LT, Falzon L, Homma K, Ezeokoli N, et al. Vitamin D Supplementation for Depressive Symptoms: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Psychosom Med. 2014;76:190–6. doi: 10.1097/PSY.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gowda U, Mutowo MP, Smith BJ, Wluka AE, Renzaho AMN. Vitamin D supplementation to reduce depression in adults: Meta-analysis of randomized controlled trials. Nutrition. 2015;31:421–9. doi: 10.1016/j.nut.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: Randomized double blind trial. J Intern Med. 2008;264:599–609. doi: 10.1111/j.1365-2796.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- 25.Yalamanchili V, Gallagher JC. Treatment with hormone therapy and calcitriol did not affect depression in older postmenopausal women: No interaction with estrogen and vitamin D receptor genotype polymorphisms. Menopause. 2012;19:697–703. doi: 10.1097/gme.0b013e31823bcec5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertone-Johnson ER, Powers SI, Spangler L, Larson J, Michael YL, Millen AE, et al. Vitamin D Supplementation and Depression in the Women's Health Initiative Calcium and Vitamin D Trial. Am J Epidemiol. 2012;176:1–13. doi: 10.1093/aje/kwr482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stokes CS, Grünhage F, Baus C, Volmer DA, Wagenpfeil S, Riemenschneider M, et al. Vitamin D supplementation reduces depressive symptoms in patients with chronic liver disease. Clin Nutr. 2016;35:950–7. doi: 10.1016/j.clnu.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen J. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1998. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 30.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.StataCorp. College Station, TX: StataCorp LP; 2013. Stata Statistical Software: Release 13. [Google Scholar]
- 32.Mozaffari-Khosravi H, Nabizade L, Yassini-Ardakani SM, Hadinedoushan H, Barzegar K. The effect of 2 different single injections of high dose of vitamin D on improving the depression in depressed patients with vitamin D deficiency: A randomized clinical trial. J Clin Psychopharmacol. 2013;33:378–85. doi: 10.1097/JCP.0b013e31828f619a. [DOI] [PubMed] [Google Scholar]
- 33.Khoraminya N, Tehrani-Doost M, Jazayeri S, Hosseini A, Djazayery A. Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. Aust N Z J Psychiatry. 2013;47:271–5. doi: 10.1177/0004867412465022. [DOI] [PubMed] [Google Scholar]
- 34.Sepehrmanesh Z, Kolahdooz F, Abedi F, Mazroii N, Assarian A, Asemi Z, et al. Vitamin D Supplementation Affects the Beck Depression Inventory, Insulin Resistance, and Biomarkers of Oxidative Stress in Patients with Major Depressive Disorder: A Randomized, Controlled Clinical Trial. J Nutr. 2016;146:243–8. doi: 10.3945/jn.115.218883. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Liu Y, Lian Y, Li N, Liu H, Li G. Efficacy of High-Dose Supplementation With Oral Vitamin D3 on Depressive Symptoms in Dialysis Patients With Vitamin D3 Insufficiency: A Prospective, Randomized, Double-Blind Study. J Clin Psychopharmacol. 2016;36:229–35. doi: 10.1097/JCP.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 36.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salim S. Oxidative Stress and Psychological Disorders. Curr Neuropharmacol. 2014;12:140–7. doi: 10.2174/1570159X11666131120230309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michel TM, Pülschen D, Thome J. The role of oxidative stress in depressive disorders. Curr Pharm Des. 2012;18:5890–9. doi: 10.2174/138161212803523554. [DOI] [PubMed] [Google Scholar]
- 39.Baser H, Can U, Baser S, Hidayetoglu BT, Aslan U, Buyuktorun I, et al. Serum total oxidant/anti-oxidant status, ischemia-modified albumin and oxidized-low density lipoprotein levels in patients with vitamin D deficiency. Arch Endocrinol Metab. 2015;59:318–24. doi: 10.1590/2359-3997000000055. [DOI] [PubMed] [Google Scholar]
- 40.Polidoro L, Properzi G, Marampon F, Gravina GL, Festuccia C, Di Cesare E, et al. Vitamin D protects human endothelial cells from H2O2 oxidant injury through the Mek/Erk-Sirt1 axis activation. J Cardiovasc Transl Res. 2013;6:221–31. doi: 10.1007/s12265-012-9436-x. [DOI] [PubMed] [Google Scholar]
- 41.Mocking RJT, Nap TS, Westerink AM, Assies J, Vaz FM, Koeter MWJ, et al. Biological profiling of prospective antidepressant response in major depressive disorder: Associations with (neuro) inflammation, fatty acid metabolism, and amygdala-reactivity. Psychoneuroendocrinology. 2017;79:84–92. doi: 10.1016/j.psyneuen.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Huang Y-N, Ho Y-J, Lai C-C, Chiu C-T, Wang J-Y. 1,25-Dihydroxyvitamin D3 attenuates endotoxin-induced production of inflammatory mediators by inhibiting MAPK activation in primary cortical neuron-glia cultures. J Neuroinflammation. 2015;12:147. doi: 10.1186/s12974-015-0370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antai-Otong D. Vitamin D: An anti-inflammatory treatment option for depression? Issues Ment Health Nurs. 2014;35:227–34. doi: 10.3109/01612840.2013.875086. [DOI] [PubMed] [Google Scholar]
- 44.Cheng J, Rui Y, Qin L, Xu J, Han S, Yuan L, et al. Vitamin D Combined with Resveratrol Prevents Cognitive Decline in SAMP8 Mice. Curr Alzheimer Res. 2017;14:820–33. doi: 10.2174/1567205014666170207093455. [DOI] [PubMed] [Google Scholar]
- 45.Kočovská E, Gaughran F, Krivoy A, Meier U-C. Vitamin-D Deficiency As a Potential Environmental Risk Factor in Multiple Sclerosis, Schizophrenia, and Autism. Front Psychiatry. 2017;8:47. doi: 10.3389/fpsyt.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res. 2014;7:69–87. doi: 10.2147/JIR.S63898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown WA. Placebo as a treatment for depression. Neuropsychopharmacol. 1994;10:265–9. doi: 10.1038/npp.1994.53. [DOI] [PubMed] [Google Scholar]
- 48.Sonawalla SB, Rosenbaum JF. Placebo response in depression. Dialogues Clin Neurosci. 2002;4:105–13. doi: 10.31887/DCNS.2002.4.1/ssonawalla. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shipowick CD, Moore CB, Corbett C, Bindler R. Vitamin D and depressive symptoms in women during the winter: A pilot study. Appl Nurs Res. 2009;22:221–5. doi: 10.1016/j.apnr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Vaziri F, Nasiri S, Tavana Z, Dabbaghmanesh MH, Sharif F, Jafari P. A randomized controlled trial of vitamin D supplementation on perinatal depression: in Iranian pregnant mothers. BMC Pregnancy Childbirth. 2016;16:239. doi: 10.1186/s12884-016-1024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mousa A, Naderpoor N, de Courten MPJ, de Courten B. Vitamin D and symptoms of depression in overweight or obese adults: A cross-sectional study and randomized placebo-controlled trial. J Steroid Biochem Mol Biol. 2017;S0960-0760:30216–9. doi: 10.1016/j.jsbmb.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Frandsen TB, Pareek M, Hansen JP, Nielsen CT. Vitamin D supplementation for treatment of seasonal affective symptoms in healthcare professionals: A double-blind randomised placebo-controlled trial. BMC Res Notes. 2014;7:528. doi: 10.1186/1756-0500-7-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zanetidou S, Murri MB, Buffa A, Malavolta N, Anzivino F, Bertakis K. Vitamin D supplements in geriatric major depression. Int J Geriatr Psychiatry. 2011;26:1209–10. doi: 10.1002/gps.2703. [DOI] [PubMed] [Google Scholar]
- 54.Marsh WK, Penny JL, Rothschild AJ. Vitamin D supplementation in bipolar depression: A double blind placebo controlled trial. J Psychiatr Res. 2017;95:48–53. doi: 10.1016/j.jpsychires.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 55.de Koning EJ, van Schoor NM, Penninx BWJH, Elders PJM, Heijboer AC, Smit JH, et al. Vitamin D supplementation to prevent depression and poor physical function in older adults: Study protocol of the D-Vitaal study, a randomized placebo-controlled clinical trial. BMC Geriatr. 2015;15:151. doi: 10.1186/s12877-015-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


