Key Points
Question
Does moderate hypothermia after decompressive hemicraniectomy reduce early mortality in patients with malignant stroke?
Findings
This randomized clinical trial investigated the effects and safety of moderate hypothermia (temperature, 33°C ± 1°C) after hemicraniectomy in 50 patients 60 years and younger who had malignant stroke. Mortality at day 14 was not significantly different between the hypothermia group and the control group; the trial was terminated early for safety reasons because of a higher rate of serious adverse events in the hypothermia group.
Meaning
Moderate hypothermia after hemicraniectomy did not improve early mortality but may be harmful to patients with malignant stroke.
This randomized clinical trial assesses whether moderate hypothermia after early hemicraniectomy reduces mortality in patients with malignant MCA stroke, compared with standard treatment.
Abstract
Importance
Moderate hypothermia in addition to early decompressive hemicraniectomy has been suggested to further reduce mortality and improve functional outcome in patients with malignant middle cerebral artery (MCA) stroke.
Objective
To investigate whether moderate hypothermia vs standard treatment after early hemicraniectomy reduces mortality at day 14 in patients with malignant MCA stroke.
Design, Setting, and Participants
This randomized clinical trial recruited patients from August 2011 through September 2015 at 6 German university hospitals with dedicated neurointensive care units. Of the patients treated with hemicraniectomy and assessed for eligibility, patients were randomly assigned to either standard care or moderate hypothermia. Data analysis was completed from December 2016 to June 2018.
Interventions
Moderate hypothermia (temperature, 33.0 ± 1.0°C) was maintained for at least 72 hours immediately after hemicraniectomy.
Main Outcomes and Measures
The primary outcome was mortality rate at day 14 compared with the Fisher exact test and expressed as odds ratio (ORs) with 95% CIs. Rates of patients with serious adverse events were estimated for the period of the first 14 days after hemicraniectomy and 12 months of follow-up. Secondary outcome measures included functional outcome at 12 months.
Results
Of the 50 study participants, 24 were assigned to standard care and 26 to moderate hypothermia. Twenty-eight were male (56%); the mean (SD) patient age was 51.3 (6.6) years. Recruitment was suspended for safety concerns: 12 of 26 patients (46%) in the hypothermia group and 7 of 24 patients (29%) receiving standard care had at least 1 serious adverse event within 14 days (OR, 2.05 [95% CI, 0.56-8.00]; P = .26); after 12 months, rates of serious adverse events were 80% (n = 20 of 25) in the hypothermia group and 43% (n = 10 of 23) in the standard care group (hazard ratio, 2.54 [95% CI, 1.29-5.00]; P = .005). The mortality rate at day 14 was 19% (5 of 26 patients) in the hypothermia group and 13% (3 of 24 patients) in the group receiving standard care (OR, 1.65 [95% CI, 0.28-12.01]; P = .70). There was no significant difference regarding functional outcome after 12 months of follow-up.
Interpretation
In patients with malignant MCA stroke, moderate hypothermia early after hemicraniectomy did not improve mortality and functional outcome compared with standard care, but may cause serious harm in this specific setting.
Trial Registration
http://www.drks.de, identifier DRKS00000623
Introduction
Large space-occupying infarction in the territory of the middle cerebral artery (MCA), also known as malignant MCA stroke, is a life-threatening neurological disease. The development of massive brain edema secondary to the ischemic injury usually leads to brain tissue shifts and transtentorial herniation within a few days.1,2,3,4,5,6 In patients 60 years and younger, early hemicraniectomy has been shown to be effective in reducing mortality and severe disability, with numbers needed to treat of 2 and 4, respectively. Despite this remarkable treatment effect, every fifth patient dies in the early stage of the disease, and every third patient is left with moderate to severe disability after hemicraniectomy.6,7,8
Therapeutic hypothermia is a potentially neuroprotective therapy with proven efficacy in clinical trials on global cerebral ischemia after cardiac arrest and animal models after focal cerebral ischemia.9,10,11,12 In focal cerebral ischemia, preclinical data and observational studies suggest that hypothermia is most effective if started early after vessel occlusion, if cooling level is moderately low (temperature, 32-33°C), and if duration of cooling is sufficiently long (>48 hours).11,12,13,14 There are only a few hypothermia studies in patients with malignant MCA stroke. In this disease, hypothermia is mainly an antiedema therapy. Although less effective than hemicraniectomy, moderate hypothermia (temperature, 32-33°C) reduced mortality to about 40% in observational studies compared with standard care.8,15 This treatment effect was consistent across different studies, settings, and durations of hypothermia (1 to 22 days).8 In a small study, the combination of hypothermia (temperature, 35°C) for 2 days in addition to hemicraniectomy did not show an increased risk of severe adverse effects and complications warranting a larger randomized clinical trial.15
Based on these data, it is suggestive that outcome in malignant MCA stroke could be further improved beyond the effects of early hemicraniectomy by simultaneous hypothermia according to an optimized protocol. Key elements of hypothermia in such a protocol are (1) early initiation after hemicraniectomy, (2) target level of cooling between 32°C and 34°C, (3) duration of hypothermia at least 72 hours, and (4) controlled induction, maintenance, and rewarming by feedback systems.8
The present trial tests the hypothesis that hypothermia in addition to hemicraniectomy could reduce mortality in patients with malignant MCA stroke. The study used a randomized and controlled approach.
Methods
Study Design
The trial was conducted at 6 German university hospitals between August 2011 and September 2016. The institutional review boards of the Friedrich-Alexander University (Erlangen, Germany) and all participating centers approved the trial protocol. The trial had an open-label, randomized, controlled design. Blinded raters obtained follow-up-information after 12 months using a structured telephone interview. Further details can be found in the previously published protocol.8
Patients
Patients were eligible if they were aged 18 to 60 years, had clinical signs of unilateral MCA infarction with a score of more than 14 in cases of nondominant hemispheric stroke or a score of more than 19 in cases of dominant hemispheric stroke, and a reduced level of consciousness on the National Institutes of Health Stroke Scale (NIHSS) as used in previous randomized clinical trials of malignant MCA stroke.6,16 On neuroimaging, unilateral ischemia had to involve at least two-thirds of the MCA territory plus the basal ganglia. All patients were treated by early hemicraniectomy within 48 hours from symptom onset. Exclusion criteria were preexisting disability exceeding a score of 1 on the modified Rankin Scale (mRS) or preexisting impairment in the activities of daily living with values below 95 on the Barthel Index. Further exclusion criteria are listed in the eMethods in Supplement 1. All patients or their legal representatives provided written informed consent prior to randomization.
Randomization
Randomization was computer generated in blocks and stratified for centers using a web-based system (https://www.randomizer.at/). Patients were assigned in a 1:1 ratio to either standard care (control group) or therapeutic hypothermia (hypothermia group).
Procedures
All patients received early hemicraniectomy within 48 hours from symptom onset and were treated on an experienced neurointensive care unit receiving the best medical treatment available, in accordance with recommendations of current guidelines and protocols of recent trials.16,17,18,19 Core body temperature was measured with bladder catheters in all patients. In the control group, the temperature was not allowed to be actively lowered beneath 36.5°C. Patients assigned to the hypothermia group received cooling with intravascular or surface cooling devices within 12 hours after hemicraniectomy to achieve a target temperature of 33.0 ± 1.0°C. Rapid induction of hypothermia with cooled saline solutions was allowed. Patients were intubated and sedated during induction and maintenance of hypothermia and during rewarming. Hypothermia was maintained for at least 72 hours, followed by controlled slow rewarming (0.05 to 0.1°C/hour).
Outcomes
The primary outcome was early mortality at day 14. Safety and feasibility end points were the rate of serious adverse events (SAEs) and treatment parameters concerning hypothermia. Pneumonia was considered an adverse event but not an SAE, because its rate in intubated patients in the intensive care unit is reported to be 70% even under normothermia.20 Further key secondary end points were stroke severity on the NIHSS at day 14, functional outcomes on the mRS and Barthel Index at 12 months, and treatment parameters concerning hemicraniectomy and treatment in the intensive care unit. Analyses of secondary end points included patients who died.
Statistical Analysis
A total of 324 patients were planned to be included in this trial based on estimated mortality rates within 14 days of 20% in the control group and 8% in the hypothermia group (1 − β = 0.80, α = .05; Fisher exact test). Because data on the treatment effect of hypothermia are weak and sample size calculation was derived from only a single small observational study, an interim analysis was planned after inclusion of 50 patients. Successive safety monitoring was conducted after the treatment of every 10th patient and on advice of the data safety monitoring board. The trial was suspended on September 9, 2015. The final follow-up was completed on September 1, 2016. Results are reported in accordance with the prespecified statistical analysis plan as intention to treat.
For mortality at day 14 and tracheostomy at day 14, odds ratios with 95% CIs were calculated via conditional likelihood and compared with the Fisher test. Pneumonia, as the adverse event of special interest, was analyzed analogously after 14 days. Incidence rates for SAE occurrence were calculated with log-transformed 95% CIs. Times from onset of symptoms to decompressive hemicraniectomy were compared using the t test. Log-rank tests and Cox regression were used to compare ventilation times in both groups for the competing risk end points end of ventilation and death during ventilation. The duration of intensive care treatment was analyzed with the log-rank test and Cox regression with the combined end point of the end of stay in intensive care (whether alive and discharged or dead in the intensive care unit). For recurring events such as days with medication while in intensive care, days with therapeutic ventilation, number of computed tomographic scans and/or magnetic resonance images while hospitalized, and days with osmotherapy, incidence rates with log-transformed 95% CIs were calculated, as well as hazard ratios, Nelson-Aalen estimators, and log-rank tests. Time-to-event end points after 12 months were analyzed using Cox proportional hazards model and log-rank tests. For SAEs, incidence rates with log-transformed 95% CIs were calculated and Cox proportional hazards model for competing risks (adverse events vs death without adverse events) was used. For functional outcome, the Wilcoxon rank sum test was used for group comparison of NIHSS scores after 14 days, Glasgow Coma Scale scores after 14 days, mRS dichotomized between 0 to 4 points vs 5 or 6 points after 12 months, and a shift analysis was applied to the mRS score after 12 months. Statistical analysis was performed using R version 1.1.423 (R Foundation for Statistical Computing). A data safety monitoring board monitored trial safety. Additional details of the statistical analysis plan are shown in Supplement 2.
Results
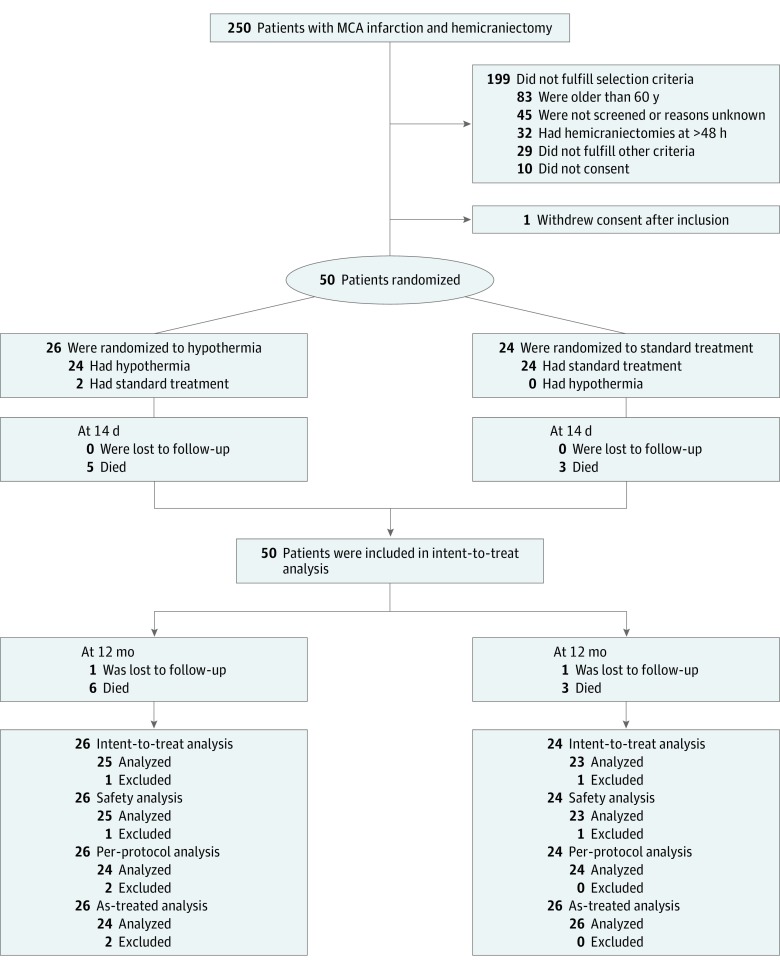
Of the projected 324 patients, 50 patients (26 in the hypothermia group and 24 in the control group) had been enrolled in the trial between August 2011 and September 2015. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is shown in Figure 1. A first safety signal in terms of a higher rate of SAEs was observed after the treatment of 34 patients. Because of the overall low number of patients included in the trial, the data safety monitoring board suggested at that point to proceed until the preplanned interim analysis. However, no trend reversal concerning safety was observed. Therefore, the data safety monitoring board recommended stopping the trial after inclusion of 50 patients and proceeding with the final analysis instead of the interim analysis.
Figure 1. CONSORT Flow Diagram.
Reasons for exclusion from per-protocol analysis were crossover to the control group in both patients; these 2 patients were allocated to the control group for the as-treated analysis. MCA indicates middle cerebral artery.
Baseline demographic and clinical characteristics are shown in Table 1. Of the 26 patients in the hypothermia group, 14 (54%) were treated with endovascular cooling devices and 10 (38%) received surface cooling. One patient additionally received intravenous cooled saline (2%). Two patients randomized to hypothermia (8%) did not receive this treatment, 1 because of a subdural hematoma after decompressive hemicraniectomy and 1 because the senior anesthetist in charge refused to induce hypothermia after randomization. There were 2 other protocol violations; in each group, 1 patient underwent hemicraniectomy after 48 hours. One patient in each treatment group was lost to follow-up at 12 months.
Table 1. Demographic and Clinical Characteristics of Patients at Baseline.
| Characteristic | Group, No. (%) | |
|---|---|---|
| Control (n = 24) | Hypothermia (n = 26) | |
| Age, median (range), y | 53 (39-60) | 51 (33-60) |
| Male | 15 (63) | 13 (50) |
| Preexisting modified Rankin scale score on admissiona | ||
| 0 | 22 (92) | 24 (92) |
| 1 | 2 (8) | 2 (8) |
| ≥2 | 0 | 0 |
| Preexisting Barthel Index score on admission, median (range)b | 100 (100-100) | 100 (95-100) |
| Site of infarction | ||
| Middle cerebral artery only | 16 (67) | 16 (62) |
| Middle cerebral artery and anterior cerebral artery | 6 (25) | 8 (31) |
| Middle cerebral artery and posterior cerebral artery | 2 (8) | 2 (8) |
| Stroke in dominant hemisphere | 11 (46) | 12 (46) |
| Glasgow Coma Scale scorec | ||
| Assessable | 12 (50) | 14 (54) |
| Median (range) | 10 (7-14) | 11.5 (8-14) |
| National Institutes of Health Stroke Scale total score on admissiond | ||
| Assessable | 23 (96) | 25 (96) |
| Median (range) | 20.5 (15-42) | 21 (15-42) |
| Hours from onset of symptoms to end point, median (range) | ||
| Randomization | 26.9 (8.6-47.9) | 30.6 (11.2-54.9) |
| Hemicraniectomy | 26.3 (7.8-55) | 31.5 (12.3-54) |
| Hypothermia | NA | 37.2 (16.3-57) |
| Adherence to assigned treatment | 24 (100) | 24 (92) |
| Comorbidities | ||
| Arterial hypertension | 10 (42) | 17 (65) |
| Diabetes mellitus | 5 (21) | 3 (12) |
| Hyperlipidemia | 4 (17) | 5 (19) |
| Present smoking | 8 (33) | 9 (35) |
| Atrial fibrillation | 2 (8) | 1 (4) |
| Recanalization treatment | ||
| Patients receiving intravenous thrombolysis | 19 (79) | 17 (65) |
| Minutes from onset to recanalization treatment, mean (SD) | ||
| Intravenous thrombolysis | 111.9 (32.6)e | 146.3 (129.5)f |
| Intraarterial thrombolysis | 585 (NA)g | 195 (106.1)h |
| Endovascular thrombectomy | 273.8 (123.7)i | 230 (96.4)j |
Abbreviation: NA, not applicable.
Scores on the modified Rankin scale range from 0 to 6, with 0 indicating no symptoms; 1, no disability despite symptoms; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; and 6, death. Persons with a score of 0, 1, or 2 are considered functionally independent.
Barthel index scores range from 0 (complete dependence) to 100 (independence) in increments of 5.
Glasgow Coma Scale scores range from 3 to 15, with lower scores indicating reduced levels of consciousness.
National Institutes of Health Stroke Scale scores range from 0 to 42, with higher scores indicating more severe neurologic impairment.
n = 13.
n = 12.
n = 1.
n = 2.
n = 5.
n = 3.
Temperature Measurements
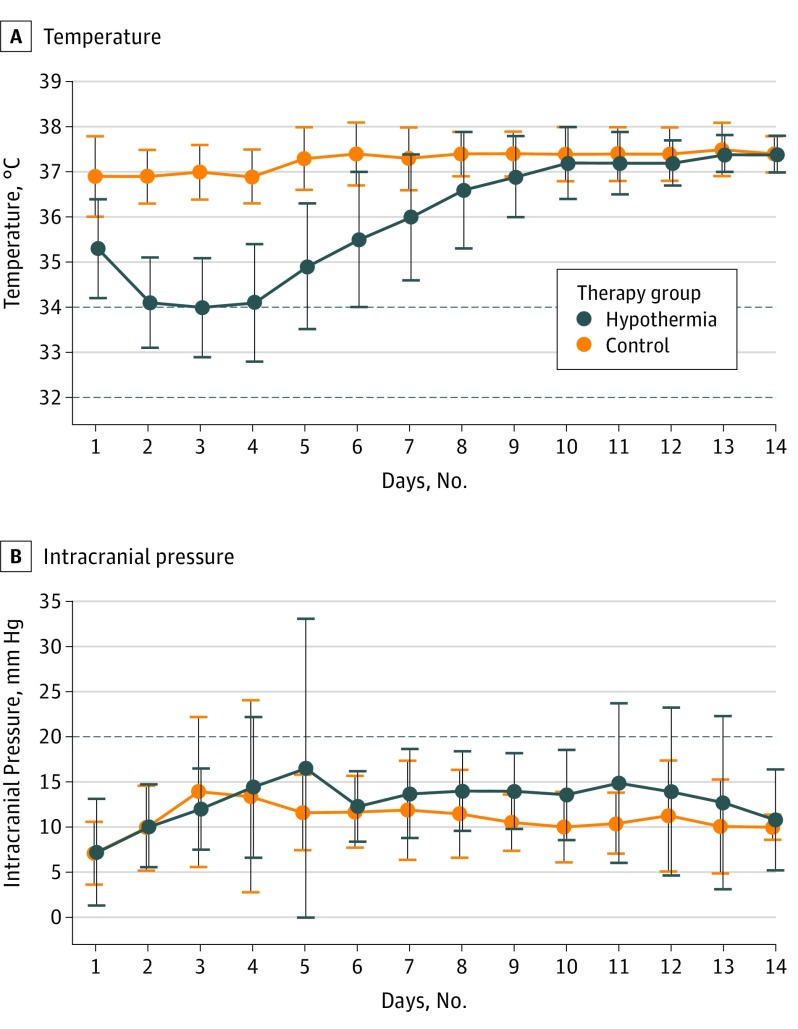
Figure 2A shows the body temperature course for both treatment groups throughout the first 14 days after randomization for the per-protocol population, excluding the 2 crossover patients. Curves are based on the mean temperature per day in each group. Patients were normothermic in both groups at the time of randomization at day 1 (control group temperature, 36.9°C ± 0.9°C; hypothermia group temperature, 36.5°C ± 1.2°C; P = .24). Hypothermia was induced after randomization at day 1. Target temperature was reached and maintained for a minimum of 72 hours in 21 of 24 patients (88%) treated with hypothermia. In 3 patients (12%), core body temperature was kept slightly higher than 34°C but did not reach the target temperature for a minimum of 72 hours (mean core body temperatures in these 3 patients were 34.1°C, 34.4°C, and 34.9°C). Median duration of hypothermia including rewarming was 7 days (interquartile range, 5-8 days) in the per-protocol population.
Figure 2. Temperature and Intracranial Pressure Curves.
Body temperatures (A) and intracranial pressure (B) course throughout the first 14 days after randomization. Curves are based on the mean temperature per day in each group; vertical bars indicate SDs. Baseline temperature was normal in both groups at day 1. Hypothermia was induced at day 1. Target temperature was reached in 89% of patients and maintained for a minimum of 72 hours in every patient receiving the assigned treatment. Intracranial pressure curves are based on the mean intracranial measurement per day in each group. Increased intracranial pressure was defined as increase over 20 mm Hg (horizontal line) for a period longer than 10 minutes.
Safety Measurements
After 14 days, SAEs were found in 12 of 26 patients (46%) in the hypothermia group and 7 of 24 patients (29%) in the control group (OR, 2.05 [95% CI, 0.56–8.00]; P = .26) (eResults in Supplement 1). Rates of SAE that were associated with temperature management were 5 of 26 patients (19%) in the hypothermia group and 0 of 24 patients in the control group (0%; P = .05). For the first 14 days after hemicraniectomy, incidence rates of SAEs per day were 0.070 in the hypothermia group (23 of 327 patient-days) and 0.036 in the control group (11 of 309 patient-days; HR, 2.19 [95% CI, 0.97-4.94]; P = .06). The SAEs were then grouped according to the Common Terminology Criteria for Adverse Events (version 4.03; https://evs.nci.nih.gov/ftp1/CTCAE) into cardiovascular disorders, nervous system disorder, respiratory disorders, and other disorders. Rates of SAEs in specific organ classes were not significantly different between the hypothermia group and control group (eTable in Supplement 1).
After 12 months, 20 of 25 patients (80%) had SAEs in the hypothermia group, and 10 of 23 (43%) had SAEs in the control group. Accordingly, incidence rates of SAEs per day were 0.006 in the hypothermia group and 0.002 in the control group (HR, 2.54 [95% CI, 1.29–5.00]; P = .005), in favor of the control group. Further data on safety measures are in Table 2.
Table 2. Mortality and Safety End Points at 14 Days and 12 Months.
| End Point | Participants, No./Total No. (%) | Odds Ratio or Hazard Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Control | Hypothermia | |||
| At 14 d | ||||
| Death | 3/24 (13) | 5/26 (19) | 1.65 (0.28-12.01)a | .70b |
| Patients with SAE | 7/24 (29) | 12/26 (46) | 2.05 (0.56-8.00)a | .26b |
| Patients with treatment-associated SAE | 0/24 | 5/26 (19) | NAa | .05b |
| At 12 mo | ||||
| Death | 3/23 (13) | 6/25 (24) | 1.55 (0.37-6.42)c | .54d |
| Patients with SAE | 10/23 (43) | 20/25 (80) | 2.51 (1.16-5.46)c | .01d |
Abbreviations: NA, not applicable; SAE, severe adverse event.
Odds ratio.
Per Fisher test.
Hazard ratio.
Per log rank test.
Early Mortality and Secondary Clinical End Points
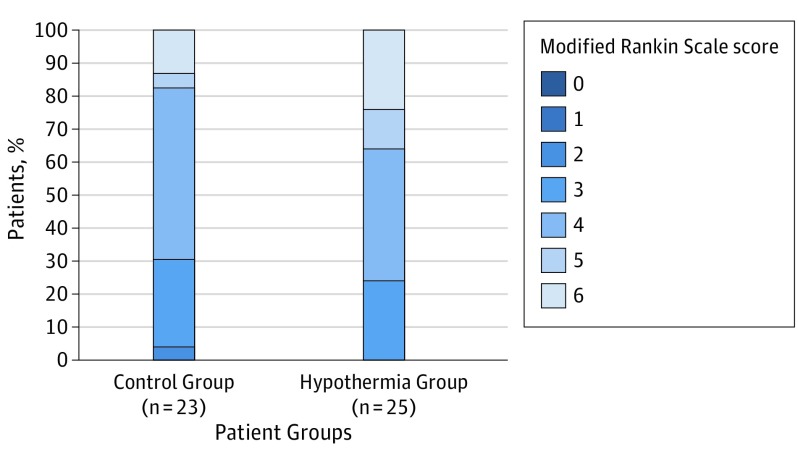
There was no significant difference for the primary end point, mortality at day 14, between both groups. Five of 26 patients (19%) in the hypothermia group and 3 of 24 (13%) in the control group had died after 14 days (OR, 1.65 [95% CI, 0.28–12.01]; P = .70). There were also no significant differences between the 2 groups concerning mortality (hypothermia group, 6 of 25 patients [24%]; control group, 3 of 23 patients [13%]; HR, 1.55 [95% CI, 0.37-6.42]; P = .54), dichotomization of functional outcome between mRS scores of 0 to 4 points vs 5 or 6 points (hypothermia group, 16 of 25 patients [64%]; control group, 19 of 23 patients [83%]; OR, 0.38 [95% CI, 0.07-1.70]; P = .20) and distribution of mRS scores after 12 months (Figure 3). If significant differences would have been achieved, the number needed to harm was 15 for mortality and 5 for poor functional outcome after 12 months (mRS score 5-6).
Figure 3. Functional Outcome at 12 Months per the Modified Rankin Scale.
A secondary end point was functional outcome at 12 months according to the modified Rankin scale. The figure shows the raw distribution of modified Rankin scores at 12 months; of the 23 participants in the control group, 1 (4%) had a score of 2, 6 (26%) had a score of 3, 12 (52%) had a score of 4, 1 (4%) had a score of 5, and 3 (13%) had a score of 6. Of the 25 participants in the hypothermia group, 6 (24%) had a score of 3, 10 (40%) had a score of 4, 3 (12%) had a score of 5, and 6 (24%) had a score of 6.
Concerning secondary clinical end points within the first 14 days, there were no significant differences between the hypothermia and control groups regarding NIHSS scores (median [interquartile range], 25 [17-37] vs 22 [16-33]; P = .46), the Glasgow Coma Scale score (median [interquartile range], 9 [4-11] vs 11 [7-12]; P = .43), and the mRS score (median [interquartile range], 5 [5-5] vs 5 [5-5]; P = .98). Rates of pneumonia were not significantly different between the hypothermia group and the control group (hypothermia group, 14 of 26 patients [54%]; control group, 15 of 24 patients [63%]; OR, 0.71 [95% CI, 0.19-2.49]; P = .74). Intracranial pressure (ICP) reached its peak at day 3 in the control group. In the hypothermia group, ICP reached a first major peak at day 5 during rewarming and a second peak at day 11 after termination of hypothermia. Although ICP values were increased in individual patients, mean ICP values were within normal ranges (<20 mm Hg) at any time until day 14. Overall ICP mean [SD] values in the control group (11.00 [1.65] mm Hg) were significantly lower than in the hypothermia group (12.89 [2.27] mm Hg; P = .007) (Figure 2).
Patients in the hypothermia group had a significantly higher risk for requiring mechanical ventilation than those in the control group (296 of 330 patient-days [90%]; 224 of 288 patient-days [78%]; HR, 1.42 [95% CI, 1.12-1.78]; P = .003) and a significantly higher risk of receiving osmotherapy (80 of 330 patient-days [24%] vs 44 of 288 patient-days [15%]; HR, 2.56 [95% CI, 1.43-4.58]; P = .001). There were no significant differences between the hypothermia group and the control group regarding other secondary end points.
Primary and key secondary end points did not change in per-protocol and as-treated analyses. Sensitivity analyses confirmed the intent-to-treat analyses.
Discussion
The trial did not meet its primary end point; patients treated with hypothermia had no benefits regarding mortality at day 14. Most of the deaths in patients with malignant MCA stroke occurred early and were mainly based on herniation owing to severe brain edema and tissue shift, as has been shown in previous clinical studies on hypothermia and randomized trials on hemicraniectomy. The treatment effect of any effective therapy so far is therefore primarily based on the reduction of early mortality rather than the improvement of the functional outcome of survivors.5,6,8,15,16 The primary end point was chosen to investigate whether the combination of 2 therapies targeting malignant brain edema and herniation is more effective to prevent early deaths than the current standard therapy of hemicraniectomy alone.
The question whether hypothermia is beneficial in malignant stroke is controversial. Hypothermia seems to be of benefit compared with best medical treatment, yet it is far less effective than hemicraniectomy.8 Since the publication of the positive trials on hemicraniectomy, hypothermia is often used in addition to hemicraniectomy in several centers.14 However, evidence for this combined treatment is weak. The negative result of our study is in contrast with the promising results of 1 small randomized controlled trial and a recent retrospective case series, both of which share methodological weaknesses.15,21
Our trial was stopped for safety reasons after enrollment of 50 patients at the time of the preplanned interim analysis. The trend toward a higher rate of SAEs within 14 days in the hypothermia group, which was first observed after treatment of 34 patients, persisted. The significantly higher rate of SAEs in the hypothermia group after 12 months of follow-up corroborates the decision to stop the trial. In addition to the lack of a positive effect on mortality, the trial thereby provides comparative evidence of potentially harmful effects of moderate hypothermia in addition to early hemicraniectomy. These negative results and the higher rate of SAEs are in accordance with negative trials on hypothermia in other severe neurological diseases as well as trials in patients who are awake with severe stroke and recent case series on malignant stroke.22,23,24,25,26,27,28,29
Our results strongly suggest that the high rates of SAEs observed in this trial may be directly associated with hypothermic treatment, although SAEs may also be attributed to prolonged sedation during hypothermia or both. These data do not allow a more detailed analysis on causality owing to the small numbers of patients.
There were only a few significant differences between the treatment groups besides the rates of SAEs. The higher risk for mechanical ventilation in the hypothermia group may certainly be attributed to the fact that patients remained sedated and intubated during hypothermic treatment. The higher risk to receive osmotherapy in the hypothermia group corresponds well to the higher mean ICP levels in the hypothermia group. The major ICP peak at day 5 in the hypothermia group suggests that rebound edema occurred despite the slow rewarming protocol, while the second peak may reflect brain edema formation postponed by initial hypothermia. Both peaks may have triggered titration of sedation and osmotherapy. These observations corroborate the conclusion that hypothermia may be detrimental in malignant MCA stroke. Hypothermia is not a stand-alone treatment but a complex intervention with prolonged sedation, mechanical ventilation, and excess osmotic therapy, which combined put patients at risk of harm.
Limitations
Despite the randomized controlled design, our study has several limitations. The small sample size, owing to the premature stoppage of the trial, might not allow the detection of significant differences concerning safety and efficacy measures. Because the trial was stopped early, these data do not warrant a formal futility statement. However, the homogenous results as well as comparable findings in other trials on hypothermia do support the validity of these observations. The open-label design of the study and the considerable number of protocol violations might have made the study prone to detection and attrition bias. On the other hand, end point assessments were conducted by blinded raters, and detection bias and attrition bias usually distort results in favor of the investigational treatment. Finally, because hypothermia is an intervention that follows biologically plausible rules, such as dose-response association, protocol violations regarding target temperature should have resulted in fewer SAEs. Thus, the analysis should have underestimated rather than overestimated the real risk of hypothermia.
Conclusions
This study suggests that moderate hypothermia does not improve survival or disability status in patients with malignant MCA stroke treated with early hemicraniectomy. Instead, the study suggests a higher rate of SAEs and probably poorer outcomes under hypothermic treatment. We cannot exclude the possibility that hypothermia may be of benefit in different settings of stroke treatment, including initiation and duration of hypothermia or target temperature or in different stroke subtypes. Currently, the use of hypothermia cannot be recommended in patients with malignant MCA stroke outside clinical trials.
eMethods.
eResults.
eTable. Severe adverse events by organ classes according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.03, at day 14.
Statistical analysis plan.
Data Sharing Statement.
References
- 1.Ropper AH, Shafran B. Brain edema after stroke: clinical syndrome and intracranial pressure. Arch Neurol. 1984;41(1):26-29. doi: 10.1001/archneur.1984.04050130032017 [DOI] [PubMed] [Google Scholar]
- 2.Silver FL, Norris JW, Lewis AJ, Hachinski VC. Early mortality following stroke: a prospective review. Stroke. 1984;15(3):492-496. doi: 10.1161/01.STR.15.3.492 [DOI] [PubMed] [Google Scholar]
- 3.Kasner SE, Demchuk AM, Berrouschot J, et al. . Predictors of fatal brain edema in massive hemispheric ischemic stroke. Stroke. 2001;32(9):2117-2123. doi: 10.1161/hs0901.095719 [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53(4):309-315. doi: 10.1001/archneur.1996.00550040037012 [DOI] [PubMed] [Google Scholar]
- 5.Vahedi K, Vicaut E, Mateo J, et al. ; DECIMAL Investigators . Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke. 2007;38(9):2506-2517. doi: 10.1161/STROKEAHA.107.485235 [DOI] [PubMed] [Google Scholar]
- 6.Vahedi K, Hofmeijer J, Juettler E, et al. ; DECIMAL, DESTINY, and HAMLET investigators . Early decompressive surgery in malignant middle cerebral artery infarction: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6(3):215-222. doi: 10.1016/S1474-4422(07)70036-4 [DOI] [PubMed] [Google Scholar]
- 7.Huttner HB, Schwab S. Malignant middle cerebral artery infarction: clinical characteristics, treatment strategies, and future perspectives. Lancet Neurol. 2009;8(10):949-958. doi: 10.1016/S1474-4422(09)70224-8 [DOI] [PubMed] [Google Scholar]
- 8.Neugebauer H, Kollmar R, Niesen WD, et al. ; DEPTH-SOS Study Group; IGNITE Study Group . Decompressive surgery plus hypothermia for space-occupying stroke (DEPTH-SOS): a protocol of a multicenter randomized controlled clinical trial and a literature review. Int J Stroke. 2013;8(5):383-387. doi: 10.1111/ijs.12086 [DOI] [PubMed] [Google Scholar]
- 9.Bernard SA, Gray TW, Buist MD, et al. . Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557-563. doi: 10.1056/NEJMoa003289 [DOI] [PubMed] [Google Scholar]
- 10.Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549-556. doi: 10.1056/NEJMoa012689 [DOI] [PubMed] [Google Scholar]
- 11.van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain. 2007;130(pt 12):3063-3074. doi: 10.1093/brain/awm083 [DOI] [PubMed] [Google Scholar]
- 12.Dumitrascu OM, Lamb J, Lyden PD. Still cooling after all these years: meta-analysis of pre-clinical trials of therapeutic hypothermia for acute ischemic stroke. J Cereb Blood Flow Metab. 2016;36(7):1157-1164. doi: 10.1177/0271678X16645112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kollmar R, Blank T, Han JL, Georgiadis D, Schwab S. Different degrees of hypothermia after experimental stroke: short- and long-term outcome. Stroke. 2007;38(5):1585-1589. doi: 10.1161/STROKEAHA.106.475897 [DOI] [PubMed] [Google Scholar]
- 14.Clark DL, Penner M, Orellana-Jordan IM, Colbourne F. Comparison of 12, 24 and 48 h of systemic hypothermia on outcome after permanent focal ischemia in rat. Exp Neurol. 2008;212(2):386-392. doi: 10.1016/j.expneurol.2008.04.016 [DOI] [PubMed] [Google Scholar]
- 15.Els T, Oehm E, Voigt S, Klisch J, Hetzel A, Kassubek J. Safety and therapeutical benefit of hemicraniectomy combined with mild hypothermia in comparison with hemicraniectomy alone in patients with malignant ischemic stroke. Cerebrovasc Dis. 2006;21(1-2):79-85. doi: 10.1159/000090007 [DOI] [PubMed] [Google Scholar]
- 16.Jüttler E, Bösel J, Amiri H, et al. ; DESTINY II Study Group . DESTINY II: decompressive surgery for the treatment of malignant infarction of the middle cerebral artery II. Int J Stroke. 2011;6(1):79-86. doi: 10.1111/j.1747-4949.2010.00544.x [DOI] [PubMed] [Google Scholar]
- 17.Wijdicks EF, Sheth KN, Carter BS, et al. ; American Heart Association Stroke Council . Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(4):1222-1238. doi: 10.1161/01.str.0000441965.15164.d6 [DOI] [PubMed] [Google Scholar]
- 18.Torbey MT, Bösel J, Rhoney DH, et al. . Evidence-based guidelines for the management of large hemispheric infarction: a statement for health care professionals from the Neurocritical Care Society and the German Society for Neuro-intensive Care and Emergency Medicine. Neurocrit Care. 2015;22(1):146-164. doi: 10.1007/s12028-014-0085-6 [DOI] [PubMed] [Google Scholar]
- 19.Ntaios G, Dziedzic T, Michel P, et al. ; European Stroke Organisation . European Stroke Organisation (ESO) guidelines for the management of temperature in patients with acute ischemic stroke. Int J Stroke. 2015;10(6):941-949. doi: 10.1111/ijs.12579 [DOI] [PubMed] [Google Scholar]
- 20.Broessner G, Beer R, Lackner P, et al. . Prophylactic, endovascularly based, long-term normothermia in ICU patients with severe cerebrovascular disease: bicenter prospective, randomized trial. Stroke. 2009;40(12):e657-e665. doi: 10.1161/STROKEAHA.109.557652 [DOI] [PubMed] [Google Scholar]
- 21.Park HS, Choi JH. Safety and efficacy of hypothermia (34°C) after hemicraniectomy for malignant mca infarction. J Korean Neurosurg Soc. 2018;61(2):267-276. doi: 10.3340/jkns.2016.1111.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews PJ, Sinclair HL, Rodriguez A, et al. ; Eurotherm3235 Trial Collaborators . Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med. 2015;373(25):2403-2412. doi: 10.1056/NEJMoa1507581 [DOI] [PubMed] [Google Scholar]
- 23.Seule MA, Muroi C, Mink S, Yonekawa Y, Keller E. Therapeutic hypothermia in patients with aneurysmal subarachnoid hemorrhage, refractory intracranial hypertension, or cerebral vasospasm. Neurosurgery. 2009;64(1):86-92. doi: 10.1227/01.NEU.0000336312.32773.A0 [DOI] [PubMed] [Google Scholar]
- 24.Mourvillier B, Tubach F, van de Beek D, et al. . Induced hypothermia in severe bacterial meningitis: a randomized clinical trial. JAMA. 2013;310(20):2174-2183. doi: 10.1001/jama.2013.280506 [DOI] [PubMed] [Google Scholar]
- 25.Legriel S, Lemiale V, Schenck M, et al. ; HYBERNATUS Study Group . Hypothermia for neuroprotection in convulsive status epilepticus. N Engl J Med. 2016;375(25):2457-2467. doi: 10.1056/NEJMoa1608193 [DOI] [PubMed] [Google Scholar]
- 26.Cooper DJ, Nichol AD, Bailey M, et al. ; POLAR Trial Investigators and the ANZICS Clinical Trials Group . Effect of early sustained prophylactic hypothermia on neurologic outcomes among patients with severe traumatic brain injury: the POLAR randomized clinical trial. JAMA. 2018;320(21):2211-2220. doi: 10.1001/jama.2018.17075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemmen TM, Raman R, Guluma KZ, et al. ; ICTuS-L Investigators . Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke. 2010;41(10):2265-2270. doi: 10.1161/STROKEAHA.110.592295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyden P, Hemmen T, Grotta J, et al. ; Collaborators . Results of the ICTuS 2 trial (Intravascular Cooling in the Treatment of Stroke 2). Stroke. 2016;47(12):2888-2895. doi: 10.1161/STROKEAHA.116.014200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider H, Krüger P, Algra A, et al. . No benefits of hypothermia in patients treated with hemicraniectomy for large ischemic stroke. Int J Stroke. 2017;12(7):732-740. doi: 10.1177/1747493017694388 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eResults.
eTable. Severe adverse events by organ classes according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.03, at day 14.
Statistical analysis plan.
Data Sharing Statement.