Abstract
Hypomethylating agents (HMAs) are the standard of care for patients with myelodysplastic syndrome (MDS). However, only around 50% of patients respond to these agents, and responses tend to be transient, with loss of response frequently happening within 2 years and being associated with very poor prognosis and limited therapeutic options. Identification of patients who will respond to HMAs is challenging. Mechanisms underlying resistance to HMAs are not clear yet. Recently, absence of response has been associated with increased cell-cycle quiescence among the hematopoietic progenitor cells. There are no standard-of-care options for patients after HMA failure. However, the increasing knowledge of MDS pathogenesis has led to the development of new potential therapies, including HMAs with longer half-life and exposure, inhibition of the antiapoptotic BCL2 protein with venetoclax or inhibition of immune-checkpoint regulatory proteins such as PD-1 or CTLA-4, innate immunity and targeting of CD33/CD3 with multiple monoclonal antibodies. In addition, multiple targeted agents are opening opportunities to treat subgroups of patients whose disease harbors mutations in TP53, IDH, FLT3, and genes involved in splicing machinery. Newer formulations of intensive chemotherapy and its different combinations may be considered a valid option in selected patients after HMA failure. Finally, decision making at the time of failure of response to HMAs should be personalized, taking into account that allogenic stem-cell transplantation remains the only therapeutic approach with curative potential in these patients. In the current review, we will focus on all the above aspects.
Keywords: azacitidine, decitabine, hypomethylating agents failure, myelodysplastic syndromes, resistance mechanisms
Introduction
Myelodysplastic syndromes (MDS) are a very heterogeneous group of clonal disorders of the bone-marrow hematopoietic stem cells (HPSCs), characterized by ineffective hematopoiesis with peripheral blood cytopenias and a higher risk for developing acute myeloid leukemia (AML).1 Several prognostic models such as the International Prognostic Scoring System (IPSS) and its revised version (IPSS-R) allow stratification of patients into risk categories based on the degree and number of their cytopenias, observed cytogenetic abnormalities, and bone-marrow blast percentage.2 The standard of care for patients with higher-risk MDS is treatment with hypomethylating agents (HMAs) azacitidine (AZA)3 and decitabine (DAC).4 In addition, although therapy in lower-risk MDS has traditionally been directed toward treatment of cytopenias with growth-factor support or lenalidomide in the setting of del(5q) MDS, HMAs have been shown as effective in lower-risk disease, with recent studies suggesting a role of early intervention using these agents.5 Although therapy with these agents has been shown to prolong survival, only 40–50% of patients respond to therapy; responses are almost universally transient, with loss of response within 2 years. Once response to these agents is lost, the prognosis is very poor and the median overall survival (OS) is 4.3 and 14 months in high- and low-risk MDS, respectively.6 In addition, there is significant heterogeneity in the nature of failure to HMA with a subset of patients experiencing progressive disease and transformation to acute myeloid leukemia, and another group of patients experiencing bone-marrow failure and progressive cytopenias. As a result of this, therapeutic needs and prognosis of these two groups of patients may likely differ. In fact, certain clinical biomarkers, such as age, performance status, complex cytogenetics (defined as >4 abnormalities), bone-marrow blast > 20%, platelet count and red cell transfusion dependency are potential predictors of outcome after failure of response to HMAs and can identify two subsets of patients with lower- or higher-risk disease, with median OS of 11.0 versus 4.5 months,7 respectively, from the time of failure.
As a result of this, development of new therapeutic strategies to prevent or overcome HMA failure is fundamental. This is particularly the case, since there are no standard-of-care options for patients with MDS who experience failure with HMAs. In this manuscript, we will review the mechanisms of failure to respond to HMAs, as well as the salvage therapeutic options for these patients.
Failure to respond to hypomethylating agents: definition and mechanisms
Primary response failure to respond to HMAs is considered in the absence of a response after at least four to six cycles of therapy, or when the MDS progresses to higher-risk categories or transforms to AML without having responded to therapy.8 Secondary failure of response to HMAs is defined as the loss of response, progression to a higher-risk category or transformation to AML in a patient who had an initial response to therapy. Although some clinical parameters and genetic mutations have weak correlations with favorable HMA response, the molecular mechanisms underlying HMA resistance are poorly understood.9,10
Several efforts have been made to identify predictive biomarkers of response to HMAs. Although therapeutic decisions don’t have to be based on molecular mutations alone, there are some molecular biomarkers associated with response to HMAs. TET2 mutations have been identified as predictive of response to AZA when present at >10% allele burden, particularly in the absence of ASXL1 mutations, without a clear knowledge of the mechanism.11,12 The expression of miR29b also has a role in the response to HMA. miR29b targets the deoxyribonucleic acid (DNA) methyltransferases (DNMTs), resulting in global DNA hypomethylation and re-expression of hypermethylated, silenced genes in AML. The overexpression of miR29b in myeloid blasts has been associated with clinical response to the hypomethylating effects of DAC.13,14
Different resistance mechanisms to HMAs have been described in literature. Since global DNA and gene-specific hypermethylation characterize MDS, the notion that hypomethylation is likely the main mechanism of action of HMAs has been traditionally accepted. However, it has been shown that there is little association between the degree of demethylation following hypomethylating treatment and hematologic response.15
Any alterations in the transport and metabolism of HMAs might cause insufficient active forms and insufficient incorporation into DNA/RNA (ribonucleic acid), resulting in resistance. Cellular transport across membranes is crucial for uptake of HMAs and depends on the presence of human nucleoside transporters (hNTs). It was observed that the presence of hNT inhibitors protected against HMA cytotoxicity, thus suggesting the importance of hNTs in manifestation of toxicity and possible utility as a biomarker of clinical response.16 Similarly, lower expression of genes involved in AZA metabolism, such as UCK1, may influence clinical response. UCK1 was determined in 57 patients with MDS who received AZA, seeing lower expression in patients without response to AZA (median 0.2 versus 0.49 for patients with response to AZA, p = 0.07), which was corroborated in vitro, where the silencing of UCK1 by siRNA led to blunted response to AZA.17 In addition, mutations of UCK2 have been associated with resistance to AZA in AZA-resistant cell lines.18 In a recent study, primary AZA resistance was associated with cell-cycle quiescence of the hematopoietic stem-cell progenitors (HSPCs).19 By performing RNA sequencing from bone-marrow CD34+ HSPCs of nonresponders, the authors could identify downregulation of cell-cycle-related genes and this finding was consistent with flow-cytometry analysis in which CD34+ cells from nonresponders were markedly quiescent. In this study, HSPCs quiescence was described as mediated by integrin alfa-5 (ITGA5) signaling, and the blockade of this integrin signaling, in combination with AZA, improved the hematopoietic potential of these cells.
Upregulation of innate immunity signaling via Toll-like receptor (TLR) signaling and nuclear-factor kappa B (NF-κB) activation,20,21 as well as of adaptive immunity molecules, such as immune-checkpoint regulators,22 has been linked to failure of response to HMAs. Overexpression of TLR2 has been described in bone-marrow CD34+ cells from patients with MDS, particularly after failure of response to HMAs, and inhibits colony formation with inhibition of TLR2 signaling restoring colony-formation capacity. In addition, programmed cell-death 1 (PD-1) and programmed cell-death ligand 1 (PD-L1) expression in HSPCs is associated with apoptosis and ineffective hematopoiesis. As will be later discussed, targeting these processes may have therapeutic potential after failure of response to HMAs.
Finally, a recent study suggests two main groups of MDS subtypes may exist, based on the HSPC architecture; one defined by expansion of the common myeloid progenitors (CMPs), and another by expansion of the granulomonocytic progenitors (GMPs). In addition, resistance to HMAs may originate from different HSPC compartments in each of these subgroups and may be a result of distinct biological mechanisms. For instance, upregulation of genes involved in promotion of cell proliferation and survival, such as BCL2, can be observed in long-term hematopoietic stem cells of patients with CMP pattern and progressive disease at the time of failure, while upregulation of genes involved in tumor necrosis factor alpha (TNF-α) is observed in lymphoid-prime multipotent progenitors of patients with GMP pattern.23
However, most of the underlying mechanisms of resistance remain unclear and are likely associated with a diversity of biological processes and, as aforementioned, dependent on specific bone-marrow-cell populations. In order to overcome this resistance, multiple new agents are currently being developed.
Novel therapies to overcome failure of response to hypomethylating agents
Multiple new potential therapeutic avenues are under development, with a number of new molecules and clinical trials specifically for patients with MDS or in studies that included AML and MDS patients (Figure 1, Table 1). As detailed below, many of these agents are capable of achieving blast clearance, but not all of them have been associated with high rates of complete hematological recovery. Although achievement of marrow complete responses (mCRs) has not been associated with shorter OS than achievement of complete responses (CRs),24 the shorter time to progression and complications associated with persistent cytopenias in patients with mCR suggests dose adjustments and adapted schedules of some of these agents may be required to determine if higher rates of high-quality responses are possible.
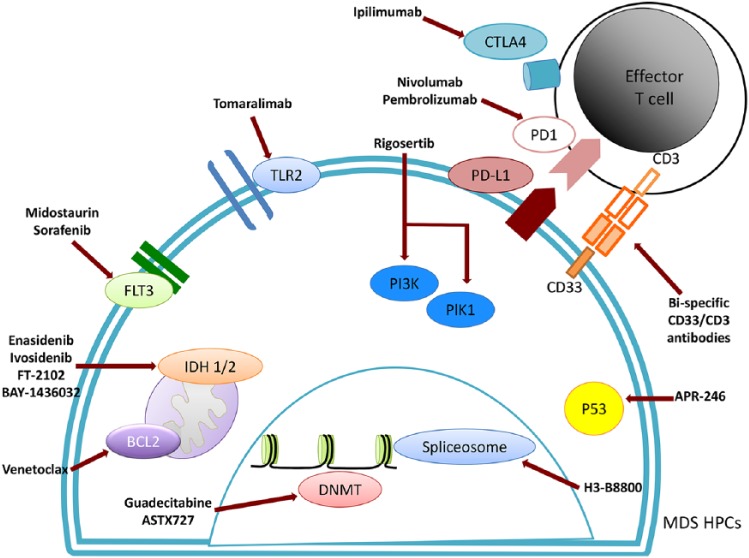
Figure 1.
Novel targets and drugs under development.
DNMT, deoxyribonucleic acid methyltransferase; MDS HPCs, myelodysplastic syndrome hematopoietic stem-cell progenitor.
Table 1.
Clinical trials evaluating new drugs for patients with MDS.
| Agent | ClinicalTrials.gov identifier | Target | Phase | n | Population | Response outcomes | Survival outcomes | Reference |
|---|---|---|---|---|---|---|---|---|
| Guadecitabine | NCT02197676 | DNMT | II |
n = 49 (TN MDS) n = 53 (R/R MDS) |
HR MDS, low-blast AML, CMML | 37% CR + mCR 32% CR + mCR |
Median OS 23.4 months Median OS 11.7 months |
Garcia-Manero et al.19 |
| ASTX727 | NCT02103478 | DNMT | I | n = 43R/R MDS: 47% | MDS, CMML (TN and R/R) | ORR 32% 5 CR, 4 mCR, 5 HI |
NA | García-Manero et al.20 |
| Pembrolizumab | NCT01953692 | PD-1 | Ib | n = 28 | Monotherapy R/R hematologic malignancies | ORR 4%3 CR, 3 HI, 3 mCR, 1 PR | Median OS 23 weeks 2-year OS 49% |
García-Manero et al.25 |
| Nivolumab | NCT02530463 | PD-1 | II | n = 15 | Monotherapy R/R MDS Addition of azacitidine allowed after 4 cycles if NR |
ORR 13% | Median OS 8 months | García-Manero et al.26 |
| Ipilimumab | NCT02530463 | CTLA-4 | II | n = 39 | Monotherapy R/R MDS Addition of azacitidine allowed after 4 cycles if NR |
ORR 35% 3 CR, 3 clearance of mutations |
Median OS 8 months | García-Manero et al.26 |
| Nivolumab + ipilimumab | NCT02530463 | PD-1, CTLA-4 | II | n = 7 | R/R MDS Addition of azacitidine allowed after 4 cycles if NR |
ORR 39% 1 CR, 1 HI |
Median OS 8.4 months | García-Manero et al.27 |
| FT-2102 | NCT02719574 | IDH1 | I |
n = 2 (TN MDS) n = 4 (R/R MDS) |
AML/MDS with IDH mutation | ORR 25% 1 CR | NA | Watts et al.28 |
| Tomaralimab | NCT02363491 | TLR2 | I/II | n = 22 | LR MDS (>50% of patients had received ⩾4 previous therapies) | ORR 50%: 6 TI and 5 had reduction of need for transfusions |
NA | García-Manero et al.29,30 |
| Rigosertib | NCT02562443 | RAS, PI3K, PLK | III |
n = 299Rigosertib = 199 BSC = 100 |
HR R/R MDS | No significant differences in response | Median OS: –Global: 8.2 versus 5.9 months –Primary failure: 8.6 versus 5.3 months Very HR: 7.6 versus 3.2 months |
García-Manero et al.31 |
| Rigosertib + AZA | NCT01926587 | RAS, PI3K, PLK | II |
n = 14 (HMAN) n = 17 (HMA R/R) |
HR MDS, RAEB-t, nonproliferative AML | ORR: 79% (HMAN) 59% (HMA R/R) |
NA | Navada et al.32 |
AML, acute myeloid leukemia; AZA, azacitidine; BSC, best supportive care; CR, complete response; CMML, chronic myelomonocytic leukemia; DNMT, deoxyribonucleic acid methyltransferase; HI, hematological improvement; HMA, hypomethylating agent; HMAN, ; HR, high risk; IDH, isocitrate dehydrogenase; LR, low risk; mCR, marrow complete response; MDS, myelodysplastic syndrome; NA, not available; NR, not reached; ORR, objective response rate; OS, overall survival; PR, partial response; R/R, relapsed/refractory; RAEB-t, refractory anemia with excess blasts in transformation; TI, transfusion independence; TN, treatment naïve.
Novel formulations of hypomethylating agents
Alterations in the transport and metabolism of AZA or DAC might lead to low active drug levels and insufficient incorporation of HMAs into DNA/RNA, resulting in resistance.9 Cytidine deaminase (CDA) is the enzyme responsible for the metabolism and clearance of cytidine analogs, decreasing AZA and DAC active forms. Therefore, higher CDA expression was found to contribute to reduced exposure to HMAs.33 New hypomethylating agents are under development, with longer half-lives, reduced toxicity, and an easier route of administration.
Guadecitabine
Guadecitabine is a next-generation hypomethylating drug administered subcutaneously, with a longer half-life and exposure than its active metabolite DAC. It is a dinucleotide antimetabolite of DAC linked to a deoxyguanosine resistant to CDA. This may result in gradual release of DAC both extra- and intracellularly, leading to more prolonged exposures to DAC. A phase II study evaluated its efficacy in high-risk MDS, chronic myelomonocytic leukemia (CMML) or low-blast count AML patients, refractory or relapsing after AZA.34 The objective response rate (ORR) was 27% and 12% in patients with primary and secondary failure, respectively, and the toxicity profile was similar to that observed in other studies and with conventional HMAs. Recently, long-term results of the phase II study were presented,35 with promising results in these patients, with CR + mCR in 32% of patients with median duration of response, and OS of almost 8 and 12 months, respectively. An ongoing phase III, randomized, open-label study comparing guadecitabine versus treatment choice for patients with MDS and CMML after HMA failure will explore its role in this disease context and its potential future approval [ClinicalTrials.gov identifier: NCT02907359].
ASTX727
ASTX727 is a unique fixed-dose combination of DAC and cedazuridine, a CDA inhibitor (E727). Cedazuridine inhibits the major mechanism by which DAC is degraded in the gut and liver, permitting the efficient delivery of DAC orally. A phase I study25 with a total of 43 MDS patients including 20 patients (47%) with relapsed disease after HMAs, demonstrated an ORR of 32%, with five CRs, four mCRs, and five hematologic improvements. In addition, eight patients achieved transfusion independence. A pharmacokinetic phase II study confirmed that the fixed-dose combination of DAC and cedazuridine emulates intravenous DAC, with a similar safety profile.36 An ongoing phase III study will provide further evidence of its potential activity [ClinicalTrials.gov identifier: NCT03306264].
Histone deacetylase inhibitors (HDACis) and other epigenetic modifiers
Histone acetylation is one of the key essential processes of epigenetic regulation, with histone modifications representing the most diverse mechanisms implicated in chromatin remodeling and regulation of gene expression.26,27 Histone deacetylases (HDACs) are a group of enzymes that reverse covalent modifications within the amino-terminal region of histone proteins, leading to gene expression deregulation. Aberrant expression and function of these regulators is common in MDS. Multiple HDACis have been explored in MDS alone or in combination with HMAs for the past decade, with underwhelming results. In a randomized, placebo-controlled, phase II study of AZA alone or in combination with pracinostat, including 102 patients with MDS, pracinostat was associated with lower CR rates (18% versus 33%, p = 0.07), with increased toxicity, and no improvement in progression-free survival (PFS) or OS.37 Several studies have explored the use of vorinostat in combination with AZA, with an ORR of 27–47%, and no benefit in terms of response and survival compared with AZA.38,39 Other HDACis, such as panobinostat or entinostat, also failed to improve response or survival outcomes compared with AZA monotherapy.40,41
Other ongoing clinical trials are exploring the potential of novel drugs targeting dimethyl lysine demethylase LSD1, such as GSK2879552 [ClinicalTrials.gov identifier: NCT02929498] or tranylcypromine [ClinicalTrials.gov identifiers: NCT02273102, NCT02717884], as well as bromodomain inhibitors such as RO6870810/TEN-010 [ClinicalTrials.gov identifier: NCT02308761] in MDS after failure to respond to HMAs.
Targeting BCL2
BCL2-like 10 (BCL2L10) is an antiapoptotic member of the BCL2 family commonly overexpressed in hematologic malignancies, acquiring antiapoptotic and therapeutic resistance. A prospective study including MDS and AML patients, showed that the overexpression of BCL2L10 in the bone-marrow mononuclear cells was significantly associated with a lower response rate to HMAs and with a shorter OS.42,43
Venetoclax (VEN) is a potent and highly selective BCL2 inhibitor, with high oral bioavailability. Several studies have evaluated the activity of VEN alone or in combination with HMAs in MDS and AML patients. As a single agent for patients with relapsed or refractory (R/R) AML, VEN has demonstrated 19% of ORR in heavily pretreated patients.44 The outcome of VEN combination regimens in R/R AML, MDS and blastic plasmocytoid dendritic-cell neoplasm were reported in a study that included 43 patients.28 In combination with VEN, the majority of patients received either DAC (53%) or AZA (19%); even received by 21 patients (68%) who had already received HMAs as a prior salvage regimen. The ORR was 21%, including two patients who successfully proceeded to allogenic stem-cell transplantation (alloSCT). Median OS was 3 months, with prolonged cytopenias as the most common complication. An ongoing phase I clinical trial is evaluating VEN alone and in combination with AZA in high-risk MDS patients after HMA failure [ClinicalTrials.gov identifier: NCT02966782].
Recently, the US Food and Drug administration (FDA) approved the combination of VEN with HMA in treatment-naïve AML elderly patients (⩾65 years) ineligible for intensive chemotherapy, excluding those patients who had received treatment with HMAs for an antecedent hematologic disorder. It was due to a phase Ib study [ClinicalTrials.gov identifier: NCT02203773] that included 145 patients who received oral VEN at 400, 800, or 1200 mg daily, in combination with DAC or AZA.45 Finally, based on the preliminary safety and efficacy data, only the dosing of 400 and 800 mg were evaluated in the expansion stage. At all VEN doses, CR + CR with incomplete count recovery (CRi) rate was 67%, with a median duration of CR + CRi of 11.3 months, and a median OS of 17.5 months. Moreover, patients with poor-risk cytogenetics and those ⩾75 years had CR + CRi rates of 60% and 65%, respectively. In the VEN 400 mg cohort, CR + CRi rate was 73%, the median duration of CR + CRi was not reached for VEN + AZA and 12.5 months for VEN + DAC, and the median OS was not reached. This combination was well tolerated without any tumor-lysis syndrome in elderly patients with AML. A phase III study of VEN 400 mg combined with AZA in adults with untreated AML ineligible for induction therapy is currently underway [ClinicalTrials.gov identifier: NCT02993523].
Immune-checkpoint regulation
Immune-regulatory proteins such as CTLA-4 and PD-1/PD-L1 play an important role inhibiting the T-cell antitumor immune response. Patients previously treated with HMAs upregulate the inhibitory checkpoint PD1/PD-L1, by reduced methylation of the PD-1 promoter in leukemia cells. For this reason, the antileukemic effect of CD8 cytotoxic T lymphocytes is significantly decreased, potentially contributing to HMA resistance.46 In addition, recent studies suggest that therapy with HMAs may lead to increased double-stranded RNA cytosolic sensing, upregulation of hypermethylated retrovirus genes and type I interferon responses, leading to apoptosis in melanoma.47,48 Although it is unknown whether this mechanism is shared in leukemia, it further supports the notion of combining HMAs with immune-checkpoint regulators and drugs targeting innate immunity signaling. As a result, multiple monoclonal antibodies against immune-checkpoint regulators are being tested in numerous clinical trials.
Pembrolizumab
Pembrolizumab is a humanized monoclonal anti-PD1 antibody (MK-3475) that blocks the interaction between PD-1 and its ligand (PD-L1). Recently, results from a phase Ib study [ClinicalTrials.gov identifier: NCT01953692] have been presented,49 including 28 MDS patients after HMA-treatment failure. Among 27 patients with evaluable response, there were no CRs and 1 patient achieved a partial response (PR), for an ORR of 4%. Among the remaining patients, best overall response was mCR in 3 patients (11%), stable disease in 14 patients (52%), and progressive disease in 9 patients (33%). Hematologic improvement was seen in 3 patients (11%). The OS rate at 24 weeks was 49%. A total of 36% of the patients experienced treatment-related adverse events, most of them grade 1 or 2, without treatment-related deaths. Preliminary data from a phase II trial combining pembrolizumab and AZA in R/R MDS patients were presented.50 Treatment was, overall, well tolerated, and of seven patients, one achieved CR, one demonstrated hematological improvement and five progressed. Therefore, the combination was relatively safe and may have antitumor activity in patients who failed HMA.
Ipilimumab (Ipi) and nivolumab (Nivo)
Ipi is a human immunoglobulin G1 (IgG1) monoclonal anti-CTLA-4 and Nivo is a humanized IgG4 anti-PD-1 monoclonal antibody. A phase II study with 35 MDS patients following failure of response to HMAs51 randomized patients to receive Nivo (15 patients) or Ipi (20 patients), both in monotherapy. ORR was 2/15 (13%) and 7/20 (35%), and the 1-year survival rate was 25% and 45% in Nivo and Ipi, respectively. Both of them reached an OS of 8 months. Moreover, 3/20 (15%) of the patients treated with Ipi achieved CR and clearance of detectable mutations, while single-agent Nivo was not associated with any CR. Ipi monotherapy was also evaluated in a phase Ib study, where 27 high-risk MDS patients that had failed to respond to HMAs were included.52 In this study, ORR was only 3.4%, as one patient achieved mCR as the best response, lasting 3 months. Seven patients achieved stable disease for ⩾46 weeks, and five patients proceeded to alloSCT. The median OS for entire cohort (censoring at time of alloSCT) was 294 days, being longer in patients who received any Ipi maintenance therapy (median OS of 400 days). Also, double immune-checkpoint-inhibitor blockade was performed in a phase II trial, combining Nivo and Ipi in R/R MDS patients.29 Of seven patients, ORR was seen in two out of seven (29%), with one CR and one hematological improvement, and OS was 8.4 months. Treatment had to be held due to rash in two patients and due to creatinine elevation in two patients. Future biological insight into the potential mechanisms explaining the higher activity of CTLA-4 inhibition compared with PD-1 blockade will be required.
Targeted therapies
TP53 mutations
Tumor-suppressor gene TP53 encodes the p53 protein, an indispensable transcription factor that regulates cell cycle and apoptosis. When MDS and AML have TP53 mutations, they associate aggressive clinical course with poor OS and resistance to conventional therapies. TP53 mutations and p53 overexpression have both emerged as independent, negative prognostic features.30 However, whether TP53 mutations predict a higher response rate to HMA therapy remains unclear, with contradictory results having been reported.53 Although a study by Welch and colleagues reported very high response rates to DAC in TP53-mutant AML and MDS,31 a study including 168 untreated MDS patients, including 23% with TP53 mutations, concluded that TP53 mutations did not influence the response to HMAs, but the duration of response was significantly shorter than in wild-type patients, with worse OS.32 Novel therapies targeting p53 as single agents, or in combination, are emerging. APR-246 is a mutant p53 activator with single-agent activity in mutant TP53 AML patients. Recently, an initial report of the results of a phase Ib/II study [ClinicalTrials.gov identifier: NCT03072043] combining APR-246 and AZA was reported.54 Eleven naïve MDS/AML patients with TP53 mutations were included. ORR was 100% with 82% CRs and 18% mCRs, accompanied by deep molecular remissions, and good tolerance to treatment. Median time to first response was 70 days (4–91). At a median follow up of 7 months, the median OS or PFS was not reached. Further follow up of the ongoing phase II and phase III trials will determine if APR-246 is capable of inducing prolonged responses and improved PFS and OS, compared with AZA.
TP53-specific molecular-targeted therapy may have broad importance in hematologic malignancy when TP53 is mutated, but further research is needed on this combination. Although the current phase II and upcoming phase III clinical trials [ClinicalTrials.gov identifier: NCT03745716] exploring the use of AZA with/without APR-246 are limited to newly diagnosed patients, the development of this targeted agent may open the opportunity of its use in patients after failure of response to HMAs.
IDH mutations
Mutations in isocitrate dehydrogenase 1 or 2 (IDH1, IDH2) lead to aberrant DNA hypermethylation, arrest differentiation of hematopoietic progenitor cells and, ultimately, promote leukemogenesis. IDH mutations are rare in myeloid malignancies, occurring in 4–12% of patients with MDS.55 Multiple IDH inhibitors are under development as single agents, and in combination with intensive chemotherapy or HMAs with promising responses, although the response may take up to 6 months. Enasidenib and ivosidenib are oral, targeted, small-molecule inhibitors of mutant IDH1 and IDH2 proteins, respectively.
Enasidenib
The FDA approved enasidenib in August 2017 in patients with R/R AML with IDH2 mutation. The approval was based on the results of a phase I/II study for patients with AML, including 30 patients with previous MDS; all of them had received prior therapy.56 Therapy with enasidenib was associated with an ORR of 40%, including 19% CRs, and a median OS of 9.3 months. However, the median time to best response was 3.7 months, with median response duration of 5.8 months. The original phase I study included 15 MDS patients,57 69% of whom had previously received HMAs, and among these, the ORR was 50%, including one CR, and the median OS was not reached after 4.7 months.
Ivosidenib
Ivosidenib is an oral, targeted, small-molecule inhibitor of mutant IDH1 enzyme, recently approved in July 2018 for patients with R/R AML and IDH1 mutation. Approval was based on a phase I clinical trial with a total of 125 patients [ClinicalTrials.gov identifier: NCT02074839], achieving an ORR of 42% with 22% of CRs, and a median OS of 8.8 months. Moreover, 21% of the patients with CRs achieved molecular remission.58 However, the median time to response was 2.8 months, and the median response duration was 8.2 months. A total of 258 patients were finally enrolled in the clinical trial, 12 of them had R/R MDS. These MDS patients were recently analyzed separately,59 with an ORR of 91.7% [95% confidence interval (CI) 61.5%, 99.8%], with five CRs (41.7%; 95% CI 15.2%, 72.3%) and six mCR (50.0%). Moreover, four of five patients became transfusion independent, and any adverse events led to permanent discontinuation of treatment.
Other inhibitors of mutant IDH1: FT-2102 and BAY-1436032
FT-2102 is an oral, highly potent, selective small-molecule inhibitor of mutant IDH1. An ongoing phase I trial is evaluating FT-2102 as a single agent in patients with MDS/AML [ClinicalTrials.gov identifier: NCT02719574]. Six enrolled patients had MDS, two were treatment naïve and four had R/R disease. Of four evaluable patients, ORR was 25%, with one CR.60 Most treatment-emergent adverse events were grade 1 or 2, with no deaths considered related to the IDH1 inhibitor. FT-2102 is being studied in combination with AZA or cytarabine. BAY-1436032 is an oral, panmutant IDH1 inhibitor active against all IDH1R132 mutation types, that showed strong antileukemic activity in two independent AML xenograft mouse models.61 Clinical development is ongoing, with a first in-man study in IDH1-mutant solid tumors.
FLT3 mutations
The FLT3 gene encodes the FLT3 tyrosine kinase receptor expressed on the surface of CD34+ HPSCs and other immature hematopoietic progenitors. The FLT3 pathway has an important role in cellular proliferation, survival, and differentiation of myeloid progenitors. FLT3 mutations occur in less than 1% of the MDS patients, increasing the incidence at the time of transformation to AML after HMA therapy, and inducing a constitutive activation of FLT3.62,63 Multiple FLT3 inhibitors have been developed in AML, with midostaurin and gilteritinib having been approved by the FDA. Nowadays, combination strategies of FLT3 inhibitors are being investigated to explore whether deeper and more durable responses can be achieved. Although there have not been any MDS specific studies, this opens the door to new potential therapies for a small subset of patients with MDS.
Midostaurin
Midostaurin is an oral multitargeted kinase inhibitor active in FLT3 mutations. It was studied in combination with AZA in 54 AML and high-risk MDS patients with an ORR of 26%, achieving one CR with minimal comorbidity.64 Similar results are published with the combination of AZA with sorafenib, another multikinase inhibitor.65 However, midostaurin has recently been approved in combination with 7+3 chemotherapy for patients with newly diagnosed AML. The FDA approved this combination due to a phase III trial where 717 newly diagnosed AML patients with FLT3 mutations were included. Patients were randomized to standard chemotherapy plus either midostaurin or placebo, concluding that OS and event-free survival (EFS) were significantly longer in the midostaurin group [hazard ratio (HR) 0.78, one-sided p = 0.009; and HR 0.78, one-sided p = 0.002, respectively].66 More novel and specific FLT3 inhibitors such as gilteritinib, which was recently approved by the FDA for the treatment of AML,67 may be a potentially more desirable FLT3 inhibitor, given its activity, with up to 18% CR in monotherapy, and better tolerability with lower frequency of myelosuppression and cytopenias.68
Splicing machinery mutations
Mutations in the spliceosomal genes SRSF2, U2AF1, ZRSR2 and SF3B1 are the most common class of mutations in patients with MDS. These mutations are always heterozygous and are exclusive with one another, suggesting that cells may tolerate only a partial deviation from normal splicing activity. Accordingly, leukemias with spliceosomal gene mutations are preferentially susceptible to additional splicing perturbations. This explains why modulation of spliceosome machinery is a new therapeutic approach in MDS and AML patients harboring spliceosome mutations. E7107 is a spliceosome inhibitor that was used to treat Srsf2P95H-mutant engineered mice, achieving a greater splicing inhibition and reduction in leukemic burden compared with Srsf2 wild type.69 A phase I study of H3-B8800, a splicing modulator, is currently ongoing for patients with previously treated MDS, AML and CMML [ClinicalTrials.gov identifier: NCT02841540].
Targeting innate immunity
TLRs are a family of pattern-recognition receptors that play a pivotal role in the innate immunity, by the activation of NF-κB and the expression of multiple cytokines. TLR2 is overexpressed in the bone-marrow CD34+ cells of MDS patients, especially after HMA therapy, resulting in ineffective hematopoiesis and, ultimately, in hematopoietic malignancies.70 Consequently, this innate immunity signaling pathway is a new potential target in MDS patients.
Tomaralimab (OPN-305)
Tomaralimab is a fully humanized antagonistic IgG4-κ monoclonal antibody that binds TLR2. In a phase I/II trial [ClinicalTrials.gov identifier: NCT02363491],71,72 tomaralimab was administered to 22 low-risk MDS patients, all of them heavily pretreated (>50% of patients had received ⩾4 previous therapies) and transfusion dependent. ORR was 50%; 6/22 (27%) patients achieved transfusion independence for two consecutive cycles and 5/22 (23%) patients achieved at least a 50% reduction in the need for transfusions. There were no dose-limiting toxicities or development of antidrug antibodies. Interestingly, a trend to increased response was observed in patients with higher TLR2 expression, with no differences in response based on cytogenetic or mutational profile.
Multikinase inhibition: rigosertib
Rigosertib is a small-molecule inhibitor of cellular signaling which acts by binding to the Ras-binding domain of multiple kinases, including RAS, PI3K and PLK. These kinases control some cellular signaling pathways often activated in hematological malignancies. Through these interactions, these proteins result in inactivation, leading to mitotic arrest and apoptosis of tumor cells.73 In a phase III trial,74 patients with high-risk MDS after failure to respond to HMAs were randomized to rigosertib plus best supportive care (BSC) versus BSC. No patients had an overall CR or PR, but 27% patients in the rigosertib group and 17% in the BSC group achieved a confirmed best bone-marrow blast response of either bone-marrow CR or bone-marrow PR. In terms of hematological improvement, there were no differences between both groups. Median OS was 8.2 months in the rigosertib group and 5.9 months in the BSC group [HR 0.87 (95% CI 0.67–1.14); p = 0.33]. Median OS improved in favor of rigosertib among very high-risk MDS patients, and in patients with primary HMA failure (median OS 7.6 versus 3.2 months, and 8.6 versus 5.3 months, respectively).
Recently, results of a phase II study of the combination of rigosertib with AZA in 17 R/R MDS patients were published, with an ORR of 59%.75 A randomized phase III trial of rigosertib [ClinicalTrials.gov identifier: NCT02562443] is under development in a high-risk MDS population to confirm the potential role of this agent in this group of patients.
Bispecific CD33/CD3 antibodies
The bispecific T-cell engaging antibodies with dual specificity are antibodies that bind both CD3 T-cell receptor and CD33, which is frequently expressed on the surface of AML blasts and leukemic stem cells.76 This creates an immune synapse, initiating lysis of CD33-expressing cells by T cells, and inducing expansion, differentiation and proliferation of T cells. AMG330 and AMV564 are two novel, bivalent, bispecific CD33/CD3-targeted immunotherapies, that are being studied in phase I clinical trials in R/R AML patients [ClinicalTrials.gov identifiers: NCT02520427 and NCT03144245, respectively]. First results from the AMV564 trial have been recently reported, in 18 R/R AML patients.77 Reduction in bone-marrow blasts, ranging from 13% to 91%, was observed in 12/18 (67%) patients, including a PR after cycle 1 in one patient, with no dose-limiting toxicity or treatment-related grade ⩾ 3 adverse events. An ongoing phase I study is evaluating its safety and activity in patients with intermediate or high-risk MDS [ClinicalTrials.gov identifier: NCT03516591].
Cytotoxic drugs and combinations
Intensive chemotherapy and its different combinations may be considered a valid option in selected patients after HMA-treatment failure. However, due to the high toxicity of these regimens, it is important to identify which patients will benefit most from induction chemotherapy. Researchers in a study including 307 MDS patients after HMA failure, randomized patients to either classical induction chemotherapy with 7+3, intermediate- to high-dose cytarabine (IDAC), or purine-nucleoside-analog (PNA) based (fludarabine, cladribine, clofarabine).78 The ORR was 41%, 64%, and 34% with IDAC and PNA induction chemotherapy, respectively, without differences in the median OS between the three groups. Age > 65, adverse cytogenetics, and the use of non-IDAC-containing regimens, were identified as factors that significantly decreased response to these cytotoxic therapies. These results support the use of induction chemotherapy as a reasonable platform to treat MDS after HMA failure in younger patients with adequate organ function and performance status, and absence of higher-risk disease features. In addition to the conventional chemotherapy regimen, other lower-intensity combinations using clofarabine in combination with low-dose cytarabine and CPX-351 can be options for this population and a subset of elderly patients with HMA failure.
Clofarabine
Clofarabine is a second-generation nucleoside analog with potential role in HMA failure. Low-dose clofarabine was studied in combination with cytarabine in a large cohort of patients with higher-risk MDS after HMA failure.79 Seventy MDS patients were included, of whom 82% had high-risk disease, 23% had therapy-related MDS, 56% had a previous response to HMAs, and 44% had primary refractory disease. ORR was 44%, with 19% CRs, and median OS was 10 months. Furthermore, 30% of the responders were able to proceed to alloSCT, 56% of whom achieved long-lasting remission. A previous response to HMAs did not affect the outcome of these patients; the presence of a complex karyotype, a platelet count < 30 × 109/l and a poor performance status being the only independent factors associated with poor survival. Treatment was generally well tolerated but was associated with relatively high rates of fever and infections that required significant supportive care.
CPX-351
A liposomal formulation of cytarabine: daunorubicin at a 5:1 molar ratio was recently approved by the FDA for secondary AML (sAML), and newly diagnosed therapy-related AML. A phase II randomized study compared CPX-351 versus 7+3.80 sAML patients treated with CPX-351 improved ORR, EFS and OS. Subsequently, these results were confirmed in a phase III randomized study [ClinicalTrials.gov identifier: NCT0169608] including 309 newly diagnosed high-risk/sAML patients treated with the liposomal formulation.81 In view of the apparent activity of this compound in AML with MDS-related changes and secondary AML, this opens the possibility of future studies using CPX-351 for patients with higher-risk disease after HMA failure.
How to treat patients with lower-risk MDS after failure to respond to HMAs:
Patients with lower-risk MDS who continue to exhibit features of low-risk disease at the time of response failure to HMAs, although classified as low risk by models such as IPSS and IPSS-R, have an OS of 12−14 months. In the absence of approved drugs, selecting adequate therapy remains a challenge.
All patients should be offered therapy within a clinical trial whenever possible. In the presence of targetable mutations (IDH1/2, SRSF2, SF3B1, U2AF1, ZRSR2), enrollment in clinical trials of enasidenib, ivosidenib or other IDH1 inhibitors/H3-B8800, should be considered. Although off-label use of enasidenib or ivosidenib could be considered, there are currently very limited data of their use in MDS and we recommend its use be limited in the context of clinical trials. For patients with no targetable mutations, clinical trials with immune-checkpoint regulators or agents targeting innate immunity could be an option. The transforming-growth-factor-beta pathway inhibitors luspatercept and sotatercept have demonstrated high clinical activity in pretreated lower-risk MDS patients with anemia refractory to erythropoietic stimulating agents and HMAs. Luspatercept treatment achieved 63% of erythroid hematologic improvement (HI-E) in 51 patients, with 38% of them achieving transfusion independence.82 Similar results were observed with sotatercept therapy, with 49% of HI-E in 74 patients.83 Lenalidomide is another therapeutic option for patients with isolated anemia and no significant thrombocytopenia, even in the absence of del(5q) aberration.84 The use of this agent in this subset of patients can induce HI-E in up to 12% patients, with a rate of progression to AML of 11% and median OS of 87 months.85 Additionally, although associated with modest- and short-lasting responses, sequential therapy with an alternative hypomethylating agent can be considered.
AlloSCT remains the only therapeutic approach with curative potential in these patients. However, it is associated with significant toxicity and mortality, thus limiting the number of patients who can potentially benefit from this therapy.10,86 Moreover, patients who have failed HMAs have higher risk of relapse after alloSCT, and consequently, of death.87 Among 125 MDS patients, the probability of relapse after alloSCT at 3 years was 56.6% for patients with failure to HMA compared with 34.2% among responders.
Median OS of 438 low-risk MDS patients after response failure to HMAs8 was 10, 28, 17, and 39 months, for patients not receiving further therapy and for those treated with conventional agents, investigational agents, and alloSCT, respectively. This suggests transplant after response failure to HMAs may be the best potential long-term therapy in these patients. Predicting which patients are most likely to benefit from transplantation is thus a central challenge. Careful planning, including adequate patient and donor selection, pretransplant therapy, timing of the transplant, and post-transplant therapy are fundamental.
How to treat patients with higher-risk MDS after HMA failure
Survival of patients with higher-risk MDS after HMA failure is short (4–6 months). This underscores the need to develop new therapies. As detailed earlier, a number of new potential agents are being developed. Adequately selecting the best therapeutic approach is fundamental to ensure the best possible clinical outcomes. Disease biology, including features such as karyotype and mutation profile, as well as patient characteristics, should be considered when selecting therapy. Clinical trials with targeted agents such as enasidenib, ivosidenib, FT-2102, H3-B8800, midostaurin or other FLT3 inhibitors should be considered in patients with IDH1/2, splicing or FLT3 mutations, respectively. Although off-label therapy with venetoclax in combination with HMA or low-dose cytarabine could be considered in patients who experience transformation to AML,88 most available data of these combinations are restricted to previously untreated patients. Moreover, given the absence of data evaluating the use of venetoclax in high-risk MDS after response failure to HMAs, we can only recommend its use in the context of a clinical trial. In the absence of a clinical trial, as in low-risk disease, sequential use of another HMA can be considered but, as previously mentioned, the likelihood of achieving a significant and long-lasting response is low. For fit patients, lower doses of chemotherapy, including purine analogs such as cladribine or clofarabine in combination with low-dose cytarabine should be considered, particularly in the subset of patients with normal karyotype and in the presence of NPM1 mutations.
AlloSCT should always be considered in these patients. Current advances in transplant technology are allowing older patients to be considered as candidates for transplant, with early data suggesting transplant with alternative donors, such as haploidentical transplantation, may be feasible, particularly in the context of clinical trials.89,90 In addition, recent studies suggest reduced-intensity conditioning may be able to achieve similar relapse-free survival (RFS) and OS in patients with MDS,91 and that comorbidity and disease-risk indices may be more relevant in outcomes than chronological age.92 In a study evaluating survival of 270 higher-risk MDS patients, median OS of the alloSCT patients was 19 months.93 Of these patients, 38% went to transplant with progressive disease after HMA failure, and 38% with stable disease, with median OS of 17 months, and not reached, respectively. Moreover, the median OS of the higher-risk MDS patients who underwent alloSCT, including the ones with progressive disease, was significantly superior to the ones that received BSC, low-dose chemotherapy, intensive chemotherapy, or investigational therapies (median OS of 4, 7.3, 8.9, and 13 months, respectively).
Recently, somatic mutations in patients with MDS at the time of transplantation have been linked to survival outcomes.94 ASXL1, RUNX1, and TP53 mutations are associated with shorter survival and shorter time to relapse after alloSCT, with TP53 mutations being particularly adverse. RAS pathway mutations at the time of transplantation have significantly elevated risk of early relapse, which may be overcome with myeloablative conditioning. Also, JAK2 mutations are associated with a higher rate of death without relapse, regardless of conditioning intensity.95 Studying somatic mutations may provide information about the outcomes in patients with MDS who are going to receive alloSCT, improving decision making. In addition, a recent study also suggests mutation clonal burden prior to transplant may also influence outcomes.96 The use of next-generation targeting sequencing, may help identify subgroups of patients who will derive the most benefit from particular conditioning regimens.
In order to try to reduce disease recurrence after transplantation, current data support the concept of post-transplant maintenance regimens, especially in patients with high-risk features. The dual activity of AZA as an antileukemic agent and inhibitor of graft versus host disease (GVHD) makes it a promising agent for post-transplant therapy.97,98 The first prospective multicenter trial using AZA as salvage therapy for relapse of myeloid malignancies after alloSCT included 30 patients, in whom an ORR of 47% achieved, with 23% CRs with severity of GVHD, as well as somewhat mild toxicities.99 A median of three courses of AZA were administered, and 73% also received donor lymphocyte infusions. Moreover, it has been shown that this treatment is particularly valuable in patients with low disease burden and in MDS patients rather than AML.97,100 Recently, results from a study of 187 patients comparing low dose of AZA maintenance versus observation were presented. Although less than 30% of the AZA patients completed the planned 12 cycles, the study showed a trend toward improvement in RFS in the patients receiving more AZA cycles.101 Maintenance with CC-486, a novel oral AZA formulation that allows extended dosing and prolonged activity, has also been explored, although with limited data on frequency of prior therapy with HMAs.98 Thirty patients in CR after alloSCT received CC-486 once daily for 7 or 14 days in a 28-day cycle for up to 12 cycles and among these, 9 had received AZA prior to transplant. Median OS was not reached at a median follow up of 19 months, and estimated 1-year survival rates were above 80%. The incidence of chronic GVHD was 10%, and only two patients discontinued the study due to a GVHD event.
Therefore, HMAs may be an important new therapy in selected patients after alloSCT, but when to initiate therapy after transplant, and how long to continue it, are still unclear aspects of this approach. In addition, future studies will have to determine whether use of these agents will benefit patients who underwent alloSCT after failure of response to HMAs and if the addition of other targeted agents may improve post-transplant outcomes in patients with MDS. The use of next-generation sequencing could help to identify patients with measurable residual disease, which might benefit most as a maintenance therapy.98
Conclusion
Once MDS patients relapse or become refractory to HMA, their outcome is poor and there are no approved standard-of-care options. Why patients become resistant to HMA is still unclear, but certain immune upregulation and gene mutations may be involved. As a result, multiple novel, targeted therapies and immune therapies are under development, and will likely open new treatment options for subsets of these patients. Therefore, treatment within a clinical trial should always be considered whenever available, for which a good knowledge of their cytogenetic and molecular characteristics is necessary. However, the only current treatment with curative potential is alloSCT. Despite being associated with significant toxicity and mortality and higher risk of relapse in the context of HMA failure, both lower- and higher-risk patients with failure to respond to HMAs should be considered for transplant, with potential post-transplant maintenance strategies. Selecting which patients may benefit the most from alloSCT remains a vitally important area of research.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Guillermo Montalban-Bravo  https://orcid.org/0000-0002-4533-5176
https://orcid.org/0000-0002-4533-5176
Contributor Information
Angela Gil-Perez, University Hospital of Guadalajara, Guadalajara, Spain.
Guillermo Montalban-Bravo, Department of Leukemia, University of Texas, MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77015, USA.
References
- 1. Nimer SD. Myelodysplastic syndromes. Blood 2008; 111: 4841–4852. [DOI] [PubMed] [Google Scholar]
- 2. Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system (IPSS-R ) for myelodysplastic syndromes. Blood 2012; 120: 2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kantarjlan H, JPJ Issa, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 2006; 106: 1794–1803. [DOI] [PubMed] [Google Scholar]
- 5. Jabbour E, Short NJ, Montalban-Bravo G, et al. Randomized phase 2 study of low-dose decitabine vs low-dose azacitidine in lower-risk MDS and MDS / MPN. Blood 2017; 130: 1514–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jabbour E, Garcia-Manero G, Batty N, et al. Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer 2010; 116: 3830–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nazha A, Sekeres MA, Komrokji R, et al. Validation of a post-hypomethylating agent failure prognostic model in myelodysplastic syndromes patients treated in a randomized controlled phase III trial of rigosertib vs. best supportive care. Blood Cancer J 2017; 7: 17–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jabbour E, Garcia-Manero G, Strati P, et al. Outcome of patients with low-risk and intermediate-1-risk myelodysplastic syndrome after hypomethylating agent failure. Cancer 2015; 121: 876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang H, Li Y, Lv N, et al. Predictors of clinical responses to hypomethylating agents in acute myeloid leukemia or myelodysplastic syndromes. Ann Hematol 2018; 97: 2025–2038. [DOI] [PubMed] [Google Scholar]
- 10. Malcovati L, Hellström-Lindberg E, Bowen D, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults : recommendations from the European LeukemiaNet. Blood 2018; 122: 2943–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bejar R, Lord A, Stevenson K, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood 2014; 124: 2705–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Itzykson R, Thepot S, Quesnel B, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Am J Hematol 2011; 117: 403–411. [DOI] [PubMed] [Google Scholar]
- 13. Miao X-R, Chen Q-B, Wei K, et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood 2016; 113: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci 2010; 107: 7473–7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Voso MT, Santini V, Fabiani E, et al. Why methylation is not a marker predictive of response to hypomethylating agents. Haematologica 2014; 99: 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Damaraju VL, Mowles D, Yao S, et al. Role of human nucleoside transporters in the uptake and cytotoxicity of azacitidine and decitabine. Nucleosides Nucleotides Nucleic Acids 2012; 31: 236–255. [DOI] [PubMed] [Google Scholar]
- 17. Valencia A, Masala E, Rossi A, et al. Expression of nucleoside-metabolizing enzymes in myelodysplastic syndromes and modulation of response to azacitidine. Leukemia 2014; 28: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sripayap P, Nagai T, Uesawa M, et al. Mechanisms of resistance to azacitidine in human leukemia cell lines. Exp Hematol 2014; 42: 294–306. [DOI] [PubMed] [Google Scholar]
- 19. Unnikrishnan A, Papaemmanuil E, Beck D, et al. Integrative genomics identifies the molecular basis of resistance to azacitidine therapy in myelodysplastic syndromes. Cell Rep 2017; 20: 572–585. [DOI] [PubMed] [Google Scholar]
- 20. Dimicoli S, Wei Y, Bueso-ramos C, et al. Overexpression of the toll-like receptor (TLR) signaling adaptor MYD88, but lack of genetic mutation in myelodysplastic syndromes. PLoS One 2013; 8: e71120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei Y, Dimicoli S, Chen R, et al. Toll-like receptor alterations in myelodysplastic syndrome. Leukemia 2013; 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang H, DiNardo C, Davanlou M, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 2013; 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ganan-Gomez I, Alfonso A, Yang H, et al. Cell-type specific mechanisms of hematopoietic stem cell (HSC) expansion underpin progressive disease in myelodysplastic syndromes (MDS) and provide a rationale for targeted therapies. Blood 2018; 132: 1798. [Google Scholar]
- 24. Alfonso Pierola A, Montalban-Bravo G, Takahashi K, et al. Impact of marrow complete response in the natural history of patients with myelodysplastic syndromes (MDS) and chronic myelomonocytic leukemia (CMML) treated with hypomethylating agents. Presented at the EHA Learning Center, Madrid, Spain, 24 June 2017, Abstract P665. [Google Scholar]
- 25. Garcia-Manero G, Odenike O, Amrein PC, et al. Successful emulation of IV decitabine pharmacokinetics with an oral fixed-dose combination of the oral cytidine deaminase inhibitor (CDAi) E7727 with oral decitabine, in subjects with myelodysplastic syndromes (MDS): final data of phase 1 study. Blood 2016; 128: 114. [Google Scholar]
- 26. Baylin SB, Jones PA. A decade of exploring the cancer epigenome — biological and translational implications. Nat Rev Cancer 2011; 11: 726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev 2014; 2: 1–19. [DOI] [PubMed] [Google Scholar]
- 28. DiNardo CD, Rausch CR, Benton C, et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol 2018; 93: 401–407. [DOI] [PubMed] [Google Scholar]
- 29. García-Manero G, Montalbán-Bravo G, Sasaki K, et al. Double immune checkpoint inhibitor blockade with nivolumab and ipilimumab with or without azacitidine in patients with myelodysplastic syndrome (MDS). Presented at the 2018 ASH Annual Meeting, Abstract 1831. [Google Scholar]
- 30. Zhang L, McGraw KL, Sallman DA, et al. The role of p53 in myelodysplastic syndromes and acute myeloid leukemia: molecular aspects and clinical implications. Leuk Lymphoma 2017; 58: 1777–1790. [DOI] [PubMed] [Google Scholar]
- 31. Welch J, Petti A, Miller C, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med 2016; 375: 2023–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takahashi K, Patel K, Bueso-Ramos C, et al. Clinical implications of TP53 mutations in myelodysplastic syndromes treated with hypomethylating agents. Oncotarget 2016; 7: 14172–14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mahfouz RZ, Jankowska A, Ebrahem Q, et al. Increased CDA expression/activity in males contributes to decreased cytidine analog half-life and likely contributes to worse outcomes with 5-azacytidine or decitabine therapy. Clin Cancer Res 2013; 19: 938–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sebert M, Bally C, Peterlin P, et al. Results of a phase II study of guadecitabine (SGI-110) in higher risk MDS, CMML or low blast count AML patients refractory to or relapsing after azacitidine (AZA) treatment. Blood 2016; 128: 347. [Google Scholar]
- 35. Garcia-Manero G, Ritchie EK, Walsh KJ, et al. Long term results of a randomized phase 2 dose-response study of guadecitabine, a novel subcutaneous (SC) hypomethylating agent (HMA), in 102 patients with intermediate or high risk myelodysplastic syndromes (MDS) or chronic myelomonocytic leukemia (CMML). Presented at the 2018 ASH Annual Meeting and Exposition, Abstract 231. [Google Scholar]
- 36. Garcia-Manero G, Griffiths EA, Roboz GJ, et al. Phase 2 dose-confirmation study of oral ASTX727, a combination of oral decitabine with a cytidine deaminase inhibitor (CDAi) cedazuridine (E7727), in subjects with myelodysplastic syndromes (MDS). Blood 2017; 130: 4274. [Google Scholar]
- 37. Garcia-Manero G, Montalban-Bravo G, Berdeja JG, et al. Phase 2, randomized, double-blind study of pracinostat in combination with azacitidine in patients with untreated, higher risk myelodysplastic syndromes. Cancer 2017; 123: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Montalban-Bravo G, Huang X, Jabbour E, et al. A clinical trial for patients with acute myeloid leukemia or myelodysplastic syndromes not eligible for standard clinical trials. Leukemia 2017; 31: 318–324. [DOI] [PubMed] [Google Scholar]
- 39. Sekeres MA, Othus M, List AF, et al. Randomized phase II study of azacitidine alone or in combination with lenalidomide or with vorinostat in higher-risk myelodysplastic syndromes and chronic myelomonocytic leukemia: North American intergroup study SWOG S1117. J Clin Oncol 2017; 35: 2745–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garcia-Manero G, Sekeres MA, Egyed M, et al. A phase 1b/2b multicenter study of oral panobinostat plus azacitidine in adults with MDS, CMML, or AML with > 30% blasts. Leukemia 2017; 31: 2799–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prebet T, Sun Z, Ketterling RP, et al. Azacitidine with or without Entinostat for the treatment of therapy-related myeloid neoplasm : further results of the E1905 North American Leukemia Intergroup study. Br J Haematol 2016; 172: 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Konopleva M, Zhao S, Hu W, et al. The anti-apoptotic genes Bcl-XL and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Br J Haematol 2002; 118: 521–534. [DOI] [PubMed] [Google Scholar]
- 43. Vidal V, Robert G, Goursaud L, et al. BCL2L10 positive cells in bone marrow are an independent prognostic factor of azacitidine outcome in myelodysplastic syndrome and acute myeloid leukemia. Oncotarget 2017; 8: 47103–47109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase 2 myelogenous leukemia. Cancer Discov 2016; 6: 1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boddu P, Kantarjian H, Garcia-Manero G, et al. The emerging role of immune checkpoint based approaches in AML and MDS. Leuk Lymphoma 2018; 59: 790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Retroviruses E, Chiappinelli KB, Pamela L, et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including article inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 2015; 162: 974–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Strick R, Strissel PL, Baylin SB, et al. Unraveling the molecular pathways of DNA-methylation inhibitors: human endogenous retroviruses induce the innate immune response in tumors. Oncoimmunology 2015; 5: e1122160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garcia-Manero G, Tallman MS, Martinelli G, et al. Pembrolizumab, a PD-1 inhibitor, in patients with myelodysplastic syndrome (MDS) after failure of hypomethylating agent treatment. Blood 2016; 128: 344. [Google Scholar]
- 50. Chien KS, Cortes JE, Borthakur G, et al. Preliminary results from a phase II study of the combination of azacitidine and pembrolizumab in patients with higher-risk myelodysplastic syndrome. Presented at the 2018 ASH Annual Meeting, Abstract 464. [Google Scholar]
- 51. Garcia-Manero G, Sasaki K, Montalbán-Bravo G, et al. A phase II study of nivolumab or ipilimumab with or without azacitidine for patients with myelodysplastic syndrome (MDS). Presented at the 2018 ASH Annual Meeting, Abstract 465. [Google Scholar]
- 52. Zeidan AM, Knaus HA, Robinson TM, et al. A multi-center phase 1 trial of ipilimumab in myelodysplastic syndrome patients following hypomethylating agent failure. Clin Cancer Res 2018; 24: 3519–3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Probst LA, Pharm D, Welch TR. Decitabine in TP53 -Mutated AML. N Engl J Med 2017; 376: 796–798. [DOI] [PubMed] [Google Scholar]
- 54. Sallman DA, DeZern A, Sweet K, et al. Phase Ib/II combination study of APR-246 and azacitidine (AZA) in patients with TP53 mutant myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). Blood 2018; 132: 3091. [Google Scholar]
- 55. DiNardo CD, Jabbour E, Ravandi F, et al. IDH1 and IDH2 mutations in myelodysplastic syndromes and role in disease progression. Leukemia 2016; 30: 980–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017; 130: 722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stein EM, Fathi AT, DiNardo CD, et al. Enasidenib (AG-221), a potent oral inhibitor of mutant isocitrate dehydrogenase 2 (IDH2) enzyme, induces hematologic responses in patients with myelodysplastic syndromes (MDS). Blood 2016; 128: 343. [Google Scholar]
- 58. DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1 -mutated relapsed or refractory AML. N Engl J Med 2018; 378: 2386–2398. [DOI] [PubMed] [Google Scholar]
- 59. DiNardo CD, Watts JM, Stein EM, et al. Ivosidenib (AG-120) induced durable remissions and transfusion independence in patients with IDH1-mutant relapsed or refractory myelodysplastic syndrome: results from a phase 1 dose escalation and expansion study. Presented at the 2018 ASH Annual Meeting, Abstract 1812. [Google Scholar]
- 60. Watts JM, Baer MR, Yang J, et al. A phase 1 dose escalation study of the IDH1m inhibitor, FT-2102, in patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS). Presented at the 2018 ASH Annual Meeting, Abstract 1453. [Google Scholar]
- 61. Heuser M, Herbst L, Pusch S, et al. Pan-mutant-IDH1 inhibitor bay-1436032 is highly effective against human IDH1 mutant acute myeloid leukemia in vivo. Blood 2016; 128: 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. El Fakih R, Rasheed W, Hawsawi Y, et al. Targeting FLT3 mutations in acute myeloid leukemia. Cells 2018; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Badar T, Patel KP, Thompson PA, et al. Detectable FLT3-ITD or RAS mutation at the time of transformation from MDS to AML predicts for very poor outcomes. Leuk Res 2015; 39: 1367–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Strati P, Kantarjian H, Ravandi F, et al. Phase I/II trial of the combination of midostaurin (PKC412) and 5-azacytidine for patients with acute myeloid leukemia and myelodysplastic syndrome. Am J Hematol 2015; 90: 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ravandi F, Alattar ML, Grunwald MR, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood 2013; 121: 4655–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 Mutation. N Engl J Med 2017; 377: 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dhillon S. Gilteritinib: first global approval. Drugs 2019; 79: 331–339. [DOI] [PubMed] [Google Scholar]
- 68. Perl AE, Altman JK, Cortes J, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia : a multicentre, first-in-human, open-label, phase 1 – 2 study. Lancet Oncol 2017; 18: 106–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee SCW, Dvinge H, Kim E, et al. Modulation of splicing catalysis for therapeutic targeting of leukemia with mutations in genes encoding spliceosomal proteins. Nat Med 2016; 22: 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wei Y, Dimicoli S, Bueso-Ramos C, et al. Toll-like receptor alterations in myelodysplastic syndrome. Leukemia 2013; 27: 1832–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Garcia-Manero G, Montalban-Bravo G, Yang H, et al. A clinical study of OPN-305, a toll-like receptor 2 (TLR-2) antibody, in patients with lower risk myelodysplastic syndromes (MDS) that have received prior hypomethylating agent (HMA) therapy. Blood 2016; 128: 227.27099149 [Google Scholar]
- 72. Garcia-Manero G, Jabbour EJ, Konopleva MY, et al. A clinical study of tomaralimab (OPN-305), a toll-like receptor 2 (TLR-2) antibody, in heavily pre-treated transfusion dependent patients with lower risk myelodysplastic syndromes (MDS) that have received and failed on prior hypomethylating agent (HMA) therapy. Presented at the 2018 ASH Annual Meeting, Abstract 798. [Google Scholar]
- 73. Navada SC, Fruchtman SM, Odchimar-Reissig R, et al. A phase 1/2 study of rigosertib in patients with myelodysplastic syndromes (MDS) and MDS progressed to acute myeloid leukemia. Leuk Res 2018; 64: 10–16. [DOI] [PubMed] [Google Scholar]
- 74. Garcia-Manero G, Fenaux P, Al-Kali A, et al. Rigosertib versus best supportive care for patients with high-risk myelodysplastic syndromes after failure of hypomethylating drugs (ONTIME): a randomised, controlled, phase 3 trial. Lancet Oncol 2016; 17: 496–508. [DOI] [PubMed] [Google Scholar]
- 75. Navada SC, Garcia-Manero G, Atallah EL, et al. Phase 2 expansion study of oral rigosertib combined with azacitidine (AZA) in patients (Pts) with higher-risk (HR) myelodysplastic syndromes (MDS): efficacy and safety results in HMA treatment naïve & relapsed (Rel)/refractory (Ref) patients. Presented at the 2018 ASH Annual Meeting, Abstract 230. [Google Scholar]
- 76. Reusch U, Harrington KH, Gudgeon CJ, et al. Characterization of CD33/CD3 tetravalent bispecific tandem diabodies (TandAbs) for the treatment of acute myeloid leukemia. Clin Cancer Res 2016; 22: 5829–5838. [DOI] [PubMed] [Google Scholar]
- 77. Westervelt P, Roboz GJ, Cortes JE, et al. Phase 1 first-in-human trial of AMV564, a bivalent bispecific (2x2) CD33/CD3 T-cell engager, in patients with relapsed/refractory acute myeloid leukemia (AML). Presented at the 2018 ASH Annual Meeting, Abstract 1455. [Google Scholar]
- 78. Ball B, Komrokji RS, Adès L, et al. Evaluation of induction chemotherapies after hypomethylating agent failure in myelodysplastic syndromes and acute myeloid leukemia. Blood Adv 2018; 2: 2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jabbour E, Faderl S, Sasaki K, et al. Phase 2 study of low-dose clofarabine plus cytarabine for patients with higher-risk myelodysplastic syndrome who have relapsed or are refractory to hypomethylating agents. Cancer 2016; 123: 629–637. [DOI] [PubMed] [Google Scholar]
- 80. Lancet JE, Cortes JE, Hogge DE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/ daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood 2014; 123: 3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lancet JE, Uy GL, Cortes JE, et al. Cpx-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol 2018; 36: 2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Platzbecker U, Germing U, Götze KS, et al. Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): a multicentre, open-label phase 2 dose-finding study with long-term extension study. Lancet Oncol 2017; 18: 1338–1347. [DOI] [PubMed] [Google Scholar]
- 83. Komrokji R, Garcia-manero G, Ades L, et al. Sotatercept with long-term extension for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes: a phase 2, dose-ranging trial. Lancet Haematol 2018; 5: e63–e72. [DOI] [PubMed] [Google Scholar]
- 84. Raza A, Reeves JA, Feldman EJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1-risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood 2008; 111: 86–93. [DOI] [PubMed] [Google Scholar]
- 85. Zeidan AM, Al Ali NH, Padron E, et al. Lenalidomide treatment for lower risk nondeletion 5q myelodysplastic syndromes patients yields higher response rates when used before azacitidine. Clin Lymphoma Myeloma Leuk 2015; 15: 705–710. [DOI] [PubMed] [Google Scholar]
- 86. Montalban-Bravo G, Garcia-Manero G, Jabbour E. Therapeutic choices after hypomethylating agent resistance for myelodysplastic syndromes. Curr Opin Hematol 2018; 25: 146–153. [DOI] [PubMed] [Google Scholar]
- 87. Festuccia M, Baker K, Gooley TA, et al. Hematopoietic cell transplantation in myelodysplastic syndromes after treatment with hypomethylating agents. Biol Blood Marrow Transplant 2017; 23: 1509–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wei A, Strickland S, Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. Epub ahead of print 20 March 2019. DOI: 10.1200/JCO.18.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kongtim P, Rondon G, Chen J, et al. Haploidentical transplantation for older patients with acute myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. Epub ahead of print 14 September 2017. DOI: 10.1016/j.bbmt.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mo X, Zhang X, Xu L, et al. Haploidentical hematopoietic stem cell transplantation for myelodysplastic syndrome. Biol Blood Marrow Transplant. 2017; 23(12): 2143–2150. [DOI] [PubMed] [Google Scholar]
- 91. Weber T, Robin M, Stelljes M, et al. Dose-reduced versus standard conditioning followed by allogeneic stem-cell transplantation for patients with myelodysplastic syndrome: a prospective randomized phase III study of the EBMT (RICMAC Trial). J Clin Oncol 2017; 35: 2157–2164. [DOI] [PubMed] [Google Scholar]
- 92. Modi D, Deol A, Kim S, et al. Age does not adversely in fluence outcomes among patients older than 60 years who undergo allogeneic hematopoietic stem cell transplant for AML and myelodysplastic syndrome. Bone Marrow Transplant 2017; 52: 1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Prébet T, Gore SD, Esterni B, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol 2011; 29: 3322–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Della Porta MG, Gallì A, Bacigalupo A, et al. Clinical effects of driver somatic mutations on the outcomes of patients with myelodysplastic syndromes treated with allogeneic hematopoietic stem-cell transplantation. J Clin Oncol 2016; 34: 3627–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lindsley RC, Saber W, Mar BG, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med 2017; 376: 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hamilton BK, Rybicki L, Hirsch C, et al. Mutation clonal burden and allogeneic hematopoietic cell transplantation outcomes in acute myeloid leukemia and myelodysplastic syndromes. Bone Marrow Transplant. Epub ahead of print 17 Jan 2019. DOI: 10.1038/s41409-019-0444-1. [DOI] [PubMed] [Google Scholar]
- 97. Schroeder T, Rautenberg C, Haas R, et al. Hypomethylating agents after allogeneic blood stem cell transplantation. Stem Cell Investig 2016; 3: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. De Lima M, Oran B, Champlin RE, et al. CC-486 maintenance after stem cell transplantation in patients with acute myeloid leukemia or myelodysplastic syndromes. Biol Blood Marrow Transplant 2018; 24: 2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schroeder T, Czibere A, Platzbecker U, et al. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia 2013; 27: 1229–1235. [DOI] [PubMed] [Google Scholar]
- 100. Craddock C, Labopin M, Robin M, et al. Clinical activity of azacitidine in patients who relapse after allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica 2016; 101: 879–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Oran B, De Lima M, García-Manero G, et al. Maintenance with 5-azacytidine for acute myeloid leukemia and myelodysplastic syndrome patients. Presented at the 2018 ASH Annual Meeting, Abstract 971. [Google Scholar]