Abstract
Backgrounds:
Despite the clinical success of taxanes, they still have limitations, such as chemoresistance. To overcome the limitations of paclitaxel, genetic alterations and targeting effects of altered genes were observed in paclitaxel-resistant cancer. Because paclitaxel-resistant cancer shows high levels of Plk1, a promising target in chemotherapy, the effectiveness of Plk1 inhibitors in paclitaxel-resistant cancer cells has been investigated.
Methods:
Paclitaxel-resistant cancer cells were developed by exposure of stepwise escalating levels of paclitaxel. Genetic alterations were detected by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and immunoblotting. Using a cell viability assay, combined targeting effects for Plk1 and androgen receptor (AR) were determined. Clinical data were analyzed to understand the relationship between Plk1 and AR in prostate cancer patients.
Results:
Treatment with Plk1 inhibitors markedly reduced the expression of MDR1, MRP1, and Plk1 in the paclitaxel-resistant cancer. Among Plk1 inhibitors, genistein, recently found as a direct Plk1 inhibitor, tended to be more effective in the paclitaxel-resistant prostate cancer than the parental cancer cells, which was related to the suppression of the AR, as well as inhibition of Plk1 activity. A combination of Plk1 inhibitors and AR antagonist bicalutamide exhibited a synergistic effect in LNCaPTXR, as well as LNCaP cells, by inhibiting Plk1 and AR. Analysis of clinical data provides evidence for the relevance between Plk1 and AR in prostate cancer patients, showing that Plk1 and AR are strong predictors of poor survival rates.
Conclusions:
We suggest that cotargeting Plk1 and AR would be effective in advanced chemoresistant prostate cancer cells to overcome the limitations associated with paclitaxel.
Keywords: androgen receptor, paclitaxel, Plk1, resistance
Introduction
Antimitotic therapies targeting microtubule dynamics, such as vinca alkaloids and taxanes, are widely used for the treatment of cancer.1–4 Taxanes are still the first choice of treatment for several solid malignant tumors, and taxanes in combination with other chemotherapy agents are standard in patients with advanced prostate cancer,5,6 breast cancer,7 ovarian cancer,3 and non-small cell lung cancer.4 Despite the clinical success of taxanes, they still have limitations, such as the acquisition of resistance and dose-dependent toxicity.1,8,9 Acquired taxane resistance is a serious clinical obstacle in effectively treating cancer patients. High expression levels of ABCB1, also known as p-glycoprotein or multidrug resistance protein 1 (MDR1), and multidrug resistance-associated protein 1 (MRP1; ABCC1) are thought to be one of the causes of paclitaxel resistance.8,10 To reduce these limitations, combination chemotherapy has been broadly investigated via in vitro experiments, in vivo studies, and clinical trials. The use of new antimitotic drugs as targeted therapies can offer the possibility to overcome some of the limitations of current antimitotic drugs.
Recently, Polo-like kinase 1 (Plk1) has drawn attention in the development of antimitotic drugs to treat cancer.11 The overexpression of Plk1 in several malignant solid tumors, including breast,12,13 colon,14 non-small cell lung,15 and prostate cancers,16,17 is correlated with tumorigenicity. Plk1 has been shown to be involved in chemoresistance, and Plk1 inhibition may overcome the drug resistance induced by several anticancer drugs, including doxorubicin,18,19 gemcitabine,20 and docetaxel.21 Plk1-targeted therapies could possibly reduce or eliminate the chemoresistance in chemotherapeutics. In addition, castration-resistant prostate cancer cells are sensitive to Plk1 inhibition by the repression of the androgen signaling pathway, according to recent studies.22,23 Because prostate cancer is an androgen-dependent disease, therapeutic approaches are directed toward androgen ablation for advanced and metastatic prostate cancer, which shows initial improvement in the patients.24,25 Taxanes are one of the therapeutic options for patients who receive androgen ablation therapies.26,27 However, the inappropriate activation of androgen receptor (AR) signaling induces a relapse with a more aggressive and castration-resistant form of prostate cancer, which does not require circulating androgens, but still depends on functional AR for tumor growth.25,28 According to the proposal of Liu and colleagues, Plk1 inhibitors might have therapeutic potential for patients with castration-resistant prostate cancer at this stage.22,23
As part of the effort to find Plk1-targeting agents, Plk1-specific inhibitors, such as volasertib, BI 2536, and GSK461364, have been developed for chemotherapeutics. We recently found genistein to be a direct inhibitor of Plk1 kinase.29 Although the majority of studies have shown that genistein induces mitotic arrest,30–33 previous studies focused on genistein as a tyrosine kinase epidermal growth factor receptor (EGFR) inhibitor,34 and did not clearly explain how genistein induced mitotic arrest as an EGFR inhibitor. The discovery that genistein is a Plk1 inhibitor, provides a mechanism for the mitotic arrest and apoptosis induced by genistein in human cancer cells.29 In addition, genistein has also been identified as a suppressor of AR expression and activity in prostate cancer.35 We hypothesized that the Plk1 inhibitor genistein would be effective in cancers with overexpression of AR and Plk1. For this, paclitaxel-resistant cancer cells were developed to test whether paclitaxel-resistant cells were sensitive to Plk1 inhibitors, because paclitaxel is a therapeutic option for patients with advanced prostate cancer and non-small cell lung cancer, and the recurrence of these cancers exhibits greater aggressiveness.26,27,36 Here, we demonstrated that treatment with Plk1 inhibitors including volasertib, BI 2536, and genistein markedly reduced the levels of MRP1, MDR1, and Plk1 in the paclitaxel-resistant cancers. Compared with other Plk1 inhibitors, genistein has suppressive effects against Plk1 and AR expression in paclitaxel-resistant prostate cancer cells. In addition, the combined effects of Plk1 inhibitors and an AR antagonist bicalutamide on cell growth in paclitaxel-resistant LNCaPTXR cells, were evaluated to investigate the possibility to overcome some of the limitations associated with current antimitotic drugs.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), Roswell Park Memorial Institute (RPMI)-1640, Minimum Essential medium (MEM), fetal bovine serum, penicillin, and streptomycin were purchased from Corning Cellgro (Manassas, VA, USA). BI 2536 and volasertib were purchased from Selleck Chemicals (Houston, TX, USA). Genistein, paclitaxel, bicalutamide, and all other chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture and establishment of paclitaxel-resistant cancer cells
Human prostate cancer LNCaP (clone FGC, #21740), DU145 (#30081), and lung cancer NCI-H460 cells (#30177) were purchased from the Korean Cell Line Bank (KCLB; Seoul, Korea) and were authenticated before being frozen by KCLB using short tandem repeats (STR). Human prostate cancer LNCaP and lung cancer NCI-H460 cells were cultured at 37°C in a 5% CO2 humidified atmosphere in RPMI-1640 medium, supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 100 units/ml penicillin, and 100 µg/ml streptomycin. Human prostate cancer DU145 cells were cultured in MEM. NCI-H460, LNCaP, and DU145 cells were exposed to stepwise escalating levels of paclitaxel to develop paclitaxel-resistant cells. The concentration of paclitaxel increased twofold at each step of resistance from 1 nM up to 20 nM. The resistant cells were established after an induction of 30 weeks of paclitaxel for NCI-H460TXR and LNCaPTXR cells, and 25 weeks for DU145TXR cells.
Cell viability assay
Cell viability was measured using a 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (Sigma-Aldrich; St. Louis, MO, USA), according to the manufacturer’s protocol and a previous report.29
Quantitative reverse transcription polymerase chain reaction
Total RNA was extracted at 48 h after exposure to volasertib, BI 2536, genistein, or bicalutamide and quantified by Nanodrop (Thermo Scientific; Wilmington, DE, USA). Next, cDNA was created with a First Strand cDNA Synthesis Kit (Thermo Scientific). After the synthesized cDNA was mixed with SYBR Green Master Mix (Roche; Mannheim, Germany) and various sets of gene-specific primers, quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed using a LightCycler Real-Time PCR system (Roche). The primer sequences used in this study are listed in Supplementary Table 4.
Fluorescence-activated cell sorting analysis
To determine the percentage of cells in each phase of the cell cycle, cells were collected by trypsinization, fixed in 75% ethanol, stained with 30 μg/ml propidium iodide solution, and subjected to fluorescence-activated cell sorting (FACS) analysis as described previously.37 Cells were sorted using a Guava easyCyteTM FACS machine (Millipore; Billerica, MA, USA), and data were analyzed with IncyteTM software (Millipore).
Immunoblot analysis
For immunoblotting, cell extracts were prepared in lysis buffer (10 mM HEPES [pH 7.4], 5 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid, 10 mM KCl, 2 mM MgCl2, 40 mM β-glycerophosphate, 1 mM dithiothreitol, 1 mM phenyl methyl sulfonyl fluoride, 25 µg/ml leupeptin, and 5 µg/ml pepstatin A) as described previously.29,38 Lysates were centrifuged at 12,000 rpm for 15 min at 4°C, and the supernatants were collected and the protein concentrations adjusted. Then, cell lysates were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis prior to immunoblot analysis. Immunoblotting was performed with anti-p-S46-TCTP (Cell Signaling Technology, 5251), anti-TCTP (Santa Cruz Biotechnology, Dallas, TX, USA), anti-p-T210-Plk1 (Cell Signaling Technology, 5472), anti-Plk1 (Millipore, Bedford, MA, USA; 05-844), and anti-actin (Sigma-Aldrich) antibodies using an Odyssey infrared imaging system (LI-COR Biosciences; Lincoln, NE, USA) and LI-COR Odyssey software.
Statistical analysis
All data are given as means ± standard deviations from at least three independent experiments. Results were analyzed for statistically significant differences using Student’s t test, and a p value <0.05 was considered statistically significant. Correlation of mRNA-mRNA pairs of the gene set in cell lines or human prostate tumors were analyzed calculating the Pearson correlation coefficient. Statistical significance is presented in figure legends: *p < 0.05; **p < 0.01; ***p < 0.001. Drug combination was analyzed by the combination index (CI) method using CompuSyn software (ComboSyn Inc., NJ, USA)39; CI<0.9 indicates synergism, CI=0.9–1.1 additivity, and CI>1.1 antagonism.
Gene set enrichment analysis and correlation analyses
The human prostate cancer patients’ microarray dataset was obtained from the NCBI-GEO database (GSE16560, GSE70769). To evaluate the correlation of the gene expression with overall survival (OS) or relapse-free survival (RFS), patients were separated into two subgroups, ‘gene expression high’ or ‘gene expression low’. Total of 281 prostate cancer patients (alive 75, dead 206) were selected (GSE16560), according to clinical stage T1-T2, Mx, and N0.40 From these 281 cases, 150 men were selected, depending on the expression levels of Plk1, AR, or prostate-specific antigen (PSA) with the top 75 samples with the highest expression and the lowest 75 samples with lowest expression of Plk1, AR, or PSA. In the GSE70769 dataset, a total of 94 prostate cancer patients were in samples in the validation cohort only, with complete analysis.41 From the 94 cases, 80 men were selected, depending on the expression levels of Plk1, AR, or PSA with the top 40 samples with the highest expression and the bottom 40 samples with the lowest expression of Plk1, AR, or PSA. A total 150 and 80 prostate cancer patients with subtype classification were used to analyze the correlation of gene expression and OS rate or RFS rate. Kaplan–Meier survival analysis and a log-rank test were used to evaluate the statistical significance of survival differences between the two groups. Correlations of mRNA expression of genes in the gene set for human prostate cancer were analyzed using the GSE70769 dataset.41 From total of 94 prostate cancer patients, we defined two subgroups, ‘gene set high’ or ‘gene set low’, depending on the expression levels of Plk1 with the top 10 samples with the highest expression and the low 10 samples with lowest expression of Plk1. Heat maps were generated using the GENE-E software (Broad Institute). GSEA was performed using the Broad Institute platform (http://www.broadinstitute.org/gsea/index.jsp). Patient samples were selected and grouped for high or low mRNA expression of the selected gene or gene set. All percentiles between the lower and upper quartiles were computed and the best performing threshold was used as cutoff ±1.5. The median was calculated over the entire dataset.
Results
Plk1 is highly expressed and activated in paclitaxel-resistant cancer cells
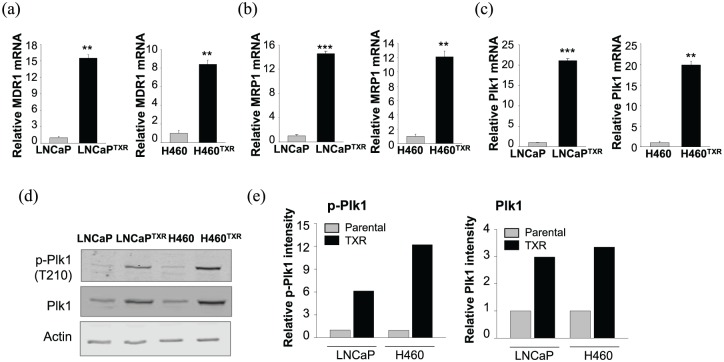
To overcome the limitations of paclitaxel, paclitaxel-resistant LNCaPTXR and NCI-H460TXR cells were established by treatment with paclitaxel as described in the Materials and Methods section. The GI50 values of paclitaxel in the LNCaPTXR cells and NCI-H460TXR cells were 130 and 204 nM, respectively, which were over 10 times higher than the GI50 values of paclitaxel in the parental LNCaP and NCI-H460 cells ( Supplementary Figure 1), indicating that the resistance indexes were over 10. Because the high expression levels of MDR1 and MRP1 are thought to be the main mechanism of chemoresistance acquisition,8,10 the mRNA levels of MDR1 (Figure 1a) and MRP1 (Figure 1b) were observed in the paclitaxel-resistant LNCaPTXR and NCI-H460TXR cells by qRT-PCR. As expected, the mRNA levels of MDR1 and MRP1 in the paclitaxel-resistant cells were over 10 times greater than those found in the parental cells.
Figure 1.
Plk1 mRNA and protein with the active form were increased in paclitaxel-resistant cancer cells. (a–c) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed to evaluate the mRNA levels of MDR1 (a), MRP1 (b), and Plk1 (c) in parental and paclitaxel-resistant LNCaP (LNCaPTXR) or NCI-H460 (H460TXR) cells. Three independent experiments were performed. The relative expression of mRNA was plotted. **p < 0.01, ***p < 0.001. (d) Cell lysates from parental and paclitaxel-resistant LNCaP (LNCaPTXR) or NCI-H460 (H460TXR) cells were prepared to determine the levels of Plk1, Plk1 phosphorylated at T210, and actin proteins using specific antibodies. (e) The relative band intensity values of p-Plk1 (left panel) and Plk1 (right panel) were quantified with LI-COR Odyssey software and plotted.
As paclitaxel induces mitotic arrest when a mitotic kinase Plk1 peaks, the levels of Plk1 mRNA were observed. The results showed that Plk1 mRNA was upregulated in paclitaxel-resistant cells compared with that of parental cells, as measured by qRT-PCR, especially in the LNCaPTXR and NCI-H460TXR cells (Figure 1c). In LNCaPTXR cells, Plk1 mRNA expression was 21-fold higher than that of the parental LNCaP cells (Figure 1c, right panel). Consistent with this finding, Plk1 mRNA of NCI-H460TXR cells was upregulated with roughly an 18-fold increase over the level measured in the parental NCI-H460 cells (Figure 1c, left panel). The mRNA level of Plk1 was over 20 times greater than that of parental cells. Thus, the paclitaxel-resistant LNCaPTXR and NCI-H460TXR cells showed high mRNA levels of Plk1 as well as MRP1 and MDR1.
Next, to observe the protein levels of Plk1 and its active form, we measured the levels of phosphorylated Plk1 (p-T210-Plk1) as well as total Plk1 (Figure 1d). In accordance with the Plk1 mRNA level (Figure 1c), the protein levels of Plk1 and its active form were higher in the LNCaPTXR and NCI-H460TXR cells compared with those in the parental LNCaP and NCI-H460 cells, as determined by immunoblot analysis using specific antibodies against Plk1 and phosphorylated Plk1 at T210, the active form of Plk1 (Figure 1, d-e), suggesting that paclitaxel-resistant cells have high levels of Plk1 mRNA, total protein, and its active form.
Elevated expressions of AR and PSA in LNCaPTXR cells were effectively reduced by treatment with the Plk1 inhibitor genistein
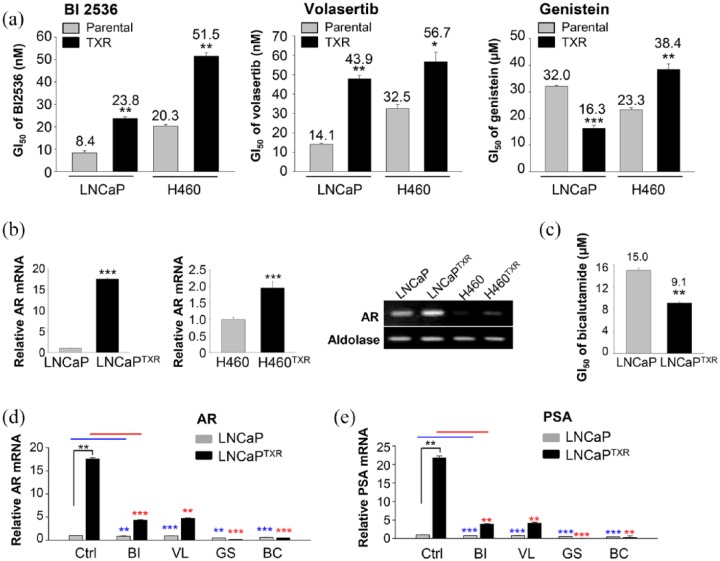
Because the paclitaxel-resistant LNCaP and NCI-H460 cells have high levels of Plk1 protein and its active form (Figure 1), the sensitivity of paclitaxel-resistant cancer cells to Plk1 inhibitors was investigated. For this, the cytotoxic effects of BI 2536, volasertib, and genistein were observed in paclitaxel-resistant cells and parental cells (Figure 2a). The cell viability after treatment with Plk1 inhibitors was evaluated in a concentration-dependent manner (Figure 2a). Although paclitaxel-resistant cells were sensitive to BI 2536, volasertib, and genistein, BI 2536 and volasertib had weak cross resistance to the paclitaxel-resistant LNCaPTXR and NCI-H460TXR cells. The ratio of GI50 values for the treatment of volasertib in NCI-H460TXR versus NCI-H460 cells was 1.7 and that for LNCaPTXR versus LNCaP cells was 3 (Figure 2a, left panel). Genistein did not show the cross resistance to the LNCaPTXR cells. It was more effective in LNCaPTXR cells compared with parental LNCaP cells when measuring with the GI50 value (Figure 2a, right panel). However, genistein had weak cross resistance in NCI-H460TXR cells. The GI50 value of genistein in NCI-H460TXR cells was approximately 1.6 times greater than that in NCI-H460 cells (Figure 2a, right panel), which was similar to the ratio of the volasertib GI50 values. These results suggested that genistein was effective against paclitaxel-resistant LNCaPTXR cells.
Figure 2.
High levels of androgen receptor (AR) and prostate-specific antigen (PSA) in paclitaxel-resistant LNCaPTXR cells were markedly reduced by treatment with genistein. (a) Cells were treated with BI 2536 (left panel), volasertib (middle panel), or genistein (right panel) in a concentration-dependent manner in LNCaP, paclitaxel-resistant LNCaP (LNCaPTXR), NCI-H460, and paclitaxel-resistant NCI-H460 (H460TXR) cells for 48 h. The numbers of viable cells were then measured by a cell viability assay. The bar graph presents the mean values of half maximal growth inhibitory concentration (GI50, nM for BI2536 and volasertib, μM for genistein). Three independent experiments were performed. *p < 0.05, **p < 0.01, ***p < 0.001. (b) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed to evaluate AR mRNA levels in LNCaP, LNCaPTXR, NCI-H460, and H460TXR cells. Relative AR mRNA levels were evaluated in LNCaPTXR cells compared with that in LNCaP cells (left panel), and that in NCI-H460TXR cells compared with in NCI-H460 cells (middle panel). Both AR and aldolase A mRNAs were detected in agarose gel after qRT-PCR (right panel). Three independent experiments were performed. (c) LNCaP and LNCaPTXR cells were grown for 48 h in the presence of 1, 10, 25, or 50 μM bicalutamide. The numbers of viable cells were then measured by cell viability assay. The bar graph presents the mean values of half maximal growth inhibitory concentration (GI50, μM) of bicalutamide in LNCaP and LNCaPTXR cells. (d-e) LNCaP and LNCaPTXR cells were grown for 48 h in the presence of 10 nM BI 2536, 15 nM volasertib, 30 µM genistein, or 14 µM bicalutamide. qRT-PCR was performed to evaluate mRNA levels of AR (d), and PSA (e) in LNCaP and LNCaPTXR cells. Three independent experiments were performed. Values are presented as the means ± standard deviations. *p < 0.05, **p < 0.01, ***p < 0.001.
Then, we wanted to understand the reason why LNCaPTXR cells are sensitive to the treatment by genistein (Figure 2a), although the levels of Plk1 were increased in both that of paclitaxel-resistant NCI-H460TXR and LNCaPTXR cells. Recent studies have shown that Plk1 inhibition represses the androgen signaling pathway in prostate cancer22,23,42 and that genistein downregulates AR in prostate cancer.35 Based on these studies, we addressed whether genistein affects to the androgen signaling pathway. For this, the expression of AR mRNA was observed in LNCaPTXR and NCI-H460TXR cells, as measured by qRT-PCR (Figure 2b). The results showed that the levels of AR mRNA in LNCaPTXR cells were approximately 16 times higher than those of the parental LNCaP cells (Figure 2b; left panel). However, the levels of AR mRNA in NCI-H460TXR cells, were only twice as high as those of the parental NCI-H460 cells (Figure 2b; middle panel). In addition, basal levels of AR mRNA in prostate cancer were much higher than those in lung cancer (Figure 2b; right panel), although AR functions in several organs, including the lung.43,44 Because AR mRNA was lower in NCI-H460 cells, the effects of genistein on AR signaling were focused on prostate cancer rather than lung cancer. To compare the effects of Plk1 inhibitors with AR antagonists on AR signaling in paclitaxel-resistant prostate cancer, the cytotoxic effects of bicalutamide, a specific AR antagonist, were measured in LNCaP and LNCaPTXR cells (Figure 2c). A cell viability assay showed that bicalutamide was more effective in the paclitaxel-resistant LNCaPTXR cells expressing high levels of AR, with a GI50 of 9.1 µM, than that of LNCaP cells (Figure 2c).
Next, to understand the effects on the expression of AR and its target, PSA, from treatment with Plk1 inhibitors or the AR antagonist bicalutamide in LNCaP and LNCaPTXR cells, qRT-PCR was performed after treatment with BI 2536, volasertib, genistein, or bicalutamide at the GI50 in LNCaP and LNCaPTXR cells (Figure 2d). The results showed that the mRNA levels of AR were reduced by treatment with BI 2536, volasertib, or bicalutamide compared with those of the control in LNCaPTXR cells (Figure 2d). Notably, by treatment with genistein, the mRNA levels of AR in the LNCaPTXR cells were markedly reduced to approximately 95-fold lower than those of the control (control 17.5 versus genistein 0.183; Figure 2d). In addition, the treatment of bicalutamide reduced the AR mRNA levels approximately 35-fold lower than those of the control (control 17.5 versus bicalutamide 0.491). The expression of PSA, a transcriptional target of AR, was effectively suppressed by treatment with genistein or bicalutamide (Figure 2e). The changes in mRNA levels of PSA were similar with the changes of AR mRNA levels in cells treated with BI 2536, volasertib, genistein, or bicalutamide (Figure 2e). Taken together, the paclitaxel-resistant LNCaPTXR cells were sensitive to the treatment of AR antagonist and Plk1 inhibitors. In particular, the Plk1 inhibitor genistein was more effective in LNCaPTXR cells expressing high levels of AR and Plk1, because of its suppression of AR expression, as well as Plk1 activity.
Paclitaxel-resistant prostate cancer cells expressing high mRNA levels of AR and PSA are sensitive to genistein and bicalutamide
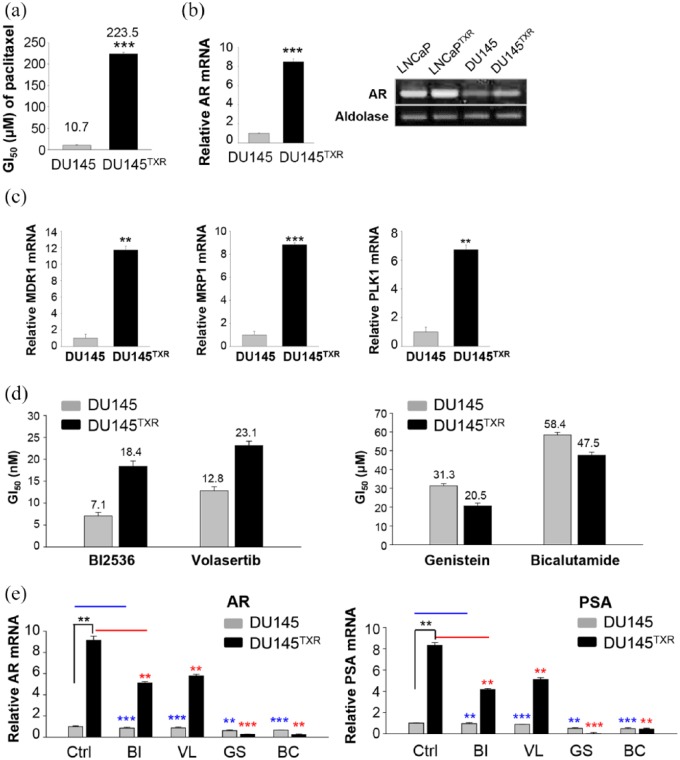
To generalize the effects of genistein in prostate cancer cells, paclitaxel-resistant DU145TXR cells were developed using DU145 cells, which are AR-positive but relatively low.45 The resistance index was over 20 because GI50 values of paclitaxel were 10.7 versus 223.5 µM in parental DU145 versus paclitaxel-resistant DU145TXR cells, respectively (Figure 3a), under the condition when mRNA levels of AR in DU145TXR cells were evaluated by qRT-PCR. AR mRNA in DU145TXR cells was upregulated by a factor of approximately eight compared with that of the parental DU145 cells (Figure 3b, left panel). Although the basal levels of AR in DU145 cells were low, acquiring the resistance against paclitaxel in DU145TXR cells, upregulated the expression of AR mRNA (Figure 3b, right panel). In addition, mRNA levels of MDR1, MRP1, and Plk1 in DU145TXR cells were higher by approximately 7–10 times compared with those of DU145 cells as expected (Figure 3c). When the cell viability after treatment with BI 2536, volasertib, genistein, or bicalutamide was evaluated in a concentration-dependent manner in both DU145 and DU145TXR cells (Figure 3d), BI 2536 and volasertib had weak cross resistance to paclitaxel-resistant DU145TXR cells, with the ratio of GI50 values for BI 2536 and volasertib in DU145TXR versus DU145 cells being 2.6 and 1.8, respectively, consistent with LNCaP TXR cells (Figure 3d, left panel). However, the ratio of GI50 values of genistein and bicalutamide in DU145TXR versus DU145 cells were 0.65 and 0.81, respectively, indicating that genistein and bicalutamide were effective against paclitaxel-resistant DU145TXR cells. Paclitaxel-resistant DU145TXR cells were sensitive to the treatment of genistein or bicalutamide with low levels of AR or PSA (Figure 3e). These data indicated that genistein, as well as the AR antagonist bicalutamide are effective in targeting the androgen signaling pathway of paclitaxel-resistant prostate cancer cells expressing high mRNA levels of AR and PSA.
Figure 3.
Genistein and bicalutamide are effective in paclitaxel-resistant DU145TXR cells expressing high mRNA levels of androgen receptor (AR) and prostate-specific antigen (PSA). Paclitaxel-resistant DU145TXR cells were developed as described in Materials and Methods. Genistein was treated in a concentration-dependent manner in DU145 and DU145TXR cells for 48 h. The numbers of viable cells were then measured by a cell viability assay. The bar graph presents the mean values of half maximal growth inhibitory concentration (GI50, μM for genistein). (b) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed to evaluate AR mRNA levels in DU145 and DU145TXR cells. The mRNA levels of AR and aldolase A were detected in agarose gel after qRT-PCR (right panel) and those of LNCaP and LNCaPTXR cells were used as positive controls. (c) qRT-PCR was performed to evaluate mRNA levels of MDR1, MRP1, and Plk1 in DU145 and DU145TXR cells. Three independent experiments were performed. (d) DU145 and DU145TXR cells were grown for 48 h in the presence of BI 2536, volasertib, genistein, or bicalutamide. The numbers of viable cells were then measured by cell viability assay. The bar graph presents the mean values of half maximal growth inhibitory concentration (GI50, μM) of each compound in DU145 and DU145TXR cells. (e) DU145 and DU145TXR cells were grown for 48 h in the presence of 10 nM BI 2536, 15 nM volasertib, 30 µM genistein, or 14 µM bicalutamide. DU145 and DU145TXR cells were grown for 48 h in the presence of 10 nM BI 2536, 15 nM volasertib, 30 µM genistein, or 14 µM bicalutamide. qRT-PCR was performed to evaluate mRNA levels of AR and PSA in DU145 and DU145TXR cells. Three independent experiments were performed. Values are presented as the means ± standard deviations. **p < 0.01, ***p < 0.001.
Paclitaxel-resistant LNCaPTXR cells underwent apoptosis more easily than parental LNCaP cells following treatment with genistein, a Plk1 inhibitor
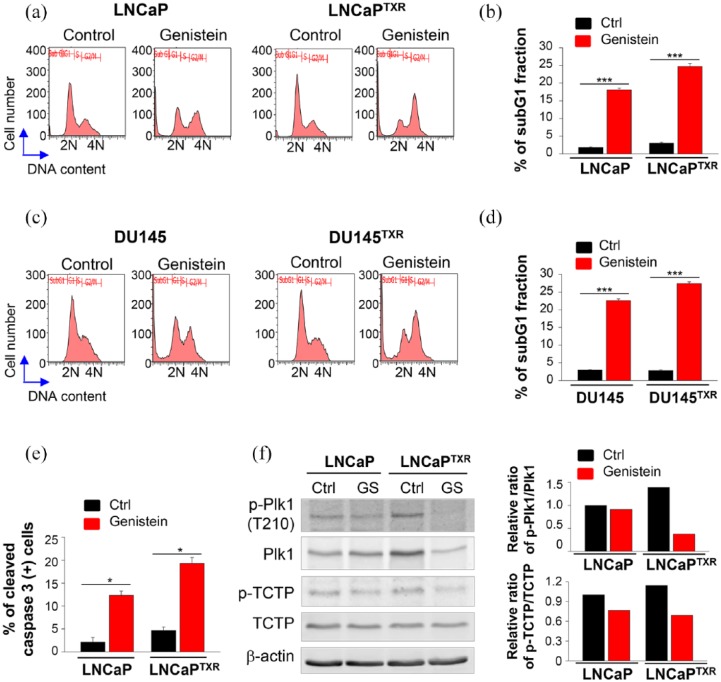
To evaluate whether genistein was effective for inducing apoptosis in paclitaxel-resistant prostate cancer cells, FACS analysis was performed to observe the apoptotic cell population. FACS analysis revealed that genistein induced mitotic arrest and DNA fragmentation, as determined by the percentage of the subG1 fraction in both LNCaP and LNCaPTXR cells (Figure 4a-and b). The percentage of the subG1 fraction in genistein-treated LNCaP and LNCaPTXR cells, was approximately 17% and 25%, respectively (Figure 4b). Consistently, the treatment of genistein induced apoptosis more effectively in DU145TXR cells than in parental DU145 cells, determined by the percentage of the subG1 fraction (Figure 4, c-d). To confirm the apoptotic cell death, immunostaining with anti-cleaved active caspase 3 was performed in genistein-treated LNCaP and LNCaPTXR cells (Figure 4e). The populations of active cleaved caspase 3-positive cells were approximately 12% and 18% of the genistein-treated LNCaP and LNCaPTXR cells, respectively (Figure 4e). These data indicated that paclitaxel-resistant prostate cancer cells were more sensitive to genistein and undergo apoptotic cell death more easily, compared with the parental prostate cancer cells, through mitotic arrest.
Figure 4.
Paclitaxel-resistant prostate cancer cells were sensitive to the treatment of genistein by reducing the activity of Plk1. (a) FACS analyses were performed on LNCaP and LNCaPTXR cells after treatment with 25 μM of genistein for 48 h. (b) The percentages of cells in the subG1 phase were measured by flow cytometry. *p < 0.05, **p < 0.01, ***p < 0.001. (c) FACS analyses were performed on DU145 and DU145TXR cells after treatment with 25 μM of genistein for 48 h. (d) The percentages of cells in the subG1 phase were measured by flow cytometry. *p < 0.05, **p < 0.01, ***p < 0.001. (e) LNCaP and LNCaPTXR cells were grown for 48 h in the presence of 25 μM genistein. Cells were fixed with 4% paraformaldehyde and stained for active caspase-3 (green) and DAPI (blue). The percentage of cells that stained positive for active caspase-3 was determined. At least 1000 cells were counted, and three independent experiments were performed. *p < 0.05, **p < 0.01, ***p < 0.001. (f) LNCaP and LNCaPTXR cells were grown for 48 h in the presence of 25 μM genistein. Lysates were analyzed by immunoblotting with anti-TCTP, anti-phospho-TCTP, anti-Plk1, anti-phospho-Plk1, and anti-actin antibodies (left panel). Ctrl, control. FACS, fluorescence-activated cell sorting; GS, genistein. The relative band intensity values of p-TCTP, TCTP, p-Plk1, and Plk1 were quantified with LI-COR Odyssey software and plotted (right panel).
Next, we wanted to understand how genistein is more effective in paclitaxel-resistant prostate cancer cells than parental cells. Because the levels of mRNA, protein, and the active form (phosphorylated form at T210) of Plk1 were higher in LNCaPTXR cells than in the parental cells (Figure 1c and d), we wanted to determine whether the activity of Plk1 in LNCaPTXR cells was inhibited more sensitively by the treatment with genistein. The levels of the phosphorylated form TCTP, an endogenous substrate of Plk1, were detected by immunoblot analysis using antibodies against p-TCTP and TCTP (Figure 4f). In LNCaPTXR cells, treatment of genistein markedly reduced the levels of phosphorylated TCTP, compared with those of LNCaP cells (Figure 4f). In addition, the levels of p-Plk1 and Plk1 were markedly reduced by treatment of genistein in LNCaPTXR cells expressing high levels of Plk1. Thus, genistein reduced the activity and expression of Plk1 more effectively in LNCaPTXR cells. Altogether, genistein induced apoptosis more effectively in paclitaxel-resistant prostate cancer cells through suppression of Plk1 activity and expression.
Plk1 inhibitors markedly suppressed the expression of MRP1, a key factor of chemoresistance, in LNCaPTXR cells
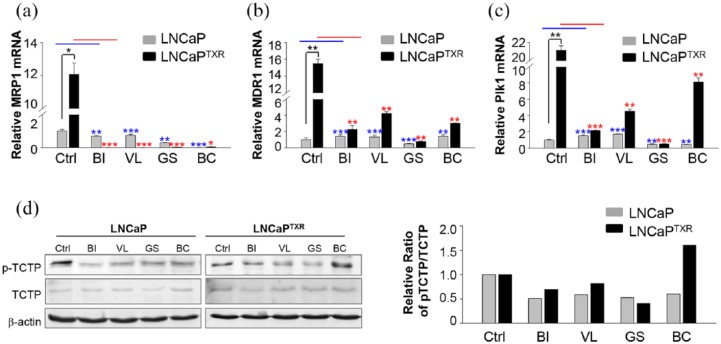
Multidrug resistance genes MRP1 and MDR1 are key factors that explain the poor response to treatment of cancer patients,46 and Plk1 inhibition overcomes the drug resistance induced by several anticancer drugs including doxorubicin,18,19 gemcitabine,20 and docetaxel.21 Because the expressions of MDR1, MRP1, and Plk1 were higher in LNCaPTXR cells than those of the parental LNCaP cells (Figure 1a–c), we wanted to observe the effects of treatment with a Plk1 inhibitor or an AR antagonist on the expression of MRP1, MDR1, and Plk1 in paclitaxel-resistant prostate cancer cells. For this, qRT-PCR for MRP1 was performed after treatment with BI 2536, volasertib, genistein, or bicalutamide at the concentration of GI50 in LNCaPTXR cells (Figure 5a). The results showed that the mRNA levels of MRP1 after treatment with BI 2536, volasertib, genistein, or bicalutamide were blocked markedly compared with those of the control in the LNCaPTXR cells (Figure 5a). The expression of MDR1 was not decreased by the treatment with BI 2536, volasertib, or bicalutamide in LNCaP cells. However, it was decreased by the treatment with genistein in LNCaP cells (Figure 5b). This reduction by genistein in LNCaP cells was observed again in LNCaPTXR cells when the cells were treated with genistein. The treatment with genistein decreased the MDR1 mRNA levels markedly compared to treatments with other compounds (Figure 5b). In addition, the expression of Plk1 was observed after the treatment with Plk1 kinase inhibitors and bicalutamide in LNCaPTXR cells (Figure 5c). The expression of Plk1 mRNA was reduced markedly by the treatment with genistein, but not greatly affected by the treatment of bicalutamide in LNCaPTXR cells.
Figure 5.
Plk1 inhibitors markedly suppressed the expression of MRP1, a key factor of chemoresistance, and the activity of Plk1 in LNCaPTXR cells. (a-c) LNCaP and LNCaPTXR cells were grown for 48 h in the presence of 10 nM BI 2536, 15 nM volasertib, 30 µM genistein, or 14 µM bicalutamide. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed to evaluate mRNA levels of MRP1 (a), MDR1 (b), and Plk1 (c) in LNCaP and LNCaPTXR cells. Three independent experiments were performed. *p < 0.05, **p < 0.01, ***p < 0.001. (d) LNCaP and LNCaPTXR cells were grown for 48 h in the presence of 10 nM BI 2536, 15 nM volasertib, or 30 µM genistein. Cell lysates of LNCaP and LNCaPTXR cells were prepared to determine the levels of TCTP, p-TCTP, and actin proteins using specific antibodies (left panel). The relative band intensity values of p-TCTP and TCTP were quantified with LI-COR Odyssey software (Li-COR Biosciences) and plotted (right panel).
To determine whether the activity of Plk1 in LNCaPTXR cells was inhibited by the treatment with Plk1 inhibitors in LNCaPTXR cells, the levels of phosphorylated TCTP, an endogenous substrate of Plk1, were detected after treatment of Plk1 inhibitors by immunoblot analysis (Figure 5d). In LNCaP and LNCaPTXR cells, treatment of Plk1 inhibitors including genistein reduced the levels of p-TCTP (Figure 5d). Of note, the treatment of Plk1 inhibitors especially genistein reduced the expression of MRP1, MDR1, and Plk1 as well as the activity of Plk1 markedly at the GI50 level in LNCaPTXR cells. Taken together, the results suggested that Plk1 inhibitors, including genistein, downregulated the expressions of MRP1, MDR1, and Plk1, as well as the activity of Plk1 in paclitaxel-resistant prostate cancer.
A combination of Plk1 inhibitors and bicalutamide was effective in paclitaxel-resistant LNCaPTXR cells
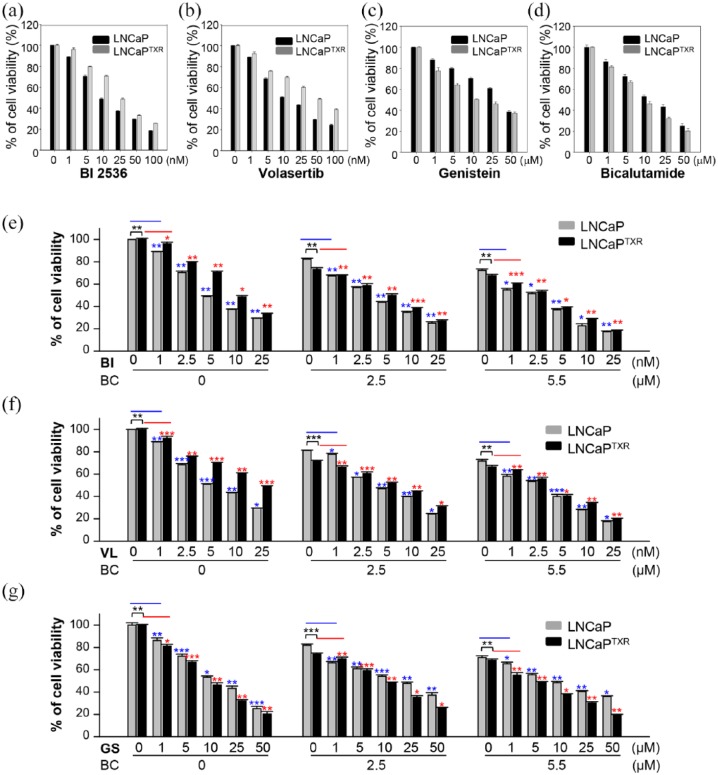
Next, for the therapeutic approach to paclitaxel-resistant prostate cancer, Plk1 inhibitors and an AR antagonist were treated as a single agent in LNCaP and LNCaPTXR cells and the half maximal inhibitory concentration (IC50) values of bicalutamide and Plk1 inhibitors, including volasertib, BI 2536, and genistein were determined (Figure 6, a-d; Supplementary Tables 1–3, Table 1). As shown in Table 1, as a single agent, IC50 values of most Plk1 inhibitors except genistein were lower in parental LNCaP cells than those in LNCaPTXR cells. LNCaPTXR cells were more sensitive to the treatment of genistein than parental LNCaP cells (Table 1). To further investigate the combined effects between Plk1 inhibitors and bicalutamide, we measured the combination index (CI). The concentration of bicalutamide was fixed at 2.5 and 5.5 μM around the values of GI70–GI80 and each Plk1 inhibitor was treated in a concentration-dependent manner in LNCaP and LNCaPTXR cells (Figure 6, e-g; Supplementary Table 1–3). In LNCaP cells, IC50 values of BI 2536, volasertib, genistein, and bicalutamide were 8.4, 14.1, 32.0, and 15.0 μM, respectively. These IC50 values of BI 2536, volasertib, and genistein, were reduced to 2.8, 3.1, and 9.9 μM, respectively, when cells were treated in combination with 5.5 μM bicalutamide. The CI between bicalutamide and Plk1 inhibitors, BI 2536, volasertib, and genistein, were 0.7002, 0.5868, and 0.6762, respectively, suggesting a synergistic effect between bicalutamide and Plk1 inhibitors in LNCaP cells (Table 1). Moreover, these synergistic effects between bicalutamide and Plk1 inhibitors were observed in paclitaxel-resistant LNCaPTXR cells as well. In LNCaPTXR cells, CI values between bicalutamide and Plk1 inhibitors, BI 2536, volasertib, and genistein, were 0.7262, 0.6818, and 0.8441, respectively (Table 1). Taken together, these results demonstrated that Plk1 inhibitors can sensitize the AR antagonist in paclitaxel-resistant prostate cancer, suggesting a synergistic effect between Plk1 inhibitors and bicalutamide.
Figure 6.
A combination of Plk1 inhibitors and bicalutamide was effective in paclitaxel-resistant LNCaPTXR cells. (a–d) LNCaP and LNCaPTXR cells were treated with BI 2536 (a), volasertib (b), genistein (c), or bicalutamide (d) in a concentration-dependent manner for 48 h. The percentages of viable cells were measured by a cell viability assay and plotted. (e–g) Combination between bicalutamide and BI 2536 (e), volasertib (f), or genistein (g) was performed in LNCaP and LNCaPTXR cells. Cells were grown for 48 h in the presence of 0, 2.5, or 5.5 µM bicalutamide with BI 2536 (BI), volasertib (VL), or genistein (GS) at the indicated concentrations in the figures. The percentages of viable cells were measured by cell viability assay. Three independent experiments were performed. Values are presented as the means ± standard deviations. *p < 0.05, **p < 0.01, ***p < 0.001.
Table 1.
The half maximal inhibitory concentration (IC50) values of bicalutamide and Plk1 inhibitors, including volasertib, BI 2536, and genistein, and the combination index (CI) in LNCaP and LNCaPTXR cells; CI<0.9 indicates synergism, CI=0.9–1.1 additivity, and CI>1.1 antagonism.
| Compounds | LNCaP cells | LNCaPTXR cells | ||
|---|---|---|---|---|
| IC50 | CI | IC50 | CI | |
| Bicalutamide | 15.0 μM | 9.1 μM | ||
| Volasertib | 14.1 nM | 43.9 nM | ||
| Volasertib (in combination 2.5 μM Bicalutamide ) | 4.2 nM | CI = 0.4647 | 6.2 nM | CI = 0.4160 |
| Volasertib (in combination 5.5 μM Bicalutamide ) | 3.1 nM | CI = 0.5868 | 3.4 nM | CI = 0.6818 |
| BI 2536 | 8.4 nM | 23.8 nM | ||
| BI 2536 (in combination 2.5 μM Bicalutamide) | 3.8 nM | CI = 0.6192 | 5.5 nM | CI = 0.5058 |
| BI 2536 (in combination 5.5 μM Bicalutamide) | 2.8 nM | CI = 0.7002 | 2.9 nM | CI = 0.7262 |
| Genistein | 32.0 μM | 16.3 μM | ||
| Genistein (in combination 2.5 μM Bicalutamide) | 19.9 μM | CI = 0.7885 | 9.2 μM | CI = 0.8401 |
| Genistein (in combination 5.5 μM Bicalutamide) | 9.9 μM | CI = 0.6762 | 3.9 μM | CI = 0.8441 |
Clinical relevance of Plk1 and AR in prostate cancer patients
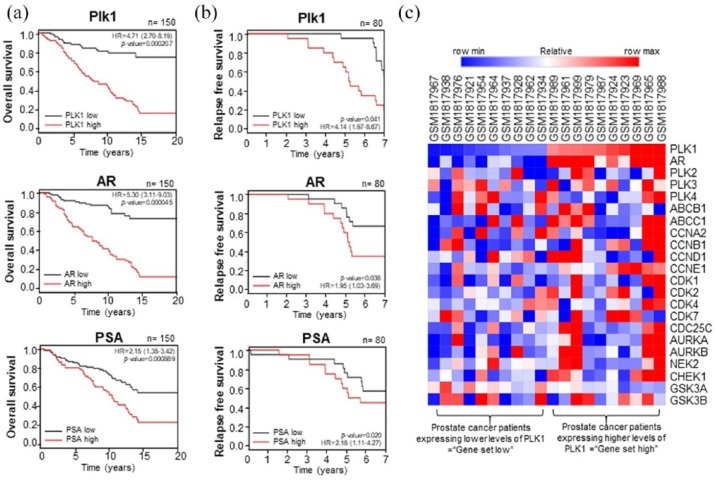
We further investigated the translational and clinical relevance of Plk1 and AR in prostate cancer patients by analyzing human prostate cancer data sets (GSE16560, GSE70769). We analyzed the correlation between the expression of Plk1, AR, and PSA, and the survival rates in prostate cancer patients (Figure 7, a-b). The expression of Plk1 mRNA and cumulative OS rates (Figure 7a) or RFS rates (Figure 7b) were inversely correlated in human prostate cancer (Figure 7, a-b), indicating high expression of Plk1 mRNA reflected the poor prognostic value in prostate cancer patients. In addition, we analyzed expression of AR in prostate cancer patients and their survival rates, because the response of prostate cancer patients is different, depending on the presence of AR. Similarly, the mRNA expression of AR or its transcriptional target PSA is inversely correlated with cumulative OS rates (Figure 7a) or RFS rates (Figure 7b) in human prostate cancer. Although the survival rates were dependent on both the levels of AR and PSA, overexpression of AR and survival rates were more correlated compared with those of PSA. Thus, the overexpression of Plk1 or AR reflects the poor prognosis in prostate cancer patients.
Figure 7.
Clinical relevance of Plk1 and androgen receptor (AR) in prostate cancer patients. (a) Kaplan–Meier plots representing the probability of cumulative overall survival (OS) in the prostate cancer patients’ samples (GSE16560) stratified according to the expression status of Plk1, AR, and prostate-specific antigen (PSA) in their primary tumors. p value reflects the significance of the correlation between gene set high and shorter survival outcome. HR, Hazard ratio. (b) Kaplan–Meier plots representing the probability of cumulative relapse-free survival (RFS) in the prostate cancer patients samples (GSE70769) stratified according to the expression status of Plk1, AR, and PSA in their primary tumors. p value reflects the significance of the correlation between gene set high and shorter survival outcome. (c) Heat map showing correlated expression of Plk1 and AR genes in prostate cancer patient samples (GSE70769) defined as ‘gene set high’ compared with tumors defined as ‘gene set low’ and the corresponding subtype classification of these tumors.
To define the correlation between Plk1 and AR gene expression, the heat map analysis was performed in prostate cancer patients’ samples (GSE70769). The tumor samples expressing high levels of Plk1 were defined as ‘gene set high’, compared with tumors expressing low levels of Plk1 defined as ‘gene set low.’. The analysis showed that the expression of mRNA between the expression of AR and Plk1 was positively correlated in prostate cancer patients (Figure 7c). Thus, analysis of clinical patients’ data provided evidence for a particular clinical relevance of our findings in AR-positive prostate cancer, showing that Plk1 is a strong predictor of poor OS and RFS in prostate cancer patients.
Discussion
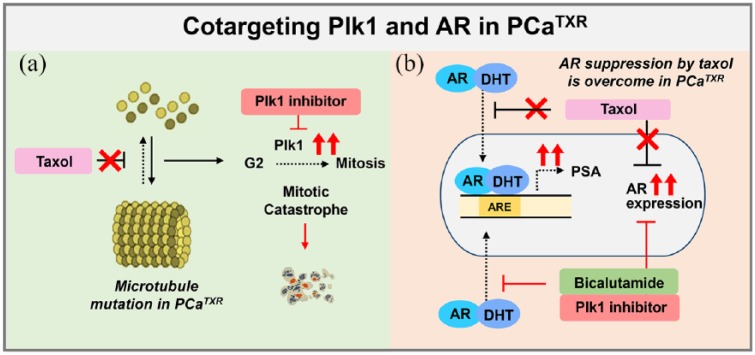
Taxanes in combination with other chemotherapy medicines are standard in patients with advanced cancers of the prostate,5,6 breast,7 ovary,3 and lung.4,8 Although there are many positive aspects of the agents, some malignancies acquire drug resistance through the expression of MDR1 or MRP1, which is thought to be the main cause of resistance to taxanes.8,47 Several mitotic targets have been studied, including the Aurora and Plk1 kinases,1 to develop the next generation of antimitotic drugs and overcome the current disadvantages. We observed the efficacy of Plk1 inhibitors in paclitaxel-resistant cells expressing high mRNA levels of MRP1, MDR1, and Plk1 and showing high Plk1 activity. Although LNCaPTXR and DU145TXR cells acquired resistance to paclitaxel, they were still sensitive to Plk1 inhibitors, which may be due to the inhibitory effect on the expression and activity of Plk1. Furthermore, paclitaxel-resistant prostate cancer cells showed high levels of AR and PSA mRNA compared with those of parental cells, which was downregulated by treatment with Plk1 inhibitors and AR antagonist (see Figure 8). Recent standard chemotherapy used in prostate cancer is docetaxel rather than paclitaxel of taxanes.48 The docetaxel-resistant mechanisms would be similar with those of paclitaxel because of the similar action mechanisms of taxanes.49
Figure 8.
Plausible model of cotargeting Plk1 and androgen receptor (AR) in paclitaxel-resistant prostate cancer. (a) Although paclitaxel-resistant cancer cells acquired resistance to paclitaxel, they are still sensitive to Plk1 inhibitors, which may be due to the inhibitory effect on the expression and activity of Plk1. (b) Paclitaxel-resistant prostate cancer cells showed high mRNA levels of AR and prostate-specific antigen (PSA) compared with those of the parental cells, which is downregulated by the AR antagonist bicalutamide. Based on these plausible mechanisms, cotargeting Plk1 and AR would be effective in the treatment of advanced prostate cancer.
According to recent studies, Plk1 inhibition represses the androgen signaling pathway in prostate cancer.22,23,42 Plk1 inhibition by BI 2536 reduced the levels of AR mRNA and potentiated the effects of androgen signaling inhibitors such as enzalutamide in LNCaP cells, indicating that Plk1 has an important role in castration-resistant prostate cancer.23 In our study, we found that the AR-positive and paclitaxel-resistant prostate cancer cells have high expression levels of Plk1 and AR, which are effectively downregulated by treatment with a single treatment of genistein or a combination of Plk1 inhibitors and an AR antagonist. This study may not reflect the AR-negative prostate cancer patients because paclitaxel-resistant AR-positive LNCaPTXR cells were mainly used. However, DU145TXR cells which are less AR-sensitive than LNCaPTXR cells, also showed the high levels of MDR1, MRP1, Plk1, and AR. DU145TXR cells were sensitive to the treatment of genistein or bicalutamide with downregulation of AR or PSA, indicating that genistein, as well as the AR antagonist bicalutamide are effective in DU145TXR cells. Clinically, the survival rates of prostate cancer patients having high expression of Plk1 or AR mRNA were lower than those of patients expressing lower levels of Plk1 or AR (See Figure 7). Thus, Plk1 and AR are useful as valuable prognostic markers in advanced prostate cancer patients.
The combination effect between Plk1 inhibitors and the AR antagonist bicalutamide was determined by CI in both prostate cancer and paclitaxel-resistant prostate cancer. The strongest synergic effects were detected in the combination between volasertib and 5.5 μM bicalutamide in both parental prostate cancer and paclitaxel-resistant prostate cancer cells with CI values of 0.5868 and 0.6818, respectively (see Table 1). When the concentration of bicalutamide was reduced to 2.5 μM, the synergistic effects were much stronger with CI values 0.4647 and 0.4160, respectively, suggesting that combination between volasertib and bicalutamide showed a strong synergistic effect for paclitaxel-resistant prostate cancer treatment. Although paclitaxel-resistant LNCaPTXR cells were sensitive to genistein, the combination between genistein and bicalutamide showed a moderate synergistic effect (CI = 0.8), which may be related with the overlapped AR inhibitory effects of genistein and bicalutamide. The combination experiments demonstrated that Plk1 inhibitors can sensitize the AR antagonist in paclitaxel-resistant prostate cancer, suggesting a synergistic effect between Plk1 inhibitors and bicalutamide. Thus, cotargeting Plk1 and AR is effective in advanced chemoresistant prostate cancers in overcoming the limitations associated with paclitaxel.
As a possible mechanism of how Plk1 regulates prostate cancer, it has been suggested that the Plk1-mediated phosphorylation of PTEN induces its stabilization and inactivation, and then the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) pathway, positively associated with AR signaling in prostate cancer,50,51 is activated.52 Based on the previous studies, the inhibition of Plk1 and AR by a Plk1 inhibitor and AR antagonist would inactivate the PI3K pathway and AR signaling pathway in paclitaxel-resistant prostate cancer cells, which inactivates the survival signaling pathway. Previous study showing that inhibition of Plk1 represses the androgen signaling pathway in castration-resistant prostate cancer,22 supports the relation between Plk1 expression and AR signaling in prostate cancer. A combination of a Plk1 inhibitor, such as volasertib, and an AR antagonist, such as bicalutamide, would be worth treating to chemoresistant prostate cancer patients. Further studies using several antimitotic drugs that have different targets, would reveal whether the clinical cross-application of multiple drugs in chemoresistant patients results in improved therapeutic efficacy.
Currently, several clinical studies with Plk1 inhibitors or genistein have been performed in prostate cancer patients. Onvansertib, a Plk1 inhibitor recently developed by Nerviano Medical Sciences, has just entered to a phase II clinical study in combination with an AR antagonist abiraterone and prednisone in metastatic castration-resistant prostate cancer (ClinicalTrials.gov identifier: NCT03414034). In the previous in vivo study using nude mouse model, cotreatment of Plk1 inhibitor BI 2536 and abiraterone synergistically inhibits the tumor growth of castration-resistant prostate cancer LuCaP35 xenograft.23 Based on this preclinical study using Plk1 inhibitor BI 2536 and abiraterone,23 this upcoming clinical study will be effective. The success of this clinical study will support that cotargeting Plk1 and AR would be effective in castration-resistant prostate cancer in vivo. In addition, several phase II clinical trials of genistein with prostate cancer patients have been examined to validate how well genistein works in treating prostate cancer patients (ClinicalTrials.gov identifier: NCT01126879), initiating androgen deprivation therapy (ClinicalTrials.gov identifier: NCT02766478), or scheduled for a prostatectomy (ClinicalTrials.gov identifier: NCT01036321). The success of these clinical studies reflects that Plk1 inhibitor genistein would be effective in prostate cancer patients, supporting our study. However, it is still needed whether cotargeting Plk1 and AR is effective in chemoresistant or castration-resistant prostate cancer in vivo as a further study.
In the present study, we have shown several findings. (1) Paclitaxel-resistant cancer cells increased the levels of Plk1 mRNA, protein, and kinase activity. (2) Genistein, a Plk1 inhibitor, effectively downregulates the expressions of MDR1 and MRP1, key factors inducing chemoresistance in paclitaxel-resistant cancer cells. (3) Paclitaxel-resistant prostate cancer cells undergo apoptotic cell death more easily than parental cells with the administration of genistein. (4) Combination between Plk1 inhibitors and bicalutamide was more effective than a single treatment in paclitaxel-resistant LNCaPTXR cells. (5) Analysis of clinical patients’ data provided evidence for a particular relevance of our findings in AR-positive prostate cancer, showing that Plk1 is a strong predictor of low survival rates with AR. These results suggest that cotargeting Plk1 and AR would be effective in the treatment of paclitaxel-resistant prostate cancer.
Supplemental Material
Supplemental material, Supplementary_Information-TAMO for Cotargeting Plk1 and androgen receptor enhances the therapeutic sensitivity of paclitaxel-resistant prostate cancer by Sol-Bi Shin, Sang-Uk Woo and Hyungshin Yim in Therapeutic Advances in Medical Oncology
Acknowledgments
We would thank to Dr. Hye Eun Byeon and Rong Xu (Hanyang University, Korea) for the technical assistance and discussions.
Footnotes
Author contributions: SS designed and performed the experiments. SW performed the experiments. HY conceived the idea, designed the experiments, and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Funding: This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (grant number NRF-2017R1A2B2012301 to HY).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Sol-Bi Shin, Department of Pharmacy, College of Pharmacy, Institute of Pharmaceutical Science and Technology, Hanyang University, Ansan, Gyeonggi-do, Korea.
Sang-Uk Woo, Department of Pharmacy, College of Pharmacy, Institute of Pharmaceutical Science and Technology, Hanyang University, Ansan, Gyeonggi-do, Korea.
Hyungshin Yim, Department of Pharmacy, College of Pharmacy, Institute of Pharmaceutical Science and Technology, Hanyang University, Ansan, Gyeonggi-do 15588, Korea.
References
- 1. Jackson JR, Patrick DR, Dar MM, et al. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nat Rev Cancer 2007; 7: 107–117. [DOI] [PubMed] [Google Scholar]
- 2. Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer 2004; 4: 253–265. [DOI] [PubMed] [Google Scholar]
- 3. Chan JK, Brady MF, Monk BJ., Weekly vs. Every-3-Week Paclitaxel for Ovarian Cancer. N Engl J Med 2016; 374: 2603–2604. [DOI] [PubMed] [Google Scholar]
- 4. Volk V, Cathomas R, Mark M, et al. Weekly carboplatin in combination with weekly paclitaxel in the treatment of metastatic non-small cell lung cancer: a single center 10-year experience. Support Care Cancer 2016; 24: 2119–2128. [DOI] [PubMed] [Google Scholar]
- 5. Kentepozidis N, Soultati A, Giassas S, et al. Paclitaxel in combination with carboplatin as salvage treatment in patients with castration-resistant prostate cancer: a Hellenic oncology research group multicenter phase II study. Cancer Chemother Pharmacol 2012; 70: 161–168. [DOI] [PubMed] [Google Scholar]
- 6. Smith DC, Tangen CM, Van Veldhuizen PJ, Jr, et al. Phase II evaluation of early oral estramustine, oral etoposide, and intravenous paclitaxel combined with hormonal therapy in patients with high-risk metastatic prostate adenocarcinoma: Southwest Oncology Group S0032. Urology 2011; 77: 1172–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoshitomi S, Taira N, Doihara H, et al. A phase 1, dose-finding and pharmacokinetic study of gemcitabine with nab-paclitaxel in patients with metastatic breast cancer. Cancer Chemother Pharmacol 2016; 78: 289–294. [DOI] [PubMed] [Google Scholar]
- 8. Orr GA, Verdier-Pinard P, McDaid H, et al. Mechanisms of Taxol resistance related to microtubules. Oncogene 2003; 22: 7280–7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schepisi G, Conteduca V, Lolli C, et al. Taxane-related nail toxicity. Lancet Oncol 2015; 16: e310–e311. [DOI] [PubMed] [Google Scholar]
- 10. Gottesman MM, Ambudkar SV. Overview: ABC transporters and human disease. J Bioenerg Biomembr 2001; 33: 453–458. [DOI] [PubMed] [Google Scholar]
- 11. Yim H, Erikson RL. Plk1-targeted therapies in TP53- or RAS-mutated cancer. Mutat Res Rev Mutat Res 2014; pii: S1383–5742(14)00019–2. [DOI] [PubMed] [Google Scholar]
- 12. Wolf G, Hildenbrand R, Schwar C, et al. Polo-like kinase: a novel marker of proliferation: correlation with estrogen-receptor expression in human breast cancer. Pathol Res Pract 2000; 196: 753–759. [DOI] [PubMed] [Google Scholar]
- 13. Weichert W, Kristiansen G, Winzer KJ, et al. Polo-like kinase isoforms in breast cancer: expression patterns and prognostic implications. Virchows Arch 2005; 446: 442–450. [DOI] [PubMed] [Google Scholar]
- 14. Weichert W, Kristiansen G, Schmidt M, et al. Polo-like kinase 1 expression is a prognostic factor in human colon cancer. World J Gastroenterol 2005; 11: 5644–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wolf G, Elez R, Doermer A, et al. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene 1997; 14: 543–549. [DOI] [PubMed] [Google Scholar]
- 16. Weichert W, Schmidt M, Gekeler V, et al. Polo-like kinase 1 is overexpressed in prostate cancer and linked to higher tumor grades. Prostate 2004; 60: 240–245. [DOI] [PubMed] [Google Scholar]
- 17. Liu XS, Song B, Elzey BD, et al. Polo-like kinase 1 facilitates loss of Pten tumor suppressor-induced prostate cancer formation. J Biol Chem 2011; 286: 35795–35800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benoit DS, Henry SM, Shubin AD, et al. pH-responsive polymeric sirna carriers sensitize multidrug resistant ovarian cancer cells to doxorubicin via knockdown of polo-like kinase 1. Mol Pharm 2010; 7: 442–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sero V, Tavanti E, Vella S, et al. Targeting polo-like kinase 1 by NMS-P937 in osteosarcoma cell lines inhibits tumor cell growth and partially overcomes drug resistance. Invest New Drugs 2014; 32: 1167–1180. [DOI] [PubMed] [Google Scholar]
- 20. Song B, Liu XS, Rice SJ, et al. Plk1 phosphorylation of orc2 and hbo1 contributes to gemcitabine resistance in pancreatic cancer. Mol Cancer Ther 2013; 12: 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakouzi NA, Cotteret S, Commo F, et al. Targeting CDC25C, PLK1 and CHEK1 to overcome Docetaxel resistance induced by loss of LZTS1 in prostate cancer. Oncotarget 2014; 5: 667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Z, Chen L, Wang H, et al. Inhibition of Plk1 represses androgen signaling pathway in castration-resistant prostate cancer. Cell Cycle 2015; 14: 2142–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Z, Hou X, Shao C, et al. Plk1 inhibition enhances the efficacy of androgen signaling blockade in castration-resistant prostate cancer. Cancer Res 2014; 74: 6635–6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med 2004; 351: 1488–1490. [DOI] [PubMed] [Google Scholar]
- 25. Taplin ME, Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem 2004; 91: 483–490. [DOI] [PubMed] [Google Scholar]
- 26. Gan L, Chen S, Wang Y, et al. Inhibition of the androgen receptor as a novel mechanism of taxol chemotherapy in prostate cancer. Cancer Res 2009; 69: 8386–8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang J, Huang H. Targeting the androgen receptor by taxol in castration-resistant prostate cancer. Mol Cell Pharmacol 2010; 2: 1–5. [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang L, Johnson M, Le KH, et al. Interrogating androgen receptor function in recurrent prostate cancer. Cancer Res 2003; 63: 4552–4560. [PubMed] [Google Scholar]
- 29. Shin SB, Woo SU, Chin YW, et al. Sensitivity of TP53-mutated cancer cells to the phytoestrogen genistein is associated with direct inhibition of Plk1 activity. J Cell Physiol 2017; 232: 2818–2828. [DOI] [PubMed] [Google Scholar]
- 30. Mukherjee S, Acharya BR, Bhattacharyya B, et al. Genistein arrests cell cycle progression of A549 cells at the G(2)/M phase and depolymerizes interphase microtubules through binding to a unique site of tubulin. Biochemistry 2010; 49: 1702–1712. [DOI] [PubMed] [Google Scholar]
- 31. Yan GR, Zou FY, Dang BL, et al. Genistein-induced mitotic arrest of gastric cancer cells by downregulating KIF20A, a proteomics study. Proteomics 2012; 12: 2391–2399. [DOI] [PubMed] [Google Scholar]
- 32. Nakayama Y, Saito Y, Soeda S, et al. Genistein induces cytokinesis failure through RhoA delocalization and anaphase chromosome bridging. J Cell Biochem 2014; 115: 763–771. [DOI] [PubMed] [Google Scholar]
- 33. Cappelletti V, Fioravanti L, Miodini P, et al. Genistein blocks breast cancer cells in the G(2)M phase of the cell cycle. J Cell Biochem 2000; 79: 594–600. [PubMed] [Google Scholar]
- 34. Akiyama T, Ishida J, Nakagawa S, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 1987; 262: 5592–5595. [PubMed] [Google Scholar]
- 35. Basak S, Pookot D, Noonan EJ, et al. Genistein down-regulates androgen receptor by modulating HDAC6-Hsp90 chaperone function. Mol Cancer Ther 2008; 7: 3195–3202. [DOI] [PubMed] [Google Scholar]
- 36. Zaniboni A, Ardizzoni A, De Marinis F, et al. Phase II study of taxol combined with ifosfamide and carboplatin in the treatment of stage IIIb-IV non-small-cell lung cancer. Am J Clin Oncol 2003; 26: 84–88. [DOI] [PubMed] [Google Scholar]
- 37. Yim H, Shin SB, Woo SU, et al. Plk1-mediated stabilization of 53BP1 through USP7 regulates centrosome positioning to maintain bipolarity. Oncogene 2017; 36: 966–978. [DOI] [PubMed] [Google Scholar]
- 38. Han YM, Woo SU, Choi MS, et al. Antiinflammatory and analgesic effects of Eurycoma longifolia extracts. Arch Pharm Res 2016; 39: 421–428. [DOI] [PubMed] [Google Scholar]
- 39. Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 2010; 70: 440–446. [DOI] [PubMed] [Google Scholar]
- 40. Sboner A, Demichelis F, Calza S, et al. Molecular sampling of prostate cancer: a dilemma for predicting disease progression. BMC Med Genomics 2010; 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ross-Adams H, Lamb AD, Dunning MJ, et al. Integration of copy number and transcriptomics provides risk stratification in prostate cancer: a discovery and validation cohort study. EBioMedicine 2015; 2: 1133–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hou X, Li Z, Huang W, et al. Plk1-dependent microtubule dynamics promotes androgen receptor signaling in prostate cancer. Prostate 2013; 73: 1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mikkonen L, Pihlajamaa P, Sahu B, et al. Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol 2010; 317: 14–24. [DOI] [PubMed] [Google Scholar]
- 44. Chang C, Lee SO, Yeh S, et al. Androgen receptor (AR) differential roles in hormone-related tumors including prostate, bladder, kidney, lung, breast and liver. Oncogene 2014; 33: 3225–3234. [DOI] [PubMed] [Google Scholar]
- 45. Alimirah F, Chen J, Basrawala Z, et al. DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: implications for the androgen receptor functions and regulation. FEBS Lett 2006; 580: 2294–2300. [DOI] [PubMed] [Google Scholar]
- 46. Leith CP, Chen IM, Kopecky KJ, et al. Correlation of multidrug resistance (MDR1) protein expression with functional dye/drug efflux in acute myeloid leukemia by multiparameter flow cytometry: identification of discordant MDR-/efflux+ and MDR1+/efflux- cases. Blood 1995; 86: 2329–2342. [PubMed] [Google Scholar]
- 47. Allen JD, Brinkhuis RF, van Deemter L, et al. Extensive contribution of the multidrug transporters P-glycoprotein and Mrp1 to basal drug resistance. Cancer Res 2000; 60: 5761–5766. [PubMed] [Google Scholar]
- 48. Puente J, Grande E, Medina A, et al. Docetaxel in prostate cancer: a familiar face as the new standard in a hormone-sensitive setting. Ther Adv Med Oncol 2017; 9: 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kroon J, Kooijman S, Cho NJ, et al. Improving taxane-based chemotherapy in castration-resistant prostate cancer. Trends Pharmacol Sci 2016; 37: 451–462. [DOI] [PubMed] [Google Scholar]
- 50. Kaarbo M, Mikkelsen OL, Malerod L, et al. PI3K-AKT-mTOR pathway is dominant over androgen receptor signaling in prostate cancer cells. Cell Oncol 2010; 32: 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011; 19: 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li Z, Li J, Bi P, et al. Plk1 phosphorylation of PTEN causes a tumor-promoting metabolic state. Mol Cell Biol 2014; 34: 3642–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Information-TAMO for Cotargeting Plk1 and androgen receptor enhances the therapeutic sensitivity of paclitaxel-resistant prostate cancer by Sol-Bi Shin, Sang-Uk Woo and Hyungshin Yim in Therapeutic Advances in Medical Oncology