Abstract
Background
Reducing obesity through effective behaviour change interventions is of key importance to prevent disabling and life-threatening conditions, particularly in individuals already at risk for morbidity.
Purpose
To assess the effects of behavioural interventions for obese adults with additional risk factors for morbidity on behaviour, weight and disease risk factors.
Methods
Systematic review of randomised controlled trials (RCTs). Three electronic databases and three journals were searched for behavioural interventions (aimed at changing dietary intake and/or physical activity (PA)) for adults (mean BMI ≧30 kg/m2; mean age ≧40 years) with risk factors for morbidity, reporting follow-up data ≧12 weeks.
Results
44 RCTs met the inclusion criteria. Behavioural outcomes, weight loss, and cardiovascular disease risk factors showed consistent modest improvements over time, especially for interventions targeting both diet and PA.
Conclusion
Behavioural interventions in at-risk populations showed positive effect tendencies on behaviour, weight, and disease risk factors. However, there is still ample room for improvement, and future research should focus on identifying the most effective means of inducing dietary and PA behaviour change in this vulnerable population.
Key Words: Systematic review, Obesity, Behavioural interventions, Diet, Physical activity
Introduction
The prevalence of obesity worldwide is both high and increasing [1]. Obesity is associated with numerous co-morbidities, including cardiovascular disease, type 2 diabetes, hypertension, and certain cancers [2]. Behavioural interventions, aimed at influencing peoples’ dietary and/or physical activity (PA) behaviour, lead to weight loss and improved obesity-related risk factor profiles in individuals with excess weight [3, 4, 5].
When studying obesity interventions, it is important to consider the influence of additional risk factors for morbidity (for further discussion of additional risk factors, see Mokdad et al. 2003 [73]) as interventions in individuals carrying these risk factors may be less effective [3]. Clinically obese populations with additional risk factors are one of the fastest growing patient populations. Consequently, it is paramount to develop an understanding of the effects of behaviour change intervention on behaviour, weight, and risk factor indicators in this population [6].
Previous systematic reviews evaluating the effects of behavioural interventions paid little attention to the most proximal target of behavioural weight loss interventions, behaviour change itself [7]. As some intervention studies may not achieve significant weight loss or improvement in health risk factors, information about behavioural change is not only relevant but essential to our appraisal of the intervention. Few systematic reviews examine the effectiveness of behaviour change interventions on behaviour, although evidence shows that dietary advice was found to lead to significant positive changes in self-reported fibre, fruit and vegetable, and saturated fat intake [8], and interventions promoting PA showed a significant moderate effect on self-reported PA [9] (for the purpose of this review dietary intake and PA are considered examples of behaviours).
This systematic review extends the evidence base for behavioural obesity treatment by addressing the lack of behavioural analysis in the scrutiny of intervention effectiveness, and focuses attention on a population in need of intervention: obese adults with additional risk factors for morbidity.
Material and Methods
Objectives
To review the effects of behavioural interventions for obese adults with at least one additional risk factor for morbidity on behaviour (diet and PA), weight, and risk factors. The effect of interventions on outcomes were compared for intervention groups (which focused on changes in diet only, PA only, or both diet and PA) against control or less intensive intervention groups.
Study Inclusion Criteria
Types of Studies: Published randomised controlled trials (RCTs) providing ≥12 weeks follow-up data after randomisation. No language limitations were specified.
Types of Participants: Individuals with a mean/median BMI ≥30 kg/m2. Studies focused on adult obesity with a mean/median age of ≥40 years as there is a rapid increase in obesity-related diseases including the metabolic syndrome [10] and type 2 diabetes [11] in middle age. At least one additional risk factor for morbidity was required as this population is in greatest need of behaviour change to prevent long-term morbidity.
Types of Interventions: Behavioural interventions aimed at changing diet and/or PA. For this review, interventions are classified by ‘diet only (D-only),’ ‘PA only (PA-only),’ or ‘diet and PA (D-PA)’.
Types of Outcome Measures: The outcomes examined in this review were behaviour (i.e. objective or self-reported measures of diet and/or PA), weight and risk factors (total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, triglycerides, systolic (SBP) and diastolic blood pressure (DBP), glycosylated haemoglobin (HbA1c), and fasting plasma glucose (FPG)).
Search Strategy for Identification of Studies
Three electronic databases (MEDLINE, EMBASE, and PsycInfo) were searched for relevant studies using a comprehensive search strategy (available upon request). Three journals (International Journal of Obesity, International Journal of Behavioural Medicine, and Obesity Research) were searched by hand. Reference lists of relevant review articles and of all included studies were searched for further studies.
Methods of the Review
Identification of RCTs: The first 200 references of RCTs were independently screened by two researchers (AA and SUD) and differences were resolved in discussion. Thereafter, the identification of studies was completed by one researcher (SUD).
Quality Assessment of Studies: Standard criteria for RCTs were used to appraise the methodological quality [12].
Data Extraction: Three researchers (AA, FFS, and SUD) extracted data for an initial three studies, and differences were resolved by discussion. Thereafter, one researcher (SUD) extracted data for behaviour, weight, and risk factors from the remaining studies. Behavioural variables were assessed by a wide variety of measures (table 1). Most studies focused on altering kilocalorie and fat intake; so these two outcomes became the main dietary focus. These measures are a proxy for change in dietary behaviour and used as dietary behavioural outcomes for the purpose of the current review. All data entry into meta-analytic software, Review Manager (Version 4.2) (clicktime.com, Inc., San Francisco, CA USA), was double checked 1 month after initial entry.
Table 1.
Details of included randomised controlled trials
| Study ID | Participants | Interventions | Outcomes |
|---|---|---|---|
| Argus-Collins 1997 [28] | Location: USA. Co-morbidity: type 2 diabetes. Sex: 52 women, 12 men. Age, mean (SD) in years: (a) 62.4 (5.9) (b) 61 (5.7). BMI, mean in kg/m2: (a) 33.9 (b) 34.9. Weight, mean (SD) in kg: (a) 93.3 (18.6) (b) 94.9 (20.1). |
(a) Group counselling intervention.Diet: <30% from fat, ~55–60% kcal from carbohydrate, 12–20% from protein.Activity: moderate physical activity ≥3 days/week.Other: weight loss of ≥4.5 kg at the rate of ≤0.9/week. (b) Usual care. Allocated: (a) 32 (b) 32. % dropout: (a) 6.25% (b) 21.9% at6 months. Possible comparisons: D-PA vs. UC. |
Follow-up(s): 3 and 6months. Outcomes: weight, total cholesterol,LDL,HDL, TGs, HbA1c, SBP, DBP, kcal, fat (% kcal), PASE. |
| Ash 2003 [17] | Location: Australia. Co-morbidity: type 2 diabetes. Sex: all men. Age, mean (SD) in years: (a) 54.3 (9.4) (b) 54.2 (7.4) (c) 54.9 (9.3). BMI, mean (SD) in kg/m2: (a) 31.2 (3.4) (b) 31.1 (3.7) (c) 32.7 (2.4). Weight, mean (SD) in kg: (a) 96.7 (11.4) (b) 97.2 (13.5) (c) 101.4 (11.9). |
Prior to randomisation all patients underwent 2 weeks of dietary stabilisation (1400–1700 kcal/day, 50% kcal from carbohydrate, 30% kcal from fat). (a) Liquid meal replacement (Modifast).Diet: 1,000 kcal/day on 4 days/week, 1,400–1,700 kcal/day on other 3 days. (b) Food provision.Diet: 6900 kj/day (1650 kcal/day, 51% of energy from carbohydrate, 20% from protein and 29% from fat). (c) Usual care. Allocated: (a) 20 (b) 17 (c) 14. % dropout: 47.1% for all groups combined at 18 months. Possible comparisons: D vs. UC. |
Follow-up(s): 12 weeks and 18 months. Outcomes: weight, total cholesterol,LDL,HDL, TGs, HbA1c, kcal. |
| Blonk 1994 [53] | Location: Holland. Co-morbidity: type 2 diabetes. Sex: (a) 18 women, 9 men (b) 16 women, 10 men. Age, mean (CI) in years: (a) 59.0 (42.0, 69.0) (b) 58.5 (29.0, 70.0). BMI, mean (CI) kg/m2: (a) 31.3 (27.2, 44.3) (b) 32.8 (27.9–45.8). Weight, mean (CI) in kg: (a) 92.3 (69.3, 120.8) (b) 87.8 (65.2, 158.3). |
(a) Comprehensive program.Diet: 500 kcal deficit, minimum intake of 1,000 kcal, 30% kcal from fat, 50–55% kcal from carbohydrate, 25g fiber, <300 mg/day cholesterol, and 15% kcal from protein.Activity: scheduled exercise sessions twice/week fading out over time. (b) Conventional programme.Diet: same as (a)Activity: scheduled exercise sessions and exercise every day at home and increase in regular daily activities. Allocated: (a) 27 (b) 26. % dropout: not given. Possible comparisons: Intensive vs. less intensive (D-PA). |
Follow-up(s): 2, 4, 6,8, 12, 14, 16, 18, 20, 22 and 24 months. Outcomes: weight, total cholesterol,LDL,HDL, TGs, HbA1c, SBP, DBP, kcal. |
| Blumenthal 2000[30] | Location: USA. Co-morbidity: hypertension. Sex: (a) 34 women, 21 men (b) 29 women, 25 men (c) 11 women, 13 men. Age, mean (SD) in years: (a) 48.5 (1.2) (b) 46.6 (1.2) (c) 47.2 (1.8). BMI, mean (SD) in kg/m2: (a) 32.1 (4.0) (b) 32.8 (4.0) (c) 32.6 (5.1). Weight, mean (SD) in kg: (a) 93.3 (17.7) (b) 95.4 (14.5) (c) 94.0 (17.3). |
(a) Weight management group.Diet: 5,021 J (1,200 kcal) for women, 6,276 J (1,500 kcal) for men, 15–20% of energy from fat.Activity: scheduled and supervised exercise sessions 4–5 times/weekOther: 0.5–1kg weight loss/week. (b) Exercise group.Activity: same as weight management group (c) Waiting list control group. Allocated: (a) 55 (b) 54 (c) 24. % dropout: (a) 16% (b) 19% (c)8% at6 months. Possible comparisons: D-PA vs. WLC; PA vs. WLC. |
Follow-up(s):6 months. Outcomes: weight, SBP, DBP, FPG, kcal, fat (g), treadmill time. |
| Burke2007 [29] | Location: Perth, Australia. Co-morbidity: hypertension (drug treated) Sex: (a) 67 women 56 men (b) 67 women, 51 men Age, mean (SD) in years: (a) 57.1 (7.2) (b) 55.5 (7.5) BMI mean (SD) kg m-2: (a) 30.4 (2.9) (b) 29.7 (2.5) Weight, mean (SD) in kg: (a) 86.7 (12.4) (b) 84.2 (10.8) |
(a) Lifestyle programme.Diet: DASH diet low in fat (<30% kcal from fat, 10% kcal from saturated fat), > fruit and vegetables, < salt and sugar, ≥ 4 fish meals/week, ≤ two standard drinks/day.Activity: accumulate≥30 min of MIPA on most days, increase incidental activity.Other: decrease baseline weight by 5–10% over 4 months. (b) Usual care. Allocated: (a) 123 (b) 118 % dropout: (a) 17% (b) 24% Possible comparisons: D-PA vs. UC. |
Follow-up(s): 4 and 12 months Outcomes: weight, SBP, DBP, kcal, fat (% kcal), time spent in PA (min/week). |
| Carels 2004 [54] | Location: USA. Co-morbidity: postmenopause. Gender: all female. Age, mean (SD) in years: (a) 55.1 (8.3) (b) 54.3 (7.8). BMI, mean in kg/m2: (a) 37.8 (5.8) (b) 35.1 (5). |
(a) Lifestyle change.Diet and Activity: LEARN program recommendations [71]. (b) Lifestyle change + Self-control skills.Diet and Activity: same as (a) (only behavioural techniques differ). Allocated: (a) 21 (b) 23. % dropout: (a) 14.3% (b) 17.4% at 12 months. Possible comparisons: Intensive vs. less intensive (D-PA). |
Follow-up(s): 6 and12 months. Outcomes: weight, total cholesterol, LDL, HDL, TGs, SBP, DBP, FPG,kcal,fat (%kcal), treadmill time (s). |
| Clark 2004 [22] | Location: UK. Co-morbidity: type 2 diabetes. Sex: 42 women, 58 men. Age, mean in years: 59.5. BMI, mean (SD) in kg/m2: (a) 32.40 (4.49) (b) 31.30 (5.01). Weight, mean in kg: not given. |
(a) Lifestyle intervention.Diet: Self selected goal(s) for lifestyle change.Activity: Self selected goal(s) for lifestyle change. (b) Usual care group. Allocated: (a) 50 (b) 50. % dropout: (a) 8% (b) 4% at 52 weeks. Possible comparisons: D-PA vs. UC. |
Follow-up(s): 3 and12 months. Outcomes: weight, total cholesterol,LDL,HDL, TGs, HbA1c, FHQ (Block fat screener), PASE. |
| Deakin 2006 [31] | Location: UK. Co-morbidity: type 2 diabetes. Sex: 152 women, 162 men. Age, mean (SD) in years: (a) 61.3 (9.7) (b) 61.8 (11.0). BMI, mean (SD) in kg/m2: (a) 30.8 (5.3) (b) 30.6 (5.7). Weight, mean (SD) in kg: (a) 83.2 (14.5) (b) 82.8 (17.6). |
(a) X-PERT programme.Diet: Recommendations based on the British Nutrition Foundation’s ‘Balance of Good Health’.Activity: Exercise on prescription scheme (individual exercise recommendations from GP). (b) Control group. Allocated: (a) 157 (b) 157. % dropout: (a) 4.5% (b) 10.2%. Possible comparisons: D-PA vs. UC. |
Follow-up(s): 4 and 14 months. Outcomes: weight, total cholesterol,LDL,HDL, TGs., HbA1c, SBP, DBP,kcal,fat (%kcal),Summary of self care activity (PA). |
| Diabetes Prevention Program, 2003 [21] | Location: USA. Co-morbidity: elevated fasting and post-load plasma glucose concentrations. Sex: (a) 737 women, 345 men (b) 710 women, 363 men (c) 747 women, 335 men. Age, mean (SD) in years: (a) 50.6 (11.3) (b) 50.9 (10.3) (c) 50.3 (10.4). BMI, mean (SD) in kg/m2: (a) 33.9 (6.8) (b) 33.9 (6.6) (c) 34.2 (6.7). Weight, mean (SD) in kg: (a) 94.1 (20.8) (b) 94.3 (19.9) (c) 94.3 (20.2). |
(a) Lifestyle intervention.Diet: 5,00–1,000 kcal/day deficit, 25% kcal from fat.Activity: ≥700 kcal/week (equivalent to 150 min of MPA).Other: 7% weight loss of initial body weight. (b) Metformin group.Other: 850 mg of metformin daily. (c) Placebo control group. Allocated: (a) 1,079 (b) 1,073 (c) 1,082. % dropout: ‘92.5% of participants had attended a scheduled visit within previous6 months’. Possible comparisons: D-PA vs. UC. |
Follow-up(s): 6, 12, 18, 24, 30, 36, 42, and 48 months. Outcomes: weight, HbAc1, FPG,kcal,fat (% kcal). |
| Djuric 2002 [32] | Location: USA. Co-morbidity: breast cancer. Sex: all women. Age, mean (SD) in years: 51.7 (8.4). BMI, mean (SD) in kg/m2: (a) 35 (1.2) (b) 35.5 (1.1) (c) 36.8 (8) (d) 34.9 (1.2). Weight, mean (SD) in kg: (a) 95.5 (5) (b) 91.4 (2.7) (c) 100.5 (5) (d) 95 (3.6). |
(a) Weight watchers group.Diet: Weight Watchers prescriptions. (b) Individualised group.Diet: 500–1,000 kcal/d deficit, 20–25% kcal from fat.Activity: 30–45 min/day of MPA most days.Other: decrease of 10% of baseline weight over 6 months. (c) Comprehensive group.Diet: Weight Watchers prescriptions.Activity: 30–45 min/day of MPA most days.Other: decrease of 10% of baseline weight over 6 months. (d) Control group. Allocated: (a) 11 (b) 13 (c) 11 (d) 13. % dropout: (a) 27.3% (b) 30.8% (c) 9.1% (d) 7.7% at 12 months. Possible comparisons: D-PA vs. UC., Intensive vs. less intensive (D-PA, D only) |
Follow-up(s): 3, 6, and12 months. Outcomes: weight, total cholesterol,LDL,HDL, TGs, FPG, kcal, fat (% kcal). |
| Edelman 2006 [33] | Location: USA. Co-morbidity: One or more of the following: diabetes, hypertension, dyslipidemia, smoking, or BMI >25 anthropometric measurements. Sex: 124 women, 30 men. Age, mean (SD) in years: (a) 52.2 (5.2) (b) 53.4 (4.8). BMI, mean (SD) in kg/m2: (a) 33.3 (7.8) (b) 34.1 (7.7). Weight, mean in kg: not given. |
(a) Personal Health Planning group.Diet and Activity: change of behaviours linked to cardiovascular risk (e.g. ‘focus of commitment to healthier behaviours’ or ‘education on the topics of nutrition, PA …’). (b) Control group. Allocated: (a) 77 (b) 77. % dropout: (a) 27.3% (b) 14.3% at 10 months. Possible comparisons: D-PA vs. UC. |
Follow-up(s): 5 and 10 months. Outcomes: weight, SBP, DBP, lipid profiles, days of exercise/week. |
| Evangelista 2006 [50] |
Location: USA. Co-morbidity: advanced heart failure. Sex: (a) 11 women, 37 men (b) 17 women, 34 men. Age, mean (SD) in years: (a) 53 (13) (b) 55 (12). BMI, mean (SD) in kg/m2: 30.5 (4.2). Weight, mean (SD) in kg: 92.8 (13.5). |
(a) Exercise intervention.Activity: graduated, low-level exercise≥4 times/week. (b) Control group. Allocated: (a) 53 (b) 51. % dropout: (a) 5.7% (b) 10.52% at6 months. Possible comparisons: PA vs. UC. |
Follow-up(s):6 months. Outcomes: weight, walking test (m/min). |
| Finish Diabetes Prevention Study, 2003 [24] | Location: Finland. Co-morbidity: impaired glucose tolerance. Sex: (a) 176 women, 81men (b) 174 women, 91 men. Age, mean (SD) in years: (a) 55 (7) (b) 55 (7). BMI, mean (SD) in kg/m2: (a) 31.4 (4.5) (b) 31.1 (4.5). Weight, mean (SD) in kg: (a) 86.7 (14.0) (b) 85.5 (14.4). |
(a) Lifestyle intervention.Diet: <30% kcal from fat, <10 kcal from saturated fat, ≥15 g / 1,000 kcal from fibre. Activity: MIPA ≥30 min/day.Other: weight reduction≥5%. (b) Control group. Allocated: (a) 265 (b) 257. % dropout: (a) 12.8% (b) 21% at 3 years. Possible comparisons: D-PA vs. UC. |
Follow-up(s): 12 and 36 months. Outcomes: weight, total cholesterol, HDL, TGs, HbA1c, FPG, kcal, fat (% kcal), LTPA (min/week). |
| Glasgow 2000 [47] | Location: USA. Co-morbidity: type 2 diabetes. Sex: (a) 45 women, 35 men (b) 46 women, 34 men (c) 38 women, 42 men, (d) 53 women, 27 men. Age, mean (SD) in years: (a) 57.4 (9.4) (b) 59.0 (9.6) (c) 60.5 (8.6) (d) 60.6 (9.5). BMI, mean in kg/m2: (a) 31.23 (b) 33.27 (c) 34.37 (d) 34.69. Weight, mean (SD) in kg: (a) 90.26 (b) 96.16 (c) 99.33 (d) 100.24. |
(a) Telephone follow-up + Community resource group.Diet: feedback on current dietary behaviour. (b) Telephone follow-up group.Diet: same as (a) (c) Community resource group.Diet: same as (a) (d) Basic group.Diet: same as (a) Allocated: (a) 80 (b) 80 (c) 80 (d) 80. % dropout: (a) 16.25% (b) 16.25% (c) 6.25% (d) 85% at6 months. Possible comparisons: D vs. UC; Intensive vs. less intensive (D only). |
Follow-up(s): 3 and 6 months. Outcomes: weight, total cholesterol, HbA1c, kcal, fat Block Fat Screener. |
| Glasgow 1996 [48] | Location: USA. Co-morbidity: type 1 or type 2 diabetes. Sex: (a) 60 women, 38 men (b) 68 women, 40 men. Age, mean (SD) in years: (a) 61.7 (12.1) (b) 63.1 (10.5). BMI mean kg m-2: (a) 30.4 (b) 30.2. Weight, mean in kg: not given. |
(a) Intervention.Diet: ≤30% calories from fat, ≤10% kcal from saturated fat. (b) Control group. Allocated: (a) 106–108 (b) 94–98. % dropout: (a) 16.7% (b) 15.3% at 12 months. Possible comparisons: D only vs. UC. |
Follow-up(s): 3 and 12 months. Outcomes: weight, total cholesterol, HbA1c, kcal, fat (% kcal). |
| Goodrick 1998 [34] | Location: USA. Co-morbidity: binge eating disorder. Sex: all women. Age, mean (SD) in years: (a) 89.04 (10.15) (b) 87.71 (9.58) (c) 86.49 (9.83). BMI, mean (SD) in kg/m2: (a) 33.50 (3.46) (b) 33.16 (3.21) (c) 32.22 (2.97). Weight, mean (SD) in kg: (a) 89.04 (10.15) (b) 87.71 (9.58) (c) 86.49 (9.83). |
(a) Dieting treatment.Diet: reducing fat (40 g/day), increasing complex carbohydrates, and eating a variety of foods.Activity: 4–5 h/week at an intensity based on training heart rate.Other: weight loss averaging 1 lb (0,454 kg) per week. (b) Non-dieting treatment.Diet: ‘gradual reductions of fat without feelings of deprivation’.Activity: home-based walking program with gradually attained goal of 4–5 h/week. (c) Control group. Allocated: (a) 79 (b) 78 (c) 62. % dropout: (a) 15.2% (b) 16.7% (c) 6.5% at 18 months for (a) and (b) and6 months for (c). Possible comparisons: D-PA vs. UC. |
Follow-up(s):6 and 18 months. Outcomes: weight, kcal/kg/day. |
| Grilo 2005 [25] | Location: USA. Co-morbidity: Binge Eating Disorder. Sex: (a) 29 women, 9 men (b) 32 women, 5 men. Age, mean (SD) in years: (a) 46.0 (9.2) (b) 46.0 (9.2) (c) 48.0 (8.2). BMI, mean (SD) in kg/m2: (a) 36.0 (6.6) (b) 33.4 (5.7) (c) 36.2 (6.6). Weight, mean in kg: not given. |
(a) Behavioural weight loss.Diet: LEARN Program for Weight Management [71]Activity: LEARN Program for Weight Management [71] (b) Cognitive behavioural therapy.Diet: Overcoming Binge Eating [72]Activity: Overcoming Binge Eating [72] (c) Control. Allocated: (a) 38 (b) 37 (c) 15% dropout: (a) 34% (b) 13% (c) 13% Possible comparisons: Intensive vs. less intensive (D-PA). |
Follow-up(s): 3 months. Outcomes: weight. |
| Hardcastle 2007 [35] |
Location: UK. Co-morbidity: CHD risk factors (hypertension, hypercholesterolemia) Sex: 240 women, 118 men. Age, mean (SD) in years: (a) 50.1 (10.5) (b) 50.41 (10.8) BMI, mean (SD) in kg/m2: (a) 33.67 (5.4) (b) 34.28 (7.0) Weight, mean (SD) in kg: (a) 93.7 (17.1) (b) 91.73 (17.2) |
(a) Counselling intervention.Diet: individualised depending on readiness to change.Activity: individualised depending on readiness to change. (b) Control group. Allocated: (a) 203 (b) 131. % dropout: (a) 38.4% (b) 29% Possible comparisons: D-PA vs. UC. |
Follow-up(s):6 months. Outcomes: weight, total cholesterol, LDL, HDL, triglycerides, SBP, DBP, fat (% kcal fat), overall PA (met/min/week). |
| Jehn 2006 [23] | Location: USA. Co-morbidity: hypertension. Sex: (a) 13 women, 9 men (b) 16 women, 7 men. Age, mean (SD) in years: (a) 53 (11) (b) 54 (8). BMI, mean (SD) in kg/m2: (a) 32.8 (5.4) (b) 34.2 (3.2). Weight, mean (SD) in kg: (a) 92.0 (14.6) (b) 97.0 (20.9). |
(a) Lifestyle group.Diet: food provision of DASH diet (18% kcal protein, 55% kcal carbohydrate, 27% kcal fat)Activity: 30–45 min of supervised, MIPA, 3 days/week.Other: weight loss goal of 4.5kg after 9 weeks. (b) Control group. Allocated: (a) 22 (b) 23. % dropout: (a) 14% (b) 0%. Possible comparisons:D-PA vs. UC. |
Follow-up(s):12 months. Outcomes: weight, fat (% kcal). |
| Joneset al. 2003 [49] | Location: Canada. Co-morbidity: type 1 or type 2 diabetes. Sex: (a) 233 women, 277 men (b) 257 women, 262 men. Age, mean in years: (a1) 54.58 (a2) 55.12 (b1) 54.86 (b2) 54.60. BMI, mean in kg/m2: (a1) 31.98 (a2) 32.22 (b1) 31.43 (b2) 31.59. Weight, mean in kg: not given. |
(a1)Intervention.Diet: healthy eating focusing on dietary fat reduction. Other: smoking cessation and regular blood glucose monitoring (free strips for self-testing provided). (a2)Intervention.Diet: healthy eating focusing on dietary fat reduction.Other: smoking cessation and regular blood glucose monitoring (no strips for self-testing provided). (b1)Control group.Other: free strips for self-testing provided. (b2)Control group. Allocated: (a1) 260 (a2) 250 (b1) 269 (b2) 250. % dropout: 33% overall at 12 months. Possible comparisons: D only vs. UC. |
Follow-up(s):12 months. Outcomes: weight, fat (% kcal). |
| Keyserling2002[36] | Location: USA. Co-morbidity: type 2 diabetes. Sex: all female. Age, mean in years: (a) 58.5 (b) 59.8 (c) 59.2. BMI mean kg/m2: (a) 36.2 (b) 34.6 (c) 36.2. Weight, mean in kg: (a) 95 (b) 91.9 (c) 95.7. |
(a) Clinic and Community intervention.Diet: 2–3 dietary goals selected according to dietary risk assessment.Activity: 2–3 activity goals selected according to PA assessment. (b) Clinical intervention.Diet: 2–3 same as (a)Activity: same as (a) (c) Control group. Allocated: (a) 67 (b)66 (c) 67. % dropout: (a) 19.4% (b) 10.6% (c) 14.9% at 12 months. Possible comparisons: D-PA vs. UC; Intensive vs. less intensive (D-PA). |
Follow-up(s):6 and12 months. Outcomes: weight, total cholesterol, HDL, HbA1c, kcal, kcal expended/day. |
| Kirk 2004 [51] | Location: UK. Co-morbidity: type 2 diabetes. Sex: 35 women, 35 men. Age, mean (SD) in years: 57.6(7.9). BMI, mean (SD) in kg/m2: 34.6(6.8). Weight, mean (SD) in kg: not given. |
(a) Exercise intervention.Activity: accumulate 30 min of MIPA most days of the week. (b) Control group. Allocated: (a) 35 (b) 35. % dropout: (a) 11.4% (b)8.6% at6 months. Possible comparisons: PA only vs. UC. |
Follow-up(s):6 months. Outcomes: weight, LDL, HDL, TGs, HbAlc, SBP, DBP, Activity counts. |
| Kirkman 1994 [37] | Location: USA. Co-morbidity: type 2 diabetes. Sex: 3 women, 272 men. Age, mean (SD) in years: (a) 63.9 ( 8.6) (b) 63.2 (8.3). % above ideal weight (SD): (a) 130.6 (23.8) (b) 130.6 (193.2). Weight, mean in kg: not given. |
(a) Intervention group.Diet and Activity: prescriptions from GP (not specified) to improve glycemic control. (b) Control group. Allocated: (a) 204 (b) 71. % dropout: not given. Possible comparisons: D-PA vs. UC. |
Follow-up(s): 12 months. Outcomes: weight, total cholesterol,LDL,HDL, TGs. |
| Laitinen 1993 [26] | Location: Finland. Co-morbidity: type 2 diabetes. Sex: 49 women, 37 men. Age, mean (SD) in years: (a) 52.2 (7) (b) 54.2 (6.5). BMI, mean (SD) in kg/m2: (a) 33.95 (5.3) (b) 33.5 (4.7). |
(a) Intervention group.Diet: planned energy restriction, ≤ 30% kcal from fat, ≤10% of kcal saturated fat, ≤300 mg/day dietary cholesterol, fatty acids ≥20% of energy unsaturated fat, and increase carbohydrates (e.g. fruits, berries, and vegetables).Activity: increase frequency of exercise sessions to 3–4/week, lasting 30–60 min each.Other: weight reduction, normoglycemia, correction of dyslipidemias, and normalisation of elevated blood pressure. (b) Usual care group. Allocated: (a) 40 (b) 46. % dropout: (a) 5% (b) 4% at 15 months. Possible comparisons: D-PA vs. UC. |
Follow-up(s): 3, 15 and 24 months. Outcomes: weight, total cholesterol, HDL, TGs, HbA1c, FPG, kcal, fat (% kcal fat). |
| Logue, 2004 [55] | Location: USA. Co-morbidity: Hypertension, elevated blood cholesterol, (oesteo) arthritis, diabetes. Sex: (a) 232 women, 97 men (b) 226 women, 110 men. Number of patients within age range (%) in years: 40–49: (a) 138 (42) (b) 129 (42); 50–59 (a) 138 (42) (b) 141 (42); 60–69 (a) 52 (16) (b)66 (20). Number of patients within BMI range (%) in kg/m2: 25–29.9 (a) 59 (18) (b) 73 (22); 30–34.5 (a) 119 (37) (b) 107 (32); 35–39 (a) 69 (21) (b) 82 (24); 40+ (a) 79 (24) (b) 74 (22). Weight, mean in kg: not given. |
(a) Intervention group.Diet: increase dietary portion control, <dietary fat, >fruits and vegetables.Activity: increase exercise, increase usual activity. (b) Augmented usual care.Diet and Activity: prescriptions by dietitian based on diet and activity recalls. Allocated: (a) 329 (b) 336. % dropout: (a) 37.8% for weight, 20.08% for other information (b) 31.3% for weight, 17.6% for other information at 24 months. Possible comparisons: Intensive vs. less intensive (D-PA). |
Follow-up(s):6, 12, 18, and 24 months. Outcomes: weight, SBP, DBP, blood lipids, kcal, kcal/kg/day. |
| Mefferd 2007 [38] | Location: USA. Co-morbidity: Breast cancer. Sex: (a) 56 Women (b) 29 women. Age, mean (SD) in years: 56.3 (8.2). BMI, mean (SD) in kg/m2: 31.0 (4.2). Weight, mean (SD) in kg: 84.7 (12.6) |
(a) Intervention group.Diet: 500–1000 kcal/day deficit. Activity: one h/d of moderate to vigorous PA. (b) Control group. Allocated: (a) 56 (b) 29. % dropout: (a) 16% (b)0%. Possible comparisons: D-PA vs. UC. |
Follow-up(s): 4 months. Outcomes: weight, total cholesterol, HDL, TGs, moderate + vigorous PA. |
| Menard 2005 [39] | Location: Canada. Co-morbidity: type 2 diabetes. Sex: (a) 9 women 27 men (b) 14 women 22 men. Age, mean (SD) in years: (a) 55.9 ( 8.6) (b) 53.7 (7.5). BMI, mean (SD) in kg/m2: (a) 32.6 (5.7) (b) 32.9 (5.5). Weight, mean (SD) in kg: (a) 93.5 (20.1) (b) 88.5 (18.5). |
(a) Intervention group.Diet: 50–55% kcal from carbohydrates, ≤30% kcal fat, ≤10% kcal from saturated fat.Activity: home based exercise sessions, 3–4 times/week, 45–55 minutes, intensity at 50–80% of maximum heart rate.Other: After 3 months pharmacological therapy was introduced in patients not able to reach treatment goals (b) Control group. Allocated: (a) 36 (b) 36. % dropout (a) 16.7% (b) 19.5% at 18 months. Possible comparisons: D-PA vs. UC. |
Follow-up(s):6, 12 and 18 months. Outcomes: weight, LDL, HDL, TGs, HbA1c, SBP, DBP, FPG, kcal, fat (g), METs. |
| Metz 2000 [18] | Location: USA. Co-morbidity: 1. hypertension/dyslipidemia or; 2. type 2 diabetes. Sex: 1. hypertension/dyslipidemia: (a) 50 women, 43 men (b) 50 women, 40 men; 2. type 2 diabetes: (a) 31 women, 25 men (b) 38 women, 25 men. Age, mean (SD) in years: 1. hypertension/dyslipidemia: (a) 54.5 (9.0) (b) 54.4 (9.5); 2. type 2 diabetes: (a) 54.6 (9.0) (b) 54.0 (9.9). BMI mean (SD) kg m-2: 1. hypertension/ dyslipidemia: (a) 33.0 (4.9) (b) 32.0 (4.2), 2. type 2 diabetes: (a) 33.0 (4.4) (b) 34.5 (4.5). Weight, mean in kg: not given. |
(a) Intervention group.Diet: 22% kcal from fat, 58% kcal from carbohydrates, 20% kcal from protein. (b) Usual care group. Allocated:1 Hypertension/dyslipidemia: (a) 93 (b) 90, 2. type 2 diabetes: (a) 56 (b) 63. % dropout: 1. hypertension/dyslipidemia: (a) 15.1% (b) 12.2% at 52 weeks, 2. Type 2 diabetes: (a) 26.8% (b) 19.0% at 52 weeks. Possible comparisons: D only vs. UC. |
Time of measurements: 12, 26 and 52 weeks. Outcomes: weight, total cholesterol,LDLHDL, TGs, HbA1c, SBP, DBP, FPG, kcal, fat (% kcal). |
| Oldroyd 2006 [40] | Location: UK. Co-morbidity: impaired glucose tolerance. Sex: (a) 19 women, 16 men (b) 10 women, 22 men. Age, mean (CI) in years: (a) 58.2 (41, 75) (b) 57.5 (41, 73). BMI, mean (SD) in kg/m2: (a) 30.4 (5.6) (b) 29.9 (4.9). Weight, mean (SD) in kg: (a) 83.3 (16.6) (b) 85.5 (14.2). |
(a) Intervention group.Diet: 30% kcal from fat, polysaturated to saturated fat ratio of 1.0, 50–55% kcal from carbohydrate, 20g/1000kcal of fibre Activity: 20–30 min of aerobic activity for 2–3 times/week. (b) Control group. Allocated: (a) 39 (b) 39. % dropout: (a) 38.5% (b) 23.1% at 24 months. Possible comparisons: D-PA vs. UC. |
Follow-up(s):6, 12 and 24 months. Outcomes: weight, total cholesterol, LDL, HbA1c, FPG, kcal, fat (g),% engaging in regular PA. |
| Pascale,1995[19] | Location: USA. Co-morbidity: 1. type 2 diabetes or 2. family history of diabetes. Sex: all women. Age, mean (SD) in years: 1. type 2 diabetes: 56.4 (8.4); 2. family history of type 2 diabetes: 42.7(8.4). BMI, mean in kg/m2: 1. type 2 diabetes (a) 36.4 (4.7) (b) 36.3 (4.2); 2. family history of type 2 diabetes (a) 35.0 (4.4) (b) 36.1 (5.6). Weight, mean (SD) in kg: 1. type 2 diabetes (a) 93.1(13.0) (b) 94.4(9.5); 2. family history of type 2 diabetes (a) 95.3(13.3) (b) 94.5(14.6). |
(a) CAL restriction group.Diet: 1,000–1,500 kcal/day, 30% of kcal from fat. (b) CAL + fat restriction group.Diet: same as (a). Allocated: 1. type 2 diabetes: (a) 22 (b) 22, 2. family history of type 2 diabetes: (a) 23 (b) 23. % dropout: 1. type 2 diabetes: (a) 27% (b) 32%, 2. family history of type 2 diabetes: (a) 43% (b) 30% at 12 months. Possible comparisons: Intensive vs. less intensive (D only). |
Follow-up(s): 16 weeks, and12 months. Outcomes: weight, total cholesterol, HDL, LDL, TGs, HbA1c, kcal, fat (%kcal). |
| Pendelton2002[58] | Location: Brisbane, Queensland, Australia. Co-morbidity: binge eating disorder. Sex: all women. Age, mean (SD) in years: 45 (8.3). BMI, mean (SD) in kg/m2: 36.2 (6.5). Weight, mean (SD) in kg: 97.2 (17.8). |
(a) CBT group.Diet: ‘establish regular and healthy eating patterns’. (b) CBT and Exercise group.Diet: same as (a).Activity: exercise three times/week ≥45 min/session. (c) CBT and Maintenance.Diet: same as (a). (d) CBT and Exercise and maintenance.Diet: same as (a).Activity: same as (b). Allocated: (a) 28 (b) 27 (c) 24 (d) 31. % dropout: (a) 39.3% (b) 25.9% (c) 16.7% (d) 22.6% at 16 months. Possible comparisons: Intensive vs. less intensive (D-PA). |
Follow-up(s): 4, 10, and 16 months. Outcomes: weight. |
| PREMIER trial, 2003 [46] | Location: USA. Co-morbidity: hypertension. Sex: (a) 174 women, 94 men (b) 154 women, 115 men (c) 172 women, 101 men. Age, mean (SD) in years: (a) 50.2 ( 8.6) (b) 50.2 (9.3) (c) 49.5 (8.8). BMI, mean (SD) in kg/m2: (a) 33.0 (5.5) (b) 33.3 (6.3) (c) 32.9 (5.6). Weight, mean (SD) in kg: not given. |
(a) Established group.Diet: ≤100 mmol/day of dietary sodium, intake of ≤30 ml/day alcohol for men and 15 ml/day for women.Activity: at least 180 minutes/week of MIPA.Other: weight loss of ≥6.8 kg if BMI ≥ 25 kg/m2. (b) Established + DASH diet.Diet: same as (a) plus ≤ 7% kcal from saturated fat, ≤ 25% of kcal from fat.Activity: ≥180 minutes/week of MIPA.Other: same as (a) (c) Advice only group.Diet: reduced-sodium diet.Activity: engaging in regular MIPA. Allocated: (a) 268 (b) 269 (c) 273. % dropout: (a)6% (b)6% (c)0% at 18 months. Possible comparisons: D-PA vs. UC, Intensive vs. less intensive (D-PA) |
Follow-up(s):6 and b18 months. Outcomes: weight, DBP, SBP, kcal, fat (% kcal), kcal/kg. |
| Reeves 2001 [41] | Location: USA. Co-morbidity: binge eating disorders Sex: all female. Number of patients within age range (n) in years: (a) 27–39 (14), 40–45 (19), 46–50 (13); (b) 27–39 (9), 40–45 (14), 46–50 (13). BMI, mean in kg/m2 (b) 33.8, (a) 31.8. Weight, mean (SD) in kg: (a) 89.36 (9.53) (b) 86.64 (14.52). |
(a) Intervention group.Diet: decrease fat intake.Activity: five 45-min walking sessions/week. (b) Waiting list control group. Allocated: (a) 59 (b) 39. % dropout: (a) 28.3% (b) 7.7% at6 months. Possible comparisons: D-PA vs. WLC. |
Follow-up(s):6 months. Outcomes: weight, kcal, fat (% kcal). |
| Samaras 1997 [20] | Location: Australia. Co-morbidity: type 2 diabetes. Sex: (a) 9 women, 4 men (b) 7 women,6 men. Age, mean (SE) in years: (a) 60.5(7.8) (b) 60.5(2.1). BMI, mean (SE) in kg/m2: (a) 32.3(1.1) (b) 35.7(1.6). Weight, mean (SD) in kg: (a) 83.0 (3.6) (b) 98.2 (3.4). |
(a) Intervention group.Activity: monthly one hour aerobic exercise classes. (b) Usual care control group. Allocated: (a) 13 (b) 13. % dropout: (a)0% (b)0% at12 months. |
Follow-up(s):6 and12 months. Outcomes: weight, total cholesterol, HDL, TGs, HbA1c, FPG, METs. |
| Southard 2003 [42] | Location: Canada. Co-morbidity: Cardiovascular disease. Sex: (a) 17 women, 36 men (b) 9 women, 42 men. Age, mean (SD) in years: (a) 61.8 (10.8) (b) 62.8 (10.6). BMI, mean (SD) in kg/m2: (a) 31.1 (6.8) (b) 29.2 (4.8). Weight, mean (SD) in kg: (a) 89 (b) 91.99. |
(a) Special intervention.Diet: dietician feedback to dietary practice.Activity: individual instructions by case managers. (b) Usual care. Allocated: (a) 53 (b) 51. % dropout: (a)6% (b)2%. Possible comparisons: D-PA vs. WLC. |
Follow-up(s):6 months. Outcomes: weight, total cholesterol, HDL, LDL, TGs, SBP, DBP, MEDFICTS (indicating fat intake), minutes of weekly exercise. |
| Tate 2003 [56] | Location: USA. Co-morbidity: one or more other risk factors for type 2 diabetes. Sex: (a) 42 women, 4 men (b) 41 women, 5 men. Age, mean (SD) in years: (a) 49.8 (9.3) (b) 47.3 (9.5). BMI, mean (SD) in kg/m2: (a) 32.5 (3.5) (b) 33.7 (3.7). Weight, mean (SD) in kg: (a) 86.2 (14.3) (b) 89.4 (12.6). |
(a) Internet counselling group.Diet: 1,200–1,500 kcal, 20% kcal from fat.Activity: ≥1000 kcal/week of PA. (b) Basic internet program.Diet: same as (a).Activity: same as (a). Allocated: (a) 46 (b) 46. % dropout: (a)0% (b)0% at12 months. Possible comparisons: Intensive vs. less intensive (D-PA) |
Follow-up(s):12 months. Outcomes: weight, FPG, fat (% kcal). |
| Tessier2000 [15] | Location: Canada. Co-morbidity: type 2 diabetes. Sex: (a) 7 women, 12 men (b) 9 women, 11 men. Age, mean (SD) in years: (a) 69.3 (4.2) (b) 69.5 (5.1). BMI, mean (SD) in kg/m2: (a) 29.4 (3.7) (b) 30.7 (5.4). Weight, mean (SD) in kg: (a) 79.4 (14.3) (b) 83.1 (18.0) |
(a) Physical exercise programme.Activity: exercise group sessions, three times/week, for 16 weeks. (b) Control group. Allocated: (a) 19 (b) 20. % dropout: (a) 21% (b) 5%. Possible comparisons: PA only vs. UC |
Follow-up(s): 4 months. Outcomes: weight, HbA1c, treadmill test (min). |
| Tudor-Locke 2004 [52] |
Location: Canada. Co-morbidity: type 2 diabetes. Sex: (a) 12 women, 12 men (b) 9 women, 14 men. Age, mean (SD) in years: (a) 52.8 (5.7) (b) 52.5 (4.8). BMI, mean (SD) in kg/m2: (a) 34.1 (6.1) (b) 32.5 (5.0). |
(a) Intervention group.Activity: self selected activity goals. (b) Waiting list control group. Allocated: (a) 30 (b) 30. % dropout: (a) 33% (b) 4% at 24 weeks. Possible comparisons: PA only vs. WLC |
Follow-up(s): 16 and 24 weeks. Outcomes: weight, SBP, DBP, total cholesterol, LDL HDL TGs, HbA1c, FPG, steps/day. |
| Toobert 2000 [27] | Location: USA. Co-morbidity: coronary heart disease Sex: all women. Age, mean (SD) in years: (a) 64 (10) (b) 63 (11). BMI, mean (SD) in kg/m2: (a) 32 (4.2) (b) 32 (5.5). Weight, mean (SD) in kg: (a) 80 (10) (b) 79 (15). |
(a) Intervention group.Diet: Reversal diet: <10% kcal from fat, 70–75% kcal from carbohydrates, 15–20% kcal from protein, 5 mg of cholesterol/day.Activity: 1 h/day, ≥3 days each week. (b) Control group. Allocated: (a) 16 (b) 12. % dropout: (a) 12.5% (b) 8.3% at 24 months. Possible comparisons: D-PA vs. WLC |
Follow-up(s): 4, 12 and 24 months. Outcomes: weight, total cholesterol, LDL HDL, TGs, SBP, DBP, kcal, fat (% kcal), Summary of self care activity (PA). |
| Toobert 2005 [43] | Location: USA. Co-morbidity: type 2 diabetes. Sex: all women. Age, mean (SD) in years: (a) 61.1 (8.0) (b) 60.7 (7.8). BMI, meam (SD) in kg/m2: (a) 35.1 (7.7) (b) 35.6 (8.8). Weight, mean (SD) in kg: (a) 92.3 (21.2) (b) 93.9 (23.8). |
(a) Mediterranean Lifestyle Program.Diet: more bread; more root vegetables, green vegetables, and legumes; more fish; less red meat (e.g., beef, lamb, pork), to be replaced by poultry; daily fruit; and avoidance of butter and cream, to be replaced by olive/canola oil or olive-/canola-based margarine.Activity: 30 min of MIPA on most days of the week, once accomplished, 1 h of MIPA/day. (b) Usual care. Allocated: (a) 163 (b) 116. % dropout:12% after6 months. Possible comparisons: D-PA vs. UC |
Follow-up(s):6 months. Outcomes: weight, total cholesterol, LDL, HDL, TGs, Hba1c, SBP, DBP, METs × duration × days baseline adjusted. |
| Villareal 2006 [16] | Location: USA. Co-morbidity: Metabolic syndrome. Sex: (a) 12 women, 5 men (b)6 women, 4 men. Age, mean (SD) in years: (a) 69 (5) (b) 71 (4). BMI, mean (SD) in kg/m2: (a) 39 (5) (b) 39 (5). Weight, mean (SD) in kg: (a) 100 (14) (b) 103 (20). |
(a) Intervention group.Diet: ≈750 kcal/day deficit, ≈30 kcal from fat, 50% kcal from carbohydrate, 20% kcal from protein.Activity: Exercise-training on 3 days/week for 90 min.Other: 1.5% loss of body weight/week, 10% weight loss after 6 months. (b) Control group. Allocated: (a) 17 (b) 10. % dropout: (a)12% (b)10%. Possible comparisons: D-PA vs. WLC |
Follow-up(s):6 months. Outcomes: weight, LDL, walking speed (m/min). |
| Wing 1985 [44] | Location: USA. Co-morbidity: type 2 diabetes. Sex: 33 women, 20 men. Age, mean (SE) in years: 55.1 (7.28). BMI, mean (SE) in kg/m2: 34.8 (5.10). Weight, mean (SE) in kg: 96.4(2.3). |
(a) Behaviour modification condition.Diet: self-selected kcal goals, <four servings of high sugar foods/week, > fiber intake.Activity: 1,000 kcal expenditure/week. (b) Nutrition education condition.Diet: ‘… given calorie goal at a level comparable to […] the behaviour modification condition’. (c) Standard care condition. Allocated: 53 overall. % dropout:6% overall at 62 weeks. Possible comparisons: D-PA vs. WLC |
Follow-up(s): 3 and 12 months. Outcomes: weight, total cholesterol, HDL, TGs, SBP, DBP, FPG. |
| Wing 1991 [57] | Location: USA. Co-morbidity: type 2 diabetes. Age, mean (SD) in years: (a) 51.2 (7.3) (b) 53.6 (7.7). BMI, mean (SD) in kg/m2: (a) 36.64 (5.77) (b) 35.68 (5.76). Weight, mean (SD) in kg: (a) 102.97 (18.5) (b) 96.84 (19.69). |
(a) Alone condition.Diet: 1,200–1,500 kcal/day.Activity: 1,000 kcal/week expenditure through exercise.Other: weight loss reward: USD 2.– for every lb lost. (b) Together condition.Diet: same as (a).Activity: same as (a)Other: same as (a).Allocated: (a) 25 (b) 24. % dropout: (a)8% (b) 17% at 12 months. Possible comparisons: Intensive vs. Less intensive (D-PA). |
Follow-up(s): 20 weeks,12 months. Outcomes: weight, HbA1c, FPG. |
| Wing 1998 [45] | Location: USA. Co-morbidity: family history of type 2 diabetes. Sex: 122 women, 32 men. Age, mean (SD) in years: (a) 45.0 (4.7) (b) 46.4 (4.5) (c) 46.3 (3.8) (d) 45.3 (4.9). BMI, mean (SD) in kg/m2: (a) 36.1 (4.1) (b) 36.0 (3.7) (c) 35.7 (4.1) (d) 36.0 (5.4). Weight, mean (SD) in kg: (a) 99.6 (13.0) (b) 99.3 (15.3) (c) 98.7 (15.9) (d) 97.4 (16.0). |
(a) Diet condition.Diet: 8,00–1,000 kcal/day, 20% of kcal from fat, gradually made more flexible with calorie goals of 1,200–1,500 kcal/day. (b) Exercise condition.Activity: gradual increase activity to 1,500 kcal/week through 5 days/week, increases of 250 kcal/week. (c) Diet-plus-exercise condition.Diet: same as (a).Activity: same as (b). (d) Usual care Allocated: (a) 37 (b) 37 (c) 40 (d) 40. % dropout: (a) 5% (b) 16% (c) 20% (d) 23% at 24 months. Possible comparisons: D-PA vs. UC, D only vs. UC, PA only vs. UC |
Follow-up(s):6, 12, and 24 months. Outcomes: weight, LDL, HDL, TGs, HbA1, SBP, DBP FPG, kcal, fat (% kcal), kcal/week. |
D-PA = Diet and PA intervention; D only = diet only intervention;DBP = diastolic blood pressure; FHQ = Food Habit Questionnaire; FPG = fasting plasma glucose; HbA1c = haemoglobin A1C; HDL = high-density lipoprotein cholesterol; LDL = low-density lipoprotein cholesterol; LTPA = leisure time physical activity; METs = metabolic equivalent of task; MIPA = moderate intensity physical activity; PA only = physical activity only interventions; PASE = physical activity scale for the elderly; SBP = systolic blood pressure; TGs = tryglycerides; UC = usual care; WLC = waiting list control.
Statistical Analysis: Where possible, a meta-analysis of the data was undertaken to determine the overall effect size. Two different effect sizes were calculated depending on the outcomes under scrutiny [13]. Dietary fat and PA outcomes were assessed as standardised mean differences (SMDs), equivalent to Hedge’s adjusted g, as both outcome variables were reported on a variety of scales (see table 1). Kilocalorie, weight and disease risk factor outcomes were reported on the same scales and were combined as mean differences (MDs). Change scores for dietary fat, kilocalorie and PA outcomes were preferred and meta-analyses used a mixture of change from baseline and final value scores [13]. Weight and disease risk factor outcomes were analysed as change scores, and missing data was imputed using methods previously described [12]. 95% confidence intervals (95% CIs) were derived for effect sizes. Degree of inconsistency across studies was assessed using I2 [14]. I2 levels of ≥25% and ≥50% were interpreted as an indicator for moderate and substantial heterogeneity respectively. It should be noted that I2 is dependent on the number of primary studies included and, in this case, there are some examples in which there are only a few primary studies. Random effects methods for combining data were used reflecting the high heterogeneity in many of the meta-analyses. Intention-to-treat data were used wherever available [13].
Results
Overall Description of Studies
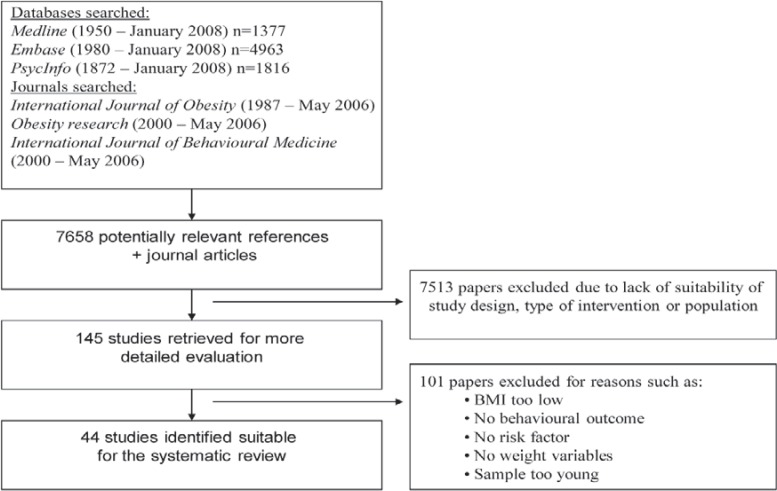
44 studies met inclusion criteria (table 1, fig. 1).
Fig. 1.
Flow diagram for locating RCTs for systematic review.
Participants: The mean age of participants was 55.0 ± 6.8 (mean ± SD) years and the mean BMI was 33.1 ± 2.2 kg/m2 ranging from 30.1 [15] to 38.8 kg/m2 [16]. In studies including participants of both genders, a small majority of women was reported (55%); eleven studies sampled women exclusively, and one study only men [17]. The majority of studies (n = 21) examined individuals with type 2 diabetes. Others included individuals with risk factors such as hypertension (n = 4), impaired glucose tolerance (n = 3), or cardiovascular disease (n = 2) (see Table 1 for details). Two studies used the same intervention for two different populations and were treated as separate studies [18, 19]. The mean number of participants was 240 ± 502 ranging from 26 [20] to 3,234 [21] with a mean dropout at completion of 16 ± 10.2%.
Intervention Setting: Most studies were conducted in the USA (n = 27). Other countries were Canada (n = 5), UK (n = 5), Australia (n = 4), Finland (n = 2), and the Netherlands (n = 1).
Study Designs: 27 trials allowed for comparison of D-PA intervention against a usual care or waiting list control group. Six comparisons of D-only and six comparisons of PA-only against usual care or waiting list control group were possible. Altogether, seven trials for D-PA and four for D-only interventions allowed comparison of more intensive against less intensive treatments. Interventions were categorised as more intense when the behaviour change components within the intervention were delivered more frequently in one of two intervention groups. Similarly, if one intervention utilised more intervention components compared with the other it was classified as more intensive.
Intervention Duration and Intensity: The modal duration of interventions was 6 months (n = 12), ranging from 2 [22, 23] to 36 months [24]. The modal duration of follow-up was 12 months (n = 15) ranging from 3 [25] to 36 months [24]. Outcomes were commonly reported at distinct points in time – 3, 6, 12, 24 and 36 months – and intervention effects are summarised for these time points. Where there were results reported for different time points, these were ascribed to the nearest time point of common reporting.
Intensity of contact varied in intervention arms ranging from one contact every 4 months [26] to twice weekly [27]. The average contact per month was 4.6 ± 6.5). High-intensity contact interventions tended to be exercise classes.
Behavioural Recommendations: Recommendations regarding dietary intake were categorised using the classifications of Avenell et al. [12]. Out of 51 different dietary treatment arms within the included trials, 20 provided general healthy eating advice (this includes studies where participants could choose their own healthy eating goals), 18 used a 600 kcal/day deficit or low fat-reducing diet, 9 used a low-calorie diet (1,000–1,600 kcal/day), two study arms used the Weight Watchers diet, one used the Ornish diet (it was felt that Weight Watchers and Ornish diets did not fit within the Avenell et al. categories [12]), and two provided no details, and one used the Ornish diet (table 1).
PA recommendations varied in intensity, type, duration, frequency and energy expenditure, and intensity was typically moderate. Few interventions reported recommending a particular type of activity, with those that did favouring walking and regular daily activities. The recommended activity duration was generally between 30 and 45 min, 3–4 times per week. Some recommendations specified targets for energy expenditure within a given period of time. Many studies employed supervised exercise classes and groups (table 1).
Quality of Trials
Randomisation: 19 trials were identified as having made a good attempt at concealment of randomisation. The remaining 25 studies stated that allocation was random without giving descriptions of procedures.
Description of Withdrawals: 21 studies provided numbers and reasons for study participant dropout, and 20 studies mentioned the numbers of withdrawals only. Three studies stated withdrawals only but did not provide further details.
Intention to Treat: 25 studies claimed to use intention-to-treat (ITT) data analysis, and 13 studies did not state ITT procedures. In 6 studies descriptions remained ambiguous.
Blinding of Outcome Assessors: The majority of trials (n = 32) did not report blinding of outcome assessors. Three studies stated that assessors were blinded, but did not provide further detail. Nine studies stated blinding assessors and described the blinding procedures.
Behaviour Change
Diet and PA Interventions versus Usual Care / Waiting List Control: 15 studies [21, 24, 26, 27, 28, 29, 30, 31, 32, 36, 39, 40, 41, 45, 46] reported kilocalorie intake which allowed for meta-analysis at 3, 6, 12, 18, 24 and 36 months (table 2). Reported decreases in favour of intervention compared with control groups were found at all time points, and significant MDs were detected at 12, 18 and 36 months. Evidence of heterogeneity in trial effects (i.e. differences in outcomes) was detected at 3 (I2 = 46.6%) and 6 (I2 = 65.4%) months. Most studies reported outcomes at 6 and 12 months. Three out of 8 studies [30, 45, 46] reported significant differences in kilocalorie intake between intervention and control groups at 6 months, and 3 out of 10 studies [21, 24, 45] at 12 months.
Table 2.
Intervention effects (95% CIs) on calorie intake, fat intake and PA in diet and PA, diet only and PA only interventions at 3, 6, 12, 18, 24, and 36 months
| Month | Diet + PA |
Diet only |
PA only |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| kcalintake |
fat intake |
PA |
kcal intake |
fat intake |
PA |
ns |
||||||
| MD | CI | SMD | CI | SMD | CI | MD | CI | SMD | CI | SMD | CI | |
| 3 | –11.6 | –160, 137 | –0.5** | –0.9, –0.2 | 0.5** | 0.3, 0.8 | –15 | –382, 352 | 0 | –0.3, 0.4 | 0.8** | –0.1,1.6 |
| 6 | –100** | –238, 39 | –0.5** | –0.9, 0 | 0.3** | 0.1,0.6 | –360 | –656, –64 | –0.4** | –1.0,0.2 | 0.7* | 0.4, 0.9 |
| 12 | –138 | –190, –86 | –0.3** | –0.5, –0.2 | 0.5** | 0.2, 0.7 | –266 | –389, –143 | –0.6** | –0.9, –0.2 | 0.7 | 0.4, 1.1 |
| 24 | –116* | –264, 32 | –1.0** | –1.7, –0.4 | 0.4** | 0,0.8 | –519 | –811, –227 | –0.8 | –1.3, –0.3 | 0.2 | –0.3, 0.7 |
| 36 | –107 | –196,–18 | –0.2 | –0.4, 0 | 0.0 | –0.2,0.2 | no data | no data | no data | |||
MD = Mean difference; SMD = standardised mean difference; CI = confidence interval.
I2 > 25%,
I2 > 50%.
N studies (participants) for MD kcal intake in D-PA trials: 4 (530), 8 (990), 10 (3,418), 3 (140), and 1 (434) at 3, 6, 12, 24, and 36 months.
N studies (participants) for SMD in fat intake in D-PA trials: 5 (624), 9 (1,469), 11 (3,514), 3 (142), and 1 (434) at 3, 6, 12, 24, and 36 months.
N studies (participants) for SMD for PA in D-PA trials: 6 (705), 12 (1,757), 10 (1,484), 4 (576), and 1 (434) at 3, 6, 12,, 24, and 36 months.
N studies (participants) for MD kcal intake in D-only trials: 1 (31), 1 (67), 5 (336), and 1 (66) at 3, 6, 12, and 24 months.
N studies (participants) for SMD in fat intake in D-only trials: 1 (67), 3 (298), and 1 (66) at 6, 12, and 24 months.
N studies (participants) for SMD in PA in PA-only trials: 2 (86), 5 (303), 3 (142), and 1 (62) at 3, 6, 12, and 24 months.
18 studies [21, 22, 23, 24, 26, 27, 28, 29, 30, 31, 32, 35, 39, 40, 41, 43, 45, 46] reported enough detail on fat intake to allow meta-analysis at 3, 6, 12, 18, 24 and 36 months (table 2). Consistent decreases in fat intake in intervention compared with control groups were found, with significant SMDs at 3, 12, 18 or 24 months. Heterogeneity was found at 3, 6, 12 and 24 months (I2 59.9–93.1%). Most studies reported outcomes at 6 and 12 months with 7 [21, 30, 35, 40, 43, 45, 46] out of 13 studies and 5 [22, 24, 27, 29, 40] out of 17 studies reporting significant between-group changes respectively. At 12 months one study found a significant decrease in fat intake in the control group compared with the intervention condition [35].
18 studies [16, 22, 24, 26, 27, 28, 29, 30, 31, 33, 34, 35, 36, 38, 39, 40, 45, 46] reported PA outcomes in enough detail to allow meta-analysis at 3, 6, 12, 18, 24 and 36 months (table 2). Positive SMDs were reported at all points in time with significant PA increases between intervention and control groups at 3, 6 and 12 months. Heterogeneity of trial effects was found at 3, 6, 12 and 24 months (I2 54.8–73%). The majority of studies reported outcomes at 6 and 12 months with 7 [16, 30, 35, 39, 40, 43, 45] out of 12 studies, and 6 [27, 31, 36, 39, 40, 45] out of 10 studies respectively inducing significant between-group PA differences.
Dietary Interventions versus Waiting List Control / Usual Care: Four studies [17, 18, 32, 45] reported dietary outcomes which could be included in meta-analysis at 3, 6, 12 and 24 months (table 2). Significant decreases in kilocalorie intake between intervention and control groups were detected at 6, 12 and 24 months. Despite significant changes in pooled outcomes at 12 months, only 1 study out of 4 [45] found a significant between-group difference in kilocalorie intake.
Changes in fat intake were reported in enough detail by 3 studies [18, 45, 47] to allow meta-analysis at 3, 6, 12, and 24 months (table 2). Significant intervention effects could be detected at 12 and 24 months. Heterogeneity was detected at 6 (I2 = 76.1%) and 12 (I2 = 53.9%) months. Significant between-group changes in fat intake were reported for all studies, except 1 at 3 months [47] and 1 at 12 months [45].
PA Interventions versus Waiting List Control / Usual Care: Seven studies [15, 20, 30, 45, 50, 51, 52] reported PA change outcomes that allowed meta-analysis at 3, 6, 12 and 24 months. Significant SMDs were found at 6 and 12 months. At most time points significant differences between intervention and control groups were reported with 2 [15, 52] out of 2 showing significant between-group PA differences at 3 months 3 [30, 45, 51] out of 5 at 6 months, and 2 [20, 45] out of 3 at 12 months.
Intensive versus Less Intensive Interventions: Three studies [19, 47, 58] allowed the investigation of an intensive against a less intensive D-only intervention. Meta-analysis at 3 and 12 months revealed no significant changes in kilocalorie intake in the one study including two samples providing enough details at those time points [19]. A significant between-group difference in favour of the intensive intervention was reported in one of the two samples. Changes in fat intake were reported to differ significantly in the one study consisting of two samples [19]. A further study of an intense dietary intervention compared with a less intense one found no effects of changes in fat intake at 3 and 6 months [47].
Ten studies [25, 32, 36, 45, 46, 53, 54, 55, 56, 58] allowed the investigation of an intensive against a less intensive D-PA intervention. None of these studies reported significant differences between groups for kilocalorie intake at any point in time. Furthermore, only one intervention significantly changed fat intake at 6 and 18 months [46], and one intervention significantly changed PA at 3 months [58] in favour of the intensive intervention.
Weight Change
Diet and PA Interventions versus Waiting List Control / Usual Care: 25 studies [16, 21, 22, 24, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46] reported weight outcomes in sufficient detail to allow meta-analysis at 3, 6, 12, 24 and 36 months (table 3). At all time points weight changes were significantly different between intervention and control groups with the exception of 18 months. Effects at all points in time were heterogeneous (I2 = 68.3–95.0%) with the exception of 24 months (fig. 2). Most studies reported outcomes at 6 and 12 months with 10 [21, 28, 30, 32, 35, 40, 42, 43, 45, 46] out of 15 at 6 months and 8 [21, 24, 26, 27, 29, 32, 40, 45] out of 15 studies reporting significant between group differences in weight respectively.
Table 3.
Mean differences (95% CIs) in weight changes from meta-analyses of RCTs comparing diet and PA, diet only and PA only interventions against usual care or waiting list control groups, and RCTs comparing intensive diet and PA, and intensive diet only interventions again less intensive diet and PA and less intensive diet only interventions
| Month | Diet and PA |
Diet only |
PA only |
Intensive diet and PA |
Intensive diet |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| MD | CI | MD | CI | MD | CI | MD | CI | MD | CI | |
| 3 | –2.8** | –4.4, –1.2 | –2.9** | –4.7, –1.2 | –0.1 | –2.0,1.8 | –1.3 | –2.4, –0.2 | –1.2** | –2.7, 0.3 |
| 6 | –3.5** | –5.1, –1.9 | –4.0** | –6.7, –1.2 | –2.7** | –4.8, –0.6 | –0.9* | –1.7, 0.0 | 0 | –2.0,2.0 |
| 12 | 2.9** | –4.3, –1.5 | –2.3** | –3.8, –0.8 | –0.3 | –2.2,1.6 | –1.2* | –2.7, 0.4 | –3.1 | –5.5, –0.6 |
| 24 | –2.8 | –3.5, –2.0 | –1.8 | –4.8, 1.2 | 1.3 | –1.0, 3.6 | –0.3 | –1.4, 0.7 | no data |
|
| 36 | –2.6 | –3.6, –1.6 | no data |
no data |
no data |
no data |
||||
MD = Mean difference; CI = confidence interval.
I2 > 25%,
I2 > 50%.
N studies (participants) for diet and PA trials: 8 (850), 15 (4,056), 15 (4,048), 3 (572), 6 (730) and 1 (434) at 3, 6, 12, 18. 24, and 36 months
N studies (participants) for D-only trials: 4 (450), 6 (486), 6 (1107), and 1 (66) at 3, 6, 12, and 24 months.
N studies (participants) for PA-only trials: 2 (86), 5 (319), 2 (83), and 1 (62) at 3, 6, 12, and 24 months.
N studies (participants) for intensive diet and PA trials: 5 (264), 7 (1323), 7 (750), 4 (957), and 2 (488) at 3, 6, 12, 18, and 24 months.
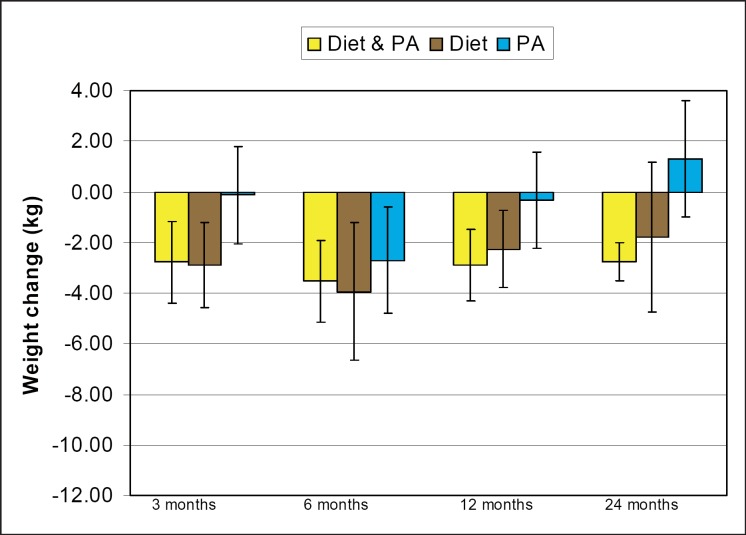
Fig. 2.
MD in weight change between intervention and control participants for diet and PA, D-only and PA-only interventions at 3, 6, 12, and 24 months.
Diet Interventions versus Waiting List Control / Usual Care: Six studies [18, 32, 45, 47, 48, 49] reported changes in weight allowing meta-analysis at 3, 6, 12, and 24 months (table 3). Differences in weight loss between intervention and control groups were significant at 3, 6, and 12 months (fig. 2). Heterogeneity in the data was found at 3, 6, and 12 months (I2 = 71.9–84.8%).
PA Interventions vs. Waiting List Control / Usual Care: Seven studies [15, 20, 30, 45, 50, 51, 52] reported weight outcomes in sufficient detail to allow meta-analysis at 3, 6, 12 and 24 months (table 3). The MD for weight change was significant only at 6 months with evidence for heterogeneity in the data (I2 = 83.5%). Few studies reported non-significant differences at 3, 12 and 24 months (fig. 2).
Intensive versus Less Intensive Interventions: 11 studies [19, 25, 32, 36, 46, 47, 53, 54, 55, 56, 57] allowed comparisons between intensive and less intensive interventions at various points in time. Intensive interventions, irrespective of whether the intervention consisted of a D-only or D-PA intervention, tended to induce greater MD for weight than the less intensive intervention groups (table 3). Significant changes were noted at 12 and 16 months for D-only and at 3 months for D-PA studies.
Risk Factor Change
Diet and PA Interventions versus Waiting List Control / Usual Care: 20 studies [21, 24, 26, 27, 28, 29, 30, 31, 32, 33, 35, 36, 37, 38, 39, 40, 42, 43, 45, 46] reported outcomes with respect to changes in at least one risk factor (table 4). Most studies provided outcome data at 3, 6, 12 and 24 months. Risk factor changes generally showed beneficial trends at various points in time. At 3 months significant changes were found in total cholesterol, triglycerides, and SBP. At 6 months DBP, SBP and FBG showed significant improvements, with both SBP and DBP also showing significant differences between intervention and control groups at 12, 24 and 36 months. Furthermore, triglycerides as well as HbA1c showed improvements at 12 months. At 18 months LDL cholesterol was found to be significantly different in only 1 study [46] and at 24 months the only measure that was significantly improved was triglycerides. The only study reporting outcomes at 36 months displayed significant HbA1c improvement [24]. Overall, significant improvements were found in all risk factors with the exception of HDL cholesterol. The most consistent improvements were found in SBP and triglycerides.
Table 4.
Mean differences (95% CIs) of total cholesterol, LDL cholesterol, HDH cholesterol, triglycerides, glycosylated haemoglobin, blood pressure, and glucose changes over time from meta-analyses of D-PA interventions
| 3 months |
6 months |
12 months |
24 months |
36 months |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MD | CI | MD | CI | MD | CI | MD | CI | MD | CI | |
| Cholesterol, mmol/l | –0.3 | –0.4, –0.1 | –0.1* | –0.3, 0.1 | –0.1* | –0.3, 0.0 | –0.1 | –0.2,0.0 | –0.2 | –0.4, 0.0 |
| LDL cholesterol, mmol/l | –0.1 | –0.2,0.1 | –0.1 | –0.2,0.0 | –0.2** | –0.3, 0.0 | 0.1 | –0.1,0.2 | No | data |
| HDL cholesterol, mmol/l | 0.0 | –0.1,0.0 | 0.0 | 0.0,0.0 | 0.0 | 0.0,0.0 | 0.0 | 0.0,0.1 | 0.0 | 0.0,0.1 |
| Triglycerides, mmol/l | –0.2 | –0.4, –0.1 | –0.1* | –0.3, 0.0 | –0.3** | –0.5, –0.1 | –0.2 | –0.4, –0.1 | –0.1 | –0.2,0.0 |
| HbA1c, % | –0.8 | –1.9, 0.3 | –0.2* | –0.5, 0.1 | –0.2 | –0.4, –0.1 | 0.0 | –0.2,0.2 | –0.2 | –0.3, –0.1 |
| DBP, mm Hg | –1.9 | –3.3, –0.6 | –2.5 | –3.3, –1.6 | –2.5 | –3.2, –1.9 | –2.7 | –3.5, –1.9 | –3.25 | –4.4, –2.1 |
| SBP, mm Hg | –5.0 | –7.0, –2.9 | –4.6** | –6.8, –2.4 | –3.7* | –5.1, –2.2 | –3.0 | –4.1, –1.9 | –2.7 | –4.1, –1.3 |
| FPG, mmol/l | 0.0 | –1.0,1.0 | –0.4 | –0.5, –2.0 | 0.1** | –0.3, 0.2 | 0.1** | –0.5, 0.4 | –0.1 | –0.2,0.0 |
MD = Mean difference; CI = confidence interval; LDL cholesterol = low density lipoprotein cholesterol; HDL cholesterol = high density lipoprotein cholesterol; DBP = diastolic blood pressure; SBP = systolic blood pressure; FPG = fasting plasma glucose.
I2 > 25%,
I2 > 50%.
N studies (participants) for total cholesterol: 5 (700), 7 (1,017), 10 (1,537), 5 (730), and 1 (434) at 3, 6, 12, 24 and 36 months.
N studies (participants) for LDL cholesterol: 5 (698), 6 (897), 8 (900), and 3 (430) at 3, 6, 12, and 24 months.
N studies (participants) for HDL cholesterol: 5 (700), 7 (1,014), 10, (1,532), 1 (63), and 1 (434) at 3, 6, 24, and 36 months.
N studies (participants) for HbA1c: 2 (359), 5 (583), 6 (1,123), 1 (61), 2 (113), and 1 (459) at 3, 6, 12, 24, and 36 months.
N studies (participants) for triglycerides: 5 (701), 6 (898), 9 (1,430), 4 (676), and 1 (434) at 3, 6, 12, 24, and 36 months.
N studies (participants) for DBP: 4 (587), 7 (1,166), 9 (3,462), 4 (2,303), and 1 (2,161) at 3, 6, 12, 24, and 36 months.
N studies (participants) for SBP: 4 (587), 7 (1,175), 9 (3,462), 4 (2,303), and 1 (2,161) at 3, 6, 12, 24, and 36 months.
N studies (participants) for FBP: 1 (241), 3 (198), 6 (804), (3 (623), and 1 (434) at 3, 6, 12, 24, and 36 months.
Diet Interventions versus Waiting List Control / Usual Care: Five studies [18, 32, 45, 47, 49] provided data for at least one risk factor, which could be analysed at 3, 6, 12 and 24 months (table 5). Risk factors generally showed tendencies towards improvement. At 3 months significant improvements occurred in total cholesterol, triglycerides, and SBP. SBP, DBP, and FBG showed no significant differences compared with controls at 6 months (table 5).
Table 5.
Mean difference (95% CIs) of total cholesterol, LDL cholesterol, HDH cholesterol, triglycerides, glycosylated haemoglobin (HbA1c), blood pressure, and glucose changes over time from meta-analyses of D-only interventions
| 3 months |
6 months |
12 months |
24 months |
|||||
|---|---|---|---|---|---|---|---|---|
| MD | CI | MD | CI | MD | CI | MD | CI | |
| Cholesterol, mmol/l | –0.3 | –0.4, –0.2 | –0.3** | –0.6,0.1 | –0.1** | –0.4, 0.2 | –0.3 | –0.6,0.0 |
| LDL cholesterol, mmol/l | –0.1 | –0.3, 0.0 | –0.1** | –0.4, 0.3 | –0.1** | –0.5, 0.3 | –0.2 | –0.5, 0.1 |
| HDL cholesterol, mmol/l | 0.0 | –0.03, 0.02 | 0.0** | –0.1,0.1 | 0.1 | 0.0,0.1 | 0.0 | –0.1,0.1 |
| Triglycerides, mmol/l | –0.3* | –0.6, –0.1 | –0.3 | –0.5, 0.0 | –0.1 | –0.4, 0.2 | –0.3 | –1.3, 0.6 |
| HbA1c, % | –0.2** | –0.5, 0.1 | –0.3** | –0.7, 0.0 | –0.1 | –0.2,0.0 | 0.00 | –0.2,0.2 |
| DBP, mm Hg | –0.8 | –2.2, 0.5 | –1.8 | –3.5 –0.1 | –0.9 | –2.4, 0.5 | 1.0 | –2.8, 4.8 |
| SBP, mm Hg | –2.9 | –5.1, –0.7 | –4.2 | –7.9, –0.5 | –0.3 | –2.9, 2.3 | 0.7 | –4.6, 6.0 |
| FPG, mmol/l | –0.8 | –2.0, 0.4 | –0.3 | –0.5, –0.1 | –0.3 | –1.1, 0.5 | 0.1 | –0.3, 0.5 |
MD = Mean difference; CI = confidence interval; LDL cholesterol = low density lipoprotein cholesterol;
HDL cholesterol = high density lipoprotein cholesterol; DBP = diastolic blood pressure; SBP = systolic blood pressure; FPG = fasting plasma glucose.
* I2 > 25%,
I2 > 50%.
N studies (participants) for total cholesterol: 3 (458), 3 (329), 5 (496), and 1 (66) at 3, 6, 12, and 24 months.
N studies (participants) for LDL cholesterol: 2 (185), 3 (329), 4 (332), and 1 (66) at 3, 6, 12, and 24 months.
N studies (participants) for HDL cholesterol: 2 (285), 3 (325), 4, (332), and 1 (66) at 3, 6, 12, and 24 months.
N studies (participants) for triglycerides: 2 (285), 3 (329), 4 (332), and 1 (66) at 3, 6, 12, and 24 months.
N studies (participants) for HbA1c: 4 (602), 4 (464), 3 (411), and 1 (66) at 3, 6, 12, and 24 months.
N studies (participants) for DBP: 2 (285), 3 (360), 3 (312), and 1 (66) at 3, 6, 12, and 24 months.
N studies (participants) for SBP: 2 (285), 3 (360), 3 (312), and 1 (66) at 3, 6, 12, and 24 months.
N studies (participants) for FBP: 2 (285), 3 (329), 4 (332), and 1 (66) at 3, 6, 12, and 24 months.
PA Interventions versus Waiting List Control / Usual Care: Six studies [15, 20, 30, 45, 51, 52] reported risk factor outcomes at least once allowing meta-analysis at 3, 6, 12, and 24 months (table 6). Changes in risk factors due to PA-only interventions were less consistent when compared with D-PA and D-only interventions, and no significant effects could be detected.
Table 6.
Mean difference (95% CIs) of total cholesterol, LDLa cholesterol, HDH cholesterol, triglycerides, glycosylated haemoglobin (HbA1c), blood pressure, and glucose changes over time from meta-analyses of PA-only interventions
| 3 months |
6 months |
12 months |
24 months |
|||||
|---|---|---|---|---|---|---|---|---|
| MD | CI | MD | CI | MD | CI | MD | CI | |
| Cholesterol, mmol/l | 0.1 | –0.3, 0.5 | –0.1 | –0.2,0.2 | –0.1 | –0.4, 0.2 | 0.2 | –0.1, 0.4 |
| LDL cholesterol, mmol/l | 0.0 | –0.3, 0.3 | –0.1 | –0.3, 0.1 | –0.1 | –0.4, 0.1 | 0.2 | –0.1, 0.5 |
| HDL cholesterol, mmol/l | 0.1 | –0.1,0.2 | 0.1 | 0.0,0.2 | 0.1 | 0.0,0.1 | 0.0 | –0.1,0.1 |
| Triglycerides, mmol/l | 0.0 | –0.9, 0.9 | –0.1 | –0.6, 0.4 | 0.0 | –0.4, 0.4 | –0.2 | 0.8, 0.5 |
| HbA1c, % | 0.0 | –1.7, 1.6 | –0.2 | –0.6,0.1 | –0.1 | –0.8, 0.5 | 0.0 | –0.2,0.2 |
| DBP, mm Hg | 0.4 | –3.6, 4.4 | –1.7 | –4.6, 1.2 | –4.0 | –8.7, 0.7 | 0.0 | –4.0, 4.0 |
| SBP, mm Hg | –1.7 | –7.9, 4.6 | –3.1 | –7.7, 1.74 | 0.0 | –6.8,6.8 | 2.4 | –4.1, 8.7 |
| FPG, mmol/l | 0.7 | –0.4, 1.8 | –0.1 | –0.4, 0.2 | 0.0 | –0.3, 0.3 | 0.2 | –0.2,0.6 |
MD = Mean difference; CI = confidence interval; LDL cholesterol = low density lipoprotein cholesterol;
HDL cholesterol = high density lipoprotein cholesterol; DBP = diastolic blood pressure; SBP = systolic blood pressure; FPG = fasting plasma glucose.
*I2 > 25%, **I2 > 50%.
N studies (participants) for total cholesterol: 1 (47), 3 (138), 3 (147), and 1 (62) at 3, 6, 12, and 24 months.
N studies (participants) for LDL cholesterol: 1 (47), 2 (105), 2 (113), and 1 (62) at 3, 6, 12, and 24 months.
N studies (participants) for HDL cholesterol: 1 (47), 3 (134), 3 (143), and 1 (62) at 3, 6, 12, and 24 months.
N studies (participants) for triglycerides: 1 (47), 3 (137), 2 (147), and 1 (62) at 3, 6, 12, and 24 months.
N studies (participants) for HbA1c: 1 (39), 3 (141), 2 (91), and 1 (62) at 3, 6, 12, and 24 months.
N studies (participants) for DBP: 1 (47), 3 (196), 1 (57), and 1 (62) at 3, 6, 12, and 24 months.
N studies (participants) for SBP: 1 (47), 3 (196), 1 (57), and 1 (62) at 3, 6, 12, and 24 months.
N studies (participants) for FBP: 1 (47), 2 (91), 2 (85), and 1 (62) at 3, 6, 12, and 24 months.
Effects of Intensive versus Less Intensive Interventions: Comparison of intensive and less intensive interventions showed a lack of significant differences with regard to risk factors.
Discussion
The current systematic review assessed intervention effects on behaviour as well as on weight and disease risk factors. When interpreting the results obtained in this review, shortcomings should be taken into account. Short-term outcomes (3 months) as well as long-term outcomes (24 months onwards) are based on a limited number of studies. Dietary and PA behaviours were reported using a variety of different measurements. In particular, difficulties with self-reported outcomes and unreliable measures have been highlighted in the literature before [8, 59] and might have had an impact on the current findings. Analyses of D-only, PA-only, and intensive versus less intensive interventions were somewhat limited due to small numbers of studies which met inclusion criteria. It should also be considered that some of our measures for heterogeneity were based on few primary studies, and this can reduce the clinical relevance of findings. The methodological quality of some of the studies that met inclusion criteria displayed room for improvement judged by study reportage. The majority of studies did not report on features such as the method of randomisation or reasons for participant dropout. Nearly half of the studies failed to report whether the analysis was intention-to-treat. Blinding of outcome assessors was rarely described.
We were unable to determine which manipulations were the active and successful ingredients within the studies [60]. We grouped the studies by their behavioural targets, but this does not specify how interventions successfully change these behaviours and why some interventions were more effective than others [61]. More research is needed to determine which specific aspects of behavioural interventions facilitate significant change in behaviour and subsequent physiological outcomes, thereby explaining some of the significant heterogeneity typically encountered in systematic review of behaviour interventions, including the current one.
The underlying model on the effect of behaviour change interventions postulates that weight, disease risk factors, and health are all influenced through mediating behavioural effects [62]. To our knowledge this review is the first one to include behavioural dietary and PA effects alongside weight and risk factor changes in obese adults carrying additional risk factors. Results indicated that behavioural interventions are successful at significantly changing behavioural outcomes to moderate degrees in both dietary and PA behaviours over consistent periods of time. The magnitude of behavioural effects was modest. The greatest reported reductions in MD of kilocalorie intake for D-PA interventions and D-only interventions were –138 kcal and –360 kcal at 12 and 6 months respectively. Given that a common aim of many dietary recommendations is the reduction of kilocalorie intake by 600 kcal/day, the observed changes suggest that many participants struggled to adhere to dietary prescriptions. Similar findings apply to modest dietary fat outcome effect sizes. A previous systematic review in overweight/obese individuals without additional risk factors found similarly modest significant effects of dietary advice on fibre, fat and saturated fat intakes [8].
An interesting pattern emerges when comparing the magnitude of behavioural intervention effects across different types of studies. Compared against respective control conditions, behavioural D-PA study effects tended to be greater in magnitude in studies aimed at changing either of the behaviours in isolation when compared with studies that focused on diet and PA at the same time. This finding suggests that behaviour change effects are greater when focusing on only one kind of behaviour, as compared with both diet and PA behaviours at the same time. However, studies focusing on dietary and PA behaviours simultaneously lead to greater long-term weight loss than D-only or PA-only studies. It might be the case that changing two behaviours at the same time decreases the magnitude of change due to participants’ limited self-regulatory resources [63]. However, longer-term behaviour change maintenance might be upheld through focusing the attention and direction of behaviour change on both behaviours that facilitate weight loss. Using dietary and PA interventions encourage a coherent change in one’s lifestyle behaviours towards behaving healthily.
Obtained weight loss findings are consistent with previous systematic reviews [3, 12]. All types of studies successfully produced some weight loss in intervention compared with control groups. Weight loss patterns over time in D-PA, D-only and PA-only studies appeared to be similar. Greatest weight loss was found at 6 months, with weight regain thereafter, echoing previous systematic review evidence [3] and underlining the difficulty of permanent lifestyle change [64] in obese adults with additional risk factors.
The magnitude of weight loss appeared to differ between intervention types. Initial weight loss was greatest for D-only interventions, PA-only interventions showed the weakest effects on weight, with the only significant effects found at 6 months. Despite superior weight loss at 6 months by D-only interventions, subsequent time points showed weight loss advantages in favour of a combination of diet and PA interventions. After 12 months the only study type found to produce significant differences in weight loss were interventions targeting changes in diet and PA.
Differences in magnitude of weight loss over time between interventions have been documented by previous research. Avenell et al. [12] found that interventions focusing on D-only led to greater MD in weight loss at 12 months compared with studies that focused on both diet and PA, with a reverse of this trend at later time points. Furthermore, a systematic review of RCTs of PA interventions found that the union of PA with dietary interventions led to a significant increase in weight loss compared with D-only interventions [65]. Previous literature has also indicated that PA-only typically has not been significantly more effective than D-only interventions in short-term weight loss [66]. These findings point to the importance of PA in the maintenance of initial, dietary induced weight loss [64, 67]. Studies using more intensive interventions produced greater weight loss. This is the case for D-PA as well as for D-only interventions.
Comparing the magnitude of weight loss in the current review to that of other published meta-analyses with most trials not recruiting individuals carrying additional risk factors [3, 12, 68, 68], it appears that the weight loss in our studies is less. This difference in the magnitude of weight loss according to the criteria of additional risk factors holds true for D-only interventions [12], PA-only interventions [65], and intensive compared with less intensive interventions [69].
With regard to changes in disease risk factors, these displayed similar patterns to weight loss outcomes. All types of interventions that could be compared with control groups demonstrated a tendency towards improving various risk factors at some point in time. However, the types of risk factors affected as well as the magnitude and consistency of risk factor change tended to differ according to intervention type. D-only interventions induced significant changes in total cholesterol, triglycerides, blood pressure and FPG in intervention compared with control conditions. Overall, changes tended to occur at 3 and 6 months, mirroring weight loss. Triglycerides seemed to show consistent beneficial trends at all points in time. Similarly, total and LDL cholesterol showed trends towards significant improvements at all time points for D-only interventions. In comparison, PA-only interventions and intensive compared with less intensive interventions were not successful at inducing risk factor changes.
The greatest changes in risk factors were achieved by interventions that targeted both diet and PA. These changes tended to peak in magnitude at 12 months. Similar to D-only interventions, triglycerides tended to show consistent positive improvements in combination interventions. Furthermore, blood pressure seemed to show consistent and mostly significant improvements over time. Unlike D-only interventions, the combination of diet and PA induced significant improvements in HbA1c.
Conclusion
Overall, the findings of this systematic review extend the evidence of behaviour change intervention effectiveness [8, 12, 70] and confirm the usefulness of this approach in populations carrying additional risk factors. Improvements in behaviour, weight, and disease risk factors were recorded for all types of reviewed behaviour change interventions. Changes tended to be greatest at around 6 months. Behavioural changes were modest and tended to be greater in studies focusing solely on a single behaviour rather than on both. Interventions focusing on diet and PA simultaneously showed the greatest improvements in terms of weight loss and disease risk factor change. However, most consistent beneficial effects over time regarding behaviour, weight and disease risk factors were found in D-PA studies. The current review suggests that behavioural interventions in at-risk populations showed positive effect tendencies. Future research should focus on identifying the most effective means of inducing dietary and PA behaviour change.
Disclosure
The authors declare that there are no conflicts of interest.
References
- 1.WHO: Obesity: preventing and managing the global epidemic. Report of a WHO consultation World Health Organization – Technical Report Series. 2000:894. [PubMed] [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiologyevaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26:968–976. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- 3.Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med. 2007;147:41–50. doi: 10.7326/0003-4819-147-1-200707030-00007. [DOI] [PubMed] [Google Scholar]
- 4.McTigue KMHarris R, Hemphill B, Lux L, Sutton S, Bunton AJ, Lohr KN. Screening and interventions for obesity in adults: summary of the evidence for the U.S Preventive Services Task Force. Ann Intern Med. 2003;139:933–949. doi: 10.7326/0003-4819-139-11-200312020-00013. [DOI] [PubMed] [Google Scholar]
- 5.Pirozzo SSummerbell C, Cameron C, Glasziou P. Advice on low-fat diets for reducing obesity. Cochrane Database Syst Rev. 2002:CD003640. doi: 10.1002/14651858.CD003640. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Health and Clinical Excellence (NICE): Obesity: The PreventionIdentification, Assessment and Management of Overweight and Obesity in Adults and Children 2006 www.nice.org.uk/nicemedia/pdf/CG43FullGuideline1.pdf. [PubMed] [Google Scholar]
- 7.Kaplan RM. Behavior as the central outcome in health care. Am Psychol. 1990;45:1211–1220. doi: 10.1037//0003-066x.45.11.1211. [DOI] [PubMed] [Google Scholar]
- 8.Brunner EJ, Rees K, Ward K, Burke M, Thorogood M. Dietary advice for reducing cardiovascular risk. Cochrane Database Syst Rev. 2007:CD002128. doi: 10.1002/14651858.CD002128.pub4. [DOI] [PubMed] [Google Scholar]
- 9.Hillsdon M, Foster C, Thorogood M. Interventions for promoting physical activity. Cochrane Database Syst Rev. 2005:CD003180. doi: 10.1002/14651858.CD003180.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckel RHGrundy SMZimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 11.Gill GVIsmail AA, Beeching NJ, Macfarlane SBJ, Bellis MA. Hidden diabetes in the UK: use of capture-recapture methods to estimate total prevalence of diabetes mellitus in an urban population. J R Soc Med. 2003;96:328–332. doi: 10.1258/jrsm.96.7.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avenell ABroom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, Smith WC, Jung RT, Campbell MK, Grant AM. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess. 2004;8:iii–iv–1–182. doi: 10.3310/hta8210. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 4.2.6. 2006 www.cochrane.org/sites/default/files/uploads/Handbook4.2.6Sep2006.pdf. [Google Scholar]
- 14.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tessier DMénard J, Fülöp T, Ardilouze J-, Roy M-, Dubuc N, Dubois M-, Gauthier P. Effects of aerobic physical exercise in the elderly with type 2 diabetes mellitus. Arch Gerontol Geriat. 2000;31:121–132. doi: 10.1016/s0167-4943(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 16.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166:860–866. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 17.Ash SReeves MM, Yeo S, Morrison G, Carey D, Capra S. Effect of intensive dietetic interventions on weight and glycaemic control in overweight men with Type II diabetes: a randomised trial. Int J Obes. 2003;27:797–802. doi: 10.1038/sj.ijo.0802295. [DOI] [PubMed] [Google Scholar]
- 18.Metz JAStern JS, Kris-Etherton P, Reusser ME, Morris CD, Hatton DC, Oparil S, Haynes RB, Resnick LM, Pi-Sunyer FX, Clark S, Chester L, McMahon M, Snyder GW, McCarron DA. A randomized trial of improved weight loss with a prepared meal plan in overweight and obese patients Impact on cardiovascular risk reduction. Arch Intern Med. 2000;160:2150–2158. doi: 10.1001/archinte.160.14.2150. [DOI] [PubMed] [Google Scholar]
- 19.Pascale RWMullen M, Wing RR, Bononi P, Butler BA. Effects of a behavioral weight loss program stressing calorie restriction versus calorie plus fat restriction in obese individuals with NIDDM or a family history of diabetes. Diabetes Care. 1995;18:1241–1248. doi: 10.2337/diacare.18.9.1241. [DOI] [PubMed] [Google Scholar]
- 20.Samaras KAshwell S, Mackintosh Am, Fleury AC, Campbell LV, Chisholm DJ. Will older sedentary people with non-insulin-dependent diabetes mellitus start exercising? A health promotion model. Diabetes Res Clin Pract. 1997;37:121–128. doi: 10.1016/s0168-8227(97)00061-2. [DOI] [PubMed] [Google Scholar]
- 21.Knowler WCBarrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark MHampson SE, Avery L, Simpson R. Effects of a tailored lifestyle self-management intervention in patients with type 2 diabetes. Br J Health Psychol. 2004;9:365–379. doi: 10.1348/1359107041557066. [DOI] [PubMed] [Google Scholar]
- 23.Jehn ML, Patt MR, Appel LJ, Miller ER., III One year follow-up of overweight and obese hypertensive adults following intensive lifestyle therapy. J Hum Nutr Diet. 2006;19:349–354. doi: 10.1111/j.1365-277X.2006.00711.x. [DOI] [PubMed] [Google Scholar]
- 24.Lindstrom J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, Uusitupa M, Tuomilehto J. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003;26:3230–3236. doi: 10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]
- 25.Grilo CM, Masheb RM. A randomized controlled comparison of guided self-help cognitive behavioral therapy and behavioral weight loss for binge eating disorder. Behav Res Ther. 2005;43:1509–1525. doi: 10.1016/j.brat.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Laitinen JHAhola IE, Sarkkinen ES, Winberg RL, Harmaakorpi-Iivonen PA, Uusitupa MI. Impact of intensified dietary therapy on energy and nutrient intakes and fatty acid composition of serum lipids in patients with recently diagnosed non-insulin-dependent diabetes mellitus. JAMA. 1993;93:276–283. doi: 10.1016/0002-8223(93)91552-2. [DOI] [PubMed] [Google Scholar]
- 27.Toobert DJGlasgow RE, Radcliffe JL. Physiologic and related behavioral outcomes from the women’s lifestyle heart trial. Ann Behav Med. 2000;22:1–9. doi: 10.1007/BF02895162. [DOI] [PubMed] [Google Scholar]
- 28.Agurs-Collins TDAdams-Campbell LL, Kumanyika SK, Ten Have TR. A randomized controlled trial of weight reduction and exercise for diabetes management in older African-American subjects. Diabetes Care. 1997;20:1503–1511. doi: 10.2337/diacare.20.10.1503. [DOI] [PubMed] [Google Scholar]
- 29.Burke V, Beilin LJ, Cutt HE, Mansour J, Williams A, Mori TA. A lifestyle program for treated hypertensives improved health-related behaviors and cardiovascular risk factorsa randomized controlled trial. J Clin Epidemiol. 2007;60:133–141. doi: 10.1016/j.jclinepi.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Blumenthal JA, Sherwood A, Gullette EC, Babyak M, Waugh R, Georgiades A, Craighead LW, Tweedy D, Feinglos M, Appelbaum M, Hayano J, Hinderliter A. Exercise and weight loss reduce blood pressure in men and women with mild hypertension: effects on cardiovascularmetabolic, and hemodynamic functioning. Arch Intern Med. 2000;160:1947–1958. doi: 10.1001/archinte.160.13.1947. [DOI] [PubMed] [Google Scholar]
- 31.Deakin TA, Cade JE, Williams R, Greenwood DC. Structured patient education: the Diabetes X-PERT Programme makes a difference. Diabetic Med. 2006;23:944–954. doi: 10.1111/j.1464-5491.2006.01906.x. [DOI] [PubMed] [Google Scholar]
- 32.Djuric ZDiLaura NM, Jenkins I, Darga L, Jen CK, Mood D, Bradley E, Hryniuk WM. Combining weight-loss counseling with the Weight Watchers plan for obese breast cancer survivors. Obes Res. 2002;10:657–665. doi: 10.1038/oby.2002.89. [DOI] [PubMed] [Google Scholar]
- 33.Edelman DOddone EZ, Liebowitz RS, Yancy Jr. WS, Olsen MK, Jeffreys AS, Moon SD, Harris AC, Smith LL, Quillian-Wolever RE, Gaudet TW. A multidimensional integrative medicine intervention to improve cardiovascular risk. J Gen Intern Med. 2006;21:728–734. doi: 10.1111/j.1525-1497.2006.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodrick GKPoston WSK, Kimball KT, Reeves RS, Foreyt JP. Nondieting versus dieting treatment for overweight binge-eating women. J Consult Clin Psych. 1998;66:363–368. doi: 10.1037//0022-006x.66.2.363. [DOI] [PubMed] [Google Scholar]
- 35.Hardcastle STaylor A, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activitydiet and CHD risk factors. Patient Educ Couns. 2008;70:31–39. doi: 10.1016/j.pec.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Keyserling TC, Samuel-Hodge CD, Ammerman AS, Ainsworth BE, Henríquez-Roldán CF, Elasy TA, Skelly AH, Johnston LF, Bangdiwala SI. A randomized trial of an intervention to improve self-care behaviors of African-American women with type 2 diabetes: impact on physical activity. Diabetes Care. 2002;25:1576–1583. doi: 10.2337/diacare.25.9.1576. [DOI] [PubMed] [Google Scholar]
- 37.Kirkman MSWeinberger M, Landsman PB, Samsa GP, Shortliffe EA, Simel DL, Feussner JR. A telephone-delivered intervention for patients with NIDDM Effect on coronary risk factors. Diabetes Care. 1994;17:840–846. doi: 10.2337/diacare.17.8.840. [DOI] [PubMed] [Google Scholar]
- 38.Mefferd KNichols JF, Pakiz B, Rock CL. A cognitive behavioral therapy intervention to promote weight loss improves body composition and blood lipid profiles among overweight breast cancer survivors. Breast Cancer Res. 2007;104:145–152. doi: 10.1007/s10549-006-9410-x. [DOI] [PubMed] [Google Scholar]
- 39.Ménard JPayette H, Baillargeon JP, Maheux P, Lepage S, Tessier D, Ardilouze Jl. Efficacy of intensive multitherapy for patients with type 2 diabetes mellitus: a randomized controlled trial. Can Med Assoc J. 2005;173:1457–1463. doi: 10.1503/cmaj.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oldroyd JCUnwin NC, White M, Mathers JC, Alberti KGMM. Randomised controlled trial evaluating lifestyle interventions in people with impaired glucose tolerance. Diabetes Res Clin Pract. 2006;72:117–127. doi: 10.1016/j.diabres.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 41.Reeves RSMcPherson RS, Nichaman MZ, Harrist RB, Foreyt JP, Goodrick GK. Nutrient intake of obese female binge eaters. JAMA. 2001;101:209–215. doi: 10.1016/S0002-8223(01)00055-4. [DOI] [PubMed] [Google Scholar]
- 42.Southard BHSouthard DR, Nuckolls J. Clinical trial of an internet-based case management system for secondary prevention of heart disease. J Cardiopulm Rehabil. 2003;23:341–348. doi: 10.1097/00008483-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Toobert DJStrycker LA, Glasgow RE, Barrera M Jr, Angell K. Effects of the Mediterranean lifestyle program on multiple risk behaviors and psychosocial outcomes among women at risk for heart disease. Ann Behav Med. 2005;29:128–137. doi: 10.1207/s15324796abm2902_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wing RREpstein LH, Nowalk MP, Koeske R, Hagg S. Behavior changeweight loss and physiological improvements in type II diabetic patients. Consult Clin Psychol. 1985;53:111–122. doi: 10.1037//0022-006x.53.1.111. [DOI] [PubMed] [Google Scholar]
- 45.Wing RR, Venditti E, Jakicic JM, Polley BA, Lang W. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care. 1998;21:350–359. doi: 10.2337/diacare.21.3.350. [DOI] [PubMed] [Google Scholar]
- 46.Elmer PJObarzanek E, Vollmer WM, Simons-Morton D, Stevens VJ, Young DR, Lin PH, Champagne C, Harsha DW, Svetkey LP, Ard J, Brantley PJ, Proschan MA, Erlinger TP, Appel LJ. Effects of comprehensive lifestyle modification on dietweight, physical fitness and blood pressure control: 18-month results of a randomized trial. Ann Int Med. 2006;144:485–495. doi: 10.7326/0003-4819-144-7-200604040-00007. [DOI] [PubMed] [Google Scholar]
- 47.Glasgow RE, Toobert Brief. DJ computer-assisted diabetes dietary self-management counseling: effects on behavior physiologicoutcomes and quality of life. Med Care. 2000;38:1062–1073. doi: 10.1097/00005650-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Glasgow RE, Toobert DJ, Hampson SE. Effects of a brief office-based intervention to facilitate diabetes dietary self-management. Diabetes Care. 1996;19:835–842. doi: 10.2337/diacare.19.8.835. [DOI] [PubMed] [Google Scholar]
- 49.Jones HEdwards L, Vallis TM, Ruggiero L, Rossi SR, Rossi JS, Greene G, Prochaska JO, Zinman B. Changes in diabetes self-care behaviors make a difference in glycemic control: the Diabetes Stages of Change (DiSC) study. Diabetes Care. 2003;26:732–737. doi: 10.2337/diacare.26.3.732. [DOI] [PubMed] [Google Scholar]
- 50.Evangelista LSDoering LV, Lennie T, Moser DK, Hamilton MA, Fonarow GC, Dracup K. Usefulness of a home-based exercise program for overweight and obese patients with advanced heart failure. Am J Cardiol. 2006;97:886–890. doi: 10.1016/j.amjcard.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 51.Kirk AMutrie N, MacIntyre P, Fisher M. Effects of a 12-month activity counselling intervention on glycaemic control and on the status of cardiovascular risk factors in people with type 2 diabetes. Diabetologia. 2004;47:821–832. doi: 10.1007/s00125-004-1396-5. [DOI] [PubMed] [Google Scholar]
- 52.Tudor-Locke CBell RC, Myers AM, Harris SB, Ecclestone NA, Lauzon N, Rodger NW. Controlled outcome evaluation of the First Step Program: a daily physical activity intervention for individuals with type II diabetes. Int J Obes. 2004;28:113–119. doi: 10.1038/sj.ijo.0802485. [DOI] [PubMed] [Google Scholar]
- 53.Blonk MCJacobs MAJM, Biesheuvel EHE, Weeda-Mannak WL, Heine RJ. Influence on weight loss in type 2 diabetic patients: little long-term benefit from group behaviour therapy and exercise training. Diabetic Med. 1994;11:449–457. doi: 10.1111/j.1464-5491.1994.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 54.Carels RADarby LA, Cacciapaglia HM, Douglass OM. Reducing cardiovascular risk factors in postmenopausal women through a lifestyle change intervention. J Womens Health. 2004;13:412–426. doi: 10.1089/154099904323087105. [DOI] [PubMed] [Google Scholar]
- 55.Logue ESutton K, Jarjoura D, Smucker W, Baughman K, Capers C. Transtheoretical model-chronic disease care for obesity in primary care: a randomized trial. Obes Res. 2005;13:917–927. doi: 10.1038/oby.2005.106. [DOI] [PubMed] [Google Scholar]
- 56.Tate DFJackvony EH, Wing RR. Effects of internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: a randomized trial. JAMA. 2003;289:1833–1836. doi: 10.1001/jama.289.14.1833. [DOI] [PubMed] [Google Scholar]
- 57.Wing RRMarcus MD, Epstein LH, Jawad A. A ‘family-based’ approach to the treatment of obese type II diabetic patients. J Consult Clin Psychol. 1991;59:156–162. doi: 10.1037//0022-006x.59.1.156. [DOI] [PubMed] [Google Scholar]
- 58.Pendleton VRGoodrick GK, Carlos Poston WS, Reeves RS, Foreyt JP. Exercise augments the effects of cognitive-behavioral therapy in the treatment of binge eating. Int J Eat Disord. 2002;31:172–184. doi: 10.1002/eat.10010. [DOI] [PubMed] [Google Scholar]
- 59.Baranowski TKlesges LM, Cullen KW, Himes JH. Measurement of outcomesmediators, and moderators in behavioral obesity prevention research. Prev Med. 2004;38:S1–S13. doi: 10.1016/j.ypmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 60.Michie SRothman AJ, Sheeran P. Advancing the science of behaviour change. Psychol Health. 2007;22:249–253. [Google Scholar]
- 61.Michie S, Abraham C. Interventions to change health behaviours: evidence-based or evidence-inspired? Psychol Health. 2004;19:29–49. [Google Scholar]
- 62.Hardeman WSutton S, Griffin S, Johnston M, White A, Wareham NJ, Kinmonth AL. A causal modelling approach to the development of theory-based behaviour change programmes for trial evaluation. Health Educ Res. 2005;20:676–687. doi: 10.1093/her/cyh022. [DOI] [PubMed] [Google Scholar]
- 63.Baumeister RF, Vohs KD, Funder DC. Psychology as the science of self-reports and finger movements whatever happened to actual behavior? Perspect Psychol Sci. 2007;2:396–403. doi: 10.1111/j.1745-6916.2007.00051.x. [DOI] [PubMed] [Google Scholar]
- 64.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 65.Shaw KGennat H, O’Rourke P, DelMar C. Exercise for overweight or obesity. Chochrane Database Syst Rev. 2006:CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Votruba SBHorvitz MASchoeller DA. The role of exercise in the treatment of obesity. Nutrition. 2000;16:179–188. doi: 10.1016/s0899-9007(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 67.Wing RR. Physical activity in the treatment of the adulthood overweight and obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S547–S552. doi: 10.1097/00005768-199911001-00010. [DOI] [PubMed] [Google Scholar]
- 68.Miller WKoceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using dietexercise or diet plus exercise intervention. Int J Obes. 1997;21:941–947. doi: 10.1038/sj.ijo.0800499. [DOI] [PubMed] [Google Scholar]
- 69.Shaw K, O’Rourke P, Del Mar C, Kenardy J. Psychological interventions for overweight or obesity. Chochrane Database Syst Rev. 2005:CD003818. doi: 10.1002/14651858.CD003818.pub2. [DOI] [PubMed] [Google Scholar]
- 70.Laederach-Hofmann KMesserli-Burgy NMeyer K. Long-term effects of non-surgical therapy for obesity on cardiovascular risk management: A weighted empirical review. J Public Health. 2008;16:21–29. [Google Scholar]
- 71.Brownell KD. The LEARN Programme for Weight Management. DallasAmerican Health Publishing. 2000 [Google Scholar]
- 72.Fairburn CG. Overcoming Binge Eating. New YorkGuilford Press. 1995 [Google Scholar]
- 73.Mokdad AH, Ford ES, Bales VS, Dietz WH, Bowman BA, Vinicor F, Marks JS. Prevalence of obesitydiabetes, and obesity-related health risk factors2001. J Am Med Assoc. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]