Abstract
Rationale: The advent of precision treatment for cystic fibrosis using small-molecule therapeutics has created a need to estimate potential clinical improvements attributable to increases in cystic fibrosis transmembrane conductance regulator (CFTR) function.
Objectives: To derive CFTR function of a variety of CFTR genotypes and correlate with key clinical features (sweat chloride concentration, pancreatic exocrine status, and lung function) to develop benchmarks for assessing response to CFTR modulators.
Methods: CFTR function assigned to 226 unique CFTR genotypes was correlated with the clinical data of 54,671 individuals enrolled in the Clinical and Functional Translation of CFTR (CFTR2) project. Cross-sectional FEV1% predicted measurements were plotted by age at which measurement was obtained. Shifts in sweat chloride concentration and lung function reported in CFTR modulator trials were compared with function–phenotype correlations to assess potential efficacy of therapies.
Measurements and Main Results: CFTR genotype function exhibited a logarithmic relationship with each clinical feature. Modest increases in CFTR function related to differing genotypes were associated with clinically relevant improvements in cross-sectional FEV1% predicted over a range of ages (6–82 yr). Therapeutic responses to modulators corresponded closely to predictions from the CFTR2-derived relationship between CFTR genotype function and phenotype.
Conclusions: Increasing CFTR function in individuals with severe disease will have a proportionally greater effect on outcomes than similar increases in CFTR function in individuals with mild disease and should reverse a substantial fraction of the disease process. This study provides reference standards for clinical outcomes that may be achieved by increasing CFTR function.
Keywords: genotype–phenotype, sweat chloride, lung function, cystic fibrosis transmembrane conductance regulator modulator
At a Glance Commentary
Scientific Knowledge on the Subject
Grouping of cystic fibrosis transmembrane conductance regulator (CFTR) variants into classes based on effects on synthesis, processing, or function demonstrated that retention of some CFTR function moderated the severity of dysfunction in the pancreas; sweat gland; and, to some extent, the lung. However, these studies relied on functional evaluation of a relatively small number of variants in each class and limited numbers of subjects, who collectively lacked adequate representation of the entire spectrum of disease.
What This Study Adds to the Field
This large-scale study revealed clinically useful correlations between CFTR genotype function and clinical features of cystic fibrosis. These correlations demonstrate that individuals with severe disease as a result of very low CFTR function could benefit the most from modulator therapies, even if the increase in function is modest. Of potentially equal importance, the study generated benchmarks of CFTR function with lung flow measurements and sweat chloride to provide points of reference for assessing CFTR modulator efficacy.
Cystic fibrosis (CF) is an autosomal recessive monogenic disorder affecting approximately 70,000 individuals worldwide (1). It is caused by variants in the cystic fibrosis transmembrane conductance regulator (CFTR), a cAMP-regulated chloride and bicarbonate channel. The advent of variant-specific small-molecule therapies, termed “CFTR modulators,” targeting CFTR (2, 3) has generated renewed interest in defining relationships between CFTR genotype, CFTR function, and CF phenotype.
CF manifests considerable allelic heterogeneity with over 2,000 CFTR variants identified to date (4) and broad clinical variability, rendering the relationship between genotype and phenotype difficult to fully elucidate (5–7). Grouping of variants into classes based on effects on synthesis, processing, or function of CFTR (8–10) demonstrated that retention of some CFTR function moderated the severity of dysfunction in the pancreas; sweat gland; and, to some extent, the lung (11–14). As functional studies of CFTR advanced (15), estimates of the relationship between level of CFTR function and phenotype were refined (16). However, these studies relied on functional evaluation of a relatively small number of variants in each class and limited numbers of subjects, who collectively lacked adequate representation of the entire spectrum of disease. The high degree of variability in CF traits necessitates large-scale studies to achieve greater granularity in the relationships between CFTR function and clinical outcomes.
To determine which individuals with CF may benefit from modulator treatment, there has been a concerted effort to evaluate function and drug response of all CFTR variants occurring in three or more individuals worldwide. Consequently, we now have measurements of the function of CFTR bearing a large set of variants that span the functional spectrum of disease (17–19). These data were paired with clinical and genetic data from 88,664 individuals with CF assembled by the Clinical and Functional Translation of CFTR (CFTR2) project, revealing robust correlations of CFTR function with key clinical outcomes. These relationships can provide benchmarks to inform expectations for response to CFTR-targeted therapies.
Methods
Clinical Data and Variant Function
Clinical data from individuals with CF were collected for the CFTR2 project, which amassed data from 88,664 individuals receiving CF care in 41 countries (Table E1 in the online supplement), as previously described (18, 20, 21). When possible, a Kulich normal residual mortality-adjusted (KNoRMA) lung disease phenotype was calculated for each individual (22), using nontransplanted lung function measures. Variants reported in at least three individuals in CFTR2 with clinical data were assigned a functional level based on in vitro studies measuring CFTR transport in Fischer rat thyroid (FRT) or CF bronchial epithelial (CFBE) cell lines (17, 18) or their presumed production of no CFTR protein (nonsense, canonical splice, frameshift, and start-loss variants, as well as exon deletions or duplications) (Table E2).
Assigning Genotypes, Genotype Function, and CF Clinical Trait Values
Individuals with a reported CFTR genotype comprised of exactly two variants having functional assignment were eligible for inclusion in analysis. A percentage of wild type (%WT) function for each CFTR variant was calculated by adjusting short-circuit current measurements of variant cell lines for amount of mRNA expression and comparing them with a WT standard curve, as previously described (17). CFTR genotype function was determined as the sum of the functional levels of the two individual variants comprising the genotype. Each genotype group analyzed included three or more individuals. Clinical traits were analyzed only if at least three subjects within the genotype group had reported clinical data for a given trait. The CF traits associated with each genotype were determined by mean value (sweat chloride, FEV1% predicted [FEV1 as a percent predicted value for age and height], KNoRMA) or percentage of individuals with a trait (pancreatic insufficiency).
Derivation of Lung Function Trajectory by CFTR Genotype Functional Grouping
Individuals in the CFTR2 dataset aged 6 years or older with an FEV1% predicted measurement (n = 42,924) were placed into CFTR genotype functional groups: less than 2%, 2% to less than 3%, 3% to less than 5%, 5% to less than 10%, 10% to less than 25%, and 25% to less than 50%. Cross-sectional FEV1% predicted measurements were plotted by age at which measurement was obtained. Lung function decrease by age was determined using locally weighted scatterplot smoothing (Lowess) or linear regression.
Derivation of the Relationship between CFTR Function and Trait after Acute Modulator Treatment
Treatment response comparisons with CFTR2-defined relationships between CFTR genotype function and phenotype were generated from published clinical trial data. Pretreatment values for sweat [Cl−] and FEV1% predicted were used when provided; otherwise, CFTR2 clinical data were used. Post-treatment values were calculated from published data. Functional measures of CFTR with and without treatment for genotypes involving gating variants were calculated from individual variants studied in FRT cell lines (23, 24). For clinical trial participants bearing a genotype containing a gating variant, total genotype function was determined by summing the function of the gating variant and of F508del, which was the most commonly reported second allele comprising the genotype in these subjects. Functional measures of CFTR with and without treatment with ivacaftor and lumacaftor for F508del were determined from CFBE cell lines (25); homozygous F508del genotype function was derived by multiplying F508del functional levels with and without treatment by 2. Baseline CFTR function for the F508del homozygous genotype treated with tezacaftor-ivacaftor and VX-445 or VX-659 was determined from CFBE cell lines (25), and fold-change treatment response was calculated from ex vivo human bronchial epithelial cells from F508del homozygous donors (26, 27). Genotypes present in fewer than three individuals in a trial were excluded.
Correlation and Statistical Analysis
The relationship between CFTR genotype function and CF traits was determined by linear regression. Pearson correlation coefficient (r) was used to determine strength of correlation. Regressions using CFTR2 data and results from clinical trials were compared using an interaction term and mixed models, with P < 0.05 as the threshold for significance. Statistical analysis was performed using Stata release 15 software (StataCorp).
Results
Deriving CFTR Function for Different CFTR Genotypes
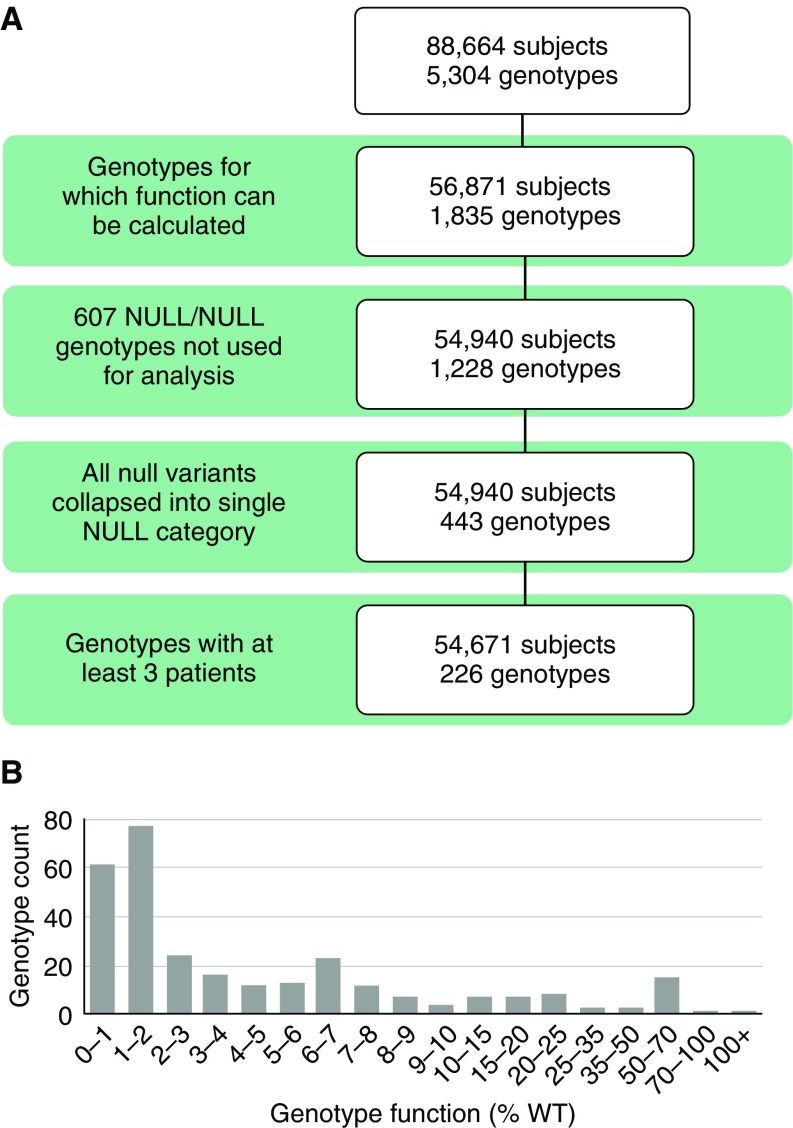
A total of 56,871 individuals carried 1 of 1,835 genotypes for which CFTR function could be assigned (Figure 1A), based on functional studies of 108 CFTR missense or in-frame insertion or deletion variants (17–19) and an assumption of no residual function for 245 null variants (Table E2). Genotypes not considered for analysis included those with non–CF-causing variants, variants for which %WT function was not assigned, complex alleles in which the combined function of two in cis variants is unclear, or nonsense variants known to escape nonsense-mediated RNA decay or operate by a different mechanism (28, 29). Of genotypes with functional assignment, 607 expected to result in no CFTR function (composed of two null variants) were excluded because clinical variation in these individuals is presumably due to factors other than CFTR genotype (4, 30). Collapsing the remaining null variants that occurred with a variant of known function into one category (because all are predicted to result in no CFTR function, thereby allowing us to consider them equivalent to each other) and removing genotypes occurring in only one or two individuals resulted in 54,671 individuals carrying 1 of 226 genotypes (Figure 1A and Table E3). Genotypes were distributed across the entire range of CFTR function (Figure 1B).
Figure 1.
Subjects, genotypes, and cystic fibrosis transmembrane conductance regulator (CFTR) function of individuals included in function–phenotype analysis. (A) Diagram of the filtering process used to select individuals for the study. The 5,304 genotypes of 88,664 subjects from the Clinical and Functional Translation of CFTR (CFTR2) project dataset were provided by contributing registries and cystic fibrosis clinics. Genotypes including only one null variant were collapsed such that any null variant could be present in trans with a nonnull variant of interest and only genotype groups with at least three individuals were included in the analysis, resulting in 226 genotypes for study. (B) Genotype function (percentage of wild type [%WT]) was assigned as the sum of the individual functions of each variant comprising the genotype. Genotype function is primarily below 10%, as expected for individuals with cystic fibrosis, though individuals with genotype function levels above this level are present in CFTR2.
Key Clinical Features of CF Exhibit a Logarithmic Relationship with CFTR Function
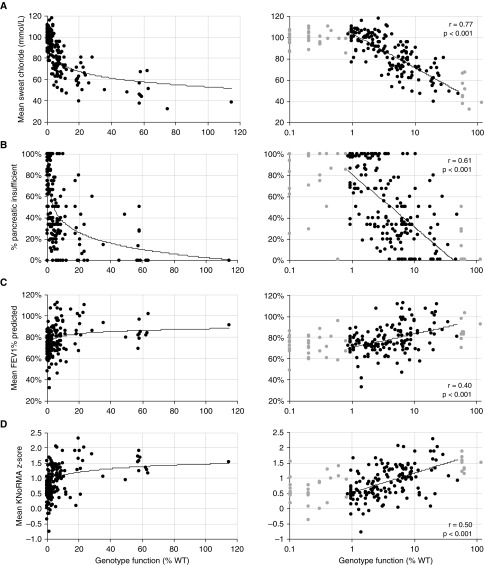
Plotting CFTR genotype function against sweat chloride concentration (sweat [Cl−]) of individuals with CF revealed a nonlinear relationship between these variables (Figure 2A, left panel), and curve fitting suggested that the interaction was logarithmic. Indeed, plotting CFTR function on a logarithmic scale revealed robust correlation with sweat [Cl−] (Figure 2A, right panel) (r = 0.77, P < 0.001). In performing correlation analysis, we restricted the analysis to genotypes generating at least 0.85% WT-CFTR function because this is the minimum function of genotypes containing the common variant F508del (17, 25) and below which estimates of percentage of WT function are considerably less certain. We also excluded genotypes generating more than 50% WT-CFTR function, because this level should be sufficient to escape disease; individuals with CF who have these genotypes likely have unidentified contributing factors.
Figure 2.
Genotype function has a nonlinear relationship with cystic fibrosis clinical traits. Left-hand panels for each cystic fibrosis clinical trait plot cystic fibrosis transmembrane conductance regulator (CFTR) genotype function as a percentage of wild type (%WT) on a linear scale against (A) the mean sweat chloride from individuals with that genotype, (B) the percentage of individuals of that genotype who are pancreatic insufficient, (C) the mean FEV1% predicted for individuals of that genotype, or (D) the mean Kulich normal residual mortality-adjusted (KNoRMA) z-score for individuals of that genotype. The best-fit line is shown. Right-hand panels show CFTR genotype function plotted on a logarithmic scale. Linear regressions for right-hand panels were performed on CFTR function between 0.85% and 50% (black data points). Trait measures from CFTR genotype function outside this range are shown in gray data points. Pearson r value for correlation and P value for deviation of slope from zero are shown for each trait.
We performed similar analyses to evaluate correlation between CFTR function and exocrine pancreatic disease (percentage of individuals with the same genotype who have pancreatic insufficiency) (Figure 2B) and lung disease (FEV1% predicted of individuals with the same genotype) (Figure 2C). To account for survival and age-dependent decline, we converted lung function measures to sex- and height-matched age-specific CF percentiles transformed to z-scores (KNoRMA) (22) (Figure 2D), measures that are based on a number of FEV1% predicted recordings over time (up to a 3-yr window; number of recordings ranged from 1 to 145) for each individual patient. As with sweat [Cl−], the relationship between CFTR genotype function and each trait appeared nonlinear. Plotting CFTR genotype function on a logarithmic scale revealed correlation with pancreatic status and lung function that was modest compared with sweat [Cl−] but statistically significant. Weighting the analysis to account for the number of individuals in each genotype group did not produce a meaningful shift in the nature or strength of the relationship between CFTR genotype function and trait (data not shown). Of note, inclusion of all data (including genotypes outside the 0.85–50% range plotted in Figure 2) generates similar degrees of correlation and significance with modest shifts in slope (Figure E1). The logarithmic relationships indicate that increases in CFTR function have proportionally greater effect on individuals with severe disease than similar increases in function in individuals with mild disease.
Lung Function of Individuals of Different Ages and Levels of CFTR Function
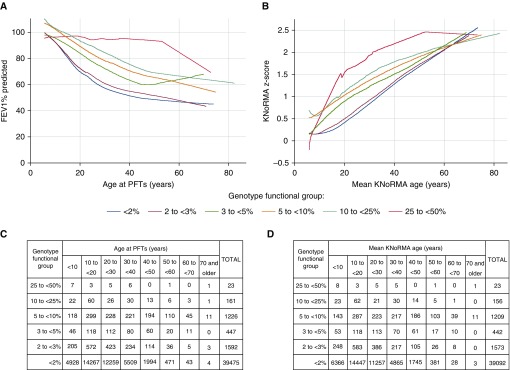
Because progressive lung disease is the major cause of morbidity and mortality in CF, we wanted to determine how CFTR genotype function affects lung function in individuals of different ages. We plotted cross-sectional FEV1% predicted measurements by the age at measurement for 42,924 individuals in CFTR2, stratified by groupings of CFTR genotype function. Functional grouping was based on published estimates for transition to pancreatic sufficiency (3% WT), reduction in sweat [Cl−] (5% WT), amelioration of lung disease (10% WT), and overlap with CFTR-related diseases (≥25% WT), acknowledging that these thresholds are approximate and likely vary among individuals (17, 31–35). Lowess revealed that higher levels of CFTR function were associated with better lung function at almost all ages (Figure 3A). Exceptions were noted in individuals over 50 years of age with 3–5% function and at over 60 years of age with less than 2% function, presumably due to survival bias (i.e., measurements of lung function are available only from living individuals or those without transplants, therefore potentially appearing falsely inflated). To address survival bias, we also plotted the mortality-adjusted lung measure (KNoRMA) stratified by the functional groupings described above (Figure 3B). As expected, KNoRMA values increased with age, reflecting the survival of those who outlive their peers with CF. Lowess revealed the same pattern observed with FEV1% predicted measures; higher levels of CFTR function were associated with higher KNoRMA values at almost all ages, with most functional groups converging around a KNoRMA z-score of 2. These results also illustrate the logarithmic relationship between CFTR genotype function and lung function in that small increases in CFTR function (e.g., 2% to <3% shifting to 3% to <5%) result in substantial shifts in cross-sectional measurements at all ages, whereas less impressive (but clinically relevant) improvements occur at higher levels of CFTR genotype function (e.g., 5% to <10% moving to 10% to <25%). Finally, although limited by a small number of subjects, individuals in the CFTR2 database who have 25% to less than 50% WT function appear to have near-normal cross-sectional FEV1% predicted measures into adulthood.
Figure 3.
Lung function by age, stratified by level of cystic fibrosis transmembrane conductance regulator (CFTR) function. (A) Locally weighted scatterplot smoothing of cross-sectional FEV1% predicted measurements from 42,924 individuals in the Clinical and Functional Translation of CFTR (CFTR2) project database, plotted by age at which measurement was taken and stratified by CFTR genotype function. (B) Locally weighted scatterplot smoothing of Kulich normal residual mortality-adjusted (KNoRMA) z-scores from 42,495 individuals in the CFTR2 database, plotted by mean age at the FEV1% predicted measures used to calculate the KNoRMA z-score and stratified by CFTR genotype function. (C) The number of patients with FEV1% predicted measurements within each genotype group at 10-year age increments are shown in the table. (D) The number of patients with KNoRMA z-scores within each genotype group at 10-year mean KNoRMA age increments are shown in the table. PFTs = pulmonary function tests.
CFTR-targeted Therapies Improve Clinical Measures to Levels Consistent with Those Observed in Individuals with Higher Lifelong Levels of CFTR Function
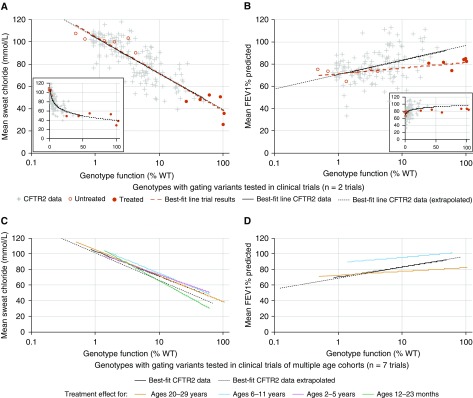
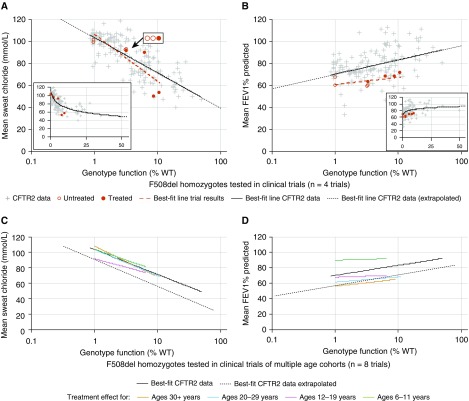
To assess the effectiveness of CFTR-targeted therapies, we compared the changes in sweat [Cl−] and lung function reported in clinical trials of CFTR modulators with those predicted using the function–phenotype correlations from CFTR2 data. Pre- and post-treatment values of sweat [Cl−] and FEV1% predicted obtained in CFTR modulator trials were plotted against CFTR function derived from cell-based studies (see Table E4 and Methods). The resulting slopes (red dashed lines in Figures 4A and 4B) were compared with the slopes derived from the sweat [Cl−] and regression of FEV1% predicted of all subjects with CF (black lines in Figures 4A and 4B). The post-treatment sweat [Cl−] observed in ivacaftor-treated individuals matched the sweat [Cl−] of individuals with higher levels of CFTR function, indicated by the overlapping slopes (Figure 4A). Post-treatment changes in lung function generated a slope that appeared to deviate from that observed at higher levels of CFTR function in the CFTR2 data (Figure 4B); however, testing using an interaction term revealed that the slopes of these lines did not differ. A mixed model approach using likelihood testing offered no advantage over simple linear regression; any apparent difference between slopes remained nonsignificant. To investigate if response to CFTR modulators in the sweat gland or lung changes as a function of age, clinical trial data from groups of individuals of varying ages (12 mo to adulthood) were compared with CFTR2 data (mean age at pulmonary function tests, 21.9 yr; SD, 11.0 yr) (Figures 4C and 4D). The regressions did not differ significantly for any age cohort studied.
Figure 4.
Treatment effect on gating variants mirrors the relationship between genotype function and phenotype determined using Clinical and Functional Translation of CFTR (CFTR2) project data. (A and B) Genotype function versus sweat chloride ([Cl−]) (A) or FEV1% predicted (B) on a semilogarithmic plot indicates that the effect of ivacaftor treatment on individuals with gating variants does not differ from the relationship shown between these variables using CFTR2 data (solid line determined from genotypes with 0.85–50% function; dotted line extrapolates this relationship <0.85% or >50% function). Genotype function and baseline sweat [Cl−] or FEV1% predicted of individuals tested in clinical trials with duration of either 24 or 48 weeks are represented by open red circles; after treatment, genotype function (determined by in vitro Fischer rat thyroid cell testing) and resulting sweat [Cl−] or FEV1% predicted are represented by filled red circles. (A and B, insets) Genotype function versus sweat [Cl−] (A, inset) or FEV1% predicted (B, inset) illustrates the nonlinear relationship between these variables. (C and D) Treatment effect from ivacaftor on sweat [Cl−] (C) or FEV1% predicted (D) stratified by age cohort (colored lines) does not differ from the relationship between these variables using CFTR2 data (all ages included [mean age, 22 yr at time of FEV1% predicted measures]). Trials included in C and D ranged in duration from 24 to 144 weeks of treatment. All comparisons were performed using an interaction term between CFTR2 data and data from clinical trials and indicated no significant difference between the regressions for each dataset. Clinical trial data plotted are detailed in Table E4 and Methods. %WT = percentage of wild type.
We also compared treatment responses of F508del homozygotes with modulator combinations in the same fashion as above. Pretreatment values for sweat [Cl−] were comparable to values from CFTR2 subjects (Figure 5A), whereas pretreatment values for FEV1% predicted were lower than those from CFTR2 subjects (Figure 5B). However, the change in each trait after treatment did not significantly differ from the slope derived from CFTR2 subjects. Analysis of responses by age group revealed no significant differences between modulator effect in F508del homozygotes and phenotypic differences in the CFTR2 population as a result of differing levels of CFTR function (Figures 5C and 5D). Recognizing that functional responses to modulators tested in clinical trials were drawn from a variety of cell types and that small numbers of subjects in trials may influence correlations, we evaluated all available clinical trial data together to determine if acute modulator response differed from the genotype–phenotype relationship derived from CFTR2 data. Treatment effect on sweat [Cl−] or FEV1% predicted did not differ from CFTR2 data when we included results from 15 trials assessing treatment response from a variety of CFTR modulators (Figure E2). Together, these comparisons illustrate that individuals in CFTR modulator trials attain sweat [Cl−] and lung function measures that approximate those of individuals with a lifetime of higher CFTR function as a consequence of their CFTR genotype.
Figure 5.
Treatment effect on F508del homozygote variants mirrors the relationship between genotype function and phenotype determined using Clinical and Functional Translation of CFTR (CFTR2) project data. (A and B) Genotype function versus sweat chloride ([Cl−]) (A) or FEV1% predicted (B) is plotted as described in Figure 4. The treatment effect of cystic fibrosis transmembrane conductance regulator (CFTR) modulators on F508del homozygotes does not differ from the relationship shown between these variables using CFTR2 data. Genotype function and baseline sweat [Cl−] or FEV1% predicted of individuals tested in clinical trials of duration 4–24 weeks are represented by open red circles; after treatment, genotype function (determined by in vitro cystic fibrosis bronchial epithelial cell or ex vivo primary cell testing) and resulting sweat [Cl−] or FEV1% predicted are represented by filled red circles. (A and B, insets) Genotype function versus sweat [Cl−] (A, inset) or FEV1% predicted (B, inset) illustrates the nonlinear relationship between these variables. (C and D) Treatment effect on sweat [Cl−] (C) or FEV1% predicted (D) stratified by age cohort (colored lines) does not differ from the relationship between these variables using CFTR2 data. Trials included in C and D ranged in duration from 4 to 24 weeks of treatment. All comparisons were performed using an interaction term between CFTR2 data and data from clinical trials and indicated no significant difference between the regressions for each dataset. Clinical trial data plotted are detailed in Table E4 and Methods. %WT = percentage of wild type.
Discussion
The availability of functional estimates for variants associated with a range of CF disease severity facilitated robust correlation analysis between CFTR genotype function and clinical features of CF. Our observation of a logarithmic relationship between CFTR function and sweat [Cl−] is consistent with prior studies of individual sweat glands (36). Nasal potential difference measures of CFTR-mediated chloride transport across nasal epithelium (another method of ascertaining CFTR function in vivo [37, 38]) also appears to exhibit a nonlinear relationship with sweat [Cl−] (16, 39). Similarly, our observation of a nonlinear relationship between CFTR function and severity of lung disease is supported by the nonlinear correlation between level of normal CFTR transcripts and FEV1% predicted in individuals carrying the 5T allele (40). Consistent with prior evidence, the correlation with sweat [Cl−] was the most robust, indicating that it is currently the best proxy measure for in vivo CFTR function (39, 41–43).
There are several plausible explanations for why the relationship between CFTR function and phenotype appears logarithmic. Like other ion channels, CFTR efficiently dissipates gradients (44, 45). Thus, having a small fraction of CFTR channels operating properly may restore close-to-normal balance of ionic concentrations, and the effect could be multiplied via CFTR’s function as a regulator of other ion channels (45). Alternatively, an increase in CFTR abundance in a few cells may allow for increased Cl− transport across epithelial tissues facilitated by intercellular ion movement via gap junctions. Coculture studies demonstrated that 10–20% of cells in human airway epithelia expressing WT-CFTR is sufficient to correct the epithelial chloride transport defect (35, 46, 47). Similarly, vector delivery of WT-CFTR to a small proportion of cells was sufficient for normal chloride transport (48) and restoration of airway surface liquid volume (34). The newly discovered pulmonary ionocyte (49, 50) supports the possibility that most CFTR-dependent chloride transport in airways may be restricted to a small fraction of cells that amplify transport by intercellular routes (35, 46, 47).
From a therapeutic perspective, a logarithmic relationship is encouraging because it implies that modest augmentation of CFTR at low functional levels can generate substantial clinical benefit. This phenomenon is consistent with results from individuals bearing the severe G551D variant, in whom moderate increases in CFTR function resulted in remarkable improvement in CF clinical measures (2, 36, 51). Precedence for a nonlinear relationship between function and phenotype is evident for other loss-of-function genetic conditions. Severe hemophilia results from plasma coagulation factor levels below 1% of normal, whereas levels of 2–5% result in moderate hemophilia and levels of 6–30% confer mild to no disease (52). This phenomenon is also observed with phenylketonuria, in which phenylalanine hydroxylase activity of 13–15% of normal level confers a mild presentation (53, 54).
Our results demonstrate that acute augmentation in CFTR function can result in improvements in clinical outcomes comparable to having a “milder” CFTR genotype since conception. This result is not surprising for sweat chloride, because the sweat gland is not believed to be damaged in CF (55). In contrast, lung disease is progressive in CF and believed to have nonreversible components such as airway loss and fibrotic replacement (56). However, our analysis suggests that the fraction of recoverable lung function may be substantial. Reports of individuals with severe CF who started ivacaftor as adults and subsequently ran marathons or climbed Mount Everest (57) illustrate this point. However, it is also possible that additional clinical trials and studies of longer duration might detect differences between lung function recovered by CFTR modulators and the phenotype that results from a lifetime of higher CFTR function.
Given the reversibility of lung disease with modulators, establishing an expected benchmark of lung function for differing levels of CFTR function is of the utmost importance: It allows more accurate predictions regarding expected lung function decline or stabilization after CFTR function augmentation. Though the CFTR2 dataset does not use individual longitudinal decline to establish these trajectories, the cross-sectional data provide insight into the relative differences among CFTR functional groups in a population whose vast majority of measurements occurred before use of modulators. Such data help inform how much recovery might be expected as CFTR function is increased so that a longer lifespan might be achieved. To this end, the benchmarking suggests that increasing CFTR function to greater than 10%—an accepted threshold below which life-limiting CF disease is expected—is associated with FEV1% predicted measurements above 80% into adulthood. However, even 10% CFTR function appears to result in a decrease in FEV1% predicted by age. This observation and previous reports associating variants conferring 10–25% function with variable expressivity of CF disease (17) suggest that further CFTR augmentation beyond this level may be necessary to maintain normal lung function. The very few individuals with genotypes conferring 25–50% CFTR function limit the ability to draw conclusions at levels above 25%, but this could be further investigated by expanding function–phenotype studies to include those not meeting diagnostic criteria for CF (i.e., CFTR-related disorders). At the other end of the spectrum, our analysis suggests that smaller increases in CFTR function (e.g., <2–5%) could produce clinically relevant improvements in lung function.
The chief limitation of this study lies in the inherent imperfection of clinical measurements, which are prone to some error and variability. Pancreatic insufficiency was designated as a discrete variable by whether an individual was receiving pancreatic enzyme replacement therapy, making our data vulnerable to skewing at small sample sizes. Individual subject FEV1% predicted measurements reported to CFTR2 are a heterogeneous group and may consist of mean, median, best, most recent, or annualized values. The requirement by many clinical trials that subjects have an FEV1% predicted value between 40% and 90% may have excluded individuals with modulator-responsive genotypes who had progressed too far in disease severity for inclusion, thereby skewing treatment response data compared with the CFTR2-derived plot (containing individuals across the range of FEV1% predicted measurements). Finally, interindividual variability, even within the same genotype, due to factors such as environment, modifier genes, and cis-regulatory variation reduces the utility of benchmarks to generalizations for groups of individuals with a certain level of CFTR function. Variance around each measure, especially for lung function, limits the confidence in predicting clinical improvements attributable to CFTR modulator therapy for an individual subject, whose response may also require assessment of CFTR augmentation on an individual level.
In summary, the extraordinary amount of data available in this large-scale study revealed clinically useful correlations between CFTR genotype function and clinical features of CF. These correlations demonstrate that individuals with severe disease as a result of very low CFTR function could benefit the most from modulator therapies, even if the increase in function is modest. Of potentially equal importance, the study generated benchmarks of CFTR function with lung flow measurements and sweat [Cl−] to provide points of reference for assessing CFTR modulator efficacy.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank all contributors to the CFTR2 project for their continued participation and Steve Rowe for his insight regarding the nature of the relationship between CFTR function and phenotype.
Footnotes
Supported by NIH grants R01DK44003 and R01HL128475 and CF Foundation grants Cuttin13A1, Cuttin15XX0, and Cuttin16IO (G.R.C.).
Author Contributions: A.F.M., K.S.R., P.R.S., and G.R.C. designed the study. C.C., J.M.C., M.C., M.H.L., C.M.P., J.M.R., and A.L.S. provided critical evaluation of methods, results, and conclusions. A.F.M., M.J.P., E.F.D.-M., T.A.E., S.T.H., Z.L., A.T.J., and N.S. generated functional data. K.S.R. and J.M.C. performed data analysis. A.F.M., K.S.R., and G.R.C. wrote the manuscript, with critique by C.C., J.M.C., M.C., M.H.L., C.M.P., J.M.R., and A.L.S.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201901-0145OC on March 19, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations–correlation with incidence data and application to screening. Hum Mutat. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wainwright CE, Elborn JS, Ramsey BW. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:1783–1784. doi: 10.1056/NEJMc1510466. [DOI] [PubMed] [Google Scholar]
- 4.Drumm ML, Ziady AG, Davis PB. Genetic variation and clinical heterogeneity in cystic fibrosis. Annu Rev Pathol. 2012;7:267–282. doi: 10.1146/annurev-pathol-011811-120900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 6.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 7.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 8.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73:1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- 9.Zielenski J, Tsui LC. Cystic fibrosis: genotypic and phenotypic variations. Annu Rev Genet. 1995;29:777–807. doi: 10.1146/annurev.ge.29.120195.004021. [DOI] [PubMed] [Google Scholar]
- 10.Estivill X. Complexity in a monogenic disease. Nat Genet. 1996;12:348–350. doi: 10.1038/ng0496-348. [DOI] [PubMed] [Google Scholar]
- 11.Koch C, Cuppens H, Rainisio M, Madessani U, Harms H, Hodson M, et al. Investigators of the ERCF. European epidemiologic registry of cystic fibrosis (ERCF): comparison of major disease manifestations between patients with different classes of mutations. Pediatr Pulmonol. 2001;31:1–12. doi: 10.1002/1099-0496(200101)31:1<1::aid-ppul1000>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 12.McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet. 2003;361:1671–1676. doi: 10.1016/S0140-6736(03)13368-5. [DOI] [PubMed] [Google Scholar]
- 13.McKone EF, Goss CH, Aitken ML. CFTR genotype as a predictor of prognosis in cystic fibrosis. Chest. 2006;130:1441–1447. doi: 10.1378/chest.130.5.1441. [DOI] [PubMed] [Google Scholar]
- 14.Goubau C, Wilschanski M, Skalická V, Lebecque P, Southern KW, Sermet I, et al. Phenotypic characterisation of patients with intermediate sweat chloride values: towards validation of the European diagnostic algorithm for cystic fibrosis. Thorax. 2009;64:683–691. doi: 10.1136/thx.2008.104752. [DOI] [PubMed] [Google Scholar]
- 15.Hirtz S, Gonska T, Seydewitz HH, Thomas J, Greiner P, Kuehr J, et al. CFTR Cl− channel function in native human colon correlates with the genotype and phenotype in cystic fibrosis. Gastroenterology. 2004;127:1085–1095. doi: 10.1053/j.gastro.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Rowe SM, Accurso F, Clancy JP. Detection of cystic fibrosis transmembrane conductance regulator activity in early-phase clinical trials. Proc Am Thorac Soc. 2007;4:387–398. doi: 10.1513/pats.200703-043BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raraigh KS, Han ST, Davis E, Evans TA, Pellicore MJ, McCague AF, et al. Functional assays are essential for interpretation of missense variants associated with variable expressivity. Am J Hum Genet. 2018;102:1062–1077. doi: 10.1016/j.ajhg.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sosnay PR, Siklosi KR, Van Goor F, Kaniecki K, Yu H, Sharma N, et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet. 2013;45:1160–1167. doi: 10.1038/ng.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottschalk LB, Vecchio-Pagan B, Sharma N, Han ST, Franca A, Wohler ES, et al. Creation and characterization of an airway epithelial cell line for stable expression of CFTR variants. J Cyst Fibros. 2016;15:285–294. doi: 10.1016/j.jcf.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 21.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 22.Taylor C, Commander CW, Collaco JM, Strug LJ, Li W, Wright FA, et al. A novel lung disease phenotype adjusted for mortality attrition for cystic fibrosis genetic modifier studies Pediatr Pulmonol 201146:857–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H, Burton B, Huang CJ, Worley J, Cao D, Johnson JP, Jr, et al. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cyst Fibros. 2012;11:237–245. doi: 10.1016/j.jcf.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Van Goor F, Yu H, Burton B, Hoffman BJ. Effect of ivacaftor on CFTR forms with missense mutations associated with defects in protein processing or function. J Cyst Fibros. 2014;13:29–36. doi: 10.1016/j.jcf.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Han ST, Rab A, Pellicore MJ, Davis EF, McCague AF, Evans TA, et al. Residual function of cystic fibrosis mutants predicts response to small molecule CFTR modulators. JCI Insight. 2018;3:121159. doi: 10.1172/jci.insight.121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies JC, Moskowitz SM, Brown C, Horsley A, Mall MA, McKone EF, et al. VX16-659-101 Study Group. VX-659-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379:1599–1611. doi: 10.1056/NEJMoa1807119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, et al. VX16-445-001 Study Group. VX-445-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379:1612–1620. doi: 10.1056/NEJMoa1807120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinzpeter A, Costa C, Sondo E, Alembik Y, Weiss L, Goossens M, et al. Rescued misprocessed protein by a truncated CFTR: analysis of the E831X mutation [abstract] Pediatr Pulmonol. 2009;44(Suppl 32):266. [Google Scholar]
- 29.Sharma N, Evans TA, Pellicore MJ, Davis E, Aksit MA, McCague AF, et al. Capitalizing on the heterogeneous effects of CFTR nonsense and frameshift variants to inform therapeutic strategy for cystic fibrosis. PLoS Genet. 2018;14:e1007723. doi: 10.1371/journal.pgen.1007723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. 2015;16:45–56. doi: 10.1038/nrg3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ooi CY, Durie PR. Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations in pancreatitis. J Cyst Fibros. 2012;11:355–362. doi: 10.1016/j.jcf.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Wilschanski M, Zielenski J, Markiewicz D, Tsui LC, Corey M, Levison H, et al. Correlation of sweat chloride concentration with classes of the cystic fibrosis transmembrane conductance regulator gene mutations. J Pediatr. 1995;127:705–710. doi: 10.1016/s0022-3476(95)70157-5. [DOI] [PubMed] [Google Scholar]
- 33.Ramalho AS, Beck S, Meyer M, Penque D, Cutting GR, Amaral MD. Five percent of normal cystic fibrosis transmembrane conductance regulator mRNA ameliorates the severity of pulmonary disease in cystic fibrosis. Am J Respir Cell Mol Biol. 2002;27:619–627. doi: 10.1165/rcmb.2001-0004OC. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Button B, Gabriel SE, Burkett S, Yan Y, Skiadopoulos MH, et al. CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol. 2009;7:e1000155. doi: 10.1371/journal.pbio.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dannhoffer L, Blouquit-Laye S, Regnier A, Chinet T. Functional properties of mixed cystic fibrosis and normal bronchial epithelial cell cultures. Am J Respir Cell Mol Biol. 2009;40:717–723. doi: 10.1165/rcmb.2008-0018OC. [DOI] [PubMed] [Google Scholar]
- 36.Char JE, Wolfe MH, Cho HJ, Park IH, Jeong JH, Frisbee E, et al. A little CFTR goes a long way: CFTR-dependent sweat secretion from G551D and R117H-5T cystic fibrosis subjects taking ivacaftor. PLoS One. 2014;9:e88564. doi: 10.1371/journal.pone.0088564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilschanski M, Dupuis A, Ellis L, Jarvi K, Zielenski J, Tullis E, et al. Mutations in the cystic fibrosis transmembrane regulator gene and in vivo transepithelial potentials. Am J Respir Crit Care Med. 2006;174:787–794. doi: 10.1164/rccm.200509-1377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon GM, Bronsveld I, Hayes K, Wilschanski M, Melotti P, Rowe SM, et al. Standardized measurement of nasal membrane transepithelial potential difference (NPD) J Vis Exp. 2018;(139):e57006. doi: 10.3791/57006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Accurso FJ, Van Goor F, Zha J, Stone AJ, Dong Q, Ordonez CL, et al. Sweat chloride as a biomarker of CFTR activity: proof of concept and ivacaftor clinical trial data. J Cyst Fibros. 2014;13:139–147. doi: 10.1016/j.jcf.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rave-Harel N, Kerem E, Nissim-Rafinia M, Madjar I, Goshen R, Augarten A, et al. The molecular basis of partial penetrance of splicing mutations in cystic fibrosis. Am J Hum Genet. 1997;60:87–94. [PMC free article] [PubMed] [Google Scholar]
- 41.Collaco JM, Blackman SM, Raraigh KS, Corvol H, Rommens JM, Pace RG, et al. Sources of variation in sweat chloride measurements in cystic fibrosis. Am J Respir Crit Care Med. 2016;194:1375–1382. doi: 10.1164/rccm.201603-0459OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang C, Balch WE. Bridging genomics to phenomics at atomic resolution through variation spatial profiling. Cell Rep. 2018;24:2013–2028, e6. doi: 10.1016/j.celrep.2018.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sontag MK. Sweat chloride: the critical biomarker for cystic fibrosis trials. Am J Respir Crit Care Med. 2016;194:1311–1313. doi: 10.1164/rccm.201606-1286ED. [DOI] [PubMed] [Google Scholar]
- 44.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79(Suppl):S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 45.Schwiebert EM, Benos DJ, Egan ME, Stutts MJ, Guggino WB. CFTR is a conductance regulator as well as a chloride channel. Physiol Rev. 1999;79(Suppl):S145–S166. doi: 10.1152/physrev.1999.79.1.S145. [DOI] [PubMed] [Google Scholar]
- 46.Farmen SL, Karp PH, Ng P, Palmer DJ, Koehler DR, Hu J, et al. Gene transfer of CFTR to airway epithelia: low levels of expression are sufficient to correct Cl- transport and overexpression can generate basolateral CFTR. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1123–L1130. doi: 10.1152/ajplung.00049.2005. [DOI] [PubMed] [Google Scholar]
- 47.Johnson LG, Olsen JC, Sarkadi B, Moore KL, Swanstrom R, Boucher RC. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet. 1992;2:21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- 48.Goldman MJ, Yang Y, Wilson JM. Gene therapy in a xenograft model of cystic fibrosis lung corrects chloride transport more effectively than the sodium defect. Nat Genet. 1995;9:126–131. doi: 10.1038/ng0295-126. [DOI] [PubMed] [Google Scholar]
- 49.Plasschaert LW, Žilionis R, Choo-Wing R, Savova V, Knehr J, Roma G, et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018;560:377–381. doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560:319–324. doi: 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mannucci PM, Tuddenham EG. The hemophilias—from royal genes to gene therapy. N Engl J Med. 2001;344:1773–1779. doi: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- 53.Okano Y, Eisensmith RC, Güttler F, Lichter-Konecki U, Konecki DS, Trefz FK, et al. Molecular basis of phenotypic heterogeneity in phenylketonuria. N Engl J Med. 1991;324:1232–1238. doi: 10.1056/NEJM199105023241802. [DOI] [PubMed] [Google Scholar]
- 54.Wettstein S, Underhaug J, Perez B, Marsden BD, Yue WW, Martinez A, et al. Linking genotypes database with locus-specific database and genotype-phenotype correlation in phenylketonuria. Eur J Hum Genet. 2015;23:302–309. doi: 10.1038/ejhg.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Munger BL, Brusilow SW, Cooke RE. An electron microscopic study of eccrine sweat glands in patients with cystic fibrosis of the pancreas. J Pediatr. 1961;59:497–511. doi: 10.1016/s0022-3476(61)80233-3. [DOI] [PubMed] [Google Scholar]
- 56.Welsh MJ, Ramsey BW, Accurso FJ, Cutting GR. Cystic fibrosis. In: Scriver CR, Beaudet AL, Valle D, Sly WS, editors. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill, Inc.; 2001. pp. 5121–5188. [Google Scholar]
- 57.Yorke H, Jamieson S. British man becomes first person with cystic fibrosis to climb Everest. The Telegraph; 2016 May 17 [accessed 2019 Jan 2]. Available from: https://www.telegraph.co.uk/news/2016/05/17/british-man-becomes-first-person-with-cystic-fibrosis-to-climb-e/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.