Abstract
Rationale: We reported a randomized trial demonstrating daily supplemental vitamin C to pregnant smokers significantly improved newborn pulmonary function tests. The current study tests these results in a new cohort using infant pulmonary function tests.
Objectives: To determine if infants of pregnant smokers randomized to daily supplemental vitamin C would have improved forced expiratory flows (FEFs) at 3 months of age compared with those randomized to placebo, and to investigate the association of the α5 nicotinic acetylcholine receptor.
Methods: A randomized, double-blind, placebo-controlled trial was conducted at three centers. Two hundred fifty-one pregnant smokers were randomized at 13–23 weeks of gestation: 125 randomized to vitamin C (500 mg/d) and 126 to placebo.
Measurements and Main Results: The primary outcome was FEF75 at 3 months of age performed with the raised volume rapid thoracic compression technique (Jaeger/Viasys). FEF50 and FEF25–75 obtained from the same expiratory curves were prespecified secondary outcomes. The infants of pregnant smokers randomized to vitamin C (n = 113) had the following FEFs at 3 months of age compared with those randomized to placebo (n = 109) as measured by FEF75 (200.7 vs. 188.7 ml/s; adjusted 95% confidence interval [CI] for difference, −3.33 to 35.64; P = 0.10), FEF50 (436.7 vs. 408.5 ml/s; adjusted 95% CI for difference, 6.10–61.30; P = 0.02), and FEF25–75 (387.4 vs. 365.8 ml/s; adjusted 95% CI for difference, 0.92–55.34; P = 0.04). Infant FEFs seemed to be negatively associated with the maternal risk alleles for the α5 nicotinic acetylcholine receptor (rs16969968).
Conclusions: Although the primary outcome of FEF75 was not improved after vitamin C supplementation to pregnant smokers, the predetermined secondary outcomes FEF50 and FEF25–75 were significantly improved. These results extend our previous findings and demonstrate improved airway function (FEF50 and FEF25–75) at 3 months of age in infants after vitamin C supplementation to pregnant smokers.
Clinical trial registered with www.clinicaltrials.gov (NCT 01723696).
Keywords: infant pulmonary function, forced expiratory flows, smoking in pregnancy, vitamin C, wheezing
At a Glance Commentary
Scientific Knowledge on this Subject
Maternal smoking during pregnancy adversely affects lung development with near lifelong decreases in pulmonary function and increased risk of wheezing, respiratory infections, and asthma. We have previously shown that adding daily supplemental vitamin C for pregnant smokers improves their infant’s newborn pulmonary function (passive respiratory compliance and time to peak tidal expiratory flow to expiratory time) measured within 72 hours of birth. We also demonstrated that the effect of maternal smoking on newborn lung function was associated with the maternal genotype for the α5 nicotinic acetylcholine receptor (rs16969968).
What This Study Adds to the Field
This randomized trial expands our previous study by demonstrating increased forced expiratory flows (a more specific measurement of airway function) at 3 months of age in the infants of pregnant smokers randomized to daily vitamin C versus placebo. The effect of maternal smoking on infant forced expiratory flows seemed to be increased with maternal risk alleles for the α5 nicotinic acetylcholine receptor genotype. Our results suggest that vitamin C supplementation in pregnant women who cannot quit smoking may be a safe, inexpensive, and simple intervention to improve the pulmonary function of their infant by blocking some of the effects of in utero smoke on lung development.
Smoking during pregnancy remains a large public health problem and is the largest preventable cause of childhood respiratory illness (1–3). Despite intensive smoking cessation initiatives, more than 50% of smokers who become pregnant continue to smoke (4, 5). This corresponds nationwide to at least 10% of American women continuing to smoke when pregnant (6) with about 450,000 exposed infants per year at an estimated annual health care cost of more than 1 billion dollars (7).
We previously reported a randomized controlled trial demonstrating that giving supplemental vitamin C (500 mg/d) to pregnant women unable to quit smoking significantly improved their offspring’s newborn pulmonary function tests (PFTs) of passive respiratory compliance and time to peak tidal expiratory flow to expiratory time (TPTEF:TE) measured within 72 hours of birth (8). We also demonstrated that the effect of maternal smoking on newborn lung function was associated with the maternal genotype for the α5 nicotinic acetylcholine receptor (nAChR) (rs16969968). This genotype is also associated with increased risk of lung cancer, chronic obstructive pulmonary disease, and increased nicotine dependence (9, 10). This trial was based on important foundation data from a nonhuman pregnant primate model that demonstrated that nicotine crosses the placenta, upregulates nicotinic receptors, and alters lung development in the offspring with decreased forced expiratory flows (FEFs) at birth (11–13). In this model, vitamin C decreased the effects of in utero nicotine on the offspring’s FEFs (14).
Although our initial study showed significantly improved PFTs in the newborns of smokers allocated to vitamin C, we did not measure FEFs because of the instability of newborn lung volumes in the first weeks of life and the required sedation to perform testing. However, FEFs are a more direct assessment of airway function, previous studies have demonstrated decreased FEFs in infants exposed to maternal smoking during pregnancy (15–18), and decreased FEFs in infancy are correlated with increased risk of respiratory disease (15, 16, 19). Therefore, the primary objective of this study was to compare the FEFs at 3 months of age in infants of pregnant smokers randomized to vitamin C (500 mg/d) versus placebo. We hypothesized that the supplemental vitamin C would improve the infant’s FEFs when compared with placebo. Some of the results of these studies were previously reported in the form of an abstract (20).
Methods
Participants
Pregnant smokers were recruited from three clinical sites: Oregon Health and Science University (OHSU), Portland, Oregon; Indiana University, Indianapolis, Indiana; and PeaceHealth Southwest Washington Medical Center, Vancouver, Washington. This study cohort was comprised of different patients than those recruited in our initial trial (8). The study protocol was approved by the three institutional review boards. All women provided written informed consent.
Inclusion criteria at randomization were: women ≥15 years old with a singleton gestation between 13 weeks and 0 days and 22 weeks and 6 days based on clinical information and confirmed by ultrasound, current cigarette smoker (≥1 cigarette in last week), English-speaking, and receiving prenatal care at surrounding clinics to the three sites. Exclusion criteria at randomization included: multiple gestation, fetal anomalies, current illicit drug use, current alcohol abuse, daily vitamin C supplementation more than 3 days/wk (not including prenatal vitamin) since last menstrual period, refusal to abstain from vitamin supplements except those from the study, history of a kidney stone, insulin-dependent diabetes, complex maternal medical conditions, participation in conflicting research projects, unable to demonstrate a stable method of communication, pregnancy by in vitro fertilization, plan to terminate pregnancy, and body mass index greater than 50 kg/m2.
Study Design and Oversight
Details of the study design were previously published (21). We conducted a randomized, double-blind, placebo-controlled study of vitamin C (500 mg/d) versus placebo in pregnant smokers.
Study staff screened women at prenatal clinics between December 2012 and June 2015 in the catchment area of the three clinical sites to identify eligible women who continued to smoke cigarettes. Staff trained in smoking cessation provided participants with brief cessation counseling consistent with the U.S. Public Health Service Clinical Practice Guideline and provided a pregnancy-specific smoking cessation pamphlet. Smoking status and counseling were documented in the study record (22). On consent, women had an ultrasound for gestational age confirmation and entered a medication adherence trial to take one placebo capsule/d for 7–21 days. If a subject returned within 21 days with the medication bottle and took at least 75% of the required placebo, she proceeded to randomization.
The vitamin C and placebo medications were manufactured in organoleptically identical tablets (Magno-Humphries Laboratories Inc.) and dispensed through the OHSU research pharmacy. Each vitamin C tablet contained 500 mg of ascorbic acid powder; the placebo tablet contained microcrystalline cellulose and 100 mg of citric acid to mimic the taste of vitamin C. The tablets were identical in appearance, size, and shape and dispensed in 100-tablet quantities during the treatment period. Participants were instructed to take one study capsule daily, at a consistent time, until delivery. After randomization, women were given a standard prenatal vitamin (Prenavite, Rugby Laboratories, 100 count) that included 60 mg of vitamin C.
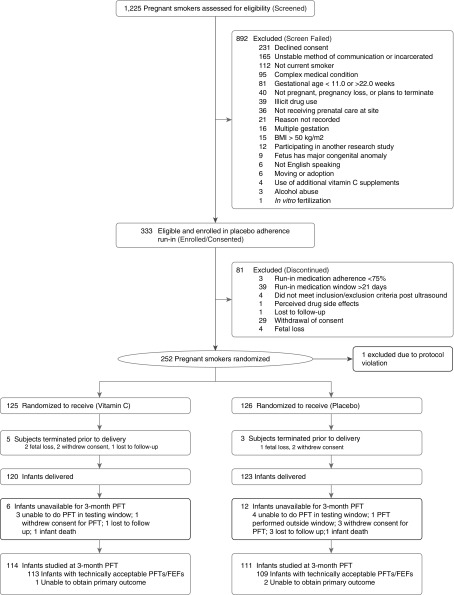
Randomization was performed at the Data Coordinating Center using a permuted block randomization stratified by gestational age at time of randomization (≤18 vs. >18 wk gestation) and clinical site. The OHSU research pharmacy prepared consecutively labeled medicine bottles to dispense, using the randomization schedule. The study medication was labeled with the study identification and a consecutive study code for the patient. The OHSU research pharmacy dispensed the study capsules to all sites. The investigators, clinicians, and patients were unaware of treatment allocation through the 3-month PFT and remain blinded. (Consolidated Standards for Reporting Trials diagram, Figure 1).
Figure 1.
Consolidated Standards for Reporting Trials diagram for randomized smokers. Enrollment, randomization, and follow-up of randomized smokers and their infants through the 3-month pulmonary function tests/forced expiratory flows. BMI = body mass index; FEF = forced expiratory flow; PFT = pulmonary function test.
Study staff met with the randomized women at each prenatal visit to collect: interval smoking histories via a standardized respiratory questionnaire (23), brief health questionnaires, medication use, and complications with review of electronic medical records. They also provided brief smoking cessation counseling. Adherence with dispensed medication (vitamin C or placebo) was assessed at each visit with pill count by study staff and at return of each medication bottle. Fasting maternal blood samples for ascorbic acid levels and maternal urine samples were obtained at randomization, and at 26 and 32 weeks of gestation.
Study Endpoints
The primary outcome was the comparison of infant FEF75, the measurement of FEF at 75% of the expired volume, at 3 months of age obtained using the raised volume rapid thoracic compression technique in offspring of pregnant smokers randomized to vitamin C versus placebo. Prespecified secondary outcomes were the FEF at 50% expired volume (FEF50), and between 25% and 75% expired volume (FEF25–75) obtained from the same expiratory curve.
Forced expiratory volumes (FEV), including FVC and FEV in the initial 0.5 seconds (FEV0.5), were obtained. Based on findings in our prior study, in a secondary analysis we evaluated the potential association of the α5 nAChR polymorphism rs16969968 (which is the α5 nAChR structural polymorphism that has the strongest link to lung disease and increased smoking) (9), in the effect of in utero smoke on infant pulmonary function.
Measurements
Infant PFT at 3 months of age
Infants were studied in the infant PFT laboratories at Doernbecher Children’s Hospital (Oregon), the James Whitcomb Riley Hospital for Children (Indiana), or at Peace Health/Southwest Medical Center (Washington) after sedation with oral chloral hydrate at 50–100 mg/kg. Each site used the same PFT equipment (Jaeger/Viasys Master Screen BabyBody). Operational procedures were rigorously followed across sites as outlined in the study’s manual of operations (18, 24). Cross-training and certification of PFT laboratories ensured the same testing techniques and acceptance criteria were applied across sites. Four experienced respiratory therapists performed all testing. All tests were reviewed by a trained, licensed respiratory therapist and reviewed for acceptability, reproducibility, and completeness. All testing was done at least 3 weeks after a respiratory illness and analyzed testing occurred within the predefined infant age of 10–26 weeks.
FEFs were obtained from FEF volume curves using the raised volume rapid thoracic compression technique with testing performed following the American Thoracic Society/European Respiratory Society criteria for performance and acceptance (24). Briefly, the lung was inflated by applying a pressure of 30 cm H2O to the airway with a face mask. An inflatable jacket was used to initiate thoracic compression at this raised volume and was maintained until residual volume was reached. Forced expiratory maneuvers were repeated with increased pressure until flow limitation was obtained. Once flow limitation was established, the maneuver was repeated over a 10–15 cm H2O range in jacket pressure until three technically acceptable curves were obtained with FEF25–75, and FVC within 10%. The best trial was chosen that was determined to be the most reliable with smooth forced expiration without evidence of early inspiration, marked flow transients, or glottic closure (18, 24).
Biomarkers and genotyping
Plasma ascorbic acid measurements were performed at the Linus Pauling Institute using high-performance liquid chromatography with coulometric electrochemical detection (25). Urine cotinine levels (26) were measured with a widely used ELISA kit following the vendor’s protocol (Calbiotech). DNA was prepared from ethylenediaminetetraacetic acid whole-blood tubes using the QIAcube and QIAamp DNA Blood Mini QIAcube Kit (Qiagen). Mothers were genotyped for rs16969968, which is the nAChR polymorphism most clearly linked to increased smoking, difficulty quitting, and lung disease (9), by real-time PCR using predesigned qPCR SNP genotyping reagents (C__26000428_20) from Thermo Fisher (Applied Biosystems). Mothers were also genotyped for GSTM1 deletion (Hs02575461_cn), GSTT1 deletion (Hs00010004_cn), and GSTP1 polymorphism (C__3237198_20). All maternal and baby DNA samples from blood were genotyped in duplicate and any samples that did not produce the same result in both replicates was repeated. As part of genotyping QC, maternal and baby genotypes were also compared for any mendelian violations.
Safety monitoring
All subjects were monitored for adverse events according to standard definition by interviewing the subject, electronic medical record review, and physical examination (including vital signs monitoring during sedation). The NIH appointed Data and Safety Monitoring Board reviewed maternal, neonatal, and infant adverse events every 3 months.
Statistical Analysis and Power
Our targeted sample size of 218 infants with successful FEFs at 3 months of age was determined to detect with 90% power, at a significance level of 0.05, increases of 15% in mean FEF75 in the vitamin C group compared with the placebo group. This was based on comparing the two means on the log scale using 0.28 as the estimate for the SD of FEF75 based on data in 155 healthy infants of smoking and nonsmoking women (27) and included allowance for 4% of the patients to be noncompliant (took <50% of their medications) as shown in our initial trial (8).
Comparisons of the two treatment groups with respect to maternal characteristics assessed after randomization, delivery outcomes, and non-PFT infant characteristics at 3 months were made using the Student’s t test for numeric variables and the Pearson chi-square test for categorical variables. Summary statistics of z-scores for PFT parameters were also calculated using the approach of Lum and colleagues (28). The statistical analyses of PFTs were based on intention to treat. We analyzed FEF75, FEF50, and FEF25–75 in infants born to mothers randomized to vitamin C versus placebo, using analysis of covariance general linear models. Included in these models were treatment arm, clinical site, and gestational age at randomization (and all interactions of these three factors) and the covariates of infant sex, maternal race, and infant length at 3 months. Analyses for FVC, FEV0.5, and FEV0.5/FVC were done using the same analysis of covariance analyses. In secondary analyses, a factor for the α5 nAChR (rs16969968) genotype (with levels for major allele homozygous, heterozygous, and minor allele homozygous) and the interaction of genotype and treatment arm were added to the previously mentioned analysis of covariance models for FEF75, FEF50, and FEF25–75.
All P values are two-sided, with significance set at P less than 0.05. Statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc.). The Data and Safety Monitoring Board reviewed results for the primary outcome when 50% of the total sample size of 3-month PFTs were completed.
Results
Characteristics of the Trial Participants
We screened 1,225 pregnant women who continued to smoke in pregnancy; 892 were excluded before consent, and 81 were excluded after the medication adherence period (Figure 1). We randomized 252 women; however, one subject was subsequently identified to have met critical exclusion criteria and therefore we analyzed 125 who received vitamin C (500 mg/d) and 126 who received placebo (Figure 1). Randomization balanced relevant baseline covariates including age, race and ethnicity, parity, and gestational age (Table 1). At study entry, the median number of cigarettes smoked per day in each group was seven, and urine cotinine was comparable in each group. Plasma ascorbic acid levels were similar at baseline between both groups (Table 1); at mid and late gestation, the vitamin C group had significantly higher ascorbic acid levels than the placebo group (Table 2). The urine cotinine levels were comparable between groups at randomization, and at mid and late gestation. Of the 243 infants at delivery, there was no significant effect of the intervention on delivery mode, birth weight, gestational age, or the incidence of prematurity (Table 2).
Table 1.
Baseline Maternal Characteristics at Randomization
| Vitamin C–treated Smokers (n = 125) | Placebo-treated Smokers (n = 126) | |
|---|---|---|
| Age, mean (SD), yr | 26.6 (5.2) | 26.4 (5.9) |
| White, n (%) | 97 (77.6) | 100 (79.4) |
| Gravida, median (IQR) | 3.0 (2.0–4.0) | 3.0 (2.0–5.0) |
| Gestational age, mean (SD), wk | 18.4 (3.0) | 18.2 (2.8) |
| BMI, mean (SD), kg/m2 | 28.6 (6.5) | 30.0 (7.3) |
| Education status, n (%) | ||
| <High school | 20 (16.0) | 31 (24.6) |
| High school or equivalent | 59 (47.2) | 40 (31.7) |
| Some college | 43 (34.4) | 49 (38.9) |
| Bachelor’s degree | 3 (2.4) | 6 (4.8) |
| Marital status, n (%) | ||
| Married | 22 (17.6) | 31 (24.6) |
| Single | 39 (31.2) | 49 (38.9) |
| Divorced | 8 (6.4) | 7 (5.6) |
| Significant other | 56 (44.8) | 39 (31.0) |
| Health insurance, n (%) | ||
| Government assistance | 112 (89.6) | 106 (84.1) |
| Private | 13 (10.4) | 18 (14.3) |
| None or self-pay | 0 (0) | 2 (1.6) |
| Cigarettes/d, median (IQR) | 7.0 (4.0–10.0) | 7.5 (4.0–10.0) |
| Urine cotinine, median (IQR), ng/ml* | 5,031 (1,885–7,058) | 5,409 (1,950–8,662) |
| Randomization plasma ascorbic acid, mean (SD), μmol/L† | 48.7 (19.1) | 49.3 (21.8) |
| Asthma, n (%) | 44 (35.2) | 38 (30.2) |
| History of substance abuse, n (%) | 20 (16.0) | 16 (12.7) |
Definition of abbreviations: BMI = body mass index; IQR = interquartile range.
Values for urine cotinine based on 121 vitamin C– and 124 placebo-treated smokers.
Values for ascorbic acid based on 119 vitamin C– and 123 placebo-treated smokers.
Table 2.
Pregnancy, Delivery, and Infant Characteristics
| Vitamin C Treated (n = 120) | Placebo Treated (n = 123) | Mean (95% CI) Difference (Vitamin C − Placebo) | |
|---|---|---|---|
| Plasma ascorbic acid | |||
| Midgestation | |||
| Women, n | 111 | 116 | |
| Mean (SD), μmol/L | 60.8 (22.6) | 41.6 (20.0)* | 19.2 (13.7 to 24.8)* |
| Late gestation | |||
| Women, n | 107 | 110 | |
| Mean (SD), μmol/L | 54.6 (23.0) | 39.6 (17.6)* | 15.0 (9.7 to 20.6) |
| Preeclampsia/hypertension, n (%)† | 8 (6.7) | 13 (10.6) | 3.9 (−10.9 to 3.1) |
| Mode of delivery, n (%) | |||
| Vaginal | 80 (66.7) | 86 (69.9) | 3.2 (−14.9 to 8.4) |
| Cesarean | 40 (33.3) | 36 (29.3) | |
| Female, n (%)† | 59 (49.2) | 61 (49.6) | 0.4 (−13.0 to 12.1) |
| Gestational age | |||
| Infants, n | 120 | 123 | |
| Mean (SD), wk | 38.7 (1.8) | 38.6 (1.7) | 0.1 (−0.3 to 0.5) |
| Birth weight, | |||
| Infants, n | 120 | 120 | |
| Mean (SD), g | 3,123 (516) | 3,078 (552) | 44.6 (−91.2 to 180.5) |
| Birth length | |||
| Infants, n | 113 | 111 | |
| Mean (SD), cm | 49.6 (3) | 49.2 (3.2) | 0.4 (−0.4 to 1.2) |
| Birth head circumference | |||
| Infants, n | 106 | 104 | |
| Mean (SD), cm | 33.7 (1.9) | 33.3 (2) | 0.4 (−0.1 to 0.9) |
| Delivered at <37 wk, n (%)† | 14 (11.7) | 11 (8.9) | −2.7 (−10.4 to 4.9) |
| IUGR, n (%)†‡ | 2 (1.7) | 4 (3.3) | 1.6 (−2.3 to 5.5) |
| Infant characteristics at 3-mo PFT | |||
| Infants, n | 114 | 111 | |
| White race, n (%)†§ | 89 (78.1) | 88 (79.3) | 1.2 (−9.5 to 11.9) |
| Female, n (%)† | 55 (48.2) | 54 (48.6) | 0.4 (−16.7 to 13.5) |
| Age, mean (SD), wk | 15.3 (3.2) | 15.3 (3.0) | 0.0 (−5.6 to 5.8) |
| Length, mean (SD), cm | 60.2 (3.2) | 60.2 (2.9) | 0.0 (−0.8 to 0.8) |
| Weight, mean (SD), kg | 6.4 (1.0) | 6.3 (1.0) | 0.1 (−0.2 to 0.3) |
| Respiratory rate, mean (SD), breaths/min | 40 (7) | 40 (7) | 0.7 (−1.1 to 2.5) |
Definition of abbreviations: CI = confidence interval; IUGR = intrauterine growth restriction; PFT = pulmonary function test.
P < 0.05, ascorbic acid levels significantly different between groups at 26- and 32-wk gestation.
Wald confidence interval.
Birth weight less than the 10th percentile for gestational age.
Maternal race used as proxy for infant race.
PFT: FEFs at 3 Months of Age
For the analysis of the primary outcome of the 3-month FEF75, 225 infants had PFTs attempted (92.6% of infants available at delivery), and 222 infants (113 in the vitamin C– and 109 in the placebo-treated group) had successful FEFs performed within the predetermined window of 10 through 26 weeks of infant age. After randomization, 12 infants in the vitamin C and 17 in the placebo group did not complete the 3-month PFTs because of fetal loss, infant death, consent withdrawal, loss to follow-up, or unsuccessful sedation (Figure 1). At the time of the attempted 3-month PFTs, infants in the vitamin C and placebo treatment groups each had a mean age of 15.3 weeks and length of 60.2 cm (Table 2).
The infants born to the smokers randomized to vitamin C had an increased FEF75 (200.7 vs. 188.7 ml/s; adjusted 95% confidence interval [CI] for difference, −3.33 to 35.64; P = 0.10) at 3 months of age, and a significantly increased FEF50 (436.7 vs. 408.5 ml/s; adjusted 95% CI, 6.10–61.30; P = 0.02) and FEF25–75 (387.4 vs. 365.8 ml/s; adjusted 95% CI, 0.92–55.34; P = 0.04) compared with those randomized to placebo (Table 3). Regarding FEVs, there was a significant difference in FEV0.5 between the groups but not for FVC or FEV0.5/FVC (Table 3).
Table 3.
Pulmonary Function Tests in Infants at 3 Months of Age
| Vitamin C Mean (SD) (n = 113) | Placebo Mean (SD) (n = 109) | Unadjusted Mean (95% CI) Difference (Vitamin C − Placebo) | Unadjusted P Value | Adjusted Mean (95% CI) Difference (Vitamin C − Placebo)* | Adjusted P Value* | |
|---|---|---|---|---|---|---|
| FEF75, ml/s | 200.7 (71.1) | 188.7 (66.4) | 12.1 (−6.1 to 30.3) | 0.19 | 16.2 (−3.3 to 35.6) | 0.10 |
| FEF50, ml/s | 436.7 (101.5) | 408.5 (94.0) | 28.1 (2.3 to 54.0) | 0.033 | 33.7 (6.1 to 61.3) | 0.02 |
| FEF25–75, ml/s | 387.4 (98.1) | 365.8 (98.8) | 21.6 (−3.8 to 47.0) | 0.096 | 28.1 (0.9 to 55.3) | 0.04 |
| FEV0.5, ml† | 180.0 (35.4) | 173.7 (32.8) | 6.3 (−2.8 to 15.4) | 0.17 | 8.6 (0.7 to 16.4) | 0.03 |
| FVC, ml | 211.2 (43.7) | 207.3 (43.3) | 3.8 (−7.7 to 15.4) | 0.51 | 5.7 (−3.7 to 15.1) | 0.24 |
| FEV0.5/FVC† | 0.80 (0.10) | 0.90 (0.10) | 0.01 (−0.01 to 0.03) | 0.18 | 0.01 (−0.01 to 0.03) | 0.20 |
Definition of abbreviations: CI = confidence interval; FEF25–75 = forced expiratory flow between 25% and 75% of the expired volume; FEF50 = forced expiratory flow at 50% of the expired volume; FEF75 = forced expiratory flow at 75% of the expired volume; FEV0.5 = forced expired volume in the initial 0.5 s.
P values and 95% CI adjusted for design factors of site; gestational age at randomization; and covariates of length, race, and sex.
Value not reported for one infant.
Maternal Genotype
The addition of maternal genotype (n = 217) for the α5 nAChR (rs16969968) into the model of treatment and the 3-month FEFs increased the effect size between the treatment arms for each FEF; however, it also increased the width of the 95% CI (Table 4). There was no significant interaction between maternal genotype and treatment group. There was a significant association of the α5 nAChR genotype in the treatment model of vitamin C versus placebo for FEF50, with infants of mothers who had two copies of the risk allele for the α5 nAChR having the lowest FEF50 in both the vitamin C– and placebo-treated subjects. Interestingly, the α5 nAChR genotype seemed to have a dose–response effect on FEF50 according to the number of functional alleles present with vitamin C treatment improving FEF50 compared with placebo at each level.
Table 4.
FEF in Infants at 3 Months of Age Incorporating α5 nAChR Genotype
| Vitamin C Mean (SD) (n = 112) | Placebo Mean (SD) (n = 105) | Adjusted Mean (95% CI) Difference without α5 Genotype (Vitamin C − Placebo)* | Adjusted P Value* | Adjusted Mean (95% CI) Difference with α5 Genotype (Vitamin C − Placebo)* | Adjusted P Value† | |
|---|---|---|---|---|---|---|
| FEF75, ml/s | 200.7 (70.0) | 188.7 (66.3) | 12.1 (−6.1 to 30.3) | 0.12 | 24.15 (−0.31 to 48.61) | 0.053 |
| FEF50, ml/s | 438.4 (100.2) | 406.4 (94.0) | 28.1 (2.3 to 54.0) | 0.016 | 47.00 (12.8 to 81.3) | 0.007 |
| FEF25–75, ml/s | 389.4 (96.3) | 363.8 (93.9) | 21.6 (−3.8 to 47.0) | 0.0493 | 40.00 (6.1 to 73.8) | 0.021 |
Definition of abbreviations: α5 nAChR = α5 nicotinic acetylcholine receptor; CI = confidence interval; FEF25–75 = forced expiratory flow between 25% and 75% of the expired volume; FEF50 = forced expiratory flow at 50% of the expired volume; FEF75 = forced expiratory flow at 75% of the expired volume.
P values and 95% CI adjusted for design factors of site; gestational age at randomization; and covariates of length, race, and sex.
P values and 95% CI adjusted for design factors of site; gestational age at randomization; and covariates of length, race, sex, and α5 nAChR genotype.
Adverse Events
Adverse events were monitored. No serious adverse events related to the intervention were reported.
Discussion
In this randomized, double-blinded multicenter clinical trial, infants delivered to pregnant smokers randomized to supplemental vitamin C (500 mg/d) did not have a significant improvement in the primary outcome of FEF75, but had a significant improvement in FEF50 and FEF25–75, which are flows obtained from the same expiratory curve. This confirms and expands our initial study (8) of improved newborn PFTs with similar findings but in a larger population with increased diversity through the measurement of FEFs, a more specific measurement of airway function. We also confirm our previous findings of the importance of the α5 nAChR in the effect of in utero smoke on pulmonary function.
We chose the primary outcome of infant FEFs to build on our strong preclinical data in nicotine exposed primates (12, 14), and to expand our initial clinical study of significantly improved passive pulmonary mechanics and TPTEF:TE in newborns of pregnant women randomized to vitamin C versus placebo (8). Several studies, including our own (2, 27–31), have demonstrated that a key effect of maternal smoking during pregnancy is a decrease in infant FEFs, which is considered a sensitive measurement of infant airway function. In contrast to older children and adults, infants have a very short rate constant for forced expiration and the entire FVC occurs in less than 1 second (24), therefore some investigators use FEV0.5 to quantify infant airway function. Among healthy infants without previous wheezing, we found that FEF75, FEF50, and FEF25–75, but not FEV0.5, were lower in infants whose mothers reported smoking during pregnancy (27). Similarly, FEF75, FEF50, and FEF25–75, but not FEV0.5 were better at detecting low airway function among infants with a history of respiratory symptoms, but asymptomatic at the PFT (32). Therefore, for the current study, we chose FEF75 as our primary outcome to assess infant airway function in the randomized groups; however, we could have just as easily chosen FEF50 and FEF25–75 as the primary outcome because they are obtained from the same expiratory curve. The summary statistics of the z-scores (28) of the FEFs of the vitamin C–and placebo-treated infants (Table 5) indicate their flows fall within normal limits (range of mean scores from −0.38 to 0.29 for all parameters) and follow the same general pattern/direction of the raw scores (i.e., the z-scores of the placebo-treated infants being lower than those of the vitamin C–treated infants).
Table 5.
Summary Table of z-Scores for PFTs in Randomized Infants
| PFT Parameter | Vitamin C (n = 113) | Placebo (n = 109) |
|---|---|---|
| FEF75, ml/s | −0.02 (1.37) | −0.24 (1.33) |
| FEF25–75, ml/s | 0.29 (1.18) | 0.02 (1.29) |
| FEV0.5, ml | −0.01 (1.07) | −0.24 (1.16) |
| FVC, ml | −0.28 (1.12) | −0.38 (1.29) |
| FEV0.5/FVC | 0.28 (0.98) | 0.10 (1.13) |
Definition of abbreviations: FEF25–75 = forced expiratory flow between 25% and 75% of the expired volume; FEF75 = forced expiratory flow at 75% of the expired volume; FEV0.5 = forced expired volume in the initial 0.5 s; PFT = pulmonary function test.
Data are given as mean (SD). Means are unadjusted for other variables. FEF50 is not included as no z-scores calculation was included for this parameter in Lum and colleagues (28).
In this study, we confirm our previous findings of the importance of the maternal α5 nAChR genotype (rs16969968) in the effect of in utero smoke on infant lung function. This common polymorphism has been associated with an increased risk of lung cancer and nicotine addiction (9). In our initial study (8), we demonstrated that newborns of mothers who had two copies of the α5 nAChR genotype risk allele had the lowest measurements of TPTEF:TE and our current study shows a similar decrease in FEF50 in relation to the number of functional α5 nAChR alleles, with vitamin C improving the FEF50 at each level. This gene environment effect may be mediated by increased smoking, increased smoking intensity, or by a direct effect on α5 nAChR receptor signaling in developing lung. This also identifies a group at particular risk of the effects of smoking during pregnancy on infant lung function and emphasizes the importance of nicotine and nicotinic signaling in mediating the effects of maternal smoking during pregnancy.
Although this study was a randomized placebo-controlled trial, there are several limitations. Our study was powered to detect a 15% vitamin C treatment effect, which we based on our previous observation of 15% lower FEFs among healthy infants tested at 11 months of age in which the history of maternal smoking during pregnancy was gathered retrospectively and not quantified (27). We were able to demonstrate significant increases of 7–9% in FEFs with vitamin C treatment at 3 months of age, showing the ability of raised volume rapid thoracic compression maneuvers to detect differences of this magnitude in infant airway function. Importantly, studies have demonstrated that decreases in FEFs in children of mothers who smoked during pregnancy are in this range and even decreases of 4–6% in FEFs are associated with increased lung disease in the children (33). Our study was also analyzed on intention to treat and there were about 10% of women who quit smoking after randomization, which may have diluted our results. Some portion of these may reflect the effect of smoking cessation counseling provided during the study.
Our results support the hypothesis that oxidative mechanisms are in part responsible for the effects of in utero nicotine on lung development. At randomization, both groups of pregnant smokers had levels of fasting ascorbic acid of 49 μmol/L that were decreased compared with levels reported for nonsmoking women (58 μmol/L) (34), consistent with the increased oxidative load associated with smoking (35). Pregnant women randomized to the supplemental vitamin C had significantly increased fasting ascorbic acid levels at 26 and 32 weeks of gestation when compared with baseline and when compared with those randomized to placebo. Importantly, these levels were within the range reported in nonsmokers (34) and demonstrate the potential impact of nutritional interventions to improve respiratory health (36, 37).
Decreased pulmonary function early in life is associated with increased respiratory illnesses early in life, and maternal smoking during pregnancy is a major contributor to these adverse respiratory outcomes (38–41). Although smoking cessation remains the top priority, it is important to recognize the reality that 50% of pregnant smokers will continue to smoke despite multiple interventions (5). Therefore, it is critical to develop early life strategies, such as prenatal vitamin C supplementation, to mitigate the effects of maternal smoking to maximize lung growth and development. Cohort studies have found that increases in adverse respiratory outcomes related to maternal smoking during pregnancy track into adulthood and may be related to subsequent onset of chronic obstructive pulmonary disease (42). This emphasizes the importance of continuing to follow the respiratory outcomes of the cohort described here. Future trials of vitamin C supplementation in pregnant smokers could investigate whether a greater treatment effect might be achieved by earlier treatment, which would cover more of the canalicular phase of lung development or more prolonged vitamin C treatment and treatment of the infant postnatally that would cover more of lung alveolarization (42, 43).
Although our primary outcome of FEF75 at 3 months of age was not improved after vitamin C supplementation to pregnant smokers, the related measures FEF50 and FEF25–75 obtained from the same forced expiratory curve were significantly improved. This extends our previous findings of improved newborn PFTs in infants of pregnant smokers randomized to vitamin C versus placebo. Our findings also suggest that vitamin C supplementation in pregnant women who cannot quit smoking may be a safe, inexpensive, and simple intervention to improve their offspring’s pulmonary function by blocking some of the effects of in utero smoke on lung development. These infants are in continued follow-up to track lung function and respiratory outcomes.
Supplementary Material
Acknowledgments
Acknowledgment
The VCSIP research team thanks the women who participated in our study. The VCSIP team also thanks and acknowledges the members of the VITEL (Vitamins for Early Lung Health) Data and Safety Monitoring Board for their advice, support, and data monitoring during the trial; and Dr. Manuel Durand for advice and careful manuscript review.
Footnotes
Supported by the NHLBI (R01 HL105447 and R01 HL105460) with cofunding from the Office of Dietary Supplements and by P51OD011092 and UG3 OD023288. Additional support from the Oregon Clinical Translational Research Institute funded by the National Center for Advancing Translational Sciences (UL1TR000128).
Author Contributions: Conception and design, C.T.M., L.E.S.-K., K.M., D.S., C.T., B.V., A.S., K.J., D.M.H., J.H., R.S., B.S.P., A.V., D.F.K., J. Mitchell, J. Metz, D.G., C.B., E.R.S., R.S.T., and C.D.M. Analysis and interpretation, C.T.M., A.V., R.S., B.S.P., D.F.K., B.K., J. Mitchell, E.R.S., R.S.T., and C.D.M. Drafting the manuscript, C.T.M., B.S.P., D.F.K., A.V., E.R.S., R.S.T., and C.D.M.
Originally Published in Press as DOI: 10.1164/rccm.201805-1011OC on December 7, 2018
Author disclosures are available with the text of this article at www.atsjournal.org
References
- 1.US Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General—Executive Summary. Atlanta, GA: Centers for Disease Control and Prevention; 2006. [PubMed] [Google Scholar]
- 2.Hayatbakhsh MR, Sadasivam S, Mamun AA, Najman JM, Williams GM, O’Callaghan MJ. Maternal smoking during and after pregnancy and lung function in early adulthood: a prospective study. Thorax. 2009;64:810–814. doi: 10.1136/thx.2009.116301. [DOI] [PubMed] [Google Scholar]
- 3.Best D Committee on Environmental Health; Committee on Native American Child Health; Committee on Adolescence. From the American Academy of Pediatrics: technical report: secondhand and prenatal tobacco smoke exposure. Pediatrics. 2009;124:e1017–e1044. doi: 10.1542/peds.2009-2120. [DOI] [PubMed] [Google Scholar]
- 4.Filion KB, Abenhaim HA, Mottillo S, Joseph L, Gervais A, O’Loughlin J, et al. The effect of smoking cessation counselling in pregnant women: a meta-analysis of randomised controlled trials. BJOG. 2011;118:1422–1428. doi: 10.1111/j.1471-0528.2011.03065.x. [DOI] [PubMed] [Google Scholar]
- 5.Schneider S, Huy C, Schütz J, Diehl K. Smoking cessation during pregnancy: a systematic literature review. Drug Alcohol Rev. 2010;29:81–90. doi: 10.1111/j.1465-3362.2009.00098.x. [DOI] [PubMed] [Google Scholar]
- 6.Tong VT, Dietz PM, Morrow B, D’Angelo DV, Farr SL, Rockhill KM, et al. Centers for Disease Control and Prevention (CDC) Trends in smoking before, during, and after pregnancy: Risk Assessment Monitoring System, United States, 40 sites, 2000-2010. MMWR Surveill Summ. 2013;62:1–19. [PubMed] [Google Scholar]
- 7.Stoddard JJ, Gray B. Maternal smoking and medical expenditures for childhood respiratory illness. Am J Public Health. 1997;87:205–209. doi: 10.2105/ajph.87.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEvoy CT, Schilling D, Clay N, Jackson K, Go MD, Spitale P, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014;311:2074–2082. doi: 10.1001/jama.2014.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bierut LJ. Convergence of genetic findings for nicotine dependence and smoking related diseases with chromosome 15q24-25. Trends Pharmacol Sci. 2010;31:46–51. doi: 10.1016/j.tips.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen LS, Baker T, Hung RJ, Horton A, Culverhouse R, Hartz S, et al. Genetic risk can be decreased: quitting smoking decreases and delays lung cancer for smokers with high and low CHRNA5 risk genotypes. A meta-analysis. EBioMedicine. 2016;11:219–226. doi: 10.1016/j.ebiom.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekhon HS, Jia Y, Raab R, Kuryatov A, Pankow JF, Whitsett JA, et al. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest. 1999;103:637–647. doi: 10.1172/JCI5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekhon HS, Keller JA, Benowitz NL, Spindel ER. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med. 2001;164:989–994. doi: 10.1164/ajrccm.164.6.2011097. [DOI] [PubMed] [Google Scholar]
- 13.Sekhon HS, Keller JA, Proskocil BJ, Martin EL, Spindel ER. Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung. Association with alpha7 nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol. 2002;26:31–41. doi: 10.1165/ajrcmb.26.1.4170. [DOI] [PubMed] [Google Scholar]
- 14.Proskocil BJ, Sekhon HS, Clark JA, Lupo SL, Jia Y, Hull WM, et al. Vitamin C prevents the effects of prenatal nicotine on pulmonary function in newborn monkeys. Am J Respir Crit Care Med. 2005;171:1032–1039. doi: 10.1164/rccm.200408-1029OC. [DOI] [PubMed] [Google Scholar]
- 15.Tager IB, Hanrahan JP, Tosteson TD, Castile RG, Brown RW, Weiss ST, et al. Lung function, pre- and post-natal smoke exposure, and wheezing in the first year of life. Am Rev Respir Dis. 1993;147:811–817. doi: 10.1164/ajrccm/147.4.811. [DOI] [PubMed] [Google Scholar]
- 16.Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med. 1988;319:1112–1117. doi: 10.1056/NEJM198810273191702. [DOI] [PubMed] [Google Scholar]
- 17.Dezateux C, Stocks J, Dundas I, Fletcher ME. Impaired airway function and wheezing in infancy: the influence of maternal smoking and a genetic predisposition to asthma. Am J Respir Crit Care Med. 1999;159:403–410. doi: 10.1164/ajrccm.159.2.9712029. [DOI] [PubMed] [Google Scholar]
- 18.Jones MH, Davis SD, Grant D, Christoph K, Kisling J, Tepper RS. Forced expiratory maneuvers in very young children. Assessment of flow limitation. Am J Respir Crit Care Med. 1999;159:791–795. doi: 10.1164/ajrccm.159.3.9803001. [DOI] [PubMed] [Google Scholar]
- 19.Martinez FD. The origins of asthma and chronic obstructive pulmonary disease in early life. Proc Am Thorac Soc. 2009;6:272–277. doi: 10.1513/pats.200808-092RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McEvoy CT, Milner KF, Schilling DG, Scherman A, Tiller A, Vuylsteke B, et al. Improved forced expiratory flows in infants of pregnant smokers randomized to daily vitamin C versus placebo [abstract] Am J Respir Crit Care Med. 2018;197:A4192. [Google Scholar]
- 21.McEvoy CT, Milner KF, Scherman AJ, Schilling DG, Tiller CJ, Vuylsteke B, et al. Vitamin C to Decrease the Effects of Smoking in Pregnancy on Infant Lung Function (VCSIP): rationale, design, and methods of a randomized, controlled trial of vitamin C supplementation in pregnancy for the primary prevention of effects of in utero tobacco smoke exposure on infant lung function and respiratory health. Contemp Clin Trials. 2017;58:66–77. doi: 10.1016/j.cct.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 23.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 24.American Thoracic Society; European Respiratory Society. ATS/ERS statement: raised volume forced expirations in infants: guidelines for current practice. Am J Respir Crit Care Med. 2005;172:1463–1471. doi: 10.1164/rccm.200408-1141ST. [DOI] [PubMed] [Google Scholar]
- 25.Frei B. Efficacy of dietary antioxidants to prevent oxidative damage and inhibit chronic disease. J Nutr. 2004;134:3196S–3198S. doi: 10.1093/jn/134.11.3196S. [DOI] [PubMed] [Google Scholar]
- 26.Benowitz NL, Hukkanen J, Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones M, Castile R, Davis S, Kisling J, Filbrun D, Flucke R, et al. Forced expiratory flows and volumes in infants. Normative data and lung growth. Am J Respir Crit Care Med. 2000;161:353–359. doi: 10.1164/ajrccm.161.2.9903026. [DOI] [PubMed] [Google Scholar]
- 28.Lum S, Bountziouka V, Wade A, Hoo AF, Kirkby J, Moreno-Galdo A, et al. New reference ranges for interpreting forced expiratory manoeuvres in infants and implications for clinical interpretation: a multicentre collaboration. Thorax. 2016;71:276–283. doi: 10.1136/thoraxjnl-2015-207278. [DOI] [PubMed] [Google Scholar]
- 29.Hanrahan JP, Tager IB, Segal MR, Tosteson TD, Castile RG, Van Vunakis H, et al. The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis. 1992;145:1129–1135. doi: 10.1164/ajrccm/145.5.1129. [DOI] [PubMed] [Google Scholar]
- 30.Hoo AF, Henschen M, Dezateux C, Costeloe K, Stocks J. Respiratory function among preterm infants whose mothers smoked during pregnancy. Am J Respir Crit Care Med. 1998;158:700–705. doi: 10.1164/ajrccm.158.3.9711057. [DOI] [PubMed] [Google Scholar]
- 31.Tager IB, Weiss ST, Muñoz A, Rosner B, Speizer FE. Longitudinal study of the effects of maternal smoking on pulmonary function in children. N Engl J Med. 1983;309:699–703. doi: 10.1056/NEJM198309223091204. [DOI] [PubMed] [Google Scholar]
- 32.Jones MH, Howard J, Davis S, Kisling J, Tepper RS. Sensitivity of spirometric measurements to detect airway obstruction in infants. Am J Respir Crit Care Med. 2003;167:1283–1286. doi: 10.1164/rccm.200204-339OC. [DOI] [PubMed] [Google Scholar]
- 33.Moshammer H, Hoek G, Luttmann-Gibson H, Neuberger MA, Antova T, Gehring U, et al. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med. 2006;173:1255–1263. doi: 10.1164/rccm.200510-1552OC. [DOI] [PubMed] [Google Scholar]
- 34.Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES) Am J Clin Nutr. 2009;90:1252–1263. doi: 10.3945/ajcn.2008.27016. [DOI] [PubMed] [Google Scholar]
- 35.Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest. 2013;144:266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 36.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA. 2016;315:362–370. doi: 10.1001/jama.2015.18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manuck TA, Levy PT, Gyamfi-Bannerman C, Jobe AH, Blaisdell CJ. Prenatal and perinatal determinants of lung health and disease in early life: a National Heart, Lung, and Blood Institute workshop report. JAMA Pediatr. 2016;170:e154577. doi: 10.1001/jamapediatrics.2015.4577. [DOI] [PubMed] [Google Scholar]
- 38.Martinez FJ, Han MK, Allinson JP, Barr RG, Boucher RC, Calverley PMA, et al. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197:1540–1551. doi: 10.1164/rccm.201710-2028PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor B, Wadsworth J. Maternal smoking during pregnancy and lower respiratory tract illness in early life. Arch Dis Child. 1987;62:786–791. doi: 10.1136/adc.62.8.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129:735–744. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 41.Neuman Å, Hohmann C, Orsini N, Pershagen G, Eller E, Kjaer HF, et al. ENRIECO Consortium. Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med. 2012;186:1037–1043. doi: 10.1164/rccm.201203-0501OC. [DOI] [PubMed] [Google Scholar]
- 42.Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1:728–742. doi: 10.1016/S2213-2600(13)70118-8. [DOI] [PubMed] [Google Scholar]
- 43.Ten Have-Opbroek AA. The development of the lung in mammals: an analysis of concepts and findings. Am J Anat. 1981;162:201–219. doi: 10.1002/aja.1001620303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.