Abstract
Aim
To examine the relation between adiposity assessment methods (percentage body fat (%BF), BMI, and waist circumference (WC)) and individual metabolic risk factors (f-insulin, HDL cholesterol, triglycerides) and a combined measure of metabolic risk.
Methods
Crosssectional study of 300 males (BMI 20.8 ± 3.0 kg/m2) and females (BMI 21.3 ± 2.9 kg/m2) 17 years of age. F-insulin and components of the metabolic syndrome defined by the International Diabetes Federation (IDF) were used as metabolic risk indicators, with samples stratified into BMI, %BF, and WC groups, respectively. Diagnostic accuracy was expressed as the area under the ROC curve (AUC).
Results
In males, diagnostic accuracy for HDL and f-insulin was poor to fair for BMI (AUC 0.70, p = 0.001; 0.60, p = 0.22), WC (0.68, p = 0.003; 0.63, p = 0.11), and %BF (0.65, p = 0.009; 0.66, p = 0.04). The diagnostic accuracy for triglycerides was greater for all three measures (BMI 0.92, WC 0.95, %BF 0.87; all p < 0.001). For females, neither test performed better than chance for f-insulin and HDL, and only %BF performed better than chance for triglycerides (0.65, p = 0.08). All three measures exhibited higher accuracy for presence of ≥2 metabolic risk factors (AUCs 0.76-0.91, p < 0.001) in both sexes.
Conclusion
%BF was not superior to BMI and WC for detecting metabolic risk in the general adolescent population.
Keywords: Adolescents, Adiposity, Anthropometry, Body composition, Metabolic risk
Introduction
In Swedish adolescents, the prevalence of overweight has tripled and obesity has quintupled over the last 3 decades [1]. Parallel to the obesity epidemic is the increase in obesity-related complications, such as insulin resistance, type 2 diabetes, hypertension, and dyslipidemia [2,3]. Adverse metabolic traits already evident in childhood track into adulthood and predict future cardiovascular risk [4]. Thus, the detection of metabolic risk markers is important already in childhood and adolescence.
BMI is widely used for assessing overweight and obesity, but can neither distinguish between fat versus muscular components of body mass nor between central and peripheral fat distribution. Therefore, BMI may be less accurate than percentage body fat (%BF) and fat distribution measurements in detecting metabolic disturbances. While BMI, waist circumference (WC), waist-hip-ratio (WHR), and/or skinfolds have been evaluated for their diagnostic accuracy in identifying excess %BF in adolescents [5–9], the ability of these measures to predict metabolic risk factors is less well known [10]. Specifically, simple measures of overall (BMI) and central fatness (WC) have rarely been directly compared with %BF measured by using more sophisticated techniques. It would thus be of interest to know whether more advanced %BF measurements perform better in detecting metabolic risk factors than BMI and WC, and hence, whether a more expensive and time-consuming procedure still is worthwhile because of its superiority in measurement.
Therefore, the aim of this study was to examine whether %BF is a more accurate indicator of elevated fasting insulin (f-insulin), adverse lipid profile, and clustering of metabolic risk factors than BMI and WC in Swedish adolescents from the general population.
Participants and Methods
Participants were a subset of the offspring of 2,342 women who participated in the Stockholm Pregnancy and Weight Development Study in 1984–1985 [11]. The present 17-year follow-up, the Stockholm Weight Development Study (SWEDES), included 481 adolescents (279 females, 202 males) and their mothers [12]. Although the dropout was substantial over the 17 years of follow-up, it appeared to be non-differential regarding maternal BMI and birth weight of the children; the BMI of the pregnant women who were initially invited did not differ between participants and non-participants in SWEDES (21.7 ± 2.8 vs. 21.5 ± 2.8 kg/m2; p = 0.10). For the children, no significant difference in birth weight could be detected between participants and non-participants (3,465 ± 504 vs. 3,453 ± 563 g; p = 0.66). Dropout analysis has been performed in a previous publication [13].
From the original sample, body composition and blood lipid data were available for 300 adolescents (166 females, 134 males), whereas body composition and f-insulin data were available for a subset of this group consisting of 245 adolescents (134 females, 111 males). No significant differences in BMI, adiposity (%BF), or WC were seen for the 300 subjects with lipid data compared to the original sample. Also, no significant differences in adiposity measurements, total and HDL cholesterol, or triglycerides were found in the subgroup with data on f-insulin. The local Ethical Committee of Huddinge University Hospital approved the study. Written informed consent was obtained from each mother and verbal assent was ascertained from each adolescent.
Measurements
Weight was measured by the BodPod® Body Composition System (Life Measurement Instruments, Concord, CA, USA) to the nearest 0.1 kg, with the subjects dressed in light underwear. Standing height was measured to the nearest 0.5 cm against a wall-mounted stadiometer. BMI was determined as kg/m2, and the adolescents were classified as normal weight, overweight, or obese using the IOTF(International Obesity Taskforce)-recommended classification system developed by Cole et al. [14]. As there were relatively few obese participants as determined by BMI, overweight and obese individuals were grouped together for comparisons between normal weight and overweight/obese groups. WC was measured in duplicate at the minimum circumference between the iliac crest and the rib cage, with subjects standing dressed in underwear, and the mean of the 2 measurements was used. Investigators were trained to make measurements as highly standardized as possible as to minimize inter- and intra-investigator variability. Central obesity was defined as ≥94 cm for males and ≥80 cm for females according to the International Diabetes Federation (IDF) definition for adolescents aged ≥16 [15].
Body composition was measured by densitometry via air-displacement plethysmography measurements using the BodPod Body Composition System. All measurements were performed in an enclosed room without windows, where a constant environment could be kept. A series of repeated measurements were performed on phantoms of known volumes for the assessment of methodological error. 2 measurements were performed on each individual in the fasting state according to manufacturer's recommendations, while wearing tight-fitting underwear, or a swimsuit, and a swim cap [16,17]. A single procedure consisted of 2 measurements of body volume. If these differed by more than 150 ml, a 3rd measurement was performed. Predicted lung volume was used for the calculation of body volume, utilizing the algorithms provided by the manufacturer. Appropriate corrections for thoracic gas volume and skin surface area artefact were applied to this raw measurement to obtain actual body volume. The final result reported by the instrumentation was calculated from the average of the raw measurements or from the average of the 2 closest measurements when 3 measurements were required. Data on body density were converted to %BF using the equation of Siri [18]. Participants were categorized into groups of normal, overfat, and obese according to published age- and sex-specific reference values [19].
Pubertal developmental stage was assessed by a medical doctor using Tanner criteria [20] during a visit to the research clinic. According to these criteria, 98% of the females and 73% of the males were post-pubertal (i.e. Tanner stage 5).
Venous blood was drawn into vacuum tubes, coagulated, centrifuged at room temperature, immediately frozen at −20 °C, and stored at −70 °C. Lipoproteins were isolated from fresh serum by a combination of preparative ultracentrifugation and precipitation with a sodium phosphotungstate and magnesium chloride solution. Serum lipoproteins were assayed by enzymatic techniques using a Monarch 2000 centrifugal analyzer (Instrumentation Laboratories, Lexington, MA, USA). Plasma glucose was determined using the glucose oxidase method on an automatic glucose analyzer. Plasma f-insulin was measured by an enzyme immunosorbent assay (ELISA) kit (Mercodia AB, Uppsala, Sweden) in a Bio-Rad Coda automated EIA analyzer (Bio-Rad Laboratories, Hercules, CA, USA).
Definitions of Selected Metabolic Risk Factors
As hyperinsulinemia has been shown to predict diabetes risk in adults as well as in children [21,22] and to correlate as well as fasting indices to insulin sensitivity [23], f-insulin was used instead of composite measures such as HOMA-IR or QUICKI. As there are no defined cut-off levels for this measure, sample-derived cut-offs were used, using the 85th percentile for each sex, in males being 9.52 µU/ml and in females 11.05 µU/ml. For the other metabolic risk factors, the IDF definitions for children and adolescents were used to determine cut-off levels [15]. A combined index of having at least 2 factors of the metabolic syndrome was also constructed.
Statistical Analyses
Statistical analyses were conducted using SPSS (version 13.0; SPSS Inc., Chicago, IL, USA) and STATA (version 9.0; StataCorp LP, College Station, TX, USA). Summary statistics used for central tendency and dispersion are means and standard deviations (SD), median, and range. Independent t-tests and ANOVA were used for the comparison of continuous data, while Pearson's Chi-square tests were used for categorical data. Normality of BMI, WC, %BF, HDL, f-insulin, glucose, and triglycerides was checked by comparison of mean and median values (table 1) and visual inspection of histograms.
To test the diagnostic accuracy of BMI, WC, and %BF in detecting adverse metabolic profiles, receiver-operating characteristics (ROC) analysis, which is a non-parametric technique, was performed. The area under the ROC curve (AUC) was used as measure of diagnostic accuracy as it incorporates the balance between sensitivity and specificity of the tests in question. Differences between ROC curves were investigated using ROCComp in STATA. Statistical significance was defined as p-values < 0.05.
Results
Individual characteristics are presented in table 1. The overall prevalence of overweight (including obesity) was 10.7% (males: 11.9%; females: 9.6%). However, according to published %BF reference values [19], approximately 30% of the sample were overfat or obese.
BMI did not differ between males and females, while males were heavier, taller, and had a larger WC, but had lower %BF (all p < 0.001). Females had significantly higher HDL and total cholesterol (TC) levels (p < 0.001) as well as higher f-insulin levels (p < 0.01), while plasma glucose was significantly higher in males than in females (p < 0.01). There were no significant differences in triglyceride levels.
Individual Risk Factors by Adiposity Group
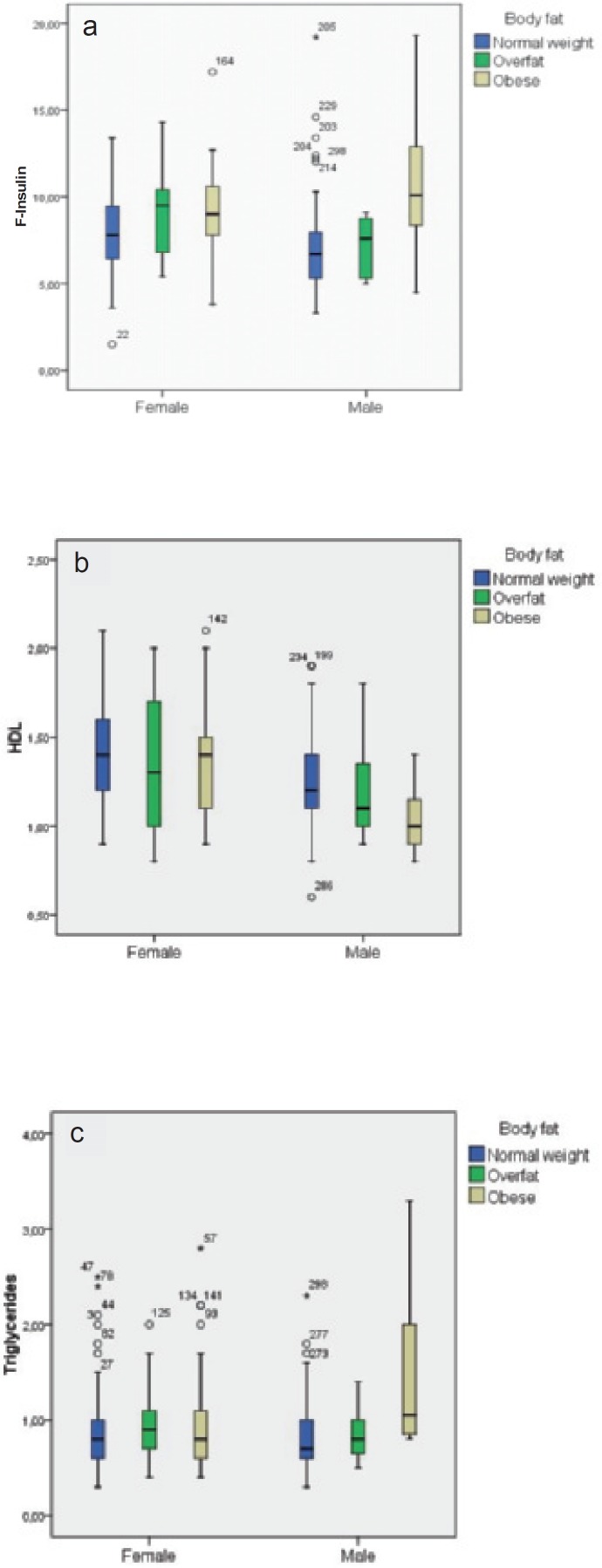
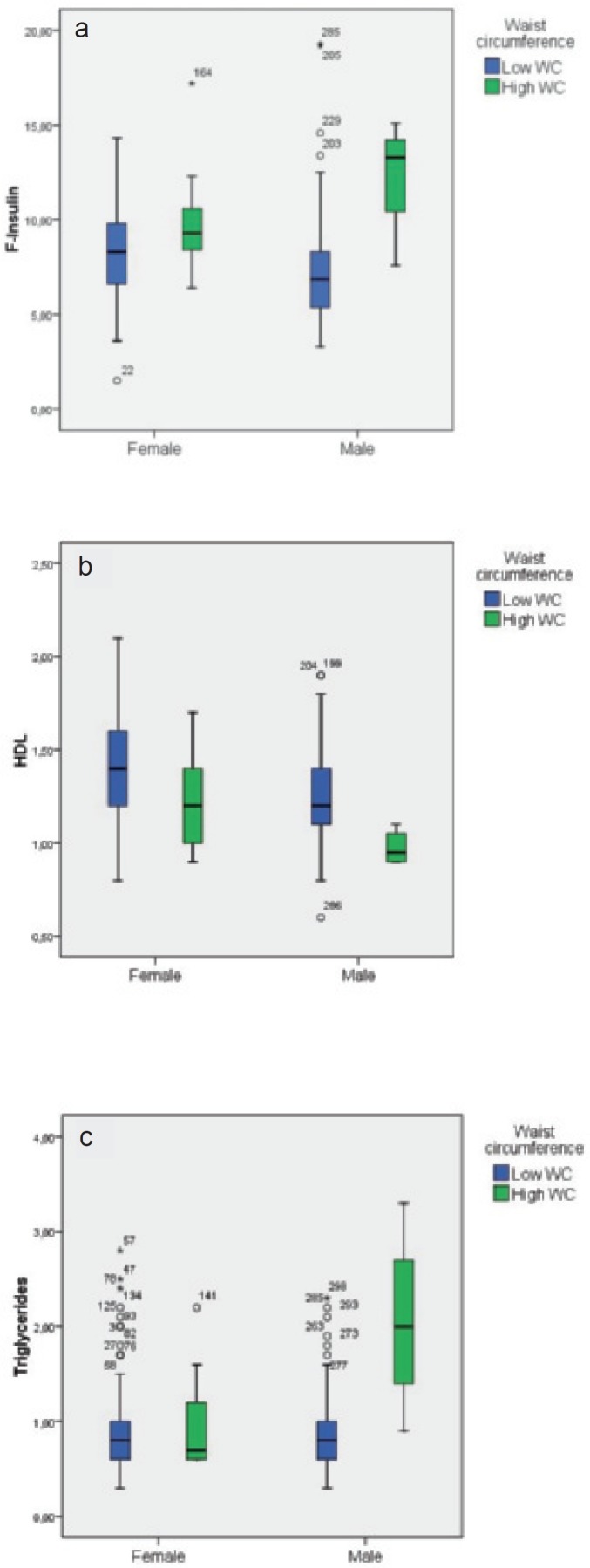
In figures 1–3, box plots are presented showing fasting insulin, HDL, and triglyceride levels stratified by sex and adiposity groups as determined by BMI, WC, and %BF, respectively. The male group with high WC was very small (n = 4), which precludes strong conclusions about this group. Compared to normal weight subjects, fasting insulin was significantly higher in overweight and obese females (p = 0.013) as determined by BMI. In overweight and obese males, HDL was significantly lower and triglycerides were significantly higher (p = 0.02 and p = 0.011, respectively).
In centrally obese females, fasting insulin was significantly higher (p = 0.022) and HDL significantly lower (p = 0.015). For centrally obese males, all three measures were significantly worse than in adolescent males without central obesity, but as stated above, this group was too small for relevant conclusions to be drawn.
Regarding obese females as determined by % BF, there were no significant differences between normal weight, overfat, and obese participants in the selected metabolic risk factors. For males, f-insulin was significantly higher in obese (p < 0.001) and overfat (p = 0.002) individuals. HDL was significantly lower in obese (p = 0.006) and triglycerides were significantly higher in overfat and obese as compared to normal weight males.
Table 2 shows the prevalence of individual factors of the IDF-defined pediatric metabolic syndrome [15] as well as the degree of clustering of risk factors. In this relatively lean sample, only 2.3% of males and 0.6% of females exhibited 3 combined risk factors, while 3.9% and 7.9% had at least 2. As both hypertension and high glucose levels were practically nonexistent, no participant displayed more than 3 risk factors.
ROC Analysis
In females, ROC analysis showed BMI, WC, and %BF to be poor or equal to chance in their ability to detect adverse levels of fasting insulin, HDL, and triglycerides (table 3). In males, these adiposity indices ranged from poor to fair in detecting higher f-insulin and low HDL as judged from AUCs, whereas the ability to find adverse triglyceride levels ranged from good to excellent (table 3).
The AUCs for BMI, WC, and %BF were of similar magnitude for both f-insulin and HDL and generally larger for triglycerides in both sexes. However, %BF did not perform significantly better than either BMI or WC for either measure of metabolic risk in males or females, with the one exception of triglycerides in females (p = 0.028). When using the combined index of ≥2 factors of the metabolic syndrome based on pediatric IDF criteria, generally high accuracy was found in both sexes and for all three measures of adiposity (table 3). For the combined index, there was no statistical difference between the three adiposity measures in diagnostic accuracy in either sex.
Discussion
In this study, we examined whether %BF measured by a sophisticated technique, i.e. air-displacement plethysmography, was superior to the simpler tests BMI and WC as indicators of adverse f-insulin and lipid profile, as well as clustering of these risk factors, in a sample of Swedish adolescents. In males, the three measures ranged from poor to fair in their diagnostic accuracy for high f-insulin and adverse HDL levels and from good to excellent in finding hypertriglyceridemia, but no measure was found to be superior. In females, %BF performed better than chance only in identifying adolescents with adverse levels of triglycerides, and in this case, %BF was significantly superior to the other two measures. Although all measures were generally weak in identifying individual risk factors, using a combined measure of metabolic risk showed higher accuracy. However, no measure was superior in finding the cluster of risk factors either.
Individual factors of the metabolic syndrome have been shown to track from childhood into adulthood [4,24], and the clustering of metabolic risk factors in childhood is known to convey an increased metabolic risk also in adulthood [25]. This warrants detecting metabolic disturbances already in childhood and adolescence, making early intervention possible and avoiding future adverse outcome. Evaluating the available tools for this purpose is thus important.
Many previous studies have evaluated BMI, WC, and/or skinfolds against %BF, i.e. in their ability to detect fatness [5–9, 26–28]. WC and BMI have been found to predict metabolic abnormalities equally well in children [27] and WC and waist-to-height-ratio to detect children at risk for metabolic and cardiovascular abnormalities [29]. Furthermore, WC has been proposed as a routine measurement to assess patients at risk for obesity-related disease [30]. Several of these studies have shown that although there is a strong correlation between BMI and %BF, specificity is generally high but sensitivity low or moderate for BMI to detect adverse adiposity levels. This has raised concerns about the misclassification of adiposity when using BMI: this may then lead to children with higher metabolic risk to remain undetected. However, few previous studies have compared %BF with other measures of adiposity as predictors of an adverse metabolic profile [10, 26–28], although early cut-offs for %BF were derived by linkage to overrepresentation of metabolic risk factors [31,32]. In post-pubertal Asian Indian children, Misra et al. [32] found that the odds ratios of hyperinsulinemia were 4.7 in overweight children, 8.0 in high %BF, 6.4 in high WC, 3.7 in high WHR, 6.8 with high triceps skinfold thickness, 8.0 with high subscapular skinfold thickness, and 10.1 with high sum of 4 skinfold thicknesses. %BF and sum of 4 skinfold thicknesses were independent predictors of hyperinsulinemia in that study. In adults, similar results to the present study have been found, with %BF showing no clear advantage to BMI and WC in predicting obesity-related metabolic risk [33].
BMI is commonly criticized for its limitation of not being able to distinguish between fat and fat-free mass as well as not providing any information on body fat distribution, which may limit its usefulness as a predictor of metabolic risk [30]. In this sample of fairly lean Swedish adolescents, %BF did not differ significantly from either BMI or WC in diagnostic accuracy for the detection of high triglyceride levels, except in females. In adolescent males, detailed body composition measurements may at best provide some additional information over and above anthropometry when predicting higher f-insulin and HDL profile, but the difference in predictive power between methods seems small in both sexes. However, adverse blood profiles related to overweight and obesity may not have developed fully in this sample, and %BF may still have predictive properties for future complications among individuals with long-standing overweight or obesity. This remains to be investigated.
We did not observe any associations between the adiposity measures and the investigated selected metabolic risk factors in adolescent females, except for triglycerides (only for %BF). For a given BMI, there was a wide variation in %BF, which could explain a low explanatory power for BMI, but not for %BF. Neither BMI nor total %BF describes the regional fat distribution, which could explain why both of these measures could be weak predictors, but WC, which estimates central obesity, did not have any significant explanatory power in females either. The reasons for the poor correlations in females are therefore not clear. However, similarly to the present study, previous studies have shown sex differences in correlations of anthropometric indices to metabolic risk factors, with stronger correlations in males than in females [26,28].
The strengths of this study were the relatively homogenous sample with regard to age and ethnicity, the availability of detailed body composition measured by densitometry as well as the collection of fasting blood samples. We could hereby evaluate detailed body composition and simple screening measures against single and clustered metabolic risk factors.
The study also had several limitations. Firstly, contrary to imaging techniques and DXA, air-displacement plethysmography does not enable analyses of regional body composition. These techniques are clearly superior to WC which we used as proxy measure for central fatness. However, these advanced imaging techniques have no potential of becoming field methods, while WC is commonly used in the clinic. For measures of total fatness, densitometry has been shown to provide estimates of similar accuracy as dual-energy X-ray absorptiometry (DXA) and hydrostatic weighing [17]. The use of densitometry for measuring body composition is superior to skinfolds and bioelectrical impedance measurements but may be regarded as inferior to imaging techniques and DXA, since these technologies provide information on regional fat stores. In this study, however, %BF estimates from densitometry did not perform better than BMI or WC as diagnostic tests of high insulin levels, adverse lipid profile, or clustering of metabolic risk factors.
Secondly, there are no generally accepted cut-off values for the metabolic risk factors we have used. On the contrary, different definitions of metabolic risk and varying cut-offs are recommended by different authors and institutions [15,34,35]. We have chosen cut-offs determined by the IDF when applicable but sample-derived cut-offs for f-insulin as there are no available reference levels for this measure. Until standardized criteria exist, this approach seems most reasonable.
Thirdly, the sample was drawn from an urban adolescent population in Sweden, which in both a national and international perspective is not heavily afflicted by obesity [1,36,37]. Therefore, there is a risk that the sample is not representative of the general population. Based on BMI, there were few overweight and obese participants in the study (overweight/obese males and females 10.4/1.5% and 7.2/2.4%, respectively). However, according to the %BF cut-offs recommended by McCarthy et al. [19], 9 and 12% of the males were overfat and obese, respectively, and in females the corresponding percentages were 17.5 and 20.5%. This inconsistency between BMI and %BF has previously been documented [9,38]. The sample was similar with respect to mean BMI (21.1 vs. 21.0 and 21.5 vs. 21.1 kg/m2 for males and females, respectively), WC (75.4 vs. 74.5 and 71.4 vs. 69.9 cm), and %BF (16.2 vs. 17.3 and 29.4 vs. 27.3) compared to the participants in a large (n = 3,142) population-based study on adolescents in Stockholm, COMPASS, with data collected in 2000–2002 [39]. When using the WC cut-offs defined by the IDF, few were defined as having adverse levels, especially among the males. However, when using proposed WC cut-offs for overweight from a Dutch population [40] (which is a population with similar prevalence of overweight and obesity as Sweden), a greater proportion of participants in this study were classified as having an adverse WC (12% of males and 13% of females). Our findings may support the use of simpler anthropometric measures used for general screening of metabolic risk in school health care and primary care settings. However, BMI, WC, and %BF may perform differently in more obese populations, such as those encountered in specialist clinics or countries with a higher prevalence of obesity. Congruent with our findings, however, the AUCs for both BMI and DXA-derived %BF as screening tests for f-insulin resistance were between 0.6 and 0.7 in obese Swedish children seeking specialized care for their obesity (Rossner et al., unpublished data).
In summary, the associations between measures of adiposity, f-insulin, and lipid profile were found to be stronger in adolescent males than females. The performance of %BF in detecting metabolic risk factors was not significantly superior to the simpler measures BMI and WC, with the exception of hypertriglyceridemia in females. Overall, all three tests did not perform better than being at best poor to fair in detecting adverse metabolic profiles, except for males, where all three anthropometric assessments detected high triglycerides with high accuracy. When grouping individual risk factors together, higher diagnostic accuracy was found. However, the results suggest that there does not appear to be any major advantage of substituting simple BMI and WC measures with more detailed assessments of body fat when detecting individuals with elevated metabolic risk.
Disclosure
None of the authors hold any financial disclosures or conflicts of interest concerning this manuscript.
Fig. 1.
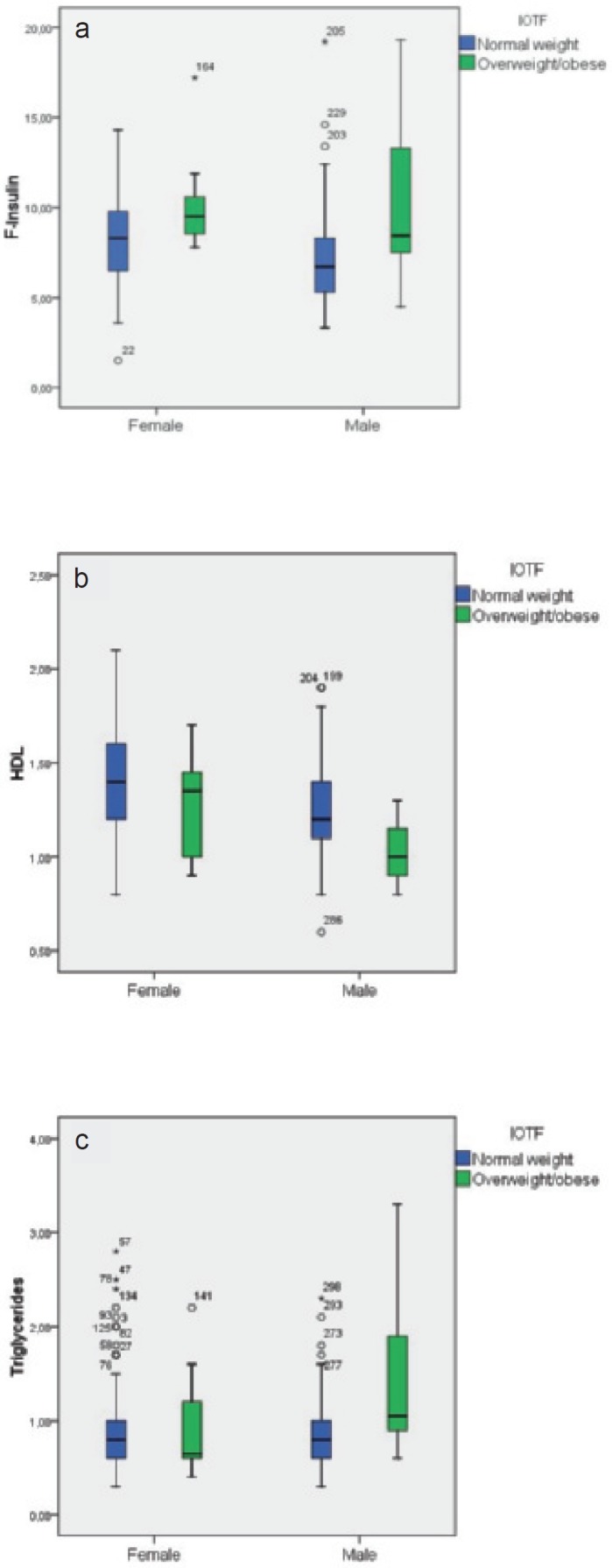
Box plots of a f-insulin, b HDL cholesterol, and c triglycerides stratified by sex and BMI groups (BMI reference proposed by Cole et al. [14]).
Fig. 2.
Box plots of a f-insulin, b HDL cholesterol, and c triglycerides stratified by sex and %BF groups (%BF reference proposed by McCarthy et al. [19]).
Fig. 3.
Box plots of a f-insulin, b HDL cholesterol, and c triglycerides stratified by sex and waist circumference groups (IDF definition, Zimmet et al. [15]).
Table 1.
Subject characteristics (mean ± SD, median, minimum–maximum); body composition measured by air-displacement plethysmography
| Male (n = 134) |
Female (n = 166) |
Total (n = 300) | |||||
|---|---|---|---|---|---|---|---|
| mean ± SD | median | Min–max | mean ± SD | median | Min–max | ||
| Age, yearsa | 16.9 ± 0.4 | 16.8 | 16.1–18.1 | 16.7 ± 0.4 | 16.7 | 15.9–17.6 | 16.8 ± 0.4 |
| Weight, kgb | 68.5 ± 11.7 | 66.0 | 47.6–107.2 | 59.5 ± 8.9 | 59.1 | 44.6–94.6 | 63.5 ± 11.2 |
| Height, mb | 1.8 ± 0.1 | 1.8 | 1.6–2.0 | 1.7 ± 0.1 | 1.7 | 1.5–1.8 | 1.7 ± 0.1 |
| BMI, kg/m2 | 20.8 ± 3.0 | 20.2 | 15.6–33.2 | 21.3 ± 2.9 | 20.7 | 16.7–33.6 | 21.1 ± 3.0 |
| Waist circumference, cmb | 75.0 ± 8.0 | 73.0 | 61.0–108.0 | 71.0 ± 7.0 | 70.0 | 58.0–100.0 | 73.0 ± 8.0 |
| %BFb | 16.0 ± 7.0 | 15.0 | 4.0–41.0 | 29.0 ± 7.0 | 29.0 | 11.0–54.0 | 23.0 ± 10.0 |
| Glucose, mmol/la, c | 4.9 ± 0.3 | 4.8 | 3.6–5.7 | 4.7 ± 0.4c | 4.7 | 3.6–6.0 | 4.8 ± 0.4 |
| F-insulin, µU/mla, c | 7.5 ± 2.9 | 6.9 | 3.3–19.3 | 8.5 ± 2.5 | 8.5 | 1.5–17.2 | 8.0 ± 2.7 |
| Total cholesterol, mmol/lb | 3.8 ± 0.6 | 3.9 | 2.6–5.5 | 4.2 ± 0.77 | 4.1 | 2.6–7.2 | 4.05 ± 0.72 |
| HDL, mmol/lb | 1.2 ± 0.3 | 1.2 | 0.6–1.9 | 1.4 ± 0.3 | 1.4 | 0.8–2.1 | 1.3 ± 0.3 |
| Triglycerides, mmol/l | 0.9 ± 0.4 | 0.8 | 0.3–3.3 | 0.9 ± 0.4 | 0.8 | 0.3–2.8 | 0.9 ± 0.4 |
| Systolic blood pressure, mm Hgd | 114.0 ± 9.0 | 115.0 | 90.0–135.0 | 108.0 ± 11.0 | 106.0 | 85.0–175.0 | 111.0 ± 11.0 |
| Diastolic blood pressure, mm Hgd | 64.0 ± 9.0 | 65.0 | 40.0–85.0 | 65.0 ± 9.0 | 65.0 | 40.0–110.0 | 65.0 ± 9.0 |
| % | % | % | |||||
|---|---|---|---|---|---|---|---|
| Overweight/obesity by BMI | 10.4/1.5 | 7.2/2.4 | 8.7/2.0 | ||||
| Overfat/obese, by % BFe | 9.0/11.9 | 17.5/20.5 | 13.7/16.7 | ||||
| Puberty passedb | 72.9 | 98.2 | 87.1 |
Significant gender difference; p < 0.01.
Significant gender difference; p < 0.001.
nmales = 111; nfemales = 134.
nmales = 128; nfemales = 165.
As defined by McCarthy et al. [19].
Table 2.
Components of the metabolic syndrome in adolescents (as defined by the International Diabetes Federation [15]); diagnosis requires central obesity plus presence of any 2 of the other 4 factors
| Components of the metabolic syndrome | Male (n = 134) | Female (n = 166) |
|---|---|---|
| Central obesitya | 4 (3%) | 17 (10.2%) |
| Triglyceridesb | 9 (6.7%) | 13 (7.8%) |
| HDLc | 33 (24.6%) | 54 (32.5%) |
| Blood pressure (BP)d | 1 (0.8%) | 1 (0.6%) |
| Glucosee | 1 (0.6%) | 3 (0.8%) |
| At least 1 of the above criteria | 27 (21.1%) | 59 (35.8%) |
| At least 2 of the above criteria | 5 (3.9%) | 13 (7.9%) |
| At least 3 of the above criteria | 3 (2.3%) | 1 (0.6%) |
Waist circumference ≥94 cm for males and ≥80 cm for females.
≥1.7 mmol/l.
<1.03 mmol/l in males and <1.29 mmol/l in females.
Systolic BP ≥130 or diastolic BP ≥85mm Hg.
Fasting glucose ≥5.6 mmol/l.
Table 3.
ROC analysis investigating BMI, WC, and %BF in predicting adverse f-insulin (defined as >85th percentile), adverse HDL cholesterol and triglycerides, and ≥2 factors of the metabolic syndrome (defined by IDF consensus report [15])
| F-insulin |
HDL cholesterol |
Triglycerides |
≥2 factors of the metabolic syndrome |
|||||
|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | p-value | AUC (95% CI) | p-value | AUC (95% CI) | p-value | AUC (95% CI) | p-value | |
| Male | ||||||||
| BMI | 0.60 (0.44–0.75) | 0.22 | 0.70 (0.60–0.80) | 0.001 | 0.92 (0.87–0.98) | <0.001 | 0.79 (0.63–0.96) | <0.001 |
| WC | 0.63 (0.48–0.82) | 0.11 | 0.68 (0.56–0.77) | 0.003 | 0.95 (0.91–0.99) | <0.001 | 0.82 (0.70–0.94) | <0.001 |
| %BF | 0.66 (0.49–0.82) | 0.04 | 0.65 (0.53–0.77) | 0.009 | 0.87 (0.77–0.98) | <0.001 | 0.81 (0.64–0.98) | <0.001 |
| Female | ||||||||
| BMI | 0.52 (0.38–0.66) | 0.79 | 0.58 (0.49–0.67) | 0.10 | 0.52 (0.37–0.66) | 0.85 | 0.88 (0.78–0.98) | <0.001 |
| WC | 0.52 (0.37–0.66) | 0.80 | 0.58 (0.48–0.67) | 0.11 | 0.58 (0.41–0.75) | 0.34 | 0.76 (0.56–0.97) | 0.01 |
| %BF | 0.54 (0.40–0.68) | 0.57 | 0.55 (0.46–0.64) | 0.32 | 0.65 (0.51–0.79) | 0.08 | 0.91 (0.82–1.00) | <0.001 |
Acknowledgements
The data collection phase of this study was funded by the European Commission, Quality of Life and Management of Living Resources, Key action 1 ‘Food, nutrition and health’ program as part of the project entitled ‘Dietary and genetic influences on susceptibility or resistance to weight gain on a high fat diet’ (QLK1-2000-00515). The analysis phase was funded by Arbetsmarknadens Forsakrings- och Aktiebolag (AFA).
Special thanks to Britta Barkeling, Catharina Grimming, Eva Hedlund, and Maria Saxer for the help and support in the study, and to the Unit for Preventive Nutrition, PrevNut, Karolinska Institutet, for the equipment support in form of the BodPod.
References
- 1.Neovius M, Rasmussen F. Place of residence and obesity in 1,578,694 young Swedish men between 1969 and 2005. Obesity (Silver Spring) 2008;16:671–676. doi: 10.1038/oby.2007.115. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 4.Berenson GS. Childhood risk factors predict adult risk associated with subclinical cardiovascular disease. The Bogalusa Heart Study. Am J Cardiol. 2002;90:3L–7L. doi: 10.1016/s0002-9149(02)02953-3. [DOI] [PubMed] [Google Scholar]
- 5.Reilly JJ, Dorosty AR, Emmett PM. Identification of the obese child adequacy of the body mass index for clinical practice and epidemiology. Int J Obes Relat Metab Disord. 2000;24:1623–1627. doi: 10.1038/sj.ijo.0801436. [DOI] [PubMed] [Google Scholar]
- 6.Sardinha LB, Going SB, Teixeira PJ, Lohman TG. Receiver operating characteristic analysis of body mass index, triceps skinfold thickness, and arm girth for obesity screening in children and adolescents. Am J Clin Nutr. 1999;70:1090–1095. doi: 10.1093/ajcn/70.6.1090. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann MB, Gubeli C, Puntener C, Molinari L. Detection of overweight and obesity in a national sample of 6–12-y-old Swiss children: accuracy and validity of reference values for body mass index from the US Centers for Disease Control and Prevention and the International Obesity Task Force. Am J Clin Nutr. 2004;79:838–843. doi: 10.1093/ajcn/79.5.838. [DOI] [PubMed] [Google Scholar]
- 8.Neovius M, Linné Y, Rossner S. BMI, waist-circumference and waist-hip-ratio as diagnostic tests for fatness in adolescents. Int J Obes Relat Metab Disord. 2005;29:163–169. doi: 10.1038/sj.ijo.0802867. [DOI] [PubMed] [Google Scholar]
- 9.Neovius MG, Linné YM, Barkeling BS, Rossner SO. Sensitivity and specificity of classification systems for fatness in adolescents. Am J Clin Nutr. 2004;80:597–603. doi: 10.1093/ajcn/80.3.597. [DOI] [PubMed] [Google Scholar]
- 10.Higgins PB, Gower BA, Hunter GR, Goran MI. Defining health-related obesity in prepubertal children. Obes Res. 2001;9:233–240. doi: 10.1038/oby.2001.27. [DOI] [PubMed] [Google Scholar]
- 11.Ohlin A, Rossner S. Maternal body weight development after pregnancy. Int J Obes. 1990;14:159–173. [PubMed] [Google Scholar]
- 12.Ekelund U, Ong K, Linné Y, et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults the Stockholm Weight Development Study (SWEDES) Am J Clin Nutr. 2006;83:324–330. doi: 10.1093/ajcn/83.2.324. [DOI] [PubMed] [Google Scholar]
- 13.Ekelund U, Ong KK, Linné Y, et al. Association of weight gain in infancy and early childhood with metabolic risk in young adults. J Clin Endocrinol Metab. 2007;92:98–103. doi: 10.1210/jc.2006-1071. [DOI] [PubMed] [Google Scholar]
- 14.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmet P, Alberti KG, Kaufman F, et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 16.Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27:1692–1697. [PubMed] [Google Scholar]
- 17.Fields DA, Goran MI, McCrory MA. Body-composition assessment via air-displacement plethysmography in adults and children a review. Am J Clin Nutr. 2002;75:453–467. doi: 10.1093/ajcn/75.3.453. [DOI] [PubMed] [Google Scholar]
- 18.Siri WE. Body composition from fluid spaces and density analysis of methods. 1961. Nutrition. 1993;9:480–491; discussion 480, 492. [PubMed] [Google Scholar]
- 19.McCarthy HD, Cole TJ, Fry T, Jebb SA, Prentice AM. Body fat reference curves for children. Int J Obes (Lond) 2006;30:598–602. doi: 10.1038/sj.ijo.0803232. [DOI] [PubMed] [Google Scholar]
- 20.Tanner JM. The measurement of maturity. Trans Eur Orthod Soc. 1975:45–60. [PubMed] [Google Scholar]
- 21.Lundgren H, Bengtsson C, Blohmé G, Lapidus L, Waldenström J. Fasting serum insulin concentration and early insulin response as risk determinants for developing diabetes. Diabet Med. 1990;7:407–413. doi: 10.1111/j.1464-5491.1990.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 22.McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Bennett PH, Knowler WC. Glucose, insulin concentrations and obesity in childhood and adolescence as predictors of NIDDM. Diabetologia. 1994;37:617–623. doi: 10.1007/BF00403382. [DOI] [PubMed] [Google Scholar]
- 23.Rössner SM, Neovius M, Montgomery SM, Marcus C, Norgren S. Alternative methods of insulin sensitivity assessment in obese children and adolescents. Diabetes Care. 2008;31:802–804. doi: 10.2337/dc07-1655. [DOI] [PubMed] [Google Scholar]
- 24.Lauer RM, Clarke WR, Mahoney LT, Witt J. Childhood predictors for high adult blood pressure. The Muscatine Study. Pediatr Clin North Am. 1993;40:23–40. doi: 10.1016/s0031-3955(16)38478-4. [DOI] [PubMed] [Google Scholar]
- 25.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Asayama K, Hayashibe H, Dobashi K, Uchida N, Kawada Y, Nakazawa S. Relationships between biochemical abnormalities and anthropometric indices of overweight, adiposity and body fat distribution in Japanese elementary school children. Int J Obes Relat Metab Disord. 1995;19:253–259. [PubMed] [Google Scholar]
- 27.Garnett SP, Baur LA, Srinivasan S, Lee JW, Cowell CT. Body mass index and waist circumference in midchildhood and adverse cardiovascular disease risk clustering in adolescence. Am J Clin Nutr. 2007;86:549–555. doi: 10.1093/ajcn/86.3.549. [DOI] [PubMed] [Google Scholar]
- 28.Misra A, Madhavan M, Vikram NK, Pandey RM, Dhingra V, Luthra K. Simple anthropometric measures identify fasting hyperinsulinemia and clustering of cardiovascular risk factors in Asian Indian adolescents. Metabolism. 2006;55:1569–1573. doi: 10.1016/j.metabol.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 29.Maffeis C, Banzato C, Talamini G. Waist-to-height ratio, a useful index to identify high metabolic risk in overweight children. J Pediatr. 2008;152:207–213. doi: 10.1016/j.jpeds.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy HD. Body fat measurements in children as predictors for the metabolic syndrome focus on waist circumference. Proc Nutr Soc. 2006;65:385–392. doi: 10.1017/s0029665106005143. [DOI] [PubMed] [Google Scholar]
- 31.Dwyer T, Blizzard CL. Defining obesity in children by biological endpoint rather than population distribution. Int J Obes Relat Metab Disord. 1996;20:472–480. [PubMed] [Google Scholar]
- 32.Williams DP, Going SB, Lohman TG, et al. Body fatness and risk for elevated blood pressure, total cholesterol, and serum lipoprotein ratios in children and adolescents. Am J Public Health. 1992;82:358–363. doi: 10.2105/ajph.82.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemmingsson E, Uddén J, Neovius M. No apparent progress in bioelectrical impedance accuracy: validation against metabolic risk and DXA. Obesity (Silver Spring) 2009;17:183–187. doi: 10.1038/oby.2008.474. [DOI] [PubMed] [Google Scholar]
- 34.Ford ES, Li C. Defining the metabolic syndrome in children and adolescents: will the real definition please stand up? J Pediatr. 2008;152:160–164. doi: 10.1016/j.jpeds.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 35.Reinehr T, de Sousa G, Toschke AM, Andler W. Comparison of metabolic syndrome prevalence using eight different definitions: a critical approach. Arch Dis Child. 2007;92:1067–1072. doi: 10.1136/adc.2006.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mårild S, Bondestam M, Bergström R, Ehnberg S, Hollsing A, Albertsson-Wikland K. Prevalence trends of obesity and overweight among 10-year-old children in western Sweden and relationship with parental body mass index. Acta Paediatr. 2004;93:1588–1595. doi: 10.1080/08035250410018265. [DOI] [PubMed] [Google Scholar]
- 37.Petersen S, Brulin C, Bergström E. Increasing prevalence of overweight in young schoolchildren in Umeå, Sweden, from 1986 to 2001. Acta Paediatr. 2003;92:848–853. doi: 10.1080/08035250310002957. [DOI] [PubMed] [Google Scholar]
- 38.Neovius M, Rasmussen F. Evaluation of BMI-based classification of adolescent overweight and obesity: choice of percentage body fat cutoffs exerts a large influence. The COMPASS study. Eur J Clin Nutr. 2008;62:1201–1207. doi: 10.1038/sj.ejcn.1602846. [DOI] [PubMed] [Google Scholar]
- 39.Rasmussen F, Eriksson M, Bokedal C, Schäfer Elinder L. Physical activity, eating habits, overweight and self-esteem among youth. COMPASS - a study in south-west Greater Stockholm (in Swedish). Center of Public Health, Stockholm County Council and National Institute of Public Health. Report 2004:1 [Google Scholar]
- 40.Fredriks AM, van Buuren S, Fekkes M, Verloove-Vanhorick SP, Wit JM. Are age references for waist circumference, hip circumference and waist-hip ratio in Dutch children useful in clinical practice? Eur J Pediatr. 2005;164:216–222. doi: 10.1007/s00431-004-1586-7. [DOI] [PubMed] [Google Scholar]