Abstract
It is hypothesized that intravenous (IV) sodium nitrite given during resuscitation of out of hospital cardiac arrest (OHCA) will improve survival. We performed a Phase 1 open-label clinical study of (IV) sodium nitrite given during resuscitation of 120 patents with OHCA from ventricular fibrillation (VF) or non-VF initial rhythms by Seattle Fire Department paramedics. A total of 59 patients received 25 mg (low) and 61 patients received 60 mg (high) of sodium nitrite during resuscitation from OHCA. Treatment effects were compared between high and low dose nitrite groups, and to all patients in a concurrent local EMS registry of OHCA. Whole blood nitrite levels were measured in 97 patients. The rate of ROSC (48% vs. 49%), re-arrest in the field (15% vs. 25%), use of norepinephrine (12% vs. 12%), first systolic blood pressure 124 ± 32 vs. 125 ± 38 mmHg), survival to discharge (23.7% vs. 16.4%) and neurologically favorable survival (18.6% vs. 11.5%) were not significantly different between the low and high nitrite groups. No significant differences in these outcomes were noted between patients who received IV nitrite as compared to concurrent registry controls. We estimate that 60 mg achieves whole blood nitrite levels between 22–38 μM 10 minutes after administration whereas 25 mg achieves a level between 9–16 μM 10 minutes after delivery. In conclusion, administration of IV nitrite is feasible and appears to be safe in patients with OHCA permitting subsequent evaluation of the effectiveness of IV nitrite for the treatment of OHCA.
Clinical Trial Registration:
ClinicalTrials.gov Identifier: NCT02987088
Introduction
Experimental animal evidence suggests that increasing nitric oxide (NO) bioavailability using pharmacologic or genetic means protects organs during ischemia-reperfusion.1 NO reduces inflammation, thrombosis, and production of reactive oxygen species (ROS), all biochemical processes which are involved in post-resuscitation injury. Importantly, NO production by the constitutively expressed isoforms of the enzyme, nitric oxide synthase (NOS 1 and 3) is inhibited during hypoxia2,3 and ischemia4–6 which also consume vascular and tissue stores of nitrite,7,8 a NOS independent reservoir of NO.9,10 These effects limit the bioavailability of NO and hence the body’s ability to protect organs from tissue damage during reperfusion. The nitrite anion (NO2-) is converted to NO during hypoxia or low pH, independent of NOS activity, making NO2- an ideal drug to increase NO bioavailability during ischemia and in reperfusion.9 Therapeutic delivery of nitrite during ischemia or at time of reperfusion is cytoprotective in animal models of cardiac arrest8,11,12 with optimal blood levels in cardiac arrest11 and other models of ischemia reperfusion injury12–15 reported as 10–20 μM in the early reperfusion period. In these reports, efficacy was maintained with nitrite levels up to 100 μM.13,15 Taken together these findings support the hypothesis that nitrite could be used as a therapy during resuscitation to reduce neurologic and cardiovascular injury and improve survival. To test this hypothesis in a clinical setting, we performed a phase 1 open-label clinical study of sodium nitrite given by paramedics during the active resuscitation of 120 patients with out-of-hospital cardiac arrest (OHCA). The major goal of this study was to determine safety of administering 25 or 60 mg IV sodium nitrite during resuscitation from OHCA and to determine whether potentially therapeutic levels of sodium nitrite of at least (10–20 μM) could be achieved with these doses within 10 minutes after administration.
Methods
The participating Emergency Medical Services (EMS) serves a population of nearly 700,000 residents in urban Seattle, WA, and responds to over 450 non-traumatic cardiac arrests annually utilizing a two-tiered response. First-tier responders (fire fighter-emergency medical technicians) are trained in high-performance cardiopulmonary resuscitation and are equipped with automated external defibrillators. Second-tier responders are paramedics who provide advanced cardiac life support including defibrillation, intubation and administration of resuscitation drugs.
Cardiac arrest was defined as being unconscious due to a sudden pulseless collapse and ROSC was defined as a return of a palpable pulse after cardiac arrest. The inclusion and exclusion criteria are detailed in Figure 1. Except for trauma, all causes of cardiac arrest were considered, including those with ventricular fibrillation or pulseless ventricular tachycardia (VF) or non-VF (defined as non-shockable rhythms) as the first recorded rhythm. Eligible patients received IV sodium nitrite after standard ACLS protocols were initiated (defibrillation, intubation, epinephrine), generally within the first several minutes after paramedic arrival.
Figure 1.
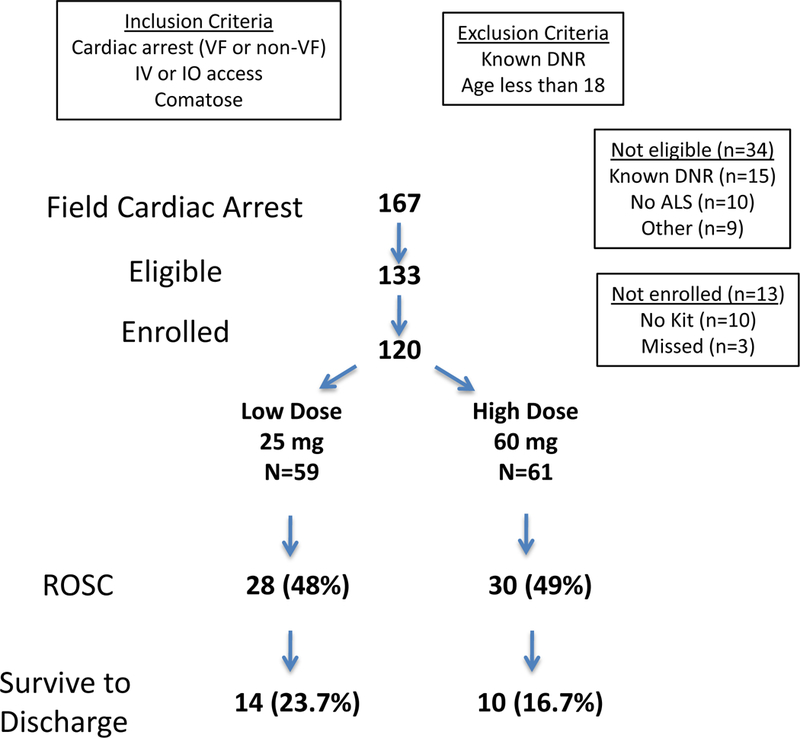
Study flow diagram describing the number of patients eligible, enrolled, and outcomes. Abbreviations: IV-intravenous, IO-intraosseous, ALS- Advanced life support, ROSC-return of spontaneous circulation, VF-ventricular fibrillation.
The 25 mg dose was chosen based on preliminary animal studies11 and a small pilot study in patients hospitalized after OHCA16. Since the present study was designed as a phase 1 study, the protocol was designed to allow for nitrite dose adjustment if necessary. After the first 30 patients were enrolled, it became clear that the 25 mg dose would not be sufficient to achieve a target level of at least 10–20 μM and pharmacokinetic modeling utilizing these measured nitrite levels suggested that a dose of 60 mg might be required. Upon review by an independent DSMB, the dose was increased to 60 mg. The first 59 enrolled patients received 25 mg IV and next 61 patients received 60 mg.
Paramedics transported patients to hospitals in the city of Seattle and provided information sheets describing the study to hospital providers. Paramedics obtained blood samples in patients at time of hospital arrival and the time of blood draw and time of nitrite dosing were recorded. In patients who did not achieve ROSC, paramedics obtained blood samples after ceasing resuscitation efforts. At one designated hospital, additional blood samples were obtained by emergency department staff within the first 2–4 h of hospitalization.
Blood was collected in nitrite preservation solution, frozen, then measured in batches of 10–15 samples using standardized methods.8 Samples were stored at -80°C and shipped overnight to the analytic lab on dry ice and whole blood nitrite levels were measured within one week. Standard pharmacokinetic (PK) analysis requires intensive sampling at early time points (e.g. 5–20 min post-dose) which could not be applied to our data set; we instead overlaid our measured whole blood nitrite data on predicted time exposure relationships based on high-resolution PK modeling of nitrite infusion in three lung transplant recipients (see Supplementary Material for complete details). Based on this study we were able to derive a best fit PK model for each subject (supplementary table 1 and figure 1) and to calculate relevant PK parameters using WinNonlin Phoenix 6.4 (Certara, Princeton, NJ) (supplementary Table 2)
We simulated the concentration-time profile as if each subject had received 25 mg or 60 mg IV nitrite as a bolus infused over 0.5 min (Figure 2 dotted lines). These models assumed that the sodium nitrite PK is linear and that OHCA subjects also follow a two-compartmental PK model with identical distribution phase kinetics as the lung transplant patients. Our purpose in doing so was to determine whether our measured values in OHCA subjects were within the range of the expected levels based on these PK models to inform dose selection.
Figure 2.
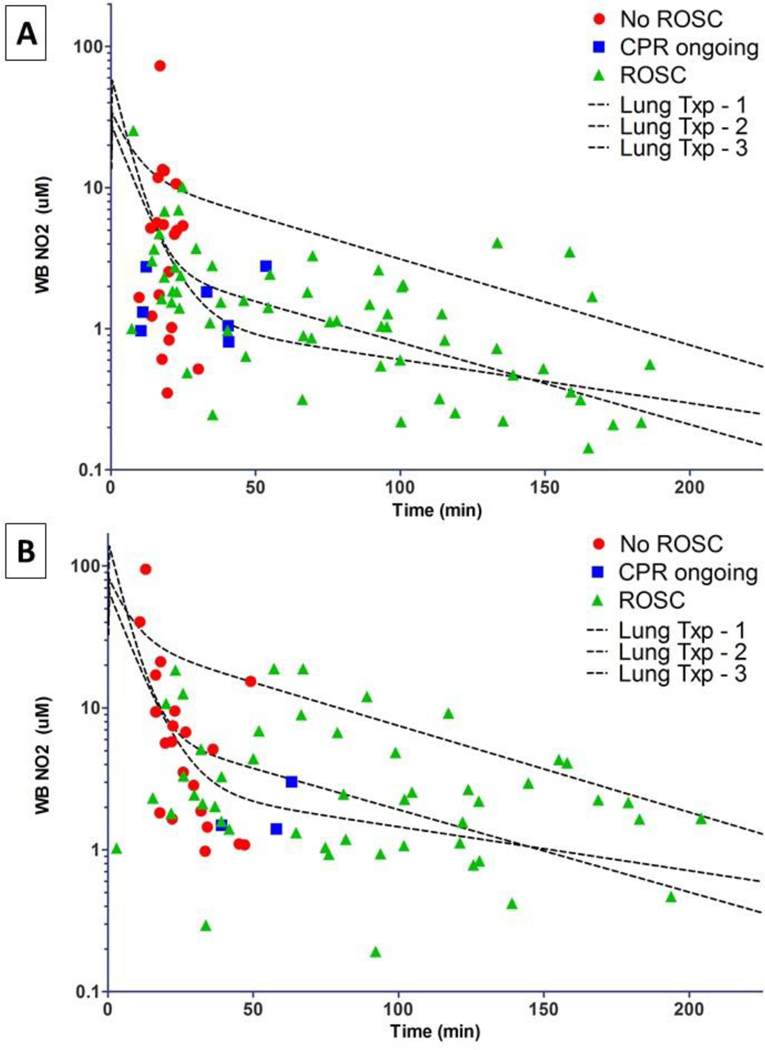
Complete whole blood nitrite levels after OHCA. The three black dotted overlay lines represent the predicted nitrite levels based upon PK parameters obtained in 3 lung transplant subjects with repeated sampling assuming a dose of 25 mg (A) or 60 mg (B) of nitrite given IV over 0.5 min. Overlaying these predicted ranges are the actual measured whole blood nitrite levels in units of μM based on the time the blood was obtained relative to the time of nitrite dosing (0 min) during CPR. The patients’ vital status at the time of blood draw is represented by the color/type of symbol used as shown in the key.
Primary safety outcomes included rate of ROSC, incidence of re-arrest, use of norepinephrine and first systolic blood pressure in the field. Data from standard run reports completed by paramedics were collected as previously described.17 Secondary outcomes focused on early hemodynamic effects from sodium nitrite (blood pressure and pressor usage), post cardiac arrest patient care characteristics (use of cooling, cardiac catherization, ICU days, troponins, and withdrawal of care) and status at hospital discharge.
The Seattle EMS maintains an ongoing prospective registry of all OHCA occurring within its jurisdiction. The registry contains demographic and other variables describing the circumstances of the event. These include age, sex, witness status, initial rhythm, ROSC, use of pressor agents and outcomes including survival and neurologic status at hospital discharge for those admitted to the hospital. For a comparison group, we included all patients who were in the registry between January 1, 2016 (11 months before the study began), and April 5, 2017 (the last day of study enrollment). We excluded registry patients who did not receive advanced life support (n=1), who awoke in the field (n=11), or were enrolled into the nitrite study. There were 355 individuals in the control or comparison group.
We planned a priori to enroll a minimum of 40 patients who achieved ROSC in the field and were transported to the ED. Safety analyses were carried out on the combined VF and non-VF groups. Using SPSS version 19.0 (SPSS Inc., Chicago IL), differences between the groups were analyzed with the Student’s t test (2 groups) or analysis of variance (3 groups) for continuous variables and the Chi-square statistic for categorical variables. Two-tailed tests were performed with alpha=0.05. Continuous values were presented as mean ± 1 standard deviation.
The trial was conducted with exception from informed consent under emergency research conditions in accordance with all applicable Federal regulations; Investigation New Drug (IND) application approved by the Federal Food and Drug Administration (FDA); approval by the Institutional Review Board (IRB) at the University of Washington and participating hospitals with oversight by an independent Data and Safety Monitoring Board (DSMB). Study personnel contacted the patient’s family as soon as feasible after enrollment to explain the study and seek written informed consent to review their medical records. Families of deceased patients were notified of their participation by mail.
Results
The study began on December 6, 2016 and the 120th patient was enrolled on April 5, 2017. During the enrollment period, one dose adjustment was made after approval from the DSMB and the higher dose was started on Jan 27, 2017.
A total of 59 patients received 25 mg IV nitrite and 61 received 60 mg during resuscitation of OHCA (Figure 1). All 120 patients had follow up data and no subjects withdrew before study completion. Whole blood nitrite levels were obtained in 97 subjects (81% of enrolled) and serial sampling was available from 41 patients (34%).
No statistically significant differences in patient characteristics were noted between the low and high dose nitrite-treated groups (Table 1). About 50% of patients who received nitrite (both high and low) during resuscitation by paramedics obtained ROSC (Table 2). Prehospital safety variables including rate of re-arrest, use of norepinephrine in the field, and first systolic blood pressures were not significantly different between the low and high nitrite group (Table 2). About half of the patients enrolled were transported to the hospital and hospital safety outcomes and hospital care characteristics were mostly similar between the low and high nitrite groups. Finally, no statistically significant difference in neurologic status or survival to discharge was noted between the two groups (Table 2).
Table 1:
Sodium nitrite dosing study-patient characteristics
| Characteristic | Low dose (n=59) |
High dose (n=61) |
|---|---|---|
| Age (years) | 60±17 | 64±17 |
| Men | 35 (59%) | 42 (69%) |
| Asystole | 29 (49%) | 26 (43%) |
| Pulseless Electrical Activity | 12 (20%) | 20 (33%) |
| Ventricular Fibrillation | 17 (29%) | 14 (23%) |
| Other | 1 (2%) | 1 (2%) |
| Time from call to first arrival (min) | 5.1±2.6 (n=59) |
4.4±1.4 (n=61) |
Table 2.
Sodium nitrite safety outcomes and early hospital outcomes
| Characteristic | Low dose (n=59) |
High dose (n=61) |
P |
|---|---|---|---|
| Any ROSC | 28 (48%) | 30 (49%) | 0.85 |
| Norepinephrine after study | 7 (12%) | 7 (12%) | 0.97 |
| Re-arrest in field (all) | 9 (15%) | 15 (25%) | 0.20 |
| Re-arrest in field (ROSC) | 9 (32%) | 15 (50%) | 0.17 |
| First systolic blood pressure | 124±32 (n=22) | 125±38 (n=22) | 0.93 |
| First systolic blood pressure in hospital | 116±33 (n=23) | 112±41 (n=19) | 0.75 |
| Transported to hospital | 34 (58%) | 32 (52%) | 0.57 |
| pH first arterial blood gas | 7.1±0.2 (n=30) | 7.1±0.2 (n=28) | 0.63 |
| Pressors in first 24 hours | 22/34 (65%) | 22/31 (71%) | 0.59 |
| Hospital cooling | 17/34 (50%) | 13/31 (42%) | 0.34 |
| First hospital temperature | 34.1±3.2 (n=30) | 35.2±1.8 (n=24) | 0.07 |
| Withdrawal of support | 13/34 (38%) | 6/29 (32%) | 0.13 |
| Re-arrest first 2 hours of hospitalization | 4/26 (15%) | 8/27 (30%) | 0.22 |
| ICU days | 7.3±8.3 (n=19) | 3.2±4.2 (n=16) | 0.15 |
| Positive troponin | 28 (48%) | 26 (43%) | 0.60 |
| Cardiac cath | 15/34 (44%) | 10/31(32%) | 0.33 |
| Percutaneous coronary intervention | 4/34 (12%) | 2/31 (6%) | 0.46 |
| Neurologic status | 0.54 | ||
| CPC 1 or 2 | 11 (19%) | 7 (12%) | |
| CPC 3 o4 | 3 (5%) | 3 (5%) | |
| Dead, other | 45 (76%) | 51 (84%) | |
| Survival to discharge | 14 (24%) | 10 (16%) | 0.32 |
We compared study participants to a registry-based cohort of 355 concurrent patients from all EMS-treated out-of-hospital cardiac arrests in Seattle during 2016 as a comparison (Table 3), who were not enrolled in this study. Demographic data were similar, however, the percentage of non-VF was higher in the high nitrite group compared to concurrent controls and low nitrite group. The time from call to first EMS arrival was longer in the low nitrite group. The rate of ROSC was lower in the nitrite groups than the control (close to 50% versus 60%) but these differences were not statistically significant; rates of field norepinephrine use were similar between the nitrite and concurrent control groups. The rates of re-arrest were not statistically significantly different between the low, high and concurrent control groups. Finally, no statistically significant differences in survival to discharge between the two nitrite groups and concurrent controls were observed.
Table 3:
Comparison of sodium nitrite groups with comparison group 2016–2017
| Characteristic | Sodium nitrite low dose (n=59) |
Sodium nitrite high dose (n=61) |
Comparison group (n=355) |
P-value |
|---|---|---|---|---|
| Age (years) | 60±17 | 64±17 | 62±17 | 0.31 |
| Men | 35 (59%) | 42 (69%) | 245 (69%) | 0.32 |
| Initial rhythm | 0.13 | |||
| Asystole | 29 (49%) | 26 (43%) | 136 (38%) | |
| Pulseless Electrical Activity | 12 (20%) | 20 (33%) | 106 (30%) | |
| Ventricular Fibrillation | 17 (29%) | 14 (23%) | 84 (24%) | |
| Other | 1 (2%) | 1 (2%) | 29 (8%) | |
| Witnessed arrest | 180 (51%) | 26 (43%) | 184 (50%) | 0.44 |
| Time from call to first arrival (min) | 5.1±2.6 | 4.4±1.4 | 4.4±21 | 0.037 |
| Any ROSC | 28 (48%) | 30 (49%) | 208 (59%) | 0.93 |
| Norepinephrine | 7 (12%) | 7 (12%) | 37 (10%) | 0.88 |
| Re-arrest in field (all cases) | 9 (15%) | 15 (25%) | 72 (20%) | 0.44 |
| Re-arrest in field (ROSC) | 9 (32%) | 15 (50%) | 72 (35%) | 0.23 |
| Survival to discharge (all) | 14 (24%) | 10 (16%) | 79 (22%) | 0.54 |
| Survival to discharge (VF) | 10 (59%) | 7 (50%) | 44 (52%) | 0.86 |
| Survival to discharge (Non VF) | 4 (10%) | 3 (6%) | 35 (13%) | 0.39 |
| Neurologic status at discharge | 0.55 | |||
| CPC 1 or 2 | 11 (19%) | 7 (12%) | 66 (19%) | |
| CPC 3 or 4 | 3 (5%) | 3 (5%) | 10 (3%) | |
| Dead, other | 45 (76%) | 51 (84%) | 279 (79%) |
Blood samples with data reflecting time of sampling relative to nitrite dosing was available on 52 subjects (n=11 without ROSC) who received 25 mg and 45 subjects (n=23 without ROSC) who received 60 mg. Whole blood nitrite levels in OHCA after 25 mg and 60 mg dosed during resuscitation are shown with ROSC/CPR status at the time of blood draw indicated (Figure 2A, 2B). This includes data from subjects in whom serial sampling was performed to capture later time points. Since paramedics obtained blood samples at hospital arrival (average time from EMS call to hospital arrival 41 ± 13 min), PK data from the early rapid distribution phase (5–10 minutes post-dose) were not available.
Overall, the nitrite levels obtained from subjects with ROSC (Figure 2; green triangles) fit reasonably well within the range of the predicted levels from our earlier PK modeling (Figure 2; dotted lines). There was a tendency for observed levels to be lower than predicted at the lower dose (25 mg; Figure 2A). In subjects who did not achieve ROSC (Figure 2; red dots) and who received either 25 or 60 mg, there was a wide range (over 100-fold) of nitrite blood levels. Based on our PK simulations from lung transplant subjects, we estimate that the 10-minute post-dose whole blood nitrite level would be between 22–38 μM in those receiving 60 mg IV and 9–16μM in those receiving 25 mg IV.
Discussion
The results from this prehospital phase 1 study suggest that sodium nitrite is not associated with significant adverse effects on rate of ROSC, field re-arrest rates, field use of norepinephrine, or first systolic blood pressure. We estimate that IV nitrite will achieve blood levels between 22–38 μM 10 minutes after a 60 mg dose or slightly lower than 9–16 μM 10 minutes after a 25 mg dose is delivered.
A major concern in using sodium nitrite for resuscitated cardiac arrest patients is the possibility of adverse hemodynamic effects since high doses of nitrite can cause hypotension. In rodent models of cardiac arrest, nitrite doses did not impact blood pressure.8,11,12 Human data on the effects of sodium nitrite during resuscitation are lacking; however, our group recently performed a small in-hospital study in which resuscitated OHCA patients received 1 or 9.6 mg IV sodium nitrite within 12 h after ROSC.16 We found no significant effect of these nitrite doses given in the post resuscitation phase on blood pressure or heart rate. Determining whether the administration of sodium nitrite during active resuscitation of OHCA is associated with significant acute effects on hemodynamics was the primary goal of this study.
In further exploratory analyses we compared the results of this phase 1 study with a concurrent registry cohort and found no statistically significant prehospital safety signal (rate of re-arrest, pressor use, rate of ROSC) even though the baseline characteristic of OHCA subjects enrolled into this study differed in that the concurrent control group had less non-VF (asystole). This finding is in agreement with other clinical studies in which sodium nitrite is given. In heart failure patients ~17.5 mg delivered over 5 min resulted in mean blood pressure reduction of only 4 mm Hg,18 and systemic administration of IV nitrite (~5 mg) over 5 min prior to coronary intervention was not associated with hypotension.19 It is noteworthy that the much higher doses we evaluated were well tolerated hemodynamically in a much sicker patient group.
Prior animal data in cardiac arrest11 and other models of ischemia reperfusion injury13,14 have demonstrated optimal nitrite mediated protection at blood levels of 10–20 μM.11,13–15 In vitro data in neuronal cell culture showed optimal neuroprotection at 10–40 μM.12 Nitrite blood levels over 150 μM, however, maybe associated with reduced neuroprotection or possibly harm.11,14 Based on these reports our goal was to provide a dose of nitrite, which would result in blood levels of at least 10–20 μM but lower than 100 μM after the initial clearance phase (i.e. 5–10 min after dosing).
Based on the fit between our measured values with PK modeling obtained from transplant patients and assumptions therein, we believe a nitrite dose in the range of 45–60 mg dose is most likely to produce 5–10 min post-dose levels of at least 10–20 μM, but less than 100 μM. These are our best estimates based on the following limitations.
First, it was not feasible to obtain blood samples within the first 10 minutes after nitrite dose administration since paramedics were actively involved in resuscitation protocols. Human PK studies during resuscitation are rare and difficult to perform20,21,22 and alternatively, have been carried out in the hospital setting11 (not in the prehospital environment). We believe that the data presented here represent the first attempt to characterize blood levels of a drug given during resuscitation in the field. Despite its limitations, these data are important to inform clinical trials since therapeutic efficacy of sodium nitrite might be diminished if a sufficient dose is not administered.
Second, PK modeling is challenging in critically ill patients, due to variable and time-sensitive changes in clearance and volume of distribution and PK associations in subjects without ROSC and during the post resuscitation period add an additional level of complexity and uncertainty. Nevertheless, there is a reasonably good correspondence between measured blood levels from OHCA patients with predicted levels obtained by PK modeling performed under more controlled circumstances in a critically ill lung transplant cohort, especially in OHCA patients who obtained ROSC in the field.
Thirdly, it is likely that nitrite levels are “consumed” during the post-resuscitation state, which adds to the complexity of PK modeling. Based on our estimates, over three times the dose of IV nitrite (60 mg vs. 17.5 mg) is required to achieve similar blood levels as those found in stable heart failure patients treated with nitrite.18 This observation is consistent with the experimental findings of whole body depletion of nitrite which result from global ischemia8,23 and also suggests that endogenous nitrite may be rapidly consumed during the early stages of global ischemia.
In conclusion, both doses of 25 or 60 mg IV sodium nitrite given during active resuscitation of OHCA are not associated with significant adverse effects on rate of ROSC, rate of re-arrest, and systolic blood pressure. Confirmation of safety and the efficacy and effectiveness of IV nitrite for the treatment of OHCA remains an important question.
Supplementary Material
Acknowledgements
Paramedics of Seattle Medic One
Data Safety Monitoring Committee: Chair: Susan Stern MD. Members: Kyra Becker, MD, Al Hallstrom, PhD, David Beiser, MD (University of Chicago), David Shavelle, MD (USC)
King County Medic One medical director: Tom Rea, MD
The following physicians helped with Human Subjects application at each medical center: Virginia Mason Medical Center: Anthony J. Gerbino, MD, Swedish Medical Center: Josh Buckler, MD
Database Entry and management: Regina LaVassaur, Lihua Yin
Source of Funding: National Heart Lung Blood Institute (NHLBI), NIH R01HL129722
Footnotes
Disclosures: None
References
- 1.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol 2006;40:16–23. [DOI] [PubMed] [Google Scholar]
- 2.Muramatsu K, Sheldon RA, Black SM, Tauber M, Ferriero DM. Nitric oxide synthase activity and inhibition after neonatal hypoxia ischemia in the mouse brain. Brain Res Dev Brain Res 2000;123:119–127. [DOI] [PubMed] [Google Scholar]
- 3.Fabian RH, Perez-Polo JR, Kent TA. Perivascular nitric oxide and superoxide in neonatal cerebral hypoxia-ischemia. American Journal of Physiology - Heart and Circulatory Physiology 2008;295:H1809–H1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iadecola C, Li J, Ebner TJ, Xu X. Nitric oxide contributes to functional hyperemia in cerebellar cortex. Am J Physiol 1995;268:R1153–1162. [DOI] [PubMed] [Google Scholar]
- 5.Elrod JW, Calvert JW, Gundewar S, Bryan NS, Lefer DJ. Nitric oxide promotes distant organ protection: evidence for an endocrine role of nitric oxide. Proc Natl Acad Sci U S A 2008;105:11430–11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serrano-Ponz M, Rodrigo-Gasque C, Siles E, Martinez-Lara E, Ochoa-Callejero L, Martinez A. Temporal profiles of blood pressure, circulating nitric oxide, and adrenomedullin as predictors of clinical outcome in acute ischemic stroke patients. Molecular medicine reports 2016;13:3724–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A 2007;104:19144–19149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP, Anderson SA, Chesley CF, Vanden Hoek TL, Gladwin MT. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation 2009;120:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 2008;7:156–167. [DOI] [PubMed] [Google Scholar]
- 10.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 2003;9:1498–1505. [DOI] [PubMed] [Google Scholar]
- 11.Dezfulian C, Alekseyenko A, Dave KR, Raval AP, Do R, Kim F, Perez-Pinzon MA. Nitrite Therapy is Neuroprotective and Safe in Cardiac Arrest Survivors. Nitric Oxide 2012;26:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dezfulian C, Kenny E, Lamade A, Misse A, Krehel N, St Croix C, Kelley EE, Jackson TC, Uray T, Rackley J, Kochanek PM, Clark RS, Bayir H. Mechanistic characterization of nitrite-mediated neuroprotection after experimental cardiac arrest. Journal of neurochemistry 2016;139:419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest 2005;115:1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, Park DK, Kim JM, Song EC, Kim M, Roh JK. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke 2006;37:2744–2750. [DOI] [PubMed] [Google Scholar]
- 15.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A 2004;101:13683–13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dezfulian C, Olsufka M, Fly D, Scruggs S, Do R, Maynard C, Nichol G, Kim F. Hemodynamic effects of IV sodium nitrite in hospitalized comatose survivors of out of hospital cardiac arrest. Resuscitation 2017;122:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim F, Nichol G, Maynard C, Hallstrom A, Kudenchuk PJ, Rea T, Copass MK, Carlbom D, Deem S, Longstreth WT Jr., Olsufka M, Cobb LA. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA 2014;311:45–52. [DOI] [PubMed] [Google Scholar]
- 18.Ormerod JOM, Arif S, Mukadam M, Evans JDW, Beadle R, Fernandez BO, Bonser RS, Feelisch M, Madhani M, Frenneaux MP. Short-Term Intravenous Sodium Nitrite Infusion Improves Cardiac and Pulmonary Hemodynamics in Heart Failure Patients. Circulation: Heart Failure 2015;8:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddiqi N, Neil C, Bruce M, MacLennan G, Cotton S, Papadopoulou S, Feelisch M, Bunce N, Lim PO, Hildick-Smith D, Horowitz J, Madhani M, Boon N, Dawson D, Kaski JC, Frenneaux M, investigators N. Intravenous sodium nitrite in acute ST-elevation myocardial infarction: a randomized controlled trial (NIAMI). European heart journal 2014;35:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barsan WG, Levy RC, Weir H. Lidocaine levels during CPR: differences after peripheral venous, central venous, and intracardiac injections. Ann Emerg Med 1981;10:73–78. [DOI] [PubMed] [Google Scholar]
- 21.McDonald JL. Serum lidocaine levels during cardiopulmonary resuscitation after intravenous and endotracheal administration. Crit Care Med 1985;13:914–915. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn GJ, White BC, Swetnam RE, Mumey JF, Rydesky MF, Tintinalli JE, Krome RL, Hoehner PJ. Peripheral vs central circulation times during CPR: a pilot study. Ann Emerg Med 1981;10:417–419. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem 2001;276:24482–24489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


