Abstract
Vector competence studies for West Nile virus (WNV) were conducted for two Culex (Culex) restuans Theobald populations Edison Park (EP) and Illinois Medical District (IMD), in Chicago, IL. The aim was to determine if there were differences between mosquito populations that contributed to the differences in WNV human cases between the two areas in 2002. Percentages of infected, disseminated and transmitting mosquitoes were estimated using a generalized linear mixed effects model including a random effect for family to account for anticipated within-family correlation. Analysis indicated that percentages of infected, disseminated and transmitting mosquitoes were not significantly different between EP and IMD (Infected, p=0.96; Disseminated, p=0.20; Transmitting, p=0.82). The within-family correlation was 0.46 (95% CI 0.28, 0.67), indicating reasonably strong tendency for WNV titers of bodies, saliva and legs within families to be similar. Overall, our results show that vector competence of Cx. restuans for WNV is not a contributing factor to the observed differences in WNV human cases between the EP and IMD areas of Chicago.
Keywords: Culex restuans, vector competence, WNV, Chicago
Introduction
Field observations and laboratory studies by a number investigators (Turell et al. 2000, Andreadis et al. 2001, Hadler et al. 2001, Turell et al. 2001, Sardelis et al. 2001, Andreadis et al. 2004, Kilpatrick et al. 2005, Ebel et al. 2005, Condotta et al. 2004, Andreadis and Armstrong 2007) support the hypothesis that Culex (Culex) restuans Theobald plays a significant role in the transmission dynamics of West Nile Virus (WNV) in eastern and mid-western United States. Since Cx. restuans populations typically peak in the spring and the early summer (Covell and Resh 1971, Reiter 1988, Geery and Holub 1989, Savage et al. 2007), the role played by this species is thought to be enzootic transmission of WNV among susceptible springtime birds. Studies by Lampman et al. (2006) reported that during the WNV outbreak in Illinois in 2002 there was an extended period of time when Cx. restuans and Culex (Culex) pipiens Linnaeus populations were equally abundant. However, during non-outbreak years, populations of Cx. restuans typically crash before the populations of Cx. pipiens explode, and the period of relatively equal population abundance for these two species is usually extremely short. This observation suggested that persistent Cx. restuans populations were associated with the WNV outbreak of 2002 in east central Illinois and probably elsewhere in the Midwest. In addition to springtime virus amplification, the fall activity of Cx. restuans populations (Reiter 1988) suggests that hibernating adults may be involved in the overwintering of WNV. Further, a study by Apperson et al. (2004) reported human blood meals detected in field-collected Cx. restuans from New Jersey, suggesting a possible limited role as a bridge vector for WNV.
In an effort to determine the biotic factors underlying the non-uniform distribution of WNV activity within the city of Chicago, we evaluated vector competence for a Cx. restuans population in an area where WNV human cases were high and compared that to another population in an area where WNV cases were low during the 2002 outbreak (Ruiz 2004). Since 2002, over 90% of the positive mosquito pools have been detected in areas where the human cases were clustered during the 2002 outbreak (unpublished data), suggesting that these areas are the foci for WNV transmission in Chicago. Geographic variations in vector competence have been described in many vector/arbovirus systems (Tesh et al. 1976, Hardy et al. 1976, Grimstad et al 1977, Tabachnik et al. 1985, Bennett et al 2002, Vaidynathan and Scott 2007) and we thought that variations in vector competence may play a role in the ecologic distribution of arbovirus activity. We hypothesized that differences in vector competence of Cx. restuans populations within two community areas, Edison Park (EP) and Illinois Medical District (IMD) (the Near West Side community area) in Chicago may contribute to the extreme variation in WNV activity observed between these two community areas. The climatic, environmental and demographic factors associated with the clustering of human cases in Chicago were investigated in detail by Ruiz et al. (2004) and Ruiz et al. (2007), but the corresponding biotic factors associated with the distribution of WNV activity in Chicago are currently not known.
Materials and Methods
Mosquito Strains.
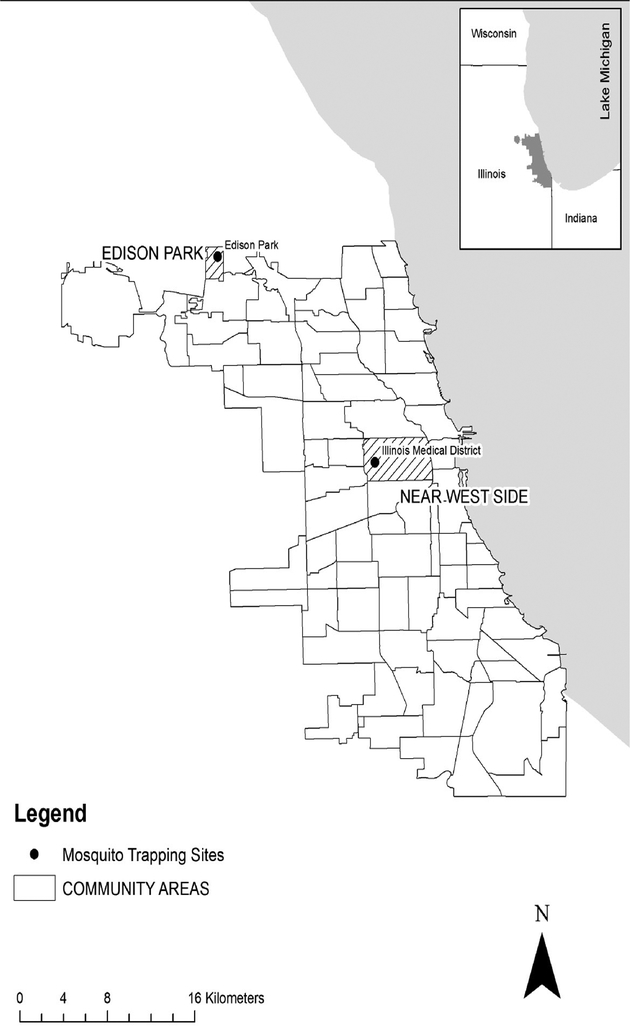
Two sites in Chicago were selected for mosquito collections on the basis of historic WNV human cases and positive mosquito pool data. Site 1, Edison Park (EP) (Fig. 1), is a residential community area on the north side of Chicago where the numbers of WNV cases and the numbers of positive mosquito pools have been consistently high since 2002. Site 2, Illinois Medical District (IMD) (Fig. 1), is a largely residential community area, called the Near West Side, approximately 3 miles west of downtown Chicago. Very few WNV positive mosquito pools have been detected in IMD and no human cases have been reported in this area. Mosquito egg rafts were collected in July and August 2008 and 2009 by using ovitraps, 12” × 18” dark blue plastic buckets baited with an infusion of water and fresh grass clippings (Lampman et al. 1996). Egg rafts were collected daily, placed individually into 2.0ml cryotubes on moist filter paper and shipped by overnight parcel to the CDC laboratory in Fort Collins.
Figure 1.
A map of community areas within the city of Chicago showing locations where Cx. restuans egg rafts were collected for the vector competence studies.
In the laboratory, each egg raft was hatched separately in 34.3 × 24.1 cm metal pans in 0.5 liters of de-ionized water. The larvae were fed on a finely ground mixture of 39.4% TetraMin® flakes (Tetra Holdings, Blacksburg, VA), 57.8% liver powder (MP Biomedicals, Solon, OH) and 8.9% brain/heart infusion (ICN Biomedicals, Aurora, OH) (Colton and Nasci 2006). Each pan received a total of 0.5 g, 0.2 g on day 0 and 0.3 g on day 2. Third instars were identified to species on basis of morphological characters using the keys of Darsie and Ward (2005). Adult mosquitoes were maintained on 5% sucrose solution and the sucrose solution was withheld 24 hrs prior to feeding the mosquitoes on a blood-virus suspension. A laboratory colony, Culex (Culex) quinquefasciatus Say Sebring strain, collected in 1988 from Harris County, Texas, was used as a control strain. Mosquito rearing was done in incubators at 28°C and 80% RH, and 16:8 h LD photoperiod.
Virus.
The WNV strain used, FCV15, was isolated from Culex (Culex) salinarius Coquillett mosquitoes collected on the southside of Chicago on 9/28/05. The virus was passaged twice in vero cells, and fresh unfrozen virus in Dulbecco’s Modified Eagle Medium (DMEM) (Mediatech, Inc., Manassas, VA) was used to infect mosquitoes in the transmission trials.
Transmission trials.
Five- to seven-day-old mosquitoes were deprived of sucrose for 24 hr and fed on a blood-virus suspension containing 108.6 PFU/ml of WNV by using a Hemotek membrane feeding system (Val Abbott Discovery Workshops, Lancs, England). Defibrinated chicken blood (Colorado Serum Company, Denver CO) was used and 3 millimolar ATP was added as a phago-stimulant and the mosquitoes were allowed to feed for 3 h. Each mosquito family raised from a single egg raft was placed in a separate cage and incubated for 15 days at 28°C, 80% RH and a 16:8 h (light:dark) cycle simulating long day photoperiod, and given ad libitum access to a diet of 5% sucrose. Blood-virus suspension samples were collected before and immediately after feeding and stored at −80°C for later virus titer assay.
At day 15 post infection, mosquitoes were sedated with triethylamine (Thermo Fisher Scientific Inc., Pittsburg, PA) allowed to salivate individually in mineral oil-filled micro tubes, immediately frozen at −80°C and later assayed for presence of WNV using real-time RT-PCR using the methods described by Lanciotti et al. (2000). We evaluated vector competence by computing infection rates (percentage of fully engorged mosquitoes with a midgut infection), dissemination rates (percentage of fully engorged mosquitoes with detectable virus in the legs) and transmission rates (percentage of fully engorged mosquitoes with detectable virus in the saliva) for each family.
Comparisons by collection site and blood meal volume.
Blood meal volume was categorized into, 1) fully engorged, abdomen fully distended by the blood meal, 2) partially engorged, abdomen partially distended, and 3) trace, barely visible blood in the midgut within no discernable distention of the abdomen, stages 5, 3 and 4, and 0 to 2 of Pillit and Jones (1972) respectively. We compared the mean body virus log-titers by site and blood meal volume using a linear mixed effects model (Pinheiro and Bates, 2000). These computations were performed in the statistical software package R (Vienna, Austria). Site, with levels EP and IMD, and blood meal volume, with levels full, partial and trace, were included in the models as a fixed effects, along with their interaction. Because individuals from the same family were reared and included in the experiment, we anticipated that within-family titers might be similar. To adjust for this anticipated correlation, a random effects term for family was included. We evaluated the possibility that the within-family, random effects variances differed by site, that the variances were the same by site, and that a random effects term was not needed (i.e., that the within-family variance was equal to 0). With all fixed effects included, models were fitted using restricted maximum likelihood (REML) and their variances compared using the restricted likelihood ratio test (RLRT). To test whether the random effect term’s variance was positive, we used the methods of Scheipl et al. (2008) to compute the p-value for the RLRT, implemented in the R package RLRsim (www.r-project.org). To test whether the random effects variances differed by site or blood meal volume, the RLRT p-value was computed using the standard chi-squared approximation. Using the variance structure thus determined, fixed effects were compared using the likelihood ratio test (LRT) after fitting with maximum likelihood (ML). The final model was then fitted using REML. All comparisons used 5% statistical significance. Ninety-five percent confidence intervals (CI) on all parameters were computed based on the normal approximation to the distributions of the REML estimators, as implemented in the R package nlme; see Pinheiro and Bates (2000) for details. To evaluate the variance structure, the linear mixed model including fixed effects for site and blood meal volume and their interaction were used.
The intraclass or within-family correlation coefficient was estimated as a measure of the strength of the association of within-family titer values. Ninety-five percent CIs for the ratio of the within-family variance to the residual variance and for the within-family correlation were computed using the method recommended in Burdick et al. (2005). Standard diagnostics were used to assess model fit, including evaluation of residual plots and of normality of predicted random effects.
We compared the proportions of mosquitoes with RT-PCR-positive legs using a similar modeling strategy. We used a generalized linear mixed model with binomial error and logit link to model the proportion of positive legs, fitted using the lme4 package in R. As above, a random effect was included for family.
Finally, because saliva titers were 0 for many individuals, we analyzed saliva titers by using a mixture distribution, with a component for a titer of 0 and one in the case that the titer was positive (continuous). Specifically, we modeled the probability that the saliva titer was positive as a logistic function of a fixed effect for site and a random effect for family-within-site, and for positive saliva titers, we used a linear model with a fixed effect for site and a random effect for family-within site. Different variances were considered for the random effects in each of the model components, as well as for the positive-saliva components by site. This mixture model was fitted with maximum likelihood using the nlmixed procedure in SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). Models selection used the likelihood ratio and Wald tests.
Results
Feeding success was low; of the ten families orally exposed to WNV from EP and IMD, seven families from each population had at least five fully engorged female Cx. restuans mosquitoes. In contrast all 4 control families had more than five fully engorged mosquitoes, which was not surprising since the Sebring Cx. quinquefasciatus colony had been in culture for several years and was well adapted to the Hemotek system used routinely in the insectary. The percentage of mosquitoes feeding in each family ranged 0 – 22% in IMD, 0 – 30% in EP, and 30% - 80% in the control Sebring strain.
In the analysis of body titers, shown in Fig. 2, we began using the full model with fixed effects for site and blood meal volume, along with a random effect for family allowing for different variances for family by site. The RLRT comparing the model with the same random effects variance for each site to that with different random effects variances by site resulted in a p-value = 0.97, indicating that only a common variance for the sites was needed. The RLRT of whether the random effects variance was positive resulted in a p-value < 0.001, indicating that this variance is indeed positive, so that the random effect for family provided an improved fit. All subsequent models therefore include a random effect for family with the same variance for each site.
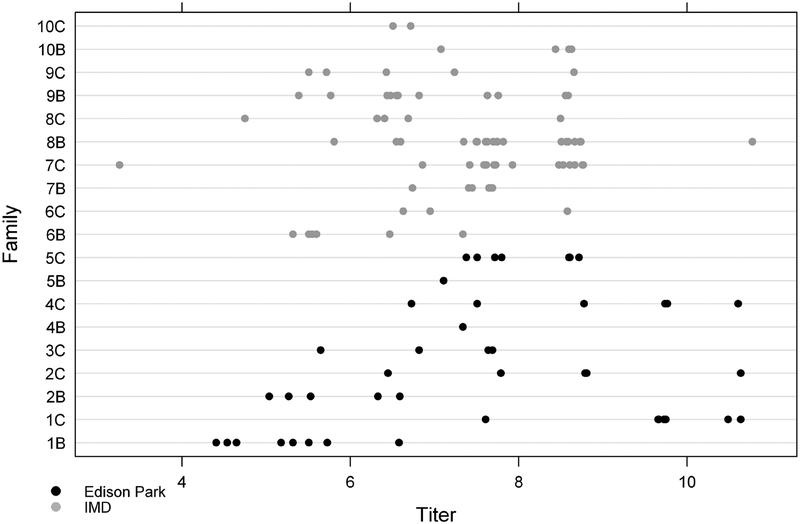
Figure 2.
WNV body titers for Cx. restuans families collected from Edison Park (EP) [dark print] and Illinois Medical District (IMD) [light print] in Chicago. The values at titer 0 have been randomly displaced to ease interpretation.
The LRT indicated no significant interaction between site and blood meal volume (p = 0.34), nor a significant difference among the three levels of blood meal volume (p = 0.65). Blood meal volume was therefore removed from the model. The estimated mean body titer for the IMD site was 7.19 (95% CI 6.50, 7.89) and for the EP site was 7.42 (95% CI 6.69, 8.15). The difference was thus estimated as −0.22 (95% CI −1.23, 0.78), indicating no statistically significant difference in body titers between the sites, in agreement with the LRT (p = 0.62). Using the reduced model with only an intercept, the family random effects variance was estimated using REML to be 0.89 (95% CI 0.42, 1.89), while the residual variance was estimated to be 1.04 (95% CI 0.84, 1.30); the ratio of these variances was thus estimated to be 0.85 (95% CI 0.39, 2.05). Finally, the within-family correlation was 0.46 (95% CI 0.28, 0.67), indicating reasonably strong tendency for titers within families to be similar. Residual analyses for the model fits indicated no significant departures from model assumptions.
The modeling for infection, dissemination and transmission rates followed a similar course, and the final models included a random effect for family, with the same variances by site. The individual rates by site (95% CIs) are reported in Table 1, along with the odds ratio comparing these by site. The likelihood ratio test comparing models for each of these measures with and without a fixed site effect showed no statistically significant difference in them by site (Infected, p=0.96; Disseminated, p=0.20; Transmitting, p=0.82). The results for the odds ratio comparisons, reported in Table 1, corroborate this conclusion.
Table 1.
Infection, dissemination and transmission rates of Culex restuans populations from Edison Park (EP) and Illinois Medical District (IMD) in Chicago, IL for West Nile.
| Population | No. Families tested | No. fed (range) | % infected (95% CI) | % disseminated (95% CI) | % transmitting (95% CI) |
|---|---|---|---|---|---|
| EP | 9 | 75 (5 – 14) | 96.9 (89.0 – 99.2) | 91.4 (81.0 – 96.4) | 42.5 (20.3 – 68.1) |
| IMD | 10 | 115 (2 – 26) | 99.1 (94.1 – 99.9) | 100.0 (85.8 – 100.0) | 40.3 (23.8 – 59.3) |
| Odds Ratio (IMD:EP) | 4.73 (0.42 – 52.81) | 3.10 (0.28 – 33.84) | 0.88 (0.25 – 3.12) |
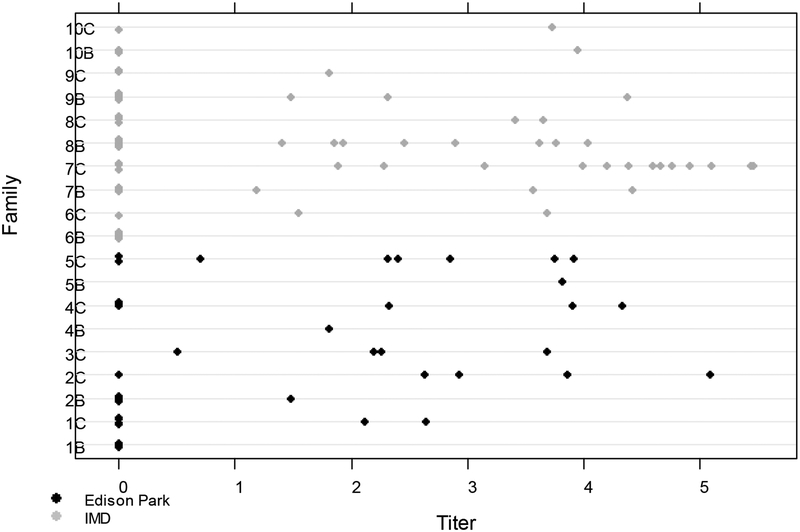
Analysis of saliva titers (Fig. 3) using the mixture distribution indicated that the family-within-site random effects between the 0 and continuous components shared the same variance (p = 0.18). Also, the variances in the normal, linear component did not differ by site (p = 0.65). Further, from the logistic model component, the probability that the titer was 0 did not depend on site (p = 0.75); similarly, from the positive, linear component, the mean titer did not depend on site (p = 0.13). Evaluation of the random effects variances indicated that they were indeed positive, thus echoing the analysis of the body titers of the existence of a relatively strong within-family correlation for the saliva titers.
Figure 3.
WNV saliva titers for Cx. restuans families collected from Edison Park (EP) (dark print) and Illinois Medical District (IMD) (light print) in Chicago.
Discussion
Although vector competence studies have been conducted for many mosquito species suspected of transmitting WNV, many of these studies have focused on establishing whether or not particular mosquito species and/or populations are competent or incompetent vectors of WNV. Although variations in vector competence have been observed among geographically distinct populations of mosquito species, few studies to date have attempted to relate vector competence to the ecology of WNV in the US. The studies by Reisen et al. (2008), reported temporal changes in WNV circulation intensity, but the circulation intensity was not positively correlated with the observed temporal variations in vector competence of the mosquito species involved. This suggested that vector competence was not a central factor to initiating increased circulation of WNV and/or to triggering WNV outbreaks. The data we present in this manuscript showed no observable variations in vector competence for WNV between the two populations of Cx. restuans collected from IMD and EP in Chicago, which suggests that differences in vector competence of Cx. restuans is not a contributing factor to the observed variations in WNV activity between these two sites (Table 1). We selected to investigate Cx. restuans because persistent Cx. restuans populations observed during 2002 and 2006 WNV outbreaks in east central Illinois (Lampman et al. 2006) were thought to play a role in initiating the WNV outbreaks. Further, Cx. restuans has repeatedly been demonstrated to be a more competent vector for WNV than the common epizootic vectors Cx. pipiens, Cx. quinquefasciatus, and Cx. nigripalpus (Sardelis et al. 2001, Ebel et al. 2005), and persistence of populations of a highly competent enzootic vector presumably leads to increased WNV circulation and ultimately WNV outbreaks.
When the body viral titers were compared between mosquitoes from IMD and EP (Fig. 2) the differences were not significant suggesting similar body viral burden in infected Cx. restuans females between the two populations. Similarly there were no significant differences in saliva viral titers between IMD and EP (Figure 3) suggesting that both populations were able to transmit WNV at the same rate. However, the body titers showed a reasonably strong tendency for siblings to have similar viral titers (Figure 2) suggesting that there is a genetic component of vector competence of Cx. restuans for WNV. Genetic control of mosquito susceptibility to pathogens has been known since the classical experiments by Huff (1929, 1931). Since then numerous studies have demonstrated genetic control of vector competence for various pathogens in different mosquito species, and linkage maps have been developed for some mosquito - pathogen systems; for a review see Beerntsen et al. (2000).
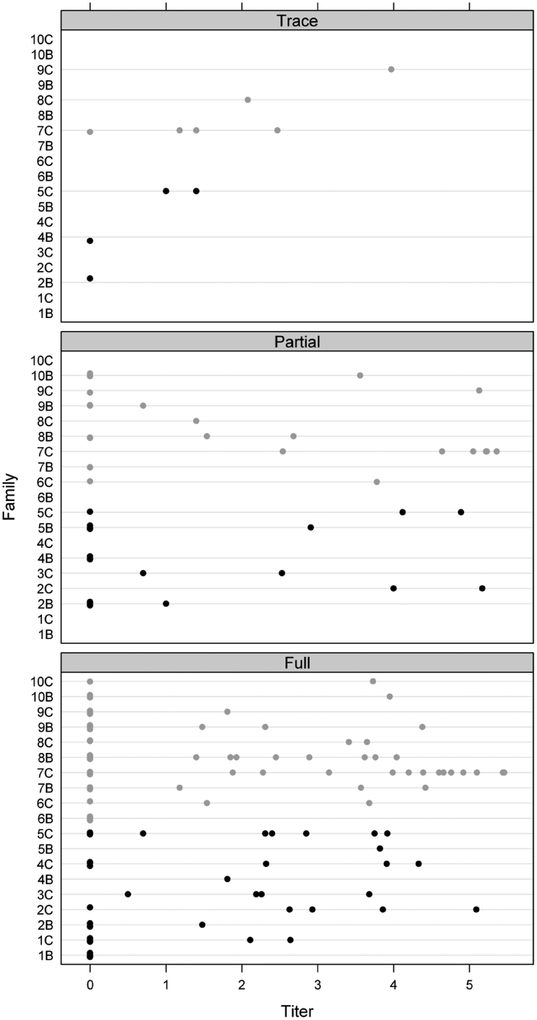
Lastly, our data showed no significant differences between body titers for WNV infected Cx. restuans females that were fully engorged, partially engorged and those that took only trace amounts of the blood infectious blood-meal (Fig. 4). This is very similar to the observations by Richards et al. (2007), who reported similar body titers for Cx. quinquefasciatus infected with different titers of WNV. Our observations suggest that mosquitoes taking small blood meals are as likely to become infected as those that feed to repletion. In contrast, earlier work by Mitchell et al. (1979) reported that 9.7% of the partially engorged Cx. pipiens were infected with St. Louis encephalitis virus, while in the same experiment 66.7% of the fully engorged were infected suggesting that the fully engorged are infected at a higher percentage. The epidemiologic importance of our observations is that erratic or interrupted feeding may increase the chances of Cx. restuans to become infected with WNV. The studies by Anderson and Brust (1995) estimated that up to 5% of Cx. restuans obtain multiple blood meals, which suggests that partial blood meals are not uncommon for Cx. restuans under field conditions. Further, Mitchell et al. (1979) pointed out that an infective partial or trace blood meal taken in late summer or fall followed by successful winter hibernation may lead to successful winter carryover. In some cases, the overwintering mosquitoes may be nulliparous because the amount of blood taken was not enough to trigger ovarian development (Mitchell et al. 1979). Clements (1999) summarized the information available on multiple blood-feeding among mosquito species and concluded that the overall importance of multiple blood feeding is complex and not yet very clear. However, by the time of his book in 1999, WNV was just recognized in the US and there were few studies on WNV in the US. There remains the possibility that multiple blood meals play a significant role in the epidemiology of WNV in the US.
Figure 4.
WNV body titers for fully, partially and trace engorged Cx. restuans collected from Chicago. The values at titer 0 have been randomly displaced to ease interpretation.
Acknowledgements
We thank Dr. Court and Claudia Blanco of the Chicago Department of Public Health (CDPH) for use of laboratory facilities. We thank Dan Markowski and Cristina Flores of VDCI and the Chicago WNV program interns for field assistance during this project. We thank Erin Borland and Andrea Peterson for help with larval rearing, and Marv Godsey and Harry Savage for laboratory assistance. This project was supported by funds from the Centers for Disease Control and Prevention (CDC).
References
- Anderson RA, and Brust RA. 1995. Field evidence for multiple host contacts during Blood feeding by Culex tarsalis, Cx. restuans, and Cx. nigripalpus. J. Med. Entomol 32: 705–710. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Anderson JF, and Vossbrinck CR. 2001. Mosquito surveillance for West Nile Virus in Connecticut, 2000: Isolation from Culex pipiens, Cx. restuans and Culiseta melanura. Journal of Emerging Infectious Diseases 7: 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadis TG, Anderson JF, Vossbrinck CR, and Main AJ. 2004. Epidemiology of West Nile virus in Connecticut: a five year analysis of mosquito data 1999–2003. Vector-borne Zoonotic Diseases 4: 360–378. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, and Armstrong PM. 2007. A two-year evaluation of elevated canopy Trapping for Culex mosquitoes and West Nile virus in an operational surveillance program in the northeastern United States. J. Am. Mosq. Control Assoc 23: 137–48. [DOI] [PubMed] [Google Scholar]
- Apperson CS, Hassan HK, Harrison BA, Savage HM, Aspen SE, Farajollahi A, Crans W, Daniels TJ, Falco RC, Benedict M, Anderson M, McMillen L, and Unnasch TR. 2004. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoonotic Dis 4: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerntsen BT, James AA, and B. M. 2000. Genetics of mosquito vector competence. Microbiol Mol Biol Rev 64: 115–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett KE, Olson KE, Munoz Mde L, Fernandez-Salas I, Farfan-Ale JA, Higgs S, Black WC, and Beaty BJ. 2002. Variation in vector competence for Dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. Am. J. Trop. Med. Hyg 67: 85–92. [DOI] [PubMed] [Google Scholar]
- Burdick RK, Borror CM, and Montgomery DC. 2005. Design and Analysis of Gague R&R Studies: Making Decisions with Confidence Intervals in Random and Mixed ANOVA Models, ASA-SIAM Series on Statistics and Applied Probability, SIAM, Philadelphia, ASA, Alexandria, VA. [Google Scholar]
- Clements AN 1999. The biology of Mosquitoes Volume 2, Sensory reception and behavior CABI Publishing. [Google Scholar]
- Colton L, and Nasci RS. 2006. Quantification of West Nile virus in the saliva of Culex species collected from the southern United States. J. Am. Mosq. Control Assoc 22: 57–63. [DOI] [PubMed] [Google Scholar]
- Condotta SA, Hunter FF, and Bidochka MJ. 2004. West Nile virus infection rates in pooled and individual mosquito samples. Vector-borne and Zoonotic Diseases 4: 198–203. [DOI] [PubMed] [Google Scholar]
- Covell CV, and Resh VH. 1971. Relative abundance of Culex pipiens and Culex restuans in catch basins in Jefferson County, Kentucky. Mosquito News 31: 73–76. [Google Scholar]
- Darsie RF Jr., and Ward RA. 2005. Identification and Geographical Distribution of The Mosquitoes of North America, North of Mexico Gainesville: University Press of Florida. [Google Scholar]
- Ebel G, Rochlin I, Longacker J, and Kramer L. 2005. Culex restuans (Diptera: Culicidae) relative abundance and vector competence for West Nile virus. Journal of Medical Entomology 43: 838–843. [DOI] [PubMed] [Google Scholar]
- Geery PR, and Holub RE. 1989. Seasonal abundance and control of Culex spp. In catch basins in Illinois. J. Am. Mosq. Control Assoc 5: 537–540. [PubMed] [Google Scholar]
- Grimstad PR, Craig GB Jr, Ross QE, and Yuill TM. 1977. Aedes triseriatus and La Crosse virus: geographic variation in vector susceptibility and ability to transmit. Am. J. Trop. Med. Hyg 26: 990–996. [DOI] [PubMed] [Google Scholar]
- Hadler J, Nelson R, McCarthy T, Andreadis T, Lis MJ, French R, Beckwith W, Mayo D, Archambault G, and Cartter M. 2001. West Nile virus surveillance in Connecticut in 2000: an intense epizootic without high risk for severe human disease. Emerg. Infect. Dis 7: 636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JL, Reeves WC, and Sjogren RD, 1976. Variations in the susceptibility of field and laboratory populations of Culex tarsalis to experimental infection with western equine encephalomyelitis virus. Am. J. Epidemiol 103: 498–505. [DOI] [PubMed] [Google Scholar]
- Huff CG 1929. The effects of selection upon susceptibility to bird malaria in Culex pipiens Linn. Ann. Trop. Med. Parasitol 23: 427–442. [Google Scholar]
- Huff CG 1931. The inheritance of natural immunity to Plasmodium cathemerium in two species of Culex. J. Prev. Med 5: 249–259. [Google Scholar]
- Kilpatrick AM, Kramer LD, Campbell SR, Alleyne EO, Dobson AP, Daszak P, 2005. West Nile Virus risk assessment and the bridge vector paradigm. Journal of Emerging Infectious Diseases 11: 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Fonseca DM, Ebel GD, Reddy MP, and Kramer LD. 2010. Spatial and temporal variation in vector competence of Culex pipiens and Culex restuans West Nile Virus risk assessment and the bridge vector paradigm. Am. J. Trop. Med. Hyg, 83: 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampman RL and Novak RJ. 1996. Oviposition Preferences of Culex pipiens and Culex restuans for infusion-baited traps. J. Am. Mosq. Control Assoc 12: 23–32. [PubMed] [Google Scholar]
- Lampman R, Slamecka M, Krasavin N, Kunkel K, and Novak R. 2006. Culex population dynamics and West Nile virus transmission in east-central Illinois. J. Am. Mosq. Control Assoc 22: 390–400. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, and Roehrig JT. 2000. Rapid detection of West Nile virus from human clinical specimens, field- collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol 38:.4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CJ, Bowen GS, Monath TP, Cropp CB, and Kerschner AJ. 1979. St. Louis encephalitis virus transmission following multiple feeding of Culex pipiens pipiens (Diptera: Culicidae) during a single gonotrophic cycle. J. Med. Entomol 16: 254–258. [DOI] [PubMed] [Google Scholar]
- Troxler S, Lalonde T, and Wilson JR, 2011. Exact logistic methods for nested binary data, Statistics in Medicine, published online 13 Jan 2011, DOI: 10.1002/sim.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilitt DR, and Jones JC. 1972. A qualitative method for estimating the degree of engorgement of Aedes aegypti adults. J. Med. Ent 9: 334–337. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, and Bates DM. 2000. Mixed-effects Models in S and S-PLUS, Springer-Verlag New York, Inc. [Google Scholar]
- R Development Core Team. 2010. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: ISBN 3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- Reisen WK, Barker CM, Fang Y, and Martinez VM. 2008. Does variation in Culex (Diptera: Culicidae) vector competence enable outbreaks of West Nile virus in California? J. Med. Entomol 45: 1126–1138. [DOI] [PubMed] [Google Scholar]
- Reiter P 1988. Weather, vector biology and arboviral recrudescence In, Monath TP (eds.), The Arboviruses; Epidemiology and Ecology American Public Health Association, Washington D.C. [Google Scholar]
- Richards SL, Mores CN, Lord CC, and Tabachnick WJ. 2007. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera; Culicidae) for West Nile virus. Vector Borne Zoonotic Dis 7: 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MO, Tedesco C, McTighe JT, Austin C, and Kitron U. 2004. Environmental and social determinants of human risk during a West Nile virus outbreak in the greater Chicago area, 2002. Int. J. Health Geogr 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MO, Walker ED, Foster ES, Haramis LD, and Kitron UD. 2007. Association of West Nile virus illness and urban landscapes in Chicago and Detroit. Int. J. Health Geogr 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage HM, Aggarwal D, Apperson CS, Katholi CR, Gordon E, Hassan HK, Anderson M, Charnetzky D, McMillen L, Unnasch EA, and Unnasch TR. 2007. Host choice and West Nile virus infection rates in blood-fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee, 2002–2003. Vector Borne Zoonotic Dis 7: 365–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, Dohm DJ, and O’Guinn ML. 2001. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg. Infect. Dis 7: 1018–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheipl F, Greven S, and Küchenhoff H. 2008. Size and power of tests for a zero random effect variance or polynomial regression in additive and linear mixed models, Computational Statistics and Data Analysis 52: 3283–3299. doi: 10.1016/j.csda.2007.10.022. [DOI] [Google Scholar]
- Tabachnick WJ, Wallis GP, Aitken TH, Miller BR, Amato GD, Lorenz L, Powell JR, and Beaty BJ. 1985. Oral infection of Aedes aegypti with yellow fever virus: geographic variation and genetic considerations. Am. J. Trop. Med. Hyg 34: 1219–1224. [DOI] [PubMed] [Google Scholar]
- Tesh RB, Gubler DJ, and Rosen L. 1976. Variation among geographic strains of Aedes albopictus in susceptibility to infection with chikungunya virus. Am. J. Trop. Med. Hyg 25: 326–335. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn M and Oliver J. 2000. Potential for New York mosquitoes to transmit West Nile virus. Am. J. Trop. Med. Hyg 2000. 62: 413–414. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Sardelis MR, Dohm DJ, and O’Guinn ML. 2001. Potential North American vectors of West Nile virus. Annals of the New York Academy of Sciences 951: 317–324. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan R, and Scott TW. 2007. Geographic variation in vector competence for West Nile virus in the Culex pipiens (Diptera: Culicidae) complex in California. Vector Borne Zoonotic Dis 7: 193–198. [DOI] [PubMed] [Google Scholar]